Abstract
We present the isotopic discrimination between paired skin and bone collagen from animals of known life history, providing a modern baseline for the interpretation of archaeological isotopic data. At present, the interpretation of inter-tissue variation (Δ(skin–bone)) in mummified remains is based on comparisons with other archaeological material, which have attributed divergence to their contrasting turnover rates, with rapidly remodelling skin collagen incorporating alterations in environmental, cultural and physiological conditions in the months prior to death. While plausible, the lack of baseline data from individuals with known life histories has hindered evaluation of the explanations presented. Our analysis of a range of animals raised under a variety of management practices showed a population-wide trend for skin collagen to be depleted in 13C by –0.7‰ and enriched in 15N by +1.0‰ relative to bone collagen, even in stillborn animals. These results are intriguing and difficult to explain using current knowledge; however, on the basis of the findings reported here, we caution any results which interpret simply on differing turnover rates. We hypothesize that there may be a consistent difference in the routing of dietary protein and lipids between skin and bone, with potentially on-site synthesis of non-essential amino acids using carbon and nitrogen that have been sourced via different biochemical pathways.
Keywords: skin, bone, archaeology, cross-disciplinary sciences, analytical chemistry, stable isotope analysis
1. Introduction
With the δ13C and δ15N of proteinaceous tissue reflecting an individual's dietary intake and physiological condition during development [1–5], the paired analysis of skin collagen—remodelling on the scale of weeks and months [6,7]—and bone collagen—on the order of years and decades [8,9]—has been used to examine short- and long-term trends in diet, migration, health and cultural practices [10–19]. While variations in amino acid composition typically result in different tissues within the same individual displaying divergent isotopic values [20–22], bone and skin collagen are both dominated by type 1 collagen and possess highly similar amino acid profiles [23–25]. Inter-tissue isotopic variation (Δ(skin–bone)) is therefore frequently attributed to their contrasting temporal resolutions.
However, with isotope ecology studies rarely focusing on the analysis of either of these tissues, much of our understanding comes from archaeological studies where skin has survived through spontaneous and anthropogenic mummification. Here, Δ(skin–bone) discrimination has been attributed to alterations in cultural and environmental conditions in the months prior to death, including substantial dietary change and/or geographical movement, as well as physiological and nutritional stress [11–19]. While these interpretations are plausible, the lack of baseline data from individuals with known life histories confounds evaluation of the explanations presented. Δ(skin–bone) discrimination in archaeological populations may be additionally influenced by differential decomposition patterns [26] and the presence of exogenous contaminants [27–29].
While considerable effort has been made to optimize the extraction and analysis of collagen from archaeological bone (i.e. [30–33]), only recently protocols for the isotopic analysis of archaeological skin have been reviewed [28,34,35]. These highlight the necessity for sample pretreatment including lipid extraction and filtration of the collagen substrate to produce samples with acceptable collagen quality indicators (collagen yield, %C, %N and C:N ratio). Many archaeological skin samples fail to meet these quality indicators due to inadequate pretreatment, often producing elevated C:N ratios (greater than 3.5) indicative of the presence of carbon-rich contaminants, such as lipids, which may artificially reduce δ13C values due to the carbon fractionation that occurs during lipid synthesis [36].
Consequently, the only modern mammalian skin sample known to have undergone pretreatment (solvent extraction of lipids; demineralization in hydrochloric acid (HCl); gelatinization of the acid-insoluble fraction in pH 3 water; filtration and freeze-drying) and produced an acceptable C:N ratio (3.3) comes from a single pig [28]. To test the null hypothesis that there is no significant difference between δ13C and δ15N values in skin and bone collagen within an individual, we report inter-tissue discrimination in 25 sheep (Ovis aries) and five pigs (Sus scrofa) with known life histories, examining the impact of sex, age and health status.
2. Materials and methods
2.1. Sample selection
Paired skin and bone samples were obtained from 25 sheep raised across three flocks in the UK. Where available, husbandry information including sex, age, date of birth, date of death and health status was obtained from the farmer.
Flock 1 (n = 14) were raised in East Yorkshire, on a diet of local grazing supplemented by hay and protein concentrates. The animals were sent for slaughter at various ages between November 2014 and December 2015. Tissue samples were collected from two late-term stillborn lambs (SH10, SH11) and lambs that had died at 2 (SH09) and 24 (SH13) days old from natural causes. Two sheep had been in poor health shortly before death. SH03—six-month-old Shetland ram—had been treated for pneumonia in the months prior to death, which had reduced the animals' appetite, weight gain and growth. SH12—5-year-old Shetland ewe—was humanely euthanized due to flystrike, a condition where parasitic flies lay eggs in the wool and the resulting maggots bury into the skin feeding off the flesh. This had severely weakened and debilitated the ewe, resulting in cessation of wool production and fleece shedding (electronic supplementary material, figure S1).
Flock 2 (n = 2) were raised in Leicestershire on a diet of local grazing supplemented with protein concentrates. SH28 was crushed by the mother at one month old, and SH29 was slaughtered at 20 months old. Samples categorized as Flock 3 (n = 10) came from an abattoir in Nottinghamshire, had been raised locally and were all aged between eight and nine months. Further husbandry information was unavailable. Paired bone and skin samples from five pigs (n = 5) were obtained from an abattoir in Nottinghamshire, for which no husbandry information was unavailable.
2.2. Sample preparation
Sample preparation was conducted at the University of York using analytical grade or above reagents, and all water was deionized and distilled.
2.2.1. Skin collagen
A methodology was adopted in line with published analyses of modern and archaeological skins for stable isotope analysis and radiocarbon dating which had produced acceptable collagen quality indicators [35]. Shortly after death, the animals were flayed, and their skins stored at −5°C prior to analysis. In an effort to remove other proteinaceous material, samples were taken from the follicle sparse belly region, and all visible signs of hair and muscle were removed. The samples were subsequently freeze dried for 48 h and ground to a powder using a ball mill (Retsch MM400). The lipids were extracted via solvent extraction dichloromethane/methanol (2 : 1 v/v) by ultrasonication for 1 h, with the supernatant removed and solvent replaced every 15 min. The skin was briefly demineralized in 0.6 M HCl at 4°C for 1 h, rinsed with distilled water and gelatinized in pH 3 0.001 M HCl for 48 h. The supernatant containing the soluble collagen was filtered (60–90 µm Ezee-Filter, Elkay Laboratories, UK), frozen and freeze dried.
2.2.2. Bone collagen
The left metacarpal in sheep, and left humerus in pigs, was removed from the carcass and stored at −20°C prior to analysis. Adhering tissues were removed, and approximately 1 g of bone sampled from the anterior mid-shaft. The bone was cleaned in distilled water, freeze dried for 48 h to remove excess moisture and then ground to a powder. The lipids were extracted as with skin via ultrasonication for 2 h, and the bone was subsequently demineralized in 0.6 M HCl at 4°C for 4 days, gelatinized, filtered, frozen and freeze dried as above. Bone and skin samples (0.8–1.1 mg) were weighed out in duplicate into 4 × 3.2 mm tin capsules (Elemental Microanalysis, Okehampton, UK) for analysis.
2.3. Stable isotope analysis
Isotope ratio determinations were carried out at the Natural Environment Research Council Life Sciences Mass Spectrometry Facility, East Kilbride, on a ThermoElectron DeltaPlusXP (Thermo Fisher Scientific, Bremen, Germany) with an Elementar Pyrocube elemental analyser (Elementar UK Ltd). Sample data were reported in standard delta per mil notation (δ‰) relative to Vienna Pee Dee Belemnite (V-PDB) (δ13C) and atmospheric air (AIR) (δ15N) international standards. Three laboratory reference materials were interspersed within the measurement run to correct for linearity and instrument drift. Each of the laboratory reference materials is checked regularly against international standards USGS40 and USGS41. Following the calculations outlined in Szpak et al. [37], the total analytical uncertainty was estimated to be ± 0.19‰ for δ13C and ± 0.20‰ for δ15N.
2.4. Statistical analysis
Statistical testing was carried out using the IBM SPSS Statistics 26 software package. Shapiro–Wilks test for normality indicated δ13C and δ15N data did not conform to a normal distribution (p < 0.01); thus, the resultant statistical tests were non-parametric in nature. Significance of differences between bone and skin values determined using a Wilcoxon signed-rank test for paired samples. Significance of differences in Δ(skin–bone) values between species, sexes and health status groups was determined using an independent t-test. The correlation between age and Δ(skin–bone) was determined through the Pearson correlation coefficient.
3. Results and discussion
3.1. Collagen quality indicators
Average collagen yields (dry mass) of 67% and 20% were obtained from skin and bone, respectively. This is well above the 2–4% threshold used to identify problematic samples [38,39]. Skin %C ranges from 41.4% to 46.1%, and %N values from 15.2% to 16.2% (table 1). Bone %C ranged from 39.6% to 47.6%, and %N from 14.9% to 16.9%, consistent with those reported in modern type I collagen-dominated tissues [38].
Table 1.
Husbandry information, isotopic values (δ13C, δ15N) and elemental composition of skin and bone samples. Sex: M = male, F = female; health: G = good, P = poor, DFM = died during first month of life; — = no data.
| skin |
bone |
Δ(skin–bone) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | species | age | sex | health | %C | %N | C/N | δ13C (‰) | δ15N (‰) | %C | %N | C/N | δ13C (‰) | δ15N (‰) | Δ13C (‰) | Δ15N (‰) |
| SH02 | sheep | 6 months | M | G | 45.5 | 16.0 | 3.3 | –25.8 | 9.2 | 43.8 | 16.1 | 3.2 | –24.7 | 8.2 | –1.1 | +1.0 |
| SH03 | sheep | 6 months | M | P | 42.5 | 16.2 | 3.3 | –26.0 | 10.0 | 39.6 | 15.7 | 3.2 | –24.2 | 7.9 | –1.7 | +2.1 |
| SH04 | sheep | 11 months | M | G | 43.8 | 16.1 | 3.2 | –25.8 | 8.4 | 41.0 | 15.2 | 3.2 | –25.0 | 7.9 | –0.8 | +0.6 |
| SH05 | sheep | 10 months | M | G | 41.9 | 16.0 | 3.1 | –25.7 | 8.4 | 42.8 | 15.8 | 3.2 | –24.6 | 7.4 | –1.1 | +1.0 |
| SH06 | sheep | 10 months | M | G | 43.2 | 16.2 | 3.1 | –26.0 | 8.9 | 40.2 | 14.9 | 3.2 | –24.6 | 7.2 | –1.4 | +1.7 |
| SH07 | sheep | 10 months | F | G | 42.9 | 16.1 | 3.1 | –25.8 | 9.0 | 41.9 | 15.6 | 3.2 | –24.7 | 8.4 | –1.2 | +0.6 |
| SH08 | sheep | 13 months | F | G | 42.9 | 15.8 | 3.1 | –26.0 | 9.7 | 43.5 | 16.2 | 3.1 | –24.8 | 8.5 | –1.2 | +1.2 |
| SH09 | sheep | 2 days | M | DFM | 42.5 | 16.1 | 3.1 | –24.7 | 8.4 | 44.9 | 16.5 | 3.2 | –23.9 | 7.7 | –0.8 | +0.7 |
| SH10 | sheep | stillborn | F | DFM | 43.6 | 15.9 | 3.2 | –25.1 | 8.7 | 43.8 | 16.0 | 3.2 | –24.4 | 7.7 | –0.7 | +1.0 |
| SH11 | sheep | stillborn | M | DFM | 43.5 | 16.0 | 3.2 | –24.9 | 8.1 | 44.9 | 16.6 | 3.2 | –23.9 | 7.4 | –1.0 | +0.7 |
| SH12 | sheep | 5 years | F | P | 43.7 | 15.2 | 3.3 | –26.0 | 9.3 | 42.1 | 15.6 | 3.2 | –24.6 | 6.4 | –1.5 | +2.9 |
| SH13 | sheep | 24 days | M | DFM | 43.2 | 16.1 | 3.3 | –23.3 | 7.6 | 44.2 | 16.0 | 3.2 | –23.3 | 7.0 | 0.0 | +0.6 |
| SH16 | sheep | 18 months | M | G | 44.2 | 15.9 | 3.2 | –25.4 | 8.0 | 45.7 | 16.9 | 3.2 | –24.5 | 6.9 | –0.9 | +1.1 |
| SH28 | sheep | 20 months | F | G | 44.7 | 15.6 | 3.4 | –25.3 | 7.3 | 45.7 | 16.8 | 3.2 | –25.2 | 5.8 | –0.1 | +1.5 |
| SH29 | sheep | 1 month | F | DFM | 43.9 | 15.3 | 3.3 | –24.7 | 8.0 | 45.1 | 16.6 | 3.2 | –24.0 | 7.1 | –0.6 | +0.9 |
| SH30 | sheep | 8–9 months | — | G | 43.6 | 15.6 | 3.3 | –25.6 | 9.7 | 44.0 | 16.1 | 3.2 | –25.3 | 8.5 | –0.2 | +1.2 |
| SH31 | sheep | 8–9 months | — | G | 41.4 | 15.4 | 3.3 | –25.5 | 9.5 | 43.6 | 16.7 | 3.2 | –25.1 | 8.0 | –0.4 | +1.4 |
| SH32 | sheep | 8–9 months | — | G | 44.3 | 15.8 | 3.1 | –25.4 | 10.1 | 44.1 | 16.8 | 3.2 | –25.0 | 9.1 | –0.5 | +1.0 |
| SH33 | sheep | 8–9 months | — | G | 43.8 | 15.5 | 3.3 | –25.6 | 10.0 | 43.5 | 16.5 | 3.2 | –25.3 | 9.0 | –0.3 | +1.1 |
| SH34 | sheep | 8–9 months | — | G | 43.5 | 15.6 | 3.3 | –25.8 | 10.0 | 43.1 | 15.8 | 3.2 | –25.3 | 8.8 | –0.4 | +1.2 |
| SH35 | sheep | 8–9 months | — | G | 43.8 | 15.5 | 3.2 | –26.0 | 10.5 | 45.0 | 16.4 | 3.2 | –25.5 | 9.4 | –0.5 | +1.1 |
| SH36 | sheep | 8–9 months | — | G | 43.6 | 15.7 | 3.3 | –25.4 | 7.4 | 44.2 | 15.7 | 3.2 | –24.8 | 7.7 | –0.7 | –0.3 |
| SH37 | sheep | 8–9 months | — | G | 43.1 | 15.3 | 3.2 | –25.9 | 7.2 | 44.6 | 16.4 | 3.2 | –24.8 | 8.2 | –1.2 | –0.9 |
| SH38 | sheep | 8–9 months | — | G | 46.1 | 15.7 | 3.3 | –26.4 | 9.1 | 45.1 | 15.8 | 3.2 | –24.9 | 6.6 | –1.4 | +2.5 |
| SH39 | sheep | 8–9 months | — | G | 43.6 | 15.5 | 3.4 | –25.8 | 9.6 | 45.1 | 15.5 | 3.2 | –25.4 | 8.0 | –0.4 | +1.6 |
| PG01 | pig | — | — | — | 43.6 | 15.8 | 3.2 | –23.3 | 4.1 | 44.3 | 16.7 | 3.2 | –23.5 | 3.5 | +0.2 | +0.6 |
| PG02 | pig | — | — | — | 43.9 | 15.9 | 3.2 | –23.3 | 4.3 | 43.9 | 16.6 | 3.1 | –23.3 | 3.5 | –0.1 | +0.7 |
| PG03 | pig | — | — | — | 42.7 | 15.7 | 3.2 | –23.0 | 5.6 | 46.4 | 15.9 | 3.2 | –22.6 | 5.0 | –0.4 | +0.6 |
| PG04 | pig | — | — | — | 43.7 | 15.8 | 3.2 | –23.0 | 5.8 | 47.6 | 15.8 | 3.2 | –22.3 | 4.6 | –0.7 | +1.1 |
| PG05 | pig | — | — | — | 43.4 | 15.8 | 3.2 | –23.6 | 4.5 | 44.2 | 16.2 | 3.2 | –23.0 | 3.5 | –0.6 | +1.0 |
Atomic C:N ratios in skin ranged from 3.10 to 3.26 (mean 3.24), and in bone from 3.14 to 3.31 (mean 3.19). Skin collagen (type I) has a theoretical C:N ratio of 3.11 [35], though in practice, integrity is often measured against the 2.9–3.5 range used for bone collagen [40]. The disparity between observed and theoretical C:N ratios in skin may indicate the presence of non-collagenous proteins such as elastin (C:N 5.8) and epithelial keratins (C:N 3.4) which account for 5% of the protein fraction [41]. Like collagen, both are glycine rich [42–44] and therefore unlikely to impact the overall isotope signal in bulk analyses.
3.2. Isotopic discrimination between paired samples
Across all individuals, skin was on average depleted in 13C by –0.7‰ (s.d. 0.5‰) and enriched in 15N by +1.1‰ (s.d. 0.7‰) relative to bone (figure 1). The mean differences in both carbon (Z = −4.70, p ≤ 0.001) and nitrogen (Z = −4.54, p ≤ 0.001) isotopic values were significant (table 2). Within sheep, skin was depleted in 13C by –0.8‰ (s.d. 0.5‰) and enriched in 15N by +1.1‰ (s.d. 0.4‰) relative to bone, of which both mean differences are significant (carbon: Z = ≤ 4.37, p ≤ 0.001; nitrogen: Z = −4.54, p ≤ 0.001). The isotopic discrimination between tissues was lower in pigs, with skin on average depleted in 13C by –0.3‰ (s.d. 0.4‰) and enriched in 15N by +0.8‰ (s.d. 0.2‰), of which only the mean difference in nitrogen values is significant (carbon: Z = −1.48, p = 0.14; nitrogen: Z = −2.02, p = 0.04).
Figure 1.
Isotope values in modern skin and bone samples. Comparison of (a) δ13C and (b) δ15N values in skin collagen and bone collagen pairings. Solid line = linear trend line with 95% CI (grey shading); dashed line = 1:1.
Table 2.
Isotopic discrimination between skin and bone in modern sheep and pigs.
| species | n | mean (‰) | min (‰) | max (‰) | s.d. |
|---|---|---|---|---|---|
| Δ13C(skin–bone) | |||||
| all | 30 | –0.7 | –1.72 | +0.17 | 0.5 |
| sheep | 25 | –0.7 | –1.72 | –0.01 | 0.5 |
| pig | 5 | –0.3 | –0.71 | +0.17 | 0.4 |
| Δ15N(skin–bone) | |||||
| all | 30 | +1.1 | +0.92 | +2.90 | 0.7 |
| sheep | 25 | +1.1 | +0.92 | +2.90 | 0.8 |
| pig | 5 | +0.8 | +0.55 | +1.13 | 0.2 |
Mean Δ13C(skin–bone) values were comparable with those observed in other modern animals (figure 2) and broadly mid-range of those in archaeological populations (–2 to +1‰). Mean Δ15N(skin–bone) values in modern animals are lower than those observed in archaeological populations. It has been speculated that the greater susceptibility of skin to chemical and structural modification in the burial environment may be responsible for the elevated skin δ15N values observed in mummified individuals [12,15,19], as peptide bond hydrolysis preferentially eliminates isotopically lighter nitrogenous compounds resulting in an enrichment in the remaining protein [35,45]. While this has the potential to influence the nitrogen isotope values, it is clear from modern animals that an enrichment of at least 2.9‰ in the skin, relative to bone, can occur even in the absence of any diagenetic explanation.
Figure 2.
Mean and range of Δ13C(skin–bone) and Δ15N(skin–bone) values reported in modern and archaeological studies. Number of paired samples in parentheses.
3.3. Species
Mean Δ13C(skin–bone) values in sheep are marginally more depleted than those in pigs (t28 = 2.1, p = 0.042) although not for Δ15N(skin–bone) values (t28 = −0.85, p = 0.41). This difference may be due in part to the imbalanced sample sizes, along with differences in their management. Pigs—of which the majority in the UK are reared indoors—often display less isotopic variation than free-ranging ruminants [46–48] as plant isotope values can vary considerably within a small area due to variable mycorrhizal activity and areas of localized defecation [49]. The sheep from Flock 1 were all raised over the same five acres but exhibit a range of 2.8‰ and 2.5‰ in skin and bone δ13C values, and 1.7‰ and 2.1‰ in skin and bone δ15N values, respectively; isotopic variation comparable with that observed by von Holstein et al. [22] in wool keratin and bone collagen from a single flock.
3.4. Sex and age
As with archaeological populations [16,18,19], the magnitude of the isotopic discrimination between skin and bone was not related to the animals' sex (carbon: t12 = 0.20, p = 0.84; nitrogen: t12 = 0.96, p = 0.35). A significant positive correlation was observed between Δ15N(skin–bone) values and age (r = 0.50, n = 25, p = 0.010); however, this was heavily influenced by the oldest individual (SH12, 5 years old, Δ15N(skin–bone) = +2.9‰), which had been in poor health prior to death (see §3.5). No correlation was found between Δ13C(skin–bone) values and age (r = −0.27, n = 25, p = 0.21).
The skins of two stillborn lambs (SH10, SH11) and a 2-day-old lamb (SH09) all showed a depletion in 13C (mean: –0.8‰) and enrichment in 15N (mean: +0.8‰) consistent with the wider population. They had received all of their nutrition from their mother in utero with the same nutritional pool supporting the development of both tissues [50]. This suggests that the variation between these tissues is present regardless of turnover rate and may be the consequence of the unequal allocation of isotopically distinct amino acids (AA) during tissue synthesis.
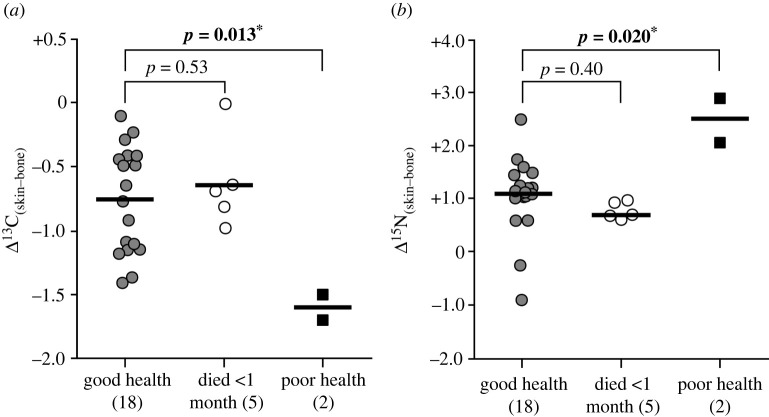
3.5. Health status
The sheep known to have been in poor health before death—SH03 (suffered from pneumonia) and SH12 (euthanized following flystrike)—had a mean depletion of –1.6‰ in skin 13C composition relative to bone (s.d. 0.2‰) and a mean enrichment of +2.5‰ in 15N (s.d. 0.6‰) (figure 3). The mean differences in both carbon and nitrogen Δ(skin–bone) values between these adults in poor health and adults in good health are significant (carbon: t18 = −2.75, p = 0.013; nitrogen: t2.6 = 2.71, p = 0.020). Values in lambs that had died less than one month old (including stillborns) were not significantly different from those in good health (carbon: t21 = −0.64, p = 0.53; nitrogen: t21 = −0.85, p = 0.40).
Figure 3.
(a) Δ13C(skin–bone) and (b) Δ15N(skin–bone) isotopic discrimination in sheep by health status. Horizontal line = group mean, number of samples in parentheses. Significance of difference between groups determined using an independent samples t-test, equal variances assumed based on Levene's test. *Statistically significant differences (p ≤ 0.05).
Physiological stress resulting from acute illness or malnutrition can alter the isotopic value of tissues [2,4,51–56]. When fatal, these are often most pronounced in tissue laid down in the final 7–14 days of life [4]. While most studies have focused on inert tissues, the rapid turnover of skin collagen may enable the recording of short-term stresses which are not visible in bone. Starvation typically results in an elevation in δ15N values as the body enters a negative nitrogen balance, with the catabolism of the body's own protein resulting in a fractionation typical of trophic level increases [2,4,51,52]. The impact of physiological stress on δ13C values is less defined [57], though a number of studies have observed a depletion in response to starvation, potentially due to nutritional ketosis and the utilization of 13C-depleted fat deposits as an energy source in the absence of dietary carbohydrate [2,4,54,55].
The catabolism of lipid reserves and proteins is a plausible explanation for the values observed in these sheep. Their poor health is known to have led to a reduction in weight, and, in the case of SH12, cessation of wool production and fleece shedding which only occurs during severe nutritional deficiency [58]. Although a very small sample size, it indicates that physiological stress in the final months of life is a potential explanation for the 13C-depleted and 15N-enriched values seen in the skins of many archaeological populations.
4. Conclusion
Contrary to the assumptions made by previous studies, we reject the hypothesis that δ13C and δ15N variation between skin and bone collagen in an individual is the result of their contrasting turnover rates. In this analysis, a variety of animals raised under different management practices, slaughtered in different years and at different ages, showed a population-wide trend for skin to have a lower δ13C and higher δ15N than bone. If the explanation was simply turnover rate, we would expect an average near zero, but with considerable variation. It is also unlikely to reflect protein or amino acid composition due to the minor variation between these tissues.
We instead postulate a consistent difference in the routing of dietary protein and lipids between skin and bone, with potentially on-site synthesis of non-essential amino acids using carbon and nitrogen that have been sourced via different biochemical pathways [59,60]. In the absence of secondary influences (i.e. dietary change, physiological stress or diagenesis), this results in skin having a lower δ13C and higher δ15N than bone. While we are unable to adequately explain this result currently, future isotope analysis of individual AAs in paired samples may provide valuable insight.
This study provides an important dataset for the interpretation of skin–bone isotopic discrimination in archaeological studies, reporting the range of values that can be expected in the absence of geographical or dietary change and diagenesis. The elevated Δ15N(skin–bone) and lower Δ13C(skin–bone) values observed in animals known to be in poor health highlight the significant potential of the isotopic analysis of skin for the study of health and palaeopathology. Similar trends have been observed in numerous mummified individuals and may indicate a period of acute physiological stress prior to death. δ15N and δ13C isotope analysis of individual AAs in skin samples may in future offer a route to exploring metabolic shifts associated with poor health, and in the case of processed skin such as parchment, offer insights into animal management and skin selection.
Supplementary Material
Ethics
No animals were raised or slaughtered for this study. Skin and bone samples were obtained as by-products of animals that had entered the food chain for human consumption. The authors were not involved in the selection or timing of the animals' slaughter, with all husbandry decisions made by the animals' registered owners. Samples were either obtained directly from UK Food Standards Agency (FSA) licensed slaughterhouses or from the animals' owner after the carcass had returned from an FSA licensed slaughterhouse.
Data accessibility
All data generated in this study are presented in the article and electronic supplementary material file.
Authors' contributions
S.P.D.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, visualization, writing—original draft, writing—review and editing; M.J.C.: conceptualization, formal analysis, funding acquisition, investigation, methodology, resources, supervision, writing—review and editing; A.J.T.H.: investigation, writing—review and editing; A.S.: investigation, writing—review and editing; J.N.: formal analysis, investigation, writing—review and editing; M.M.A.: conceptualization, formal analysis, investigation, methodology, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
At the time of writing, Professor Matthew Collins and Dr Michelle Alexander are Board Members of Royal Society Open Science but were not involved in the review or assessment of the paper.
Funding
This research was supported by NERC Life Sciences Mass Spectrometry Facility grant no. EK259–14/15. S.P.D. was supported by the AHRC White Rose College of Arts and Humanities Doctoral Training Partnership (Award Ref. 1489527). M.J.C is supported by the Danish National Research Foundation-DNRF128.
References
- 1.Tieszen LL, Fagre T. 1993. Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissues. In Prehistoric human bone: archaeology at the molecular level (eds Lambert JB, Grupe G), pp. 121-155. Berlin, Germany: Springer. [Google Scholar]
- 2.Mekota A-M, Grupe G, Ufer S, Cuntz U. 2006. Serial analysis of stable nitrogen and carbon isotopes in hair: monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 20, 1604-1610. ( 10.1002/rcm.2477) [DOI] [PubMed] [Google Scholar]
- 3.O'Connell TC, Kneale CJ, Tasevska N, Kuhnle GGC. 2012. The diet-body offset in human nitrogen isotopic values: a controlled dietary study. Am. J. Phys. Anthropol. 149, 426-434. ( 10.1002/ajpa.22140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuberger FM, Jopp E, Graw M, Püschel K, Grupe G. 2013. Signs of malnutrition and starvation—reconstruction of nutritional life histories by serial isotopic analyses of hair. Forensic Sci. Int. 226, 22-32. ( 10.1016/j.forsciint.2012.10.037) [DOI] [PubMed] [Google Scholar]
- 5.Webb EC, et al. 2017. The influence of varying proportions of terrestrial and marine dietary protein on the stable carbon-isotope compositions of pig tissues from a controlled feeding experiment. STAR: Sci. Technol. Archaeol. Res. 3, 28-44. ( 10.1080/20548923.2016.1275477) [DOI] [Google Scholar]
- 6.el-Harake WA, Furman MA, Cook B, Nair KS, Kukowski J, Brodsky IG. 1998. Measurement of dermal collagen synthesis rate in vivo in humans. Am. J. Physiol. 274, 586-591. ( 10.1152/ajpcell.1998.274.3.C586) [DOI] [PubMed] [Google Scholar]
- 7.Babraj JA, Cuthbertson DJR, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. 2005. Collagen synthesis in human musculoskeletal tissues and skin. Am. J. Physiol. Endocrinol. Metab. 289, 864-869. ( 10.1152/ajpendo.00243.2005) [DOI] [PubMed] [Google Scholar]
- 8.Hedges REM, Clement JG, Thomas CDL, O'Connell TC. 2007. Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808-816. ( 10.1002/ajpa.20598) [DOI] [PubMed] [Google Scholar]
- 9.Matsubayashi J, Tayasu I. 2019. Collagen turnover and isotopic records in cortical bone. J. Archaeol. Sci. 106, 37-44. ( 10.1016/j.jas.2019.03.010) [DOI] [Google Scholar]
- 10.Vogel JC. 1978. Isotopic assessment of the dietary habits of ungulates. S. Afr. J. Sci. 74, 298. [Google Scholar]
- 11.White CD, Schwarcz HP. 1994. Temporal trends in stable isotopes for Nubian mummy tissues. Am. J. Phys. Anthropol. 93, 165-187. ( 10.1002/ajpa.1330930203) [DOI] [PubMed] [Google Scholar]
- 12.Iacumin P, Bocherens H, Mariotti A, Longinelli A. 1996. An isotopic palaeoenvironmental study of human skeletal remains from the Nile Valley. Palaeogeogr. Palaeoclim. Palaeoecol. 126, 15-30. ( 10.1016/s0031-0182(96)00067-3) [DOI] [Google Scholar]
- 13.Iacumin P, Bocherens H, Chaix L, Marioth A. 1998. Stable carbon and nitrogen isotopes as dietary indicators of ancient Nubian populations (Northern Sudan). J. Archaeol. Sci. 25, 293-301. ( 10.1006/jasc.1997.0206) [DOI] [Google Scholar]
- 14.Shelnut N. 2006. Before the Inca: prehistoric dietary transitions in the Argentine Cuyo. MA Thesis, University of South Florida, Tampa, FL. [Google Scholar]
- 15.Finucane BC. 2007. Mummies, maize, and manure: multi-tissue stable isotope analysis of late prehistoric human remains from the Ayacucho Valley, Perú. J. Archaeol. Sci. 34, 2115-2124. ( 10.1016/j.jas.2007.02.006) [DOI] [Google Scholar]
- 16.Williams LJ. 2008. Investigating seasonality of death at Kellis 2 cemetery using solar alignment and isotopic analysis of mummified tissues. PhD Thesis, The University of Western Ontario, London, Ontario, Canada. [Google Scholar]
- 17.Norris AL. 2009. Age as a factor in inter-tissue spacing of stable carbon isotope values in juvenile human remains from the Dakhleh Oasis, Egypt. MA Thesis, University of Central Florida, Orange County, FL. [Google Scholar]
- 18.Cadwallader L. 2013. Investigating 1500 years of dietary change in the Lower Ica valley, Peru using an isotopic approach. PhD Thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- 19.Basha WA, Chamberlain AT, Zaki ME, Kandeel WA, Fares NH. 2016. Diet reconstruction through stable isotope analysis of ancient mummified soft tissues from Kulubnarti (Sudanese Nubia). J. Archaeol. Sci: Rep. 5, 71-79. ( 10.1016/j.jasrep.2015.10.036) [DOI] [Google Scholar]
- 20.O'Connell TC, Hedges REM, Healey MA, Simpson AHRW. 2001. Isotopic comparison of hair, nail and bone: modern analyses. J. Archaeol. Sci. 28, 1247-1255. ( 10.1006/jasc.2001.0698) [DOI] [Google Scholar]
- 21.Becker BH, Newman SH, Inglis S, Beissinger SR. 2007. Diet–feather stable isotope (δ15N and δ13C) fractionation in common Murres and other seabirds. Condor. 2007, 451-456. ( 10.1093/condor/109.2.451) [DOI] [Google Scholar]
- 22.von Holstein ICC, Hamilton J, Craig OE, Newton J, Collins MJ. 2013. Comparison of isotopic variability in proteinaceous tissues of a domesticated herbivore: a baseline for zooarchaeological investigation. Rapid Commun. Mass Spectrom. 27, 2601-2615. ( 10.1002/rcm.6725) [DOI] [PubMed] [Google Scholar]
- 23.Wenstrup RJ, Murad S, Pinnell SR. 1991. Collagen. In Physiology, biochemistry, and molecular biology of the skin (ed. Goldsmith LA), pp. 481-508. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Delmas PD, Malaval L. 1993. The proteins of bone. In Physiology and pharmacology of bone (eds Mundy GR, Martin TJ), pp. 673-724. Berlin, Germany: Springer. [Google Scholar]
- 25.Veysey EC, Finlay AY. 2010. Aging and the skin. In Brocklehurst's textbook of geriatric medicine and gerontology, (eds Fillit HM, Rockwood K, Woodhouse K), pp. 133-137, 7th edn. Philadelphia, PA: W.B. Saunders. [Google Scholar]
- 26.Keenan SW, DeBruyn JM. 2019. Changes to vertebrate tissue stable isotope (δ15N) composition during decomposition. Sci. Rep. 9, 9929. ( 10.1038/s41598-019-46368-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White CD, Longstaffe FJ, Law KR. 1999. Seasonal stability and variation in diet as reflected in human mummy tissues from the Kharga Oasis and the Nile Valley. Palaeogeogr. Palaeoclim. Palaeoecol. 147, 209-222. ( 10.1016/S0031-0182(98)00168-0) [DOI] [Google Scholar]
- 28.Cockitt J, Lamb A, Metcalfe R. 2020. An ideal solution? Optimising pretreatment methods for artificially mummified ancient Egyptian tissues. Rapid Commun. Mass Spectrom. 34, e8686. ( 10.1002/rcm.8686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.France CAM, Kaczkowski RA, Kavich GM, Epitropou A. 2020. The effects of cyclododecane and subsequent removal on δ15N, δ13C, and δ18O values in collagen and bioapatite of a modern bone. J. Archaeol. Sci.: Rep. 31, 102367. ( 10.1016/j.jasrep.2020.102367) [DOI] [Google Scholar]
- 30.Longin R. 1971. New method of collagen extraction for radiocarbon dating. Nature 230, 241-410. ( 10.1038/230241a0) [DOI] [PubMed] [Google Scholar]
- 31.Brown TA, Nelson DE, Vogel JS, Southon JR. 1988. Improved collagen extraction by modified 412 Longin method. Radiocarbon 30, 171-177. ( 10.1017/s0033822200044118) [DOI] [Google Scholar]
- 32.Collins MJ, Galley P. 1998. Towards an optimal method of archaeological collagen extraction: the influence of pH and grinding. Anc. Biomol. 2, 209-223. [Google Scholar]
- 33.Guiry EJ, Szpak P. 2020. Quality control for modern bone collagen stable carbon and nitrogen isotope measurements. Methods Ecol. Evol. 11, 1049-1060. ( 10.1111/2041-210x.13433) [DOI] [Google Scholar]
- 34.Brock F, Geoghegan V, Thomas B, Jurkschat K, Higham TFG. 2013. Analysis of bone ‘collagen’ extraction products for radiocarbon dating. Radiocarbon 55, 445-463. ( 10.1017/s0033822200057581) [DOI] [Google Scholar]
- 35.Doherty S, Alexander MM, Vnouček J, Newton J, Collins MJ. 2021. Measuring the impact of parchment production on skin collagen stable isotope (δ13C and δ15N) values. STAR: Sci. Technol. Archaeol. Res. 7, 1-12. ( 10.1080/20548923.2020.1868132) [DOI] [Google Scholar]
- 36.DeNiro MJ, Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197, 261-263. ( 10.1126/science.327543) [DOI] [PubMed] [Google Scholar]
- 37.Szpak P, Metcalfe JZ, Macdonald RA. 2017. Best practices for calibrating and reporting stable isotope measurements in archaeology. J. Archaeol. Sci.: Rep. 13, 609-616. ( 10.1016/j.jasrep.2017.05.007) [DOI] [Google Scholar]
- 38.Ambrose SH. 1990. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 17, 431-451. ( 10.1016/0305-4403(90)90007-R) [DOI] [Google Scholar]
- 39.van Klinken GJ. 1999. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687-695. ( 10.1006/jasc.1998.0385) [DOI] [Google Scholar]
- 40.DeNiro MJ. 1985. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317, 806-809. ( 10.1038/317806a0) [DOI] [Google Scholar]
- 41.McKee TJ, Perlman G, Morris M, Komarova SV. 2019. Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci. Rep. 9, 10542. ( 10.1038/s41598-019-46896-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein GD, Boucek RJ. 1960. Collagen and elastin of human dermis. J. Invest. Dermatol. 35, 227-229. ( 10.1038/jid.1960.109) [DOI] [PubMed] [Google Scholar]
- 43.Fuchs E. 1983. Evolution and complexity of the genes encoding the keratins of human epidermal cells. J. Invest. Dermatol. 81, 141-144. ( 10.1111/1523-1747.ep12540922) [DOI] [PubMed] [Google Scholar]
- 44.Strnad P, Usachov V, Debes C, Gräter F, Parry DAD, Omary MB. 2011. Unique amino acid signatures that are evolutionarily conserved distinguish simple-type, epidermal and hair keratins. J. Cell Sci. 124, 4221-4232. ( 10.1242/jcs.089516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks GL, Brenna JT. 2005. 15N/14N position-specific isotopic analyses of polynitrogenous amino acids. Anal. Chem. 77, 1013-1019. ( 10.1021/ac048903o) [DOI] [PubMed] [Google Scholar]
- 46.Kelly S, Heaton K, Hoogewerff J. 2005. Tracing the geographical origin of food: the application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 16, 555-567. ( 10.1016/j.tifs.2005.08.008) [DOI] [Google Scholar]
- 47.Matthews KR, Boner M, Young R. 2011. Authentication of country of origin of pork and pig meat using isotope reference analysis. Kenilworth, UK: Agricultural and Horticultural Development Board. [Google Scholar]
- 48.Camin F, Bontempo L, Perini M, Piasentier E. 2016. Stable isotope ratio analysis for assessing 455 the authenticity of food of animal origin. Compr. Rev. Food Sci. Food Saf. 15, 868-877. ( 10.1111/1541-4337.12219) [DOI] [PubMed] [Google Scholar]
- 49.Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 33, 507-559. ( 10.1146/annurev.ecolsys.33.020602.095451) [DOI] [Google Scholar]
- 50.Lübcker N, Whiteman JP, Newsome SD, Millar RP, de Bruyn PJN. 2020. Can the carbon and nitrogen isotope values of offspring be used as a proxy for their mother's diet? Using foetal physiology to interpret bulk tissue and amino acid δ15N values. Conserv. Physiol. 8, coaa060. ( 10.1093/conphys/coaa060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobson KA, Alisauskas RT, Clark RG. 1993. Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. Condor 95, 388-394. ( 10.2307/1369361) [DOI] [Google Scholar]
- 52.Hatch KA, Sacksteder KA, Treichel IW, Cook ME, Porter WP. 1995. Early detection of catabolic state via change in 13C/12C ratios of blood proteins. Biochem. Biophys. Res. Commun. 212, 719-726. ( 10.1006/bbrc.1995.2030) [DOI] [PubMed] [Google Scholar]
- 53.Fuller BT, Fuller JL, Sage NE, Harris DA, O'Connell TC, Hedges REM. 2004. Nitrogen balance and δ15N: why you're not what you eat during pregnancy. Rapid Commun. Mass Spectrom. 18, 2889-2896. ( 10.1002/rcm.1708) [DOI] [PubMed] [Google Scholar]
- 54.Beaumont J, Montgomery J. 2016. The great Irish famine: identifying starvation in the tissues of victims using stable isotope analysis of bone and incremental dentine collagen. PLoS ONE 11, e0160065. ( 10.1371/journal.pone.0160065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaumont J, Montgomery J, Buckberry J, Jay M. 2015. Infant mortality and isotopic complexity: new approaches to stress, maternal health, and weaning. Am. J. Phys. Anthropol. 157, 441-457. ( 10.1002/ajpa.22736) [DOI] [PubMed] [Google Scholar]
- 56.Baković M, Vreča P, Mayer D. 2017. Case of fatal starvation: can stable isotope analysis serve to support morphological diagnosis and approximate the length of starvation? J. Forensic Sci. 62, 258-264. ( 10.1111/1556-4029.13244) [DOI] [PubMed] [Google Scholar]
- 57.Doi H, Akamatsu F, González AL. 2017. Starvation effects on nitrogen and carbon stable isotopes of animals: an insight from meta-analysis of fasting experiments. R. Soc. Open Sci. 4, 170633. ( 10.1098/rsos.170633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheeke PR, Dierenfeld ES. 2010. Comparative animal nutrition and metabolism. Wallingford, UK: Centre for Agriculture and Bioscience International (CABI). [Google Scholar]
- 59.Jim S, Jones V, Ambrose SH, Evershed RP. 2006. Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. Br. J. Nutr. 95, 1055-1062. ( 10.1079/bjn20051685) [DOI] [PubMed] [Google Scholar]
- 60.Newsome SD, Wolf N, Peters J, Fogel ML. 2014. Amino acid δ13C analysis shows flexibility in the routing of dietary protein and lipids to the tissue of an omnivore. Integr. Comp. Biol. 54, 890-902. ( 10.1093/icb/icu106) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are presented in the article and electronic supplementary material file.