Abstract
Compact CRISPR-Cas9 systems that can be packaged into an adeno-associated virus (AAV) show promise for gene therapy. However, the requirement of protospacer adjacent motifs (PAMs) restricts the target scope. To expand this repertoire, we revisited and optimized a small Cas9 ortholog derived from Streptococcus pasteurianus (SpaCas9) for efficient genome editing in vivo. We found that SpaCas9 enables potent targeting of 5′-NNGYRA-3′ PAMs, which are distinct from those recognized by currently used small Cas9s; the Spa-cytosine base editor (CBE) and Spa-adenine base editor (ABE) systems efficiently generated robust C-to-T and A-to-G conversions both in vitro and in vivo. In addition, by exploiting natural variation in the PAM-interacting domain, we engineered three SpaCas9 variants to further expand the targeting scope of compact Cas9 systems. Moreover, mutant mice with efficient disruption of the Tyr gene were successfully generated by microinjection of SpaCas9 mRNA and the corresponding single guide RNA (sgRNA) into zygotes. Notably, all-in-one AAV delivery of SpaCas9 targeting the Pcsk9 gene in adult mouse liver produced efficient genome-editing events and reduced its serum cholesterol. Thus, with distinct PAMs and a small size, SpaCas9 will broaden the CRISPR-Cas9 toolsets for efficient gene modifications and therapeutic applications.
Keywords: CRISPR, SpaCas9, base editor, Acr, AAV, Pcsk9
Graphical abstract
Zhanjun Li and colleagues revisited and optimized a small Cas9 ortholog derived from Streptococcus pasteurianus (SpaCas9) for efficient genome editing in vivo with distinct 5′-NNGYRA-3′ PAMs. All-in-one AAV delivery of SpaCas9 targeting the Pcsk9 gene in adult mouse liver produced efficient genome-editing events and reduced serum cholesterol.
Introduction
The CRISPR-Cas9 system can induce powerful genome manipulation in various organisms and is thus a versatile genome-editing platform in biotechnology and medicine.1,2 Cas9, derived from Streptococcus pyogenes (SpCas9), is the most widely used variant for genome editing because of its high efficiency and simple NGG protospacer adjacent motif (PAM) requirement. However, SpCas9 (1,368 amino acids [aa]) and its single guide RNA (sgRNA) sequence are too large to be packaged together into a single adeno-associated virus (AAV) vector for efficient delivery into cells in vivo.3,4 The current solution is to pack SpCas9 and its sgRNA separately to circumvent the limited cargo capacity of the AAV vector.5 To overcome this limitation, researchers have identified several small Cas9 orthologs for in vivo genome editing, including SaCas9 (1,053 aa),3 Nme1Cas9 (1,082 aa),6 Nme2Cas9 (1,082 aa),7 CjeCas9 (984 aa),8 St1Cas9 (1,121 aa),9 and SauriCas9 (1,061 aa),4 (Table S1). These compact orthologs have been repurposed for in vivo editing via single AAV delivery and have promoted the development of gene therapy, but they are restrictive due to the intricate PAM requirements. Therefore, exploration of new small Cas9 nucleases with distinctive PAMs to broaden the nuclease repertoire covering all possible PAM sequences is urgently needed.
In this study, we revisited the properties of Streptococcus pasteurianus Cas9 (SpaCas9), a small Cas9 ortholog identified previously that has not been fully explored and utilized for in vivo genome editing.3,10 The previously reported SpaCas9 showed relatively low activity and a restrictive NNGTGA PAM and thus has not been widely used.3 Here, we demonstrated that the optimized SpaCas9 system enables efficient in vivo editing at endogenous sites with distinct 5′-NNGYRA-3′ PAMs. Importantly, all-in-one AAV delivery of compact SpaCas9 together with its gRNA results in efficient genome editing in adult mice, showing promise for gene therapy in the future.
Results
Determination of the NNGYRA PAM sequences recognized by SpaCas9
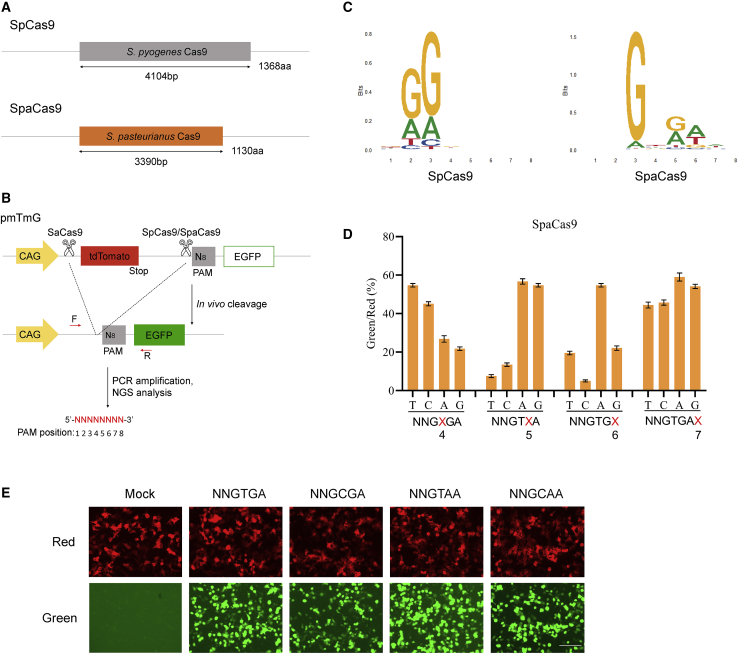
SpaCas9 is a type II-A Cas9 ortholog with a smaller size (1,130 aa) than conventional SpCas9 (Figure 1A). The PAM sequences of SpaCas9 were identified as NNGTGA using in vitro cleavage.3 However, many Cas9 orthologs identified by library cleavage in vitro or in bacteria often do not function well in mammalian cells.3,11,12 Therefore, we fully delineated the functional PAMs of SpaCas9 in human cells using a recently developed positive screening system termed PAM-DOSE (PAM-definition by observable sequence excision).13 First, a library of pmTmG plasmids containing a randomized 8-bp sequence (5′-NNNNNNNN-3′) was generated (Figure 1B). After the cleavage of SpCas9 (as a control) and SpaCas9, the tandem dimer Tomato (tdTomato) cassette was excised, and the CAG promoter drove the expression of the EGFP gene.13 Cleaved products were PCR amplified and subjected to deep sequencing to identify PAM sequences recognized by SpaCas9, which revealed that SpaCas9 recognizes NNGNRA (R = A/G) as a PAM (Figure 1C).
Figure 1.
PAM sequence analysis for SpaCas9
(A) Schematic representation of SpCas9 and SpaCas9. (B) Schematic showing the pmTmG reporter assay for characterization of PAM sequences recognized by Cas9s. An 8N (5′-NNNNNNNN-3′) library of PAM sequences was introduced into the constructs. In the presence of a functional PAM, cleavage-mediated tdTomato cassette excision was performed, which led to the expression of EGFP. (C) Sequence logo of recognized PAMs based on next-generation sequencing (NGS) results. (D) Comparison of SpaCas9 cleavage efficiency of different PAMs by flow cytometry analysis (FCA). The different PAMs were designed based on the optimal NNGTGA PAM sequence. Red or green represents the tdTomato or EGFP signal, respectively. (E) Representative fluorescence microscopy images of SpaCas9 PAM analysis. Scale bar, 200 μm. Error bars indicate the SEM (n = 3).
To further verify the PAM specificity of SpaCas9, we performed pmTmG reporter assays by co-transfecting plasmids encoding SpaCas9, its sgRNA, and reporter plasmids containing variable PAM sequences based on an optimal AGGTGA PAM (Figure S1). Reporter assays showed that SpaCas9 efficiently cleaved target sites containing PAMs of NNGTGA, NNGCGA, NNGTAA, and NNGCAA (NNGYRA [Y = C/T, R = A/G]) in human cells (Figures 1D and 1E). Compared with previously reported NNGTGA PAM, the NNGYRA PAMs are more general, indicating that four times more target sites are now theoretically available for SpaCas9 in human cells.
Improved efficiency by optimizing SpaCas9 and sgRNA
The lack of widespread use of SpaCas9 in the past is partly attributed to its relatively low activity.3 We thus attempted to improve its activity by optimizing the SpaCas9 architecture and its sgRNA. First, we reoptimized the human codon-optimized open reading frame (ORF) of SpaCas9 using codon usages from GenScript and flanked it with bipartite nuclear localization signals (bpNLSs), which have been demonstrated to substantially improve the expression levels of base editors (BEs)14 (Figure S2A). As a result, reoptimized SpaCas9 showed a 1.4-fold increase in editing efficiency (mean 52% versus 38%) compared to that of previously reported SpaCas9 systems3 using pmTmG reporter assays (Figure S2B). The previous sgRNA structure (version1, v1)3 has a truncated duplex compared with the innate bacterial CRISPR RNA (crRNA)-transactivating crRNA (tracrRNA) duplex and contains a continuous sequence of uracils (Us) as the pause signal for RNA polymerase III, which could potentially reduce transcription efficiency15,16 (Figure 2A). Therefore, we attempted to customize the sgRNA scaffold to maximize nuclease activity. We introduced a suitable Watson-Crick base pair into the initial sgRNA scaffold to disrupt consecutive Us and altered the duplex length (v2−v5) (Figure 2A). We determined that the extension of the duplex had no obvious effect, but the disruption of consecutive Us markedly increased editing efficiency in both the reporter assay and endogenous loci (Figures 2B and 2C). The best-performing sgRNA scaffold architecture (v5) was achieved by disrupting both regions of consecutive Us (Figure 2D).
Figure 2.
Optimized SpaCas9 sgRNA improves efficiency in human cells
(A) Schematic diagram of six designed versions of the sgRNA scaffold. The tetraloop and consecutive Us are shown in green and yellow, respectively. (B) Comparison of the efficiency of different scaffolds by reporter assays. Red or green represents the tdTomato or EGFP signal, respectively. (C) Comparison of the efficiency of different scaffolds by two endogenous target sites. (D) sgRNA structure of scaffold v5 was obtained using the mfold web server. (E) Comparison of the efficiency of variable spacer lengths by reporter assays. (F) Comparison of the efficiency of variable spacer lengths by two endogenous target sites. Error bars indicate the SEM (n = 3).
In addition, the length of the spacer in the sgRNA differs among Cas9 orthologs and affects target activity.3,8 To test the optimal spacer length requirement for SpaCas9, we created a series of sgRNAs targeting the pmTmG reporter and two endogenous sites with variable lengths. An additional guanine was added to the 5′-terminal sequence of each sgRNA to guarantee that the U6 promoter properly initiates transcription. We found that the ideal spacer length is 21 nucleotide (nt; G + 20), and activity decreased upon other lengths (Figures 2E and 2F). Collectively, these results validated that the codon-optimized SpaCas9 system with scaffold v5 and 21-nt spacer length is a potent genome-editing platform.
Efficient genome editing and base editing in human cells by SpaCas9
To thoroughly attest to the efficacy of SpaCas9 for genome editing, we co-transfected the optimized SpaCas9 system with a panel of 16 endogenous sites into HEK293T cells (Figure 3A). SpaCas9 generated indels at all 16 endogenous sites with varied editing efficiencies depending on the targets (Figures 3B and S3). Notably, endogenous sites containing all four NNGYRA PAMs could be efficiently edited by SpaCas9, consistent with the results of exogenous reporter assays (Figure 3B). Through side-by-side comparisons in overlapping regions, SpaCas9 showed comparable efficiency to other small Cas9s, including representative SaCas9 and SauriCas9 (Figure S4). In addition, the Cell Counting Kit 8 (CCK-8) assay suggested that the viability of the 293T cell line, which stably expresses SpaCas9, was not significantly different from that of wild-type (WT) cells (Figure S5).
Figure 3.
Genome editing of SpaCas9 and derived base editors in human cells
(A) Schematic representation of the optimal architecture of SpaCas9 and its sgRNA. (B) Genome editing with SpaCas9 with all NNGYRA PAMs in 16 endogenous loci. (C, top) Schematic of the Spa-CBE-rA1. (Bottom) Efficient C-to-T conversions in 12 endogenous loci. (D, top) Schematic of the Spa-ABE, including Spa-ABEmax (using TadA version 7.10) and Spa-ABE8e (using TadA version 8e). (Bottom) Efficient A-to-G conversions in 11 endogenous loci. The highest C-to-T/A-to-G editing frequency within the editing window represents the base-editing frequency at each site. Error bars indicate SEM (n = 3).
Base editing is a revolutionary technology based on the CRISPR-Cas9 platform that can induce targeted base conversions without generating DNA double-strand breaks (DSBs) or requiring a donor template.17,18 Cytosine BE (CBE) and adenine BE (ABE), a combination of Cas9 nickase (nCas9) and cytidine deaminase or an evolved adenine deaminase, respectively, enable the conversion of C · G to T · A or A · T to G · C base pairs, respectively.19,20 A limitation is that BEs are highly dependent on a proper PAM adjacent to the target base.20 To test whether SpaCas9 could be employed for base editing, we replaced the SpCas9 nickase (D10A) in BE4max with SpaCas9 nickase (D10A) to create Spa-CBE-rA1 (Figure 3C). Our data indicated that Spa-CBE-rA1 can induce robust C-to-T conversions in human cells (Figures 3C and S6). In addition, we created Spa-CBE-eAID, which is more efficient than Spa-CBE-rA1 in target sites in the GC context (Figure S7), consistent with our previous reports.21,22 We also generated Spa-ABEmax and Spa-ABE8e to mediate the conversion of A to G in genomic DNA. Potent editing efficiencies were observed by Spa-ABE8e rather than Spa-ABEmax, consistent with the statement that the ABEmax architecture is not fully compatible with compact Cas9s9,22,23 (Figures 3D and S8). Additionally, efficient editing mediated by SpaCas9 and Spa-BEs was validated in HepG2 and A549 cells (Figure S9). Taken together, these data further indicated that SpaCas9 is an efficient genome-editing system and can be used to expand the targeting range of BEs.
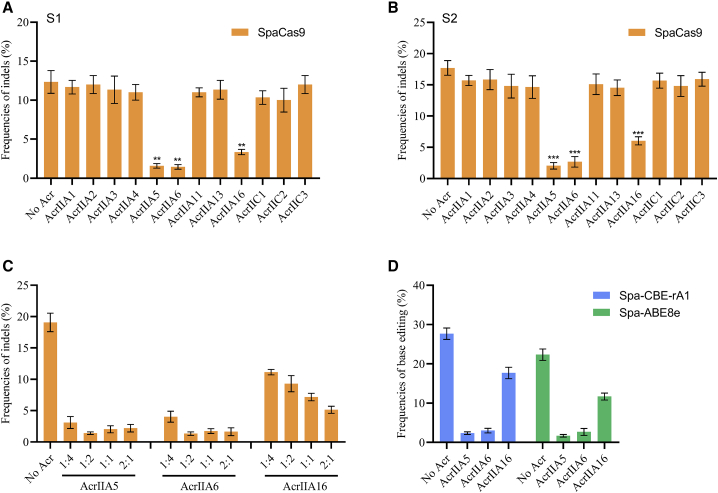
Anti-CRISPR (Acr)IIA5, AcrIIA6, and AcrIIA16 can be used as off switches for SpaCas9
To combat a potent CRISPR-Cas9 system, mobile genetic elements (MGEs) have acquired genes encoding inhibitors known as Acr proteins.24 Acr, as a natural brake for CRISPR-Cas technologies, has been widely used to control genome editing in mammalian cells and organisms.24 To test Acr inhibition of SpaCas9, we performed SpaCas9 and sgRNA transfections (targeting S1 and S2) in HEK293T cells in the presence or absence of 12 representative Acr proteins (AcrIIA1−6, -11, -13, and -16 and AcrIIC1−3). As a result, AcrIIA5, AcrIIA6, and AcrIIA16 were shown to significantly inhibit SpaCas9 genome editing, whereas others showed no effect (Figures 4A and 4B). Moreover, we observed robust inhibition presented by AcrIIA5 and AcrIIA6, suggesting that they have high potential against SpaCas9, whereas AcrIIA16 exhibited more moderate inhibition of SpaCas9 with an obvious dose-dependent effect (Figure 4C). Similar inhibitory effects were observed in both the Spa-CBE and Spa-ABE systems, indicating that Acr can also be used to regulate the activity of Spa-BEs (Figure 4D). These results suggested that the AcrIIA5, AcrIIA6, and AcrIIA16 proteins can be used as off switches for SpaCas9 applications.
Figure 4.
SpaCas9 is inhibited by anti-CRISPR (Acr) proteins in human cells
(A and B) Genome editing of SpaCas9 in the presence of the 12 previously described Acr families. Plasmids expressing SpaCas9, sgRNA, and each Acr (1:1:1) were co-transfected into HEK293T cells. (C) Inhibition of SpaCas9 is dose dependent for three Acrs at the S2 site. The ratio of Acr to SpaCas9 ranges from 1:4 to 2:1. (D) Base editing of Spa-CBE-rA1 (targeting S2) and Spa-ABE8e (targeting S4) in the presence of three Acrs. Error bars indicate the SEM (n = 3).
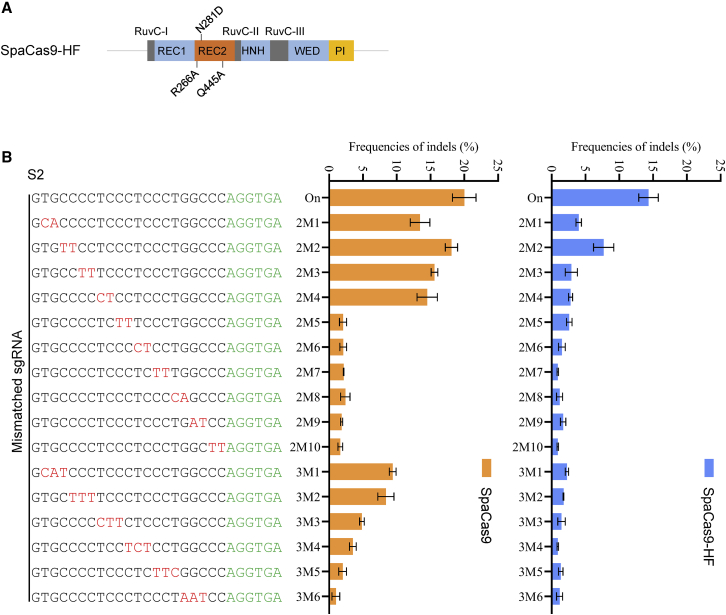
Engineered high-fidelity SpaCas9 variant
The off-target effects of the CRISPR-Cas9 system remain a major bottleneck that precludes safe and reliable applications in genome editing.25 Thus, we attempted to develop a high-fidelity SpaCas9 variant. Recently, two high-fidelity versions of SaCas9 have been reported: SaCas9-HF (R245A/N413A/N419A/R654A)26 and efSaCas9 (N260D or Q414A).27 R266, N281, and Q445 in SpaCas9, equivalent to R245, N260, and Q414 in SaCas9, are located in the REC2 domain, which interacts with the RNA-DNA heteroduplex (Figures 5A and S10). In addition, we observed the equivalent aa residues of R266 and Q445 forming polar contacts within the target DNA strand in the crystal structure of St1Cas9,28 which has 65% sequence identity with SpaCas9 (Figure S10). Therefore, we selected R266A, N281D, and Q445A to first create single mutants of SpaCas9. To evaluate the specificity of these mutant variants, we generated a panel of sgRNAs targeting S2 with dinucleotide and trinucleotide mismatches (Figure 5B). All three single mutants only showed improved sensitivity to mismatches in PAM-proximal 2M3 and 2M4 (Figure S11A). We thus further generated double mutants (R266A/N281D and Q455A/N281D) and triple mutants (R266A/N281D/Q445A) of SpaCas9. Notably, all of them displayed further improved specificity (Figures 5B and S11B). Thus, the mutant (R266A/N281D/Q445A), which slightly reduced on-target efficiency but significantly improved specificity in mismatched sgRNAs, was referred to as high-fidelity SpaCas9 (SpaCas9-HF) (Figure 5B).
Figure 5.
Engineering a high-fidelity SpaCas9 variant
(A) Schematic of designed SpaCas9-HF. R266A, N281D, and Q445A are all located in the REC2 domain. (B) Comparison of the tolerance of SpaCas9 and SpaCas9-HF for mismatched sgRNAs. Mismatched sgRNAs that differed from the S2 site by two or three nucleotides. Mismatched nucleotides and the PAM sequence are shown in red and green, respectively. Error bars indicate the SEM (n = 3).
Engineering SpaCas9 variants to expand the targeting range
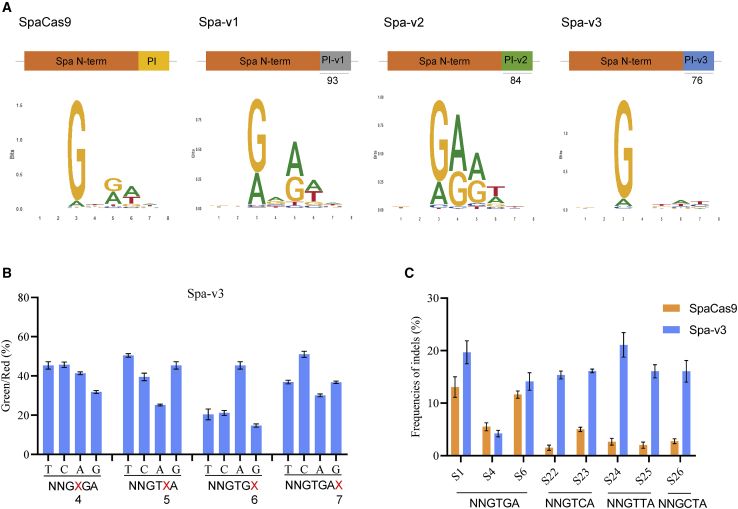
The PAM-interacting domains (PIDs) are located at the C terminus of most type II Cas9s. It has been demonstrated that Cas9 PAM specificity can be altered by mutations in PIDs.29,30 Cas9 PIDs also evolve such that closely related orthologs recognize distinct PAMs in natural selective pressure.7 Recently, some novel Cas9 homologs have been identified based on characterized structural homologs, such as Nme2Cas9,7 St1Cas9 variants,9 and SauriCas9.4 Encouraged by the excellent performance of SpaCas9, we attempted to excavate new SpaCas9 homologs with different PAMs to further broaden the targeting scope. After protein sequence alignment, the top 20 Cas9s with >87% overall identity to SpaCas9 were selected for further analysis (Figure S12A). The crystal structure of St1Cas9 showed that Q1084, K1086, and M1049 interact with the third, fourth, fifth, and sixth bases of the NNRGAA PAM, respectively.9,28 Structural homology modeling revealed that SpaCas9 shares 65% sequence identity with St1Cas9, and Q1084, K1086, and M1049 in St1Cas9 correspond to R1088, I1090, and M1053 in SpaCas9, respectively. We then selected three Cas9 homologs from the top 20 Cas9s that were different from SpaCas9 in the crucial positions of 1088, 1090, and 1053 (Figure S12B). Importantly, they share >98% identity in the N terminus of SpaCas9 and diverge mostly within the C-terminal PID, implying that they potentially recognize different PAM sequences.
We tested whether swapping the PID of SpaCas9 with the three selected homologs could reprogram PAM specificity. Three new SpaCas9 variants were constructed and termed Spa-v1, Spa-v2, and Spa-v3 (Figure 6A). Subsequently, the PAM sequences were determined by the pmTmG plasmid library previously mentioned.13 Unlike NNGYRA of SpaCas9, Spa-v1 and Spa-v2 showed NNRARA and NNRRRT PAMs, respectively (Figure 6A). The less stringent preference in the third position is consistent with the critical R1088Q aa change (Figure S6B). Surprisingly, Spa-v3 exhibited a markedly expanded PAM of NNGNNA (Figure S6B). We thus further explored the PAM preference of Spa-v3 in detail using a reporter assay, which revealed that Spa-v3 can efficiently target PAMs of NNGHBA (H = T/C/A, B = T/C/G). In addition, it showed a slight preference for thymine in the seventh position, which is different from SpaCas9 (Figure 6B). Spa-v3 also efficiently cleaved endogenous target sites with NNGTCA, NNGTTA, and NNGCTA PAMs, which are almost inaccessible to SpaCas9 (Figure 6C). In summary, these results validated that rationally engineered SpaCas9 variants generate efficient genome editing with an expanded targeting scope, further broadening the compact Cas9 toolsets.
Figure 6.
Engineering SpaCas9 variants to expand the targeting range
(A, top) Schematic of constructed SpaCas9 variants obtained by swapping the PID with SpaCas9. The PIDs of Spa-v1, -v2, and -v3 have 93%, 84%, and 76% sequence identity with that of SpaCas9, respectively. (Bottom) Sequence logo of recognized PAMs based on NGS results. (B) Comparison of Spa-v3 cleavage efficiency of different PAMs using reporter assays. The different PAMs were designed based on the optimal NNGTGA PAM sequence. (C) Comparison of the efficiency between SpaCas9 and Spa-v3 in 8 endogenous sites with different PAMs. Error bars indicate the SEM (n = 3).
Efficient genome editing in mice induced by SpaCas9
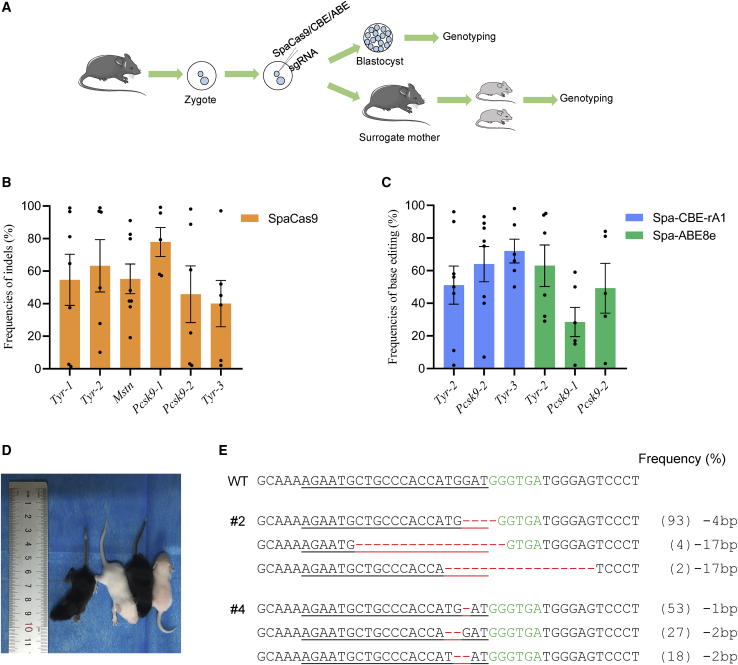
To evaluate the feasibility and efficiency of SpaCas9 in mice, we selected six target sites with NNGYRA PAMs from three genes (Tyr, Mstn, and Pcsk9). Genome editing was conducted in mouse zygotes using microinjection of SpaCas9-encoding mRNA and the appropriate sgRNA as previously reported.19,31 The mouse zygotes were cultured in vitro to blastocysts after injection and then genotyped (Figure 7A). Notably, all six target sites showed efficient editing, with an average efficiency ranging from 40.0% to 77.9% (Figures 7B and S13; Table S2). Encouraged by the results of the pilot study, we further tested the efficiency of Spa-BEs in mouse zygotes. As a result, both Spa-CBE and Spa-ABE induced robust C-T (from 51.1% to 72.0%) and A-G (from 28.5% to 63.0%) conversions (Figures 7C and S14; Table S2), respectively. The editing frequencies were variable, which may be because the blastocysts were collected and genotyped individually. The rate of successful microinjections influences gene-editing outcomes.
Figure 7.
SpaCas9 can induce efficient genome editing in mice
(A) Workflow for genome editing in mouse zygotes or to obtain F0 mice. (B) Average frequencies of indels at six target sites using SpaCas9 in mouse blastocysts. Data are presented as the mean ± SEM (n = ∼6 blastocysts). (C) Average frequencies of base editing by Spa-CBE-rA1 and Spa-ABE8e in mouse blastocysts. Data are presented as the mean ± SEM (n = ∼6 blastocysts). (D) Photograph of four F0 mice at 1 week. Tyr mutant mice exhibited a systemic albino phenotype. (E) Alignments of edited sequences of two mutant F0 mice. The targeted sequence is underlined. The PAM site and indels are shown in green and red, respectively. The column on the right indicates frequencies of mutant alleles. WT, wild-type.
Subsequently, we attempted to examine the practicality of SpaCas9 at the animal level by generating Tyr mutant mice to mimic human oculocutaneous albinism (OCA).32 The injected zygotes were transferred to surrogate mice. Four pups were obtained, and two of them (50%) carried the desired mutations at the Tyr-1 target site (Figures 7D and 7E). Consistent with the homozygous genotypes, both mutants exhibited a completely albino phenotype compared with the WT littermates (Figure 7D). In addition, no apparent off-target mutations were detected at potential off-target (POT) sites in the mutant mice (Figure S15A). Consequently, this mouse model has recapitulated human OCA disease symptoms, underscoring the potential of the SpaCas9 system in efficiently generating disease models in animals.
In vivo genome editing using all-in-one AAV-SpaCas9
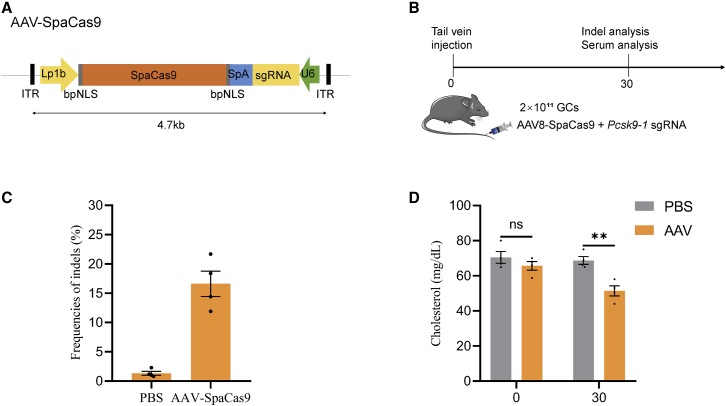
The small size allows the SpaCas9 system to fit into an all-in-one AAV vector for in vivo delivery. To test whether effective SpaCas9 genome editing can be achieved via single AAV delivery, we cloned a liver-specific promoter (Lp1b)-driven SpaCas9 expression cassette and U6-driven sgRNA in the opposite orientation into an AAV vector backbone (Figure 8A). With the use of hepatotropic AAV serotype 8, we targeted Pcsk9, a therapeutically relevant gene involved in cholesterol homeostasis.3 The disruption of Pcsk9 has been demonstrated to reduce blood cholesterol levels and to attenuate the risk of cardiovascular disease.33,34 The tail veins of the mice in the two groups were injected with 2 × 1011 AAV-SpaCas9 genome copies (GCs) targeting Pcsk9 or PBS as a negative control (Figure 8B). Serum was collected at 0 and 30 days post-injection for analysis, and all mice were euthanized at 30 days post-injection (Figure 8B). 1 month after administration, we observed an average of 16.6% indel formation at the Pcsk9-1 site in whole liver tissue (Figure 8C). Low off-target mutations (∼1%) were detected at the predicted POT sites in the edited mice by deep sequencing (Figure S15B). In addition, we observed significantly reduced serum cholesterol levels in the AAV-SpaCas9 group, whereas the mice treated with PBS maintained normal levels of cholesterol at 30 days post-injection (Figure 8D). Moreover, the serum alanine aminotransferase (ALT) or albumin (ALB) levels were not significantly changed in the AAV-SpaCas9 group, suggesting no signs of toxicity or liver damage after AAV-SpaCas9 expression (Figure S16). These data confirmed that SpaCas9 can be delivered by a single AAV vector as an effective genome-editing system in vivo.
Figure 8.
In vivo genome editing of SpaCas9 via all-in-one AAV delivery
(A) Representation of the designed AAV-SpaCas9 vector. SpA, synthetic polyadenylation sequence. (B) Experimental timeline of AAV-SpaCas9 tail-vein injections and analyses. (C) Frequencies of indels at Pcsk9-1 targets in liver tissue following injection of AAV at 2 × 1011 total genome copies (n = 4 mice). (D) Reduced serum cholesterol levels in mice injected with AAV-SpaCas9 compared to the PBS controls.
Discussion
In this study, we identified a new SpaCas9 that can be utilized for efficient genome editing in vivo. The editing efficiency of the SpaCas9 system was dramatically improved through reasonable optimization of codon usage, sgRNA scaffolds, and spacer length. We further validated that SpaCas9 and relevant BEs can efficiently rewrite the genome in both human cells and mice. Efficient Acr protein inhibitors and an engineered SpaCas9-HF variant with better specificity were also developed for precise regulation and applications. Moreover, SpaCas9 was shown to be an effective in vivo genome-editing platform using an all-in-one AAV vector. Our study provided a comprehensive analysis and characterized SpaCas9 as a versatile and robust in vivo genome-editing tool.
Although several small Cas9 nucleases have been used for genome editing via single AAV delivery, the range of targetable sequences remains limited because of the PAM requirements. We demonstrated that SpaCas9 recognized a novel PAM of NNGYRA, which is different from those of currently used small Cas9s, including SaCas9 (NNGRRT),3 Nme1Cas9 (NNNNGATT),6 Nme2Cas9 (NNNNCC),7 CjeCas9 (NNNVRYAC),8 St1Cas9 (NNRGAA),9 and SauriCas9 (NNGG).4 Therefore, SpaCas9 further expands the PAM compatibility of small Cas9 orthologs and improves the flexibility of therapeutic applications. In addition, we discovered that a further expanded targeting scope can be achieved by rationally engineering SpaCas9 variants with altered PIDs from their homologs. The approach used in this report can be applied to other Cas9 orthologs to broaden their targeting range. Thus, structure-guided natural variations or artificial mutagenesis in PID are a worthwhile direction to continue exploring novel Cas9 orthologs with more flexible PAM sequences.
We have validated that both SpaCas9-mediated CBEs and ABEs can induce robust C-to-T and A-to-G base editing in endogenous target sites. In addition to BEs, other Cas9-derived tools are also limited by PAM requirements. Recently, some new precise editing tools, including glycosylase BEs (GBEs) that cause C-to-G transversions,35, 36, 37 dual BEs that simultaneously catalyze both cytosine and adenine base conversions,38, 39, 40, 41 APOBEC (apolipoprotein B mRNA editing catalytic polypeptide-like)-Cas9 fusions that induce predictable multi-nucleotide deletions (AFIDs),42 and prime editing (PE) technology that can directly write new genetic information into a specified site, were reported.43,44 SpaCas9 could be used as a new scaffold to further augment the versatility of these precise genome-editing systems in the future.
In summary, we developed a SpaCas9 system that induced efficient genome editing in vivo with a distinct NNGYRA PAM. Importantly, the compact size of SpaCas9 enables it to be packaged into an all-in-one AAV vector for efficient delivery and genome editing in vivo. The SpaCas9 system is a promising tool for both basic research and therapeutic applications in the future.
Materials and methods
Animals
C57/BL6 mice and Institute for Cancer Research (ICR) mice were obtained from the Laboratory Animal Center of Jilin University (Changchun, China). All animal studies were conducted under the guidance of the Animal Welfare and Research Ethics Committee at Jilin University.
Plasmid construction
The previously reported SpaCas9 was obtained from Addgene (#68322). The pmTmG reporter plasmid was a kind gift from Feng Gu and co-workers.13 The optimized SpaCas9, Spa-CBEs, and Spa-ABEs were synthesized by Genscript Biotech (China). The DNA fragments of PIDs from three SpaCas9 homologs were synthesized and cloned into SpaCas9 to construct Spa-v1, -v2, and -v3 by Genscript Biotech (Nanjing). Plasmid site-directed mutagenesis was performed using the Fast Site-Directed Mutagenesis Kit (Tiangen, China). All of the site-directed mutation primers are listed in Table S3. The sequences of plasmids are listed in the Supplemental sequence.
Cell culture and transfection
HEK293T, HepG2, and A549 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (HyClone, China) and incubated at 37°C in an atmosphere of 5% CO2. The cells were seeded in 24-well plates and transfected using Hieff Trans Liposomal Transfection Reagent (Yeasen, China) according to the manufacturer’s instructions. After 72 h, the cells were collected and used for genotyping by TIDE45 and EditR.46 All target sites and primers used for genotyping are listed in Tables S4 and S5.
pmTmG reporter assay
The pmTmG reporter assay was carried out as previously reported.13 Briefly, HEK293 cells were co-transfected with SpaCas9, sgRNA, and pmTmG reporter plasmids (1:1:1) using Hieff Trans Liposomal Transfection Reagent. After 72 h of incubation, the fluorescent images were imaged with a microscope (ts100; Nikon, Tokyo, Japan). Cells were harvested for editing quantification by flow cytometry. Quantification was based on the relative fluorescent frequencies of green/red.
Cell viability assay
The 293T cell line stably expressing SpaCas9 was constructed by using a piggyBac transposase as previously reported.47 WT 293T cells and 293T cells stably expressing SpaCas9 were seeded in 96-well plates (10,000 cells per well) and then cultured with 200 μL of DMEM in 5% CO2 at 37°C. Then, 20 μL of CCK-8 solution (Meilunbio, China) was added to each well, and the cells were cultured for 30 min. The absorbance was measured at 450 nm with a microplate analyzer from 24 h to 72 h.
mRNA and gRNA preparation
The SpaCas9, Spa-CBE-rA1, and Spa-ABE8e plasmids were linearized with NotI and transcribed in vitro using the HiScribe T7 ARCA (Anti-Reverse Cap Analog) mRNA kit (NEB). mRNA was purified using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol. The sgRNA oligos were annealed into the pUC57-Spa sgRNA expression vector containing a T7 promoter. The sgRNAs were then amplified and transcribed in vitro using the MAXIscript T7 kit (Ambion) and purified using the miRNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol.
Microinjection of mouse zygotes and genotyping
Briefly, a mixture of mRNA (50 ng/μL) and sgRNA (30 ng/μL) was co-injected into the cytoplasm of pronuclear-stage zygotes. Each group was injected with an average of approximately 15 zygotes to test the base editing efficiency. The injected zygotes were transferred to potassium simplex optimized medium (KSOM) for culture at 37°C, 5% CO2, and 100% humidity. Then, the injected single zygote was collected at the blastocyst stage. Genomic DNA was extracted in embryo lysis buffer (1% Nonidet P-40 [NP-40]) at 56°C for 60 min and then at 95°C for 10 min in a Bio-Rad PCR Amplifier. Then, the extracted products were amplified by PCR (95°C, 5 min for predegeneration, 42 cycles of [95°C, 30 s; 58°C, 30 s; 72°C, 30 s] 72°C, 5 min for extension) and determined by Sanger sequencing. The genomic DNA of newborn mice was extracted from ear clips and analyzed by PCR genotyping. All primers used for genotyping are listed in Table S3.
Off-target assay
The potential off-target sites for each gRNA were predicted to analyze site-specific edits according to COSMID48 (https://crispr.bme.gatech.edu/). All primers for the off-target assay are listed in Table S6.
AAV injection and processing
Production of AAV8-SpaCas9 vectors was performed by Packgene Biotech (China). The titer of the produced AAV was 2 × 1012 GC/mL. For AAV vector injections, 8-week-old female ICR mice were injected with 2 × 1011 GCs per mouse via the tail vein, with the sgRNA targeting a validated Pcsk9-1 site. Mice were fasted overnight for 12 h before blood collection from the mandibular vein at 0 and 30 days. The levels of serum total cholesterol, ALB, and ALT were measured using a Catalyst One Chemistry Analyzer (IDEXX, USA) following the manufacturer’s protocol. Mice were euthanized 30 days after vector administration, and liver tissues were collected for analysis.
Statistical analysis
All data are expressed as mean ± SEM of at least three individual determinations for all experiments. Data were analyzed by Student’s t test via GraphPad prism software 8.0.1. The probability value was that smaller than 0.05 (p < 0.05) was considered as statistically significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Acknowledgments
The authors thank Peiran Hu and Nannan Li for assistance at the Embryo Engineering Center for critical technical assistance. This study was financially supported by the National Key Research and Development Program of China Stem Cell and Translational Research (2019YFA0110700), Program for Changjiang Scholars and Innovative Research Team in University (no. IRT_16R32), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030501 and XDA16030503), and Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104004).
Author contributions
Z. Liu, L.L., and Z. Li conceived and designed the experiments. Z. Liu and S.C. performed the experiments. Z. Liu, S.C., and W.X. analyzed the data. Y.S. and J.L. contributed reagents/materials/analysis tools. Z. Liu and Z. Li wrote the paper. All authors have read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.06.013.
Contributor Information
Liangxue Lai, Email: lai_liangxue@gibh.ac.cn.
Zhanjun Li, Email: lizj_1998@jlu.edu.cn.
Supplemental information
References
- 1.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knott G.J., Doudna J.A. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361:866–869. doi: 10.1126/science.aat5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S., et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Z., Wang S., Zhang C., Gao N., Li M., Wang D., Wang D., Liu D., Liu H., Ong S.G., et al. A compact Cas9 ortholog from Staphylococcus Auricularis (SauriCas9) expands the DNA targeting scope. PLoS Biol. 2020;18:e3000686. doi: 10.1371/journal.pbio.3000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Li J., Song C.Q., Tran K., Mou H., Wu P.H., Tai P.W.L., Mendonca C.A., Ren L., Wang B.Y., et al. Cas9-mediated allelic exchange repairs compound heterozygous recessive mutations in mice. Nat. Biotechnol. 2018;36:839–842. doi: 10.1038/nbt.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibraheim R., Song C.Q., Mir A., Amrani N., Xue W., Sontheimer E.J. All-in-one adeno-associated virus delivery and genome editing by Neisseria meningitidis Cas9 in vivo. Genome Biol. 2018;19:137. doi: 10.1186/s13059-018-1515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edraki A., Mir A., Ibraheim R., Gainetdinov I., Yoon Y., Song C.Q., Cao Y., Gallant J., Xue W., Rivera-Pérez J.A., Sontheimer E.J. A Compact, High-Accuracy Cas9 with a Dinucleotide PAM for In Vivo Genome Editing. Mol. Cell. 2019;73:714–726.e4. doi: 10.1016/j.molcel.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E., Koo T., Park S.W., Kim D., Kim K., Cho H.-Y., Song D.W., Lee K.J., Jung M.H., Kim S., et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017;8:14500. doi: 10.1038/ncomms14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agudelo D., Carter S., Velimirovic M., Duringer A., Rivest J.F., Levesque S., Loehr J., Mouchiroud M., Cyr D., Waters P.J., et al. Versatile and robust genome editing with Streptococcus thermophilus CRISPR1-Cas9. Genome Res. 2020;30:107–117. doi: 10.1101/gr.255414.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X., Seebeck T., Feng Y., Jiang Y., Davis G.D., Chen F. Improving CRISPR-Cas9 Genome Editing Efficiency by Fusion with Chromatin-Modulating Peptides. CRISPR J. 2019;2:51–63. doi: 10.1089/crispr.2018.0036. [DOI] [PubMed] [Google Scholar]
- 11.Hirano S., Abudayyeh O.O., Gootenberg J.S., Horii T., Ishitani R., Hatada I., Zhang F., Nishimasu H., Nureki O. Structural basis for the promiscuous PAM recognition by Corynebacterium diphtheriae Cas9. Nat. Commun. 2019;10:1968. doi: 10.1038/s41467-019-09741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorova I., Arseniev A., Selkova P., Pobegalov G., Goryanin I., Vasileva A., Musharova O., Abramova M., Kazalov M., Zyubko T., et al. DNA targeting by Clostridium cellulolyticum CRISPR-Cas9 Type II-C system. Nucleic Acids Res. 2020;48:2026–2034. doi: 10.1093/nar/gkz1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang L., Yang F., He X., Xie H., Liu X., Fu J., Xi H., Lu X., Liu C., Song Z., et al. Efficient cleavage resolves PAM preferences of CRISPR-Cas in human cells. Cell Regen. (Lond.) 2019;8:44–50. doi: 10.1016/j.cr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koblan L.W., Doman J.L., Wilson C., Levy J.M., Tay T., Newby G.A., Maianti J.P., Raguram A., Liu D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018;36:843–846. doi: 10.1038/nbt.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B., Gilbert L.A., Cimini B.A., Schnitzbauer J., Zhang W., Li G.-W., Park J., Blackburn E.H., Weissman J.S., Qi L.S., Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang Y., Jia G., Choi J., Ma H., Anaya E., Ye C., Shankar P., Wu H. Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol. 2015;16:280. doi: 10.1186/s13059-015-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Badran A.H., Bryson D.I., Liu D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z., Chen M., Chen S., Deng J., Song Y., Lai L., Li Z. Highly efficient RNA-guided base editing in rabbit. Nat. Commun. 2018;9:2717. doi: 10.1038/s41467-018-05232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees H.A., Liu D.R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z., Shan H., Chen S., Chen M., Zhang Q., Lai L., Li Z. Improved base editor for efficient editing in GC contexts in rabbits with an optimized AID-Cas9 fusion. FASEB J. 2019;33:9210–9219. doi: 10.1096/fj.201900476RR. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z., Chen S., Jia Y., Shan H., Chen M., Song Y., Lai L., Li Z. Efficient and high-fidelity base editor with expanded PAM compatibility for cytidine dinucleotide. Sci. China Life Sci. 2021 doi: 10.1007/s11427-020-1775-2. Published online January 6, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Richter M.F., Zhao K.T., Eton E., Lapinaite A., Newby G.A., Thuronyi B.W., Wilson C., Koblan L.W., Zeng J., Bauer D.E., et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020;38:883–891. doi: 10.1038/s41587-020-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino N.D., Pinilla-Redondo R., Csörgő B., Bondy-Denomy J. Anti-CRISPR protein applications: natural brakes for CRISPR-Cas technologies. Nat. Methods. 2020;17:471–479. doi: 10.1038/s41592-020-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Y., Chu A.H.Y., Bao S., Hoang D.A., Kebede F.T., Xiong W., Ji M., Shi J., Zheng Z. Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc. Natl. Acad. Sci. USA. 2019;116:20969–20976. doi: 10.1073/pnas.1906843116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie H., Ge X., Yang F., Wang B., Li S., Duan J., Lv X., Cheng C., Song Z., Liu C., et al. High-fidelity SaCas9 identified by directional screening in human cells. PLoS Biol. 2020;18:e3000747. doi: 10.1371/journal.pbio.3000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Zhang H., Xu X., Wang Y., Chen W., Wang Y., Wu Z., Tang N., Wang Y., Zhao S., et al. Catalytic-state structure and engineering of Streptococcus thermophilus Cas9. Nat. Catal. 2020;3:813–823. [Google Scholar]
- 29.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P., Li Z., Peterson R.T., Yeh J.R., et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimasu H., Shi X., Ishiguro S., Gao L., Hirano S., Okazaki S., Noda T., Abudayyeh O.O., Gootenberg J.S., Mori H., et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–1262. doi: 10.1126/science.aas9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D., Xu J., Zhu T., Fan J., Lai L., Zhang J., Chen Y.E. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J. Mol. Cell Biol. 2014;6:97–99. doi: 10.1093/jmcb/mjt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathi R.K., Strunk K.M., Giebel L.B., Weleber R.G., Spritz R.A. Tyrosinase gene mutations in type I (tyrosinase-deficient) oculocutaneous albinism define two clusters of missense substitutions. Am. J. Med. Genet. 1992;43:865–871. doi: 10.1002/ajmg.1320430523. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Raghavan A., Chen T., Qiao L., Zhang Y., Ding Q., Musunuru K. CRISPR-Cas9 Targeting of PCSK9 in Human Hepatocytes In Vivo-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2016;36:783–786. doi: 10.1161/ATVBAHA.116.307227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadwick A.C., Wang X., Musunuru K. In Vivo Base Editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a Therapeutic Alternative to Genome Editing. Arterioscler. Thromb. Vasc. Biol. 2017;37:1741–1747. doi: 10.1161/ATVBAHA.117.309881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D., Li J., Li S., Xin X., Hu M., Price M.A., Rosser S.J., Bi C., Zhang X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021;39:35–40. doi: 10.1038/s41587-020-0592-2. [DOI] [PubMed] [Google Scholar]
- 36.Kurt I.C., Zhou R., Iyer S., Garcia S.P., Miller B.R., Langner L.M., Grünewald J., Joung J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021;39:41–46. doi: 10.1038/s41587-020-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Park J.E., Paa P., Rajakumar P.D., Chew Y.T., Manivannan S.N., Chew W.L. Precise and programmable C:G to G:C base editing in genomic DNA. bioRxiv. 2020 doi: 10.1101/2020.07.21.213827. [DOI] [Google Scholar]
- 38.Grünewald J., Zhou R., Lareau C.A., Garcia S.P., Iyer S., Miller B.R., Langner L.M., Hsu J.Y., Aryee M.J., Joung J.K. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat. Biotechnol. 2020;38:861–864. doi: 10.1038/s41587-020-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakata R.C., Ishiguro S., Mori H., Tanaka M., Tatsuno K., Ueda H., Yamamoto S., Seki M., Masuyama N., Nishida K., et al. Base editors for simultaneous introduction of C-to-T and A-to-G mutations. Nat. Biotechnol. 2020;38:865–869. doi: 10.1038/s41587-020-0509-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Zhu B., Chen L., Xie L., Yu W., Wang Y., Li L., Yin S., Yang L., Hu H., et al. Dual base editor catalyzes both cytosine and adenine base conversions in human cells. Nat. Biotechnol. 2020;38:856–860. doi: 10.1038/s41587-020-0527-y. [DOI] [PubMed] [Google Scholar]
- 41.Xie J., Huang X., Wang X., Gou S., Liang Y., Chen F., Li N., Ouyang Z., Zhang Q., Ge W., et al. ACBE, a new base editor for simultaneous C-to-T and A-to-G substitutions in mammalian systems. BMC Biol. 2020;18:131. doi: 10.1186/s12915-020-00866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S., Zong Y., Lin Q., Zhang H., Chai Z., Zhang D., Chen K., Qiu J.L., Gao C. Precise, predictable multi-nucleotide deletions in rice and wheat using APOBEC-Cas9. Nat. Biotechnol. 2020;38:1460–1465. doi: 10.1038/s41587-020-0566-4. [DOI] [PubMed] [Google Scholar]
- 43.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Q., Zong Y., Xue C., Wang S., Jin S., Zhu Z., Wang Y., Anzalone A.V., Raguram A., Doman J.L., et al. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020;38:582–585. doi: 10.1038/s41587-020-0455-x. [DOI] [PubMed] [Google Scholar]
- 45.Brinkman E.K., Chen T., Amendola M., van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kluesner M.G., Nedveck D.A., Lahr W.S., Garbe J.R., Abrahante J.E., Webber B.R., Moriarity B.S. EditR: A Method to Quantify Base Editing from Sanger Sequencing. CRISPR J. 2018;1:239–250. doi: 10.1089/crispr.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schertzer M.D., Thulson E., Braceros K.C.A., Lee D.M., Hinkle E.R., Murphy R.M., Kim S.O., Vitucci E.C.M., Calabrese J.M. A piggyBac-based toolkit for inducible genome editing in mammalian cells. RNA. 2019;25:1047–1058. doi: 10.1261/rna.068932.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cradick T.J., Qiu P., Lee C.M., Fine E.J., Bao G. COSMID: A Web-based Tool for Identifying and Validating CRISPR/Cas Off-target Sites. Mol. Ther. Nucleic Acids. 2014;3:e214. doi: 10.1038/mtna.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.