Abstract
Objective
Bladder cancer is the 9th cause of human urologic malignancy and the 13th of death worldwide. Increased collagen cross-linking, NIDOGEN1 expression and consequently stiffness of extracellular matrix (ECM) may be responsible for the mechanotransduction and regulation of transcriptional co-activator with PDZ-binding motif (TAZ) and transforming growth factor β1 (TGF-β1) signaling pathways, resulting in progression of tumorigenesis. The present study aimed to assess whether type 1 collagen expression is associated with TAZ nuclear localization.
Materials and Methods
In this case-control study, real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemical analysis were performed to evaluate the activation of the TAZ pathway in patients with bladder cancer (n=40) and healthy individuals (n=20). The ELISA method was also conducted to measure the serum concentrations of TGF-β1. Masson’s trichrome staining was carried out to histologically evaluate the density of type 1 collagen.
Results
Our findings that the expression levels of COL1A1, COL1A2, NIDOGEN1, TAZ, and TGF-β1 genes were overexpressed in patients with bladder cancer, and their expression levels were positively associated with the grade of bladder cancer. The immunohistochemical analysis demonstrated that the nuclear localization of TAZ was markedly correlated with high-grade bladder cancer. We also found that TAZ nuclear localization was substantially higher in cancerous tissues as compared with normal bladder tissues. Masson's trichrome staining showed that the tissue density of type I collagen was considerably increased in patients with bladder cancer as compared with healthy subjects.
Conclusion
According to our findings, it seems the alterations in the expression of type I collagen and NIDOGEN1, as well as TAZ nuclear localization influence the progression of bladder cancer. The significance of TGF-β1 and TAZ expression in tumorigenesis and progression to high-grade bladder cancer was also highlighted. However, a possible relationship between TGF-β1 expression and the Hippo pathway needs further investigations.
Keywords: Bladder Cancer, Cancer, Collagen Type 1, Signal Transduction, Transforming Growth Factor β1
Introduction
According to epidemiological studies, bladder cancer is ranked as the 9th leading cause of human urologic malignancy, and the 13th cause of death in the world (1). Despite efforts made in the development of cancer treatment methods, including surgery, chemotherapy, and radiotherapy, to date, there is no cure for advanced stages of bladder cancer, and the recurrence after transurethral resection (TR) is still the main concern in patients with bladder cancer who underwent surgery (2). Genome-wide association studies have shown an undeniable role of signal transduction pathways in the development of bladder tumorigenesis (3).
The extracellular matrix (ECM) is a combination of proteins with structural and functional roles, providing a natural scaffold for cells, tissues, and organs (4). Its proteins consisted of fibrillar proteins, such as elastic fibers and various types of collagens, non-fibrillar proteins such as glycosaminoglycans, NIDOGEN1, and proteoglycans (5). Recently, recent studies have focused on how the modulation of the ECM can be sensed by cancer cells (6). A major contributor to ECM-dependent mechanotransduction is the increased collagen cross-linking that enhances matrix stiffness. Moreover, it is well known that type I collagen is a main structural protein in the ECM, which is altered in tumorigenesis (7). It has been also reported that nidogens including NIDOGEN1 and 2 as a link protein interact with ECM various proteins such as collagens, and play a functional role in matrix strengthening and stiffening (8). The increased collagen cross-linking and subsequently, ECM stiffness is thought to be responsible for cancer cell growth and renewal (7). However, its precise signaling pathway has not been fully elucidated.
The Hippo pathway has emerged as a substantial signal transduction pathway that transduces signals to impact the proliferation and differentiation of cancer cells (9, 10). In bladder cancer, enhanced nuclear accumulation of transcriptional co-activator with PDZ-binding motif (TAZ) promotes the oncogenic activity of the cells and incites cell growth. Besides, the overexpression of nuclear TAZ increases the stemness of cancer cells (11, 12). The activity of TAZ in tumor initiation relies on binding to a transcription factor, named the TEAD family (11). Correspondingly, the complex of TAZ-TEAD is able to upregulate the expression of genes involved in tumorigenesis and cancer development (13). In the nucleus, TAZ also acts as a transcriptional factor to activate transforming growth factor β1 (TGF-β1) and regulate the SMAD transcription factor (14). TGF-β1 comprises a family of secreted proteins that promote the development of various types of cancer, such as bladder cancer (15, 16). Recent findings suggested that mechanical stimuli caused by ECM stiffness are required for the activation of TGF-β1 to make this protein soluble (17). TGF-β1 can exert pro-oncogenic activity through the regulation of the nuclear localization of the smad2/3-smad4 complex. The cross-talk between TGF-β1 and the Hippo pathway has been addressed in previous studies (18). Nuclear TAZ dictates the nuclear localization of SMADs and acts as a nuclear retention factor for this family of proteins to regulate their oncogenic activity (19, 14). However, whether a link between the Hippo and TGF-β1/SMAD pathways plays a pivotal role in bladder cancer development needs further investigations (20). In this study, we have examined the association of type I collagen and TAZ activity with the degree of bladder cancer malignancy.
Materials and Methods
Patients and study design
In this case-control study, TR of the bladder tissue was obtained from 40 patients with bladder malignancy as well as 20 patients with benign prostatic hyperplasia (BPH) with normal bladder function from Shahid Beheshti Hospital of the Hamadan University of Medical Sciences (Iran) between 2018 and 2019. All cancerous bladder tissues were examined by a trained and expert pathologist to rank the degree of malignancy (cases) and identification of normal bladder tissues (control) according to the relevant guidelines and criteria for grading and diagnosis (21). Fresh frozen tissues were used to analyze the gene expression using real-time polymerase chain reaction (PCR). The paraffin-embedded tissue blocks were applied for immunohistochemistry (IHC) and Masson’s trichrome staining. Blood samples were also collected, and their serum samples were separated to determine the serum levels of TGF-β1 in both cases and control individuals.
Real-time polymerase chain reaction
For the relative estimation of mRNA expression, total RNA was extracted from fresh frozen in liquid nitrogen tissue samples using the AccuZol Total RNA Extraction Kit (Bioneer, Korea), and then cDNA was synthesized based on the manufacturer’s recommendations using a commercially available kit (Fermentas, Thermo Fisher Scientific, Waltham, USA). The real-time PCR was carried out using RealQ Plus 2×Master Mix Green (Amplicon, Denmark) on the LightCycler® 96 Instrument (Roche, Life Science, Sandhofer, Germany). Briefly, PCR reactions were conducted at 20 µl as final volume, containing SYBR Green master mix (10 µl), 1 µl cDNA, and 2 µl of specific primers, and 7 µl ddH2 O. The PCR reactions were set at the following program: 95°C (5 minutes), followed by 40 cycles at 95°C (30 seconds), 57°C (30 seconds), and 72°C (40 seconds). To analyze the relative gene expression data for mRNA, we used the Livak-Schmittgen (22) equation to calculate the 2−ΔΔCT, which compares two values in the exponent, then the obtained data were used for further analysis of gene expression levels. Specific primers were designed using the Primer-BLAST tool available from the NCBI for target genes sequences in the GenBank databases (www.ncbi.nlm.nih.gov). The primer sets (forward and reverse) for real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) are presented in supplementary Table 1.
Immunohistochemical analysis
The immunohistochemical analysis was performed on the tissues section at a thickness of 4 μm to determine the subcellular localization of TAZ using a specific monoclonal antibody. Briefly, after deparaffinization and rehydration of tissue sections, the antigen retrieval process was carried out via boiling the tissue sections in citrate buffer at pH=6.00 for 15 minutes and followed by the cool-down process at room temperature. Then, the endogenous peroxidase was blocked using hydrogen peroxide. After incubation with a primary specific Anti-TAZ antibody (ab110239, at a dilution of 1:200) overnight at 4°C, prepared sections were incubated with an appropriate secondary antibody available in the immunoperoxidase staining commercial kit (PVP1000D, Mouse/Rabbit PolyVue Plus™ HRP/DAB Detection System, Diagnostic BioSystems) to track the localization of the TAZ protein. Tumor cells with more than 20% positivity for nuclear localization of TAZ were considered positive samples. For the analysis of cytoplasmic TAZ expression, a semi-quantitative ranking method was utilized to rank the specimens from 0 to 3. Tissue samples with scores between 2 and 3 were considered positive in terms of the cytoplasmic expression of TAZ.
Measurement of the serum level of TGF-β1
The serum levels of TGF-β1 were measured by the enzyme-linked immunosorbent assay (ELISA) method carried out using a commercially available kit (Quantikine® ELISA, R&D Systems, DB100B) based on the manufacturer provided protocol.
Masson’s trichrome staining
For the determination of collagen density, bladder tissue samples were fixed in 10% formalin and paraffin-embedded. Then, tissue samples were cut into a section with 5 µm thickness, and MT staining was performed to evaluate the collagen density using light microscopy. Briefly, after washing the tissue sections in distilled water, sections were re-fixed in the Bouin’s solution for 60 minutes. After rinsing in distilled water, sections were incubated in Weigert’s iron hematoxylin (10 minutes). After staining with the Biebrich scarlet-acid fuchsin solution (15 minutes), the phosphomolybdic-phosphotungstic acid solution was used to differentiate the tissue sections for 15 minutes. The differentiated tissues were directly stained with aniline blue and then washed using distilled water. Finally, tissue slides were differentiated in 1% acetic acid solution (4 minutes). After dehydration, tissue slides were cleared and mounted on slides using the mounting media.
Statistical analysis
The analysis of collected data was analyzed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to determine whether the data were normally distributed. t test was performed to analyses the difference between the mean of the obtained data with normal distribution. To analyses, the data with non-normal distribution, the Mann-Whitney U test was applied to compare the difference between the values of experimental groups. The correlation between different variables was calculated by Spearman’s and Pearson’s correlation coefficient tests. The χ2 test was also applied for categorical data. The P<0.05 was considered as a statistical significance difference.
Ethical considerations
All procedures carried out in this study involving human participants were in accordance with the Ethical standards of the Institutional and/or National Research Committee of Baqiyatallah University of Medical Sciences (IR. BMSU.REC.1397.120). Informed consent was obtained from all participants involved in the study.
Results
Characteristics of participants
The demographic characteristics of participants are presented in Table 1. All of the participants were Iranian, and the median age was 67 years (45-86), of which 45-55% of them were over 67 years old. Nearly 7% of patients were smokers, and 30% were non-smokers. Approximately 62.5% of patients had low-grade bladder cancer, while 37.5% has high-grade bladder cancer. In addition, the size of the bladder tumor was determined in patients. About 60% of patients had a tumor size of less than 2cm, while 40% had a tumor size of larger than 2 cm.
Table 1.
Clinic-pathological parameters of bladder cancer patients (n=40)
|
| ||
|---|---|---|
| Characteristics | Cases (%) | |
|
| ||
| Age (Y) | ||
| ≦67a | 18 (45.0) | |
| >67 | 22 (55.0) | |
| Smoking | ||
| Yes | 28 (70.0) | |
| No | 12 (30.0) | |
| Tumor size (cm) | ||
| ≦2.0a | 24 (60.0) | |
| >2.0 | 16 (40.0) | |
| Histologic grade | ||
| Low grade | 25 (62.5) | |
| High grade | 15 (37.5) | |
| TAZ expression | ||
| Nuclear positive | 29 (72.5) | |
| Cytoplasmic positive | 17 (42.5) | |
|
| ||
a;Median
The overexpression of type I collagen in bladder cancer
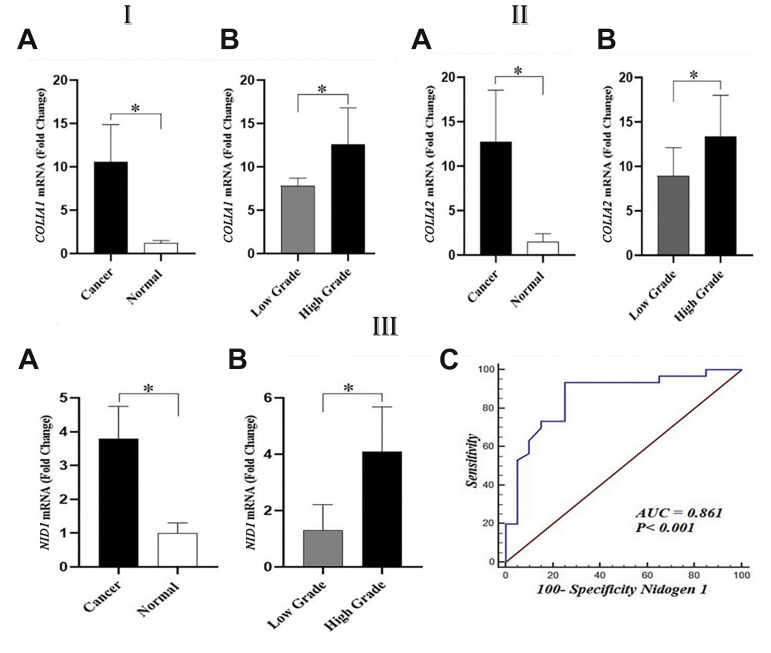
The expression of type 1 collagen was analyzed in bladder cancer and normal bladder tissues. COL1A1 and COL1A2 genes were evaluated in cancerous bladder tissue samples and normal bladder tissues collected from BPH patients. Our findings revealed that both the expression levels of COL1A1 (P<0.001) and COL1A2 (P<0.001) genes were significantly higher in bladder cancer than in normal bladder tissues. Further analyses revealed that COL1A1 and COL1A2 were positively associated with high-grade bladder cancer. These two genes were assessed in various grades of bladder cancer to investigate their association with the grade of bladder cancer. Our results demonstrated that patients with high-grade bladder cancer had dramatically higher levels of COL1A1 (P=0.021) and COL1A2 (P=0.036) expression than those with low-grade bladder cancer, indicating a positive relationship between the grading of bladder cancer with the expression rate of type I collagen (Fig .1I, II).
Fig.1.
Gene expression of collagen type 1 and NIDOGEN1 (NID1) in bladder cancer (25 low grade and 15 high grade) and normal bladder tissue (n=20). IA. COL1A1 gene expression was lower in bladder normal than cancerous tissues. IB. COL1A1 gene expression analysis showed markedly higher in high-grade bladder cancer as compared to low-grade bladder cancer tissues. IIA. COL1A2 gene expression was significantly higher in bladder cancerous tissues that than normal bladder tissues. IIB. COL1A2 gene expression was markedly higher in high-grade bladder cancer as compared to low-grade bladder cancer tissues. IIIA. NID1 gene expression was significantly higher in cancerous tissues than that bladder normal tissues. IIIB. NID1 gene expression in low- and high-grade bladder cancer tissues revealed that high-grade bladder cancer had significantly higher NID1 gene expression as compared to the low-grade. IIIC. ROC of NID1 mRNA level in normal and cancerous bladder tissues showed that the NID1 expression might be a possible tumor marker for bladder cancer. ROC; Receiver operating characteristic, AUC; Area under the curve, *; P<0.05 in all comparisons.
Expression of NIDOGEN1 in human bladder cancer
For the assessment of the role of NIDOGEN1 in tumorigenesis and tumor progression, the gene expression of NIDOGEN1 was analyzed in bladder cancerous and normal bladder tissue samples using qRT-PCR. Our findings showed that NIDOGEN1 expression was significantly (P<0.05) increased in bladder cancerous tissue when compared with normal bladder tissues (Fig .1IIIA). The correlation of NIDOGEN1 expression with the grading of bladder cancer tissue was also studied and showed no significant (P=0.061) association between the expression of this gene and the grades of bladder cancer (Fig .1IIIB). Additionally, receiver operating characteristic (ROC) curve analysis was showed that the expression levels of the NIDOGEN1 gene expression might be a possible tumor marker for bladder cancer. Our findings demonstrated that the area under the curve (AUC) for NIDOGEN1 between bladder cancerous and normal bladder tissue was 0.861 (95% Confidence interval, 0.734- 0.942) when 40 cancerous bladder tissues and 20 normal bladder tissue were compared (Fig .1IIIC).
The correlation of high-grade bladder cancer with collagen density
MT staining was performed to examine the collagen density in various bladder tissues and then semi-quantified using ImageJ software. Images obtained from Masson’s trichrome staining are displayed in Figure 2. The results indicated that cancerous bladder tissues had significantly (P<0.0001) a higher rate of collagen density in comparison with normal bladder tissues (Fig .2D). The collagen density was also evaluated in both grades (low and high) bladder cancer groups. Interestingly, our findings demonstrated that bladder cancer tissues with high-grade properties had significantly (P<0.0001) a higher degree of collagen density in compression with low-grade bladder cancerous tissues (P<0.001, Fig .2E).
Fig.2.
Masson’s trichrome (MT) staining for collagen density in different groups (scale bar: 60 µm). A. Representative image for normal bladder tissue. B. Representative image for low-grade bladder cancer; less collagen bundles had grown into the bladder tissue (arrow). C. High-grade bladder cancer, many collagen bundles had grown into the bladder tissue (arrows). D. Collagen density in bladder normal and cancerous tissues. E. Collagen density in low- and high-grade bladder cancer tissues. *; P<0.05 in all comparisons.
TAZ expression in bladder cancer and normal bladder tissue
As shown in Figure 3IA, the TAZ mRNA expression was statistically (P=0.038) higher in bladder cancerous tissues than that in normal bladder tissue samples. Moreover, TAZ expression has a significant correlation with high-grade bladder cancer. The results also revealed that the gene expression of TAZ was considerably (P=0.46) elevated in high-grade tumors in comparison with low-grade cancerous tissues (Fig .3IB). For the assessment of the relationship between TAZ expression and collagen density, the correlation between TAZ expression and the mRNA expression of COL1A1 and COL1A2 genes was analyzed. As expected, a positive correlation was found between TAZ expression and collagen density. Our results demonstrated that the mRNA expression of TAZ was significantly correlated with expression levels of COL1A1 (Fig .3IC) and COL1A2 (Fig .3ID) genes.
Fig.3.
TAZ gene expression and Immunohistochemistry staining in bladder cancer (25 low-grade and 15 high-grade) and normal bladder tissues (n=20). IA. TAZ gene expression was considerably higher in bladder cancer as compared to the normal bladder tissues. IB. High-grade bladder cancer showed a markedly higher TAZ gene expression than that low-grade bladder cancer tissues. TAZ mRNA expression showed a positive correlation between and IC. COL1A1 and ID. COL1A2 mRNA levels. II. Representative images from IHC staining of YAP (x720); the nuclear TAZ expression (blue arrow), cytoplasmic TAZ expression (red arrow) and non-nuclear TAZ expression (black arrow). *; P<0.05 in all comparisons.
The relationship between TAZ nuclear localization and high-grade bladder cancer
The immunohistochemical analysis using a specific antibody showed the nuclear and cytoplasmic localization of the TAZ protein in human bladder cancerous and normal tissues. Images obtained from IHC staining are depicted in Figure 3II. Among bladder cancerous tissue samples, 72.5% (29/40) and 42.5% (17/40) of bladder cancerous tissues were positive for nuclear and cytoplasmic localization of TAZ, respectively. Moreover, the results showed that 20% (4/20) and 0% of normal bladder tissues were positive for nuclear and cytoplasmic localization of TAZ. The correlation of the subcellular localization of the TAZ protein with the grade of bladder cancer was assessed by the immunohistochemical analysis. Our findings demonstrated that TAZ nuclear localization was positively associated with high-grade bladder tumors. Notably, the results demonstrated that all of the cancerous tissues with high-grade tumors were positive for nuclear localization of TAZ; however, that cytoplasmic localization of TAZ was not statistically correlated with the grades of bladder cancer (Table 2).
Table 2.
Association between histologic grade and clinic-pathological characteristics of patients with bladder cancer
|
| ||||
|---|---|---|---|---|
| Characteristics | Histologic grade | P valuea | ||
| Low (%) | High (%) | |||
|
| ||||
| Age (Y) | 0.412 | |||
| ≦67b | 10 (40.0) | 8 (53.3) | ||
| >67 | 15 (60.0) | 7 (46.7) | ||
| Smoking | 0.722 | |||
| Yes | 17 (68.0) | 11 (73.3) | ||
| No | 8 (32.0) | 4 (26.7) | ||
| Tumor size (cm) | 0.008 | |||
| ≦2.0b | 19 (76.0) | 5 (33.3) | ||
| >2.0 | 3 (24.0) | 10 (66.7) | ||
| Nuclear TAZ | ||||
| Positive | 14 (56.0) | 15 (100.0) | 0.003 | |
| Negative | 11 (44.0) | 0 (0.0) | ||
| Cytoplasmic TAZ | ||||
| Positive | 11 (44.0) | 6 (40.0) | 0.804 | |
| Negative | 14 (56.0) | 9 (60.0) | ||
|
| ||||
a;Chi-square test and b ; Median.
The association of TGF-β1 expression with the development of bladder cancer and its grading
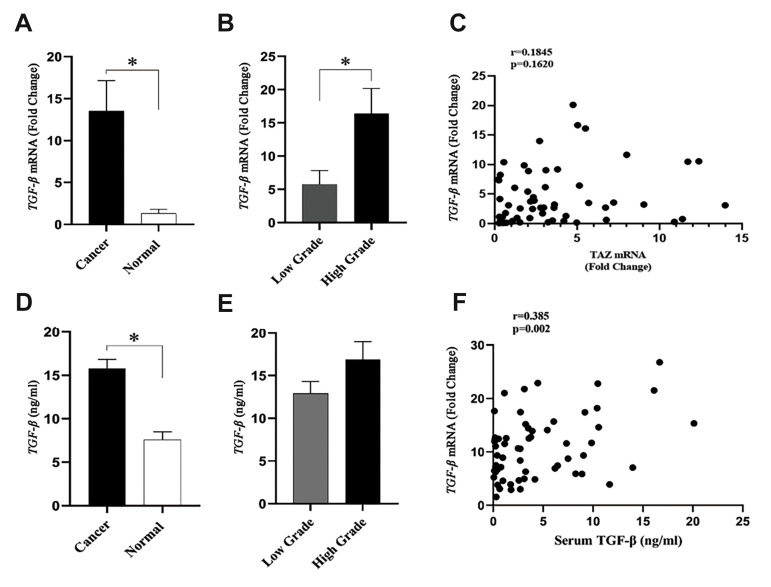
The gene expression of TGF-β1, at the level of the gene and protein, was analyzed in normal and cancerous bladder tissues to examine whether TGF-β1 expression is linked to tumor development. As shown in Figure 4, the results indicated that the TGF-β1 gene was significantly (P<0.001) higher in bladder cancerous tissues in comparison to normal bladder tissue samples. Cancerous tissues with high-grade bladder cancer had a significantly (P<0.05) higher rate of TGF-β1 expression (three times) in comparison with those with low-grade bladder cancer. The change in the gene expression of TGF-β1 was confirmed by the analysis of the protein expression of this gene. Patients with normal bladder tissues had a significantly (P=0.039) lower degree of TGF-β1 expression compared with those with bladder cancer. The results demonstrated that the serum level of TGF-β1 was higher in high-grade bladder cancer patients as compared with low-grade bladder cancer. However, such a difference in the serum level of TGF-β1 was not statistically (P>0.05) significant between high and low-grade bladder cancer. The serum level of TGF-β1 was positively correlated with the rate of the gene expression of TGF-β1 (Fig .4F). The results demonstrated that there was no considerable association between the gene expression of TGF-β1 and TAZ proteins (Fig .4C).
Fig.4.
TGF-β gene and protein expression in bladder cancer (25 low-grade and 15 high-grade) and normal bladder tissues (n=20). A. TGF-β gene expression was significantly increased in bladder cancer as compared to the normal bladder tissues. B. High-grade bladder cancer showed a higher TGF-β gene expression than that low-grade bladder cancer tissues. C. TGF-β gene expression was positively correlated with TAZ mRNA expression level. D. Serum TGF-β level was considerably elevated in patients with bladder cancer as compared to the healthy subjects. E. Serum TGF-β levels was not statistically different between patients with low- and high-grade bladder cancer. F. There was a positive correlation between TGF-β mRNA level and serum TGF-β levels. *; P<0.05 in all comparisons.
Discussion
Bladder cancer is a commonly distributed cancer that affects the urinary tract. According to the grading system of the world health organization (WHO), bladder cancer is histopathologically classified into three grades as follows: papillary urothelial neoplasms of low malignant potential (PUNLMP), low-grade, and high-grade urinary tumors (23).
The alterations in basal membrane and ECM components play a crucial role in the progression of bladder cancer and its prognosis. A large number of studies have reported that the modulation and increased components of the ECM may participate in the bladder tumor development and cancer cell growth, while the precise molecular pathway underlying these events has not been fully understood. Recently, it has been revealed that type I collagen is one of the main components of the ECM microenvironment, which is associated with epithelial tumorigenesis (7).
Herein, we attempted to analyze the expression of type 1 collagen and its relationship with bladder tumorigenesis. Our results demonstrated that COL1A1 and COL1A2 genes were highly expressed in bladder cancerous tissue compared to normal bladder tissues, and their expression levels were associated with bladder cancer with high-grade properties. Masson’s trichrome staining showed the increased expression of collagen in bladder cancerous tissue as compared with normal bladder tissues. In line with the results of Masson’s trichrome staining, tissues with high-grade bladder cancer had a higher rate of the collagen density in compression with bladder cancer tissues with low-grade properties. In agreement with our study, other similar studies reported the same expression of type I collagen in various cancer types, including colon (24) and bladder cancer (7). Also, increased collagen cross-linking and elevated components have been previously reported to be positively associated with cancer progression (6, 25). Due to the importance of type I collagen in ECM components, these results implicate a role for tumor microenvironment in the development of bladder cancer. Moreover, we demonstrated that NIDOGEN1, as an essential part of the ECM, is overexpressed in bladder cancer.
Other studies showed a positive relationship between the gene expression of NIDOGEN1 and the grades of bladder cancer. Parallel with an increase in the gene expression of type 1 collagen, NIDOGEN1 was markedly expressed in high-grade cancerous bladder tissues when compared with low-grade bladder tumor samples. One possible mechanism for such a direct relationship is that the ECM is overexpressed in cancerous bladder tissue, especially in high-grade bladder tumor. The excessive amounts of the ECM at the proximity of cancer cells increase the stiffness of the ECM and contribute to cancer progression (26). Besides, the stiffened ECM interacts with cell surface receptors, such as integrins, which convert mechanical force into biochemical signaling pathways, such as the Hippo pathway and, as a consequence, stimulates the proliferation and growth of cancer cells (27). The ROC curve analysis revealed that NIDOGEN1 expression could be a possible tumor marker for bladder tumor.
Given the significance of the modulation of the ECM in cancer development, changes in the composition of the ECM may influence the activity of multiple signaling pathways, such as the Hippo pathway (28). The Hippo pathway, as a critical regulator of cancer development, affects cell fate through its central components, YAP/ TAZ, and nuclear localization (29). We showed that the gene expression of TAZ is upregulated in bladder cancer. We also investigated the association between the type I collagen expression and subcellular localization of TAZ to determine whether there is a possible link between the modulation of the ECM and TAZ expression. Elevated expression of type I collagen was positively associated with TAZ expression.
It has been shown that cancer cells can sense external mechanical cues generated by ECM components through several contact points, including integrin-based cell-matrix (focal) adhesions, stretch-modulated ion channels, and cell-cell junctions (30). As previously reported, increased collagen cross-linking, and substrate rigidity can delicately control the subcellular localization of TAZ. In line with the present study, recent findings showed that TAZ is transported into the cytoplasm when the cells grow in a soft matrix, whereas the stiffened matrix led to nuclear localization of TAZ and stimulates the expression of some genes involved in cell division and progression (31).
In addition to regulating the cytoplasmic/nuclear localization of TAZ, ECM modulation, and its rigidity can also control the gene expression of TAZ in cancer cells. In the current research, it was shown that TAZ expression was positively correlated with the expression of the main proteins of the ECM in bladder cancer, such as type I collagen. Also, our findings demonstrated that TAZ gene expression was positively associated with tumor grading, and patients with higher grades of bladder cancer had a higher rate of TAZ expression compared to low-grade bladder cancer. It is worth mentioning that the relationship between TAZ activity and higher tumor grading scores in various types of cancer has previously been reported in several studies (32). It has been reported that in the case of TAZ overexpression, this protein is localized to the nucleus and interacts with TEAD to induce high-grade tumors (33).
Interestingly, our findings revealed that most patients with high-grade bladder cancer were positive for TAZ nuclear localization, and there was a positive relationship between TAZ nuclear localization and tumor grading. In agreement with our findings, Xie et al. showed that YAP/ TAZ activity in the nucleus promotes the expression of oncogenic factors and contributes to cancer progression (34). Recently, it has been reported that TAZ activity is necessary for the transduction of tumorigenic phenotypes induced by TGF-β1 in cancer cells (11).
TGF-β1 plays an intricate role in cancer development, and it also can suppress the oncogenic factors at the early stages of tumor development; however, it is also capable of enhancing the late stages of tumorigenesis (35). Moreover, TGF-β1 promotes the production of ECM in cancerous tissues (18). In this study, a significant elevation for the expression of the TGF-β1 gene and protein e in bladder cancer tissue were observed as compared with normal bladder tissues. As expected, in patients with high-grade bladder tumors, the expression of TGF-β1 was markedly higher than those with low-grade tumors. Collectively, these findings suggest that TGF-β1 could play a significant role in cancer progression. In line with the present study, similar results were reported in previous investigations performed on bladder cancer (36- 39). A possible mechanism by which TGF-β1 promotes cancer progression is the activation of SMADs and their accumulation in the nucleus to cooperate with TAZ (11). It is well-known that activated TAZ increases the expression of genes involved in tumorigenesis (40). However, our findings revealed no significant association between the expression of TAZ and TGF-β1, and further studies with a large sample size would be needed to clarify the relationship between these two proteins and their impact on the progression of tumors.
Conclusion
Our study showed the significance of the alterations of ECM components and TAZ nuclear localization and their correlation with tumor grading. We also highlighted the importance of the expression of type 1 collagen, as well as the expression of TGF-β1 and TAZ, and also subcellular localization of TAZ in bladder cancer progression. However, further studies are needed to clarify the possible relationship between TGF-β1 and the Hippo pathway.
Acknowledgements
This work was supported by the Baqiyatallah University of Medical Sciences (Grant No: 97000066). The authors would like to Acknowledge the Hamadan University of Medical Sciences. There are no conflicts of interest.
Authors’ Contributions
H.Gh., H.M.H., S.A.M.; Participated in study design, drafting, and data statistical analysis. H.Gh., M.H; Contributed to all experimental analyses of the study. S.H.M.B.; Performed bladder cancer patients selection and bladder tissue sample collection. All authors read and approved the final manuscript.
References
- 1.Stojnev S, Krstić M, Čukuranović Kokoris J, Conić I, Petković I, Ilić S, et al. Prognostic impact of canonical TGF-β signaling in urothelial bladder cancer. Medicina (Kaunas) 2019;55(6):302–302. doi: 10.3390/medicina55060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell CM, Lebastchi AH, Borza T, Spratt DE, Morgan TM. The role of transurethral resection in trimodal therapy for muscle-invasive bladder cancer. Bladder Cancer. 2016;2(4):381–394. doi: 10.3233/BLC-160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartsch G, Mitra AP, Cote RJ. Expression profiling for bladder cancer: strategies to uncover prognostic factors. Expert Rev Anticancer Ther. 2010;10(12):1945–1954. doi: 10.1586/era.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagheban EM, Rezazadeh M, Altarihi T, Kazemi S. ultrastructure of huamn endometrial epitelial cells cultured on extracellular matrix and plastic. Cell J. 2003;5(19):145–153. [Google Scholar]
- 5.Aitken KJ, Bägli DJ. The bladder extracellular matrix.Part I: architecture, development and disease. Nat Rev Urol. 2009;6(11):596–611. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 6.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38–38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks M, Mo Q, Krasnow R, Ho PL, Lee YC, Xiao J, et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget. 2016;7(50):82609–82619. doi: 10.18632/oncotarget.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav VK, Lee TY, Hsu JBK, Huang HD, Yang WCV, Chang TH. Computational analysis for identification of the extracellular matrix molecules involved in endometrial cancer progression. PLoS One. 2020;15(4):e0231594–e0231594. doi: 10.1371/journal.pone.0231594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad T, Nouri K, van Rensburg HJJ, Maritan SM, Wu L, Hao Y, et al. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene. 2020;39(2):334–355. doi: 10.1038/s41388-019-0988-y. [DOI] [PubMed] [Google Scholar]
- 10.Xia J, Zeng M, Zhu H, Chen X, Weng Z, Li S. Emerging role of Hippo signalling pathway in bladder cancer. J Cell Mol Med. 2018;22(1):4–15. doi: 10.1111/jcmm.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289(19):13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouri K, Azad T, Ling M, van Rensburg HJJ, Pipchuk A, Shen H, et al. Identification of celastrol as a novel YAP-TEAD Inhibitor for cancer therapy by high throughput screening with ultrasensitive YAP/ TAZ-TEAD biosensors. Cancers (Basel) 2019;11(10):1596–1614. doi: 10.3390/cancers11101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad T, Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun. 2018;9(1):1061–1076. doi: 10.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The crumbs complex couples cell density sensing to hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Hung TT, Wang H, Kingsley EA, Risbridger GP, Russell PJ. Molecular profiling of bladder cancer: involvement of the TGF-β pathway in bladder cancer progression. Cancer Lett. 2008;265(1):27–38. doi: 10.1016/j.canlet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Huang R, Li H, Wang B, Chen Y, Chen S, et al. Secreted TGF-beta-induced protein promotes aggressive progression in bladder cancer cells. Cancer Manag Res. 2019;11:6995–7006. doi: 10.2147/CMAR.S208984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinz B. Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11(2):120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 18.Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y, et al. TGF-β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209(3):479–494. doi: 10.1084/jem.20111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khajehahmadi Z, Mohagheghi S, Nikeghbalian S, Geramizadeh B, Khodadadi I, Karimi J, et al. Downregulation of hedgehog ligands in human simple steatosis may protect against nonalcoholic steatohepatitis: Is TAZ a crucial regulator? IUBMB Life. 2019;71(9):1382–1390. doi: 10.1002/iub.2068. [DOI] [PubMed] [Google Scholar]
- 20.Mohseni R, Arab Sadeghabadi Z, Karimi J, Gholami H, Ghasemi H, Ghadimipour HR, et al. Chlorella vulgaris supplementation attenuates the progression of liver fibrosis through targeting TGF-β- signaling pathway in the CCl4-induced liver fibrosis in rats. Toxin Rev. 2019:1–9.
- 21.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Colombel M, Soloway M, Akaza H, Böhle A, Palou J, Buckley R, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol. 2008;7(10):618–626. [Google Scholar]
- 24.Vellinga TT, den Uil S, Rinkes IH, Marvin D, Ponsioen B, Alvarez- Varela A, et al. Collagen-rich stroma in aggressive colon tumors induces mesenchymal gene expression and tumor cell invasion. Oncogene. 2016;35(40):5263–5271. doi: 10.1038/onc.2016.60. [DOI] [PubMed] [Google Scholar]
- 25.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11–11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seewaldt V. ECM stiffness paves the way for tumor cells. Nat Med. 2014;20(4):332–333. doi: 10.1038/nm.3523. [DOI] [PubMed] [Google Scholar]
- 27.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemi H, Mousavibahar SH, Hashemnia M, Karimi J, Khodadadi I, Mirzaei F, et al. Tissue stiffness contributes to YAP activation in bladder cancer patients undergoing transurethral resection. Ann N Y Acad Sci. 2020;1473(1):48–61. doi: 10.1111/nyas.14358. [DOI] [PubMed] [Google Scholar]
- 29.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588(16):2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 30.HicksBerthet J, Varelas X. Integrin-FAK-CDC42-PP1A signaling gnaws at YAP/TAZ activity to control incisor stem cells. Bioessays. 2017;39(10):1–16. doi: 10.1002/bies.201700116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 32.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cellrelated traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Skinner M. Cancer stem cells: TAZ takes centre stage. Nat Rev Cancer. 2012;12(2):82–83. doi: 10.1038/nrc3210. [DOI] [PubMed] [Google Scholar]
- 34.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61(24):8917–8923. [PubMed] [Google Scholar]
- 35.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, et al. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112(7):1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Azayzih A, Gao F, Goc A, Somanath PR. TGFβ1 induces apoptosis in invasive prostate cancer and bladder cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated activation of caspases. Biochem Biophys Res Commun. 2012;427(1):165–170. doi: 10.1016/j.bbrc.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20(6):1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Hau AM, Al-Ahmadie HA, Harwalkar J, Shoskes AC, Elson P, et al. Transforming growth factor-β is an upstream regulator of mammalian target of rapamycin complex 2-dependent bladder cancer cell migration and invasion. Am J Pathol. 2016;186(5):1351–1360. doi: 10.1016/j.ajpath.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei H, Kamat AM, Aldousari S, Ye Y, Huang M, Dinney CP, et al. Genetic variations in the transforming growth factor beta pathway as predictors of bladder cancer risk. PLoS One. 2012;7(12):e51758–e51758. doi: 10.1371/journal.pone.0051758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19(18):4925–4930. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]