Summary
Dynamic tuning of terahertz (THz) wave is vital for the development of next generation THz devices. Utilization of solar energy for tuning THz waves is a promising, eco-friendly, and sustainable way to expand THz application scenarios. Ti2O3 with an ultranarrow bandgap of 0.1eV exhibits intriguing thermal-induced metal-insulator transition (MIT), and possesses excellent photothermal conversion efficiency. Herein, Ti2O3 film was fabricated by a two-step magnetron sputtering method, and exhibited an excellent photothermal conversion efficiency of 90.45% and demonstrated temperature-dependent THz transmission characteristics with a wideband at 0.1–1 THz. We supposed to combine photothermal conversion characteristics with temperature-dependent THz transmission properties of Ti2O3 film, which could introduce solar light as the energy source for tuning THz waves. Our work will provide new sight for investigating MIT characteristics of Ti2O3 at THz regime and exhibit huge potential in the application of tuning terahertz waves in outdoor scenarios in the future.
Subject areas: Radiation physics, Radiation sources, Nanotechnology
Graphical abstract

Highlights
-
•
Multilayer (TiO2-Ti)n films were annealed to prepare Ti2O3 film
-
•
Ti2O3 film exhibited an excellent photothermal conversion efficiency of 90.45%
-
•
The temperature-dependent THz transmission properties with a wideband at 0.1–1 THz
-
•
THz wave could be dynamically modulated by a sunlight-controlled system
Radiation physics; Radiation sources; Nanotechnology
Introduction
Terahertz (THz) spectral band (30–3000μm) belongs to one of the most promising regions of the electromagnetic spectrum which is located between microwave and infrared regions. In recent years, THz technologies have achieved unprecedentedly rapid development due to the outstanding optoelectronic properties of THz waves. Terahertz wave is very promising in biological detection applications because of its low invasiveness, non-destructive, and non-poisonous interaction with biological tissues (Ahmadivand et al., 2020; Smolyanskaya et al., 2018). It has also been applied in non-destructive testing due to its penetrative power through a number of highly relevant materials in industry and science (Smolyanskayaet al., 2018). Meanwhile, THz technologies are expected to meet the ever-increasing demand for high-capacity wireless communications for their much larger available bandwidth (Federici and Moeller, 2010; Koenig et al., 2013; Nagatsuma et al., 2016). What's more, terahertz spectroscopy can probe charge carrier dynamics in semiconductors, spin, and lattice oscillations in solids (Batignani et al., 2018; Cocker et al., 2021). As a result, there has been a huge demand for tunable devices at THz frequencies, as they provide tremendous opportunities for a wide range of THz applications.
To achieve the function of dynamically tuning THz waves in devices, THz waves need to be controlled, including switching, modulating, phase shifting etc (Chen et al., 2018; Manjappa et al., 2019; Zhang et al., 2015). Perovskites, two-dimensional materials and phase transition materials have been extensively used to realize these functions because of their tunable optical properties (Chen et al., 2018; Pitchappa et al., 2019; Spaldin and Ramesh, 2019). Generally, dynamic tuning of THz wave is realized by external artificial excitation sources, such as electric field, temperature, and laser (Cong et al., 2014; El Fatimy et al., 2016; Zhang et al., 2012). With the development of THz technologies, efficient utilization of solar energy to regulate THz waves is a promising tuning approach. Nevertheless, introducing solar energy as the tuning of THz waves energy source has not been reported to our knowledge.
Recently, Ti2O3 has attracted widespread attention in many research fields because it not only exhibits intriguing metal-insulator transition but also has an ultranarrow bandgap of 0.1eV(Huang et al., 2020; Li et al., 2019; Zeng et al., 2019). Specifically, Ti2O3 exhibits excellent photothermal conversion performance due to its narrow bandgap, which is capable of harvesting light across solar spectra (Wang et al., 2017). Ti2O3 has also exhibited high performance in mid-infrared photodetection in conjunction with graphene taking advantage of its highly efficient broadband absorption and the fast carrier transport in graphene/Ti2O3interface (Yu et al., 2018). In addition, Ti2O3 has attracted considerable attention because it exhibits intriguing metal-insulator transition (MIT) in the temperature range of 300–450K, which is accompanied by significant changes in optical, electric, and magnetic properties (Honig and Reed, 1968; Li et al., 2019; Nandi et al., 2020). During the transition, the changes in optical, electric and magnetic properties can be attributed to the lattice parameter alteration (Yan et al., 2012). With the variation of lattice, the crossing of bands at different points in the Brillouin zone results in the disappearance of an energy gap (Vanzandt et al., 1968). All these properties make Ti2O3 a promising candidate for applications in tunable optoelectronic devices. Particularly, the metal-insulator transition characteristics of Ti2O3 are similar to another transition metal oxide, VO2, which has attracted great attention and exhibits advanced THz modulation properties(Liang et al., 2019; Wang et al., 2019). However, to our knowledge, the research on Ti2O3 in the terahertz regime has not been reported so far.
In this work, Ti2O3 film was fabricated on SiO2 substrate by a two-step magnetron sputtering method. The Ti2O3 film exhibits excellent photothermal conversion performance with conversion efficiency of 90.45%. Besides, the THz transmission characteristics were investigated through a thermal-induced MIT of Ti2O3 film. Apparently, the photothermal conversion and thermal-induced MIT of Ti2O3 film can be related. Therefore, we supposed to combine photothermal conversion characteristics with temperature-dependent THz transmission properties of Ti2O3 film, which could realize sunlight-tuning THz waves. This work opens up the new concept of investigating the MIT transition of Ti2O3 and would meet the requirements of tuning of THz waves in outdoor scenarios in the future.
Results
Fabrication and material characterizations of the Ti2O3 film
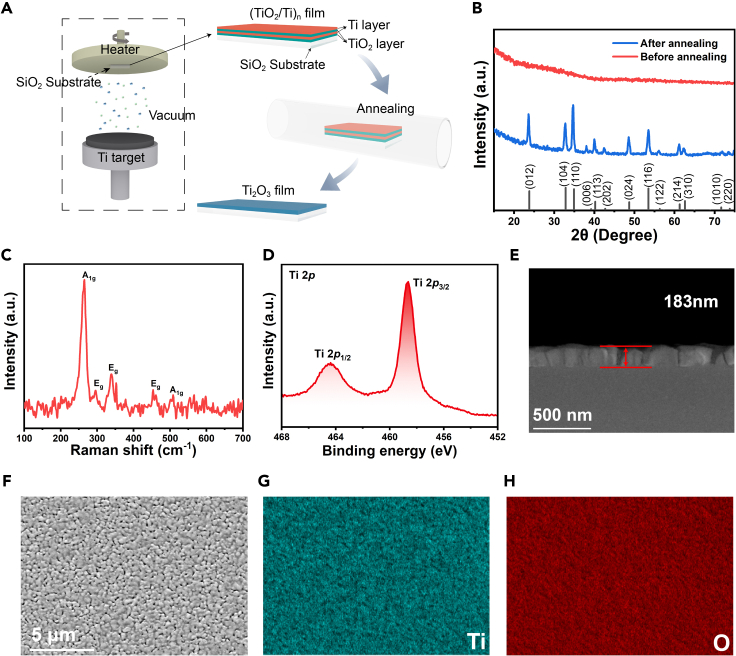
In our experiments, Ti2O3 films were prepared by a two-step magnetron sputtering method, as illustrated schematically in Figure 1A. Briefly, the (TiO2-Ti)n films were initially obtained by magnetron sputtering, and the appropriate proportion of TiO2 and Ti was obtained by controlling the presence or absence of oxygen in the sputtering process. Subsequently, the (TiO2-Ti)n films were annealed to obtain the Ti2O3 films. To further investigate the microstructural characteristics and recognize the chemical conversion during fabrication, we examined the morphology, phase, elementary mapping, and composition of the Ti2O3 film (seeSTAR Methods).
Figure 1.
Fabrication and structural characterizations of the Ti2O3film
(A) Schematic diagram of the fabrication processes of Ti2O3 film.
(B) XRD pattern of Ti2O3 film before and after annealing, respectively.
(C) Raman spectrum of the Ti2O3 film.
(D) XPS of the Ti2O3 film, depicting the Ti 2p peak.
(E) Cross-sectional SEM image of Ti2O3 film with thickness of 183 nm.
(F–H) SEM images of Ti2O3 film with thicknesses of 183 nm and its corresponding elemental mapping images: Ti, O, elements.
Grazing incidence X-ray diffraction (GIXRD) was used to examine the crystalline structure and phase composition of the prepared film on SiO2 substrate before and after annealing. Figure 1B shows the XRD patterns of TiO2-Ti films prepared by magnetron sputtering. The film shows no detectable diffraction peaks, illustrating that the film was amorphous due to the low mobility of atoms at the low deposition temperature. As shown in Figure 1B after annealing at 1050°C for 2h, the XRD pattern of annealed film shows obvious diffraction peaks, which are indexed to Ti2O3 in a standard card (PDF#71-1055). No other peaks were found, which indicates that the prepared Ti2O3 film is of high purity and free of impurities. We also investigated the effect of proportion of TiO2 and Ti on film composition after annealing, shown in Figure S2. The results indicated that the higher the ratio of TiO2 to Ti, the higher the oxidation degree of the final composition of the film would be. Conversely, the lower the ratio of TiO2 to Ti, the lower the oxidation degree of the final composition of the film would be. Moreover, the Raman spectra of as-prepared film are shown in Figure 1C. As shown in Figure 1C, for the as-prepared Ti2O3 film, the Raman peaks at 296.2 cm−1, 338.6 cm−1, and 453.3 cm−1, can be assigned to the Eg modes, and the peak at 264.3 cm−1 and 508.3 cm−1, can be assigned to the A1g modes (Li et al., 2018; Wang et al., 2017). All of the observed peaks can be identified as Eg and A1g modes of Ti2O3, indicating the high crystallization and phase purity of the Ti2O3film (Li et al., 2018). X-ray photoelectron spectroscopy (XPS) measurements were carried out to elucidate the surface elemental composition and chemical states of the Ti2O3 film. As shown in Figure 1D, the 2p spectra of the Ti at 458.63 and 464.53 eV are the spin orbits caused by the double peaks of Ti 2p3/2 and Ti 2p1/2. The positions of the XPS peaks are consistent with those of corundum Ti2O3 single crystals, indicating a dominant Ti valence state of 3+ (Li et al., 2018).
The surface morphologies of Ti2O3 film were investigated using FESEM and the thickness of the film was examined by cross-sectional SEM. Figure 1E shows the cross-sectional SEM images of Ti2O3 film, with the thickness of approximately183 nm. We also performed the thickness of film with a deposition time of 30 min before and after annealing. There is almost no change in the thickness of the film before and after annealing and the deposition rate of film is approximately 3 nm/min. As shown in Figure 1F, the surface morphologies of Ti2O3 film are relatively compact, and the distribution of particle size is not very uniform. This difference in crystal size is possibly due to grain growth during annealing. The energy-dispersive X-ray spectroscopy (EDS) shown in Figures 1G and 1H was utilized to confirm the elemental composition of the same region in the corresponding SEM image (Figure 1F). As can be seen, the elements of Ti and O are homogeneously distributed on the surface of the film, indicating the uniformity of the composition of the film.
Photothermal conversion performance of Ti2O3 film
Subsequently, the photothermal conversion performance of Ti2O3 film was investigated. Figure 2A presents the UV-vis-NIR absorption spectra (200–2000 nm) of Ti2O3 film and TiO2 film for contrast. As can be seen, the Ti2O3 film shows excellent absorption features in the region of visible and NIR spectrum, which is in contrast to TiO2 which only absorbs light in the UV region. Next, we used commercial simulated sunlight (0.5 W/cm2) as the light source, and used an infrared camera to record the surface temperature of the films under illumination to evaluate the photothermal effect performance of the Ti2O3 film. Upon the arrival of the equilibrium temperature of films, which was determined when the film temperature fluctuation was less than 2°C, the light was shut down and cooling process was initialized. Figure 2B shows the total temperature profile of films in response to photothermal heating and subsequently natural cooling. As can be seen, there was a sharp temperature rise of the Ti2O3 film as soon as the simulated solar light was turned on, which is an indicator of instantaneous heat convection within the Ti2O3 film. The surface temperature of Ti2O3 film stabilized at 95°C after 3 min of solar light illumination. By contrast, under the identical illumination condition, the surface temperature of TiO2 film was heated to about 42°C. Inset in Figure 2B presents the IR photo of the Ti2O3 film and TiO2 film after 10 min of solar light illumination. The temperature change (ΔT) of TiO2 film is about 23.5°C; however, the ΔT of Ti2O3 film is about 76.5°C, which is about 3.3 times more than the TiO2 film. In our case, Ti2O3 film with the substantial enhancement of visible and near-infrared light absorption and the excellent photothermal performance might be attributed to the narrow-bandgap structure which enables Ti2O3 film to absorb the full solar spectrum (Wang et al., 2017).
Figure 2.
Photothermal conversion performance of Ti2O3film
(A) UV-vis-NIR absorption spectra of Ti2O3 film and TiO2 film.
(B) Surface temperatures of TiO2 film and Ti2O3 film under solar illumination of 0.5 W/cm2 as a function of illumination time tested by an IR camera. Inset in (B) is the IR photograph of Ti2O3 film and TiO2 film under illumination from a solar simulator.
(C) The temperature relationship against time with or without 800 nm laser irradiation. Inset in (C) is the IR photograph of Ti2O3 film with 808 nm laser irradiation at 3 W cm−2.
(D) Fitting plots of the temperature curve under 808 nm laser irradiation by Equation (6).
To further evaluate the photothermal conversion efficiency of Ti2O3 film, the Ti2O3 film was exposed to an 808 nm laser at a power density of 3 W cm−2 recording its temperature change. We used near-infrared 808nm laser as the light source, and used an infrared camera to record the surface temperature of the film under irradiation. As shown in Figure 2C, upon irradiation, the temperature of the surface of the Ti2O3 film rapidly increases due to the local heat generation on the surface by photothermal conversion. Under the laser irradiation of 3 W cm−2, a stable surface temperature of Ti2O3 film at 142°C is achieved in 5 min. Inset in Figure 2C presents the IR photo of the Ti2O3 film after 10 min of 808 nm laser irradiation. The Ti2O3 film was irradiated with NIR laser for 10 min, followed by turning off the laser for cooling the film to room temperature naturally. In this case, the increased temperature (ΔT) of Ti2O3 film is about 121.4°C. The photothermal conversion efficiency η of Ti2O3 film was calculated from the thermal profile of Figure 2C via the energy balance function (Bi et al., 2020; Ren et al., 2015, 2017; Wang et al., 2017):
| (Equation 1) |
where m and Cp are the mass and heat capacity, respectively. The suffix “i” of m and Cp refers to SiO2 substrate and Ti2O3 film. T is the temperature, and t is time. is the photothermal energy absorbed by Ti2O3 film. Qsurr is thermal energy lost to the surroundings. Details of the calculation are given in the experimental section. According to the equation, the photothermal conversion efficiency of Ti2O3 film was calculated to be 90.45% under 808 nm laser irradiation.
MIT characteristics of the Ti2O3 film
We carried out in-situ temperature-dependent XRD measurement under vacuum to investigate the thermal-induced MIT characteristics of the Ti2O3 film. Figure 3A shows the temperature-dependent XRD profiles that range from 30 to 200 K at a heating rate of 5°C/min with a holding time of 3 min at each temperature. As can be seen, the XRD profiles of Ti2O3 film at different temperatures are analogous, which indicates that the crystal structure of Ti2O3 has not changed. However, the positions of some peaks on the XRD spectrum have shifted. The shift of the diffraction peaks is not unidirectional, but it shifts on both the lower as well as higher 2θ values. Figure 3B shows the XRD profiles of (110) and (006) planes of Ti2O3 film at different temperatures. As can be seen, the (110) plane of Ti2O3 shifts to a higher diffraction angle and the (006) plane of Ti2O3 shifts to a lower diffraction angle with the increase of temperature. Generally, the right shift of the XRD peak position indicates the decrease of the interplanar spacing, and the corresponding left shift indicates the increase of the interplanar spacing. Meanwhile, the change of interplanar spacing is related to the lattice parameters. Ti2O3 has a corundum structure, and its interplanar spacing calculation equation is:
| (Equation 2) |
Figure 3.
Thermal-induced MIT characterizations of the Ti2O3 film
(A and B) In-situ temperature-dependent XRD profiles of Ti2O3 film ranging from 30°C to 200°C and (B) magnified versions of XRD data depicted in 32°–42°.
(C) The temperature-dependent sheet resistance measured on the Ti2O3 film upon heating and cooling.
Therefore, in our case, the peak position of (110) plane shifts to a higher diffraction angle and the peak position of (006) plane shifts to a lower diffraction angle with an increase of temperature, indicating the lattice parameter “a” decreases and the lattice parameter “c” increases, respectively. This result reveals the variation of lattice parameters during the temperature-induced MIT transition process of Ti2O3 film, which exhibits similar MIT transition characteristics of Ti2O3particles (Rice and Robinson, 1976).
Next, to investigate the thermal-induced MIT characteristics of Ti2O3 film, we carried out temperature-dependent square resistance measurement. Figure 3C shows the square resistance of Ti2O3 film against temperature from 25°C to 200°C. As can be seen, when the temperature increased from 25°C to 200°C, the square resistance of the Ti2O3 thin film decreased from 390.2 Ω/□ to 86 Ω/□. Alternatively, the square resistance increased from 85.9 Ω/□ to 391.4 Ω/□ with a temperature reduction from 200°C to 25°C. In this case, the temperature-dependent resistivity characteristic indicates the thermal-induced MIT of the Ti2O3 film, and the change of resistivity can be attributed to the change in electronic structure of Ti2O3 caused by temperature-induced lattice deformation (Honig, 1968; Vanzandtet al., 1968; Yoshimatsu et al., 2020). Notably, the resistance change curves during heating and cooling almost coincide, indicating that there is no hysteresis during the metal-insulator transition. Compared with VO2, which exhibits the hysteresis loop of the square resistance against temperature (Liang et al., 2019; Zhu et al., 2020), Ti2O3 film is expected to achieve an extensive range of optoelectronic applications. The mid-infrared transmittance properties of the Ti2O3 film on SiO2 substrate were analyzed.
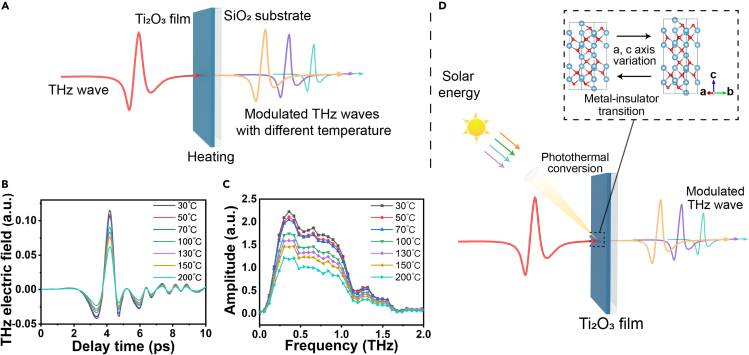
THz transmission characteristics of the Ti2O3 film
With the behavior of temperature-dependent resistance for the Ti2O3 film, which would be quite suitable for application in dynamic electronic devices specially due to the sensitivity of the THz wave to the resistivity of the transmission medium, this kind of material with a dynamic change of resistivity would be available for the tuning of the THz transmission (Shi et al., 2016; Zhu et al., 2018). To investigate the THz transmission properties of the Ti2O3 film, a THz time-domain spectroscopy combined with a heating system was used to measure the THz transmission tuning properties of Ti2O3 film during MIT. Figure 4A shows the schematic of the THz time-domain system. The THz wave penetrates the center of the Ti2O3 film vertically. By heating the film to different preset temperatures under air atmosphere, the THz transmission time-domain signals were recorded and then Fourier transformed to the frequency-domain spectra. As shown in Figure 4B, the amplitude of THz transmission time-domain signals decreased gradually with increasing temperature and the transmission amplitude tuning depth can reach around 45.8%. Theoretically, the decrease in the THz transmission amplitude could be attributed to the increased absorption and reflection loss by free electrons (Mackenzie et al., 2018; Morimoto et al., 2019). In our case, the decrease in the THz transmission amplitude was caused by the increase in conductivity of Ti2O3 film during the MIT transition, which is consistent with the tendency of resistivity vs temperature curves in Figure 3C. Using a fast Fourier transform of the time-domain signals, we were able to obtain the THz frequency-domain spectra of the Ti2O3 film. As shown in Figure 4C, the THz transmission frequency-domain signals amplitude decreased monotonically with the increase of temperature. Similarly, the decrease in the THz transmission amplitude was ascribed to the decrease in resistivity of Ti2O3 film during the MIT transition. The signal intensity in the frequency-domain spectrum also indicated the temperature-induced MIT of Ti2O3 film. In addition, the tuning of THz transmission presents a wideband characteristic across the 0.1–1.1 THz range.
Figure 4.
THz characterizations of the Ti2O3 film
(A) Schematic of the THz wave transmitted through the Ti2O3 film deposited on SiO2 substrate.
(B and C) THz transmission time-domain spectra and (C) frequency-domain spectra of Ti2O3 film upon heating from 30°C to 200°C.
(D) Schematic illustration of tuning THz waves scenario under sunlight illumination.
Naturally, considering the excellent photothermal conversion properties and temperature-dependent THz transmission characteristics of Ti2O3 film, we envisage combining these two features. As a prospect, let us consider a sunlight-controlled tuning of THz waves system with Ti2O3 film, which will expand opportunities to use THz wave scenarios in the future. As shown in Figure 4D, under the illumination of sunlight, Ti2O3 film could absorb solar energy to increase its temperature due to the excellent photothermal conversion performance. Subsequently, the metal-insulator transition was triggered by the variation of temperature. Finally, the tuning of THz amplitude was realized by the sunlight illumination. As for the application scenario, a sunlight-controlled THz wave switch could be utilized in outdoor. And the sunlight intensity can be adjusted through lens to control the MIT process of Ti2O3 film, so that THz amplitude can be modulated. Besides, due to the sensitivity of Ti2O3 film to light, the variation of THz amplitude also reflects the change of light intensity. By collecting changes in terahertz signals, the corresponding devices can be controlled to respond to changes in ambient light intensity. This kind of utilizing solar energy to modulate THz waves amplitude requires no external energy sources, which could save energy or be utilized in emergencies. This kind of tuning THz waves method would expand the application range of terahertz technologies, and exploit a novel route toward achieving dynamic tuning of THz waves.
Conclusions
In summary, we demonstrated a two-step magnetron sputtering method for the fabrication of Ti2O3 film, investigated its photothermal conversion performance, and then demonstrated the THz transmission properties of Ti2O3 film across this MIT transition. The fabricated Ti2O3 film exhibited an excellent photothermal conversion efficiency of 90.45%. Moreover, theTi2O3 film can achieve a THz tuning depth of nearly 45.8% with a wideband at 0.1–1THz owing to the change in conductivity of Ti2O3 film during the MIT transition. Therefore, based on the excellent photothermal conversion properties and temperature-dependent THz transmission characteristics of Ti2O3 film, we envisage applying the high-performance photothermal conversion characteristics to tuning of THz waves, which could realize solar light-tuning THz waves. Our work not only discloses the thermal-induced MIT transition of Ti2O3 through THz transmission characteristics but also opens a venue for expansion of THz application scenarios.
Limitations of the study
In this paper, Ti2O3 polycrystalline film was fabricated on SiO2 substrate by a two-step magnetron sputtering method. We have demonstrated the application potential of Ti2O3 film in the field of sunlight-tuning THz waves. Therefore, the performance of the Ti2O3 film on devices needed to be evaluated. Compared with polycrystalline film, epitaxial films with highly ordered atomic arrangement demonstrate unusual optical or electrical properties. Further work needs to be done to investigate the performance of Ti2O3 epitaxial film. Besides, the deposition process for the high-quality epitaxial films and the performance optimization of Ti2O3 film will be the direction of our continued research.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Ti Target (99.995%) | ZhongNuo Advanced Material Technology Co.,Ltd | http://www.znxc.com/ |
| SiO2 single crystal substrates (110) | HeFei Crystal Technical Material Co.,Ltd | http://www.ctmwafer.com/ |
| Argon (Ar) (99.999%) | Sichuan Qiaoyuan Gas Co.,Ltd | CAS: 7440-37-1 |
| Oxygen (O2) (99.99%) | Sichuan Qiaoyuan Gas Co.,Ltd | CAS: 7782-44-7 |
| Ethanol | Chengdu Chron Chemicals Co.,Ltd | CAS: 64-17-5 |
| Other | ||
| GIXRD, Rigaku D Max 2500 Pc Powder X Ray Diffractometer | Rigaku | https://www.rigaku.com/products/xrd/smartlab |
| XPS, Thermo Scientific K-Alpha Surface Analysis | Thermal Fisher Scientific | https://www.thermofisher.cn/cn/zh/home.html?cid=ebz_bus_sbu_r04_cn_0se_bdk_bz_pur_gene_aBrand_pc_hp_051021_title |
| UV-Visible Spectrophotometer, Shimadzu UV-3600i Plus | Shimadzu | https://www.shimadzu.com.cn/an/spectro/uv/uv-3600iplus/index.html |
Resource availability
Lead contact
Further information and requests for resources and materials should be directed to the lead contact, Wanxia Huang (huangwanxia@scu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Method details
Fabrication of film
The preparation processes of Ti2O3 film were divided into two main stages: firstly, the (TiO2-Ti)n films were fabricated by magnetron sputtering, and then the prepared films were annealed to obtain the Ti2O3 films. Specifically, as schematically illustrated in Figure 1A, the (TiO2-Ti)n films were deposited by magnetron sputtering, in which the argon plasma was used to systematically bombard a titanium target (purity = 99.995%) in the presence or absence of oxygen to form the TiO2 and Ti layer respectively. In the magnetron sputtering process, in every five-minute, the oxygen presence and oxygen absence time were 210 and 90 seconds respectively. Single crystal SiO2(001) sheets were used as substrates, which were cleaned by sonication in acetone, ethanol and deionized(DI) water in sequence for 30 minutes each, and dried in an oven at 80°C for 30 min. After loading the substrates into the sputtering chamber, the distance between the target and substrates was set as 10 cm, and then the chamber was heated to 100°C to remove residual water and evacuated to a base pressure of below 8 × 10−4 Pa. A flow of 200 sccmAr was used to keep the deposition pressure maintained at 0.30 Pa. The target material was presputtered for 5 minutes before deposition to remove surface oxides and contaminants. Thereafter, the TiO2-Ti films were placed in a molybdenum boat and annealed at 1050°C in argon (purity of 99.999%) at a flow rate of 400 sccm for 2 h and cooled down to room temperature naturally to obtain the Ti2O3 film.
Material characterizations
Grazing incidence X-ray diffraction(GIXRD) patterns were recorded using a Rigaku D/Max-2500/PC setup with a Cu Kα (0.15418 nm) radiation. In-situ temperature-dependent XRD profiles of films were measured with a BrukerD8 Advance diffractometer, using CuKa radiation. Raman measurements were carried out with a confocal micro-Raman system (Horiba Aramis/ JobinYvonHR800) at ambient conditions, using a solid state laser (excitation wavelength is 532 nm). X-ray photoelectron spectroscopy (XPS) analysis was also performed to characterize the electronic structure and Ti-O hybridization on the Ti2O3 films' surfaces. Scanning electron microscopy (SEM) images and corresponding element mapping/EDS spectrum were obtained on a field-emission microscope (Inspect F50, FEI Company). UV-vis-NIR absorption spectra were collected by UV-3600 plus Shimadzu UV-vis-NIR spectrophotometer at room temperature. The resistance variation of the films with temperature was investigated by using the four-point probe method. The THz transmission characteristics of the transparent Ti2O3 film were measured by the terahertz time-domain spectroscopy (THz-TDS) system.
Calculation of the photothermal conversion efficiency
The photo-thermal conversion efficiency η of Ti2O3 film was calculated via the energy balance function(Bi et al., 2020; Ren et al., 2015, 2017; Wang et al., 2017):
| (Equation 3) |
wherem and Cp are the mass and heat capacity, respectively. The suffix “i” of m and Cp refers to SiO2 substrate and Ti2O3 film. T is the temperature, and t is time. is the photothermal energy absorbed by Ti2O3 film:
| (Equation 4) |
Where Iis the laser power, Aλ is the absorbance of Ti2O3 film at the wavelength of 808 nm, and η is the photothermal conversion efficiency of Ti2O3 film. Qsurr is thermal energy lost to the surroundings:
| (Equation 5) |
Where h is the heat transfer coefficient, s is the cross-sectional area perpendicular to thermal conduction, and ΔT is the changed temperature, which is referred to T-Tsurr.
When the temperature is stabilized, = 0, so the equation can be:
| (Equation 6) |
The temperature vs. time after removal of the incident light is given by eq:
| (Equation 7) |
whereTmax and Tsurr are the Ti2O3 film temperature and ambient temperature of the surrounding, and B = .
B can be acquired from fitting the heating curve data after removal of the incident laser in Figure 2C. As shown in Figure 2D, the value of B was found to be 0.02845. Mass of Ti2O3 film was1.6 × 10−4g.Cp of Ti2O3 was 0.786J g−1 K−1. Mass of SiO2 substrate was 0.22g. Cp of SiO2 substrate was 0.745J g−1 K−1. So we can get hs equals 4.663 mW/K.
According to the Equations (2), (4), and (6), the photothermal conversion efficiency η can be expressed as following:
| (Equation 8) |
where was 121.4 K. I was 588.75 mW where the diameter of light spot was 0.5 cm. was 1.081. Thus the photo-thermal conversion efficiency of Ti2O3 film was calculated to be 90.45% under 808 nm laser irradiation.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos61771327, 11704358, and U20A20212), NSAF (Nos. U1930123, and U1730246), Distinguished Young Scholars of Sichuan Province (No.2020JDJQ0008), Foundation of President of China Academy of Engineering Physics (YZJJLX2018001), National Key Research and Development Project (2020YFA0714001), Science and Technology project of Sichuan Province (No. 2018GZ0328), and the Fundamental Research Funds for the Central Universities.
Author contributions
Y.C. conceived the study and wrote the manuscript. H.Z. carried out the THz transmission experiments. W.H. supervised the project. All authors made a substantial contribution to the discussion of the content and reviewed the manuscript before submission.
Declaration of interests
The authors declare no competing interests.
Published: January 21, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103661.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ahmadivand A., Gerislioglu B., Ahuja R., Mishra Y.K. Terahertz plasmonics: the rise of toroidalmetadevices towards immunobiosensings. Mater. Today. 2020;32:108–130. doi: 10.1016/j.mattod.2019.08.002. [DOI] [Google Scholar]
- Batignani G., Fumero G., Kandada A.R.S., Cerullo G., Gandini M., Ferrante C., Petrozza A., Scopigno T. Probing femtosecond lattice displacement upon photo-carrier generation in lead halide perovskite. Nat. Commun. 2018;9:1971. doi: 10.1038/s41467-018-04367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D.G., Li Y.Y., Yao Y.B., Tao T., Liang B., Lu S.G. Preparation and characterizations of flexible photothermalTi2O3-PVAnanocomposites. J. Alloys Compounds. 2020;825:153998. doi: 10.1016/j.jallcom.2020.153998. [DOI] [Google Scholar]
- Chen Z., Chen X., Tao L., Chen K., Long M., Liu X., Yan K., Stantchev R.I., Pickwell-MacPherson E., Xu J.-B. Graphene controlled Brewster angle device for ultra broadband terahertz modulation. Nat. Commun. 2018;9:4909. doi: 10.1038/s41467-018-07367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker T.L., Jelic V., Hillenbrand R., Hegmann F.A. Nanoscale terahertz scanning probe microscopy. Nat. Photon. 2021;15:558–569. doi: 10.1038/s41566-021-00835-6. [DOI] [Google Scholar]
- Cong L., Xu N., Gu J., Singh R., Han J., Zhang W. Highly flexible broadband terahertz metamaterial quarter-wave plate. Laser Photon. Rev. 2014;8:626–632. doi: 10.1002/lpor.201300205. [DOI] [Google Scholar]
- El Fatimy A., Myers-Ward R.L., Boyd A.K., Daniels K.M., Gaskill D.K., Barbara P. Epitaxial graphene quantum dots for high-performance terahertz bolometers. Nat. Nanotechnol. 2016;11:335. doi: 10.1038/nnano.2015.303. [DOI] [PubMed] [Google Scholar]
- Federici J., Moeller L. Review of terahertz and subterahertz wireless communications. J. Appl. Phys. 2010;107:111101. doi: 10.1063/1.3386413. [DOI] [Google Scholar]
- Honig J.M. Nature of electrical transition in Ti2O3. Rev. Mod. Phys. 1968;40:748. doi: 10.1103/RevModPhys.40.748. [DOI] [Google Scholar]
- Honig J.M., Reed T.B. Electrical properties OF TI2O3 single crystals. Phys. Rev. 1968;174:1020. doi: 10.1103/PhysRev.174.1020. [DOI] [Google Scholar]
- Huang H., Wang C., Li Q., Wang R., Yang Y., Muhetaer A., Huang F., Han B., Xu D. Efficient and full-spectrum photothermaldehydrogenation of ammonia borane for low-temperature release of hydrogen. Adv. Funct. Mater. 2020:2007591. doi: 10.1002/adfm.202007591. [DOI] [Google Scholar]
- Koenig S., Lopez-Diaz D., Antes J., Boes F., Henneberger R., Leuther A., Tessmann A., Schmogrow R., Hillerkuss D., Palmer R., et al. Wireless sub-THz communication system with high data rate. Nat. Photon. 2013;7:977–981. doi: 10.1038/nphoton.2013.275. [DOI] [Google Scholar]
- Li Y., Weng Y., Zhang J., Ding J., Zhu Y., Wang Q., Yang Y., Cheng Y., Zhang Q., Li P., et al. Observation of superconductivity in structure-selected Ti2O3 thin films. Npg Asia Mater. 2018;10 doi: 10.1038/s41427-018-0050-5. [DOI] [Google Scholar]
- Li Y., Yu Z.G., Wang L., Weng Y., Tang C.S., Yin X., Han K., Wu H., Yu X., Wong L.M., et al. Electronic-reconstruction-enhanced hydrogen evolution catalysis in oxide polymorphs. Nat. Commun. 2019;10:3149. doi: 10.1038/s41467-019-11124-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Jiang Y., Guo J., Li N., Qiu W., Yang H., Ji Y., Luo S.-N. Van der Waals heteroepitaxialVO2/mica films with extremely low optical trigger threshold and large THz field modulation depth. Adv. Opt. Mater. 2019;7:1900647. doi: 10.1002/adom.201900647. [DOI] [Google Scholar]
- Mackenzie D.M.A., Whelan P.R., Boggild P., Jepsen P.U., Redo-Sanchez A., Etayo D., Fabricius N., Petersen D.H. Quality assessment of terahertz time-domain spectroscopy transmission and reflection modes for graphene conductivity mapping. Opt. Express. 2018;26:9220–9229. doi: 10.1364/oe.26.009220. [DOI] [PubMed] [Google Scholar]
- Manjappa M., Solanki A., Kumar A., Sum T.C., Singh R. Solution-processed lead iodide for ultrafast all-optical switching of terahertz photonic devices. Adv. Mater. 2019;31:1901455. doi: 10.1002/adma.201901455. [DOI] [PubMed] [Google Scholar]
- Morimoto T., Nagai M., Minowa Y., Ashida M., Yokotani Y., Okuyama Y., Kani Y. Microscopic ion migration in solid electrolytes revealed by terahertz time-domain spectroscopy. Nat. Commun. 2019;10:2662. doi: 10.1038/s41467-019-10501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsuma T., Ducournau G., Renaud C.C. Advances in terahertz communications accelerated by photonics. Nat. Photon. 2016;10:371–379. doi: 10.1038/nphoton.2016.65. [DOI] [Google Scholar]
- Nandi S., Tripathi R., Das Adhikary G., Kumar P., Misra A. Ultrahigh infrared photoresponse in titanium sesquioxide at mott-insulator transition. Adv. Mater. Inter. 2020:2001091. doi: 10.1002/admi.202001091. [DOI] [Google Scholar]
- Pitchappa P., Kumar A., Prakash S., Jani H., Venkatesan T., Singh R. Chalcogenide phase change material for active terahertz photonics. Adv. Mater. 2019;31:1808157. doi: 10.1002/adma.201808157. [DOI] [PubMed] [Google Scholar]
- Ren H., Tang M., Guan B., Wang K., Yang J., Wang F., Wang M., Shan J., Chen Z., Wei D., et al. Hierarchical graphenefoam for efficient omnidirectional solar-thermal energy conversion. Adv. Mater. 2017;29:1702590. doi: 10.1002/adma.201702590. [DOI] [PubMed] [Google Scholar]
- Ren W.Z., Yan Y., Zeng L.Y., Shi Z.Z., Gong A., Schaaf P., Wang D., Zhao J.S., Zou B.B., Yu H.S., et al. A near infrared light triggered hydrogenated black TiO2 for cancer photothermaltherapy. Adv. Healthc. Mater. 2015;4:1526–1536. doi: 10.1002/adhm.201500273. [DOI] [PubMed] [Google Scholar]
- Rice C.E., Robinson W.R. Structural-changes associated with semiconductor-to-metal transition IN TI2O3. Mater. Res. Bull. 1976;11:1355–1359. doi: 10.1016/0025-5408(76)90045-3. [DOI] [Google Scholar]
- Shi Q., Chai G., Huang W., Shi Y., Huang B., Wei D., Qi J., Su F., Xu W., Lu T. Fabrication of nanocrystalline lambda-Ti3O5 with tunable terahertz wave transmission properties across a temperature induced phase transition. J. Mater. Chem. C. 2016;4:10279–10285. doi: 10.1039/c6tc03108k. [DOI] [Google Scholar]
- Smolyanskaya O.A., Chernomyrdin N.V., Konovko A.A., Zaytsev K.I., Ozheredov I.A., Cherkasova O.P., Nazarov M.M., Guillet J.P., Kozlov S.A., Kistenev Y.V., et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog.Quan.Electron. 2018;62:1–77. doi: 10.1016/j.pquantelec.2018.10.001. [DOI] [Google Scholar]
- Spaldin N.A., Ramesh R. Advances in magnetoelectricmultiferroics. Nat. Mater. 2019;18:203–212. doi: 10.1038/s41563-018-0275-2. [DOI] [PubMed] [Google Scholar]
- Vanzandt L.L., Honig J.M., Goodenou J.B. Resistivity and magnetic order IN TI2O3. J. Appl. Phys. 1968;39:594. doi: 10.1063/1.2163536. [DOI] [Google Scholar]
- Wang J., Li Y.Y., Deng L., Wei N.N., Weng Y.K., Dong S., Qi D.P., Qiu J., Chen X.D., Wu T. High-performance photothermal conversion of narrow-bandgapTi2O3 nanoparticles. Adv. Mater. 2017;29:1603730. doi: 10.1002/adma.201603730. [DOI] [PubMed] [Google Scholar]
- Wang R.-k., Wang H., An Z.-q., He J.-s., Zhang C.-l., Pan G.-p., Li X. Two-step hydrothermal growth of a thin film of vanadium dioxide on sapphire with large terahertz modulation depth. J. Appl. Phys. 2019;125:163104. doi: 10.1063/1.5050640. [DOI] [Google Scholar]
- Yan W.W., Fang M., Liu M., Kang S.H., Wang R.N., Zhang L.D., Liu L. Semiconductor-metal transition of titanium sesquioxidenanopowder. J. Appl. Phys. 2012;111:123509. doi: 10.1063/1.4729801. [DOI] [Google Scholar]
- Yoshimatsu K., Hasegawa N., Nambu Y., Ishii Y., Wakabayashi Y., Kumigashira H. Metallic ground states of undopedTi2O3 films induced by elongated c-axis lattice constant. Sci. Rep. 2020;10:22109. doi: 10.1038/s41598-020-79182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.C., Li Y.Y., Hu X.N., Zhangg D.L., Tao Y., Liu Z.X., He Y.M., Haque M.A., Liu Z., Wu T., Wang Q.J. Narrow bandgap oxide nanoparticles coupled with graphene for high performance mid-infrared photodetection. Nat. Commun. 2018;9:4299. doi: 10.1038/s41467-018-06776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P., Chen M.F., Jiang S.X., Li Y.F., Xie X., Liu H., Hu X.Y., Wu C., Shu H.B., Wang X.Y. Architecture and performance of the novel sulfur host material based on Ti2O3microspheres for lithium-sulfur batteries. ACS Appl. Mater. Inter. 2019;11:22439–22448. doi: 10.1021/acsami.9b05874. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhou J., Park Y.-S., Rho J., Singh R., Nam S., Azad A.K., Chen H.-T., Yin X., Taylor A.J., Zhang X. Photoinduced handedness switching in terahertz chiral metamolecules. Nat. Commun. 2012;3:942. doi: 10.1038/ncomms1908. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Qiao S., Lang S., Wu Z., Yang Z., Feng Z., Sun H., Zhou Y., Sun L., Chen Z., et al. Gbps terahertz external modulator based on a composite metamaterial with a double-channel heterostructure. Nano Lett. 2015;15:3501–3506. doi: 10.1021/acs.nanolett.5b00869. [DOI] [PubMed] [Google Scholar]
- Zhu H.-F., Du L.-H., Li J., Shi Q.-W., Peng B., Li Z.-R., Huang W.-X., Zhu L.-G. Near-perfect terahertz wave amplitude modulation enabled by impedance matching in VO2 thin films. Appl. Phys. Lett. 2018;112:081103. doi: 10.1063/1.5020930. [DOI] [Google Scholar]
- Zhu H.F., Li J., Du L.H., Huang W.X., Liu J.B., Zhou J.H., Chen Y.F., Das S., Shi Q.W., Zhu L.G., Liu C.L. A phase transition oxide/graphene interface for incident-angle-agile, ultrabroadband, and deep THz modulation. Adv. Mater. Inter. 2020:2001297. doi: 10.1002/admi.202001297. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.