Abstract
Selective regimes favouring the evolution of functional specialization probably affect covariation among phenotypic traits. Phalanges of most tetrapods develop from a conserved module that constrains their relative proportions. In geckos, however, biomechanical specializations associated with adhesive toepads involve morphological variation in the autopodium and might reorganize such modular structures. We tested two hypotheses to explain the modular architecture of hand bones in geckos, one based on developmental interactions and another incorporating functional associations related to locomotion, and compared the empirical support for each hypothetical module between padded and padless lineages. We found strong evidence for developmental modules in most species, which probably reflects embryological constraints during phalangeal formation. Although padded geckos exhibit a functional specialization involving the hyperextension of the distal phalanges that is absent in padless species, the padless species are the ones that show a distal functional module with high integration. Some ancestrally padless geckos apparently deviate from developmental predictions and present a relatively weak developmental module of phalanges and a strongly integrated distal module, which may reflect selective regimes involving incipient frictional adhesion in digit morphology. Modularity of digit elements seems dynamic along the evolutionary history of geckos, being associated with the presence/absence of adhesive toepads.
Keywords: autopodium, phalanges, morphology, toepad, modularity
1. Introduction
Organisms have variable degrees of cohesion among traits. Such variation may result in divergent modular body organization derived from a diversity of genetically, developmentally and/or functionally integrated processes [1,2]. Divergent integration expressed within and between modules may have important evolutionary consequences because it constrains to different extents the range of morphological variation to be further selected [3]. Reorganization of a modular architecture might be elicited by changes in the magnitude of integrative forces within units, for example owing to changes in environmental pressures acting across generations [4,5]. This idea is supported by empirical evidence of modular serial homologues that evolved novel functional specialization coupled with changes in magnitudes of trait correlation [1], as illustrated by the evolution of tetrapod limbs associated with the diversification of modes of locomotion [6–8].
Some authors have suggested that limbs in tetrapods are derived from a single ancestral developmental module [9], but developmental variation expressed in each limb segment (stylopodium, zeugopodium and autopodium) is hypothesized to match functional specialization [6,9–12]. For example, strong covariation is reported among limb segments in quadrupedal animals, in which fore and hind limbs perform similar functions [6]. In non-quadrupedal mammals, however, selective regimes related to specific locomotor activities, including flying and walking bipedally, favoured functional decoupling of fore and hind limbs associated with a reduction of the overall integration among elements [6,7,11]. Such morphological divergence possibly includes a reduction in the local developmental factors shared by the limbs, as functional specialization probably involves the reorganization of the ancestral modular architecture into smaller and highly integrated sub-modules. The evolution of digits involving particular developmental networks [10] has been interpreted as a novel semi-independent distal limb module in which morphology and integration are also susceptible to environmental interactions [13].
The origin of digits in the evolutionary history of vertebrates seems associated with novel functional and developmental patterns [14] that probably reflect a modular individualization of the autopodial structure driven by selective pressures that prevailed in the ancestral environments [13,15]. Among most tetrapod lineages, a deeply conserved pattern of length proportionality within phalanges of a digit is observed, ranging from a nearly equal-sized phenotype to a large-to-small gradient of phalangeal lengths [13]. Such range seems determined by the developmental modularization of autopodial features, where morphological proportions of phalanges strongly covary in response to developmental disturbances, encompassing: (i) a module that is developmentally semi-autonomous from metapodial bones (metacarpals in the forelimb and metatarsals in the hind limb), and (ii) a phalangeal-tip module [13]. The range of phalangeal proportions observed in most tetrapods is also probably associated with functional constraints in specific ecological settings [13]. Although scarce, exceptions for such a constrained pattern of variation have been detected in extant lineages, including Squamata (lizards and snakes). Lizards emerge as an excellent model system to evaluate modularity in the autopodium also owing to the ecological diversity and the remarkable variation in limb morphology reported in several clades [16–20].
The lineage Gekkota comprises a wide range of autopodium phenotypes that do not always reflect the model of morphological pattern derived from developmental modularity synthesized by Kavanagh et al. [13]. Geckos are renowned for the presence of sub-digital adhesive toepads, which have independently evolved several times in the lineage, often associated with remarkable climbing abilities [17,21,22]. In these lizards, pad evolution seems associated with several anatomical modifications in the autopodium, including specific shape and size patterns of the phalanges [16,17,23,24]. Biomechanical specialization during locomotion in padded geckos also involves distoproximal hyperextension of the digits [23,25]. Most tetrapods release digits from the surface in a proximal-to-distal mode [23,26], resulting in a precise correspondence between functional and developmental modules [13], while digital hyperextension of padded geckos starts distally, and pad detachment is followed by wrist/ankle lift-off [23,26,27]. Current models that assume phalanges as deriving from a single developmental module imply strong integration among these bones, an assumption that is challenged by the functional implications of hyperextension in padded geckos. In fact, a phenotype of ‘large-small-large’ proportions has been reported in padded gecko species that exhibit an excessive reduction of the antepenultimate phalanx [22,28], suggesting that pad evolution might have elicited reorganization of a modular architecture that is different from the developmental model suggested for other tetrapods [13]. Functional reorganization of the ancestral developmental module of the autopodium might then have been favoured by the selection of a distal module that executes particular functions in specific ecological contexts (e.g. arboreality).
Here, we evaluate whether functional implications of adhesive toepad evolution influenced the modular phalangeal architecture in geckos. We predict that conserved developmental modules in Tetrapoda [13] are shared by all gecko species, but correlations will be lower in padded species because of distoproximal hyperextension, which does not occur in most tetrapods. Specifically, the degree of modularity (i.e. how much correlations within-modules surpass those between-modules) of developmental modules is expected to be weaker in padded geckos than in padless species, while stronger modularity of functional modules is expected in padded geckos. To test these hypotheses, we established two hypothetical models based on different theoretical backgrounds to explain the modular pattern of the anterior autopodium, one strictly based on developmental processes, and the other incorporating developmental and functional relationships to explain autopodial modularization. We explore the degree of modularity of the developmental and functional models for each species, and also use comparative methods to test whether the presence of toepads is associated with higher degrees of developmental and/or functional modularity. Investigation of trait covariation at several hierarchical levels coupled with the functional diversity of geckos provides an excellent context to address how evolutionary processes might drive phenotypic diversity in living organisms.
2. Material and methods
(a) . Study sample
We compiled a morphological dataset from digital X-rays obtained on preserved specimens of 18 gecko species and two outgroups available at herpetological collections; samples ranged from 16 to 32 individuals for each species (490 specimens; electronic supplementary material, List S1). We focused on a monophyletic radiation of three families—Sphaerodactylidae, Phyllodactylidae and Gekkonidae—which probably comprise several independent origins of adhesive toepads [17]. Specimens were radiographed using Faxitron LX-60 (specimens from Brazilian collections) and Solus-Schall (specimens from the Natural History Museum in the UK) X-ray systems. The anterior autopodia were flattened on a support plate parallel to the X-ray platform and fixed with adhesive tape to avoid limb rotation and parallax error. Measurements were preferably taken at the right side of each specimen. When the right autopodium was damaged, we assumed left-right symmetry and measured the left side. Osteological distances were measured from radiographs using ImageJ version 1.45s [29]. Fifteen variables were compiled for each individual representing metacarpal and phalangeal lengths of digits III, IV and V (electronic supplementary material, figure 1 and table S1). We measured three digits exhibiting the same phalangeal formula across all sampled species (digit III = 4, digit IV = 5, digit V = 3). We measured digit V in 13 gecko species for a previous publication [16], and new measurements for digits III and IV were acquired from the same X-ray images used before. Digit I was not measured because its developmental identity differs from that of the remaining digits [30]; digit II was also excluded because it was damaged in several specimens and inclusion would have restricted sample size. Species were classified as padded and padless according to the presence or absence of digital adhesive toepads.
(b) . Phenotypic matrices: similarity, repeatability and overall integration
After measuring the osteological elements in the specimens, we performed basic descriptive statistical analyses to verify data reliability and presence of outliers. All statistical analyses were conducted in the R programming environment ([31]; see detailed explanation in the electronic supplementary material, methods S1 and also figure S1). We applied linear models to account for the sex effect on trait means when appropriate (detailed in the electronic supplementary material, methods S2 and also tables S2–S5) and used their residuals to estimate for each species: (i) the variance/covariance matrices (V/CV, using the function CalculateMatrix, from EvolQG R package; [32]), and (ii) the correlation matrices (using the function cov2cor).
We then performed pairwise comparisons among matrices of all species to infer similarity patterns using the method of random skewers (RS; [33–35]). Inference of similarity based on the RS method essentially indicates the evolutionary resemblance between two matrices in their response to random selection [35]. We used 1000 random selection vectors with lengths standardized to 1.0; the resulting index is interpreted as any correlation and varies from −1 (opposite responses to random selection) to 1 (similarity in response to random selection is fully shared between matrices; see [35]). We recognize that some authors question reliance of the RS method on the distribution of cosines [36], but criticisms raised are restricted to dimensionalities (only 2 or 3) lower than the 15 dimensions we use here [37].
In order to account for sampling errors in matrix estimates and to determine matrix reliability, matrix repeatability was inferred using a bootstrap resampling test of self-correlation [38]. We used the function BootstrapRep from EvolQG [32], which randomly resamples individuals with replacement, re-estimates the matrix and then computes the average similarity (using the RS function) between the resampled matrices and the original matrix. To account for differences in matrix similarity among species with different sample sizes, we adjusted matrix similarity by matrix repeatability using the following equation: radj = robs/(t1t2)0.5, where radj is the adjusted similarity, robs is the original similarity, and t1 and t2 are the repeatabilities of the two matrices to be compared. We expected higher matrix similarity within padded or within padless species than between padded and padless species.
Body size is frequently the factor that explains most of trait variation resulting from allometric relationships that derive from growth [4,39]. Allometry probably enhances integration among all traits because it is a global integration factor [40], so growth effects may obscure the identification of modular patterns [41]. Therefore, we constructed residual correlation matrices by removing variation in the matrices associated with the size component for each species and tested modularity models on these residual matrices. Size was inferred from raw data using an eigenanalysis of species matrices: when one of the eigenvectors showed all trait loadings positive or negative, we assumed overall variation in the vector for all traits to increase or decrease in length; it was therefore a vector associated with size variation [41]. In all gecko species, the first eigenvector was size-related and accounted for 35.71% to 88.12% of variation. The variation associated with this size-related eigenvector was removed from the species V/CV matrices using the function RemoveSize from EvolQG R package [32]. By removing variation associated with allometric size, several correlations previously positive in the original matrices became negative. Therefore, many positive associations between traits were originally related to growth effects. For the correlations that remained positive in residual matrices, we infer that local developmental and/or functional processes might be strong enough to contribute to the positive association between traits despite global integrating effects of allometric growth.
(c) . Inferences on modularity
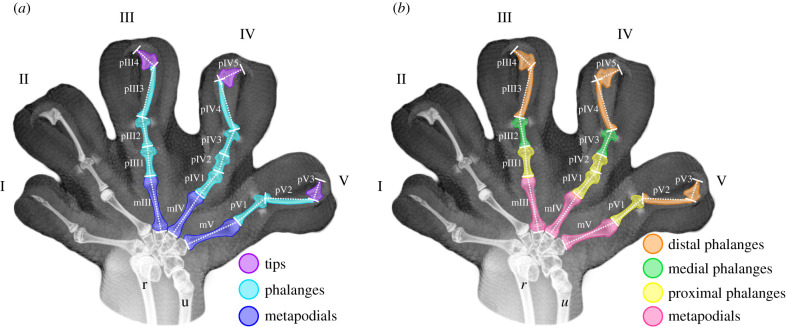
To test whether hypothetical models of modularity are good predictors to describe correlation patterns for each gecko species, we established two theoretical models to explain the autopodium modular pattern (see the electronic supplementary material). The first model was named ‘developmental model’ and strictly expressed the developmental hypothesis proposed by Kavanagh et al. [13], predicting that all phalanges (except for the tip, which is the distal-most one) develop from a single module that is different from that of the metapodium (figure 1). The second model was named ‘functional model’ and incorporated functional implications of locomotion to explain modularity in the autopodium. This model assumes the metacarpals as a single functional module but also attributes biomechanical explanations for phalangeal variation. It is important to highlight that both modularity models presume the metapodium as a separate module, so that integration in this region would reflect the developmental and functional relationships previously described in the literature [13]. The functional modularity model incorporated functional implications of distoproximal hyperextension into the strictly developmental hypothetical model and was expected to better adjust to padded species—which perform such movement. Phalanges in the functional modularity model would be structured in three modules (figure 1): (i) proximal module, which is not directly involved in hyperextension; (ii) medial module (phalanges adjacent to the region of distoproximal hyperextension that are reduced in some gecko lineages [28]); and (iii) distal module (phalanges that actively perform distoproximal hyperextension). Although we refer to this modularity hypothesis as a ‘functional model’, we clarify that this model might also be interpreted as a ‘mixed development-functional model’ because it also predicts that terminal elements will vary differently from more proximal elements, similarly to the developmental model (although the number of terminal elements in each hypothetical module differs between models; figure 1).
Figure 1.
X-rays from the anterior autopodium of Thecadactylus rapicauda, illustrating measurements obtained in digits III, IV and V (dashed white lines), and coloured representations of each hypothetical module tested in the developmental ((a): tip, phalangeal, metapodial) and the functional ((b): distal, medial, proximal and metapodial) models of digit modularity. Codes correspond to: r = radius; u = ulna; mIII = third metacarpal; pIII1/2/3/4 = digit III phalanges; mIV = fourth metacarpal; pIV1/2/3/4/5 = digit IV phalanges; mV = fifth metacarpal; pV1/2/3 = digit V phalanges. (Online version in colour.)
Theoretical modularity matrices were assembled through attribution of value = 1 when a given correlation was enclosed within the module, or a value = 0 if it was not a component of that module [38,41]. We evaluated the empirical support for each module separately, but also for a model that combined all hypothetical modules of each hypothesis (developmental and functional) in a single matrix (named ‘total integration’), using the function TestModularity from EvolQG R package [32]. Theoretical matrices were correlated with the observed residual correlation matrices, and significance was verified using the Mantel test, which compares the original correlation between matrices with a random distribution that accounts for the non-independence of trait correlations using matrix permutations [38]. It is important to highlight that the theoretical matrices do not correspond to a literal representation of the patterns expected to be observed in nature, when two traits would exhibit either complete correlation (1) or none (0). These matrices actually provide a simplified tool to evaluate if average correlation is higher when traits belong to the same hypothetical module than the average correlation when traits belong to different modules [33].
To infer the degree of modularity, we calculated the difference between AVG+ (the average squared correlation within-modules) and AVG- (the average squared correlation between-modules), which is named ‘AVG difference’ [33,42]. We repeated the procedure for each module of both hypotheses, as well as for the matrix of total integration of ‘development’ and ‘functional’ modules. To account for sampling error on AVG difference, we used the Monte Carlo method to resample the observed correlation matrix of each species 1000 times using a multivariate normal distribution with a vector of zero means, the empirical correlation matrix and sample size as parameters, specifically for each species (function rmvnorm from Mvtnorm R package [43]). We then used the 1000 resampled matrices to estimate the interval of two standard deviations from the average that accounts for the 95% confidence interval of AVG differences distributions and interpreted values as being significant when intervals did not contain zero. We also inferred the sampling error using bootstrapping to resample 1000 times the residuals obtained from the linear models, with replacement and using the original sample size, which does not assume multivariate normal distribution of the traits. A pattern is considered modular when the significant values of AVG difference are positive because integration within modules (AVG+) is higher than that between modules (AVG−). Therefore, the higher the AVG difference, the higher degree of modularity inferred [42].
(d) . Pad presence and modular signal analyses
Results from the modularity tests were used to investigate associations between the degree of modularity and the presence of toepads. In agreement with the modularity analyses just described, these subsequent analyses were also performed on size-corrected data. The AVG differences calculated for all hypothetical modules were included as dependent variables in phylogenetic generalized least-squares (PGLS) analyses, and morphological classification as ‘padded’ or ‘padless’ phenotype constituted independent variables. Even though analysis to reconstruct ancestral covariance matrices along with a phylogeny are now available (e.g. [44]), AVG difference is a single metric that describes the phenomenon we are interested in, that is, the degree of modularity, with probably low uncertainty because it corresponds to a difference in average correlations, which is better estimated than individual correlations. Therefore, we considered it appropriate to reconstruct AVG difference along with the phylogeny to investigate its relationship with the presence of toepads. We used the R package ape [45] to calculate the structure of phylogenetic covariation between species assuming that traits evolved under a Brownian motion model. Association between the metacarpal module and the presence of toepads was tested only once because these elements correspond to the same hypothetical module in the developmental and functional models tested here. We used the phylogenetic hypothesis proposed by Tonini et al. [46], which comprises branch lengths for all species sampled in our study. A polytomy in the node of the genus Hemidactylus was solved based on the topology proposed by Pyron et al. [47]. We first performed a PGLS using all species sampled (function gls from R package nlme [48]) and then removed the outgroups Sphenodon punctatus and Tropidurus catalanensis in order to verify if results are sensitive to taxonomic sampling (i.e. within Gekkota or in a broader evolutionary scenario).
3. Results
(a) . Species phenotypic matrices
Analyses performed using a subset of 12 gecko species and one outgroup suggested that sexual dimorphism does not significantly affect trait means in geckos, but does so in the iguanian T. catalanensis (electronic supplementary material, tables S2–S5). Therefore, subsequent analyses using the gecko dataset were implemented without correcting for sex, while such correction was applied in the outgroup (see the electronic supplementary material, methods S2). Repeatability in residual correlation matrices ranged from 0.85 to 0.96 (electronic supplementary material, table S6), which indicates a high confidence in the matrix estimation. Values of similarity were high among all correlation matrices (average correlation of 0.86, in which the lowest value was 0.69 and all comparisons had a p-value < 0.01), which indicates that the pattern of correlation is similar and stable among all studied species (see the electronic supplementary material, table S6). On average, the values of similarity between correlation matrices of padless species were higher than those of padded geckos (0.91 and 0.83, respectively).
(b) . Tests of modularity
Prior to the phylogenetic comparative analyses, we detected that the degree of modularity was variable among the modules tested, as well as among species (electronic supplementary material, table S7). The significance of the degree of modularity is also affected by the resampling method employed (electronic supplementary material, table S7). Therefore, we considered a module having a robust modularity when both distributions derived from Monte Carlo and bootstrap resampling exhibited significant values.
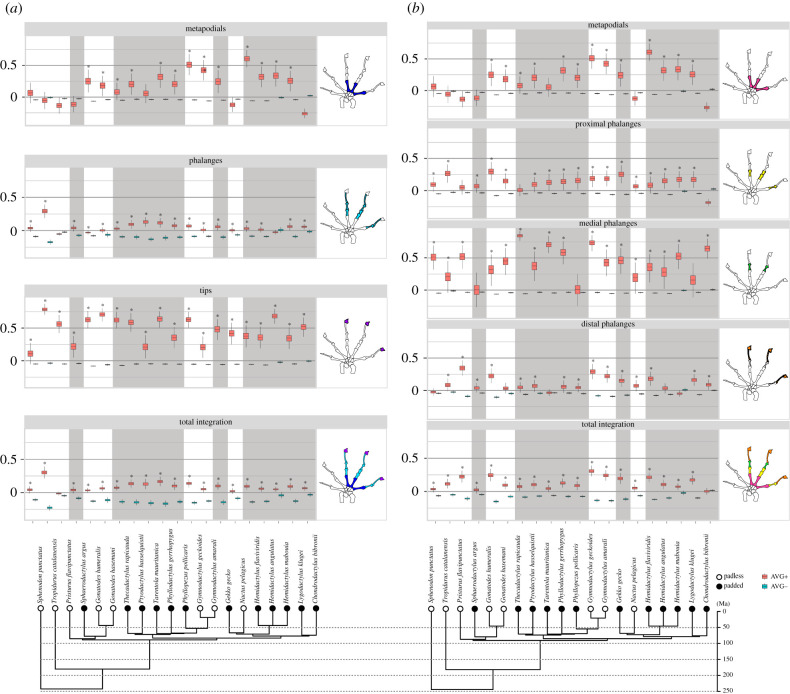
The developmental hypothesis was strongly supported for most species, as shown by the average differences of integration values within modules (AVG+) and between modules (AVG−) in the total integration analyses (figure 2a). Compared to some gecko species, the outgroups S. punctatus and T. catalanensis exhibited relatively strong support for the phalangeal developmental module and weak support for the distal functional module, corroborating the expectation that an ancestral phalangeal module of development would be retained in our analyses. The phalangeal module of development was supported in almost all geckos, although showing the modularity signal relatively weaker in comparison with the mean of other modules (AVG difference = 0.13), such as the tips (figure 2a). The tip module exhibited a high modular signal (mean AVG difference = 0.52) for almost all species except for S. punctatus. This is the case because all average correlations between-modules (AVG−) are slightly negative whereas all average correlations within the tip module are highly positive, indicating that local processes related to tip development are strong. The metacarpal module is common to both hypotheses of modularity tested, and although most species exhibited strong modular signal among the metacarpal bones, five geckos had low values for within-module integration (AVG+) among the metapodials, indicating no evidence that they form their own module in these taxa (mean AVG difference = 0.21).
Figure 2.
Average correlations within (AVG+; red boxplots) and between (AVG−; blue bloxplots) modules of the developmental (a) and the functional (b) models, and asterisks indicate a significant difference between them. Adjacent autopodia illustrate which bones belong to each module. Correlations highlighted in grey indicate pad-bearing geckos (black dots in the topology), and those in white correspond to padless species (white dots in the topology). Time-calibrated phylogeny extracted from Tonini et al. 2016 [46], age of clades in million years. (Online version in colour.)
The total integration of the functional model was also supported for most species (figure 2b), although the modular signal was, in general, not as strong as identified for the developmental model. Most species showed strong modularity in the phalangeal modules (proximal, medial and distal), with the highest modularity degree for the medial module (mean AVG difference = 0.45). We did not identify clear differences between padless and padded animals for the medial nor the proximal phalangeal modules (mean AVG difference = 0.18) in the functional model. In general, the distal module (mean AVG difference = 0.16) was not as modular as the medial module, a result especially pronounced among padless geckos. In the padless outgroup, however, the distal module exhibited a relatively low degree of modularity in comparison to padless geckos.
(c) . Phylogenetic comparative analysis
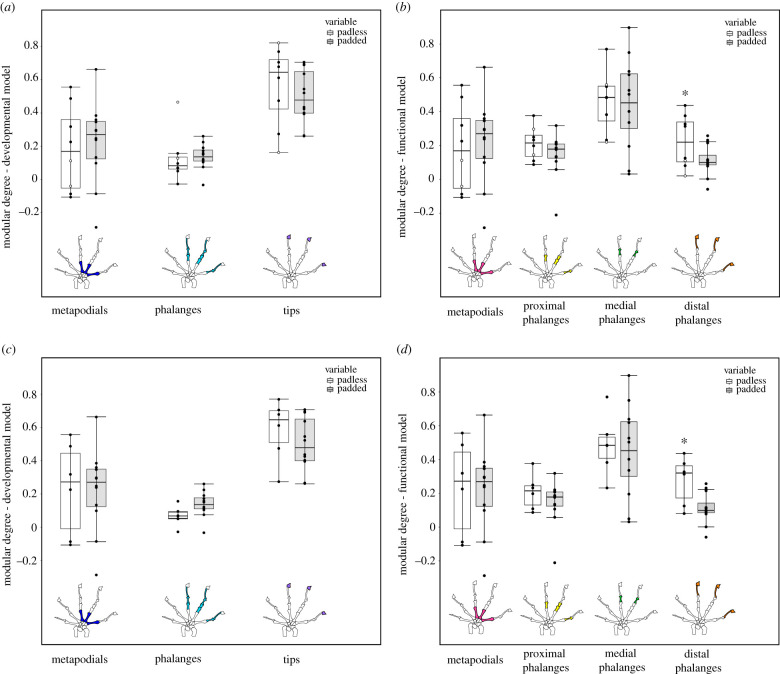
We identified divergence in modularity between padded and padless species, the later involving a functional module comprising the distal phalanges. This novel module was detected in geckos without toepads in both PGLS analyses, and divergence was more pronounced after removing the outgroups (S. punctatus and T. catalanensis; figure 3; electronic supplementary material, tables S7 and S8). In the developmental hypothesis, the difference between pad-bearing and padless geckos was more apparent when we excluded the two outgroup species from the PGLS analysis (figure 3; electronic supplementary material, table S8). Although the difference was only marginally significant (p-value = 0.06), analysis performed only with geckos suggests that padded species tend to have more integrated phalanges than the padless ones.
Figure 3.
Modular degree (AVG difference) of padless (white) and padded (grey) species, for each module of the developmental and functional modularity hypotheses. (a) and (b) include all sampled species (white dots indicate the outgroups Sphenodon punctatus and Tropidurus catalanensis); (c) and (b) comprise geckos only. Significant differences (p < 0.05) indicated by an asterisk. (Online version in colour.)
4. Discussion
Autopodium evolution played essential roles during the occupation of novel habitats [14,49], this structure being a model system to investigate associations between morphological evolution and functional diversity. The present study evaluates how the evolution of specialized locomotor traits—the presence of adhesive toepads in geckos—relates to the modular architecture of the autopodium. We investigated whether developmental and functional modularity may have changed during the evolutionary history of three major families of Gekkota. Our results corroborate that the developmental model of digit formation (see [13]) is a strong hypothesis to explain the modularity of hand bones for most species, regardless of the presence of adhesive toepads. Functional modules also exhibited strong modularity signal both in padded and padless geckos. However, although padded geckos exhibit a functional specialization that includes hyperextension of the distal phalanges during setal attachment and release, the padless species are the ones that show a distal functional module, contradicting our expectation that this distal module would exhibit a greater modular degree in padded geckos. Together, these results indicate that the autopodium has a variable modular architecture associated with the evolution of adhesive toepads during the radiation of three Gekkota families.
We identified similar correlation patterns of autopodial bones among all studied species of geckos, in agreement with expectations from comparisons of homologues sharing similar genetic architecture. Analogous to what has been proposed for cranial stability of the covariance structure in vertebrates [33,34,42,50,51], the conservation of correlation patterns in the autopodium is probably maintained by a similar pattern of multivariate stabilizing selection associated with developmental trait interactions in geckos. Phalangeal development comprises a high integration among adjacent segments during early stages of phalangeal condensation [13,52], a process established before the development of toepads [53]. Early fundamental morphogenic processes channel the pattern of variation accessible to selection, so development is often phylogenetically conserved and the phenotypic structure of covariation is stable [4,33,51,54]. This is also a reasonable explanation for why the development-based model prevailed over the functional model for most of the gecko species regardless of the presence of adhesive toepads and may also explain why developmental modules were evident even in species where functional modules showed highly modular signals. However, the conserved patterns of covariation and correlation among hand bones identified in geckos do not imply invariable mean morphology of phalangeal size and proportions in this lineage. Autopodial elements of geckos display a variety of shapes and sizes, as exemplified by the extremely reduced intermediate phalanx of Hemidactylus [22,28] and the overall shorter phalangeal proportions in pad-bearing species [24]. Such mean morphological variation across species is possible even though species show conserved developmental modularity and may occur because integration among autopodium elements is not complete, allowing some independent variation of single elements across species. Although development predominantly explains autopodial correlations in geckos, this is not a rule for all species, and functional modules also seem associated with the modular architecture in this lineage.
In Gekkota, adhesive toepads independently evolved several times in association with many unique anatomical and biomechanical features [17,22,24,55], and different toepad origins probably encompassed unique changes in the autopodium modular architecture. Functional limb specializations in tetrapods associated with non-quadrupedal locomotion often result in a decrease in the overall integration between the fore and hindlimbs, eventually including partitions of the ancestral developmental module [6,12,56,57]. In geckos, the presence of toepads is associated with locomotor specializations of the digits [23,58,59] and therefore a similar result was expected. However, the modular pattern detected in pad-bearing geckos is characterized by functional distal elements that show reduced integration, while the developmental module of phalanges in these species exhibits a trend of relatively stronger integration. We identified this same pattern in the padless outgroups. These patterns suggest that selection related to functional specialization of phalanges was not strong enough to modify the developmental interactions in the same way as observed, for example, in the limb bones of primates, which modular structure has changed in response to locomotor specialization [12,56]. Likewise, a partition of ancestral modules was not detected in other cases of drastic morphological change associated with functional specialization, as highlighted by the conserved modular organization observed in the mammalian skull [33]. Thus, divergent phenotypes with different functional advantages may comprise similar modular architectures, reflecting shared development, but differing in average phenotypes even when an evolutionary innovation is present, such as adhesive toepads.
We acknowledge our dataset represents only a fraction of the overall Gekkota diversity, so the pattern of conserved developmental modules and higher integration of the distal functional module observed in padless species might not fully capture the complex evolutionary history of gains and losses of adhesive toepads in the group. Inclusion of additional species is challenged by the relatively large numbers of individuals per species requested by modularity analyses. Current literature comprising ancestral reconstructions of toepad evolution in Gekkota (see [17]) supports our premise that all padless lineages studied here probably evolved from padless ancestors and, accordingly, that the origin of adhesive toepads might have disrupted the high integration between distal elements eventually present in ancestral lineages of Gekkota. Complementary comparisons between species from sister-clades (electronic supplementary material, table S7 and figures S2 and S3) endorse such interpretation for most—but not all—padless species (for example, in Sphaerodactylidae, the padless Gonatodes hasemani exhibits a modular signal in the distal phalanges as low as that of the padded Sphaerodactylus argus). Further analyses with larger sampling may confirm whether the presence of a distal module of phalanges is a derived condition across padless geckos.
In climbing geckos that bear pads, the two distal-most phalanges (penultimate phalanx and the tip) are often shorter than in non-climbing geckos, which probably facilitates distoproximal hyperextension of padded digits [16]. These same phalanges also comprise lower modular signal in padded geckos (this study), a result that contrasts with the higher modularity of distal phalanges we detected in the padless Gonatodes, Pristurus and Gymnodactylus. The distal autopodium region in padless lizards often contributes to increased functional limb length during steady sprinting and promotes grasping stability at different orientations [16,60,61]. Padless species usually exhibit penultimate phalanges that are curved, establishing with the ungual phalanx a distally arched region above the substratum that allows efficient claw grasping [17,55,61] through interaction with the region of intermediate phalanges [55,62,63]. The padless geckos we studied lack a complex adhesive system but may exhibit friction enhancing sub-digital filaments [55,62,64]. Therefore, sub-digital filaments positioned in this region of intermediate phalanges may enhance friction and traction during locomotion and, especially in Gonatodes humeralis, may provide adhesive ability even in the absence of distoproximal hyperextension [25]. The strong signal we detected in the padless distal module may therefore reflect the coordinated function of these elements during grasping, in contrast with the weaker integration of distal phalanges detected in geckos that actively perform distoproximal hyperextension. Padded gecko species apparently evolved functionally specialized distal phalanges able to perform a novel biomechanical function (distoproximal hyperextension) while retaining an ancestral developmentally conserved modular pattern.
5. Conclusion
The present study describes the modular architecture of the autopodium in geckos, evaluating for the first time, to our knowledge, possible associations between modularity among osteological elements and the evolution of complex adhesive toepads. Our results indicate that the modular architecture of the autopodium in Gekkota reflects both development and function associated with the absence or presence of complex sub-digital adhesive systems. We corroborated the hypothesis that most geckos retained the ancestral developmental modules in the autopodium, but also identified an unexpected pattern. Specifically, we showed that the functional specialization associated with toepad evolution did not result in a new highly integrated module of novel biomechanical significance. Moreover, some padless species that do not perform distoproximal hyperextension show a remarkable modularity in the distal elements of the autopodium that diverges from the ancestral phalangeal pattern. This may reflect responses to specific selective regimes involving anatomical rearrangements of the sub-digital spinules during the occupation of novel microhabitats. In Gekkota, locomotion involving incipient frictional setae seems to prompt adjustments in the autopodial modular architecture that increase its integration within a functional distal module, at the same time that biomechanical innovation comprising distoproximal hyperextension evolved without the partitioning of an ancestral developmentally conserved modular pattern. The modularity of digit elements has therefore been dynamic throughout the evolutionary history of geckos, providing support for an integrative perspective of evolutionary processes shaping morphological diversity during multiple independent origins of complex adhesive systems.
Supplementary Material
Acknowledgements
We acknowledge the curators and technicians at the following herpetological collections for loans and/or support during technical visits for data acquisition: Patrick Campbell (Natural History Museum, UK); Selma Torquato (Museum of Natural History, Federal University of Alagoas, Brazil); Rejâne Silva and Gabriel Passos (Museum of Zoology, Federal University of Bahia, Brazil); Hussam Zaher and Carolina Mello (Museum of Zoology, University of São Paulo, Brazil); Paulo Passos, Ronaldo Fernandes and Pedro Pinna (National Museum, Federal University of Rio de Janeiro, Brazil) and Guarino Colli and Isis Arantes (Herpetological Collection of the University of Brasília, Brazil). We also thank Flávio Bockmann and Ricardo MC Castro for space and technical support in the Ichthyology Laboratory at Ribeirão Preto (University of São Paulo, Brazil).
Contributor Information
Priscila S. Rothier, Email: priscila.de-souza-rothier-duarte@edu.mnhn.fr.
Tiana Kohlsdorf, Email: tiana@usp.br.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.c59zw3r74 [65]. Codes are described in the electronic supplementary material S2.
Authors' contributions
P.S.R.: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, writing—original draft and writing—review and editing; M.N.S.: formal analysis, methodology, validation and writing—review and editing; G.M.: methodology, validation, writing—review and editing; A.H.: investigation, visualization and writing—review and editing; T.K.: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, validation, visualization, writing—original draft and writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Capes and FAPESP student grant nos (2012/18269-3, 2014/26287-7 and 2016/03953-7) to P.S.R., a FAPESP postdoctoral grant no. (2015/19556-4) to M.N.S. and thematic FAPESP grant nos. (2015/07650-6 and 2020/14780-1) to T.K.
References
- 1.Wagner GP, Pavlicev M, Cheverud JM. 2007. The road to modularity. Nat. Rev. Genet. 8, 921-931. ( 10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 2.Klingenberg CP. 2008. Morphological integration and developmental modularity. Ann. Rev. Ecol. Evol. Syst. 39, 115-132. ( 10.1146/annurev.ecolsys.37.091305.110054) [DOI] [Google Scholar]
- 3.Goswami A, Smaers JB, Soligo C, Polly PD. 2014. The macroevolutionary consequences of phenotypic integration: from development to deep time. Phil. Trans. R. Soc. B 369, 20130254. ( 10.1098/rstb.2013.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raff RA. 1996. The shape of life: genes, development, and the evolution of animal form, 1st edn. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Melo D, Porto A, Cheverud JM, Marroig G. 2016. Modularity: genes, development, and evolution. Ann. Rev. Ecol. Evol. Syst. 47, 463-486. ( 10.1146/annurev-ecolsys-121415-032409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young NM, Hallgrímsson B. 2005. Serial homology and the evolution of mammalian limb covariation structure. Evolution 59, 2691-2704. ( 10.1111/j.0014-3820.2005.tb00980.x) [DOI] [PubMed] [Google Scholar]
- 7.Schmidt M, Fischer MS. 2009. Morphological integration in mammalian limb proportions: dissociation between function and development. Evolution 63, 749-776. ( 10.1111/j.l558-5646.2008.00583.x) [DOI] [PubMed] [Google Scholar]
- 8.Polly D. 2007. Limbs in mammalian evolution. In Fins into limbs: evolution, development, and transformation (ed. Hall BK), pp. 245-268. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 9.Ruvinsky I, Gibson-Brown JJ. 2000. Genetic and developmental bases of serial homology in vertebrate limb evolution. Development 5244, 5233-5244. ( 10.1242/dev.127.24.5233) [DOI] [PubMed] [Google Scholar]
- 10.Shubin N, Tabin C, Carroll S. 1997. Fossils, genes and the evolution of animal limbs. Nature 388, 639-648. ( 10.1038/41710) [DOI] [PubMed] [Google Scholar]
- 11.Young NM. 2013. Macroevolutionary diversity of amniote limb proportions predicted by developmental interactions. J. Exp. Zool. Part B 320, 420-427. ( 10.1002/jez.b.22516) [DOI] [PubMed] [Google Scholar]
- 12.Young NM, Wagner GP, Hallgrímsson B. 2010. Development and the evolvability of human limbs. Proc. Natl Acad. Sci. USA 107, 3400-3405. ( 10.1073/pnas.0911856107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavanagh KD, Shoval O, Winslow BB, Alon U, Leary BP, Kan A, Tabin CJ. 2013. Developmental bias in the evolution of phalanges. Proc. Natl Acad. Sci. USA 110, 18 190-18 195. ( 10.1073/pnas.1315213110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shubin NH. 2002. Origin of evolutionary novelty: examples from limbs. J. Morphol. 252, 15-28. ( 10.1002/jmor.10017) [DOI] [PubMed] [Google Scholar]
- 15.Shubin NH, Davis MC. 2004. Modularity in the evolution of vertebrate appendages. In Modularity in development and evolution (eds G Schlosser, GP Wagner), pp. 429-442. Chicago, IL: University of Chicago Press. [Google Scholar]
- 16.Rothier PS, Brandt R, Kohlsdorf T. 2017. Ecological associations of autopodial osteology in Neotropical geckos. J. Morphol. 278, 290-299. ( 10.1002/jmor.20635) [DOI] [PubMed] [Google Scholar]
- 17.Russell AP, Gamble T. 2019. Evolution of the gekkotan adhesive system: does digit anatomy point to one or more origins? Integr. Comp. Biol. 59, 131-147. ( 10.1093/icb/icz006) [DOI] [PubMed] [Google Scholar]
- 18.McGlothlin JW, Kobiela ME, Wright HV, Mahler DL, Kolbe JJ, Losos JB, Brodie ED. 2018. Adaptive radiation along a deeply conserved genetic line of least resistance in Anolis lizards. Evol. Lett. 2, 310-322. ( 10.1002/evl3.72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolbe JJ, Revell LJ, Szekely B, Brodie ED, Losos JB. 2011. Convergent evolution of phenotypic integration and its alignment with morphological diversification in Caribbean Anolis ecomorphs. Evolution 65, 3608-3624. ( 10.1111/j.1558-5646.2011.01416.x) [DOI] [PubMed] [Google Scholar]
- 20.Aerts P, van Damme R, Vanhooydonck B, Zaaf A, Herrel A. 2000. Lizard locomotion: how morphology meets ecology. Netherlands J. Zool. 50, 261-277. ( 10.1163/156854200X00126) [DOI] [Google Scholar]
- 21.Autumn K, Peattie AM. 2002. Mechanisms of adhesion in geckos. Integr. Comp. Biol. 42, 1081-1090. ( 10.1093/icb/42.6.1081) [DOI] [PubMed] [Google Scholar]
- 22.Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM. 2012. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE 7, e39429. ( 10.1371/journal.pone.0039429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell AP. 2002. Integrative functional morphology of the gekkotan adhesive system (Reptilia: Gekkota). Integr. Comp. Biol. 42, 1154-1163. ( 10.1093/icb/42.6.1154) [DOI] [PubMed] [Google Scholar]
- 24.Zhuang MV, Russell AP, Higham TE. 2019. Evolution of pedal digit orientation and morphology in relation to acquisition and secondary loss of the adhesive system in geckos. J. Morphol. 280, 1582-1599. ( 10.1002/jmor.21051) [DOI] [PubMed] [Google Scholar]
- 25.Higham TE, Gamble T, Russell AP. 2017. On the origin of frictional adhesion in geckos: small morphological changes lead to a major biomechanical transition in the genus Gonatodes. Biol. J. Linn. Soc. 120, 503-517. ( 10.1111/bij.12897) [DOI] [Google Scholar]
- 26.Biewener AA, Patek SN. 2003. Movement on land. In Animal locomotion, pp. 61-88. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Russell AP. 1975. A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia: Gekkonidae). J. Zool. 176, 437-476. ( 10.1111/j.1469-7998.1975.tb03215.x) [DOI] [Google Scholar]
- 28.Russell AP. 1977. The phalangeal formula of Hemidactylus Oken, 1817 (Reptilia, Gekkonidae): a correction and a function explanation. Zentralblatt fur Veterinarmedizin Reihe C-Journal of Veterinary Med. Ser. C- Anatomia Histologia Embryologia 6, 332-338. ( 10.1111/j.1439-0264.1977.tb00443.x) [DOI] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner GP. 2014. Digits and digit identity. In Homology, genes, and evolutionary innovation. pp. 356-382. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32.Melo D, Garcia G, Hubbe A, Assis AP, Marroig G. 2015. EvolQG - an R package for evolutionary quantitative genetics. F1000Research 4, 925. ( 10.1101/026518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porto A, de Oliveira FB, Shirai LT, de Conto V, Marroig G. 2009. The evolution of modularity in the mammalian skull I: morphological integration patterns and magnitudes. Evol. Biol. 36, 118-135. ( 10.1007/s11692-008-9038-3) [DOI] [Google Scholar]
- 34.Cheverud JM. 1996. Quantitative genetic analysis of cranial morphology in the cotton-top (Sagainus oedipas) and saddle-back (S. fuscicollis) tamarins. J. Evol. Biol. 9, 5-42. ( 10.1046/j.1420-9101.1996.9010005.x) [DOI] [Google Scholar]
- 35.Cheverud JM, Marroig G. 2007. Comparing covariance matrices: random skewers method compared to the common principal components model. Genet. Mol. Biol. 30, 461-469. ( 10.1590/S1415-47572007000300027) [DOI] [Google Scholar]
- 36.Rohlf FJ. 2017. The method of random skewers. Evol. Biol. 44, 542-550. ( 10.1007/s11692-017-9425-8) [DOI] [Google Scholar]
- 37.Machado FA, Zahn TMG, Marroig G. 2018. Evolution of morphological integration in the skull of Carnivora (Mammalia): changes in Canidae lead to increased evolutionary potential of facial traits. Evolution 72, 1399-1419. ( 10.1111/evo.13495) [DOI] [PubMed] [Google Scholar]
- 38.Cheverud JM, Wagner GP, Dow MM. 1989. Methods for the comparative analysis of variation patterns. Syst. Zool. 38, 201-213. ( 10.2307/2992282) [DOI] [Google Scholar]
- 39.Marroig G. 2007. When size makes a difference: allometry, life-history and morphological evolution of capuchins (Cebus) and squirrels (Saimiri) monkeys (Cebinae. Platyrrhini). BMC Evol. Biol. 7, 20. ( 10.1186/1471-2148-7-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bookstein F, Mitteroecker P. 2014. Comparing covariance matrices by relative eigenanalysis, with applications to organismal biology. Evol. Biol. 41, 336-350. ( 10.1007/s11692-013-9260-5) [DOI] [Google Scholar]
- 41.Porto A, Shirai LT, de Oliveira FB, Marroig G. 2013. Size variation, growth strategies, and the evolution of modularity in the mammalian skull. Evolution 67, 3305-3322. ( 10.1111/evo.12177) [DOI] [PubMed] [Google Scholar]
- 42.Simon MN, Marroig G. 2017. Evolution of a complex phenotype with biphasic ontogeny: contribution of development versus function and climatic variation to skull modularity in toads. Ecol. Evol. 7, 10 752-10 769. ( 10.1002/ece3.3592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genz A, Bretz F, Miwa T, Mi X, Leisch F, Scheipl F, Hothorn T. 2008. mvtnorm: multivariate normal and t distributions. R package version 0.9-2. See http://CRAN.R-project.org/package=mvtnorm.
- 44.Caetano DS, Harmon LJ. 2017. ratematrix: an R package for studying evolutionary integration among several traits on phylogenetic trees. Methods Ecol. Evol. 8, 1920-1927. ( 10.1111/2041-210X.12826) [DOI] [Google Scholar]
- 45.Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526-528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 46.Tonini JFR, Beard KH, Ferreira RB, Jetz W, Pyron RA. 2016. Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 204, 23-31. ( 10.1016/j.biocon.2016.03.039) [DOI] [Google Scholar]
- 47.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93. ( 10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2021. nlme: linear and nonlinear mixed effects models. R package version 3.1-152. See https://CRAN.R-project.org/package=nlme.
- 49.Hall BK. 2007. Fins into limbs: evolution, development, and transformation. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 50.Melo D, Marroig G. 2015. Directional selection can drive the evolution of modularity in complex traits. Proc. Natl Acad. Sci. USA 112, 470-475. ( 10.5061/dryad.3cb81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marroig G, Cheverud JM. 2001. A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of New World monkeys. Evolution 55, 2576-2600. ( 10.1111/j.0014-3820.2001.tb00770.x) [DOI] [PubMed] [Google Scholar]
- 52.Kavanagh K. 2020. Developmental plasticity associated with early structural integration and evolutionary patterns: examples of developmental bias and developmental facilitation in the skeletal system. Evol. Dev. 22, 196-204. ( 10.1111/ede.12323) [DOI] [PubMed] [Google Scholar]
- 53.Khannoon ER. 2015. Developmental stages of the climbing gecko Tarentola annularis with special reference to the claws, pad lamellae, and subdigital setae. J. Exp. Zool. Part B 324, 450-464. ( 10.1002/jez.b.22630) [DOI] [PubMed] [Google Scholar]
- 54.Sears K, Maier JA, Sadier A, Sorensen D, Urban DJ. 2017. Timing the developmental origins of mammalian limb diversity. Genesis 56, e23079. ( 10.1002/dvg.23079) [DOI] [PubMed] [Google Scholar]
- 55.Russell AP, Baskerville J, Gamble T, Higham TE. 2015. The evolution of digit form in Gonatodes (Gekkota: Sphaerodactylidae) and its bearing on the transition from frictional to adhesive contact in gekkotans. J. Morphol. 276, 1311-1332. ( 10.1002/jmor.20420) [DOI] [PubMed] [Google Scholar]
- 56.Rolian C. 2009. Integration and evolvability in primate hands and feet. Evol. Biol. 36, 100-117. ( 10.1007/s11692-009-9049-8) [DOI] [Google Scholar]
- 57.Kelly EM, Marcot JD, Selwood L, Sears KE. 2019. The development of integration in marsupial and placental limbs. Integr. Org. Biol. 1, oby013. ( 10.1093/iob/oby013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins CE, Russell AP, Higham TE. 2015. Subdigital adhesive pad morphology varies in relation to structural habitat use in the Namib day gecko. Funct. Ecol. 29, 66-77. ( 10.1111/1365-2435.12312) [DOI] [Google Scholar]
- 59.Higham TE, Russell AP, Niewiarowski PH, Wright A, Speck T. 2019. The ecomechanics of gecko adhesion: natural surface topography, evolution, and biomimetics. Integr. Comp. Biol. 59, 148-167. ( 10.1093/icb/icz013) [DOI] [PubMed] [Google Scholar]
- 60.Irschick DJ, Jayne BC. 1999. Comparative three-dimensional kinematics of the hindlimb for high-speed bipedal and quadrupedal locomotion of lizards. J. Exp. Biol. 202, 1047-1065. ( 10.1242/jeb.202.9.1047) [DOI] [PubMed] [Google Scholar]
- 61.Zani PA. 2000. The comparative evolution of lizard claw and toe morphology and clinging performance. J. Evol. Biol. 13, 316-325. ( 10.1046/j.1420-9101.2000.00166.x) [DOI] [Google Scholar]
- 62.Peattie AM. 2008. Subdigital setae of narrow-toed geckos, including a Eublepharid (Aeluroscalabotes felinus). Anat. Rec. 291, 869-875. ( 10.1002/ar.20706) [DOI] [PubMed] [Google Scholar]
- 63.Koppetsch T, Böhme W, Büsse S, Gorb SN. 2020. Comparative epidermal microstructure anatomy and limb and tail osteology of eyelid geckos (Squamata: Eublepharidae): implications of ecomorphological adaptations. Zoologischer Anzeiger 287, 45-60. ( 10.1016/j.jcz.2020.05.005) [DOI] [Google Scholar]
- 64.Russell A. 1976. Some comments concerning interrelationships among gekkonine geckos. In Morphology and biology of reptiles (eds Ad'A Bellairs, CB Cox). Linnean Society Symposium Series No. 3, pp. 217-244. London, UK: Academic Press. [Google Scholar]
- 65.Rothier PS, Simon MN, Marroig G, Herrel A, Kohlsdorf T. 2022. Data from: Development and function explain the modular evolution of phalanges in gecko lizards. Dryad Digital Repository. ( 10.5061/dryad.c59zw3r74) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rothier PS, Simon MN, Marroig G, Herrel A, Kohlsdorf T. 2022. Data from: Development and function explain the modular evolution of phalanges in gecko lizards. Dryad Digital Repository. ( 10.5061/dryad.c59zw3r74) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.c59zw3r74 [65]. Codes are described in the electronic supplementary material S2.