Abstract
Purpose
While the incidence of Barrett’s neoplasia has been increasing in Western countries, the disease remains rare in Asian countries. Therefore, very few studies have investigated the endoscopic treatment for Barrett’s neoplasia in Korea. Endoscopic submucosal dissection (ESD) enables en bloc and complete resection of gastrointestinal neoplastic lesions. This study aimed to evaluate the therapeutic outcomes of ESD for Barrett’s neoplasia in a single center in Korea and to examine the predictive factors for incomplete resection.
Materials and Methods
We conducted a retrospective observational study of 18 patients who underwent ESD for superficial Barrett’s neoplasia (dysplasia and early cancer) between January 2010 and December 2019 at Pusan National University Hospital. The therapeutic outcomes of ESD and procedure-related complications were analyzed.
Results
En bloc resection, complete resection, and curative resection were performed in 94%, 72%, and 61% of patients, respectively. Histopathology (submucosal or deeper invasion of the tumor) was a significant predictive factor for incomplete resection (P=0.047). Procedure-related bleeding and stenosis were not observed, whereas perforation occurred in one case. During the median follow-up period of 12 months (range, 6–74 months), local recurrence occurred in 2 patients with incomplete resection, one patient underwent repeat ESD, and the other patient received concurrent chemoradiotherapy. The 3-year overall and disease-specific survival rates were 73% and 93%, respectively.
Conclusions
ESD seems to be an effective and safe treatment for superficial Barrett’s neoplasia in Korea. Nevertheless, the suitability of ESD for Barrett’s cancer cases should be determined considering the high risk of deep submucosal invasion.
Keywords: Barrett’s esophagus, Neoplasm, Endoscopic submucosal dissection, Adenocarcinoma
INTRODUCTION
Barrett’s esophagus (BE) is a premalignant condition caused by gastroesophageal reflux disease, with the risk of progression to esophageal adenocarcinoma ranging from 0.07% to 0.8% [1,2]. Recently, the incidence of Barrett’s neoplasia has increased considerably in Western countries [3,4]; however, this condition remains rare in Asian countries [5]. Although relevant data for Asian countries are scarce, Barrett’s neoplasia is expected to increase in Korea because of the westernization of lifestyle, including diet, and the decreased prevalence of Helicobacter pylori infection [6]. Endoscopic resection is the preferred treatment modality in patients with early-stage Barrett’s neoplasia (high-grade dysplasia and adenocarcinoma limited to the mucosa) [7,8]. In Western countries, endoscopic mucosal resection (EMR) for visible lesions combined with radiofrequency ablation for residual Barrett’s epithelium is the treatment of choice for Barrett’s neoplasia [7,9]. However, piecemeal lesion resection by EMR may overlook important histopathological findings and is, thus, a risk factor for local recurrence [10]. In addition, subsquamous adenocarcinoma frequently recurs [11].
Endoscopic submucosal dissection (ESD) is a widely accepted therapeutic modality for neoplastic lesions in the gastrointestinal tract and increases the en bloc and complete resection rates. ESD is also known to be effective for superficial esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma [12,13]. Recently, therapeutic outcomes of ESD for superficial Barrett’s neoplasia have been reported, mainly in Western countries and Japan [14,15]. In Korea, ESD is the preferred treatment modality for complete tumor resection; however, reports on ESD outcomes for Barrett’s neoplasia are limited. Therefore, the aim of the present study was to evaluate the therapeutic outcomes of ESD for superficial Barrett’s neoplasia and to examine the predictive factors for incomplete resection.
MATERIALS AND METHODS
Patients
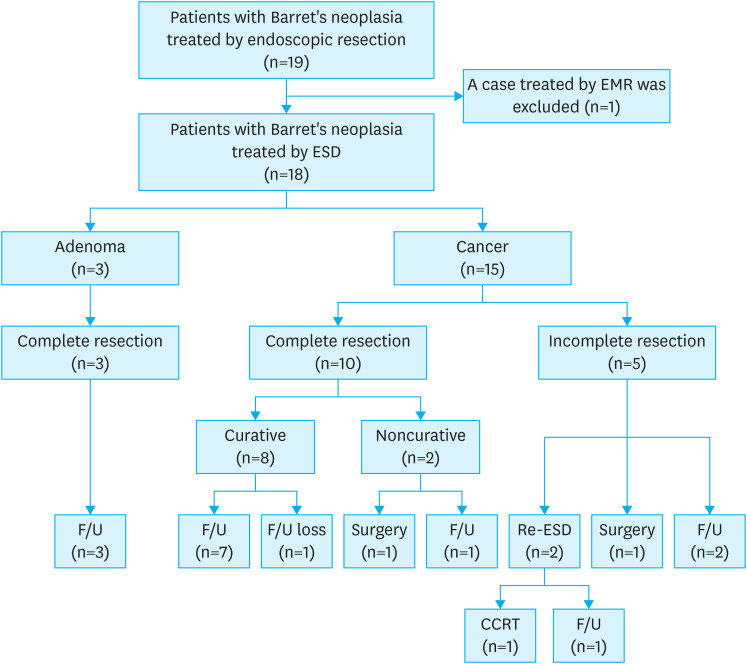
We retrospectively investigated the data of all patients who underwent endoscopic resection for esophageal and gastric neoplasia at Pusan National University Hospital (Busan, Korea) between January 2010 and December 2019. Nineteen patients with Barrett’s neoplasia (dysplasia and early cancer) underwent endoscopic resection. Among the 19 patients, one patient had undergone EMR and was excluded from the study. Hence, 18 patients who underwent ESD for Barrett’s neoplasia were included in the analysis (Fig. 1). All patients with Barrett’s cancer underwent chest and abdominal computed tomography (CT) before ESD to examine lymph node status or distant metastases. Endoscopic ultrasonography (EUS) was also performed to exclude submucosal invasion in selected cancer cases. Before the procedure, all patients consented to undergo ESD after explanation of the benefits and risks, including ESD-related complications and the possible need for additional surgical treatment. Written informed consent was obtained from all the patients prior to the procedure. This retrospective study protocol was reviewed and approved by the Institutional Review Board of Pusan National University Hospital (H-2005-017-091).
Fig. 1. Flowchart of the patients included in the study.
EMR = endoscopic mucosal resection; ESD = endoscopic submucosal dissection; F/U = follow-up; CCRT = concurrent chemoradiotherapy.
Diagnosis of BE and neoplasia
The esophagogastric junction was defined as the proximal end of the gastric folds in the absence of hiatal hernia or the distal end of the esophageal palisade vessels in the presence of hiatal hernia [16]. The endoscopic diagnosis of BE was established during the observation of the columnar epithelium on the proximal side of the esophagogastric junction [17]. BE was subdivided as follows: long-segment BE (LSBE; maximum length ≥3 cm) and short-segment BE (SSBE; length <3 cm) [7]. Barrett’s neoplasia was defined as dysplasia or adenocarcinoma that was endoscopically confirmed in the BE or pathologically confirmed in the resected specimen by the presence of esophageal submucosal glands, squamous islands, or a double layer of the muscularis mucosae within the lesion or on the anal side [17].
Assessment of tumor location and macroscopic shape
The tumor location was classified based on the hemispheric direction. In the forward view, a clock-face orientation of the endoscope (with the lesser curvature of the stomach in contiguity with the 12 o’clock position of the esophagogastric junction) was used for hemispheric direction: the left hemisphere was from 6 to 12 o’clock; right hemisphere, from 12 to 6 o’clock. The macroscopic shape of lesions was classified according to the Paris endoscopic classification of superficial neoplastic lesions as follows [18]: type I (protruding), type IIa (slightly elevated), type IIb (flat), type IIc (slightly depressed), and type III (excavated). Moreover, all lesions were further classified into 3 types: elevated (I, IIa), flat (IIb), and depressed (IIc, III).
Other endoscopic assessment
The presence or absence of reflux esophagitis, hiatal hernia, and atrophic gastritis was evaluated. Reflux esophagitis was graded using the Los Angeles classification [19]. Hiatal hernia was defined as a circular extension of the gastric mucosa above the diaphragmatic hiatus with an axial length of >2 cm [20]. Atrophic gastritis was evaluated using the Kimura-Takemoto classification [21]; the extent of atrophy was classified into 2 types: closed type (the atrophic border was present on the lesser curvature of the stomach) and open type (the atrophic border was no longer limited to the lesser curvature but extended into the anterior and posterior walls of the stomach). Gastric corpus and antrum biopsy samples were obtained for the evaluation of H. pylori infection using the rapid urease test.
ESD
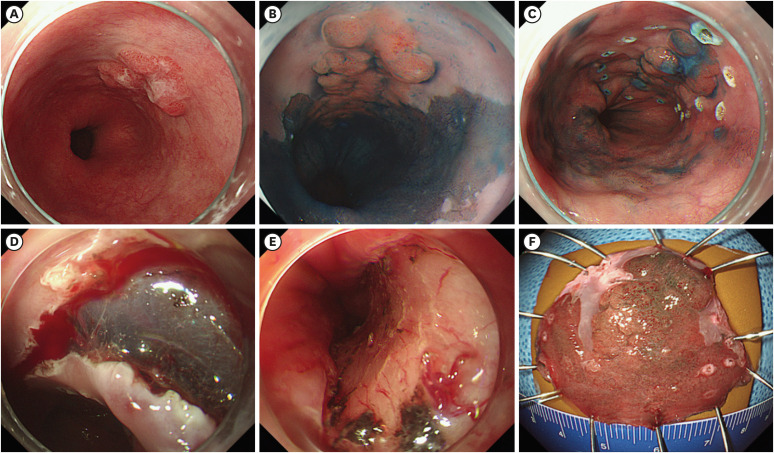
Three experienced endoscopists (K.G.H., L.B.E., and S.G.A.) performed the ESD procedures using a single-channel endoscope (GIF-Q260 or GIF-H260; Olympus Co., Ltd., Tokyo, Japan). A representative case is shown in Fig. 2. The procedures were performed under conscious sedation and cardiorespiratory monitoring. Midazolam (5–10 mg) and meperidine (25 mg) were administered intravenously for sedation, and propofol was administered as needed during the procedure. Argon plasma coagulation was used to mark the borders of the lesion, which could be detected by conventional endoscopy or indigo carmine chromoendoscopy. Subsequently, a saline solution (0.9% saline mixed with a small amount of epinephrine and indigo carmine) was injected submucosally around the lesion. A circumferential mucosal incision was made outside the marking dots with a dual knife (Olympus Co., Ltd.) and/or an IT knife (Olympus Co., Ltd.). Thereafter, submucosal dissection was performed using the knives to completely resect the lesion. An electrosurgical system (Erbotom VIO 300D; ERBE, Tübingen, Germany) was used during marking, mucosal incision, submucosal dissection, and hemostasis. After resection of the lesions, preventive coagulation was performed for exposed vessels with the Coagrasper or hot biopsy forceps.
Fig. 2. Endoscopic submucosal dissection procedure. (A) A nodular, elevated lesion is observed at the long-segment Barrett’s esophagus. (B) After chromoendoscopy with indigo carmine, the lesion becomes more clearly visible. (C) Circumferential marking is performed around the tumor using argon plasma coagulation. (D) Mucosal incision and submucosal dissection are performed using a Dual knife and an IT knife. (E) The lesion is completely removed. (F) Resected specimen.
All patients underwent post-procedural chest radiography on the day of the procedure to check for perforation or aspiration pneumonia. Proton pump inhibitors and sucralfate were administered to promote ulcer healing, prevent procedure-related bleeding, and relieve pain. Patients without complications or serious symptoms were allowed to start food intake on the day after the procedure and were discharged within 3–4 days.
Histopathologic evaluation of resected specimens
Resected specimens were fixed in formalin and serially sectioned at 2-mm intervals to assess tumor involvement in the horizontal and vertical margins. The presence of intestinal metaplasia, tumor size, degree of differentiation, depth of invasion, and lymphovascular invasion were evaluated microscopically according to the Vienna classification [22] and the Japanese classification of esophageal cancer [17]. Histopathological diagnosis was made by 2 pathologists specializing in gastroenterology (L.S. and P.D.Y.).
Therapeutic outcomes of ESD
The primary outcome measure was the success of ESD, that is, en bloc resection, complete resection, and curative resection rates. The secondary outcome measures were procedure time, procedure-related complications, and the local recurrence rate. En bloc resection was defined as resection in a single piece and complete resection as a successful en bloc resection with the horizontal and vertical margins histopathologically free of tumors. Curative resection was defined as a complete resection that fulfilled the following histopathologic criteria: negative horizontal margin, negative vertical margin, absent lymphovascular invasion, and depth of submucosal invasion ≤500 μm [23,24]. Procedure time was defined as the time from tumor margin marking to lesion resection completion, and procedure-related bleeding, as bleeding after ESD requiring endoscopic re-evaluation or blood transfusion. Perforation was diagnosed endoscopically during the ESD procedure or by the presence of free air on chest radiography after the procedure. Procedure-related stenosis was confirmed when a standard 9- to 10-mm-diameter endoscope could not pass through the esophagogastric junction.
Follow-up
When curative resection was achieved, follow-up endoscopy was performed 6 months after ESD and annually thereafter. Moreover, in cancer cases with curative resection, chest and abdominal CT and measurements of blood tumor markers were performed 6 months after ESD and annually thereafter. In cancer cases with non-curative resection, such as those with deep submucosal invasion, a positive vertical margin, or lymphovascular invasion, an additional esophagectomy with lymph node dissection is strongly recommended for curative resection. In cancer cases with non-curative resection due to a positive horizontal margin, additional ESD was recommended; however, when patients refused additional surgical intervention, follow-up endoscopy with biopsies and chest and abdominal CT was performed 1–2 months and 4–6 months after ESD.
Data for long-term analysis (recurrence and survival rates) were collected from electronic medical records and calculated from the date of index ESD. Overall survival included deaths from any cause, whereas disease-specific survival included deaths from Barrett’s cancer; patients who died of intercurrent diseases were considered “withdrawn alive.” Follow-up data on recurrence and death were obtained until March 2020.
Statistical analysis
Variables are expressed as median (range) and simple proportions. Factors affecting incomplete resection after ESD were assessed using the χ2 test or Fisher’s exact test. The Kaplan–Meier method was used to estimate long-term survival outcomes. A P-value <0.05 was considered statistically significant, and statistical analyses were performed with SPSS version 25.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
The clinicopathological characteristics of the 18 patients with superficial Barrett’s neoplasia are summarized in Table 1. There were 15 men and 3 women with a median age of 60 years (range, 37–79 years). The body mass index was <23 kg/m2 in 9 patients, 23–25 kg/m2 in 5, and >25 kg/m2 in 4, with a median body mass index of 23.0 kg/m2 (range, 17.3–28.7 kg/m2). The hemispheric direction was left in 3 patients and right in 15 patients. Macroscopically, 15 lesions were elevated, and 3 were depressed. The tumor size was ≤10 mm in 4 lesions, 11–20 mm in 11, and >20 mm in 3, with a median size of 16 mm (range, 5–35 mm). Reflux esophagitis and hiatal hernia were observed in 3 patients and 12 patients, respectively. Sixteen patients had SSBE, and 2 had LSBE. H. pylori infection was present in 3 patients, and open-type atrophic gastritis was found in 2 patients. Histopathologic diagnoses of the lesions were dysplasia in 3 lesions and cancer in 15.
Table 1. Clinicopathologic characteristics of patients with superficial Barrett’s neoplasia.
| Characteristics | Value | ||
|---|---|---|---|
| Median age (range, yr) | 60 (37–79) | ||
| Sex | |||
| Male | 15 (83) | ||
| Female | 3 (17) | ||
| Body mass index (kg/m2)* | |||
| <23 | 9 (50) | ||
| 23–25 | 5 (22) | ||
| ≥25 | 4 (28) | ||
| Hemispheric direction | |||
| Right | |||
| 12 to 3 o’clock | 12 (67) | ||
| 3 to 6 o’clock | 3 (17) | ||
| Left | |||
| 6 to 9 o’clock | 0 (0) | ||
| 9 to 12 o’clock | 3 (17) | ||
| Macroscopic morphology | |||
| Elevated | 15 (83) | ||
| Depressed | 3 (17) | ||
| Tumor size (mm)† | |||
| ≤10 | 4 (22) | ||
| 11–20 | 11 (61) | ||
| >20 | 3 (17) | ||
| Reflux esophagitis | |||
| Absent | 15 (83) | ||
| Present | 3 (17) | ||
| Hiatal hernia | |||
| Absent | 6 (33) | ||
| Present | 12 (67) | ||
| Barrett’s esophagus | |||
| Short segment (<3 cm) | 16 (89) | ||
| Long segment (≥3 cm) | 2 (11) | ||
| Helicobacter pylori infection | |||
| Absent | 15 (83) | ||
| Present | 3 (17) | ||
| Atrophic gastritis | |||
| Closed type | 16 (89) | ||
| Open type | 2 (11) | ||
| Histopathology | |||
| Dysplasia | |||
| Low grade | 1 (6) | ||
| High grade | 2 (11) | ||
| Adenocarcinoma‡ | |||
| Mucosal cancer | 8 (44) | ||
| Submucosal cancer | 6 (33) | ||
| Advanced cancer | 1 (6) | ||
Data are expressed as number (%).
*The median body mass index was 23.0 kg/m2 (range, 17.3–28.7 kg/m2).
†The median tumor size was 16 mm (range, 5–35 mm).
‡Eight tumors were well-differentiated, 6 were moderately differentiated, and one was mixed with moderately differentiated and poorly differentiated lesions.
Outcomes of ESD
The therapeutic outcomes of ESD for BE are presented in Table 2. The en bloc resection rate was 94% (17/18). Piecemeal resection occurred in one patient with cancer, and the histopathologic result showed horizontal involvement with tumor cells. Of the 17 en bloc resected lesions, 4 had positive margins (horizontal involvement with tumor cells in 2, vertical involvement with tumor cells in 1, and both horizontal and vertical involvement with tumor cells in 1). Thus, the complete resection rate was 72% (13/18). Two of the 13 completely resected tumors had deep submucosal invasion (>500 µm from the muscularis mucosa). Accordingly, the curative resection rate was 61% (11/18). The median procedure time was 38 minutes (range, 14–84 minutes).
Table 2. Therapeutic outcomes of endoscopic submucosal dissection.
| Characteristics | Value | |
|---|---|---|
| En bloc resection | 17 (94) | |
| Complete resection | 13 (72) | |
| Curative resection* | 11 (61) | |
| Causes of incomplete resection | 5 (28) | |
| Horizontal involvement | 4 | |
| Vertical involvement† | 2 | |
| Median procedure time (range, min) | 38 (14–84) | |
| Procedure-related complications | ||
| Bleeding | 0 (0) | |
| Perforation | 1 (6) | |
| Stenosis | 0 (0) | |
| Local recurrence‡ | 2 (13) | |
| Distant metastasis | 0 (0) | |
| Median follow-up after procedure (range, mon) | 12 (6–74) | |
Data are expressed as number (%).
*Two completely resected cancers had deep submucosal invasion (>500 µm from the muscularis mucosa).
†Horizontal and vertical involvement and lymphovascular invasion were observed in one case.
‡Three patients were excluded because of loss to follow-up (n=1) or surgical resection (n=2).
Of the 15 cases of Barrett’s cancer, 8 (53%) had mucosal cancers, 6 (40%) had submucosal cancers, and 1 (7%) had advanced cancer infiltrating the proper muscle layer. Of the 6 submucosal cancer cases, 5 had deep submucosal invasion. Based on the analysis of the therapeutic outcomes of ESD in 15 cases of Barrett’s cancers, the en bloc resection, complete resection, and curative resection rates were 93% (14/15), 67% (10/15), and 53% (8/15), respectively.
Table 3 shows the factors associated with incomplete resection. Histopathology was significantly associated with incomplete resections. The incomplete resection rate for lesions containing submucosal or deeper cancer was significantly higher than that for lesions containing mucosal cancer or dysplasia (57% [4/7] vs. 9% [1/11], P=0.047). Hemispheric direction, macroscopic shape, tumor size, hiatal hernia, and BE length were not associated with incomplete resection.
Table 3. Factors for incomplete resection after endoscopic submucosal dissection.
| Factors | Complete resection (n=13) | Incomplete resection (n=5) | P-value | |
|---|---|---|---|---|
| Hemispheric direction | 0.172 | |||
| Left | 1 (33) | 2 (67) | ||
| Right | 12 (80) | 3 (20) | ||
| Macroscopic morphology | 1.000 | |||
| Elevated | 11 (73) | 4 (27) | ||
| Non-elevated | 2 (67) | 1 (33) | ||
| Tumor size (mm) | 1.000 | |||
| ≤20 | 11 (73) | 4 (27) | ||
| >20 | 2 (67) | 1 (33) | ||
| Hiatal hernia | 1.000 | |||
| Absent | 4 (67) | 2 (33) | ||
| Present | 9 (75) | 3 (25) | ||
| Barrett’s esophagus | 0.575 | |||
| Short segment (<3 cm) | 11 (69) | 5 (31) | ||
| Long segment (≥3 cm) | 2 (100) | 0 (0) | ||
| Histopathology | 0.047 | |||
| Dysplasia/Mucosal cancer | 10 (91) | 1 (9) | ||
| Submucosal or deeper cancer | 3 (43) | 4 (57) | ||
Data are expressed as number (%).
No procedure-related bleeding or stenosis was observed (Table 2). However, perforation occurred in 1 patient (6%) with severe alcoholic hepatitis and was treated successfully with antibiotics and restricted oral intake after clipping during the ESD procedure.
Local recurrence
All 3 dysplasias were completely resected. Of the 7 non-curative cancer lesions, 5 were deep submucosal cancers, one was advanced cancer, and one was mucosal cancer (Table 4). Additional surgical resection was recommended in 6 patients with deep submucosal or advanced cancer. However, 3 patients did not undergo additional esophagectomy with lymph node dissection because of poor performance status, advanced age, or refusal to undergo further surgery. The final histopathology in 2 patients who underwent additional surgery showed no residual tumor or lymph node metastasis.
Table 4. Clinicopathologic characteristics of Barrett’s cancers non-curatively resected by ESD.
| Patient No. | Sex/Age | Macroscopic morphology | Tumor size (mm) | Invasion depth of tumor | Horizonal involvement | Vertical involvement | Lymphovascular invasion | Additional treatment | Recurrence | Follow-up period (mon) | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/69 | IIa | 11 | MM | + | - | - | Re-ESD | No | 78 | Yes |
| 2 | M/60 | I | 17 | SM2 | - | - | - | Esophagectomy | NA | 16 | Yes |
| 3 | M/43 | IIa+IIc | 15 | SM2 | + | - | - | Follow-up loss | NA | 107 | Yes |
| 4 | M/75 | IIc | 18 | SM2 | - | + | - | No | No | 48 | Yes |
| 5 | M/79 | IIa | 22 | SM2 | - | - | - | No | No | 6 | Yes |
| 6 | M/74 | IIa+IIc | 21 | SM2 | + | - | + | Re-ESD → CCRT | Yes | 8 | No |
| 7 | M/66 | IIa | 20 | MP | + | - | - | Esophagectomy | NA | 97 | Yes |
MM = muscularis mucosa; ESD = endoscopic submucosal dissection; SM2 = deep submucosa; NA = not applicable; CCRT = concurrent chemoradiation; MP = muscularis propria.
Fifteen of the 18 patients who underwent ESD were followed up for >6 months (Fig. 1). During the median follow-up period of 12 months (range, 6–74 months), recurrences occurred in 2 patients. The lesion recurred 1 month after ESD in one patient who previously had an incompletely resected mucosal cancer and 5 months after ESD in one patient who previously had incompletely resected deep submucosal cancer with lymphovascular invasion. The former was successfully treated with additional ESD, and no recurrence was noted. The latter received concurrent chemoradiotherapy, but died of pneumonia after 3 months.
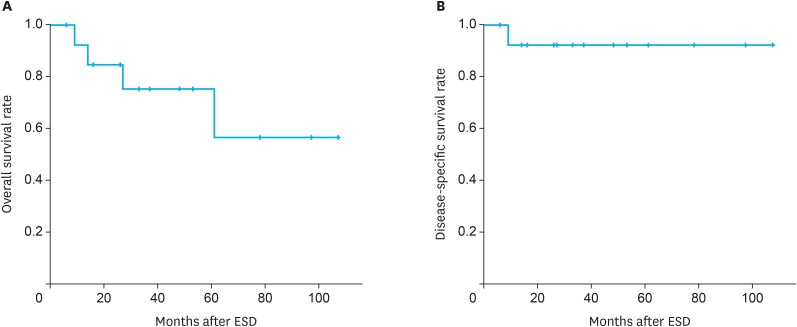
All 15 patients who underwent ESD were eligible for the analysis of survival status, cause of death, and date of death. During the median observation period of 33 months (range, 6–107 months), 3 patients died from causes other than esophageal cancer (i.e., alcoholic hepatitis and cardiac causes), and one patient died from pneumonia occurring after chemoradiotherapy for recurrent cancer. The 3-year overall and disease-specific survival rates were 73% and 93%, respectively (Fig. 3).
Fig. 3. Survival rates of patients who underwent ESD for superficial Barrett’s cancer. (A) Overall survival rate. (B) Disease-specific survival rate.
ESD = endoscopic submucosal dissection.
DISCUSSION
Our data demonstrated the technical feasibility and safety of ESD for superficial Barrett’s neoplasia: en bloc resection, complete resection, and curative resection rates were acceptable (94%, 72%, and 61%, respectively), and procedure-related complications occurred in only one patient. Complete tumor resection by ESD is influenced by histopathology (mainly by tumor invasion depth). These results provide endoscopists with useful information for assessing the technical feasibility of ESD for Barrett’s neoplasia before the procedure.
In this study, Barrett’s neoplasia was predominant in men, and the incidence of hiatal hernia, reflux esophagitis, and atrophic gastritis (open type) in patients with Barrett’s neoplasia was 67%, 17%, and 11%, respectively, which is consistent with the results of a previous study in Japan [25]. In most cases, Barrett’s neoplasia was located in the right hemisphere, which is consistent with the data of previous studies in Western countries and Asia [25,26,27]. Regarding the macroscopic shape of Barrett’s neoplasia, elevated lesions are common in SSBE and flat/depressed lesions in LSBE [25,28]. In our study, SSBE was predominant; thus, almost all lesions had an elevated morphology.
Barrett’s neoplasia resection using ESD is difficult because of the anatomical characteristics of the esophagogastric junction, including a narrow lumen, fibrosis, poor accessibility, movability of the lesion during respiration, and esophageal peristalsis [13]. These factors could affect ESD outcomes, such as en bloc resection, complete resection rates, and procedure-related complications. However, studies on the outcomes of ESD for Barrett’s neoplasia are relatively scarce compared with those on other organs, such as the stomach and colon. Barrett’s neoplasia has been treated with EMR, radiofrequency ablation, cryotherapy, or surgery, and data on the therapeutic outcomes are mainly from Western countries. Thus, based on the aforementioned information, we investigated several factors affecting the therapeutic outcomes of ESD in 18 patients with BE. The en bloc, complete, and curative resection rates in our study were similar to those reported in previous studies [14,15,25,26,29]. Generally, the complete resection rate of ESD for Barrett’s neoplasia is lower than that (85%–92%) for esophageal squamous epithelial neoplasia [12,30,31]. This finding could be explained by the aforementioned anatomical characteristics of the esophagogastric junction, difficulty in predicting the margins, and tumor invasion with conventional endoscopy due to coexistent reflux or inflammation, and the high frequency of deep submucosal invasion in Barrett’s cancer [13,32,33]. Moreover, in our study, among 15 cancer cases, complete resection and curative resection rates were 67% and 53%, respectively. EUS was performed in 5 selected cases prior to ESD, of which 2 had submucosal invasion. However, in EUS, maintaining adequate water filling is difficult when the tumor is located near the esophagogastric junction, which in turn affects the ultrasound transducer perpendicular to the lesion. This results in poor visualization and pseudo-thickening of the esophageal wall layers [34,35]. In addition, a recent meta-analysis showed that the sensitivity and specificity of EUS in predicting submucosal or deeper cancers were 56% and 89%, respectively [36]. These limitations may have resulted in an underestimation of the invasion depth. In our study, the frequency of submucosal or deeper invasion was 47% (7/15).
Furthermore, we attempted to determine predictive factors for incomplete resection. Hemispheric direction, macroscopic shape, tumor size, hiatal hernia, and BE length were not associated with incomplete resection. The complete resection rate significantly decreased in submucosal or deeper cancer (P=0.047), which is consistent with the results of previous studies showing that the invasion depth of the tumor (i.e., presence of submucosal cancer relative to absence) is an independent factor for incomplete resection [26,28].
In our study, procedure-related bleeding and stenosis were not observed, whereas perforation occurred in 1 patient (6%), which is similar to previous studies [14,25,33]. The perforation was managed successfully with endoscopic clipping and not with surgery. A risk factor for stenosis after ESD in esophageal neoplasia is circumferential involvement of >3/4 of the whole lumen [14]. A circumferential mucosal defect of >3/4 was not observed in any of our cases. These results suggest that ESD is a technically safe therapeutic modality for BE.
Recent long-term follow-up studies showed that the local recurrence rate after EMR for Barrett’s cancer was 6%–15%, whereas no local recurrence was observed in patients who achieved curative resection with ESD [37,38,39]. In our study, local recurrence occurred in 2 cases with incomplete resection. In LSBE, which is more frequently observed in Western countries, metachronous neoplasia may develop from residual BE; however, in Asian countries, including Korea and Japan, most BE cases are SSBE [27,40]. A recent study in Japan reported a 5-year cumulative incidence of metachronous cancer of 1.1% [38]. Therefore, the incidence of metachronous Barrett’s neoplasia in Asian countries is lower than that in Western countries (9%–27%) [10,14]. This suggests that local tumor resection by ESD could be a useful treatment modality for Barrett’s neoplasia in Asian countries. In our study, metachronous neoplasia was not observed in any case; however, the follow-up period was short.
ESD for Barrett’s cancer showed favorable long-term outcomes. Although the overall survival rate was only 73%, the disease-free survival rate was high (93%). The patient with Barrett’s cancer who died had non-curative resection, and the cause of death was pneumonia as a chemoradiotherapy-related adverse event. The survival rate in our study was consistent with that of previous studies on ESD for Barrett’s cancer [25,38]. Given the higher comorbidity of esophagectomy in patients with Barrett’s cancer, ESD is a safe, non-invasive treatment option for Barrett’s cancer, especially in elderly patients.
This study has several limitations. First, this was a single-center retrospective study, and thus is subject to biases inherent in retrospective observational studies. Second, patient selection for ESD was based on the clinical judgment of endoscopists at the time of treatment, with consideration of the patients’ needs. Finally, the number of patients was relatively small, and the follow-up period was short. In addition, the outcomes of ESD for BE were not excellent compared with the results of a recent study [38]. This could be explained by the fact that most endoscopists lack knowledge and experience in the diagnosis and treatment of Barrett’s neoplasia in Korea, and the subsequent results that the proportion of submucosal cancer is relatively high compared to that of dysplasia and mucosal cancer. Thus, given the increased interest in Barrett’s neoplasia, similar to early gastric cancer, further large-scale, prospective, multi-center studies with a long follow-up period are needed to elucidate the outcomes of ESD for Barrett’s neoplasia.
In summary, this is the first series of ESD for superficial Barrett’s neoplasia in Korea, where the incidence of Barrett’s neoplasia is extremely low. Our results demonstrated a high rate of complete resection and a low rate of procedure-related complications. Although technically challenging, ESD may be a safe and effective endoscopic treatment for patients with Barrett’s neoplasia. Nevertheless, ESD should be carefully applied in Barrett’s cancer cases wherein mucosal invasion is suspected during pre-treatment workup because of the high frequency of deep submucosal invasion.
Footnotes
Funding: This study was supported by the Medical Research Center Program through a National Research Foundation Grant funded by the Korean Government (NRF-2015R1A5A2009656).
- Conceptualization: K.G.H.
- Data curation: J.D.C., L.B.E., L.M.W.
- Formal analysis: J.D.C., K.G.H., L.S., P.D.Y.
- Methodology: K.G.H., B.D.H.
- Supervision: S.G.A.
- Validation: B.D.H.
- Writing - original draft: J.D.C. and K.G.H.
- Writing - review & editing: J.D.C., K.G.H., L.B.E., L.M.W., B.D.H., S.G.A., L.S., P.D.Y.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 2.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645–654. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 4.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99:860–868. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 5.Goda K, Singh R, Oda I, Omae M, Takahashi A, Koike T, et al. Current status of endoscopic diagnosis and treatment of superficial Barrett’s adenocarcinoma in Asia-Pacific region. Dig Endosc. 2013;25(Suppl 2):146–150. doi: 10.1111/den.12093. [DOI] [PubMed] [Google Scholar]
- 6.Wu JC. Gastroesophageal reflux disease: an Asian perspective. J Gastroenterol Hepatol. 2008;23:1785–1793. doi: 10.1111/j.1440-1746.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Falk GW, Iyer PG, Gerson LB American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 9.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 10.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 11.Titi M, Overhiser A, Ulusarac O, Falk GW, Chak A, Wang K, et al. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett's esophagus. Gastroenterology. 2012;143:564–566.e1. doi: 10.1053/j.gastro.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo DC, Kim GH, Park DY, Jhi JH, Song GA. Long-term outcome after endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma: a single-center study. Gut Liver. 2014;8:612–618. doi: 10.5009/gnl13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JK, Kim GH, Lee BE, Park CH, Jeon HK, Baek DH, et al. Endoscopic submucosal dissection for esophagogastric junction tumors: a single-center experience. Surg Endosc. 2018;32:760–769. doi: 10.1007/s00464-017-5735-2. [DOI] [PubMed] [Google Scholar]
- 14.Chevaux JB, Piessevaux H, Jouret-Mourin A, Yeung R, Danse E, Deprez PH. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett’s neoplasia. Endoscopy. 2015;47:103–112. doi: 10.1055/s-0034-1390982. [DOI] [PubMed] [Google Scholar]
- 15.Kagemoto K, Oka S, Tanaka S, Miwata T, Urabe Y, Sanomura Y, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc. 2014;80:239–245. doi: 10.1016/j.gie.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–330. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14:1–36. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–SS43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 19.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong JH, Kim GH, Lee BE, Choi MK, Moon JY, Ryu DY, et al. Endoscopic grading of gastroesophageal flap valve helps predict proton pump inhibitor response in patients with gastroesophageal reflux disease. Scand J Gastroenterol. 2011;46:789–796. doi: 10.3109/00365521.2011.579154. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Satoh K, Ido K, Taniguchi Y, Takimoto T, Takemoto T. Gastritis in the Japanese stomach. Scand J Gastroenterol Suppl. 1996;214:17–20. doi: 10.3109/00365529609094509. [DOI] [PubMed] [Google Scholar]
- 22.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manner H, Pech O, Heldmann Y, May A, Pauthner M, Lorenz D, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc. 2015;29:1888–1896. doi: 10.1007/s00464-014-3881-3. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, et al. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2017;52:800–808. doi: 10.1007/s00535-016-1275-0. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu T, Fujisaki J, Omae M, Yamasaki A, Horiuchi Y, Ishiyama A, et al. Treatment outcomes of endoscopic submucosal dissection for adenocarcinoma originating from long-segment Barrett’s esophagus versus short-segment Barrett’s esophagus. Digestion. 2018;97:316–323. doi: 10.1159/000486197. [DOI] [PubMed] [Google Scholar]
- 26.Subramaniam S, Chedgy F, Longcroft-Wheaton G, Kandiah K, Maselli R, Seewald S, et al. Complex early Barrett’s neoplasia at 3 Western centers: European Barrett’s Endoscopic Submucosal Dissection Trial (E-BEST) Gastrointest Endosc. 2017;86:608–618. doi: 10.1016/j.gie.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Chung JW, Lee GH, Jung HY, Choi KD, Song HJ, Choi KS, et al. Clinicopathologic characteristics of Barrett’s cancer in Korea. Gut Liver. 2008;2:193–198. doi: 10.5009/gnl.2008.2.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osumi H, Fujisaki J, Omae M, Shimizu T, Yoshio T, Ishiyama A, et al. Clinicopathological features of Siewert type II adenocarcinoma: comparison of gastric cardia adenocarcinoma and Barrett’s esophageal adenocarcinoma following endoscopic submucosal dissection. Gastric Cancer. 2017;20:663–670. doi: 10.1007/s10120-016-0653-x. [DOI] [PubMed] [Google Scholar]
- 29.Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;47:113–121. doi: 10.1055/s-0034-1391086. [DOI] [PubMed] [Google Scholar]
- 30.Park HC, Kim DH, Gong EJ, Na HK, Ahn JY, Lee JH, et al. Ten-year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J Intern Med. 2016;31:1064–1072. doi: 10.3904/kjim.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimamura Y, Ikeya T, Marcon N, Mosko JD. Endoscopic diagnosis and treatment of early esophageal squamous neoplasia. World J Gastrointest Endosc. 2017;9:438–447. doi: 10.4253/wjge.v9.i9.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui A, Kuribayashi Y, Nomura K, Tanaka T, Toba T, Yamada A, et al. Conventional white light endoscopic features of small superficial Barrett’s esophageal adenocarcinoma. Digestion. 2016;93:47–52. doi: 10.1159/000441764. [DOI] [PubMed] [Google Scholar]
- 33.Anders M, Lucks Y, El-Masry MA, Quaas A, Rösch T, Schachschal G, et al. Subsquamous extension of intestinal metaplasia is detected in 98% of cases of neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:405–410. doi: 10.1016/j.cgh.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Jang YS, Lee BE, Kim GH, Park DY, Jeon HK, Baek DH, et al. Factors associated with outcomes in endoscopic submucosal dissection of gastric cardia tumors: a retrospective observational study. Medicine (Baltimore) 2015;94:e1201. doi: 10.1097/MD.0000000000001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung JI, Kim GH, I H, Park DY, Kim TK, Cho YH, et al. Clinicopathologic factors influencing the accuracy of EUS for superficial esophageal carcinoma. World J Gastroenterol. 2014;20:6322–6328. doi: 10.3748/wjg.v20.i20.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qumseya BJ, Brown J, Abraham M, White D, Wolfsen H, Gupta N, et al. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett’s esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc. 2015;81:865–874.e2. doi: 10.1016/j.gie.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Anders M, Bähr C, El-Masry MA, Marx AH, Koch M, Seewald S, et al. Long-term recurrence of neoplasia and Barrett’s epithelium after complete endoscopic resection. Gut. 2014;63:1535–1543. doi: 10.1136/gutjnl-2013-305538. [DOI] [PubMed] [Google Scholar]
- 38.Abe S, Ishihara R, Takahashi H, Ono H, Fujisaki J, Matsui A, et al. Long-term outcomes of endoscopic resection and metachronous cancer after endoscopic resection for adenocarcinoma of the esophagogastric junction in Japan. Gastrointest Endosc. 2019;89:1120–1128. doi: 10.1016/j.gie.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660.e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Hongo M. Review article: Barrett’s oesophagus and carcinoma in Japan. Aliment Pharmacol Ther. 2004;20(Suppl 8):50–54. doi: 10.1111/j.1365-2036.2004.02230.x. [DOI] [PubMed] [Google Scholar]