Abstract
Linear IgA bullous dermatosis (LABD) is a mucocutaneous autoimmune blistering disease affecting both adults and children. It is caused by IgA antibodies targeting multiple antigens along the basement membrane zone, leading to disruption of dermoepidermal junction and development of bullous lesions which often presents in characteristic arrangement. Although most LABD cases have been reported to be idiopathic, different triggers have been described, including several drugs and infection. However, the occurrence of vaccine-induced cases of LABD is not widely known and accepted due to the few reports available. We present two cases of LABD occurred following different triggers, rising the suspicion for a possible pathogenetic role of vaccines.
Keywords: adverse reaction, autoimmune blistering disease, dermatology, linear IgA bullous dermatosis, papillomavirus, vaccines
Introduction
Linear Immunoglobulin A bullous dermatosis (LABD) is a rare acquired autoimmune subepithelial bullous disease characterized by linear deposition of IgA along the basement membrane zone (BMZ) targeting several autoantigens. 1 The clinical presentation is heterogeneous and can mimic other blistering diseases such as dermatitis herpetiformis and bullous pemphigoid. However, characteristic cutaneous lesions consist of tense arciform bullae in ‘string-of-pearls’ configuration. LABD can affect both adults and children, with similar histological and immunopathological features. 2 However, LABD generally shows spontaneous resolution in children but not in adults. Incidence also differs between adults and children, even changing among countries. 3
Most cases of childhood LABD are idiopathic, but drugs and infections were reported as potential triggering factors. In adults, malignancies and influenza vaccination also may act as triggers.4,5 We will present two cases of LABD occurred following different triggers, both rising the suspicion for a possible pathogenetic role of vaccines.
Case reports
Case 1
A 1-year-old male child was admitted for severe blistering eruption. One week before he was administered amoxicillin-clavulanic-acid for upper airway infection, while 2 weeks earlier he received anti-measles, -mumps and -rubella vaccination. On physical examination, tense bullous lesions of about 3–10 mm in diameter, most in ‘string-of-pearls’ configuration, appeared on upper limbs, buttocks and abdomen, associated with erythema and crusts (Figure 1). The child appeared restless and distressed for intense itch. Lesional and perilesional biopsies were taken from the buttocks for histology and direct immunofluorescence (DIF). Histology showed subepidermal blistering with neutrophilic dermal infiltration, while DIF revealed linear IgA and C3 deposition along the BMZ (Figure 1). Indirect immunofluorescence (IIF) on human salt-split-skin substrate was negative. Clinical features and DIF supported the diagnosis of LABD of childhood. Amoxicillin-clavulanic acid was discontinued while topical steroid treatment was started, considering parents rejection for systemic therapies. Complete resolution was obtained within a week, without local side effects. The topical therapy was continued for 4 weeks. One month later, a recurrence of mild-itchy blisters occurred on buttocks and limbs following further intake of amoxicillin for a new upper airway infection. Considering the exacerbation after drug re-administration, the Naranjo Score applied for amoxicillin-clavulanic acid was 5, stating the association as probable. The recurrence was controlled simply suspending the drug intake without adjunctive therapies.
Figure 1.
(a–d) Tense bullous lesions, some of which in the typical ‘string-of-pearls’ configuration, in periorificial regions, upper limbs and abdomen. (e) Linear IgA deposition associated with linear C3 deposition along the BMZ of the perilesional skin.
Case 2
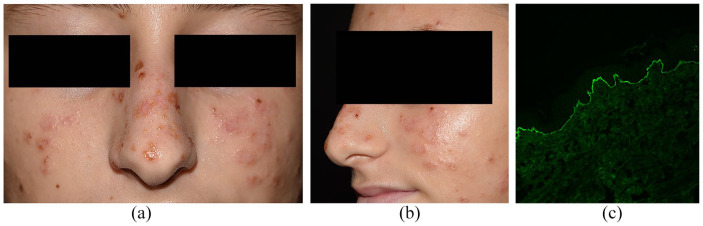
A 14-year-old girl was admitted for a face eruption appeared 1 week before. Vesicles and small blisters on normal to slightly erythematous skin were located on forehead, cheeks, nose, ears, forearms and dorsal surface of hands (Figure 2a and b). Most of the lesions were grouped in ‘string-of-pearls’ forms, clinically compatible with LABD. Some honey-coloured crusts raised the suspicion of bacterial superinfection. The girl was otherwise healthy, with no recent history of medications. Three weeks before, she got -6, -11, -16, -18 human papillomavirus (HPV) vaccine injection (Gardasil). Perilesional skin biopsy was taken in preauricular area: DIF showed linear IgA deposition along the BMZ (Figure 2c), confirming the diagnostic hypothesis.
Figure 2.
(a, b) Vesicles and blistering lesions on cheeks, nose and forehead in ‘string-of-pearls’ distributions, with some yellowish crusts. (c) Linear IgA deposition along BMZ at DIF performed on perilesional skin biopsy.
The patient started systemic steroid therapy contemporary with topical retamipulin, getting progressive improvements with complete resolution within 3 weeks.
Discussion
LABD is one of the most common bullous disorders in children and occurs usually at the age of approximately 4–5 years old2,3,6–8 while few cases are reported in new-borns.9–12 The clinical heterogeneity of the disease can lead to diagnostic difficulties for differential diagnosis with other bullous diseases, such as bullous pemphigoid and dermatitis herpetiformis, even in cases with blisters in ‘string-of-pearls’ arrangements.13,14 Conversely, LABD can manifest atypical clinical pictures, such as the prurigo-like variant. 15 Mucosal involvement is less frequent2,16; however, previous reports of mucosal or mucosal-dominant LABD could represent misdiagnosed cases of mucous membrane pemphigoid (MMP). 17
Although most cases of LABD are idiopathic, infections and drugs may represent the trigger. Several medications were reported as responsible for drug-induction in adults including antibiotics, 16 non-steroidal anti-inflammatory drugs (NSAIDs), antiretrovirals, diuretics, ACE-inhibitors, anti-epileptics, statins, amiodarone and cyclosporine. 3 By contrast, in children drug-induced LABD has been related to antibiotics and NSAIDs only.18,19
Amoxicillin-clavulanic acid was described as possible trigger in different cases including two paediatric patients of 2 and 9 years old respectively, and one in a 62-year-old woman.20,21 Clinical presentation of these cases and, more in general, of drug-induced LABD, consists of classic tense bullae often in annular disposition, but toxic-epidermal-necrolysis-like pattern has been reported in up to 20% of cases in a French study.22,23 The median interval from start of drug intake to the onset of LABD ranges from 7 to 9 days to 3 weeks and in 20% rapid resolution following discontinuation of the offending drug can be observed, even without specific treatments.9,23 However, the particularity of our case 1 is related to the possible association of LABD with anti-measles, -mumps and -rubella vaccination. Similar reports are not currently available in literature. One case of LABD shortly after receiving influenza vaccination has been described in a 54-year-old woman, 24 while two paediatric cases occurred 2 weeks after varicella and influenza vaccination are reported. 25
While the possible causative role of amoxicillin was suggested by an involuntary re-challenge test and the Naranjo score, it was not possible to clearly demonstrate that the vaccination was not able to induce the disease, considering the compatible timing. In the second case, the association between LABD and vaccination appears clearer, due to the absence of concomitant therapies. Moreover, another case of LABD following quadrivalent HPV vaccination has been reported, with a latency time of 3 weeks, similarly to our case. 24 Regarding the physiopathology, one hypothesis implies vaccinations as non-specific activators of the immune response, leading to exacerbations of pre-existent autoimmunity or the development of de novo autoimmune diseases. Autoimmunity can also be induced by molecular mimicry between vaccine antigens and BMZ proteins. 26
Conclusions
To date, the role of vaccinations in LABD induction has little evidence, indeed it is shared only by few case reports. However, the growing number of reports should make clinicians aware of this association, which appears more frequent than a simple coincidence. Possible causative agents should be carefully searched in patients with LABD, since their removal alone may be effective. Further warnings and population-based studies would reveal useful in improving the knowledge of this phenomenon, in particular about the incidence. Moreover, the attention should be raised in 2021 considering the global vaccination plan for COVID-19, which will involve billions of subjects in a short period.
Footnotes
Authors’ Note: Lavinia Quintarelli, Alice Verdelli, and Marzia Caproni are now affiliated to Rare Dermatological Diseases Unit, Section of Dermatology, Azienda USL Toscana Centro, Florence, Italy.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Alberto Corrà  https://orcid.org/0000-0003-0903-4998
https://orcid.org/0000-0003-0903-4998
References
- 1. Cozzani E, Di Zenzo G, Gasparini G, et al. (2020) Autoantibody profile of a cohort of 54 Italian patients with linear IgA bullous dermatosis: LAD-1 denoted as a major auto-antigen of the lamina Lucida subtype. Acta Dermato-Venereologica 100(4): adv00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Genovese G, Venegoni L, Fanoni D, et al. (2019) Linear IgA bullous dermatosis in adults and children: A clinical and immunopathological study of 38 patients. Orphanet Journal of Rare Diseases 14(1): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fortuna G, Marinkovich MP. (2012) Linear immunoglobulin A bullous dermatosis. Clinics in Dermatology 30(1): 38–50. [DOI] [PubMed] [Google Scholar]
- 4. Kartan S, Shi VY, Clark AK, et al. (2017) Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: Characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment. American Journal of Clinical Dermatology 18(1): 105–126. [DOI] [PubMed] [Google Scholar]
- 5. Alberta-Wszolek L, Mousette AM, Mahalingam M, et al. (2009) Linear IgA bullous dermatosis following influenza vaccination. Dermatology Online Journal 15(11): 3. [PubMed] [Google Scholar]
- 6. Denguezli M, Ben Nijma B, Nouira R, et al. (1994) IgA linear bullous dermatosis in children. A series of 12 Tunisian patients. Annales de Dermatologie et de Venereologie 121: 888–892. [PubMed] [Google Scholar]
- 7. Kharfi M, Khaled A, Karaa A, et al. (2010) Linear IgA bullous dermatosis: The most frequent dermatosis of children. Dermatology Online Journal 16: 2. [PubMed] [Google Scholar]
- 8. Kong YL, Lim YL, Chandran NS. (2015) Retrospective study on autoimmune blistering disease in paediatric patients. Pediatric Dermatology 32(6): 845–852. [DOI] [PubMed] [Google Scholar]
- 9. Mazurek MT, Banihani R, Wong J, et al. (2018) Uncomplicated neonatal linear IgA bullous dermatosis: A case report. Journal of Cutaneous Medicine and Surgery 22(4): 431–434. [DOI] [PubMed] [Google Scholar]
- 10. Hruza LL, Mallory SB, Fitzgibbons J, et al. (1993) Linear IgA bullous dermatosis in a neonate. Pediatric Dermatology 10(2): 171–176. [DOI] [PubMed] [Google Scholar]
- 11. Kishida Y, Kameyama J, Nei M, et al. (2004) Linear IgA bullous dermatosis of neonatal onset: Case report and review of the literature. Acta Paediatrica 93(6): 850–852. [PubMed] [Google Scholar]
- 12. Julapalli MR, Brandon KL, Rosales CM, et al. (2012) Neonatal linear immunoglobulin a bullous dermatosis: A rare presentation. Pediatric Dermatology 29(5): 610–613. [DOI] [PubMed] [Google Scholar]
- 13. Raposo I, Machado S, Sampaio R, et al. (2017) Infantile bullous pemphigoid with “string of pearls sign”. Dermatology Online Journal 23(7): 13030/qt3fw4d2t2. [PubMed] [Google Scholar]
- 14. Giacaman A, Bauzá A, Olea JM, et al. (2018) Annular paraneoplastic bullous pemphigoid mimicking linear IgA bullous dermatosis in a 40-year-old patient. Journal der Deutschen Dermatologischen Gesellschaft 16(4): 482–484. [DOI] [PubMed] [Google Scholar]
- 15. Torchia D, Caproni M, Del Bianco E, et al. (2006) Linear IgA disease presenting as prurigo nodularis. The British Journal of Dermatology 155: 479–480. [DOI] [PubMed] [Google Scholar]
- 16. Kelly SE, Frith PA, Millard PR, et al. (1988) A clinicopathological study of mucosal involvement in linear IgA disease. The British Journal of Dermatology 119(2): 161–170. [DOI] [PubMed] [Google Scholar]
- 17. Torchia D, Caproni M, Fabbri P. (2007) Linear IgA dermatosis with exclusive mucosal involvement or mucous membrane pemphigoid? Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 104(2): 151–152. [DOI] [PubMed] [Google Scholar]
- 18. Sarikaya Solak S, Ficicioglu S. (2019) Cephalosporin induced linear IgA dermatosis in a child: Case report and literature review. Dermatologic Therapy 32(4): e12927. [DOI] [PubMed] [Google Scholar]
- 19. Gatto A, Guerriero C, Moretta G, et al. (2017) Linear IgA bullous dermatosis in a two-year-old child: Possible association with aspirin. European Journal of Dermatology 27(4): 417–418. [DOI] [PubMed] [Google Scholar]
- 20. Ho JC, Nq PL, Tan SH, et al. (2007) Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatric Dermatology 24(5): E40–E43. [DOI] [PubMed] [Google Scholar]
- 21. Santos-Juanes J, Coto Hernández R, Trapiella L, et al. (2007) Amoxicillin-associated linear IgA bullous dermatosis. Journal of the European Academy of Dermatology and Venereology 21(7): 992–993. [DOI] [PubMed] [Google Scholar]
- 22. Dellavalle RP, Burch JM, Tayal S, et al. (2003) Vancomycin associated linear IgA bullous dermatosis mimicking toxic epidermal necrolysis. Journal of the American Academy of Dermatology 48: S56–S57. [DOI] [PubMed] [Google Scholar]
- 23. Garel B, Ingen-Housz-Oro S, Afriat D, et al. (2019) Drug-induced linear immunoglobulin A bullous dermatosis: A French retrospective pharmacovigilance study of 69 cases. British Journal of Clinical Pharmacology 85(3): 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikeya S, Urano S, Tokura Y. (2012) Linear IgA bullous dermatosis following human papillomavirus vaccination. European Journal of Dermatology 22(6): 787–788. [DOI] [PubMed] [Google Scholar]
- 25. Díaz MS, Morita L, Ferrari B, et al. (2019) Linear IgA bullous dermatosis: A series of 17 cases. Actas Dermo-Sifiliograficas 110(8): 673–680. [DOI] [PubMed] [Google Scholar]
- 26. Koenig HC, Sutherland A, Izurieta HS, et al. (2011) Application of the immunological disease continuum to study autoimmune and other inflammatory events after vaccination. Vaccine 29: 913–919. [DOI] [PubMed] [Google Scholar]