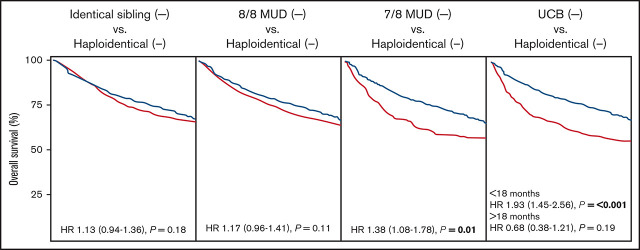
Key Points
Haploidentical HCT is the preferred alternate donor approach for adults with ALL.
Haploidentical transplantation had similar survival compared with fully HLA-matched donor HCT but with reduced GVHD.
Visual Abstract
Abstract
The role of haploidentical hematopoietic cell transplantation (HCT) using posttransplant cyclophosphamide (PTCy) for acute lymphoblastic leukemia (ALL) is being defined. We performed a retrospective, multivariable analysis comparing outcomes of HCT approaches by donor for adults with ALL in remission. The primary objective was to compare overall survival (OS) among haploidentical HCTs using PTCy and HLA-matched sibling donor (MSD), 8/8 HLA-matched unrelated donor (MUD), 7 /8 HLA-MUD, or umbilical cord blood (UCB) HCT. Comparing haploidentical HCT to MSD HCT, we found that OS, leukemia-free survival (LFS), nonrelapse mortality (NRM), relapse, and acute graft-versus-host disease (aGVHD) were not different but chronic GVHD (cGVHD) was higher in MSD HCT. Compared with MUD HCT, OS, LFS, and relapse were not different, but MUD HCT had increased NRM (hazard ratio [HR], 1.42; P = .02), grade 3 to 4 aGVHD (HR, 1.59; P = .005), and cGVHD. Compared with 7/8 UD HCT, LFS and relapse were not different, but 7/8 UD HCT had worse OS (HR, 1.38; P = .01) and increased NRM (HR, 2.13; P ≤ .001), grade 3 to 4 aGVHD (HR, 1.86; P = .003), and cGVHD (HR, 1.72; P ≤ .001). Compared with UCB HCT, late OS, late LFS, relapse, and cGVHD were not different but UCB HCT had worse early OS (≤18 months; HR, 1.93; P < .001), worse early LFS (HR, 1.40; P = .007) and increased incidences of NRM (HR, 2.08; P < .001) and grade 3 to 4 aGVHD (HR, 1.97; P < .001). Haploidentical HCT using PTCy showed no difference in survival but less GVHD compared with traditional MSD and MUD HCT and is the preferred alternative donor HCT option for adults with ALL in complete remission.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative therapy for acute lymphoblastic leukemia (ALL) and has been shown to be superior to intensive chemotherapy alone in some studies.1,2 The UKALL XII/ECOG 2993 study compared an adult chemotherapy backbone or chemotherapy followed by myeloablative autologous HCT (auto-HCT) with myeloablative allo-HCT in patients with ALL age 15 to 59 years. An overall survival (OS) benefit was seen in patients with standard-risk ALL with a donor primarily because of higher rate of relapse in the no donor group than in the combined chemotherapy and auto-HCT groups.1 A meta-analysis of 13 trials comparing allo-HCT to chemotherapy with or without auto-HCT concluded that the benefit of allo-HCT for patients with ALL in first complete remission (CR1) was limited to patients younger than age 35 years.3 Recent studies have also shown that allo-HCT in CR1 yields outcomes similar to those in pediatric-inspired chemotherapy in patients who are minimal residual disease (MRD) negative but improves outcomes for patients who are MRD positive.4 For these MRD-positive patients, who benefit most from allo-HCT in CR1, donor availability is especially important because haploidentical HCT or umbilical cord blood (UCB) HCT may shorten the time to allo-HCT and promote the higher cure rates observed with traditional fully HLA-matched donor allo-HCT.
The optimal donor for allo-HCT based on existing data seems to be a matched sibling donor (MSD) or an 8/8 HLA-matched unrelated donor (MUD) if an MSD is unavailable. A recently published study by the Center for International Blood and Marrow Transplant Research (CIBMTR) compared outcomes of traditional donor (MSD or MUD) HCT and 7/8 HLA-MUD HCT for adults with ALL. Compared with MSD HCT, MUD HCT yielded similar survival outcomes whereas the alternative 7/8 HLA-MUD HCT had inferior survival.5 For patients without a related or unrelated donor, haploidentical HCT using posttransplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis is now a common alternative transplant modality with demonstrated efficacy in ALL.6-8 In addition, despite having outcomes that are inferior to those for MSD and MUD HCT, 7/8 HLA-MUD and UCB HCT remain alternative graft sources for adult patients with ALL who do not have a fully HLA-matched donor.
Although comparative data to support the use of haploidentical HCT as a reasonable alternative to traditional MSD and MUD allo-HCT for acute myeloid leukemia (AML) are increasing,9-13 comparative data for ALL are more limited. Recent retrospective, comparative studies using the European Society for Blood and Marrow Transplantation (EBMT) registry have found no differences in outcomes between haploidentical HCT using PTCy and MSD, MUD, and mismatched UD (MMUD) HCT.14,15 The BMT-CTN 1101 study compared the results of parallel phase 2 studies of reduced-intensity conditioning (RIC) haploidentical HCT using PTCy and UCB HCT in lymphoma and acute leukemia. The study found no difference in the primary end point of progression-free survival at 2 years but found increased nonrelapse mortality (NRM) and decreased overall survival (OS) with UCB HCT compared with haploidentical HCT with PTCy.16 Taken together, previous studies have shown no significant differences in OS when comparing haploidentical HCT to MSD, MUD, or MMUD HCT and a superior alternative donor approach among haploidentical HCT with PTCy, 7/8 HLA-matched UD HCT, and UCB HCT for adult ALL specifically has not been established.
This retrospective, multivariable study was designed to compare OS, leukemia-free survival (LFS), relapse, and NRM among adult patients with ALL undergoing postremission therapy with haploidentical HCT using PTCy compared with MSD HCT, MUD HCT, 7/8-HLA MUD HCT, or UCB HCT. We hypothesized that haploidentical HCT using PTCy would result in similar OS compared with MSD, MUD, and UCB HCT and superior OS compared with 7/8 HLA-MUD HCT in adults with ALL undergoing first allo-HCT in CR. Results from this study further define the role of haploidentical HCT for ALL in first or subsequent remissions.
Patients and methods
Patients
All patient data were generated from the CIBMTR patient registry. Eligible patients were age 18 years or older with a diagnosis of ALL in first, second, or third or greater CR undergoing first allo-HCT from 2013 through 2017. Patients must have had an allo-HCT from a haploidentical, HLA-MSD, 8/8 HLA-MUD, 7/8 HLA-MUD, or UCB donor.17 Patients undergoing haploidentical HCT that did not use PTCy-based GVHD prophylaxis were excluded as were those receiving ex vivo T-cell depletion or CD34 selection. Also excluded were patients without consent to research, from embargoed centers, with no follow-up forms, alive with <3 months of follow-up, or receiving infrequently observed conditioning regimens. MRD testing methods and positivity were as reported from CIBMTR sites. MRD testing methods included flow cytometry (75%), molecular methods (76%), and cytogenetics (62%), with 74% of patients being evaluated with more than 1 method. Data on MRD testing methods was missing for 7% of patients. The study was approved by the Institutional Review Board of the National Marrow Donor Program.
Study objectives
The primary objective was to compare OS after HCT among the following donor-transplant groups: haploidentical HCT using PTCy, MSD HCT, MUD HCT, 7/8 HLA-MUD HCT, and UCB HCT. Secondary objectives included comparing the LFS, relapse, NRM, grade 2 to 4 and grade 3 to 4 acute GVHD (aGVHD) rates,18 and chronic GVHD (cGVHD) rates19 among the groups. We also performed 2 planned sensitivity analyses restricting the analysis to myeloablative conditioning20 with peripheral blood as a source for hematopoietic stem cells for non–cord blood donor types and to US centers only. We also determined causes of death in each group.
Statistical analysis
This was a retrospective, 5-cohort comparative study from the CIBMTR. Patient-, disease-, and transplant-related factors were compared among the 5 transplant groups using χ2 test for categorical variables and Mann-Whitney U test for continuous variables. The outcomes that were analyzed were OS, LFS, cumulative incidence (CI) of relapse, NRM CI, rate of aGVHD, and rate of cGVHD. OS was the time from transplantation to death as a result of any cause, with surviving patients censored at the last time they were reported alive. LFS was the time to leukemia relapse or death as a result of any cause, with surviving patients censored at the last time they were reported alive and were leukemia free. NRM was summarized by the CI estimate of death in CR with relapse as a competing risk. Relapse was summarized by the CI estimate with treatment-related mortality as a competing risk. Probabilities of OS and LFS were calculated by using the Kaplan-Meier estimator. CI curves were created to present relapse and NRM with time to relapse and time to NRM as competing risks.
To adjust for the differences in baseline characteristics, Cox proportional hazards regression was used to compare the main treatment groups. First, variables to be considered in the multivariable models were selected. Variables considered were donor type, recipient age, Karnofsky performance status, sex, HCT-CI score,21 race, ALL lineage, Philadelphia chromosome (Ph)-BCR-ABL1 status, cytogenetic risk, remission status, MRD status for CR1, time from diagnosis to HCT for CR1, conditioning intensity, donor-recipient sex match, donor-recipient cytomegalovirus (CMV) serostatus, year of transplant, and transplantation center. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariables. When the test indicated differential effects over time (nonproportional hazards), models were constructed that broke the posttransplant time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint. The proportionality assumptions were further tested. A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for treatment groups. Factors that were significant at a 5% level were kept in the final model. The potential interactions between main effect and all significant risk factors were tested. Adjusted probabilities of LFS and OS and adjusted CI estimates were generated from the final regression models stratified on treatment and weighted averages of covariable values using the pooled sample proportion as the weight function. These adjusted probabilities estimated likelihood of outcomes in populations with similar prognostic factors. With haploidentical HCT using PTCy as the baseline comparison group (independent testing, no multiple testing considered, no differences in patient characteristics adjusted, assuming all patients had at least a 2-year follow-up), the power test for 2-year OS probability was based on a two-sided test with a significance level of 5%: haploidentical HCT using PTCy vs (1) MSD HCT, 80% power to detect at least a difference of 8%; (2) MUD HCT, 80% power to detect at least a difference of 8%; (3) 7/8 HLA-MUD HCT, 80% power to detect at least a difference of 11%; and (4) UCB HCT, 80% power to detect at least difference of 10%.
Results
Patients
Between 2013 and 2017, a total of 4201 patients in 5 HCT cohorts were eligible: 393 haploidentical HCT using PTCy, 1627 MSD HCT, 1646 MUD HCT, 230 7/8 HLA-matched UD HCT, and 305 UCB HCT. Cohorts were well matched for age, sex, Karnofsky performance status, HCT-CI, immunophenotype, cytogenetic risk, Ph-BCR-ABL1 status, disease status, MRD status at transplantation, and recipient CMV serostatus. Notable differences between groups included race, time from diagnosis to HCT (CR1 only), conditioning regimen intensity, donor age, graft source for non–cord blood (peripheral blood or bone marrow), GVHD prophylaxis modality, and the use of in vivo T-cell depletion. PTCy-based GVHD prophylaxis was used in 5% of MSD HCT, 4% of MUD HCT, and 13% of 7/8 HLA-MUD HCT. Compared with other groups, haploidentical HCT using PTCy had the lowest percentage of non-Hispanic White patients (43% vs 49%-74%), was more likely to use RIC (42% vs 17%-25%), and was more likely to use bone marrow as the graft source (41% vs 14%-29%) (Table 1).
Table 1.
Patient characteristics
| Characteristic | Donor and HCT group | ||||
|---|---|---|---|---|---|
| Haploidentical | Matched sibling | 8/8 HLA-MUD | 7/8 HLA-MUD | UCB | |
| No. of patients | 393 | 1627 | 1646 | 230 | 305 |
| No. of centers | 92 | 206 | 181 | 90 | 79 |
| Median follow-up, mo (range) | 24 (3-67) | 26 (3-72) | 35 (3-74) | 35 (3-64) | 35 (3-64) |
| Median recipient age, y (range) | 41 (18-74) | 42 (18-75) | 43 (18-77) | 38 (18-70) | 37 (18-70) |
| Karnofsky performance status (%) | |||||
| ≥90 | 233 (59) | 1046 (64) | 995 (60) | 163 (71) | 196 (64) |
| <90 | 152 (39) | 542 (33) | 629 (38) | 65 (28) | 107 (35) |
| Missing | 8 (2) | 39 (2) | 22 (1) | 2 (<1) | 2 (<1) |
| No. of male recipients | 214 (54) | 969 (60) | 976 (59) | 134 (58) | 176 (58) |
| HCT-CI score | |||||
| 0 | 81 (21) | 592 (36) | 405 (25) | 53 (23) | 89 (29) |
| 1 | 57 (15) | 221 (14) | 224 (14) | 35 (15) | 42 (14) |
| 2 | 61 (16) | 228 (14) | 266 (16) | 37 (16) | 46 (15) |
| 3+ | 194 (49) | 552 (34) | 745 (45) | 104 (45) | 125 (41) |
| Missing | 0 | 34 (2) | 6 (<1) | 1 (<1) | 3 (<1) |
| Race/ethnicity* | |||||
| Hispanic White | 87 (22) | 246 (15) | 136 (8) | 42 (18) | 64 (21) |
| Non-Hispanic White | 170 (43) | 846 (52) | 1226 (74) | 113 (49) | 150 (49) |
| Black | 59 (15) | 74 (5) | 53 (3) | 19 (8) | 27 (9) |
| Asian | 22 (6) | 111 (7) | 64 (4) | 10 (4) | 22 (7) |
| Other/not specified | 55 (14) | 350 (22) | 167 (10) | 46 (20) | 42 (14) |
| Immunophenotype | |||||
| T cell | 25 (6) | 201 (12) | 186 (11) | 27 (12) | 36 (12) |
| B cell | 330 (84) | 1316 (81) | 1319 (80) | 185 (80) | 246 (81) |
| Not specified | 38 (10) | 110 (7) | 141 (9) | 18 (8) | 23 (8) |
| Cytogenetic risk score † | |||||
| Normal | 91 (23) | 320 (20) | 335 (20) | 52 (23) | 63 (21) |
| Poor | 222 (56) | 750 (46) | 855 (52) | 101 (44) | 154 (50) |
| Missing/not tested/other | 80 (21) | 557 (34) | 456 (28) | 77 (33) | 88 (29) |
| Ph/BCR-ABL1-positive | 152 (46) | 562 (43) | 614 (47) | 80 (43) | 122 (50) |
| Remission status | |||||
| CR1, MRD positive | 112 (28) | 513 (32) | 509 (31) | 58 (25) | 78 (26) |
| CR1, MRD negative | 143 (36) | 644 (40) | 697 (42) | 85 (37) | 124 (41) |
| CR1, MRD missing | 14 (4) | 145 (9) | 59 (4) | 6 (3) | 10 (3) |
| CR2 | 105 (27) | 296 (18) | 334 (20) | 62 (27) | 74 (24) |
| ≥CR3 | 19 (5) | 29 (2) | 47 (3) | 19 (8) | 19 (6) |
| Time from diagnosis to HCT (CR1 only) (mo) | |||||
| 0-5 | 130 (48) | 842 (65) | 744 (59) | 56 (38) | 93 (44) |
| 6-11 | 115 (43) | 388 (30) | 463 (37) | 81 (54) | 102 (48) |
| ≥12 | 24 (9) | 72 (6) | 58 (5) | 12 (8) | 17 (8) |
| Conditioning regimen | |||||
| MAC, TBI-based | 163 (41) | 984 (60) | 950 (58) | 139 (60) | 217 (71) |
| MAC, chemotherapy-based | 63 (16) | 323 (20) | 312 (19) | 51 (22) | 11 (4) |
| RIC/NMA | 167 (42) | 316 (19) | 383 (23) | 39 (17) | 76 (25) |
| Missing | 0 | 4 (<1) | 1 (<1) | 1 (<1) | 1 (<1) |
| Donor/recipient sex | |||||
| Female donor/male recipient | 82 (21) | 415 (26) | 244 (15) | 42 (18) | 161 (53) |
| other donor/recipient | 311 (79) | 1212 (74) | 1396 (85) | 188 (82) | 137 (45) |
| Missing | 0 | 0 | 6 (<1) | 0 | 7 (2) |
| Donor/recipient CMV serostatus | |||||
| +/+ | 206 (52) | 859 (53) | 506 (31) | 90 (39) | 0 |
| +/− | 31 (8) | 144 (9) | 197 (12) | 25 (11) | 0 |
| −/+ | 83 (21) | 287 (18) | 553 (34) | 64 (28) | 0 |
| −/− | 72 (18) | 306 (19) | 382 (23) | 50 (22) | 0 |
| UCB–/recipient+ | 0 | 0 | 0 | 0 | 200 (66) |
| UCB–/recipient– | 0 | 0 | 0 | 0 | 100 (33) |
| Missing | 1 (<1) | 31 (2) | 8 (<1) | 1 (<1) | 5 (2) |
| Median donor age, y (range) | 35 (10-74) | 41 (9-75) | 28 (18-60) | 31 (19-60) | NA |
| Graft source | |||||
| Bone marrow | 160 (41) | 230 (14) | 316 (19) | 67 (29) | — |
| Peripheral blood | 233 (59) | 1397 (86) | 1330 (81) | 163 (71) | — |
| GVHD prophylaxis | |||||
| CNI + MTX ± others | 0 | 1107 (68) | 1165 (71) | 162 (70) | 7 (2) |
| CNI + MMF ± others | 0 | 236 (15) | 191 (12) | 18 (8) | 265 (87) |
| CNI + others | 0 | 118 (7) | 141 (9) | 13 (6) | 6 (2) |
| CNI alone | 0 | 66 (4) | 58 (4) | 5 (2) | 14 (5) |
| PTCy + CNI ± MMF | 393 (100) | 75 (5) | 73 (4) | 29 (13) | 2 (<1) |
| Other prophylaxis | 0 | 17 (1) | 13 (<1) | 2 (<1) | 10 (3) |
| Missing | 0 | 8 (<1) | 5 (<1) | 1 (<1) | 1 (<1) |
| In vivo T-cell depletion | |||||
| Antithymocyte globulin | 5 (1) | 76 (5) | 561 (34) | 116 (50) | 39 (13) |
| Alemtuzumab | 0 | 33 (2) | 62 (4) | 6 (3) | 0 |
| None | 388 (99) | 1505 (93) | 1010 (61) | 105 (46) | 265 (87) |
| Missing | 0 | 13 (<1) | 13 (<1) | 3 (1) | 1 (<1) |
BCR-ABL, breakpoint cluster region-Abelson murine leukemia; CMV, cytomegalovirus; CNI, calcineurin inhibitor; MAC, myeloablative conditioning; MMF, mycophenolate mofetil; MTX, methotrexate; NA, not applicable; NMA, non-myeloablative.
Other/not specified: Native American (n = 30), Pacific Islander (n = 20), non-resident of the United States (n = 291), not specified (n = 156), Hispanic, excluding White Hispanic (n = 213).
CIBMTR cytogenetics criteria definition: Poor: Ph+/t(9:22)/BCR-ABL1, t(4:11), 11q23/MLL/KMT2A, hypodiploid (<45), t(8:14), complex (≥3 abnormalities), iAMP21; normal: without any abnormality; other: abnormality count of 1 or 2 abnormalities.
OS and LFS
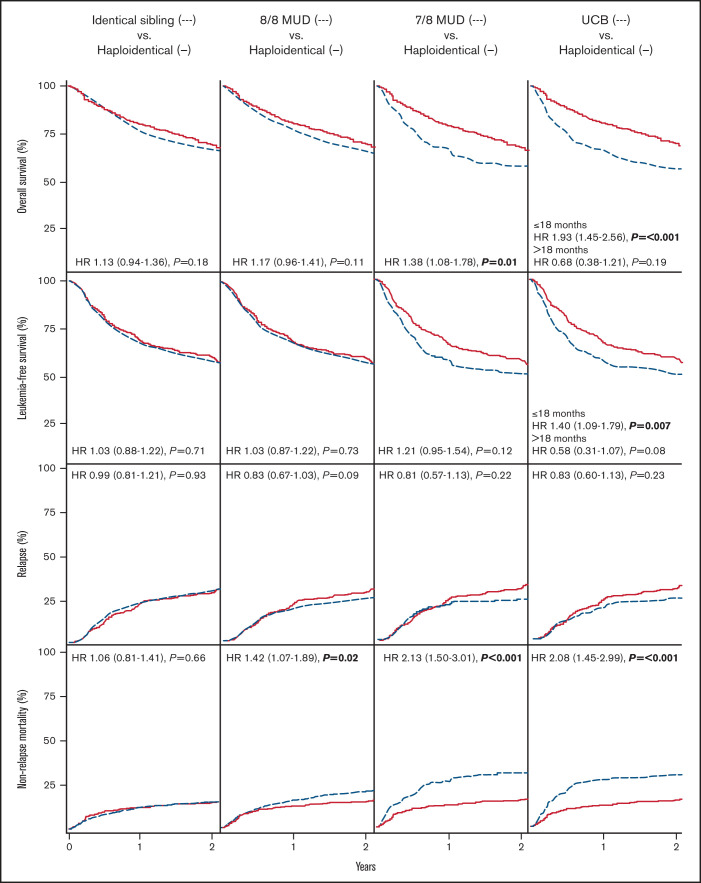
In multivariable analysis, compared with haploidentical HCT, MSD HCT and MUD HCT had similar OS (hazard ratio [HR], 1.13; P = .18 and HR, 1.17; P = .11, respectively) and LFS (HR, 1.03 [P = .71] and HR, 1.03 [P = .73], respectively). In contrast, 7/8 HLA-MUD HCT had inferior OS and similar LFS when compared with haploidentical HCT (OS: HR, 1.38 [P = .01]; LFS: HR, 1.21 [P = .12]). UCB HCT had inferior OS before 18 months (HR, 1.93; P ≤ .001) and similar OS after 18 months (HR, 0.68; P = .19) when compared with haploidentical HCT. In addition, LFS before 18 months was inferior to UCB HCT (HR, 1.40; P = .007) and was similar after 18 months (HR, 0.58; P = .08). Other multivariable factors associated with decreased OS included HCT in CR2+, older age, female donor to male recipient, Ph-BCR-ABL1 negativity, and CMV-seronegative donor to CMV-seropositive recipient for MSD HCT vs haploidentical HCT; CR2+, older age, non-Asian race, HCT-CI 3+, and Ph-BCR-ABL1 negativity for MUD HCT vs haploidentical HCT; CR2+ for 7/8 HLA-MUD HCT vs haploidentical HCT; and CR2+ and myeloablative chemotherapy (vs myeloablative total body irradiation [TBI]) for UCB HCT vs haploidentical HCT. Multivariable survival outcomes are summarized in Tables 2-5 and Figure 1. Univariable outcomes are summarized in supplemental Table 3.
Table 2.
Multivariable analysis for HLA-MSD HCT vs haploidentical HCT, 2013-2017
| Covariate | No. | HR | 95% Confidence interval | P |
|---|---|---|---|---|
| OS | ||||
| Main effect | ||||
| Haploidentical HCT | 393 | Reference | ||
| MSD HCT | 1627 | 1.13 | 0.94-1.36 | .18 |
| Remission status | ||||
| CR1 | 1571 | Reference | ||
| CR2+ | 449 | 1.86 | 1.58-2.19 | <.001 |
| Age (y) | <.001 | |||
| 18-29 | 572 | Reference | ||
| 30-39 | 367 | 0.97 | 0.77-1.22 | .78 |
| 40-49 | 432 | 1.30 | 1.05-1.60 | .02 |
| 50-59 | 417 | 1.49 | 1.21-1.85 | <.001 |
| 60-69 | 232 | 2.07 | 1.63-2.63 | <.001 |
| Donor/recipient sex match | ||||
| Other than F/M | 1523 | Reference | ||
| F/M | 497 | 1.29 | 1.10-1.51 | .002 |
| Ph-BCR-ABL1 status | .007 | |||
| Negative | 932 | Reference | ||
| Positive | 714 | 0.78 | 0.66-0.92 | .003 |
| T-cell-ALL/unspecified subtype | 374 | 1.02 | 0.84-1.24 | .83 |
| Donor/recipient CMV serostatus | .02 | |||
| +/+ | 1065 | Reference | ||
| +/− | 175 | 0.81 | 0.62-1.05 | .11 |
| −/+ | 370 | 0.76 | 0.62-0.93 | .007 |
| −/− | 378 | 0.84 | 0.69-1.01 | .07 |
| LFS | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MSD HCT | 1583 | 1.03 | 0.88-1.22 | .71 |
| Disease status | ||||
| CR1 | 1528 | Reference | ||
| CR2+ | 436 | 1.93 | 1.67-2.23 | <.001 |
| Conditioning regimen | ||||
| MAC-TBI | 1116 | Reference | ||
| MAC-chemotherapy | 376 | 1.35 | 1.15-1.60 | <.001 |
| RIC/NMA | 470 | 1.50 | 1.28-1.76 | <.001 |
| NRM | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MSD HCT | 1583 | 1.06 | 0.81-1.41 | .66 |
| Remission status | ||||
| CR1 | 1528 | Reference | ||
| CR2+ | 436 | 1.52 | 1.17-1.98 | .002 |
| Age (y) | <.001 | |||
| 18-29 | 553 | Reference | ||
| 30-39 | 353 | 0.66 | 0.44-0.99 | .04 |
| 40-49 | 422 | 1.19 | 0.86-1.65 | .28 |
| 50-59 | 411 | 1.59 | 1.17-2.16 | .003 |
| 60-69 | 225 | 2.10 | 1.49-2.96 | <.001 |
| Donor/recipient sex match | ||||
| Other than F/M | 1479 | Reference | ||
| F/M | 485 | 1.54 | 1.22-1.94 | <.001 |
| Relapse | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MSD HCT | 1583 | 0.99 | 0.81-1.21 | .93 |
| Remission status | ||||
| CR1 | 1528 | Reference | ||
| CR2+ | 436 | 2.25 | 1.89-2.68 | <.001 |
| Conditioning regimen | ||||
| MAC-TBI | 1116 | Reference | ||
| MAC-chemotherapy | 376 | 1.40 | 1.14-1.72 | .001 |
| RIC/NMA | 470 | 1.53 | 1.26-1.87 | <.001 |
| aGVHD, grade 2-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| MSD HCT | 1545 | 0.92 | 0.77-1.11 | .40 |
| aGVHD, grade 3-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| MSD HCT | 1545 | 1.09 | 0.79-1.50 | .59 |
| cGVHD | ||||
| MSD vs haploidentical HCT for donor/recipient sex match, other | 1.37 | 1.12-1.69 | .003 | |
| MSD vs haploidentical HCT for donor/recipient sex match, F/M | 2.59 | 1.68-3.99 | <.001 | |
| Age (y) | .002 | |||
| 18-29 | 563 | Reference | ||
| 30-39 | 361 | 1.13 | 0.93-1.37 | .24 |
| 40-49 | 428 | 1.37 | 1.14-1.64 | <.001 |
| 50-59 | 413 | 1.17 | 0.95-1.43 | .14 |
| 60-69 | 228 | 1.57 | 1.21-2.03 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 333 | Reference | ||
| White non-Hispanic | 1006 | 0.75 | 0.63-0.89 | .001 |
| Black | 132 | 0.93 | 0.70-1.23 | .61 |
| Asian | 130 | 0.79 | 0.59-1.07 | .13 |
| Other/not specified | 392 | 0.66 | 0.53-0.82 | <.001 |
| Donor/recipient sex match | ||||
| Other than F/M | 1501 | Reference | ||
| F/M | 492 | 0.73 | 0.47-1.14 | .17 |
| Conditioning regimen | ||||
| MAC-TBI | 1132 | Reference | ||
| MAC-chemotherapy | 380 | 0.94 | 0.79-1.11 | .46 |
| RIC/NMA | 478 | 0.74 | 0.61-0.90 | .002 |
F, female; M, male.
Table 5.
Multivariable analysis for UCB HCT vs haploidentical HCT, 2013-2017
| Covariate | No. | HR | 95% Confidence interval | P |
|---|---|---|---|---|
| OS | ||||
| UCB HCT vs haploidentical HCT (mo) | ||||
| ≤18 | 1.93 | 1.45-2.56 | <.001 | |
| >18 | 0.68 | 0.38-1.21 | .19 | |
| Remission status | ||||
| CR1 | 481 | Reference | ||
| CR2+ | 217 | 1.62 | 1.27-2.07 | <.001 |
| Karnofsky performance status (%) | ||||
| <90 | 259 | Reference | ||
| ≥90 | 429 | 0.81 | 0.64-1.04 | .10 |
| Conditioning regimen | ||||
| MAC-TBI | 380 | Reference | ||
| MAC-chemotherapy | 74 | 2.14 | 1.45-3.14 | <.001 |
| RIC/NMA | 243 | 1.22 | 0.93-1.59 | .15 |
| LFS | ||||
| UCB HCT vs haploidentical HCT (mo) | ||||
| ≤18 | 1.40 | 1.09-1.79 | .007 | |
| >18 | 0.58 | 0.31-1.07 | .08 | |
| Remission status | ||||
| CR1 | 469 | Reference | ||
| CR2+ | 203 | 1.59 | 1.27-1.99 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 144 | Reference | ||
| White non-Hispanic | 310 | 0.86 | 0.65-1.13 | .27 |
| Black | 83 | 1.33 | 0.93-1.89 | .12 |
| Asian | 41 | 0.55 | 0.31-0.97 | .04 |
| Other/not specified | 94 | 0.94 | 0.65-1.36 | .74 |
| Conditioning regimen | ||||
| MAC-TBI | 364 | Reference | ||
| MAC-chemotherapy | 72 | 1.77 | 1.23-2.55 | .002 |
| RIC/NMA | 235 | 1.51 | 1.19-1.91 | <.001 |
| NRM | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| UCB HCT | 291 | 2.08 | 1.45-2.99 | <.001 |
| Karnofsky performance status (%) | ||||
| <90 | 247 | Reference | ||
| ≥90 | 416 | 0.65 | 0.46-0.90 | .01 |
| Conditioning regimen | ||||
| MAC-TBI | 364 | Reference | ||
| MAC-chemotherapy | 72 | 1.96 | 1.16-3.32 | .01 |
| RIC/NMA | 235 | 0.88 | 0.59-1.29 | .51 |
| Relapse | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| UCB HCT | 291 | 0.83 | 0.60-1.13 | .23 |
| Remission status | ||||
| CR1 | 469 | Reference | ||
| CR2+ | 203 | 1.88 | 1.40-2.53 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 144 | Reference | ||
| White non-Hispanic | 310 | 0.99 | 0.68-1.45 | .98 |
| Black | 83 | 1.51 | 0.95-2.39 | .08 |
| Asian | 41 | 0.55 | 0.26-1.19 | .13 |
| Other/not specified | 94 | 0.73 | 0.43-1.25 | .25 |
| Conditioning regimen | ||||
| MAC-TBI | 364 | Reference | ||
| MAC-chemotherapy | 72 | 1.64 | 0.99-2.71 | .05 |
| RIC/NMA | 235 | 2.01 | 1.47-2.74 | <.001 |
| aGVHD, grade 2-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| UCB HCT | 285 | 1.83 | 1.46-2.30 | <.001 |
| aGVHD, grade 3-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| UCB HCT | 285 | 1.97 | 1.35-2.88 | <.001 |
| cGVHD | ||||
| Main effect | ||||
| Haploidentical HCT | 393 | Reference | ||
| UCB HCT | 297 | 1.13 | 0.86-1.47 | .38 |
| Conditioning regimen | ||||
| MAC-TBI | 375 | Reference | ||
| MAC-chemotherapy | 71 | 1.11 | 0.72-1.72 | .64 |
| RIC/NMA | 243 | 0.65 | 0.49-0.87 | .003 |
| HCT-CI | .05 | |||
| 0 | 169 | Reference | ||
| 1 | 98 | 0.60 | 0.39-0.92 | .02 |
| 2 | 105 | 0.91 | 0.63-1.31 | .60 |
| 3+ | 317 | 0.68 | 0.50-0.91 | .01 |
Figure 1.
OS, LFS, CI of relapse, and CI of NRM comparing haploidentical HCT with posttransplant cyclophosphamide to matched sibling, 8/8 HLA-MUD, 7/8 HLA-MUD, or UCB HCT.
Table 3.
Multivariable analysis for 8/8 HLA-MUD HCT vs haploidentical HCT, 2013-2017
| Covariate | No. | HR | 95% Confidence interval | P |
|---|---|---|---|---|
| OS | ||||
| Main effect | ||||
| Haploidentical HCT | 393 | Reference | ||
| MUD HCT | 1646 | 1.17 | 0.96-1.41 | .11 |
| Remission status | ||||
| CR1 | 1534 | Reference | ||
| CR2+ | 505 | 1.79 | 1.53-2.10 | <.001 |
| Age (y) | <.001 | |||
| 18-29 | 545 | Reference | ||
| 30-39 | 364 | 1.03 | 0.81-1.30 | .82 |
| 40-49 | 391 | 1.38 | 1.11-1.71 | .004 |
| 50-59 | 382 | 1.55 | 1.24-1.93 | <.001 |
| 60-69 | 357 | 1.85 | 1.48-2.31 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 223 | Reference | ||
| White non-Hispanic | 1396 | 0.95 | 0.75-1.21 | .68 |
| Black | 112 | 1.33 | 0.94-1.87 | .11 |
| Asian | 86 | 0.44 | 0.26-0.75 | .002 |
| Other/not specified | 222 | 1.02 | 0.74-1.39 | .92 |
| HCT-CI | .01 | |||
| 0 | 486 | Reference | ||
| 1 | 281 | 1.01 | 0.79-1.30 | .91 |
| 2 | 327 | 1.03 | 0.81-1.30 | .84 |
| 3+ | 939 | 1.25 | 1.04-1.50 | .02 |
| Ph-BCR-ABL1 status | ||||
| Negative | 883 | Reference | ||
| Positive | 766 | 0.82 | 0.70-0.96 | .02 |
| T-ALL/unspecified subtype | 390 | 1.03 | 0.85-1.24 | .77 |
| LFS | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MUD HCT | 1618 | 1.03 | 0.87-1.22 | .73 |
| Remission status | ||||
| CR1 | 1509 | Reference | ||
| CR2+ | 490 | 1.74 | 1.51-1.99 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 217 | Reference | ||
| White non-Hispanic | 1379 | 0.97 | 0.78-1.19 | .76 |
| Black | 105 | 1.33 | 0.98-1.82 | .07 |
| Asian | 84 | 0.57 | 0.37-0.87 | .01 |
| Other/not specified | 214 | 0.94 | 0.71-1.24 | .67 |
| Conditioning regimen | ||||
| MAC-TBI | 1097 | Reference | ||
| MAC-chemotherapy | 363 | 1.46 | 1.24-1.73 | <.001 |
| RIC/NMA | 539 | 1.61 | 1.39-1.87 | <.001 |
| NRM | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MUD HCT | 1618 | 1.42 | 1.07-1.89 | .02 |
| Remission status | ||||
| CR1 | 1509 | Reference | ||
| CR2+ | 490 | 1.33 | 1.06-1.67 | .01 |
| Age (y) | <.001 | |||
| 18-29 | 539 | Reference | ||
| 30-39 | 356 | 0.86 | 0.62-1.20 | .37 |
| 40-49 | 382 | 1.30 | 0.97-1.76 | .08 |
| 50-59 | 372 | 1.61 | 1.20-2.15 | .001 |
| 60-69 | 350 | 1.82 | 1.36-2.44 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 217 | Reference | ||
| White non-Hispanic | 1379 | 0.79 | 0.58-1.09 | .15 |
| Black | 105 | 1.04 | 0.63-1.73 | .87 |
| Asian | 84 | 0.35 | 0.16-0.74 | .006 |
| Other/not specified | 214 | 0.98 | 0.66-1.47 | .93 |
| Relapse | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| MUD HCT | 1618 | 0.83 | 0.67-1.03 | .09 |
| Remission status | ||||
| CR1 | 1509 | Reference | ||
| CR2+ | 490 | 2.20 | 1.84-2.64 | <.001 |
| Sex | ||||
| Male | 1168 | Reference | ||
| Female | 831 | 0.81 | 0.68-0.97 | .02 |
| Race/ethnicity | ||||
| White Hispanic | 217 | Reference | ||
| White non-Hispanic | 1379 | 1.04 | 0.78-1.39 | .77 |
| Black | 105 | 1.59 | 1.06-2.37 | .02 |
| Asian | 84 | 0.75 | 0.44-1.26 | .27 |
| Other/not specified | 214 | 0.88 | 0.60-1.29 | .52 |
| Conditioning regimen | ||||
| MAC-TBI | 1097 | Reference | ||
| MAC-chemotherapy | 363 | 1.57 | 1.25-1.98 | <.001 |
| RIC/NMA | 539 | 1.83 | 1.50-2.23 | <.001 |
| aGVHD, grade 2-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| MUD HCT | 1553 | 1.17 | 0.98-1.41 | .09 |
| Conditioning regimen | ||||
| MAC-TBI | 1042 | Reference | ||
| MAC-chemotherapy | 367 | 0.86 | 0.72-1.04 | .11 |
| RIC/NMA | 519 | 0.81 | 0.68-0.95 | .01 |
| aGVHD, grade 3-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| MUD HCT | 1553 | 1.59 | 1.15-2.20 | .005 |
| Race/ethnicity | ||||
| White Hispanic | 217 | Reference | ||
| White non-Hispanic | 1318 | 0.65 | 0.47-0.90 | .009 |
| Black | 109 | 0.90 | 0.53-1.53 | .69 |
| Asian | 80 | 0.29 | 0.12-0.68 | .005 |
| Other/not specified | 205 | 0.67 | 0.43-1.06 | .08 |
| cGVHD | ||||
| MUD vs haploidentical for donor/recipient sex match, other | 1.38 | 1.14-1.68 | .001 | |
| MUD vs haploidentical for donor/recipient sex match, F/M | 2.91 | 1.87-4.52 | <.001 | |
| Remission status | ||||
| CR1 | 1528 | Reference | ||
| CR2+ | 501 | 0.81 | 0.69-0.95 | .009 |
| Donor/recipient sex match | ||||
| Other than F/M | 1707 | Reference | ||
| F/M | 322 | 0.69 | 0.44-1.08 | .10 |
Table 4.
Multivariable analysis for 7/8 HLA-MUD HCT vs haploidentical HCT, 2013-2017
| Covariate | No. | HR | 95% Confidence interval | P |
|---|---|---|---|---|
| OS | ||||
| Main effect | ||||
| Haploidentical HCT | 393 | Reference | ||
| 7/8 HLA-MUD HCT | 230 | 1.38 | 1.08-1.78 | .01 |
| Remission status | ||||
| CR1 | 418 | Reference | ||
| CR2+ | 205 | 1.82 | 1.41-2.34 | <.001 |
| LFS | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| 7/8 HLA-MUD | 227 | 1.21 | 0.95-1.54 | .12 |
| Remission status | ||||
| CR1 | 414 | Reference | ||
| CR2+ | 194 | 1.84 | 1.46-2.33 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 124 | Reference | ||
| White non-Hispanic | 277 | 0.95 | 0.71-1.28 | .73 |
| Black | 75 | 1.33 | 0.92-1.94 | .13 |
| Asian | 32 | 0.50 | 0.25-0.97 | .04 |
| Other/not specified | 100 | 0.70 | 0.48-1.03 | .07 |
| Conditioning regimen | ||||
| MAC-TBI | 295 | Reference | ||
| MAC-chemotherapy | 111 | 1.29 | 0.94-1.75 | .11 |
| RIC/NMA | 201 | 1.46 | 1.12-1.89 | .005 |
| NRM | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| 7/8 HLA-MUD HCT | 227 | 2.13 | 1.50-3.01 | <.001 |
| Donor/recipient CMV serostatus | ||||
| +/+ | 287 | Reference | ||
| +/− | 55 | 0.40 | 0.18-0.86 | .02 |
| −/+ | 143 | 0.78 | 0.51-1.19 | .25 |
| −/− | 121 | 0.56 | 0.34-0.92 | .02 |
| Relapse | ||||
| Main effect | ||||
| Haploidentical HCT | 381 | Reference | ||
| 7/8 HLA-MUD HCT | 227 | 0.81 | 0.57-1.13 | .22 |
| Remission status | ||||
| CR1 | 414 | Reference | ||
| CR2+ | 194 | 2.39 | 1.76-3.25 | <.001 |
| Race/ethnicity | ||||
| White Hispanic | 124 | Reference | ||
| White non-Hispanic | 277 | 0.94 | 0.64-1.39 | .76 |
| Black | 75 | 1.24 | 0.76-2.02 | .38 |
| Asian | 32 | 0.36 | 0.14-0.93 | .03 |
| Other/not specified | 100 | 0.58 | 0.34-0.99 | .05 |
| Conditioning regimen | ||||
| MAC-TBI | 295 | Reference | ||
| MAC-chemotherapy | 111 | 1.60 | 1.05-2.44 | .03 |
| RIC/NMA | 201 | 2.09 | 1.49-2.95 | <.001 |
| aGVHD, grade 2-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| 7/8 HLA-MUD HCT | 216 | 1.33 | 1.02-1.73 | .04 |
| Conditioning regimen | ||||
| MAC-TBI | 288 | Reference | ||
| MAC-chemotherapy | 107 | 0.68 | 0.47-0.98 | .04 |
| RIC/NMA | 196 | 0.68 | 0.51-0.92 | .01 |
| aGVHD, grade 3-4 | ||||
| Main effect | ||||
| Haploidentical HCT | 376 | Reference | ||
| 7/8 HLA-MUD HCT | 216 | 1.86 | 1.23-2.80 | .003 |
| cGVHD | ||||
| Main effect | ||||
| Haploidentical HCT | 393 | Reference | ||
| 7/8 HLA-MUD HCT | 230 | 1.72 | 1.34-2.20 | <.001 |
Relapse and NRM
In multivariable analysis, MSD HCT had similar relapse (HR, 0.99; P = .93) and NRM (HR, 1.06; P = .66) compared with haploidentical HCT. Compared with haploidentical HCT, relapse was not significantly different with MUD HCT (HR, 0.83; P = .09), 7/8 HLA-MUD HCT (HR, 0.81; P = .22), or UCB HCT (HR, 0.83; P = .23). NRM, however, was significantly higher with MUD HCT (HR, 1.42; P = .02), 7/8 HLA-MUD HCT (HR, 2.13; P ≤ .001), or UCB HCT (HR, 2.08; P ≤ .001) compared with haploidentical HCT. Notably, myeloablative conditioning using TBI significantly reduced the risk of relapse across all donor HCT cohorts. Multivariable relapse and NRM analyses are summarized in Tables 2-5 and Figure 1. Univariable analyses are summarized in supplemental Table 3.
GVHD
Multivariable analysis revealed either reduced or similar rates of severe aGVHD and cGVHD with haploidentical HCT using PTCy relative to other HCT cohorts. Compared with haploidentical HCT, MSD HCT had similar CIs of grade 2 to 4 and grade 3 to 4 aGVHD (HR, 0.92 [P = .40] and HR, 1.09 [P = .59], respectively) but increased CI of cGVHD (HR, 2.59; P < .001 for female-male donor-recipient sex match; HR 1.37; P = .003 for other donor-recipient sex match). MUD HCT had a similar CI of grade 2 to 4 aGVHD (HR, 1.17; P = .09), an increased CI of grade 3 to 4 aGVHD (HR, 1.59; P = .005), and an increased CI of cGVHD (HR, 1.38; P = .001). 7/8 HLA-MUD HCT had an increased CI of grade 2 to 4 aGVHD (HR, 1.33; P = .04), grade 3 to 4 aGVHD (HR, 1.86; P = .003), and cGVHD (HR, 1.72; P < .001). UCB HCT was associated with an increased CI of grade 2 to 4 and grade 3 to 4 aGVHD (HR, 1.83 [P < .001] and HR, 1.97; [P < .001], respectively) with a similar CI of cGVHD (HR, 1.13; P = .38). Multivariable GVHD analyses are summarized in Tables 2-5.
Causes of death
Death from ALL was more common with haploidentical HCT (48%) and HLA-identical sibling HCT (52%) compared with other HCT cohorts (31%-38%). Death from GVHD accounted for 5% of deaths after haploidentical HCT compared with 12% to 24% in other HCT cohorts. Similar rates of death from infection were observed when comparing haploidentical HCT (21%) to other HCT cohorts (17%-23%). Other causes of death were also similar among the cohorts (detailed summary in Table 6).
Table 6.
Causes of death by cohort
| Characteristic | Haploidentical | MSD | MUD | 7/8 HLA-MUD | UCB |
|---|---|---|---|---|---|
| No. of deaths | 132 | 564 | 625 | 103 | 130 |
| Cause of death | |||||
| ALL | 64 (48) | 293 (52) | 240 (38) | 33 (32) | 40 (31) |
| Graft failure | 1 (<1) | 4 (<1) | 1 (<1) | 3 (3) | 3 (2) |
| GVHD | 7 (5) | 81 (14) | 126 (20) | 25 (24) | 16 (12) |
| Infection | 28 (21) | 98 (17) | 126 (20) | 21 (20) | 30 (23) |
| Idiopathic pneumonia | 4 (3) | 5 (<1) | 7 (1) | 0 | 5 (4) |
| Acute respiratory distress syndrome | 3 (2) | 7 (1) | 9 (1) | 0 | 4 (3) |
| Organ failure | 8 (6) | 31 (5) | 53 (8) | 9 (9) | 19 (15) |
| Organ toxicity | 0 | 4 (<1) | 1 (<1) | 2 (2) | 0 |
| Secondary malignancy | 2 (2) | 4 (<1) | 4 (<1) | 2 (2) | 2 (2) |
| Hemorrhage | 3 (2) | 4 (<1) | 4 (<1) | 1 (<1) | 2 (2) |
| Accident or suicide | 0 | 0 | 3 (<1) | 0 | 0 |
| Vascular | 0 | 2 (<1) | 1 (<1) | 0 | 2 (2) |
| Other known | 11 (8) | 23 (4) | 39 (6) | 7 (7) | 6 (5) |
| Unknown | 1 (<1) | 8 (1) | 11 (2) | 0 | 1 (<1) |
Sensitivity analyses
To address 2 potential sources of bias, we performed 2 sensitivity analyses for OS, LFS, relapse, and NRM, restricting the study population to either the most common modalities of myeloablative conditioning with peripheral blood as a source of hematopoietic stem cells or to US centers for better completion of follow-up at 2 years. When restricted to myeloablative conditioning and peripheral blood stem cell source, outcomes were similar to those for the full population except that decreased OS with 7/8 HLA-MUD compared with haploidentical HCT was no longer statistically significant (HR, 1.39; P = .07; supplemental Tables 1, 4, 6-9; supplemental Figures 1-4). When restricted to US centers only, outcomes were also similar except there was a decreased risk of relapse (HR, 0.76; P = .02) but inferior OS (HR, 1.23; 95% confidence interval, 1.00-1.50; P = .05) with MUD compared with haploidentical HCT (supplemental Tables 2, 5, 10-13; supplemental Figures 1-4).
Discussion
Haploidentical HCT is a growing allo-HCT modality for ALL that has expanded allo-HCT to patients without traditional HLA-matched related or unrelated donors, especially those of mixed race or ethnicity. The choice of alternative donors for allo-HCT in ALL is an area of ongoing research, debate, and clinical interest. In addition, the relative benefits of haploidentical HCT compared with traditional MSDs and MUDs is just being defined. In this study, we demonstrated that haploidentical HCT using PTCy resulted in OS similar to that in traditional MSD and MUD allo-HCT but with less GVHD. In addition, we found superior OS compared with alternative 7/8 HLA-MUD and UCB HCT. The superior survival seen with haploidentical HCT using PTCy compared with 7/8 HLA-MUD HCT and UCB HCT was likely due to reduced NRM related to reduced GVHD with haploidentical HCT. Notably, rates of infection were similar among the 5 cohorts, suggesting that delayed immune reconstitution with haploidentical HCT in the adult ALL population did not translate into increased infection-related mortality.
Previous smaller retrospective studies comparing haploidentical HCT to MSD, MUD, and MMUD HCT found no differences in disease-free survival, relapse, NRM, aGVHD, or cGVHD. Recently, Shem-Tov et al14 performed a retrospective multi-institution comparison of 136 ALL patients undergoing haploidentical HCT with 809 patients with ALL receiving MUD HCT and 289 patients with ALL receiving 9/10 HLA-MUD HCT. This smaller study found no differences in OS, LFS, relapse, NRM, aGVHD, or cGVHD among the groups.14 Similarly, a larger study comparing 487 haploidentical HCTs to 974 MUD HCTs for ALL found no difference in any outcome, including aGVHD and cGVHD.15 Our study expands on and contrasts these studies with a large contemporary population that showed significant differences in major outcomes between haploidentical HCT using PTCy to all other major donor sources. This study helps clarify the role of haploidentical HCT in adult ALL and expands our knowledge of the expected benefits of haploidentical HCT relative to other donor HCT approaches. Importantly, our study supports haploidentical HCT with PTCy as the preferred HCT approach for patients who do not have an MSD or MUD.
Similar to previous studies,22-25 our results show that myeloablative conditioning using TBI compared with myeloablative chemotherapy or RIC/non-myeloablative conditioning significantly reduced the risk of relapse and improved LFS across all donor HCT cohorts. The recently published Phase III FORUM study randomly assigned 417 children and young adults ages 4 to 21 years with ALL to either myeloablative TBI-based or myeloablative chemotherapy-based conditioning before MSD, MUD, or MMUD allo-HCT. Patients in the TBI arm had improved OS, improved event-free survival, less relapse, and improved NRM.23 In adults with ALL, a retrospective EBMT registry study comparing TBI-based to chemotherapy-myeloablative conditioning for MSD, MUD, or MMUD allo-HCT found better OS, LFS, and relapse incidence with TBI-based conditioning,24 although the OS benefit in adults has not been seen across all retrospective studies.22,25 In this study, the benefit of myeloablative conditioning using TBI on reducing relapse improved OS only in haploidentical HCT and UCB HCT comparisons, suggesting that these modalities may derive more benefit from TBI. Overall, our study supports current recommendations26 for using myeloablative TBI for conditioning in allo-HCT for adult ALL because of the reduced risk of relapse with similar or improved OS, but further study is warranted on optimal conditioning regimens across donor HCT types for adult ALL.
The primary reason for decreased NRM with haploidentical HCT compared with MUD HCT, 7/8 MMUD HCT, and UCB HCT seems to be significantly decreased rates of severe aGVHD and cGVHD with haploidentical HCT using PTCy. Death from GVHD was substantially higher in the non-haploidentical HCT cohorts and reduced quality of life from GVHD-related complications, although not assessed in this study, with other donor sources may be an additional reason to pursue haploidentical HCT with PTCy in the ALL population. On the basis of its success in haploidentical HCT, PTCy GVHD prophylaxis is being studied in MSD, MUD, and MMUD HCT. Existing studies evaluating alternative GVHD prophylaxis with PTCy for MSD and UD HCT27-30 have consistently found low rates of cGVHD, and these approaches may produce relative benefits similar to those seen with haploidentical HCT in this study for reducing GVHD and NRM. However, the impact of these approaches on relapse in the setting of fully HLA-matched donor HCT will need to be closely evaluated.
Although HRs for relapse favored non-haploidentical HCT modalities except HLA-identical sibling (HR, 0.81-0.83), this finding was not statistically significant and did not lead to inferior OS or LFS with haploidentical HCT using PTCy. When restricted to sites in the United States only, relapse was significantly higher with haploidentical HCT using PTCy compared with MUD HCT (HR, 0.76; 95% confidence interval, 0.61-0.96; P = .02), which raised some concern that relapse may be higher in some settings with haploidentical HCT, although in the same comparison, haploidentical HCT showed significantly better OS because of substantially lower NRM. A larger future study and longer follow-up are needed to evaluate whether the large and significant reduction in aGVHD and cGVHD and death from GVHD with haploidentical HCT may be associated with a small increased risk of relapse after HCT. Non-severe aGVHD and cGVHD have previously been associated with reduced relapse,31 and this study suggests that reducing GVHD with haploidentical HCT may have an impact on relapse. Consistent with this, MSD HCT and haploidentical HCT had similar rates of aGVHD and nearly identical risk of relapse (HR, 0.99).
A strength of this study is the large number patients and international centers, which allows us to generalize the results, especially to centers in the United States. In addition, the large sample size in each cohort allowed adequate power to detect meaningful differences in outcomes between the HCT approaches. One limitation of this study is that it is retrospective. A prospective randomized study to better control for numerous variables would be needed to confirm our findings and address some limitations. For instance, the impact on outcomes from large centers favoring certain donor HCT modalities could influence the results. Another limitation is lack of standardized testing and definitions for MRD in data collected from sites. We found no differences in OS based on the CIBMTR definitions of MRD before HCT in contrast to a recent EBMT registry report.24 However, well-defined MRD positivity before allo-HCT has been shown to predict poor outcomes with increased relapse and reduced survival after allo-HCT for ALL.32-40 Reasons for our findings could be heterogeneity in testing, definitions of MRD used at different CIBMTR sites, and possibly a lack of sensitivity of MRD for predicting outcomes in a real-world setting. Another limitation of our study was an inability to evaluate the impact of central nervous system and extramedullary ALL on outcomes because the centers did not report these data. Follow-up for this study was also relatively short, given that haploidentical HCT has only come into widespread use in the last 5 years. Finally, our analysis was restricted to patients undergoing haploidentical HCT using PTCy, and our conclusions may not extend to alternate haploidentical HCT approaches. Approaches that use in vivo T-cell depletion or in vitro T-cell depletion and CD34+ cell selection have shown promising outcomes in ALL that seem to be comparable or possibly superior to MSD and MUD allo-HCT.41-48 High-quality comparative studies are needed that compare well-matched populations undergoing T-cell replete haploidentical HCT using PTCy with approaches using in vivo T-cell depletion or in vitro T-cell depletion and CD34+ cell selection.
Our findings support haploidentical HCT using PTCy as the preferred alternative donor HCT for ALL given the superior OS seen relative to 7/8 HLA-MUD and UCB HCT. Our data also suggest that OS is not different with haploidentical HCT using PTCy compared with traditional MSD and MUD HCT but with a reduced risk of GVHD. Although longer follow-up and confirmatory studies are needed, from this analysis haploidentical HCT seems to be an acceptable HCT option for all adult patients with ALL in remission that lacks anti-donor–specific HLA antibodies. To overcome the major causes of failure of haploidentical HCT uncovered in this study, future studies that aim to prevent relapse and reduce infectious death may further improve outcomes after haploidentical HCT. Future studies with longer follow-up will also be needed to definitively establish the role of haploidentical HCT using PTCy at different stages of ALL remission, particularly in the era of effective salvage treatments such as bispecific T-cell engagers, antibody-drug conjugates, and cellular therapies.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Cancer Institute (U24CA076518 and R01CA215134), the National Heart, Lung, and Blood Institute (R01HL130388 and UG1HL06924), the National Institute of Allergy and Infectious Diseases (R01AI128775), Health Resources and Services Administration (HHSH250201700006C and HHSH250201700007C), and Office of Naval Research (N00014-20-1-2705 and N00014-20-1-2832) (all to CIBMTR), by the Biomedical Advanced Research and Development Authority, and by Actinium Pharmaceuticals, Adienne, Allovir, Amgen, Angiocrine Bioscience, Astellas Pharma, bluebird bio, Boston Children’s Hospital, Bristol Myers Squibb, Be the Match Foundation, Celgene, CSL Behring, CytoSen Therapeutics, Daiichi Sankyo, Dana-Farber Cancer Institute, ExcellThera, Fate Therapeutics, Gamida-Cell, Genentech, Incyte, Janssen/Johnson & Johnson, Jazz Pharmaceuticals, Kiadis Pharma, Kite Pharma, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Medical College of Wisconsin, Merck Sharp & Dohme, Millennium Pharmaceuticals, Miltenyi Biotec, National Marrow Donor Program, Novartis Pharmaceuticals, Omeros, OncoImmune, Orca Biosystems, Pfizer, Pharmacyclics, Sanofi Genzyme, St. Baldrick’s Foundation, Stanford University, Stemcyte, Takeda Oncology, Takeda Pharma, Vor Biopharma, and Xenikos.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: M.J.W. and L.M. conceived and designed the study; CIBMTR provided financial support and collected and assembled the data; M.J.W., L.M., M.d.L., W.S., M.-J.Z., and N.E.-M. analyzed the data; M.J.W., L.M., and M.d.L. prepared the first draft of the manuscript; and M.J.W, L.M., M-J.Z.,H-L.W., N.E-M., D.I.M., A.S.A-H., L.M., N.C., D.R., R.P.G., S.M.G., M.C., A.M., S.G., V.R.B., S.S.P., F.V.M., Y.I., S.M.B., E.C., N.P., M.A.K-D., H.M.L., S.G., C.B., M.A.D.P., R.C., B.N.S., K.B., R.M., B.W., U.B., M.A., A.B., H.S.M., J.A.Y., I.A., N.F., H.L., H.A-A., E.K.W., M.S., M.D.S., M.v.d.P., M.R.G., J.L.L., R.T.K., J.M., R.M., J-Y.C., J.W.L., C.O.F., M.M.K., L.E.W., U.G., S.N., R.F.O., L.F.V., A.S., O.R., B.D.F., J.C., H.C., S.C., T.N., S.S., B.G., L.A.B-L., G.C.H., M.d.L., M.L., P.K., C.S.H., M.B.A., D.J.W., and W.S. interpreted the data, helped revise the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: L.M. received speaker fees from Incyte and Takeda and research funding from Pfizer. D.R. received personal fees from AbbVie, Agios, AROG, Bayer, Celgene, Gilead, Incyte, Jazz Pharmaceuticals, Kadmon, Kite Pharma, Morphosys, Mustang, Novartis, Pfizer, Sanofi, Seattle Genetics, Amgen, Acrobiotech, UCART, Chimerix, and Pharmacyclics, and personal fees and other from Celltrion/Teva and Stemline (outside the submitted work). R.P.G. served as a consultant for BeiGene, Kite Pharma, Fusion Pharma, LaJolla NanoMedical, Mingsight Pharmaceuticals, and CStone Pharmaceuticals, served as a medical director for FFF Enterprises, is a partner in AZCA, served on the Board of Directors for RakFond Foundation for Cancer Research Support, and served on a scientific advisory board for Antegene Biotech and StemRad. V.R.B. received personal fees from Agios, Takeda, Omeros, Partner Therapeutics, Partnership for Health Analytic Research, CSL Behring, Rigel Pharmaceuticals, Genentech, grants and personal fees from Incyte and AbbVie, grants from Jazz, National Marrow Donor Program, and Tolero Pharmaceuticals, other from Oncoceutics and Novartis, and grants and other from Pfizer (outside the submitted work). S.S.P. received personal fees from Kite Pharma (outside the submitted work). Y.I. received personal fees from Novartis, Janssen, and Meiji Seika Pharma (outside the submitted work). E.C. received other from the Amgen Oncology Executive Advisory Council (outside the submitted work). M.A.K.-D. received other from Daiichi Sankyo (outside the submitted work). S.G. received personal fees from Seattle Genetics, Kite Pharma, Kadmon, Sanofi, Bristol Myers Squibb, Astellas, and Daiichi Sankyo (outside the submitted work). R.C. received grants from Merck and Vanda Pharmaceuticals, grants and personal fees from Amgen, Kite Pharma/Gilead, and Pfizer, and other from Seagen (outside the submitted work). H.L. received grants from Bristol Myers Squibb and Karyopharm and personal fees from Agios (outside the submitted work). M.R.G. received personal fees from AbbVie, Agios, Amgen, Cardinal Health, Bristol Myers Squibb, Daiichi Sankyo, Merck, Pfizer, Premier, Karius, Astellas, Trovagene, Stemline, and Gilead, other from Forma Therapeutics, Genentech/Roche, and Janssen, and personal fees and other from Incyte (outside the submitted work). J.L.L. received personal fees from AbbVie and other from Onconova (outside the submitted work). J.M. received other from AlloVir HCP, Juno Therapeutics, Kite Pharma/Gilead, and Magenta Therapeutics (outside the submitted work). J.-Y.C. received other from Agios, AbbVie, Otsuka, and Race Oncology (outside the submitted work). R.F.O. received personal fees from AstraZeneca (outside the submitted work). A.S. received clinical trial salary support from Vertex Pharmaceuticals and CRISPR Therapeutics, Novartis provided funding to his institution, and he received personal consultancy fees from Spotlight Therapeutics (outside the submitted work). J.C. received personal fees from Jazz Pharmaceuticals, Daiichi-Sankyo, Pfizer, Amgen, Allovir (outside the submitted work) and owns stock in Actinium Pharmaceuticals, bluebird bio, Dynavax Pharma, Atyr Pharmac, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart, and Veru. T.N. received other from Novartis and Karyopharm (outside the submitted work). S.S. received personal fees from Janssen Pharmaceutical (outside the submitted work). L.A.B.-L. is a member of the Transplant Advisory Board for Luminex. G.C.H. received other from Incyte, Jazz Pharmaceuticals, Morphosys, Alexion Pharmaceuticals, Karyopharm Therapeutics, Pharmacyclics, AstraZeneca, Astellas Pharma, Falk Foundation, and Takeda (outside the submitted work). M.d.L. received grants from Pfizer and Celgene and personal fees from Kadmon, Pfizer, Incyte, and Bristol Myers Squibb (outside the submitted work). P.K. received other from Amgen, Ziopharm, Pfizer, Kite Pharma, Novartis, and Jazz (outside the submitted work). C.S.H. received other from Sellas (outside the submitted work). The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Wieduwilt, University of Oklahoma Health, Stephenson Cancer Center, 800 NE 10th St, Oklahoma City, OK 73104; e-mail: matthew-wieduwilt@ouhsc.edu.
References
- 1.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. [DOI] [PubMed] [Google Scholar]
- 2.Pidala J, Djulbegovic B, Anasetti C, Kharfan-Dabaja M, Kumar A. Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission. Cochrane Database Syst Rev. 2011(10):CD008818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta V, Richards S, Rowe J; Acute Leukemia Stem Cell Transplantation Trialists’ Collaborative Group . Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121(2):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhédin N, Huynh A, Maury S, et al. ; GRAALL group . Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486-2496. [DOI] [PubMed] [Google Scholar]
- 5.Segal E, Martens M, Wang HL, et al. Comparing outcomes of matched related donor and matched unrelated donor hematopoietic cell transplants in adults with B-Cell acute lymphoblastic leukemia. Cancer. 2017;123(17):3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo X-D, Xu L-P, Zhang X-H, et al. Haploidentical hematopoietic stem cell transplantation in adults with Philadelphia-negative acute lymphoblastic leukemia: no difference in the high- and low-risk groups. Int J Cancer. 2015;136(7):1697-1707. [DOI] [PubMed] [Google Scholar]
- 7.Santoro N, Ruggeri A, Labopin M, et al. Unmanipulated haploidentical stem cell transplantation in adults with acute lymphoblastic leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srour SA, Milton DR, Bashey A, et al. Haploidentical transplantation with post-transplantation cyclophosphamide for high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23(2):318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashey ZA, Zhang X, Brown S, et al. Comparison of outcomes following transplantation with T-replete HLA-haploidentical donors using post-transplant cyclophosphamide to matched related and unrelated donors for patients with AML and MDS aged 60 years or older. Bone Marrow Transplant. 2018;53(6):756-763. [DOI] [PubMed] [Google Scholar]
- 11.Rashidi A, Hamadani M, Zhang M-J, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3(12):1826-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brissot E, Labopin M, Ehninger G, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104(3):524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz J, Galimard JE, Labopin M, et al. ; Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) . Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shem-Tov N, Peczynski C, Labopin M, et al. Haploidentical vs. unrelated allogeneic stem cell transplantation for acute lymphoblastic leukemia in first complete remission: on behalf of the ALWP of the EBMT. Leukemia. 2020;34(1):283-292. [DOI] [PubMed] [Google Scholar]
- 15.Al Malki MM, Yang D, Labopin M, et al. Comparing transplant outcomes in ALL patients after haploidentical with PTCy or matched unrelated donor transplantation. Blood Adv. 2020;4(9):2073-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs EJ, O’Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137(3):420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009; 15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aristei C, Santucci A, Corvò R, et al. ; Italian TBI Working Group . In haematopoietic SCT for acute leukemia TBI impacts on relapse but not survival: results of a multicentre observational study. Bone Marrow Transplant. 2013;48(7):908-914. [DOI] [PubMed] [Google Scholar]
- 23.Peters C, Dalle JH, Locatelli F, et al. ; EBMT Paediatric Diseases Working Party . Total body irradiation or chemotherapy conditioning in childhood ALL: A multinational, randomized, noninferiority phase III study. J Clin Oncol. 2021;39(4):295-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlu J, Labopin M, Niittyvuopio R, et al. The role of measurable residual disease (MRD) at time of allogeneic hematopoietic cell transplantation in adults with acute lymphoblastic leukemia transplanted after myeloablative conditioning. A study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(3):S7. [Google Scholar]
- 25.Kebriaei P, Anasetti C, Zhang MJ, et al. ; Acute Leukemia Committee of the CIBMTR . Intravenous busulfan compared with total body irradiation pretransplant conditioning for adults with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2018;24(4):726-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFilipp Z, Advani AS, Bachanova V, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: Updated 2019 evidence-based review from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25(11):2113-2123. [DOI] [PubMed] [Google Scholar]
- 27.Grosso D, Carabasi M, Filicko-O’Hara J, et al. Low nonrelapse mortality after HLA-matched related 2-step hematopoietic stem cell transplantation using cyclophosphamide for graft-versus-host disease prophylaxis and the potential impact of non- cyclophosphamide-exposed T cells on outcomes. Biol Blood Marrow Transplant. 2020;26(10):1861-1867. [DOI] [PubMed] [Google Scholar]
- 28.Mielcarek M, Furlong T, O’Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127(11):1502-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moiseev IS, Pirogova OV, Alyanski AL, et al. Risk-adapted GVHD prophylaxis with post-transplantation cyclophosphamide in adults after related, unrelated, and haploidentical transplantations. Eur J Haematol. 2018;100(5):395-402. [DOI] [PubMed] [Google Scholar]
- 30.Kwon M, Bailén R, Pascual-Cascón MJ, et al. Posttransplant cyclophosphamide vs cyclosporin A and methotrexate as GVHD prophylaxis in matched sibling transplantation. Blood Adv. 2019;3(21):3351-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeshurun M, Weisdorf D, Rowe JM, et al. The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv. 2019;3(4):670-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachanova V, Burke MJ, Yohe S, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant. 2012;18(6):963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Garcia J, Serrano J, Serrano-Lopez J, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant. 2013;48(3):396-402. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Slack R, Jorgensen JL, et al. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2014;14(4):319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26(11):2367-2374. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Hao S, Diong C, et al. Pre-transplant achievement of negativity in minimal residual disease and French-American-British L1 morphology predict superior outcome after allogeneic transplant for Philadelphia chromosome positive acute lymphoblastic leukemia: an analysis of Southeast Asian patients. Leuk Lymphoma. 2015;56(5):1362-1369. [DOI] [PubMed] [Google Scholar]
- 37.Lussana F, Intermesoli T, Gianni F, et al. Achieving molecular remission before allogeneic stem cell transplantation in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Impact on relapse and long-term outcome. Biol Blood Marrow Transplant. 2016;22(11):1983-1987. [DOI] [PubMed] [Google Scholar]
- 38.Nagafuji K, Miyamoto T, Eto T, et al. Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with Ph-negative ALL: results of a prospective study (ALL MRD2002 Study). J Hematol Oncol. 2013;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logan AC, Vashi N, Faham M, et al. Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant. 2014;20(9):1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao XS, Liu YR, Xu LP, et al. Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with ALL receiving unmanipulated haploidentical allografts. Am J Hematol. 2019;94(5):512-521. [DOI] [PubMed] [Google Scholar]
- 41.Lang P, Greil J, Bader P, et al. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis. 2004;33(3):281-287. [DOI] [PubMed] [Google Scholar]
- 42.Federmann B, Bornhauser M, Meisner C, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica. 2012;97(10):1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang P, Teltschik HM, Feuchtinger T, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165(5):688-698. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liu QF, Xu LP, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: A biologically phase III randomized study. Clin Cancer Res. 2016;22(14):3467-3476. [DOI] [PubMed] [Google Scholar]
- 45.Han LJ, Wang Y, Fan ZP, et al. Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2017;179(1):120-130. [DOI] [PubMed] [Google Scholar]
- 46.Nagler A, Kanate AS, Labopin M, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin for graft-versus-host disease prevention in haploidentical transplantation for adult acute lymphoblastic leukemia. Haematologica. 2021;106(6):1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang YJ, Wang Y, Xu LP, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv M, Jiang Q, Zhou DB, et al. Comparison of haplo-SCT and chemotherapy for young adults with standard-risk Ph-negative acute lymphoblastic leukemia in CR1. J Hematol Oncol. 2020;13(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.