Abstract
Objective
Whereas prostate cancer (PrCa) may be unresponsive or moderately responsive to radiation therapy (RT)- most common modality for treatment of PrCa- patients must receive a high dose of RT In order to achieve appropriate tumour control. However, this increase in radiation dose may lead to severe adverse effects in normal tissues. Sensitization of PrCa to radiation provides an alternate approach to improve the therapeutic efficacy of RT. This study aims to assess the radiosensitisation effect of apigenin (Api) on a prostate cancer cell line (LNCaP).
Materials and Methods
In this experimental study, LNCaP cells were treated with 0-80 µM Api to investigate its effect on LNCaP cell viability and determine its half-maximal inhibitory concentration (IC50). Next, the cells were divided into four groups: i. Control, ii. Cells treated with the IC50 concentration of Api, iii. Cells treated with 2 Gy ionizing radiation (IR), and cells co-treated with Api and IR. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, real-time polymerase chain reaction (PCR), and an Annexin V-FITC/PI assay were performed to assess cell survival, Bax and Bcl-2 expressions, and presence of apoptosis and necrosis.
Results
Api inhibited cell survival in a dose-dependent, but not time-dependent manner. Cells treated with Api had increased amounts of early apoptosis, late apoptosis, and secondary necrosis compared to the control group. This group also had decreased Bcl-2 gene expression and up-regulated Bax gene expression. Co-treatment with Api and IR significantly inhibited cell survival, and increased early apoptosis, late apoptosis and secondary necrosis compared to the other groups. There was a significant decrease in Bcl-2 gene expression along with up-regulation of Bax gene expression, and Bax/Bcl-2 ratio changes that favoured apoptosis.
Conclusion
Api inhibited PrCa cell survival and induced apoptosis as a single agent. In addition, Api significantly sensitized the LNCaP cells to IR and enhanced radiation-induced apoptosis.
Keywords: Apigenin, Apoptosis, LNCaP, Prostate Cancer, Radiation
Introduction
Prostate cancer (PrCa) is the second most prevalent malignancy and the fifth major risk for male mortality worldwide. In 2018, about 1.3 million new cases were recorded worldwide, which comprised 7.1% of all cancer cases in men (1-3). In Iran, the pooled age-standardized incidence rate was 87 per million and it is one of the ten most cancers (4, 5). Conventional therapy for PrCa includes surgery, chemotherapy, hormone therapy, immune therapy, and radiation therapy (RT). RT is one of the most effective and common treatment modalities to treat localized PrCa. However, PrCa is only moderately responsive or sometimes unresponsive to the cytotoxic effects of RT (6). Radiation doses are generally limited to <80 Gy for PrCa, and an increased dose may not show efficacy for local tumour control. Increasing RT doses are associated with urinary and bowel adverse effects (7, 8). The results of studies show that radiation resistance of PrCa may result in the expressions of multiple pivotal genes that regulate apoptosis, cell proliferation, and cell cycle pathways (8-11). Hence, a strategy for targeting the molecular pathways that play a crucial role in radiation resistance may sensitize PrCa to radiation. This strategy may lead to decreased RT adverse effects by employing lower RT doses or at least increase the five-year survival rate at the current RT dose.
Bcl-2 is a member of an anti-apoptotic protein family that regulates apoptosis in both normal and abnormal cells. It is the second most frequently considered genetic aberration and is correlated with PrCa resistance to RT (9). Bcl-2 is a pro-survival protein that has an essential role in PrCa radiation resistance and is correlated with tumour aggressiveness. The PrCa cell line, LNCaP, overexpresses Bcl-2 (8, 9). Therefore, attempts to inhibit its expression could sensitize this cell line to ionizing radiation (IR).
4′, 5, 7-trihydroxyflavone (apigenin [Api]) is a well-known flavonoid family member and a natural component of many fruits and vegetables. Api has anti-inflammatory and anti-carcinogenic effects (12-14). The results of several studies show that the micromolar concentrations of Api lead to inhibition of viability, apoptosis induction, and suppression of Bcl-2 expression in the bout androgen-sensitive PrCa cell line. (LNCaP) and androgen-insensitive PrCa cell lines (PC-3 and DU-145). Api can cause overexpression of Bax (15-17). Api selectively reduces cell viability and induces apoptosis in the PC-3, DU-145 and LNCaP cell lines without affecting normal cells (18). Increased Bcl-2 expression in PrCa is correlated with radiation resistance; therefore, Api may have a radiation sensitizer effect on PrCa. The current study is the first study that aims to investigate the effects of Api on the therapeutic efficacy of radiation by targeting Bcl-2 and Bax pathway enhanced radiation-induced apoptosis and assess the ability of Api to sensitize the LNCaP cells to current or lower radiation doses.
Materials and Methods
Cell culture and treatment
In this experimental study, the human PrCa cell lines were obtained from Pasteur Institute of Iran (Tehran, Iran) and subsequently cultured in RPMI 1640 medium (Gibco, Rockville, USA) supplemented with 10% FBS (Gibco, Rockville, USA) and 1% penicillin/streptomycin. The cells were grown in T-75 flasks at 37°C and 5% CO2 . Api (<95% purity, Nanochemia Salamat Company, Karaj, Iran) was dissolved in dimethyl sulfoxide (DMSO, Kiazist Co., Hamedan, Iran) and stored as a 10 mM stock solution. The stock solution was diluted to the desired concentration directly in the culture medium. The maximum final concentration of DMSO was 0.1%. We also added 0.1% DMSO to the control cells and those that only received 2 Gy IR for each of the experiments. The cells were divided into four groups: untreated (control), 2 Gy ionizing radiation (IR), Api (half-maximal inhibitory concentration [IC50], and the combination of Api (IC50 concentration) and 2 Gy IR. For the combination therapy, we added the IC50 concentration of Api to the cell cultures two hours before irradiation (2 Gy). The cell cultures were irradiated with one fraction of 2 Gy X-rays at room temperature using a 6 MV LINAC (Elekta, Stockholm, Sweden) at a 200 cGy/minutes dose rate (gantry angle: 180º, collimator angle: 0º, field size: 30×30 cm2, SSD: 98 cm, depth: 2 cm). In order to account for full backscatter, we placed a 5 cm slab phantom on the surface of the well plate. The Ethical Commitee of Tehran University of Medical Sciences (IR. TUMS.MEDICINE.REC.1398.534).
Half-maximal inhibitory concentration determination
The cells were plated in 96-well plate at a density of 1×104 cells per well in 100 µl of complete culture medium and allowed to attach overnight before the treatments. The medium was removed 24 hours after plating and replaced by fresh medium that contained different concentrations (up to 80 µM) of Api in RPMI 1640 medium. We assessed toxicity and cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Kiazist Co., Hamedan, Iran) and determined the IC50 dose 24 and 48 hours after treatment with the various concentrations of Api.
Cell viability assessment
The cells were seeded in 96-well plates at a density of 1×104 cells per well and allowed to attach overnight before the treatments. Briefly, at 24 and 48 hours after treatment, both toxicity and viability of the LNCaP cells were evaluated by the MTT assay (Kiazist Co., Hamedan, Iran). For the viability assessment, we removed the culture medium and washed the cells with phosphate-buffered saline (PBS, Merck Biosciences, Nottingham, UK). Next, we added 100 µl of complete culture medium that contained 0.5 mg/mL MTT to each well of the 96-well plates. The plates were incubated for 3 hours at 37°C. Subsequently, solubilizing solution was added and the plates were incubated in the dark at room temperature for 30 minutes. Absorbance of the samples was measured by an automatic microplate reader (BioTek, VT, USA) at 540 nm.
Apoptosis assay
An Annexin V-FITC/PI Assay kit (Merck Biosciences, Nottingham, UK) was used to detect apoptotic and necrotic cells. The cells were cultured at a density of 2×105 cells per well in a six-well plate. Briefly, at 24 and 48 hours after treatment, the cells were detached and centrifuged at 300 g for 5 minutes, and subsequently washed twice with PBS. Diluted Annexin V binding solution was added for a final cell concentration of 1×105 cells/ml and 100 µl of the resultant cell suspension was transferred to a new tube. Next, we added 5 µl of Annexin conjugated V- fluorescein isothiocyanate (FITC) and 5 µl of propidium iodide (PI) to the cell suspensions and incubated them for 15 minutes at room temperature in the dark. Then, 400 µl diluted Annexin V binding solution was added and the samples were assessed by a flow cytometer (BD Biosciences, USA). FITC and PI have been exited at 488 nm wavelength. FITC emission and PI were detected at 525 nm and 650 nm, respectively. Annexin V/PI negative cells indicate healthy cells, whereas Annexin V positive and PI negative populations represent cells in the early stages of apoptosis, and Annexin V/PI positive cells are in late apoptosis/secondary necrosis. Therefore, Annexin V positive cells are the sum of early apoptosis and late apoptosis/secondary necrosis.
Gene expression
The cells were plated at 2×105 cells per well in six-well plates. Then, RNA was extracted 24 and 48 hours after treatment with a GeneALL kit (Biotech, Korea) and StepOne™ thermal cycler (Applied Biosystems, USA). For checking RNA concentration and its purity, Nanodrop (thermo Fisher, USA) was used. A cDNA Synthesis kit was used to reverse transcribe 1 µg total RNA to cDNA to check the RNA concentration and its purity. Then, real-time polymerase chain reaction (PCR) was conducted as follows: one cycle that activated the Taq polymerase enzyme and separated the two-stranded DNA of the primary pattern for 5 minutes at 95°C, followed by 40 cycles for 15 seconds at 95°C, 30 seconds at 60°C, and finally 30 seconds at 72°C. SYBR Green PCR Master Mix (Biotech, Korea) was used for the real-time PCR analysis. The Bcl-2 and Bax gene expressions were normalized to the reference control (Gapdh) gene. The relative expression ratio (R) of the target gene was calculated based on E and the crossing point (CP) deviation of an unknown sample versus the control, and was expressed and compared to the reference gene.
Table 1.
List of gene primers
|
| ||
|---|---|---|
| Gene | Primer sequences (5ˊ-3ˊ) | Accession number |
|
| ||
| Bax | F: CCTGTGCACCAAGGTGCCGGAACT | XM_017027077 |
| R: CCACCCTGGTCTTGGATCCAGCCC | ||
| Bcl-2 | F: TTGTGGCCTTCTTTGAGTTCGGTG | XM_017025917 |
| R: GGTGCCGGTTCAGGTACTCAGTCA | ||
| Gapdh | F: ACCCAGAAGACTGTGGATGG | NM_001289745 |
| R: TCTAGACGGCAGGTCAGGTC | ||
|
| ||
Combination index
The Bliss Independence method was used to calculate the combination index (CI) as an effect-based strategy. By this method, the effect of combining of two drugs (Eab) was compared directly with the effects of its individual components (EA and EB). This model assumes that drugs act individually and do not interfere with each other. The observed combined effect is represented as a probability (0<EAB<1); therefore, the CI can be calculated as CI=(EA+EB-EBEA)/EAB. This model is one of the most common models used to evaluate the combined effects of drugs (17).
Statistical analysis
The data are presented as mean ± standard error of the mean (SEM) from at least three different experiments with four samples per group. Statistical analysis was performed using the unpaired t test with GraphPad software (version 8, San Diego, CA, USA). Statistical significance was set at P<0.05.
Results
Radiation combined with apigenin significantly reduced cell survival
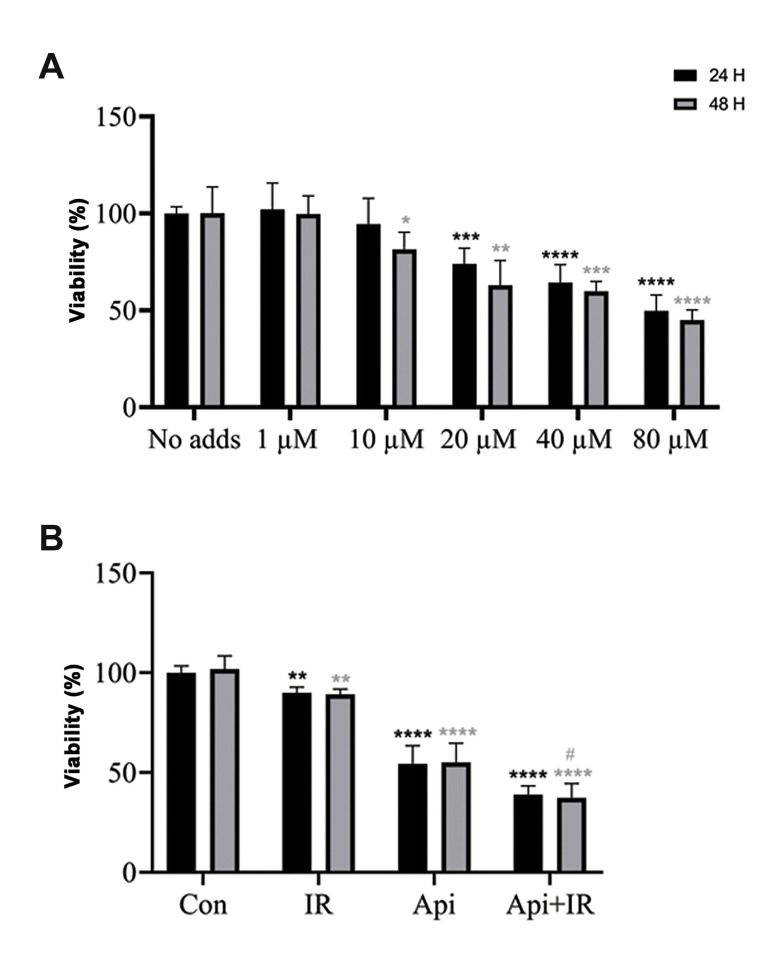
Figure 1A shows the effect of different concentrations of Api on the LNCaP cell line after treatment for 24 and 48 hours, and their post-treatment viability according to the MTT assay. Figure 1A shows that Api inhibited cell growth in a dose-dependent manner, but was not time-dependent. The 65 μM concentration of Api induced 50% inhibition of cell growth 24 hours after treatment. Figure 1B shows that the combination of 65 µM Api and 2 Gy IR caused a significant decrease in viability compared to the individual treatments with Api and 2 Gy IR. The combination of Api and radiation also did not appear to have a time-dependent inhibition of the LNCaP cells. The CI, which was based on Bliss Independence for co-treatment of LNCaP cells by Api and radiation, was 0.837 at 24 hours and 0.753 at 48 hours.
Fig.1.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess the effect of apigenin (Api), 2 Gy ionizing radiation (IR) and their combination on LNCaP cell viability at 24 and 48 hours after treatment. A. Viability of cells treated with Api. B. Viability of individual API and IR treatments, and their combination. Data are shown as % of the untreated control group. Mean ± standard error of the mean (SEM) are obtained from five independent repetitions. **; P<0.01, ***; P<0.001, ****; P<0.0001 versus the control at 24 hours after treatment, *; P<0.05, **; P<0.01, ***; P<0.001, ****; P<0.0001 versus the control at 48 hours after treatment, #; P<0.05 versus Api at 48 hours after treatment, and h; hours.
Co-treatment with Api and radiation caused apoptosis and necrosis
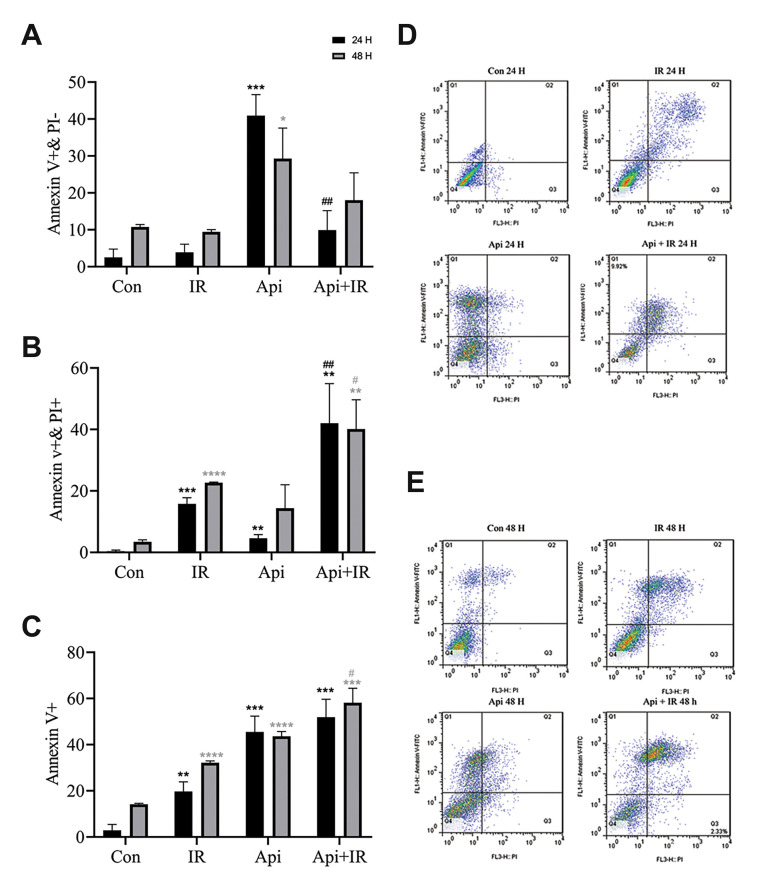
Flow cytometric analysis of LNCaP cells that were treated with 65 µM Api showed significant increase in early apoptosis (Fig .2A). Co-treatment of Cells with the combination of 65 µM Api and 2 Gy irradiation showed a significant increase in late apoptosis/secondary necrosis compared to cells which treated with API or IR (Fig .2B).
Fig.2.
Flow cytometry analysis of the effect of 65 µM Apigenin (Api), 2 Gy ionizing radiation (IR), and their combination on necrosis and apoptosis induction. A. The percentage of early apoptosis induction. B. The percentage of late apoptosis/secondary necrosis induction. C. The percentage of the combination of early apoptosis and late apoptosis/secondary necrosis cell fractions. D. Apoptosis dot-plot 24 hours after treatment. E. Apoptosis dot-plot 48 hours after treatment. Data are shown as % of the untreated control group. Mean ± standard error of the mean (SEM) are obtained from three independent repetitions. **; P<0.01, ***; P<0.001 versus the control 24 hours after treatment. *; P<0.05, **; P<0.01, ***; P<0.001, ****;P<0.0001 versus the control 48 hours after treatment, ##; P<0.01 versus Api 24 hours after treatment, #; P<0.05, versus Api 48 hours after treatment, PI; propidium iodide, h; Hours, and Con; Control.
Figure 2C shows that at 24 hours after co-treatment of the cells with Api and IR, the difference between the combined early apoptosis and late apoptosis/secondary necrosis cell fraction (Annexin v+) (51.9%) was not significant compared to those treated with Api alone (41.52%). However, 48 hours after co-treatment cells with Api and IR the combination of early apoptosis and late apoptosis/secondary necrosis cells fraction (Annexin v+) (58.9%) is significantly more than cells just treated with Api (45.9%).
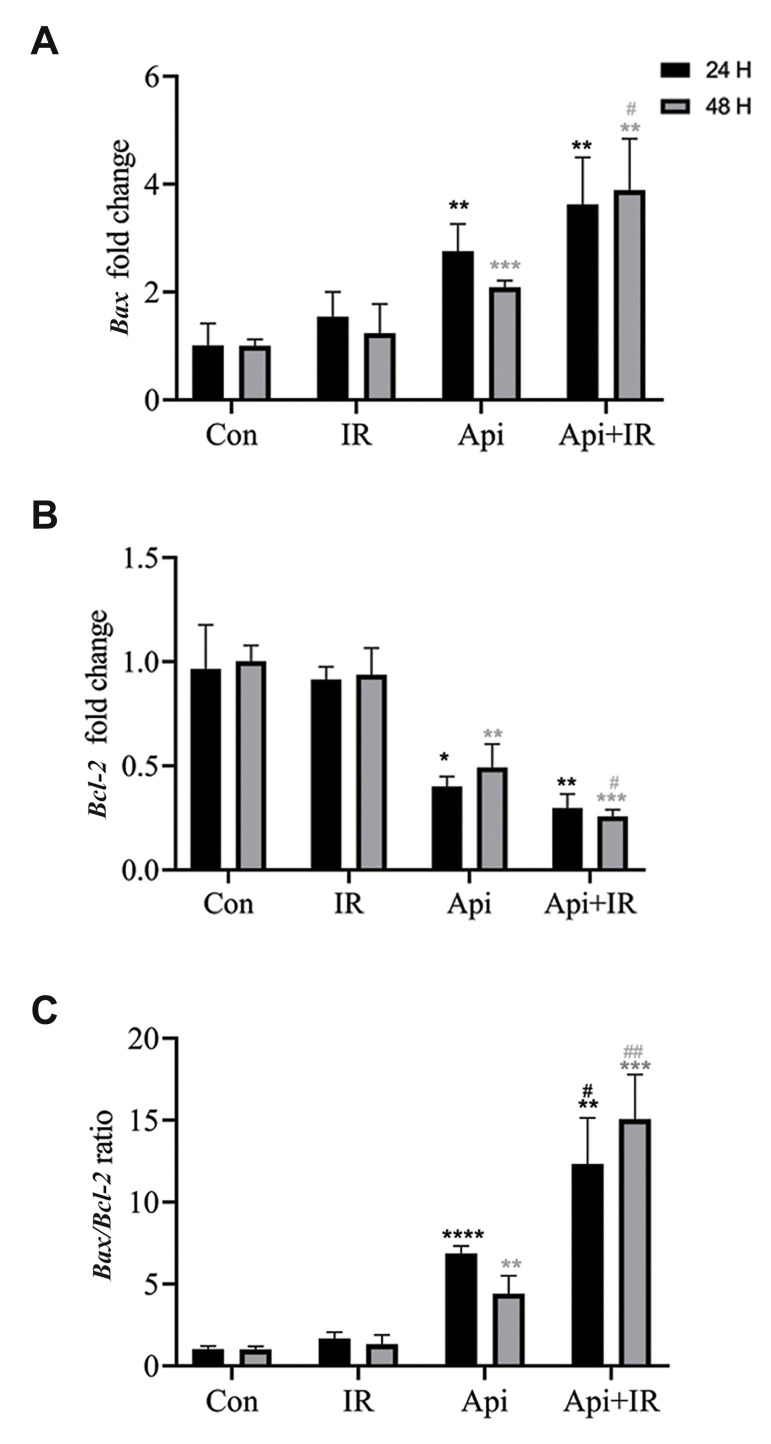
Bax gene up-regulation and inhibition of Bcl-2 gene expression
Real-time PCR analysis of LNCaP cells treated with 65 µM Api showed a significant increase in Bax gene expression (Fig .3A) and a decrease in Bcl-2 gene expression (Fig .3B). Consequently, the Bax/Bcl-2 ratio favoured apoptosis (Fig .3C). The effect of Api on expressions of these genes was not time-dependent. However, the Bax/ Bcl-2 ratio significantly increased over time. Next, we investigated the Bax and Bcl-2 gene expressions 24 and 48 hours after combined treatment of the cells with Api and IR. Figure 3A, B show that the Bax and Bcl-2 gene expressions 24 hours after co-treatment of the LNCaP cells with Api and IR was not significantly different compared to those treated with Api alone. However, 48 hours after co-treatment with Api and IR, there was a significant increase in Bax gene expression and a significant decrease in Bcl-2 gene expression compared to cells treated with either Api or IR. Figure 3C shows a significant change in the Bax/Bcl-2 ratio after the combination therapy that favoured apoptosis.
Fig.3.
Real-time polymerase chain reaction (PCR) assessment of the effects of Apigenin (Api), ionizing radiation (IR), and their combination on Bax and Bcl-2 gene expressions, and changes in the Bax/Bcl-2 ratio at 24 and 48 hours after treatment. A. Bax fold-change. B. Bcl-2 fold-change. C. Bax/Bcl-2 ratio change. Data are shown as % of the untreated control group. Mean ± standard error of the mean (SEM) obtained from three independent repetitions.*; P<0.05, **; P<0.01, ****; P<0.0001 versus the control 24 hours after treatment, **; P<0.01, ***; P<0.001 versus the control 48 hours after treatment, #; P<0.05 versus Api 24 hours after treatment, #; P<0.05, ##; P<0.01 versus Api 48 hours after treatment, Con; Control, and h; Hours.
Discussion
In this study, Api reduced the viability of PrCa cells. Api reduced the viability of LNCaP cells in µM concentration. The IC50 concentration of Api for LNCaP cells is 65 µM. Treatment of PrCa by 2 Gy IR and 65 µM of Api showed that, at 24 hours, the CI of this treatment based on the Bliss Independence method was 0.85 and it was 0.72 after 48 hours. Previous study proposed that a CI between 0.85-1.05 could not be considered to show any synergistic effects for combination therapy (19). Our findings indicated that 24 hours after co-treatment of LNCaP with Api and radiation, no significant changes in the viability of cells was observed in comparison to those treated with Api alone. However, 48 hours after co-treatment of PrCa with Api and radiation showed a significant decrease in PrCa cell viability compared to Api or radiation alone.
Co-treatment of LNCaP cells with Api and IR led to a change in the apoptotic pattern compared to cells treated by Api alone. The cells co-treated with Api and IR had a higher percentage of late apoptosis/secondary necrosis, whereas cells treated by Api alone had a higher percentage of early apoptosis. The apoptosis/necrosis pattern for cells that treated with only IR was similar to the cells co-treated with Api and IR.
At 24 hours after co-treatment of the cells with Api and IR, we noted that the difference between the combination of early apoptosis and late apoptosis/secondary necrosis cell fraction with those treated by Api alone was not significant despite the significant difference between their apoptosis/necrosis pattern. However, flow cytometry analysis of Annexin V-FITC 48 hours after co-treatment with Api and IR showed that the combination of the early apoptosis and late apoptosis/secondary necrosis cell fractions was significantly more than those treated with Api alone, which was in agreement with our findings from the MTT assay.
We evaluated Bcl-2 gene expression, as a critical inhibitor of apoptosis, following irradiation and treatment with Api. Bcl-2 is an upstream effector gene in the apoptotic pathway and that is a potent suppressor of apoptosis (18). Bcl-2 has been shown to form a heterodimer with the pro-apoptotic Bax and might neutralize its pro-apoptotic effects. The ratio of Bax/Bcl-2 is a crucial factor that plays an essential role in determining whether cells undergo apoptosis or not. Bcl-2 is the second most frequent genetic aberration that has a correlation with radiation resistance in PrCa. LNCaP cells have high expression of Bcl-2 (7). Since Bcl-2 plays a crucial role in apoptosis, we studied the time-dependent effects of Api on its expression level in LNCaP cells alone or combined with radiation. We observed that Api significantly inhibited Bcl-2 gene expression and up-regulated Bax gene expression. The expressions of these genes was not as time-dependent as the results of the MTT and fluorescence tests. Co-treatment of LNCaP cells with both Api and IR showed that was a significant suppression of Bcl-2 gene expression and up-regulation of Bax gene expression compared to cells that treated individually by Api or IR 48 hours after combination therapy. However, at 24 hours after combination therapy, we observed no significant change in gene expressions compared to cells that were treated with Api.
The decrease in Bcl-2 protein levels and increase in Bax protein levels enhanced radiation sensitivity and increased apoptosis induction. The combination therapy of Api and radiation increased Bax gene expression compared to treatment with Api or radiation alone. One of the limitations of this study is that the protein levels of Bcl-2 and Bax were not measured. However, the results of gene expression were consistent with the enhancement of apoptosis induction, and it could be concluded that this co-treatment, by increasing the protein levels of Bax and decreasing Bcl-2 protein levels, most likely enhanced radiation-induced apoptosis and sensitized PrCa to radiation. Thus, this strategy might lead to a decrease in adverse effects caused by RT by employing lower doses of radiation or at least increasing the five-year survival rate at the current dose. Of note, based on the daily dietary intake of flavonoids, the concentrations used in this study are physiologically achievable and not toxic in humans (20). More in vitro studies, including a study on an androgen-insensitive PrCa cell line (PC-3 or DU-145) and an investigation of the clonogenic formation ability of PrCa after co-treatment with Api, in addition to an in vivo study would be needed to confirm the radiosensitisation effect of Api on PrCa.
Conclusion
Here, we successfully demonstrated that co-treatment of LNCaP cells with Api and IR significantly increased the Bax/Bcl-2 gene expression ratio in favour of apoptosis. Api could potentiate radiation-induced apoptosis and cause a decrease in LNCaP cell viability. Api and IR have a synergic effect, and Api is a potent radiosensitiser of LNCaP cells.
Acknowledgements
This research was supported by Tehran University of Medical Sciences, Tehran, Iran (grant 98-02-30-43065). The authors declare that there is no conflict of interest.
Authors’ Contributions
A.R.Sh., M.N., M.T.B.; Contributed to the study conception and design. Gh.G., Sh.A., M.T.B.; Conducted the experimental work, data and statistical analysis, and data interpretation. Gh.G., Sh.A.; Supervised the study. M.T.B.; Drafted the manuscript, which was revised by M.N. and Gh.G. All authors read and approved the final manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer today. Available from: http://gco.iarc.fr/today/home. (16 Nov 2019).
- 4.Moradi A, Zamani M, Moudi E. A systematic review and meta-analysis on incidence of prostate cancer in Iran. Health Promot Perspect. 2019;9(2):92–98. doi: 10.15171/hpp.2019.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farhood B, Geraily G, Alizadeh A. Incidence and mortality of various cancers in Iran and compare to other countries: a review article. Iran J Public Health. 2018;47(3):309–316. [PMC free article] [PubMed] [Google Scholar]
- 6.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23(8):1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 7.Bonkhoff H. Factors implicated in radiation therapy failure and radiosensitization of prostate cancer. Prostate Cancer. 2012;2012:593241–593241. doi: 10.1155/2012/593241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palacios DA, Miyake M, Rosser CJ. Radiosensitization in prostate cancer: mechanisms and targets. BMC Urol. 2013;13:4–4. doi: 10.1186/1471-2490-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosser CJ, Gaar M, Porvasnik S. Molecular fingerprinting of radiation resistant tumors: can we apprehend and rehabilitate the suspects? BMC Cancer. 2009;9:255–255. doi: 10.1186/1471-2407-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakozy C, Grignon DJ, Sarkar FH, Sakr WA, Littrup P, Forman J. Expression of bcl-2, p53, and p21 in benign and malignant prostatic tissue before and after radiation therapy. Mod Pathol. 1998;11(9):892–899. [PubMed] [Google Scholar]
- 11.Scherr DS, Vaughan ED, Wei J, Chung M, Felsen D, Allbright R, et al. BCL-2 and p53 expression in clinically localized prostate cancer predicts response to external beam radiotherapy. J Urol. 1999;162(1):12–16. doi: 10.1097/00005392-199907000-00003. discussion 16-17. [DOI] [PubMed] [Google Scholar]
- 12.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30(1):233–245. [PubMed] [Google Scholar]
- 14.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23(4):519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 15.Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35(2):452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissey C, O’Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RWG. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63(2):131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 17.Shukla S, Fu P, Gupta S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis. 2014;19(5):883–894. doi: 10.1007/s10495-014-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287(4):914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 19.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3(3):e00149–e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollman PCH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37(9-10):937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]