Abstract
Prenatal HIV screening is critical to eliminate mother-to-child (MTC) HIV transmission. Although Massachusetts (MA) has near-zero MTC transmission rates, recent trends in statewide prenatal HIV testing are unknown. This study examined variations in prenatal HIV screening across race/ethnicity, socioeconomic status, and prenatal care settings in MA, in the period following national and state-level changes in guidance encouraging routine prenatal HIV testing.
According to the MA Pregnancy Risk Assessment Monitoring System (PRAMS) data, 68.3% of pregnant women in MA were screened for HIV between 2007 and 2016. There were significant differences in prenatal screening rates across race/ethnicity, with 83.38% of Black non-Hispanic (NH), 85.5% of Hispanic women, and 62.4% of White NH women reporting being tested for HIV at some point during their pregnancy (P <.0001). Multivariate regression found that differences in screening were explained by race/ethnicity, Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) status, prenatal care site, type of insurance, nativity, and marital status. Annual rates of prenatal HIV screening did not change significantly in MA from 2007 to 2016 (P = .27).
The results of the analysis revealed that prenatal HIV screening rates differ based on race/ethnicity, with higher rates in Black NH and Hispanic women when compared to White NH women. The racial disparities in prenatal HIV screening and lack of universal screening in MA raises questions about the effectiveness of the state's approach.
Keywords: maternal HIV screening, HIV testing, Massachusetts, human immunodeficiency virus, perinatal transmission, perinatal HIV transmission, prenatal HIV testing, mother-to-child HIV transmission
Introduction
In 2006, the centers for disease control (CDC) introduced guidelines highlighting the importance of HIV screening for all individuals in a health care setting with an emphasis on prenatal HIV screening in all women. 1 The CDC recommended that HIV screening should be included as a part of routine prenatal tests and should be done for all pregnant women and introduced an “opt-out” strategy where all women are tested unless they specifically request not to be tested. 1
The Comprehensive Care Workgroup for the Elimination of Perinatal HIV Transmission, a working group sponsored by the CDC from 2010 to 2017, focused on identifying factors that contribute to mother-to-child (MTC) HIV transmission and creating a comprehensive strategy to reduce MTC transmission. This group found that routine prenatal HIV screening is a critical component to reduce MTC HIV transmission across the United States. 2 They recommended that a multipronged strategy be used to reduce MTC HIV transmission, with robust prenatal HIV screening programs playing a significant role. Further recommendations focused on screening in high-risk populations (women who inject drugs and women who are non-US born), comprehensive support during and postpregnancy, and widespread integration and promotion of HIV prevention into prenatal care services. 2
Although national testing recommendations exist, individual state legislatures can supersede these protocols. In 2012, the Massachusetts (MA) State Legislature amended MGL 111 70F which changed MA's state law regarding written informed consent for HIV testing. Prior to this amendment, written, informed consent was needed to perform HIV testing, and MGL 111 70F amended the law to allow for verbal consent. Following this amendment, the Massachusetts Department of Public Health (MDPH) released a clinical advisory update, a tool commonly used to communicate with providers but that holds no force or consequence, that encouraged prenatal, maternal, and newborn caregivers to routinely offer HIV screening for all pregnant women in MA with a particular emphasis that it be performed “without reference to their risk profile." 3 This recommendation suggested that clinicians provide repeated offers for voluntary HIV testing to pregnant women who are at “continued risk” or who declined testing earlier in their pregnancy. 3 HIV testing is performed only with verbal consent in accordance with the state law. This differs from the 2006 CDC recommendation for an “opt-out” policy that does not require informed consent.3,4
Identifying pregnant women who are HIV positive in time to administer medical intervention is critical to reduce the risk of HIV transmission to the fetus. In 2013, the incidence rate of HIV in the United States was 1.8 per 100 000 live births. 5 Although this represents a significant improvement from the high incidence of 43.1 cases of perinatal acquired HIV per 100 000 live births in 1992, it is still above the CDC's public health goal of 1 case per 100 000 live births. 6 Moreover, the prevalence of MTC HIV transmission has significantly decreased with the introduction of antiretroviral prophylaxis, strict test and treat protocols, and education on safe infant feeding. 1 For example, in high-resource settings, perinatal HIV transmission occurs at a rate of 1% to 2% with the use of medical interventions and preventative measures. 7 Yet, without medical intervention, perinatal transmission occurs at a rate of 15 to 40%. 7 Even late-stage interventions, such as infant prophylaxis with a dual- or triple-nucleoside regimen, can be effective at reducing perinatal transmission of HIV. 8
In MA, MTC transmission of HIV has steadily decreased since 1999, when the promotion of universal screening of pregnant women began. From 2006 to 2011, there were 14 reported cases of MTC transmission of HIV, while from 2012 to 2017, there were three reported cases of MTC transmission of HIV. 9 Although increasingly rare, transmissions still occur.
One study examining rates of prenatal HIV screening in Pennsylvania, a state that has a policy similar to MA around HIV testing (opt-in), found that women who received PNC in resident or community clinics were significantly more likely to be screened than those who received prenatal care in a private practice setting. 10 Additionally, women who were told that HIV screening was standard practice were significantly more likely to accept prenatal HIV screening, and patient perception of the health care providers’ attitude regarding prenatal HIV screening was influential in whether or not women accepted screening. In contrast, a study examining the potential impacts of an opt-out policy reported that states with opt-out policies have had increases in rates of HIV screening in the prenatal population. 11 Still, limited research exists that examines the outcomes in high-resource “opt-in” states, such as MA, when compared with high-resource states that have opt-out policies.
Study Contribution
The aim of this study is to examine patterns of prenatal HIV screening in MA around the time of the amendment to MGL 111 70F and the subsequent clinical advisory update. The study draws on a statewide self-reported survey of women with a recent live birth—the MA Pregnancy Risk Assessment Monitoring System (PRAMS)—to assess whether MA has moved closer to universal prenatal HIV screening by recommending universal HIV screening to all pregnant women regardless of race or risk profile.
Methods
Study Population
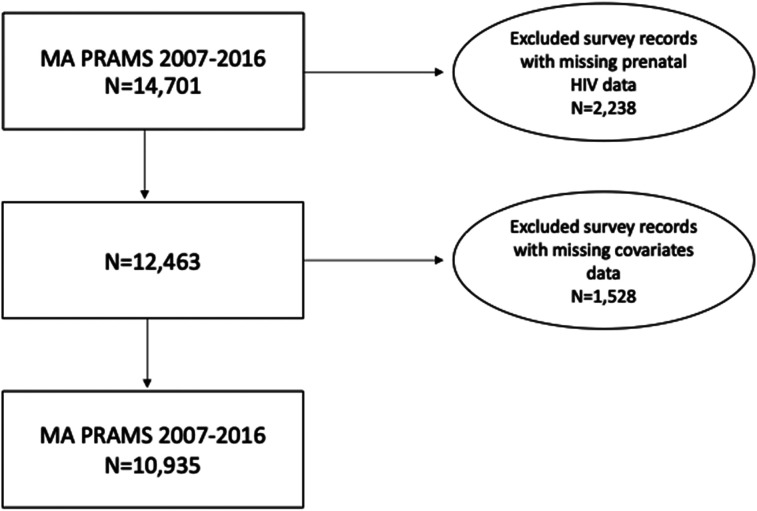
This study utilized 2007 to 2016 data from MA PRAMS. PRAMS is an ongoing collaborative surveillance project between the CDC and state health departments designed to collect self-reported population-based state-level data on maternal attitudes and experiences before, during, and shortly after pregnancy to better understand maternal and child health outcomes. MA PRAMS uses a stratified sampling methodology, sampling disproportionately from four racial and Hispanic ethnic groups: White non-Hispanic (NH); Black NH; Hispanic; and other NH. All but White NH mothers are oversampled to improve precision in examining disparities by race and ethnicity. Using birth certificates, MA PRAMS conducts a random sample of MA resident women with a live birth. The self-reported survey data is weighted using selected maternal demographics to account for nonresponse and complex survey design. Between 2007 and 2016, 14 701 MA resident women responded to the PRAMS survey with a weighted response rate of 65.6%. Informed consent was obtained from all participants. The final study sample had 10 935 women after excluding data from the other NH race group, missing data on prenatal HIV screening and race/Hispanic ethnicity (n = 2238), as well as missing data on selected covariates (n = 1528) (Figure 1).
Figure 1.
Flowchart of PRAMS participants and final study population.
Abbreviation: PRAMS, Pregnancy Risk Assessment Monitoring System.
Measures
The exposure analyzed was maternal race/ethnicity, which was obtained using birth certificates and was categorized as follows: (1) White NH; (2) Black NH; (3) Hispanic; and (4) Asian NH.
Our primary outcome was prenatal HIV screening. PRAMS respondents were asked whether at any time during their most recent pregnancy or delivery they have had a test for HIV. The outcome variable was categorized as binary (yes/no).
Covariates
We accounted for potential confounders by determining the distribution of various maternal covariates according to race/ethnicity and prenatal HIV screening status. Maternal age (<20, 20-24, 25-29, 30-34, 35-39, or 40 + years), education (less than high school, high school diploma/GED, some college, or completed college education), maternal nativity (US-born/not US-born), marital status (not married/married), and prenatal care site (private physician's office, hospital clinic, community health center, and other) were obtained from birth certificates. Family income based on the annual federal poverty level (FPL) and household size (≤100%, 101%-200%, or 201% or greater), health insurance during pregnancy (pubic, private, other, or none), and Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) participation (yes/no) were all derived from PRAMS.
Analytic Approach
Bivariate analyses examined the association of prenatal HIV screening with select maternal characteristics. Multivariate logistic regression models assessed the independent effect of race/ethnicity on prenatal HIV screening. Adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) were calculated for this association controlling for age, education, FPL, insurance during pregnancy, prenatal care site, WIC participation, nativity, and marital status. These covariates were chosen based on their significant association with both our exposure and outcome (chi-square P-value < .05). All analyses were conducted using SAS v9.4 and SUDAAN v11.0. Joinpoint v4.6 was used to examine year-to-year changes in prenatal testing rates between 2007 and 2016.
Ethical Approval and Informed Consent
This study received ethical approval from the Commonwealth of MDPH’s Institutional Review Board (approval no. 1302522-2). According to the MDPH policy, informed consent is not required for research presenting minimal risk and involving procedures that do not require written consent performed outside of a research setting.
Results
The final study sample included 10 935 women after excluding data from other NH race groups, missing data on prenatal HIV screening and race/Hispanic ethnicity (n = 2238), as well as missing data on selected covariates (n = 1528) (Figure 1). The final sample was comprised of 66.9% of self-identified NH White women, 9% NH Black women, 15.8% Hispanic women, and 8.3% NH Asian women. Among NH White women, the majority (36.6%) was aged 30 to 34 years and 58.4% had a college degree. 70.8% of NH White women included in the sample were living at or above 201% of FPL, 71.9% had private health insurance during their pregnancy, and 78.9% received prenatal care at a physician's office. The majority of Black NH mothers in the final study sample were aged 30 to 34 years (28.4%), with some college education (38.6%), living at or below 100% of the FPL (45.6%), on public insurance during their pregnancy (66%), received prenatal care (PNC) at a private physician's office (45.8%). Among Hispanic mothers, the majority were aged 25 to 29 years (28.7%), with a high school diploma (32.3%), living at or below 100% of the FPL, on public insurance (58.5%), received PNC at private physician's office (44%). During 2007 and 2016, 68.3% of women reported receiving prenatal HIV screening. Black NH and Hispanic women had higher rates of HIV screening (83.3% and 85.5%, respectively) when compared to White NH women (62.4%) (P < .0001) (Table 1).
Table 1.
Selected Characteristics of Women With Live Births by Race/Ethnicity in Massachusetts (MA), Pregnancy Risk Assessment Monitoring System (PRAMS), 2007 to 2016 (n = 10,935).
| Race/Ethnicity | ||||||
|---|---|---|---|---|---|---|
| All |
White, non-Hispanic n = 3473 |
Black, non-Hispanic n = 2373 |
Hispanic n = 2776 |
Asian, non-Hispanic n = 2313 |
P value | |
| % | % | % | % | % | ||
| Overall | 66.9% | 9.0% | 15.8% | 8.3% | ||
| Prenatal HIV Screening | < . 0001 | |||||
| No | 31.7% | 37.6% | 16.7% | 14.5% | 32.4% | |
| Yes | 68.3% | 62.4% | 83.3% | 85.5% | 67.6% | |
| Maternal age (years) | < . 0001 | |||||
| < 20 | 3.8% | 2.5% | 5.5% | 9.3% | 1.3% | |
| 20 to 24 | 14.5% | 11.8% | 19.7% | 26.7% | 6.9% | |
| 25 to 29 | 25.2% | 24.0% | 26.7% | 28.7% | 26.8% | |
| 30 to 34 | 33.8% | 36.6% | 28.4% | 21.9% | 39.1% | |
| 35 to 39 | 18.6% | 20.6% | 15.0% | 10.9% | 21.5% | |
| ≥ 40 | 4.1% | 4.4% | 4.6% | 2.6% | 4.4% | |
| Maternal education | < . 0001 | |||||
| < High school | 8.0% | 3.8% | 9.6% | 26.0% | 5.8% | |
| High school diploma | 20.6% | 17.8% | 28.5% | 32.3% | 12.9% | |
| Some college | 22.3% | 20.1% | 38.6% | 27.4% | 12.4% | |
| ≥ College graduate | 49.1% | 58.4% | 23.3% | 14.3% | 68.9% | |
| Federal poverty level (FPL) | < . 0001 | |||||
| ≤ 100% FPL | 25.2% | 15.5% | 45.6% | 58.5% | 18.2% | |
| 101%-200% FPL | 16.6% | 13.8% | 28.4% | 22.7% | 15.5% | |
| ≥ 201% FPL | 58.2% | 70.8% | 25.6% | 18.9% | 66.3% | |
| Health insurance during pregnancy | <.0001 | |||||
| Public | 36.8% | 25.4% | 66.0% | 73.9% | 26.6% | |
| Private | 60.4% | 71.9% | 31.3% | 23.1% | 70.2% | |
| Other | 2.1% | 2.3% | 1.7% | 1.7% | 2.0% | |
| None | 0.7% | 0.5% | 1.0% | 1.3% | 1.2% | |
| Prenatal care site | ||||||
| Private physician's office | 69.1% | 78.9% | 45.8% | 44.0% | 63.2% | . 2691 |
| Hospital clinic | 17.5% | 12.9% | 32.6% | 27.7% | 19.4% | |
| Community health center | 8.0% | 2.8% | 15.4% | 24.4% | 11.2% | |
| Other | 5.4% | 5.5% | 6.2% | 3.9% | 6.3% | |
| WIC participant | ||||||
| No | 64.6% | 77.8% | 28.9% | 24.4% | 73.5% | |
| Yes | 35.4% | 22.2% | 71.1% | 75.6% | 26.5% | |
| Maternal nativity | < . 0001 | |||||
| Non US-born | 28.0% | 9.6% | 52.1% | 64.0% | 81.0% | |
| US-born | 72.0% | 90.4% | 47.9% | 36.0% | 19.0% | |
| Marital status | < . 0001 | |||||
| Not married | 33.0% | 25.6% | 55.6% | 61.4% | 13.7% | |
| Married | 67.0% | 74.4% | 44.4% | 38.6% | 86.3% | |
Table 2 shows the distribution of selected demographic characteristics by prenatal HIV screening status. Compared to women who did not receive prenatal HIV screening, higher proportions of women being tested were observed among those who were Black NH (11.0% vs 4.8%) and Hispanic (19.8% vs 7.3%), and lower proportions of women being tested were observed among those who were White NH (61.1% vs 79.5%). The proportions of women being tested also varied by age, education, FPL, health insurance during pregnancy, site of PNC services, WIC participation status, nativity, and marital status.
Table 2.
Selected Characteristics of Women With Live Births by Prenatal HIV Screening status in Massachusetts (MA), Pregnancy Risk Assessment Monitoring System (PRAMS), 2007 to 2016 (n = 10,935).
| Prenatal HIV Screening | |||
|---|---|---|---|
|
No n = 2856 |
Yes n = 8079 |
P value | |
| % | % | ||
| Maternal race/ethnicity | < . 0001 | ||
| White, non-Hispanic | 79.5% | 61.1% | |
| Black, non-Hispanic | 4.8% | 11.0% | |
| Hispanic | 7.3% | 19.8% | |
| Asian, non-Hispanic | 8.5% | 8.2% | |
| Maternal age (years) | < . 0001 | ||
| < 20 | 2.0% | 4.6% | |
| 20 to 24 | 8.7% | 17.2% | |
| 25 to 29 | 22.9% | 26.3% | |
| 30 to 34 | 39.3% | 31.2% | |
| 35 to 39 | 23.2% | 16.5% | |
| ≥ 40 | 4.0% | 4.2% | |
| Maternal education | < . 0001 | ||
| < High school | 3.7% | 10.0% | |
| High school diploma | 13.8% | 23.8% | |
| Some college | 18.4% | 24.1% | |
| ≥ College graduate | 64.1% | 42.2% | |
| Federal poverty level (FPL) | < . 0001 | ||
| ≤ 100% FPL | 13.1% | 30.8% | |
| 101%-200% FPL | 13.1% | 18.3% | |
| ≥ 201% FPL | 73.7% | 50.9% | |
| Health insurance during pregnancy | < . 0001 | ||
| Public | 21.2% | 44.1% | |
| Private | 76.2% | 53.1% | |
| Other | 2.0% | 2.1% | |
| None | 0.6% | 0.7% | |
| Prenatal care site | |||
| Private physician's office | 79.0% | 64.5% | < . 0001 |
| Hospital clinic | 11.2% | 20.5% | |
| Community Health center | 3.5% | 10.1% | |
| Other | 6.3% | 4.9% | |
| WIC participant | < . 0001 | ||
| No | 81.2% | 56.9% | |
| Yes | 18.8% | 43.1% | |
| Maternal nativity | < . 0001 | ||
| Non US-born | 20.0% | 31.7% | |
| US-born | 80.0% | 68.3% | |
| Marital status | < . 0001 | ||
| Not married | 18.3% | 39.8% | |
| Married | 81.7% | 60.2% | |
Our crude logistic regression analysis showed that prenatal HIV screening was significantly and independently associated with maternal race/ethnicity, age, education, FPL, insurance status, prenatal care site, WIC participation, nativity, and marital status. Through multivariate logistic modeling, we found that race/ethnicity, WIC participation, site of PNC, nativity, and marital status remained statistically significantly associated with prenatal HIV screening. When compared to White NH mothers, Black NH and Hispanic mothers were 1.12 times and 1.13 times more likely to receive prenatal HIV testing, respectively, (P < .01). When compared to non-WIC mothers, those on WIC were 1.11 times
more likely to receive prenatal HIV testing (P < .01). Compared to those mothers who received PNC at a private physician's office, mothers who received PNC at a hospital clinic and community health center were 1.15 times and 1.16 times more likely to receive prenatal HIV testing, respectively (P < .01). Compared to US-born mothers, foreign-born mothers were 1.06 times more likely to receive prenatal HIV testing (P < .01). Compared to married mothers, unmarried mothers were 1.15 times more likely to receive prenatal HIV testing (P < .01) (Table 3).
Table 3.
Association Between Prenatal HIV Screening and Maternal Race/Ethnicity in Massachusetts (MA), Pregnancy Risk Assessment Monitoring System (PRAMS), 2007 to 2016 (n = 10,935).
| HIV Screening | ||
|---|---|---|
| Crude RR (95% CI) | Adjusted RR (95% CI) | |
| Maternal race/ethnicity | ||
| White, non-Hispanic | Ref. | Ref. |
| Black, non-Hispanic | 1.34 (1.29-1.38)** | 1.12 (1.07-1.17)** |
| Hispanic | 1.37 (1.33-1.41)** | 1.13 (1.08-1.18)** |
| Asian, non-Hispanic | 1.08 (1.04-1.13)** | 1.02 (0.97-1.07) |
| Maternal age (years) | ||
| < 20 | 1.17 (1.10-1.25)** | 0.96 (0.86-1.08) |
| 20 to 24 | 1.14 (1.09-1.19)** | 1.01 (0.95-1.08) |
| 25 to 29 | Ref. | Ref. |
| 30 to 34 | 0.89 (0.85-0.93)** | 0.98 (0.93 −1.02) |
| 35 to 39 | 0.85 (0.80-0.90)** | 0.95 (0.90-1.00) |
| ≥ 40 | 0.98 (0.89-1.06)** | 1.04 (0.96-1.13) |
| Maternal education | ||
| < High school | 1.46 (1.39-1.52)** | 1.08 (1.00-1.18) |
| High school diploma | 1.34 (1.29-1.40)** | 1.07 (1.00-1.14) |
| Some college | 1.26 (1.21-1.32)** | 1.05 (1.00-1.10) |
| ≥ College graduate | Ref. | Ref. |
| Federal poverty level (FPL) | ||
| ≤ 100% FPL | 1.39 (1.35-1.44)** | 0.99 (0.92-1.07) |
| 101%-200% FPL | 1.25 (1.20-1.31)** | 0.98 (0.92-1.04) |
| ≥ 200% FPL | Ref. | Ref. |
| Health insurance during pregnancy | ||
| Public | 1.36 (1.32-1.41)** | 1.04 (0.98-1.10) |
| Private | Ref. | Ref. |
| Other | 1.16 (1.03-1.31)** | 1.02 (0.91-1.15) |
| None | 1.22 (1.03-1.46)** | 0.99 (0.81-1.21) |
| Prenatal care site | ||
| Private physician's office | Ref. | Ref. |
| Hospital clinic | 1.25 (1.20-1.30)** | 1.15 (1.10-1.19)** |
| Community Health center | 1.35 (1.30-1.41)** | 1.16 (1.10-1.23)** |
| Other | 0.99 (0.90-1.08)** | 0.98 (0.91-1.06) |
| WIC participant | ||
| No | Ref. | Ref. |
| Yes | 1.38 (1.34-1.43)** | 1.11 (1.05-1.17)** |
| Maternal nativity | ||
| Non US-born | 1.19 (1.16-1.23)** | 1.07 (1.03-1.12)** |
| US-born | Ref. | Ref. |
| Marital status | ||
| Not married | 1.34 (1.30-1.39)** | 1.16 (1.10-1.21)** |
| Married | Ref. | Ref. |
Abbreviations: CI, confidence interval; RR, risk ratio; Ref, reference. *P < .05; **P < .01.
Prenatal HIV Screening Trends Over Time
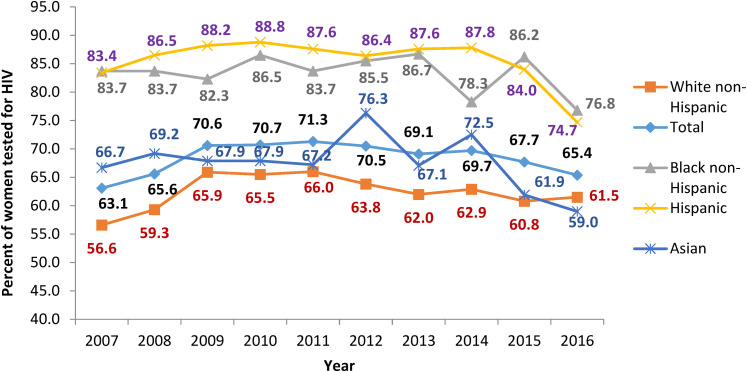
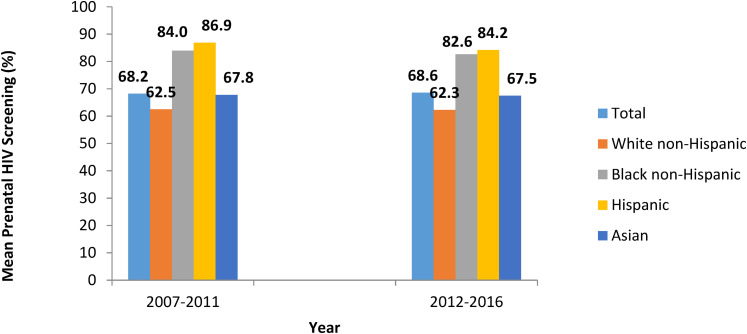
From 2007 to 2016, the overall trend of prenatal HIV screening did not change significantly (P = .27) (Figure 2). The prevalence of prenatal HIV screening from 2012 to 2016, following the amendment of MA's Law MGL 111 70F and subsequent clinical advisory, was not significantly different than the prevalence from 2007 to 2011 (Figure 3). This may indicate that the amendment was not impactful at increasing prenatal HIV screening, that communication of the amendment via the clinical advisory update did not reach its intended audience, or that policy change without a campaign to educate providers and encourage adoption may not be sufficient to change clinician behavior.
Figure 2.
Trends of prenatal HIV screening in Massachusetts (MA), PRAMS, 2007 to 2016.
Abbreviation: PRAMS, Pregnancy Risk Assessment Monitoring System.
Figure 3.
Prevalence of prenatal HIV screening in Massachusetts (MA) during 2007 to 2011 and 2012 to 2016 (post law change and clinical advisory update release), PRAMS.
Abbreviation: PRAMS, Pregnancy Risk Assessment Monitoring System.
Reasons for Refusing HIV Testing:
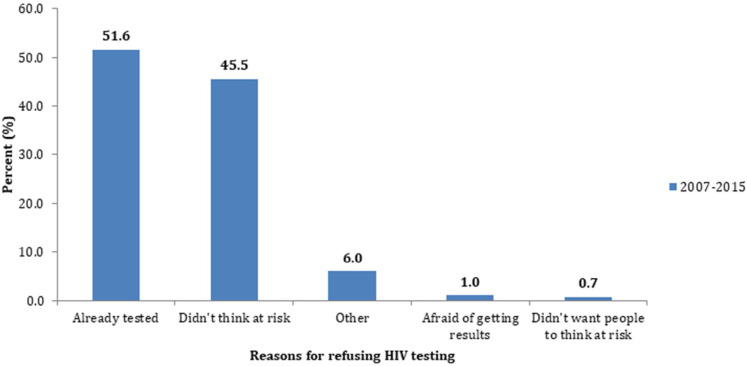
Data regarding why women refused prenatal HIV testing was only available from 2012 to 2015 due to a change to the PRAMS questionnaire (n = 1222). Of those respondents, 51.6% of women reported that they had already been tested, 45.5% did not think they were at risk, 6% reported “other reasons,” 1% reported that they were afraid of getting results, and <1% reported that they didn’t want others to think they were at risk (Figure 4). No further data were available regarding precisely when those who reported prior testing had last been tested for HIV.
Figure 4.
Reasons for refusing HIV testing, PRAMS, 2007 to 2015.
Abbreviation: PRAMS, Pregnancy Risk Assessment Monitoring System.
Discussion
This study examined the prevalence of prenatal HIV screening in MA from 2007 to 2016. Unlike prior studies, the study examines prenatal testing data both pre and post the 2012 law change and release of the subsequent clinical advisory update issued by the MA Department of Public Health.
The 2012 amendment to MA's Law MGL 111 70F and subsequent clinical advisory update did not coincide with an increase in the rates of HIV screening in women receiving prenatal care in MA. The average rate of screening between 2012 and 2016—after the law change—did not differ significantly from the rate during the pre-implementation phase of 2007 to 2011. However, over the entire study period, significant discrepancies in prenatal HIV screening were seen by race/ethnicity, with higher rates of Black NH and Hispanic women being tested when compared to their white counterparts. Additionally, rates of screening were higher in mothers that received WIC, were foreign born, were not married, and received prenatal care at a hospital clinic or community health center. These findings underscore the differences in HIV screening among pregnant women in MA, and while this trend reflects progress toward eliminating MTC transmission of HIV, our results highlight that a systematic gap in screening exists.
The substantially lower rates of prenatal screening in White women, particularly White women that receive their prenatal care in a private physician's office, highlights differences in testing practices. However, it is unclear whether White women in private care settings were more likely to refuse testing, or were not offered testing at all. The average rate of HIV offering between 2007 and 2016 was 79.1% (data are not shown). Among those who were offered an HIV test, the refusal rate between 2007 and 2015 was about 10.9% (the refusal rate was calculated based on 2007-2015 data due to a change to the PRAMS questionnaire beginning in 2016). The reasons underlying why some women refused to be screened also warrant further research. In the present study, most women refused prenatal HIV screening because they had already been tested (40.5%) or did not believe they were at risk (37.6%).
The high rates of non-testing during pregnancy runs counter to CDC and international recommendations for universal screening during this period to eliminate MTC transmission. Elimination of perinatal transmission requires universal application of available interventions to be effective. The inconsistent delivery of routine prenatal screening has the potential to result in some HIV-positive women missing the appropriate linkages to care, treatment, and education. Black individuals in the United States have historically had a greater annual incidence and prevalence rate of HIV infection, however, in MA, 23% of women living with HIV are White, 46% are Black NH, and 29% are Hispanic or Latina. 12 Thus, it is critical to screen all women for sexually transmitted infections, including HIV, during the prenatal period and beyond.
Therefore, a national goal to eliminate perinatal transmission should focus on screening all women regardless of risk profile. As Nesheim et al. explains a comprehensive universal testing strategy is necessary to further prevent any of the already relatively low numbers of cases. 5 Across states, the difference in the number of cases stems from the consistency of the testing/treatment protocol. The cost of treating a child born with HIV is more than $270,000 over their lifetime. 5 Thus, eliminating 100 cases would save nearly $30 million in health spending for perinatally infected individuals. 5
Strengths and Limitations
Strengths of the current study include a robust 10-year timeframe of PRAMS data, which includes an oversampling of racial/ethnic minorities. Additionally, the PRAMS data that was analyzed is state-specific and includes both pre and postclinical advisory information to substantiate the findings. However, the study also has limitations that include (a) selection for the study was limited to mothers of live-born infants, but the population of interest is all expectant mothers and (b) PRAMS is administered in English and Spanish only, therefore the experiences of women who use other languages may not be well described in this data set.
Additional limitations include recall bias, and whether women that participated in PRAMS were able to accurately recall whether they were offered or accepted HIV screening during their prenatal care visits or at delivery. As PRAMS is a standardized survey, it may not adequately capture whether HIV screening was offered in a way that considered the woman's health literacy and cultural background, which may influence if the patient understood that they were being offered an HIV test, and why they were being offered the test. Finally, it is impossible to discern how provider factors, such as the racial or cultural concordance with a patient might play a role as it pertains to the context of the screening conversation during the prenatal care visit. Future research on differential rates of HIV screening is warranted to examine the influence of provider factors on the context of the screening conversation and incorporate the role of women's voices in informing and updating HIV screening policies across the state.
Implications
The implications of our findings provide a reflexive rationale to the guidance issued by CDC which stated the importance of universal prenatal HIV screening. With only 68.3% of women reporting that they received prenatal HIV testing during their most recent pregnancy, it is likely that MA has not yet achieved routine, universal prenatal testing. The inconsistent delivery of routine prenatal testing across public and private clinical settings creates the potential to result in some HIV-positive women missing the appropriate linkages to care, treatment, and education. Despite Black NH women historically having a greater risk of HIV infection, in MA, 51% of women living with HIV are Hispanic or Latina and White NH. 12 Thus, it is critical to obtain informed verbal consent and consistently test all women for sexually transmitted infections, including HIV, during the prenatal period and beyond.
Conclusion
MA has not yet reached the goal of universal prenatal HIV screening with 68.3% of women reporting that they received prenatal HIV screening during their most recent pregnancy from 2007 to 2016. Black NH and Hispanic women were more likely to receive prenatal HIV screening than White NH women. Our findings suggest that race and socioeconomic status may influence the likelihood that pregnant women receive HIV testing as part of their prenatal care. Further research is required to assess whether patients are actively refusing HIV testing, or if testing was not offered.
Acknowledgments
The authors would like to acknowledge Ms Marina Magicheva, Dr Hafsatou Diop, and H. Dawn Fukuda, ScM, Director of the Office of HIV/AIDS, Bureau of Infectious Disease and Laboratory Sciences, Massachusetts Department of Public Health for their content review of the manuscript.
Footnotes
Declaration of Conflicting Interests: The authors received no financial support for the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ndidiamaka Amutah-Onukagha https://orcid.org/0000-0003-1194-9927
Tonia J. Rhone https://orcid.org/0000-0001-5420-2220
References
- 1.Branson B. M., Handsfield H. H., Lampe M. Aet al. (2006). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morbidity and Mortality Weekly Report: Recommendations and Reports, 55(RR14):1–17. Retrieved from Google Scholar. [PubMed] [Google Scholar]
- 2.Andrews M, Storm D, Burr Cet al. et al. Perinatal HIV service coordination: closing gaps in the HIV care Continuum for pregnant women and eliminating perinatal HIV transmission in the United States. Public Health Rep. 2018;133(5):532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach J., Smith L. A. (n.d.). Clinical Advisory Update: Routine HIV Screening of all Pregnant Women in Massachusetts. February, 2012. [Google Scholar]
- 4.Nesheim S, Taylor A, Lampe Met al. et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012;130(4):738. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016; vol. 28. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2017. Accessed April 2019. [Google Scholar]
- 6.Nesheim S., Wiener J., Fitz Harris L., Lampe M., Weidle P. (2017). Brief report: estimated incidence of perinatally acquired HIV infection in the United States, 1978–2013. J Acquir Immune Defic Syndr. (1999), 76(5), 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong, Garcia, Olszewski, Statton, Bryant Borders, Grobman, & Cohen. (2012). Perinatal HIV testing and diagnosis in illinois after implementation of the perinatal rapid testing initiative. Am J Obstet Gynecol. 207(5), 401.e1–401.e6. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen-Saines K., Watts D. H., Veloso V. G., et al. NICHD HPTN 040/PACTG 1043 protocol team (2012). Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 366(25), 2368–2379. 10.1056/NEJMoa1108275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massachusetts Department of Public Health, HIV/AIDS Surveillance Program, Data as of 01/01/18.
- 10.Kennedy MR, Meyn LA, Reeves MF, Wiesenfeld HC. Universal prenatal HIV screening: are we there yet? Int J STD AIDS. 2011;22(4):194–198. doi: 10.1258/ijsa.2011.010200 [DOI] [PubMed] [Google Scholar]
- 11.Wocial L., Cox E. (2013). An ethical analysis of Opt-Out HIV screening for pregnant women. Journal of Obstetric, Gynecologic, & Neonatal Nursing, 42(4), 485–491. [DOI] [PubMed] [Google Scholar]
- 12.Massachusetts Department of Public Health, Bureau of Infectious Disease and Laboratory Sciences. 2018. Massachusetts HIV/AIDS Epidemiologic Profile, The Massachusetts HIV/AIDS Epidemic at a Glance. Published September 2018. Accessed April 2019.