Abbreviations
- ATM
ataxia telangiectasia mutated
- BER
base excision repair
- BRCA
breast cancer susceptibility gene
- DDR
DNA damage response
- DR
direct repair
- ERCC6
ERCC excision repair 6, chromatin remodeling factor; LIG3, DNA ligase 3
- FA
Fanconi anemia
- HR
homologous recombination
- MMR
mismatch repair
- MSH3
mutS homolog 3
- NER
nucleotide excision repair
- NGS
next‐generation sequencing
- NHEJ
nonhomologous end joining
- PALB2
partner and localizer of BRCA2
- PARP
poly(adenosine diphosphate‐ribose) polymerase
- PDAC
pancreatic ductal adenocarcinoma
- PD‐L1
programmed death ligand 1
- POLE
DNA polymerase epsilon, catalytic subunit
- PRKDC
protein kinase, DNA‐activated, catalytic subunit
- OS
overall survival
- RAD50
RAD50 double strand break repair protein
- REV3L
REV3 like, DNA directed polymerase zeta catalytic subunit
- SMARCA4
SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4
- TLS
translesion synthesis
- TMB
tumor mutation burden
- TP53BP1
tumor protein p53 binding protein 1
To the editor,
Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis among all common malignant solid tumors, with a 5‐year overall survival (OS) rate of less than 10% [1]. Few effective targets for anticancer therapy have been confirmed in pancreatic cancer. Recently, it was substantiated that pancreatic cancer patients carrying deleterious mutations of the DNA damage response (DDR) genes are more likely to benefit from platinum‐based chemotherapy [2] and poly(adenosine diphosphate‐ribose) polymerase (PARP) inhibitor [3]. The DDR genes are involved in the activation of cell cycle checkpoints and DNA repair pathways that maintain genomic integrity and prevent the generation of potentially deleterious mutations which have been identified as potential novel therapeutic targets in a variety of tumors [4]. The classical DDR pathways are divided into eight categories: direct repair (DR), mismatch repair (MMR), base excision repair (BER), and nucleotide excision repair (NER), representing pathways involved in DNA single‐strand break repair, Fanconi anemia (FA), translesion synthesis (TLS), nonhomologous end joining (NHEJ), and homologous recombination (HR), representing pathways involved in DNA double‐strand break repair [4]. Some DNA damage sensor genes linked to DDR‐associated pathways defined as others are also considered crucial [5]. It has been reported that approximately 10% of Caucasian pancreatic cancer patients carry a pathogenic germline mutation in pancreatic cancer‐susceptible genes, of which breast cancer susceptibility gene 1/2 (BRCA1/2) and ataxia telangiectasia mutated (ATM) are the most common [6]. However, the characteristics of DDR genes and DDR pathways in Chinese PDAC patients have not been elucidated.
To depict the molecular landscape of DDR mutations in 1080 PDAC patients, we evaluated 209 DDR‐related genes and 9 DDR functional pathways covered by our next‐generation sequencing (NGS) panels (Supplementary file), which were referenced in previous reports [5, 6]. Among the 1080 PDAC patients, 339 (31.4%) had DDR‐related gene mutations (Figure 1A). Of the 339 patients with DDR gene mutations, 251 (74.0%) had only somatic DDR gene mutations, 32 (9.4%) had only germline DDR gene mutations, and 56 (16.5%) had both. A total of 307 (28.4%) patients with 169 somatic mutant DDR genes and 88 (8.1%) patients with 19 germline mutant DDR genes were evaluated (Figure 1B). A total of 88 (8.1%) patients had more than one somatic DDR gene mutations. Among the 9 functional pathways of DDR genes, we observed that HR, FA, and others were the most commonly mutated pathways in our PDAC patients (Figure 1C). The most frequently mutated pathway was HR, being detected in 156 (14.4%) patients.
FIGURE 1.
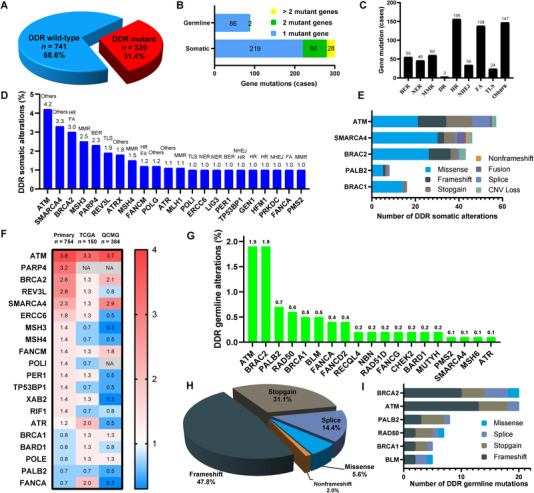
DDR‐related genes and DDR functional pathways in the 1080 Chinese PDAC patients. (A) The frequency of DDR mutations. (B) The number of patients with somatic or germline mutant genes. (C) The number of patients with mutations in the 9 DDR functional pathways. (D) The mutation types of somatic DDR genes. (E) The mutation types of representative somatic DDR‐related genes. (F) Heatmap showing the frequency comparison of somatic DDR mutant genes without CNV alterations among Chinese patients with primary PDAC (n = 754) and the Western cohorts from The Cancer Genome Atlas (TCGA, n = 150) and Queensland Centre for Medical Genomics (QCMG, n = 384). The DDR genes are listed in the order of mutation frequency in our cohort. The gradient color from red to blue represents the mutation frequency from high to low. NA denotes not detected. (G) The frequency of germline mutations in representative DDR genes. (H) The mutation types of germline DDR genes. (I) The mutation types of representative germline DDR‐related genes. Abbreviations: DDR, DNA damage response; PDAC, pancreatic ductal adenocarcinoma; DR, direct repair; MMR, mismatch repair; BER, base excision repair; NER, nucleotide excision repair; FA, Fanconi anemia; TLS, translesion synthesis; NHEJ, nonhomologous end joining; HR, homologous recombination; CNV, copy number variation.
In the analysis of somatic DDR gene mutations, the most frequently mutated representative somatic DDR genes in specific DDR pathways are listed in Figure 1D, including ATM (others, 4.2%), SWI/SNF‐related, matrix‐associated, actin‐dependent regulator of chromatin, subfamily A, member 4 (SMARCA4, others, 3.3%), BRCA2 (FA and HR, 3.0%), mutS homolog 3 (MSH3, MMR, 2.5%), PARP4 (BER, 2.3%), REV3‐like, and DNA‐directed polymerase zeta catalytic subunit (REV3L, TLS, 1.9%). ERCC excision repair 6, chromatin‐remodeling factor (ERCC6) and DNA ligase 3 (LIG3) (1.0%) were the most frequently observed somatic DDR mutant genes in the NER pathway. Tumor protein p53‐binding protein 1 (TP53BP1) and protein kinase, DNA‐activated, catalytic subunit (PRKDC, 1.0%) were the most commonly observed somatic DDR mutant genes in the NHEJ pathway. In our cohort, only 22 genes had somatic mutational frequencies greater than 1%. We also listed the mutation types of several representative somatic DDR mutant genes (Figure 1E). Compared with Western public data from The Cancer Genome Atlas and Queensland Centre for Medical Genomics, REV3L, ERCC6, MSH3, MSH4, and TP53BP1 with somatic mutations were more often detected in our Chinese cohort (primary only without copy number variations), while BRCA1 and DNA polymerase epsilon, catalytic subunit (POLE) were more likely to be observed in the Western cohorts (Figure 1F). To help characterize the role played by germline DDR mutations in our cohort, we investigated the germline DDR deficiency in these 1080 patients. A total of 19 genes with DDR germline mutations were detected in our cohort (Figure 1G). The most prevalent germline mutations were commonly seen in BRCA2 (1.9%), ATM (1.9%), partner and localizer of BRCA2 (PALB2, 0.7%), RAD50 double‐strand break repair protein (RAD50, 0.6%), and BRCA1 (0.5%). The most common mutation type was frameshift (47.8%), followed by stopgain (31.1%, Figure 1H). We also demonstrated the mutation types of these representative germline DDR mutant genes (Figure 1I).
To explore the potential relationship between DDR deficiency and immunotherapy, we investigated the relationship between DDR mutations and clinicopathological features including tumor mutation burden (TMB) level and programmed death‐ligand 1 (PD‐L1) expression which were reported to predict the efficacy of immunotherapy in a variety of tumors, including pancreatic cancer [7]. We observed that the age at diagnosis of patients with PDAC was not associated with somatic and germline DDR mutations (Supplementary Figure S1A). In patients with PDAC, metastatic lesions involved significantly more DDR mutations than primary lesions (36.0% vs. 27.5%, P = 0.005, Supplementary Figure S1B). Then, we confirmed that patients with mutant DDR genes had a significantly higher TMB level than did patients with wild‐type DDR genes (68.0% vs. 32.0%, P < 0.001, Supplementary Figure S1C). We also noticed that except for the NER, MMR, and TLS pathways, the other DDR pathway mutations were significantly related to higher TMB levels (all P < 0.01), especially the FA and HR pathways (both P < 0.001). We further examined the underlying relationship between DDR mutations and PD‐L1 expression. Interestingly, DDR genes in the FA (P < 0.023) and HR pathways (P < 0.048) were significantly associated with PD‐L1 expression in PDAC patients, while DDR mutant status and other DDR pathways had no association with PD‐L1 expression (Supplementary Figure S1D, S1E).
A prior study, investigating whether the biological differences between sporadic and germline mutations in PDAC patients were associated with survival benefits, demonstrated that germline HR alteration carriers had a significantly longer overall survival (OS) than non‐carriers [8]. Beyond ATM, BRCA1/2, and PALB2, the clinical significance of other DDR family genes or DDR pathways has not been elucidated. The patients with potential DDR deficiency who could benefit from platinum‐based regimens or PARP inhibitors warrant further clinical exploration. In addition, the development of other specific and potent DDR inhibitors (such as ATM inhibitors) for anti‐DDR deficiency therapy is under investigation [9]. A comprehensive understanding of the characteristics of DDR deficiency in pancreatic cancer patients could help to screen more suitable populations who would benefit from these compounds in the future.
A previous study based on a western population demonstrated that nivolumab plus nab‐paclitaxel and gemcitabine in patients with advanced PDAC did not show a survival benefit [10]. However, we found that TMB‐H and PD‐L1 expression were significantly associated with mutations of HR and FA pathway‐related genes in Chinese PDAC patients, suggesting that some DDR mutant subgroups of Chinese PDAC patients may potentially benefit from immunotherapy, which warrants further studies based on molecular screening.
Taken together, this study revealed the mutational characteristics of 209 DDR genes and 9 DDR pathways in 1080 PDAC patients from China. We found that 28.4% of the patients had somatic DDR gene mutations and 8.1% had germline DDR deficiency. With the increasing applications of NGS and the development of novel agents, we believe that the treatment targeting DDR mutations will benefit more PDAC patients in the future.
DECLARATIONS
AUTHOR CONTRIBUTIONS
LX and LWW designed the study. XFZ, TBM, BZ, SQC, LX, and LWW created the study methodology. YBL, DLF, YWS, XBZ, QL, XFZ, TBM, HYX, JJC, FJ, DQC, YW, JH, QX, SML, MY, JYM, JYY, YCW, and XZ acquired samples and collected clinical data. BZ, SQC, and YZB processed samples and generated experimental data. XFZ, TBM, BZ, SQC, YZB, LX, and LWW designed and conducted the statistical analysis plan. XFZ, TBM, BZ, SQC, YXW, LX, and LWW analyzed and interpreted data. XFZ and SQC wrote the manuscript. XFZ and LWW obtained funding. LWW and LX supervised the study. All authors reviewed and approved the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All patients signed consent forms before participating in this research. The study protocol was reviewed and approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital (protocol No.2016089).
AVAILABILITY OF DATA AND MATERIALS
The Cancer Genome Atlas and Queensland Centre for Medical Genomics studies are available at www.cbioportal.org. Data and materials can be provided upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
FUNDING
This work was supported by a senior investigator LWW's fundings from the Innovation Group Project of Shanghai Municipal Health Commission (2019CXJQ03), National Natural Science Foundation of China (81874048), Shanghai Municipal Commission of Health and Family Planning (2018ZHYL0223), Fostering Fund of Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine (PYIV‐17‐001), Shanghai Municipal Commission of Health and Family Planning Grant (2018ZHYL0223), Clinical Research Plan of SHDC (No. SHDC2020CR1035B), Shanghai Key Clinical Speciality (Oncology), Shanghai leading talents project, Innovative research team of high‐level local universities in Shanghai. Also supported by XFZ's grant from Clinical plus Excellence Project (2020ZYA003) from Shanghai Nucleic Acid Chemistry and Nanomedicine Key Laboratory. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We owe thanks to the patients in our study and their family members. We also appreciate Dr. Yidi Sun for her suggestions and comments on this work.
XF. Zhang and TB. Mao contributed equally to this work.
Contributor Information
Lei Xiong, Email: simon.t@3dmedcare.com.
Liwei Wang, Email: liweiwang@shsmu.edu.cn.
REFERENCES
- 1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008‐2020. [DOI] [PubMed] [Google Scholar]
- 2. Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122(3):333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA‐mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearl LH, Schierz AC, Ward SE, Al‐Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15(3):166‐80. [DOI] [PubMed] [Google Scholar]
- 5. Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23(1):239‐254.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood LD, Yurgelun MB, Goggins MG. Genetics of Familial and Sporadic Pancreatic Cancer. Gastroenterology. 2019;156(7):2041‐2055. [DOI] [PubMed] [Google Scholar]
- 7. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan‐tumor genomic biomarkers for PD‐1 checkpoint blockade‐based immunotherapy. Science. 2018;362(6411):eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yadav S, Kasi PM, Bamlet WR, Ho TP, Polley EC, Hu C, et al. Effect of Germline Mutations in Homologous Recombination Repair Genes on Overall Survival of Patients with Pancreatic Adenocarcinoma. Clin Cancer Res. 2020;26(24):6505‐6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020;21(3):e135‐e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG, et al. Open‐label, Phase I Study of Nivolumab Combined with nab‐Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res. 2020;26(18):4814‐4822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The Cancer Genome Atlas and Queensland Centre for Medical Genomics studies are available at www.cbioportal.org. Data and materials can be provided upon reasonable request to the corresponding author.


