Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder with very limited treatment options. Stem cells have been raised as a new treatment modality for these patients. We have designed a single-center, prospective, open-label, and single arm clinical trial to assess the safety, feasibility, and rather efficacy of administrating allogeneic adipose-derived mesenchymal stromal cells (Ad-MSCs) in ALS patients. We enrolled 17 patients with confirmed ALS diagnosis with ALS Functional Rating Scale-Revised (ALSFRS-R) (<24) and predicted forced vital capacity (FVC) (<40)%. Allogeneic Ad-MSCs were transplanted intravenously for all patients. Follow-ups were done at 24 hours, 2, 4, 6, and 12 months after cell infusion by checking adverse events, laboratory tests, and clinically by ALSFRS-R and FVC. Patients were also followed five years later and ALSFRS-R score was recorded in the survived individuals. There was no report of severe adverse events related to cell infusion. Two patients experienced dyspnea and chest pain 36 and 65 days after cell infusion due to pulmonary emboli. The progressive decrease in ALSFRS-R and FVC levels was recorded and three patients died in the first year. During five years follow up, despite a notable decrease in functional scores, 5 patients survived. Intravenous (IV) infusion of allogeneic Ad-MSCs in ALS patients is safe and feasible. The survival rate of the patients is more than IV autologous MSCs (Registration number: IRCT20080728001031N26).
Keywords: Allogeneic Cells, Amyotrophic Lateral Sclerosis, Autologous Cell Transplantation, Mesenchymal Stromal Cells, Stem Cells
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a devastating and rapidly progressive, incurable neurodegenerative disorder in which motor neurons (MNs) are affected in the primary motor cortex, brainstem, and spinal cord (1).
This disease results in muscle weakness, muscle atrophy, fasciculation, spasticity, and paralysis, finally leading to death usually within 3-5 years after the onset of clinical symptoms. 90% of all cases are considered to be sporadic, while the remaining 10% of patients suffer from familial ALS, where in approximately 20% of them the symptoms initiates after mutations in the gene encoding superoxide dismutase 1 (SOD1) (1).
Currently there is no efficient and approved curative treatment for ALS. The first available drug, Riluzole, extends survival time by an average of 3 months. Edaravone has been recently approved in Japan and US by Food and Drug Administration (FDA), slowing down disease progression in a subgroup of patients without respiratory contention (1). Cell therapy can be a potential therapeutic option for ALS, although numerous scientific, technical, ethical, and regulatory challenges remain to be addressed. There are two main cell types used for cell-based therapy in ALS patients. Mesenchymal stromal cells (MSCs), such as bone marrow-MSCs (BM-MSCs), adipocyte-MSCs (Ad-MSCs), and hematopoietic stem cells (HSCs), such as CD133+ and CD34+ progenitor cells from bone marrow and umbilical cord blood cells (CB) respectively. The second cell type includes neural stem cells, such as fetal origin spinal cord and olfactory scabbard glial cells (OECs). There are ongoing discussions over which cell types have the most regenerative potential in this case (2).
BM-MSCs are a type of adult stem cells with the capability to self-renew and differentiate into various cell types (3). MSCs can improve ALS impairments by using multiple mechanisms, such as secretion of rich trophic factors, immunomodulation by increased expression of interleukin-10 (IL-10), and transforming growth factor beta-1 (TGF-β1). Moreover, MSCs can improve neural function in a defective zone of the central nervous system (4). In an animal model of ALS, it has been shown that administration of MSCs in SOD1/G93A mice restored motor neurons, extended life span, and improved motor function by the secretion of growth factors, immunomodulatory effects, and depletion of oxidative stress (5).
In previous studies, stem cell transplantation in ALS patients was performed via different routes such as intrathecal (IT) (6), intraspinal (7), intravenous (IV), intraventricular (6), intracortical (8), and intra-arterial (9) injections.
Studies have shown that stem cells isolated from ALS patients -or other types of neurodegenerative disorders- have reduced trophic factor secretion and immunomodulatory potential compared to the stem cells derived from healthy individuals. It is assumed that production of various trophic factors, plasticity, and migration ability are significantly reduced in stromal cells derived from ALS patients (ALS-SCs), suggesting that ALS-SCs have reduced capacity as trophic mediators and perhaps less beneficial effects in autologous cell therapy (10).
Additionally, allogeneic cells can be isolated from healthy donors and cultured in advance, so their application would be more reasonable and convenient. On the other hand, these cells can be a practical ‘off the shelf’ therapeutic agent (11).
In this study, adipose-derived allogeneic source of MSCs, obtained from healthy donors, were infused intravenously. Donors were healthy relatives of the patients, (younger than patients, sex-matched or sex-mismatched). The donors were tested for hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), human T-cell lymphotropic virus type 1 and 2 (HTLV1 and 2), and they were negative. Referring to our previous study, this approach is safe and more feasible in ALS patients compared to the IT injection (12). The main aim of the study was the safety assessment of IV injection of allogeneic MSCs in these patients.
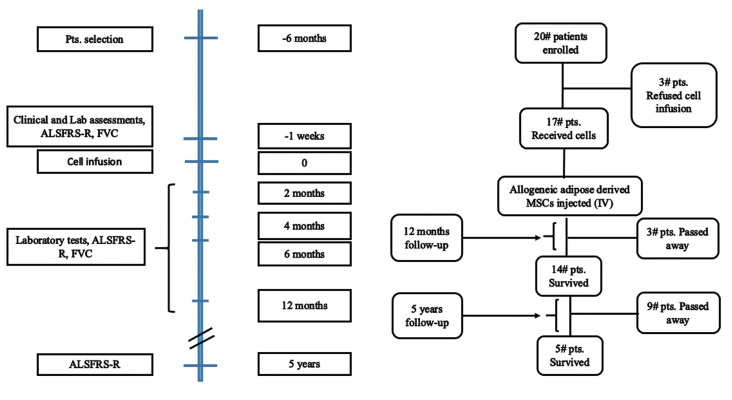
The trial was designed as a single-center, prospective, open-label, single-arm study without a placebo control group. We conducted this clinical trial at Royan Institute in collaboration with the Neurology Department of Mostafa Khomeini Hospital, Tehran, Iran. The outcome of this study was to assess the safety and efficacy of IV administration of allogeneic adipose derived MSCs in patients with ALS. The study protocol and informed consent form (ICF) were approved by Royan Institute Ethics Committee (EC/93/1084). This study was registered at the National Institutes of Health (NIH) clinical trial site (www.clinicaltrials.gov) with identification number "NCT02492516" and Iranian Registry of Clinical Trials "IRCT20080728001031N26". Figure 1 shows the study flowchart and timeline for follow-up and interventions.
Fig.1.
Study flow diagram. Different time points for follow-up. The total number of patients enrolled in the study and during follow-up. Laboratory tests which were performed: routine blood tests and serum biochemistry and liver function tests as well as renal functional tests. Pts; Patients, FVC; Forced vital capacity, and ALSFRS-R; ALS Functional Rating Scale-Revised.
A total number of 20 patients, 18-65 years old, with an approved diagnosis of sporadic ALS enrolled in the study from December 2013 to June 2015. Demographic data is listed in Table 1. Eligible patients are ones diagnosed with ALS more than 6 months ago, with an ALS Functional Rating Scale (ALSFRS-R) score ≥24, and predicted forced vital capacity (FVC) ≥40%.
Table 1.
Patients’ demographic data
|
| ||||||
|---|---|---|---|---|---|---|
| Patient ID | Age (Y) | Gender | Disease onset | Disease duration (m) | ALSFRS-R (baseline) | FVC (baseline) % |
|
| ||||||
| 1 | 35 | Male | Limb | 17 | 37 | 50 |
| 2 | 25 | Male | Limb | 90 | 37 | 43 |
| 3 | 53 | Female | Limb | 21 | 33 | 82.6 |
| 4 | 44 | Female | Limb | 44 | 36 | 57 |
| 5 | 30 | Male | Limb | 46 | 45 | 107 |
| 6 | 35 | Male | Limb | 15 | 26 | 96 |
| 7 | 56 | Female | Bubar | 24 | 30 | 106 |
| 8 | 46 | Female | Limb | 30 | 39 | 53 |
| 9 | 50 | Female | Bubar | 8 | 34 | 54.41 |
| 10 | 36 | Female | Limb | 26 | 29 | 41 |
| 11 | 55 | Male | Bubar | 9 | 34 | 58 |
| 12 | 67 | Male | Limb | 30 | 36 | 57.7 |
| 13 | 63 | Female | Limb | 22 | 30 | 48.2 |
| 14 | 52 | Male | Limb | 19 | 37 | 111.3 |
| 15 | 71 | Male | Bubar | 4 | 39 | 100 |
| 16 | 56 | Male | Bubar | 20 | 43 | 101 |
| 17 | 61 | Male | Limb | 26 | 36 | 41 |
|
| ||||||
The mean age of patients was 49.12 ± 13.32 years old. The mean disease duration was 26.5 ± 19.8 months. The range of FVC was 41 to 111.3 percent before cell infusion. FVC; Forced vita capacity, and ALSFRS-R; ALS Functional Rating Scale-Revised.
ALSFRS-R contains 12 functional items concerning bulbar function (speech-salivation-swallowing), upper limb (handwriting-cutting food and handling utensils-dressing and hygiene), lower limb (walking-climbing stairs), trunk (turning in bed and adjusting bed cloths), and respiratory functions (dyspnea-orthopnea-respiratory insufficiency). Each item is scored from 0 (unable to) to 4 (normal) (13).
All patients were under treatment with Riluzole, at a dose of 100 mg, twice per day. Exclusion criteria were any concomitant neurological, psychiatric or systemic diseases or receiving any corticosteroids, immunoglobulin, or immunosuppressant during the last 6 months.
Following local anesthesia, under sterile conditions, 100 ml of subcutaneous adipose tissue of a healthy donor, was isolated by lipoaspiration and sent to the Royan Institute’s Cell Therapy Center.
Adipose tissue was washed with warm PBS (Gibco, Germany, 10010023) containing antibiotics and cut to 1 to 2 mm pieces using a razor blade in a 15 cm dish. We added the 0.2% collagenase enzyme (Gibco, Germany, 17018-029) in a volume equal to adipose tissue. Enzyme and the adipose tissue were mixed for 15-30 minutes in the incubator and stamped every 5 minutes. The enzyme was neutralized using 10% FBS (HyClone, Logan, UT, USA, SH30070.03IR). The mixture was centrifuged for 15 minutes at 2000 rpm. The stromal vascular fractions (SVFs) was filtered through 70 micrometers filter (Corning, USA, 431751). And the number of MNCs (mononuclear cells) was counted by the NucleCounter®NC200TM. After cell counting, 106 MNCs were seeded in a T-150 flask (TPP, Switzerland, 90151) containing DMEM-F12 medium (Gibco, Germany, 12634) enriched with 10% FBS. The flasks were incubated in the standard condition for 3 days. Then, the supernatant was transferred to a new flask. The flasks were refreshed every 4 days for 3 weeks.
A. Flow Cytometry: To ensure the purity of the isolated mesenchymal cells, we analyzed the cells in each passage using a flow cytometric method for specific surface markers. Thus, in P0 to P3 passages, positive markers of CD105 (Endoglin, BD PharmingenTM, USA), CD90 (BD, PharmingenTM, USA), CD44 (BD PharmingenTM, USA), CD73 (BD PharmingenTM, USA) and negative markers of CD45, CD34 (BD PharmingenTM, USA) were assessed through flow cytometry. The flow analysis data related to the AD-MSC is included in the supplementary file.
B. Differentiation potential of Ad-MSCs: One of the other methods for verifying mesenchymal cells is to investigate the differentiation potential of these cells. Ad-MSCs cultured in chondrogenic, osteogenic, and adipogenic permissive media to evaluate their multi-potential capacity.
Preparation of mesenchymal cells for transplantation: After cultivation and proliferation of mesenchymal stromal cells in a good manufacturing practice (GMP) facility, they were harvested under enzymatic treatment.
A total number of 20 ALS patients who had been diagnosed more than 6 months prior to this study, were referred to the neurology clinic of Royan Institute and enrolled in the study. Three patients out of twenty refused to receive MSCs transplantation and 17 patients received allogeneic MSCs. First, the patients were provided with an informed consent to be signed.
Magnetic resonance imaging (MRI), Electromyographynerve conduction velocity (EMG-NCV), and spirometry tests were performed for all patients. ALSFRS-R form was filled out for all patients. Patients received IV infusion of Ad-MSCs in OR (2×106 cells/kg). The cell suspension was 10 mL for each patient and gradually infused in five minutes.
After cell infusion, each patient was monitored for 6 hours. Then, the follow-up was performed at 24 hours, 2, 4, 6, and 12 months after injection. All patients received routine medications, i.e., Riluzole (100 mg) twice a day.
The assessments included the comprehensive physical examination, taking medical history about any new symptoms, ALSFRS-R, FVC, laboratory analysis (liver, kidney, thyroid function, serology, virology, urine analysis, and culture). These assessments were performed 4 weeks before cell therapy and also 2, 4, 6, and 12 months after cell infusion. Brain and spinal cord MRIs were performed one week before cell transplantation in order to confirm the diagnosis.
Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). In the present study, continuous variables were expressed as mean ± standard error (SE). Repeated-measures ANOVA was used to assess the effects of treatment and time (months) and treatment-by-time interactions on ALSFRS-R and FVC. All statistical tests were two-sided and P<0.05 was considered statistically significant.
This cell infusion did not cause any severe adverse events. Immediate adverse events were detected in some cases including, two patients who reported sinuous tachycardia (heart rate: 150 ppm) during infusion that was improved by IV administration of propranolol. One of them experienced headache and vertigo after injection lasting for 24 hours.
One female patient experienced hypertension (blood pressure: 180/100 mmHg) and tachycardia immediately after cell infusion. IV propranolol was prescribed and her condition was stabilized within 4 hours.
One patient complained of left side chest pain three weeks after cell infusion. Chest X-ray showed an obstruction in the left pleural corner confirming pulmonary emboli. The patient underwent warfarin therapy, physiotherapy, and noninvasive positive pressure ventilation (NIPPV). Furthermore, percutaneous endoscopic gastrostomy (PEG) insertion was performed for this patient five months after transplantation.
One patient reported dyspnea 2 to 3 weeks after cell infusion which was initially suspected as emboli. However, bacterial pneumonia was diagnosed for him and his condition was stabilized after standard treatment.
Another patient suffered from pleural effusion and pulmonary arrest two months after cell infusion who referred to the cardiopulmonary resuscitation (CPR) unit. Pulmonary emboli were also confirmed for this patient. He underwent a tracheostomy and a NG (nasogastric) tube was inserted for him. This patient passed away seven months after cell infusion. Another patient needed PEG insertion about four months after cell infusion. Two other patients died before completing 12 months follow-up period. Table 2 shows adverse events during follow-up times up to 12 months after cell infusion and also after five years.
Table 2.
Adverse events reported during the first year of study
|
| |||
|---|---|---|---|
| AE type | Patients (n) | Time after injection | Outcome |
|
| |||
| Hypertension | 1 | 10-15 minutes | Improved 4 hours after using IV propranolol |
| Tachycardia | 3 | 10-15 minutes | Improved after using IV propranolol |
| Headache | 1 | 10 minutes | Improved |
| Dizziness | 1 | 10 minutes | Improved |
| Dyspnea/tachypnea | 2 | 2 weeks | Improved |
| Chest pain | 2 | 3 weeks/2 months | Improved |
| PEG insertion | 2 | 5 months/4 months | NA |
| Pulmonary emboli | 2 | 3 weeks/2 months | Death |
| Pneumonia | 1 | 2 weeks | Improved |
| Death | 3 | 7 months/9 months/6 months | NA |
|
| |||
Three patients passed away during the 12 months follow-up. Two death were as a result of pulmonary emboli and the third one was due to the lethal nature of the disease. IV; Intravenous and NA: Not applicable.
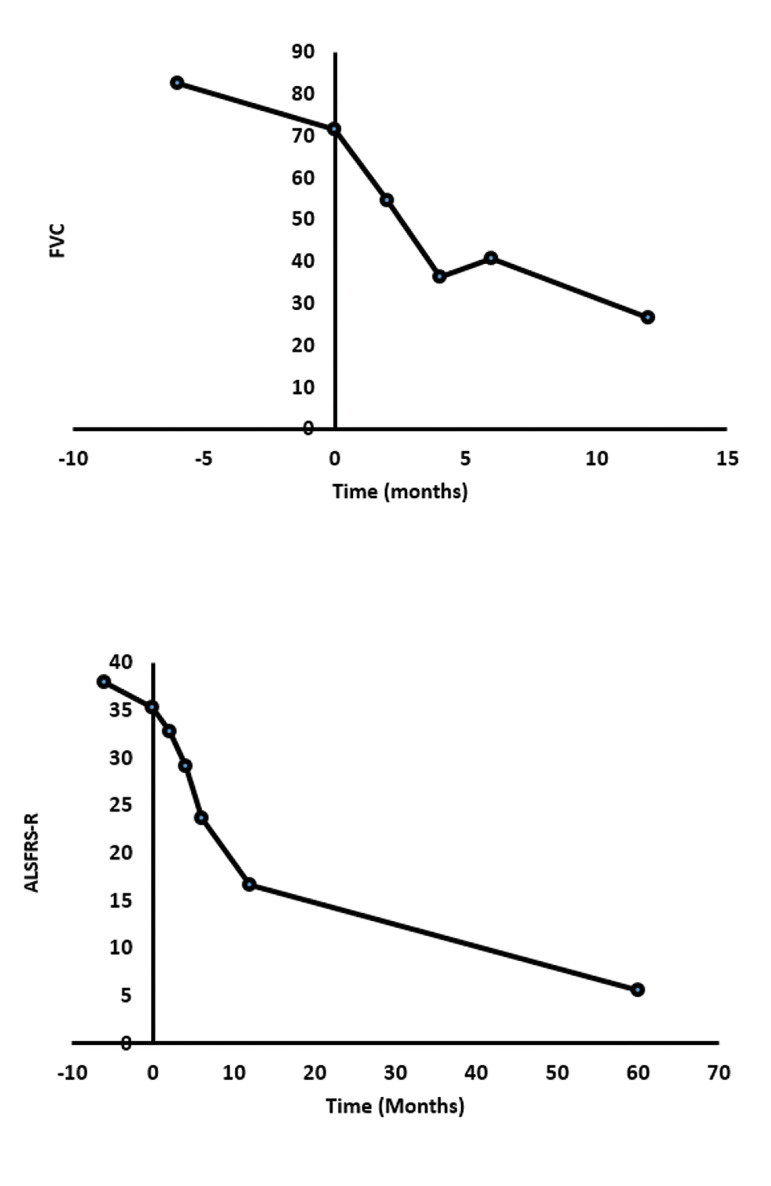
ALSFRS-R and FVC levels decreased in all patients during 12 months follow-up which indicated disease progression (Fig .2).
Fig.2.
Trends of forced vital capacity (FVC) and revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R) percent during 12 months follow-up. FVC percent declined rapidly up to 4 months after infusion, but we can see a moderate elevation between months 4 and 6. After 6 months, the progression of respiratory disturbances was observed. ALSFRS-R score, significantly decreased after infusion. Data are mean ± SEM.
The only point is that between 4 and 6 months of follow-up, the mean FVC score was increased slightly suggesting that a booster dose of injection may be necessary for these patients.
We also followed patients up for five years after transplantation. Fortunately, five patients survived, and unfortunately, nine more patients passed away. The ALSFRS-R scores were recorded for the remained patients who demonstrated a decreasing trend with a modified slope (Fig .2).
ALS is a progressive neurodegenerative disorder in which neuro- inflammation plays an important role. Stem cells have been raised as a potential treatment modality for this disease. MSCs with their anti-inflammatory effects are one of the best and most useful candidates. MSCs prevent apoptosis in neurons and oligodendrocytes using their paracrine effect and releasing trophic and anti-apoptotic factors, resulting in providing a neuroprotective microenvironment (1).
Moreover, adult MSCs are less prone to genetic abnormalities and malignant transformation during multiple passages (3).
Several preclinical studies have proved that the intrathecal, intraspinal, intravenous, or combined (intraspinal and intravenous) administration of MSCs (either single or repeated applications) are safe procedures (14-16). MSCs application can postpone deterioration of the motor neuron function that can make a bridge for ALS patients in the future. Moreover, this intervention can increase the survival rate in symptomatic transgenic animals. MSCs have anti-inflammatory effects, and can induce the secretion of specific cytokines and growth factors that contributes to cell survival rather than cell replacement (17).
Since the bone marrow puncture is an invasive procedure for these patients and considering the potential advantages of allogeneic stem cells in the literature (18), we preferred allogeneic MSC-based therapy. MSCs were isolated from the SVFs of adipose tissue from a healthy donor.
Autologous MSCs are a great therapeutic option for treating many chronic diseases; however, cell expansion makes the procedure slow and expensive. Application of allogeneic MSCs would be more feasible, cost-benefit, and logistically doable, and more convenient. The important disadvantage of using these cells can be the possibility of alloreactivity. However, MSCs, are immune-privileged cells. As a result, non-matched MSCs are better tolerated than other cell types. There are no reports for alloreactivity of MSCs in animal studies, and transplanted MSCs showed the same results for autologous and allogeneic cells (18).
Recently, Barczewska et al. (19, 20), have injected allogeneic Wharton’s jelly-derived MSCs in patients with ALS intrathecaly, and reported that it was safe and more effective in some patients.
Adipose tissue-derived stem cells (Ad-MSCs), like other stem cells are capable of self-renewing and differentiating into various cells. Ad-MSCs are abundant, available, and easily obtained by liposuction with minimal morbidity. A total number of 1-10% of nucleated cells in adipose tissue are Ad-MSCs, whereas only 0.01% of nucleated cells in bone marrow (BM) are MSCs. Moreover, donor age does not influence the phenotype and function of Ad-MSCs, but in elderly individuals, it negatively affects the function of BM-MSCs (21).
There are lots of clinical studies that have shown the feasibility, safety, and efficacy of stem cells injection in patients with motor neuron diseases through different mechanisms (8, 9, 22, 23).
The optimal route of stem cell administration in generaland particularly MSCs-in patients with neurological diseases remains disputable. Some investigators have asserted that IV injection may be a competent route because MSCs apply their immunomodulating effects systemically and may also migrate through the blood to the damaged tissues in CNS after drawing inflammatory signals (23-25).
A possible challenge of the IV administration of MSCs is that most of the injected cells into the blood will be trapped into the lungs, lymph nodes, and other tissues at the beginning (23).
In our phase I, IIa open-label clinical trial, allogeneic Ad-MSCs were administered intravenously for 17 patients with ALS to evaluate the feasibility, safety, and efficacy of this method. Patients were followed for 12 months after cell infusion. We did not detect any severe adverse event during 12 months follow-up. After that for long-term follow-up, the five years’ survival rate was evaluated. Two patients experienced pulmonary emboli which may not be definitely related to the cell infusion because according to the previous articles, venous thromboembolism is common in patients with ALS, particularly those with leg weakness and reduced mobility (35%/year) (26-28). The other point is that we didn’t have any control group to compare the rate of emboli between the two groups. However, we are going to design another study with similar conditions and inject 5000 IU heparin 30 minutes before cell infusion to decrease the risk of DVT or emboli in patients.
Five-year follow-up of these patients showed progression in different aspects of the disease and nine patients passed away within the four years. According to the high mortality rate of this disease (the average lifespan after diagnosis is 3-5 years) (29, 30), in our current study, a total number of 12 out of 17 patients died after five years. Meanwhile, even though the mean basic ALSFRS-R score of the patients was similar to our previous study, the survival rate was increased in the current study (12).
All twelve patients died after five years in the previous study. In our previous study, patients received autologous BM-MSC, IV and intrathecaly. ALSFRS-R score decreased rapidly in almost all patients. The mean ALSFRS-R in the five survived patients was 18 ± 4.243.
Two young patients who were at the first stages of the disease at the time of infusion reported almost good scores after five years. Perhaps younger patients with an early diagnosis can benefit more from stem cell therapy.
Overall, we can conclude that administrating allogeneic Ad-MSCs is a rather safe and feasible procedure in patients with ALS. Our study did not prove how effective this procedure could be in terms of reversing or relenting the disease progression.
Being open-label and also the low number of patients were limitations of the current study. Some patients showed temporary mild improvement in some symptoms such as muscle atrophy, and also hand dysfunction, but since this trial was not blinded or placebo-controlled, these effects cannot be interpreted as a positive response to MSCs therapy. Designing a study with more participants and a blinded design can help researchers to evaluate the benefits of MSCs therapy in these patients in the future.
Acknowledgements
This work financial supported by Royan Institute. There is no conflict of interest in this study.
Authors’ Contributions
S.M.N., N.A., M.V.; Developed the concept and designed the study. S.K., L.A.; Drafted the manuscript which was revised by S.M.N., and M.V. L.S.; Contributed in data preparing. S.M., V.A., N.J.; Contributed in MSC preparation and deliver it to the hospital. A.Gh.; Contributed in data analysis and interpreting. S.-E.H.; Contributed in admission of patients and injecting the product intravenously for them. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
References
- 1.Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, Voříšek I, et al. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–658. doi: 10.3727/096368916X693716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13(1):1–16. doi: 10.1186/s12967-014-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HY, Kim H, Oh KW, Oh SI, Koh SH, Baik W, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells. 2014;32(10):2724–2731. doi: 10.1002/stem.1770. [DOI] [PubMed] [Google Scholar]
- 4.Nabavi SM, Arab L, Jarooghi N, Bolurieh T, Abbasi F, Mardpour S, et al. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J. 2018;20(4):592–592. doi: 10.22074/cellj.2019.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31(3):395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Baek W, Kim YS, Koh SH, Lim SW, Kim HY, Yi HJ, et al. Stem cell transplantation into the intraventricular space via an Ommaya reservoir in a patient with amyotrophic lateral sclerosis. J Neurosurg Sci. 2012;56(3):261–263. [PubMed] [Google Scholar]
- 7.Blanquer M, Pérez-Espejo MA, Martínez-Lage JF, Iniesta F, Martinez S, Moraleda JM. A surgical technique of spinal cord cell transplantation in amyotrophic lateral sclerosis. J Neurosci Methods. 2010;191(2):255–257. doi: 10.1016/j.jneumeth.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Huang H, Zhang J, Zhang F, Liu Y, Xi H, et al. Short-term outcome of olfactory ensheathing cells transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):961–966. [PubMed] [Google Scholar]
- 9.Moviglia GA, Moviglia-Brandolino MT, Varela GS, Albanese G, Piccone S, Echegaray G, et al. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(1_suppl):57–63. doi: 10.3727/096368912X633770. [DOI] [PubMed] [Google Scholar]
- 10.Cho GW, Noh MY, Kim HY, Koh SH, Kim KS, Kim SH. Bone marrow- derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cells Dev. 2010;19(7):1035–1042. doi: 10.1089/scd.2009.0453. [DOI] [PubMed] [Google Scholar]
- 11.Mei L, Shen B, Ling P, Liu S, Xue J, Liu F, et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS One. 2017;12(4):e0176107–e0176107. doi: 10.1371/journal.pone.0176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabavi SM, Arab L, Jarooghi N, Bolurieh T, Abbasi F, Mardpour S, et al. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J. 2019;20(4):592–598. doi: 10.22074/cellj.2019.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function.BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169(1-2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 14.Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91–91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossein-Khannazer N, Torabi S, Hosseinzadeh R, Shahrokh S, Asadzadeh Aghdaei H, Memarnejadian A, et al. Novel cell-based therapies in inflammatory bowel diseases: the established concept, promising results. Hum Cell. 2021;34(5):1289–1300. doi: 10.1007/s13577-021-00560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vosough M, Moossavi S, Mardpour S, Akhlaghpoor S, Azimian V, Jarughi N, et al. Repeated intraportal injection of mesenchymal stem cells in combination with pioglitazone in patients with compensated cirrhosis: a clinical report of two cases. Arch Iran Med. 2016;19(2):131–136. [PubMed] [Google Scholar]
- 17.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karantalis V, Schulman IH, Balkan W, Hare JM. Allogeneic cell therapy: a new paradigm in therapeutics. Circ Res. 2015;116(1):12–15. doi: 10.1161/CIRCRESAHA.114.305495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barczewska M, Grudniak M, Maksymowicz S, Siwek T, Ołdak T, Jezierska-Woźniak K, et al. Safety of intrathecal injection of Wharton’s jelly-derived mesenchymal stem cells in amyotrophic lateral sclerosis therapy. Neural Regen Res. 2019;14(2):313–318. doi: 10.4103/1673-5374.243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barczewska M, Maksymowicz S, Zdolińska-Malinowska I, Siwek T, Grudniak M. Umbilical cord mesenchymal stem cells in amyotrophic lateral sclerosis: an original study. Stem Cell Rev Rep. 2020;16(5):922–932. doi: 10.1007/s12015-020-10016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrabani D, Mehrabani G, Zare S, Manafi A. Adipose-derived stem cells (ADSC) and aesthetic surgery: a mini review. World J Plast Surg. 2013;2(2):65–70. [PMC free article] [PubMed] [Google Scholar]
- 22.Appel SH, Engelhardt JI, Henkel JS, Siklos L, Beers DR, Yen AA, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71(17):1326–1334. doi: 10.1212/01.wnl.0000327668.43541.22. [DOI] [PubMed] [Google Scholar]
- 23.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39(3):229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue M, Honmou O, Oka S, Houkin K, Hashi K, Kocsis JD. Comparative analysis of remyelinating potential of focal and intravenous administration of autologous bone marrow cells into the rat demyelinated spinal cord. Glia. 2003;44(2):111–118. doi: 10.1002/glia.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beam DM, Courtney DM, Kabrhel C, Moore CL, Richman PB, Kline JA. Risk of thromboembolism varies, depending on category of immobility in outpatients. Ann Emerg Med. 2009;54(2):147–152. doi: 10.1016/j.annemergmed.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Elman LB, Siderowf A, Houseman G, Kelley M, McCluskey LF. Venous thrombosis in an ALS population over four years. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(4):246–249. doi: 10.1080/14660820510043226. [DOI] [PubMed] [Google Scholar]
- 28.Gladman M, Dehaan M, Pinto H, Geerts W, Zinman L. Venous thromboembolism in amyotrophic lateral sclerosis: a prospective study. Neurology. 2014;82(19):1674–1677. doi: 10.1212/WNL.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 30.Yedavalli VS, Patil A, Shah P. Amyotrophic lateral sclerosis and its mimics/variants: a comprehensive review. J Clin Imaging Sci. 2018;8:53–53. doi: 10.4103/jcis.JCIS_40_18. [DOI] [PMC free article] [PubMed] [Google Scholar]