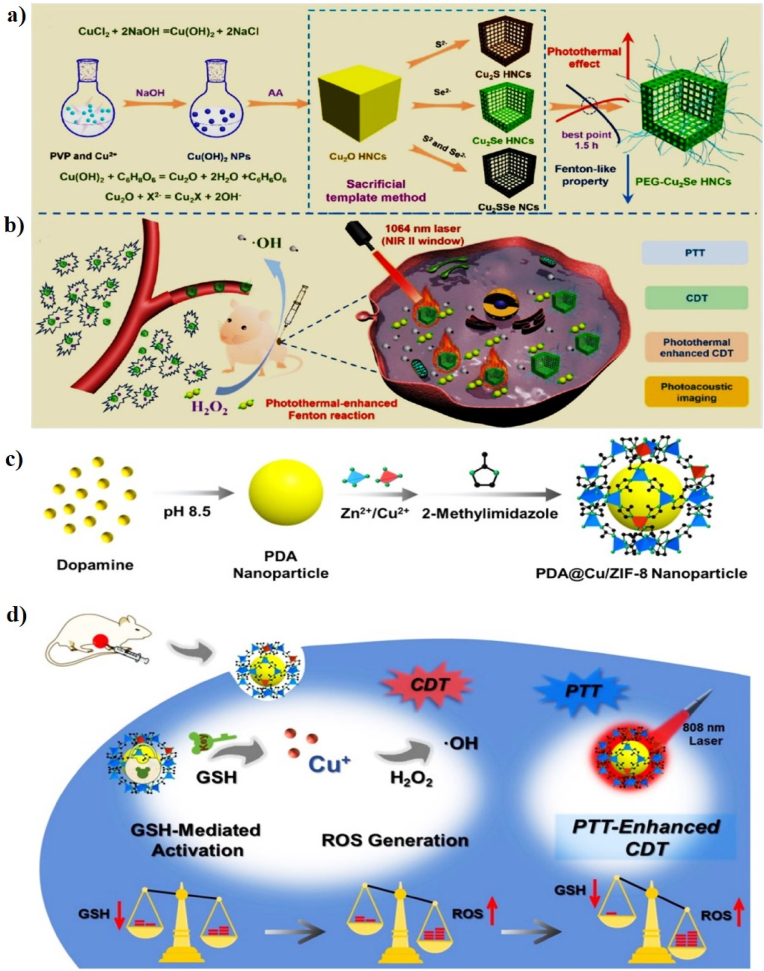
Abstract
Photothermal (PT)-enhanced Fenton-based chemodynamic therapy (CDT) has attracted a significant amount of research attention over the last five years as a highly effective, safe, and tumor-specific nanomedicine-based therapy. CDT is a new emerging nanocatalyst-based therapeutic strategy for the in situ treatment of tumors via the Fenton reaction or Fenton-like reaction, which has got fast progress in recent years because of its high specificity and activation by endogenous substances. A variety of multifunctional nanomaterials such as metal-, metal oxide-, and metal-sulfide-based nanocatalysts have been designed and constructed to trigger the in situ Fenton or Fenton-like reaction within the tumor microenvironment (TME) to generate highly cytotoxic hydroxyl radicals (•OH), which is highly efficient for the killing of tumor cells. However, research is still required to enhance the curative outcomes and minimize its side effects. Specifically, the therapeutic efficiency of certain CDTs is still hindered by the TME, including low levels of endogenous hydrogen peroxide (H2O2), overexpression of reduced glutathione (GSH), and low catalytic efficacy of Fenton or Fenton-like reactions (pH 5.6–6.8), which makes it difficult to completely cure cancer using monotherapy. For this reason, photothermal therapy (PTT) has been utilized in combination with CDT to enhance therapeutic efficacy. More interestingly, tumor heating during PTT not only causes damage to the tumor cells but can also accelerate the generation of •OH via the Fenton and Fenton-like reactions, thus enhancing the CDT efficacy, providing more effective cancer treatment when compared with monotherapy. Currently, synergistic PT-enhanced CDT using multifunctional nanomaterials with both PT and chemodynamic properties has made enormous progress in cancer theranostics. However, there has been no comprehensive review on this subject published to date. In this review, we first summarize the recent progress in PT-enhanced Fenton-based CDT for cancer treatment. We then discuss the potential and challenges in the future development of PT-enhanced Fenton-based nanocatalytic tumor therapy for clinical application.
Keywords: Nanomaterials, Fenton reaction, Chemodynamic therapy, Photothermal therapy, Combination therapy
Graphical abstract
Highlights
-
•
This review summarizes recent progress in nanomaterials for PT-enhanced CDT.
-
•
PT-enhanced CDT as an emerging new modality for tumor-specific therapy.
-
•
The mechanism of PT-enhanced Fenton-based nanocatalytic tumor therapy is provided.
-
•
Propose the major challenges and prospects of nanomaterials for PT-enhanced CDT.
1. Introduction
Cancer remains a major threat to human health worldwide because of the intense physical pain, high cytotoxicity, toxic side effects, and poor therapeutic efficiency associated with traditional therapeutic strategies, such as surgery, chemotherapy (CHT), and radiotherapy [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Numerous studies have attempted to explore new therapeutic strategies, such as photothermal therapy (PTT), chemodynamic therapy (CDT), photodynamic therapy (PDT), sonodynamic therapy (SDT), immunotherapy (IMT), and starvation therapy (ST), with high efficacy and reduced side effects [[15], [16], [17], [18], [19], [20], [21]]. New therapeutic strategies have been successfully used as an interesting alternative to traditional therapeutic strategies, and they are integrated with diagnostic imaging techniques, which are promising for cancer diagnosis and treatment [22,23]. However, monotherapy (e.g., PTT, CDT, PDT, SDT, IMT, or ST alone) cannot maximize the therapeutic efficiency because it is insufficient to effectively eliminate all of the tumor tissue, especially for large tumors [24]. Therefore, there are still many challenges to be overcome for the development of new strategies in the preclinical and clinical research of tumors to move away from monotherapy to combination cancer therapy (e.g., PT-enhanced CDT) to improve the therapeutic efficacy [[25], [26], [27], [28]].
CDT is an emerging nanocatalyst-based therapeutic strategy for tumor-specific therapy that has been developed over the last five years [[29], [30], [31]]. It is well known that CDT is highly selective for tumors and can greatly reduce side effects in normal tissues because it can induce tumor cell death through protein, DNA, and lipid damage upon the decomposition of H2O2 to generate highly toxic reactive oxygen species (ROS) such as •OH via intratumoral metal ion (e.g., Fe2+, Cu2+, Co2+, etc.)-mediated Fenton or Fenton-like reactions [[32], [33], [34]]. Compared with other therapeutic modalities, CDT is initiated by endogenous stimuli, which is exceedingly selective and logical, as well as highly sensitive to the concentration of catalytic ions and the Fenton or Fenton-like reaction in the TME (e.g. H2O2, GSH, and pH value) [35]. In addition, CDT has several unique advantages, such as high tumor specificity, stimulus activation, lower systemic toxicity, and the ability to regulate the TME, for cancer treatment [36,37]. Despite its potential, the use of CDT alone is limited by issues such as the low content of endogenous H2O2 within the tumor, low catalytic efficacy of the Fenton or Fenton-like reactions in the mildly acidic TME (pH 5.6–6.8), and the overexpression of reduced glutathione (GSH) in the TME, which reduces •OH productivity and the therapeutic efficiency of CDT [[38], [39], [40]]. In particular, the neutral pH and mildly acidic pH (pH 5.6–6.8) of the TME are always weakened and non-beneficial for the Fenton reaction, which is only effective under relatively strong acidic conditions (pH 3.0–5.0) [41,42]. However, the low generation rate of the •OH in the mildly acidic TME limits the therapeutic efficacy of CDT [24,25,28,43]. Thus, increasing the production of •OH in the mildly acidic TMEs is critical for the further development of CDT.
Combination cancer therapy has emerged and achieved satisfactory therapeutic effects, which can produce significant synergistic effects and minimize adverse effects [24,44,45]. PT-based combination cancer therapies, such as PT-enhanced CDT [[46], [47], [48]], PT-enhanced PDT [49,50], and chemo-PT-enhanced PDT [26,51], have received a significant amount of research attention because of their outstanding super-additive effect. Among these, synergistic PT-enhanced CDT has recently emerged as a promising therapeutic strategy for cancer therapy due to its high selectivity, low toxicity, and negligible invasiveness [[52], [53], [54], [55]]. In addition, PT-enhanced CDT has been utilized in combination with CHT, PDT, SDT, IMT, and ST to increase tumor treatment performance [56]. It has been recognized that the integration of more therapeutic modalities such as PT-enhanced CDT with CHT, PDT, SDT, IMT, and ST, and diagnostic imaging techniques into one nanoplatform will further enhance the tumor suppression effect because of their synergistic effects [53,56].
Based on the basic principle that evaluated temperature can accelerate the reaction rate of Fenton or Fenton-like reactions, increasing the temperature in the tumor area will be an efficient strategy to enhance the efficacy of the Fenton or Fenton-like reactions with an increased yield of •OH, which has been generally employed because of its practicability and simplicity [24,27,41,42,48,[57], [58], [59]]. As previously reported tumor-heating strategies, PTT is a new type of cancer treatment strategy that converts near-infrared (NIR) light energy (700–1300 nm) into heat to kill cancer cells [[60], [61], [62]]. PTT has several advantages: the NIR wavelength is located in the “biological transparency window,” which is minimally absorbed by biological tissue, blood, or water; PTT is minimally invasive, highly selective, has a high spatial resolution, deep penetration, and low toxicity, which utilizes PT conversion to generate local heating for the ablation of tumors [29,30,63]. More interestingly, the PT effect on the tumor area not only causes damage to the tumor cells but can also accelerate the formation of ROS, such as •OH, thus enhancing the CDT efficacy, providing more effective cancer treatment [64]. Therefore, a reasonable combination of PTT with other therapeutic modalities (CDT) using multifunctional nanomaterials is highly desirable toward enhancing therapeutic efficiency [52,[65], [66], [67], [68]]. In addition, there is also an urgent need to develop multifunctional nanomaterials with both PT and chemodynamic properties for PT-enhanced CDT, which can help toward the development of highly effective combination cancer therapies [24,33,69].
To date, a variety of multifunctional nanomaterials, such as metal-, metal oxide-, and metal sulfide-based nanomaterials, have been widely studied for PT-enhanced CDT because of their strong NIR absorption, photothermal conversion efficiency (PCE) properties, biocompatibility, and chemodynamic properties [47,[70], [71], [72]]. In addition, a variety of multifunctional nanomaterials with diagnostic imaging properties have been widely used to improve imaging quality, which has excellent NIR absorption properties, magnetic properties, and other unique physicochemical properties, leading to more efficient and promising applications in diagnostic imaging [45,73]. Therefore, combination cancer therapy allows precise diagnosis and highly efficient tumor therapy, which has the potential to overcome the aforementioned drawbacks. In this review, we summarize the recent progress in multifunctional nanomaterials for PT-enhanced Fenton-based nanocatalytic tumor therapy.
2. Fenton or Fenton-like reaction in PT-enhanced CDT
Recently, various metal-based nanomaterials have been developed for CDT such as Fe, Cu, Co, Mn, Cr, Ag, etc [56]. CDT is generally based on Fenton or Fenton-like reactions and is used to trigger the in situ Fenton or Fenton-like reaction within the TME to produce a large amount of toxic •OH to kill cancer cells, and is a promising anticancer therapy with high specificity and minimal invasiveness [[74], [75], [76], [77], [78]]. Up to now, a lot of iron (Fe)-based nanocatalysts (e.g., Fe, Fe3O4, FeS2, etc.) with different nanostructures or formulations have been developed as a Fenton agent for CDT, which represent highly efficient Fenton reagents that trigger the intratumoral Fenton chemical reaction, resulting in extremely high nanocatalytic efficacy as well as high biocompatibility [74,77,78]. The specific Fenton reaction is commonly based on Fe2+ or Fe3+, which can produce •OH by catalyzing the decomposition of H2O2, resulting in the destruction of proteins, lipids, and DNA, allowing efficient tumor-specific treatment without producing significant side effects in healthy cells [74,[79], [80], [81], [82], [83], [84], [85]]. The relevant equations (Eqs. (1), (2))) are shown below:
| Fe2++H2O2 → Fe3++•OH + OH− | (1) |
| Fe3++H2O2 → Fe2++•OOH + OH+ | (2) |
Apart from Fe-based nanocatalysts, other metal-based nanocatalysts based on Cu, Co, Mn, Cr, and Ag have also been developed as Fenton-like agents for CDT, which (e.g., Cu+, Co2+, Mn2+, Cr3+, and Ag+) can also catalyze the in situ decomposition of acidic H2O2 into highly toxic •OH via Fenton-like reactions leading to the specific destruction of tumor cells [33,[86], [87], [88], [89], [90], [91], [92]]. For example, Cu + or Cu2+ can also act as a Fenton-like catalyst for CDT, which can more easily react with H2O2 to generate •OH in the TME [33]. The copper (Cu)-based catalytic reactions are shown in Equations (3), (4).
| Cu++H2O2 → Cu2++•OH + OH− | (3) |
| Cu2++H2O2 → Cu++•OOH+H+ | (4) |
Fenton or Fenton-like reactions catalyzed by metal-based nanocatalysts commonly require mild acidic pH conditions (pH 3.0–5.0) for higher reaction rates. However, the pH of the TME is in the range of 5.6–6.8, which leads to the low catalytic efficacy of the Fenton or Fenton-like reactions [74,79,81]. In addition, the low concentration of H2O2 in the TME (<100 μM) generates low concentrations of •OH, which also hinders the effect of CDT [93,94]. Based on the basic principle that increasing the tumor tissue temperature by PTT can expedite the reaction rate of the Fenton or Fenton-like reactions, it can potentially produce a sufficient amount of the toxic •OH to induce tumor cell death [84,85]. In particular, the mildly acidic TME (pH 5.6–6.8) has a significantly less effective role in the Fenton reaction without PT treatment [95,96]. Therefore, PT-enhanced Fenton-based nanocatalytic tumor therapy can effectively enhance the reaction kinetics of the classical Fenton reaction [93,94]. Numerous nanomaterials are capable of catalyzing the Fenton reaction with NIR laser irradiation for the PT-enhanced Fenton reaction in CDT against cancer [97,98].
3. Multifunctional nanomaterials for PT-enhanced CDT
In recent years, numerous nanomaterials, such as metal (Fe, Cu, Au, etc.)-, metal oxide (WO3, MoOχ, MnO2, etc.)- and metal sulfide (Cu2−xS, CoS2, MoS2, etc.)-based nanomaterials with both PT and chemodynamic properties, have been developed for PT-enhanced CDT due to their excellent biocompatibility (Table 1) [27,48,87,[99], [100], [101]]. Multifunctional nanomaterial-based contrast agents have been widely used to improve imaging quality, and contrast agents have excellent near-infrared (NIR) absorption properties, magnetic properties, and other unique physicochemical properties, leading to more efficient and promising applications in multimodal imaging, such as photoacoustic imaging (PAI), fluorescence imaging (FLI), magnetic resonance imaging (MRI), and computed tomography (CT) [45,73,102]. During CDT, Fenton or Fenton-like reactions can be triggered by various metal-based nanocatalysts [36,56,103]. Numerous nanomaterials have been used to catalyze the decomposition of H2O2 to produce •OH, which destroys tumors by triggering intratumoral Fenton or Fenton-like reactions during CDT [[104], [105], [106]]. However, the mildly acidic TME decreases the •OH productivity [107,108]. Thus, there is an urgent need to improve the speed of •OH production in the mildly acidic TME [109]. Most importantly, the tumor-heating effect of PTT may accelerate the generation of •OH, thus enhancing the efficacy of CDT [[110], [111], [112], [113], [114], [115]]. The combination of PT-enhanced CDT is highly desirable for generating a synergistic combination cancer therapy because the heat accelerates Fenton chemistry [110].
Table 1.
Summary of multifunctional nanomaterials for PT-enhanced CDT.
| Nanomaterials | Composition | Size (nm) | % PCE (η) | Irradiation conditions | Temp (°C) | Cell line/animal model | Treatment/imaging | Ref |
|---|---|---|---|---|---|---|---|---|
| Fe-based nanomaterials | FeS2-PEG | 180–200 nm | 28.6% | 808 nm, 1.5 W/cm2, 5 min | 50 | MCF-7, and 4T1 cells; 4T1 tumor-bearing mouse | In vitro and in vivo PTT-CDT/MRI | [27] |
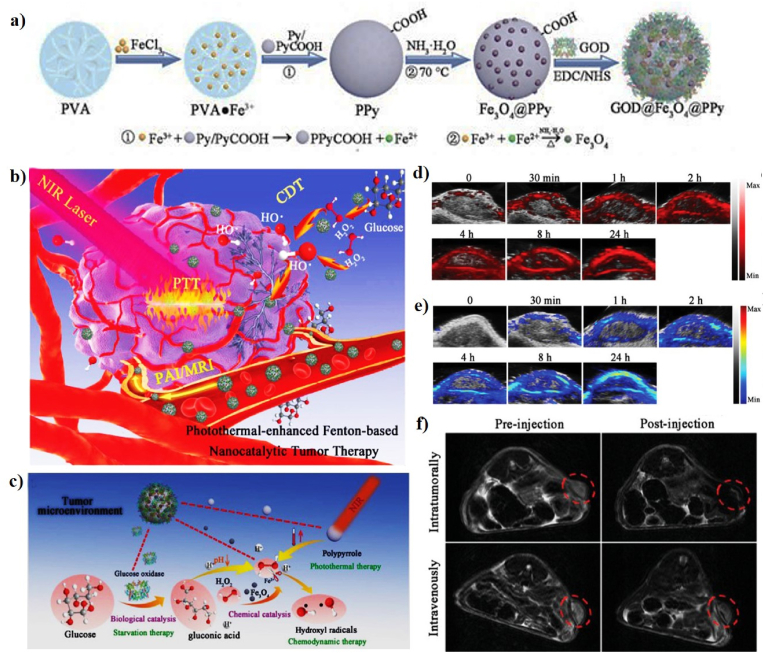
| Fe3O4@PPy@GOD NCs | 163.5 nm | 35.1 and 66.4% | 808 nm or 1064, 1.0 W/cm2, 15 min | 59 | L929, 4T1, HeLa, and HUVEC cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI/MRI | [59] | |
| PSAF NCs | 69.5 nm | 19.21% | 808 nm, 2.0 W/cm2, 10 min | 45 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT | [116] | |
| BSA-CuFeS2 NPs | 4.9 ± 0.9 nm | 38.8% | 808 nm, 1.5 W/cm2, 5 min | 63 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/FLI/MRI | [109] | |
| PMFG | 12–20 nm | – | 808 nm, 0.8 W/cm2, 5 min | 45 | MCF-7 cell line | In vitro PTT-CDT | [25] | |
| Nb2C-IO-CaO2 | 150 nm | 32.1% | 1064 nm, 1.5 W/cm2, 10 min | 54.4 | HUVEC and 4T1 cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT | [117] | |
| AFP NPs | 242.3 nm | – | 808 nm, 1.0 W/cm2, 10 min | 47.7 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [118] | |
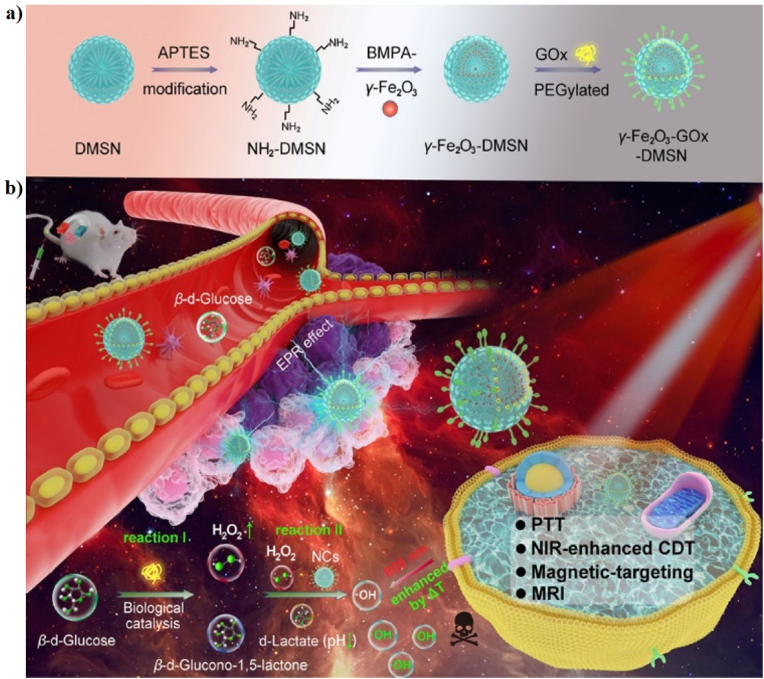
| γ-Fe2O3-GOx-DMSN NCs | 130 nm | 49.8% | 808 nm, 1.0 W/cm2, 5 min | 50 | HeLa and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI | [119] | |
| FP NRs | 180 nm | 56.6% | 1064 nm, 0.5 W/cm2, 10 min | 55.7 | HeLa, L929, and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo SDT-PTT-CDT/PAI/MRI | [48] | |
| EA-Fe@BSA NPs | 13.84 ± 2.53 | 31.9% | 808 nm, 1.0 W/cm2, 15 min | 41 | HCT116 and HUCEC cell lines; HCT116 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI | [110] | |
| BSO–FeS2 NPs | 7.27 ± 1.43 nm | 49.5% | 808 nm, 1.0 W/cm2, 5 min | 45 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PDT/PAI | [120] | |
| RLR NPs | 200 nm | 26.8%, | 808 nm, 1.5 W/cm2, 5 min | 49 | MDA-MB-231 cell line; MDA-MB-231 tumor-bearing mice | In vitro and in vivo chemo-PTT-CDT/MRI/FLI | [121] | |
| FeS2@RBCs | 185.2 nm | 30.2% | 1064 nm, 1.0 W/cm2, 5 min | 25.3 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI/FLI | [122] | |
| Fe-POM | 12.9 nm | 51.4% | 1060 nm, 1.0 W/cm2, 10 min | 50 | HUVEC and HeLa cell lines; HeLa tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [123] | |
| PB@FePt-HA-g-PEG | 150 nm | 28.14% | 808 nm, 1.5 W/cm2, 10 min | 68.3 | L02 and MCF-7 cell lines; MCF-7 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI/CT | [124] | |
| FMO | 450 nm | 48.5% | 808 nm, 0.7 W/cm2, 5 min | 50 | HeLa, and L929 cell lines; HeLa tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI | [125] | |
| CFMG hydrogel | – | – | 808 nm, 1.0 W/cm2, 5 min | 49.5 | A375 and C2C12 cell lines; A375 tumor-bearing mice | In vitro and in vivo PTT-CDT | [126] | |
| FeO/MoS2-BSA | 150 nm | 56% | 1064 nm, 0.75 W/cm2, 5 min | 52 | HeLa and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI | [127] | |
| F-BS NCs | 80 nm | 23.46% | 808 nm, 1.2 W/cm2, 5 min | 59.1 | L929 and HeLa cell lines; HeLa tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [128] | |
| Fe(III)-GA/GOx@ZIF-Azo | 3 nm | 65.3% | 808 nm, 1.54 W/cm2, 10 min | 46 | MCF-7 cell line; MCF-7 tumor-bearing mice | In vitro and in vivo PTT-CDT/FLI | [129] | |
| Cu-based nanomaterials | CP NCS | 22 nm | 27% | 1064 nm, 0.75 W/cm2, 5 min | 51 | L929, HeLa, and U14 cell lines; U14-tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI/PAI | [130] |
| PEG-Cu2Se HNCs | 86.89 ± 19.93 nm | 50.89% | 1064 nm, 0.75 W/cm2, 5 min | 58.4 | HUVEC and 4T1 cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [41] | |
| SC@G NSs | 60.94 nm | 46.3% | 1064 nm, 1.0 W/cm2, 5 min | 47.5 | 293T and 4T1 cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [131] | |
| PDA@Cu/ZIF-8 NPs | 50 nm | – | 808 nm, 1.0 W/cm2, 10 min | 70 | MCF-7, A549, and MDA-MB-231 cell lines; MCF-7 tumor-bearing mice | In vitro and in vivo PTT-CDT | [72] | |
| CuO@AuCu-TPP | 255 nm | 37.9% | 808 nm, 1.0 W/cm2, 5 min | 51.6 | HeLa cell line; HeLa tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI/CT | [132] | |
| Cu9S8 NPs | ∼18.05 nm | – | 808 nm, 0.5 W/cm2, 5 min | 43 | 4T1 and HUVEC cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [64] | |
| CuO@CNSs-DOX | 15 nm | 10.14% | 808 nm, 2.0 W/cm2, 10 min | 60.3 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo Chemo-PTT-CDT/PAI | [133] | |
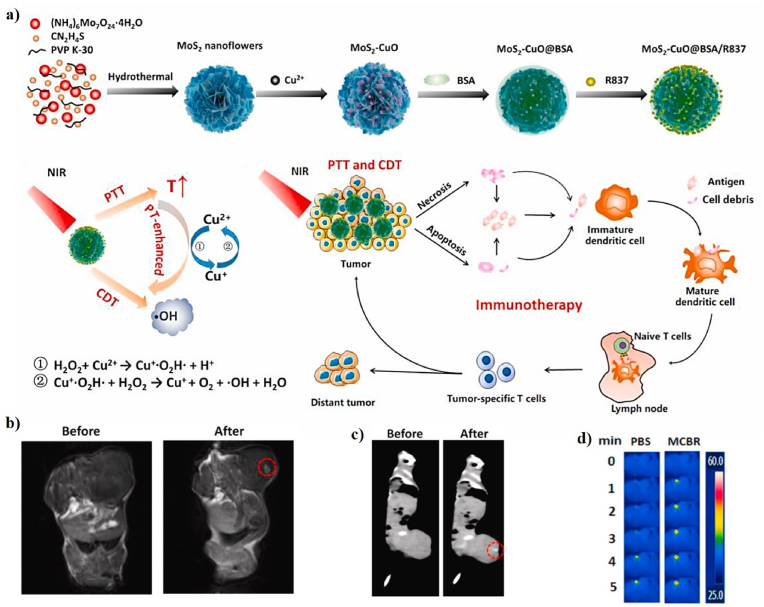
| MCBR | 130 nm | 24.6% | 808 nm, 1.5 W/cm2, 5 min | 75 | CT26 cell line; CT26 tumor-bearing mice | In vitro and in vivo PTT-CDT/IMT/CT/MRI | [47] | |
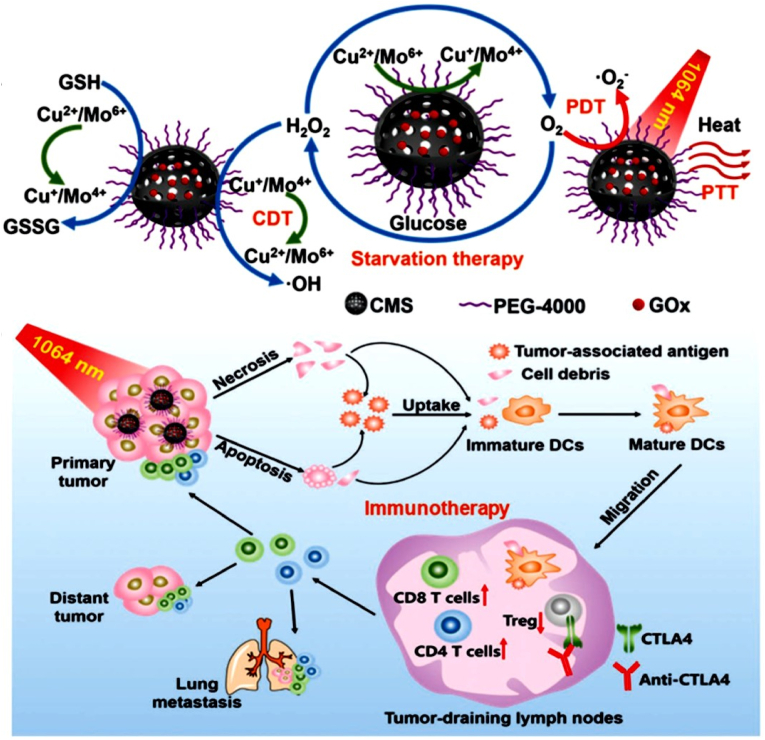
| PEG-CMS@GOx | 5.88 nm | 63.27% | 1064 nm, 0.48 W/cm2, 5 min | 52 | HeLa, L929, and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo PTT-CDT/PDT/IMT/PAI | [134] | |
| PFN | 22 nm | 30.17% | 1064 nm, 1.0 W/cm2, 5 min | 55 | Panc02 cell line; Panc02 tumor-bearing mice | In vitro and in vivo PTT-CDT/MRI | [135] | |
| Gold-based nanomaterials | CAANSs | 52 nm | – | 808 nm, 2.4 W/cm2, 10 min | 68.4 | HeLa cell line | In vitro chemo-PTT | [136] |
| Au@HCNs | 275 ± 0.355 nm | 26.8% | 808 nm, 2.0 W/cm2, 10 min | 52.9 | CT26 cell line; CT26 tumor-bearing mice | In vitro and in vivo PTT-CDT | [137] | |
| Au@MnO2 | 25 nm | 23.6% | 808 nm, 1.0 W/cm2, 15 min | 42 | 4T1 cell line; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI/MRI | [43] | |
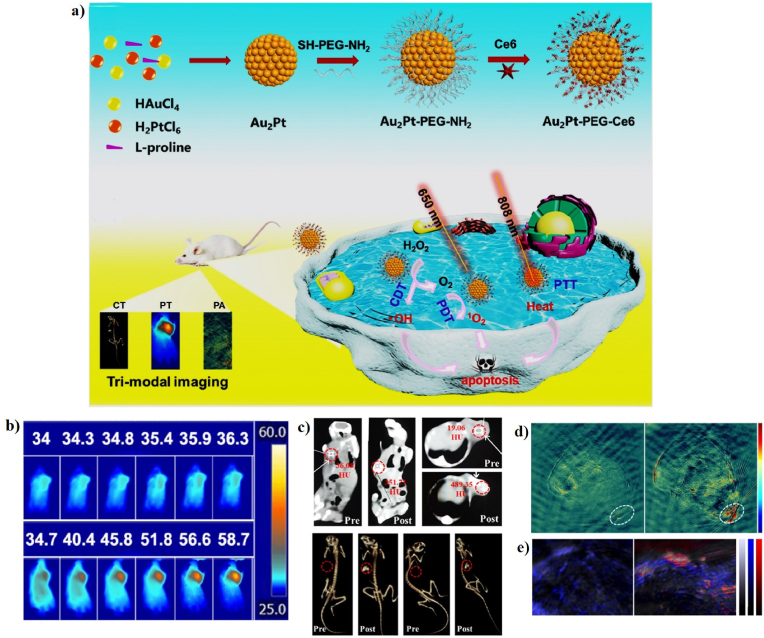
| Au2Pt-PEG-Ce6 | 42 ± 3 nm | 31.5 | 808 nm, 1.0 W/cm2, 5 min; 650 nm, 0.259 W/cm2, 5 min | 58.7 | HeLa, L929, and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo PTT/PDT/CDT/CT/PAI | [138] | |
| Metal oxide-based nanomaterials | WO3−x@γ-PGA NPs | 5.73 ± 0.93 nm | 25.8% | 1064 nm, 0.5 W/cm2, 5 min | 46 | HUVEC and 4T1 cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [24] |
| DCDMs | 123 nm | 51.5% | 808 nm, 1.0 W/cm2, 10 min | 58.7 | L929, HeLa, and U14 cell lines; U14 tumor-bearing mice | In vitro and in vivo PTT-CDT/FLI/CT/MRI | [139] | |
| HMCMs | 80 nm | 23.5% | 808 nm, 1.0 W/cm2, 8 min | 54 | MCF-7, MDA-MB-231, and MGC-803 cell lines; MCF-7 tumor-bearing mice | In vitro and in vivo PTT-CDT/FLI | [46] | |
| Metal sulfide-based nanomaterials | CoS2 NCs | 19.79 ± 5.2 nm | 60.4% | 808 nm, 1.0 W/cm2, 10 min | 55.4 | 4T1 and HUEVC cell lines; 4T1 tumor-bearing mice | In vitro and in vivo PTT-CDT/PAI | [42] |
3.1. Iron-based nanomaterials for PT-enhanced CDT
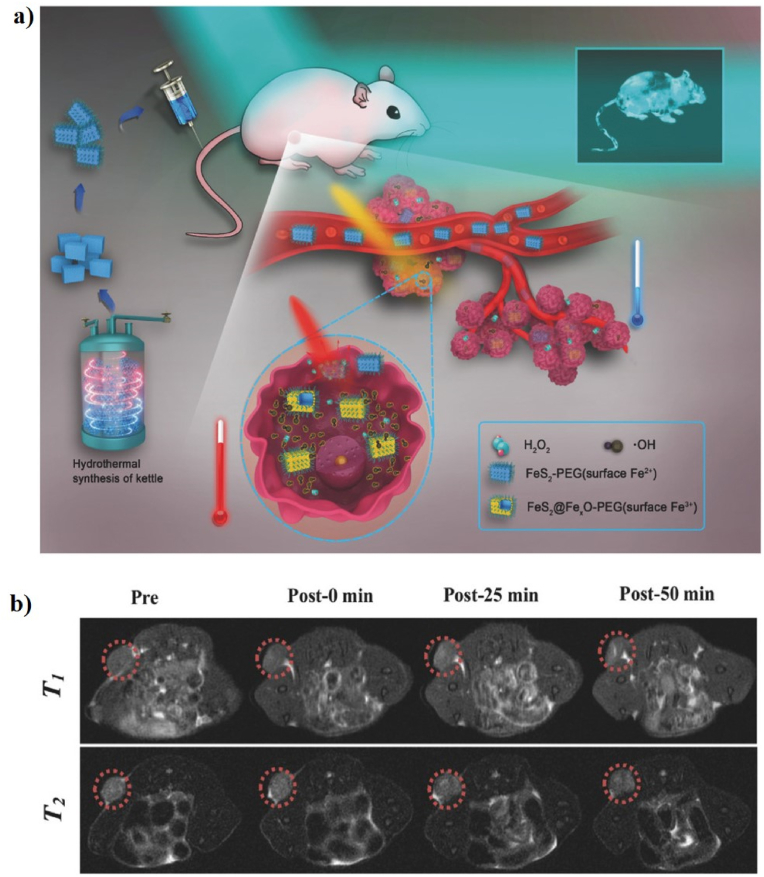
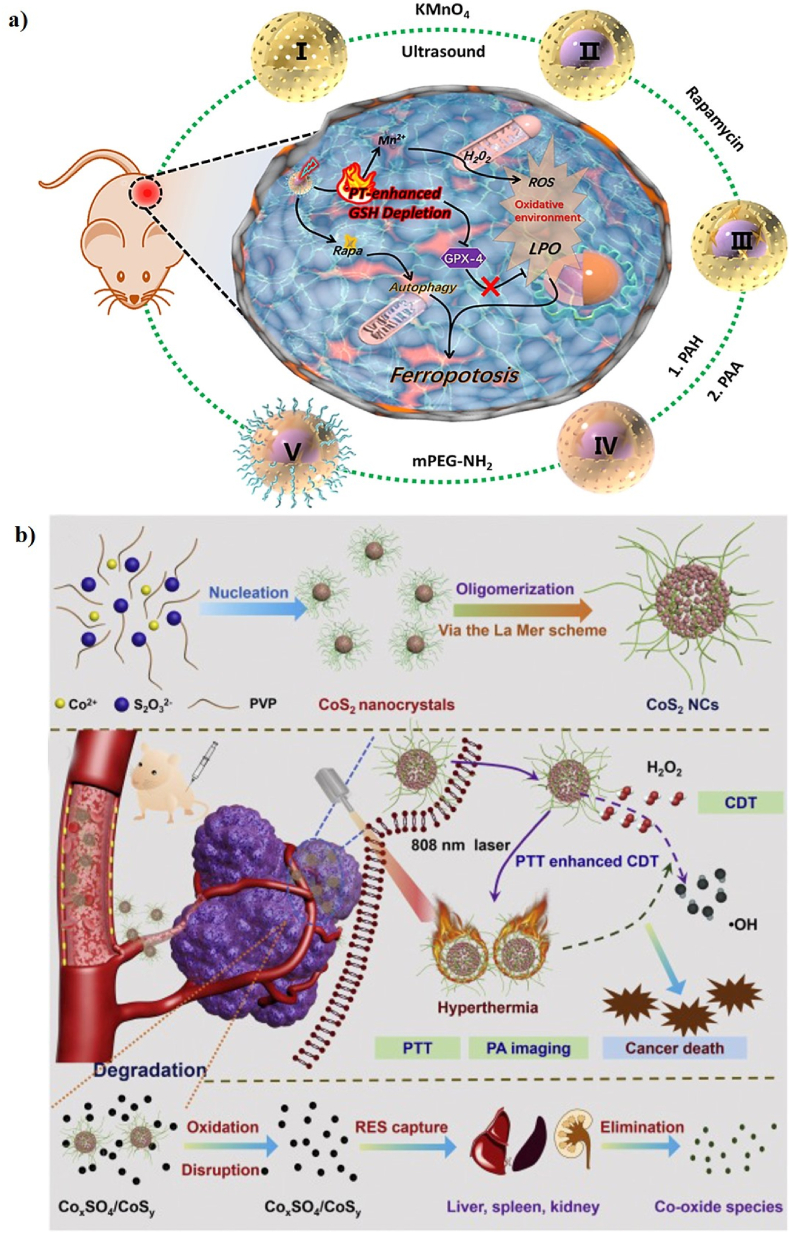
Iron (Fe)-based nanomaterials have been widely used in versatile biomedical applications, such as drug delivery, tissue engineering, PTT, PDT, CDT, SDT, ST, and multimodal imaging [[140], [141], [142], [143], [144], [145]]. Some formulations of iron-based nanomaterials have been approved by the United States (US) Food and drug administration (FDA) for use in humans as therapeutics for iron deficiency, drug carriers for cancer therapy, and as contrast agents in MRI [81,[146], [147], [148], [149]]. Recently, iron-based nanomaterials have attracted a lot of attention as antiferromagnetic pyrite Fenton nanocatalysts for cancer therapy [81]. For example, Tang et al. first reported the use of antiferromagnetic pyrite polyethylene glycol (FeS2-PEG) nanocubes for self-enhanced MRI and PT-enhanced CDT (Fig. 1a). Iron pyrite (FeS2) ion has been successfully used in a new era of cancer treatment because it can catalyze the production of ROS. PEG is a synthetic and biocompatible polymer that has been used in various applications, such as PEGylation, drug delivery, cancer therapy, and wound healing. FeS2 nanocubes were modified with SH-PEG to enhance their biocompatibility and solubility. More interestingly, the localized heat generated by FeS2-PEG via the PT effect, which accelerates the intratumoral Fenton reaction to efficiently generate •OH to kill tumor cells, implies that the synergistic effect of PTT and CDT into a single nanomaterial can be used as a “one-for-all” formulation. FeS2-PEG is cube-shaped with an average diameter of 180–200 nm. The PCE (η) values of FeS2-PEG and FeS2@FeꭓO-PEG are 28.6 and 29.5%, respectively. The in vitro PT-enhanced CDT effect of FeS2-PEG shows that the heat generated by the PT effect can accelerate and enhance the amount of •OH formed, suggesting that increased temperature can simultaneously induce ionization and improve the production of •OH. 4T1 tumor-xenografted mice were intratumorally injected with FeS2-PEG. The tumor-bearing mice treated with FeS2-PEG and laser irradiation were almost completely inhibited, implying the potential of FeS2-PEG as a PTT/CDT agent for cancer therapy. The T1-and T2-weighted magnetic resonance (MR) signal intensities showed a significant increase of 56.38 and 2.49%, respectively, which resulted in the synergism of the self-enhanced MRI-guided PT-enhanced CDT (Fig. 1b) [27].
Fig. 1.
(a) A schematic illustration of the preparation of FeS2-PEG for self-enhanced MRI and PT-enhanced CDT. (b) In vivo T1-and T2-weighted MR images of tumor-bearing mice after intratumoral injection with FeS2-PEG (the red dotted circles). Reprinted with permission of Ref. [27]. Copyright 2017 Wiley.
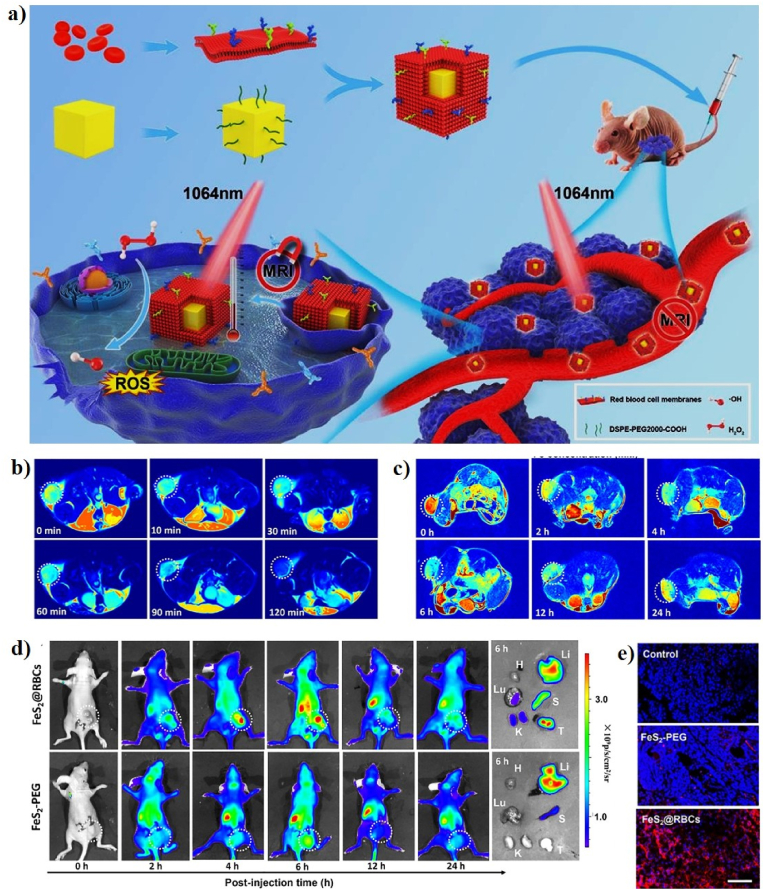
As a representative FeS2-based Fenton nanocatalyst in CDT, She et al. designed red blood cell membrane (RBC)-coated FeS2 for effective PT-enhanced CDT using an FDA-approved 1064 nm laser at 1.0 W/cm2 (Fig. 2a). FeS2 was synthesized by a facile solvothermal reaction and FeS2 was surface-modified with DSPE-PEG2000 to improve the water solubility (FeS2-PEG). RBC was finally coated on the surface of FeS2-PEG to form FeS2@RBCs. FeS2 has a cubic shape with an average diameter of 100–120 nm. The hydrodynamic size of FeS2@RBCs slightly increases from 168.3 nm (FeS2-PEG) to 185.2 nm, and their zeta potential of FeS2@RBCs was −10.3 mV because of the negative charge of RBCs. The PCE (η) of FeS2@RBCs was 30.2%. The synergetic effect of PT-enhanced CDT using FeS2@RBCs has been widely demonstrated in vitro and in vivo. The in vitro PT-enhanced CDT effect of FeS2@RBCs has been evaluated in 4T1 cells under laser irradiation, which showed a stronger inhibition rate of 4T1 cells when exposed to 1064 nm laser irradiation, suggesting the good synergistic PT-enhanced CDT antitumor effect upon 1064 nm laser irradiation. CDT relies on the Fe (FeS2@RBCs)-mediated Fenton reaction to induce cancer cell ferroptosis by catalyzing the decomposition of H2O2 into highly toxic •OH, which is only produced only in the TME without damaging healthy cells due to the high amount of H2O2 present in the tumor area. PT heating in the TME can significantly improve the Fenton reactivity and produce a high amount of •OH, which can effectively inhibit tumor growth with a clinically approved 1064 nm laser. In addition, FeS2@RBCs can be used for diagnosis and MRI-guided PTT/CDT. 4T1 tumor-bearing mice were intravenously and intratumorally injected with FeS2@RBCs to explore the MRI signal change in the TME, which shows the TME-enhanced capability of FeS2@RBCs as an MRI contrast agent for imaging-guided therapy (Fig. 2b, c). Furthermore, 4T1 tumor-bearing mice were intravenously injected with the cyanine5 amine dye (Cy5)-modified FeS2@RBCs for in vivo FLI. The Cy5-FeS2@RBCs exhibit strong fluorescent signals in the TME 6 h post-injection, indicating that FeS2@RBCs have superior accumulation at the tumor site, which is more beneficial for PT-enhanced CDT (Fig. 2d, e) [122].
Fig. 2.
(a) A schematic illustration of the preparation of FeS2@RBCs for PT-enhanced CDT. In vivo T2-weighted MR images of tumor-bearing mice at various time points before and after intratumoral (b) and intravenous (c) injection of FeS2@RBCs. (d) In vivo FLI of tumor-bearing mice at various time points before and after intravenous injection of Cy5-modified FeS2@RBCs and ex vivo FLI images of the tumors and main organs collected from the treated mice 6 h post-injection. (e) Confocal images of tumor tissues 6 h post-injection. DAPI and Cy5 are shown in blue and red, respectively. Reprinted with permission of Ref. [122]. Copyright 2020 Elsevier Ltd.
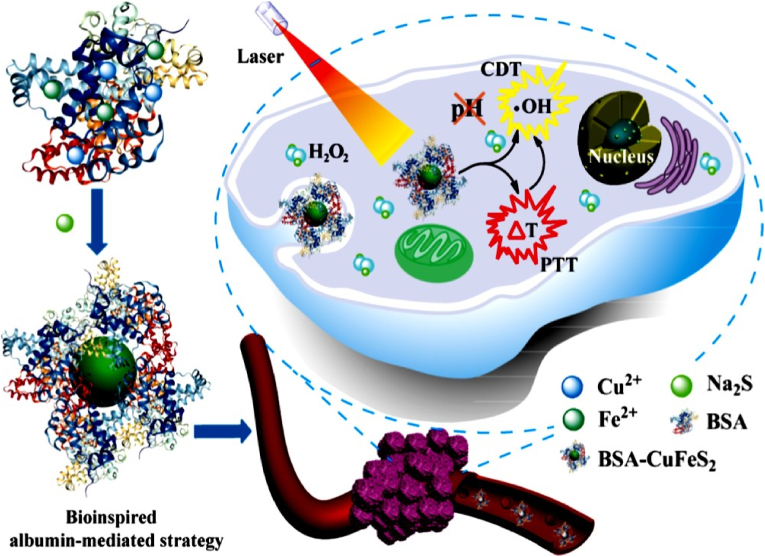
Currently, several ultra-small Fe-based nanomaterials have been developed as a multifunctional theranostic platform, which has a pH-independent Fenton reaction property to produce •OH and photothermal conversion efficiency for PT-enhanced CDT [109]. The ultra-small nanomaterials can be passed through the kidneys, allowing them to be eliminated more quickly from the body and increase long-term biosafety [150,151]. For example, Chen et al. synthesized ultra-small bovine serum albumin (BSA)-modified chalcopyrite NPs (BSA-CuFeS2 NPs) for PT-enhanced CDT (Fig. 3). BSA is a protein that has been utilized as a stabilizer to bind Fe and Cu ions due to the high affinity of carboxyl groups and surfactants. Na2S∙9H2O was then added to BSA-containing solutions to change the black color, implying the formation of BSA-CuFeS2 NPs. The particles of BSA-CuFeS2 NPs were mono-dispersed with an average size of 4.9 ± 0.9 nm. The PCE (η) value of the BSA-CuFeS2 NPs reached 38.8%, indicating their potential as PTT agents. BSA-CuFeS2 NPs exhibit pH-independent Fenton properties, which can produce a large amount of •OH in the weakly acidic TME. Subsequently, the heat generated by BSA-CuFeS2 NPs can enhance the •OH-generating efficacy, which is a synergistic effect in favor of efficient tumor growth inhibition. The FLI of 4T1 tumor-bearing mice was intravenously injected with BSA-CuFeS2 NPs at different time points. The fluorescence signal of the tumor area at 30 min post-injection increased 8.8-fold when compared with that in the control group, suggesting a high passive accumulation of BSA-CuFeS2 NPs at the tumor site. MR imaging showed a dramatic dark effect at the tumor site and the tumor MR signal rapidly decreased to 43.2%, indicating that the BSA-CuFeS2 NPs can serve as highly efficient MRI contrast agents to guide tumor ablation in vivo. More interestingly, the tumor treated with PTT/CDT was almost completely inhibited post-injection of the BSA-CuFeS2 NPs, suggesting that the BSA-CuFeS2 NPs exhibit the MRI-guided synergistic PT-enhanced CDT for highly efficient tumor ablation [109].
Fig. 3.
A schematic representation of the fabrication of BSA-CuFeS2 NPs for PT-enhanced CDT. Reprinted with permission of Ref. [109]. Copyright 2019 American Chemical Society.
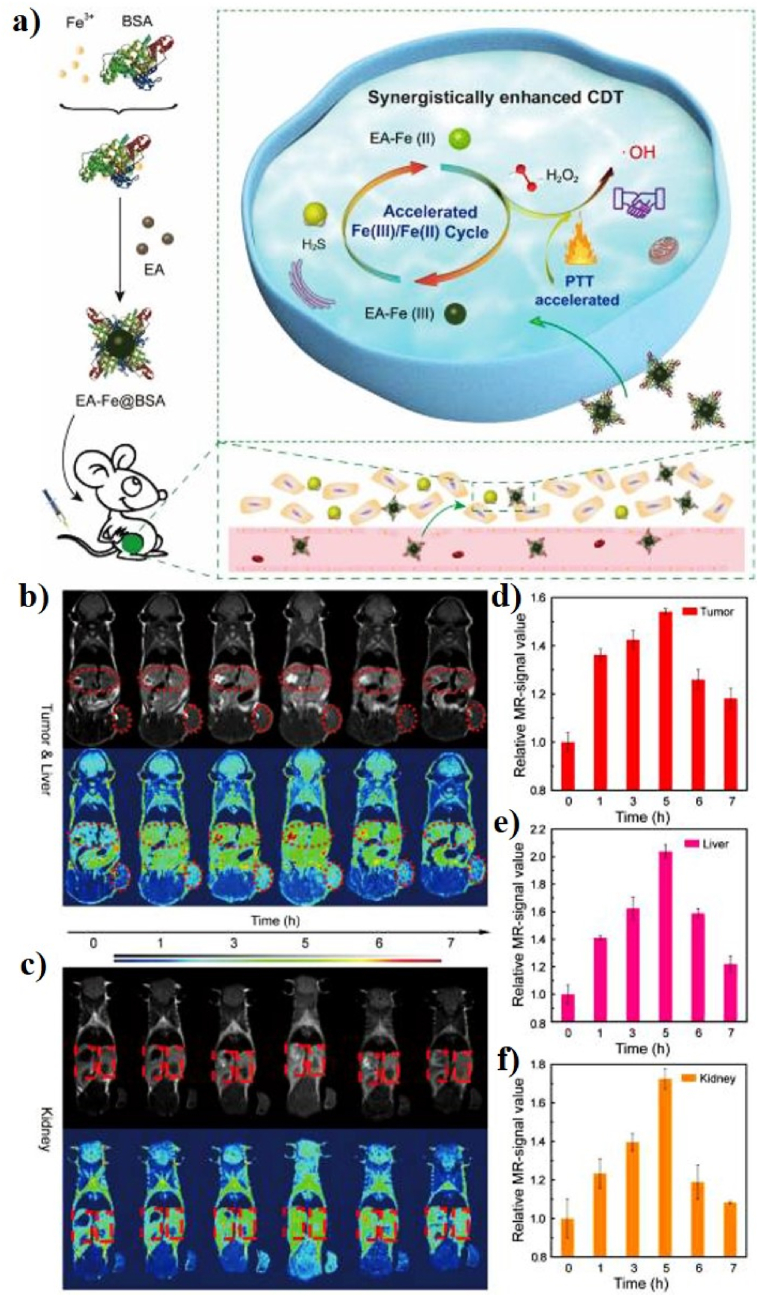
Another study was designed the ultra-small Fe-based nanomaterials for PT-enhanced CDT. In 2002, Tian et al. constructed ultra-small ellagic acid (EA)-Fe BSA (EA-Fe@BSA) NPs as a CDT agent for PTT to synergistically enhance CDT (Fig. 4a). EA-Fe@BSA was prepared via the simple assembly of EA, Fe(III), and BSA for endogenous hydrogen sulfide (H2S) and PT-enhanced CDT efficiency. The EA-Fe@BSA NPs show a monodispersed spherical morphology with an average diameter of 13.84 ± 2.53 nm and the PCE (η) of EA-Fe@BSA NPs before and after reaction with sodium hydrosulfide (NaHS) was ∼31.9 and 31.2%, respectively. The in vitro therapeutic efficacy of the EA-Fe@BSA NPs accelerates the production of •OH upon the addition of NaHS and increases the temperature by the heat generated via PT. NaHS was chosen to induce endogenous H2S to explore the H2S-stimulated CDT efficacy, which induced a greater conversion of Fe(III) in the EA-Fe@BSA NPs to Fe(II) and enhanced the CDT efficacy. The EA-Fe@BSA NPs exhibit obvious T1-weighted MRI both in vitro and in vivo, indicating their potential application as MRI contrast agents (Fig. 4b–f). More importantly, the PT heating effect of the EA-Fe@BSA NPs also accelerated the production of •OH via Fenton reactions in the tumor area. Even though H2S-enhanced CDT alone cannot inhibit the tumor, the synergy of H2S-enhanced CDT and PT-enhanced CDT almost completely inhibits and destroys the tumor, demonstrating outstanding enhanced CDT efficacy upon H2S and PTT acceleration [110].
Fig. 4.
(a) Schematic illustration of the synthesis of EA-Fe@BSA NPs for PT-enhanced CDT. In vivo MR images of the tumor and liver (b), and kidney (c) after intravenous injection of EA-Fe@BSA NPs. The corresponding MR signal intensities of the tumor (d), liver (e), and kidney (f) images. Reprinted with permission of Ref. [110]. Copyright 2020 IVYSPRING International Publisher.
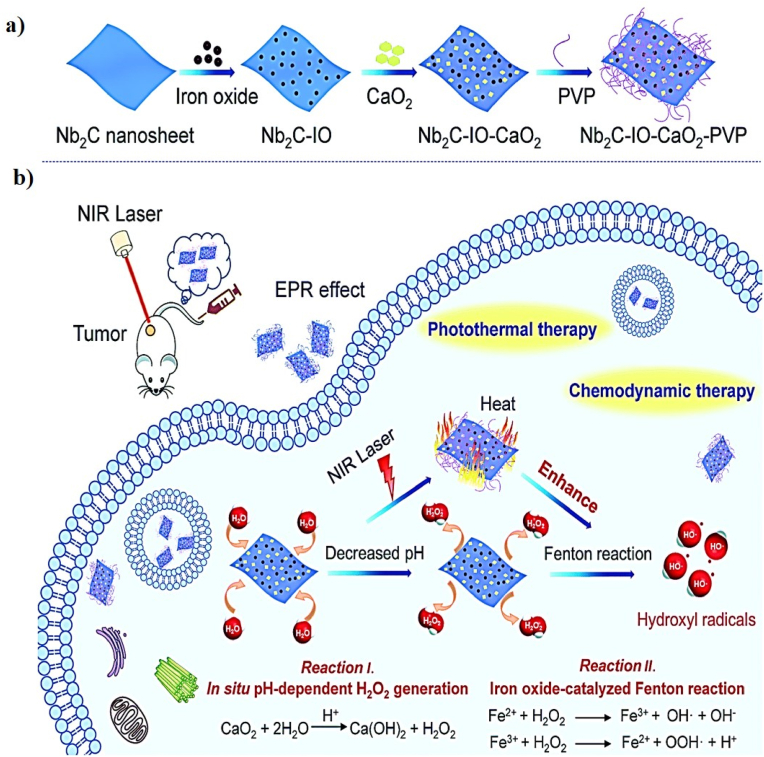
Iron oxide nanomaterials have broad potential in theranostic applications because of their excellent biocompatibility, photothermal conversion efficiency, and magnetic properties, which can be used as Fenton nanocatalysts for cancer therapy [152,153]. Similarly, Gao et al. reported the use of iron oxide (IO)-loaded niobium carbide (Nb2C) MXene nanosheets (Nb2C-IO) conjugated with calcium peroxide (CaO2) NPs to form two-dimensional (2D) composite nanosheets (Nb2C-IO-CaO2) for PT-enhanced CDT (Fig. 5a, b). The Nb2C and CaO2 NPs have average hydrodynamic sizes of 150 and 8 nm, respectively, and the zeta potential of Nb2C-IO-CaO2 is −5.3 mV. The PCE (η) of Nb2C-IO-CaO2 nanosheets, which act as potent and durable PT agents for PT-enhanced CDT, was 32.1%, which was higher than that of commonly reported photothermal agents, such as Fe3O4@CuS NPs (19.2%) [154] and Fe3O4 nanoclusters (20.8%) [155]. Nb2C-IO-CaO2 employs CaO2 as a potent H2O2 supplier to sustain the IO NP-mediated catalytic Fenton reaction to produce a high amount of •OH to induce tumor cell death. In addition, the Nb2C nanosheets also serve as PT agents to convert NIR light into heat, which can greatly improve the intratumoral •OH yield via Fenton reactions, leading to a more satisfactory therapeutic effect. Nb2C-IO-CaO2 exhibits significantly higher cytotoxicity after laser irradiation, and the maximum apoptosis was 93.3% in 4T1 cells, resulting in PT-enhanced ROS generation by the Fenton reaction produced by Nb2C-IO-CaO2 with self-supplied H2O2. The in vivo synergistic PT-enhanced CDT efficiency of Nb2C-IO-CaO2 was evaluated and the tumor temperature of mice injected with Nb2C-IO-CaO2 rapidly increased to 54.4 °C upon laser irradiation at 1064 nm, leading to the complete inhibition of tumor growth, implying that Nb2C-IO-CaO2 has promising potential as a tumor therapeutic agent for PT-enhanced CDT [117].
Fig. 5.
A schematic illustration of (a) the preparation of Nb2C-IO-CaO2 nanosheets and (b) PT-enhanced CDT utilizing Nb2C-IO-CaO2 nanosheets. Reprinted with permission of Ref. [117]. Copyright 2019 Royal Society of Chemistry.
Also, iron oxide-based Fenton nanocatalysts have excellent catalytic ability to generate •OH. For instance, Zhang et al. synthesized a novel iron oxide (FeO)/molybdenum disulfide (MoS2) modified with BSA (FeO/MoS2-BSA) for PT-enhanced CDT (Fig. 6a). 2D MoS2 nanosheets have been used as photoablation agents because of their strong NIR absorbance. In addition, MoS2 nanosheets have a peroxidase-mimicking ability, allowing them to catalyze the decomposition of H2O2 to produce •OH. FeO/MoS2 was synthesized via electrostatic interactions and then modified with BSA to enhance their biocompatibility. MoS2 has an average size of 150 nm, and FeO has an average size of 8 ± 3 nm. The PCE (η) of the FeO/MoS2-BSA nanocomposite at 1064 nm was calculated to be 56%, indicating its excellent PT performance. The Mo4+ ions on the surface of the MoS2 nanosheets can accelerate the conversion of Fe3+ into Fe2+, while Fe2+ ions can decompose H2O2 to produce a large amount of •OH via Fenton reactions, which can be further improved by the PT effect. The in vitro therapeutic effect of FeO/MoS2-BSA on HeLa cells was confirmed using calcein-AM and propidium iodide (PI) staining, which showed a stronger red fluorescence after 1064 nm laser irradiation, indicating the destruction of the HeLa cells by •OH and PT heat. The in vivo PT efficiency of the FeO/MoS2-BSA nanocomposites on the U14 tumor xenograft model was demonstrated using an IR thermal camera (Fig. 6b). The temperature of the tumor area was increased to 52 °C upon 1064 laser irradiation for 10 min and the treated tumors were thoroughly ablated after the 5th day of treatment, implying that synergistic PT-enhanced CDT has high anti-cancer efficiency. Furthermore, the MR signal of the tumor area was observed with an increase in the concentration of FeO/MoS2-BSA, confirming that FeO/MoS2-BSA can act as a promising MRI contrast agent (Fig. 6c) [127].
Fig. 6.
(a) A schematic illustration of the fabrication of FeO/MoS2-BSA for PT-enhanced CDT. (b) IR thermal images of tumor-bearing mice upon intravenous injection of normal saline and FeO/MoS2-BSA, followed by 1064 nm laser irradiation at 0.75 W/cm2 for 10 min. (c) In vivo MR imaging of tumor-bearing mice at various time points after intravenous injection of FeO/MoS2-BSA. Reprinted with permission of Ref. [127]. Copyright 2020 Springer.
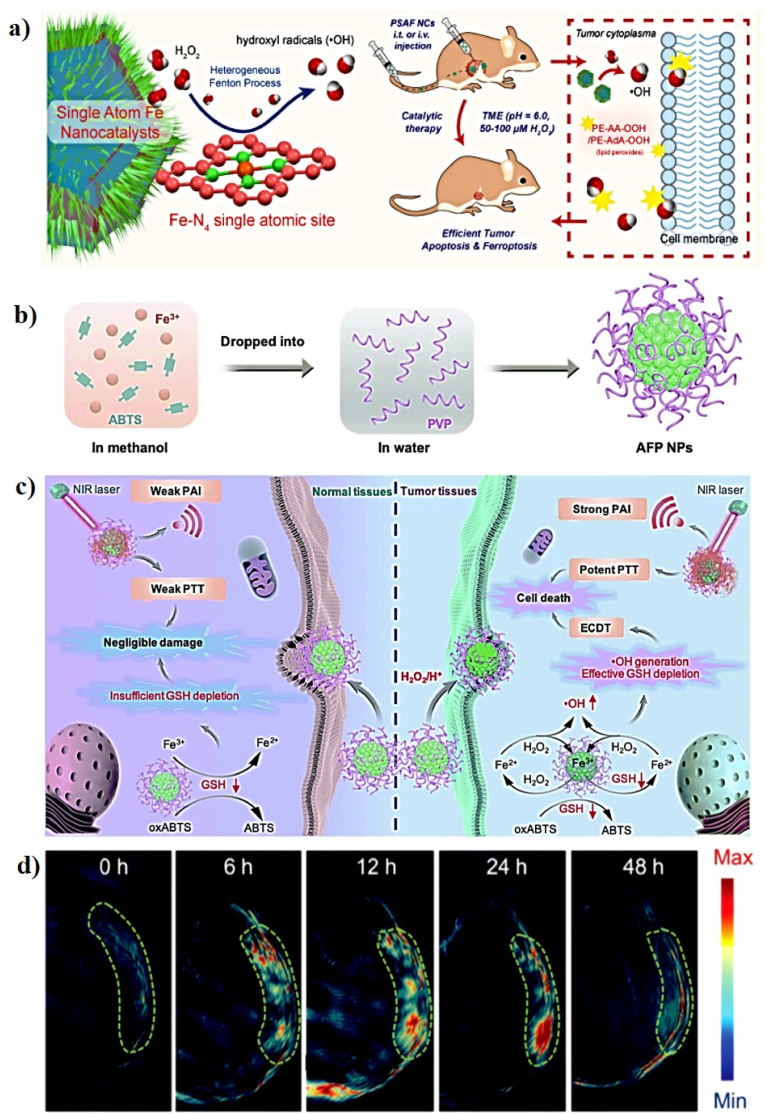
A number of Fe-based nanomaterials showed great potential for PT-enhanced CDT because of their photothermal conversion efficiency and •OH generation capacity via Fenton reaction [156]. In 2019, Huo et al. fabricated PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs) for highly efficient tumor inhibition via the Fenton reaction (Fig. 7a). Single-atom Fe catalysts (SAF NCs) have evolved to become highly efficient electrocatalysts, which will enable improved therapeutic efficiency in tumor-specific nanocatalytic therapy. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] (DSPE-PEG-NH2) is a linear heterobifunctional PEGylation reagent with a hydrophobic structure DSPE and amino-terminated PEG chain. The SAF NCs were PEGylated with DSPE-PEG-NH2 via hydrophobic-hydrophobic interactions after vigorous sonication to form the PSAF NCs. The SAF NCs exhibit a uniform dodecahedral geometry with an average diameter of ∼69.5 nm. When the PSAF NC aqueous solution (200 μg/mL) was irradiated with an 808 nm laser at 2.0 W/cm2 for 10 min, the temperature increased by 45 °C with high PT stability and strong PCE (19.21%). The in vitro therapeutic effects of PSAF NCs against 4T1 cells were evaluated, and cells treated with PSAF NCs + H2O2 exhibited a strong inhibitory effect. It is clear that the Fenton reaction catalyzed by PSAF NCs also resulted in the production of a large amount of toxic •OH and localized apoptosis and ferroptosis of tumor cells when exposed to H2O2 in the acidic TME, which can be effectively enhanced by the mild-PT effect. The in vivo catalytic therapeutic performance of PSAF NCs was evaluated and the PT-enhanced CDT of tumors could fully destroy the tumors in mice on the 5th and 9th days post-injection of PSAF NCs with laser irradiation [116].
Fig. 7.
(a) A schematic illustration of the preparation of PSAF NCs for PT-enhanced CDT. Reprinted with permission of Ref. [116]. Copyright 2019 American Chemical Society. (b) A schematic illustration of the synthesis procedure for AFP NPs. (c) Proposed mechanism for AFP NPs in the PT-enhanced Fenton-based CDT of tumor and normal tissues. (d) In vivo PA signals of the tumor area at various time points after intravenous injection of AFP NPs. Reprinted with permission of Ref. [118]. Copyright 2020 Royal Society of Chemistry.
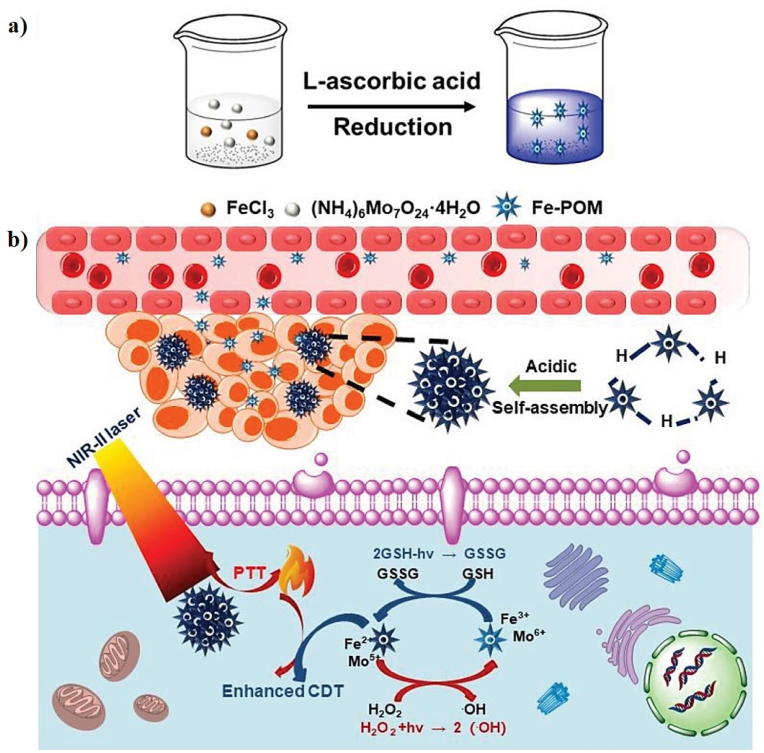
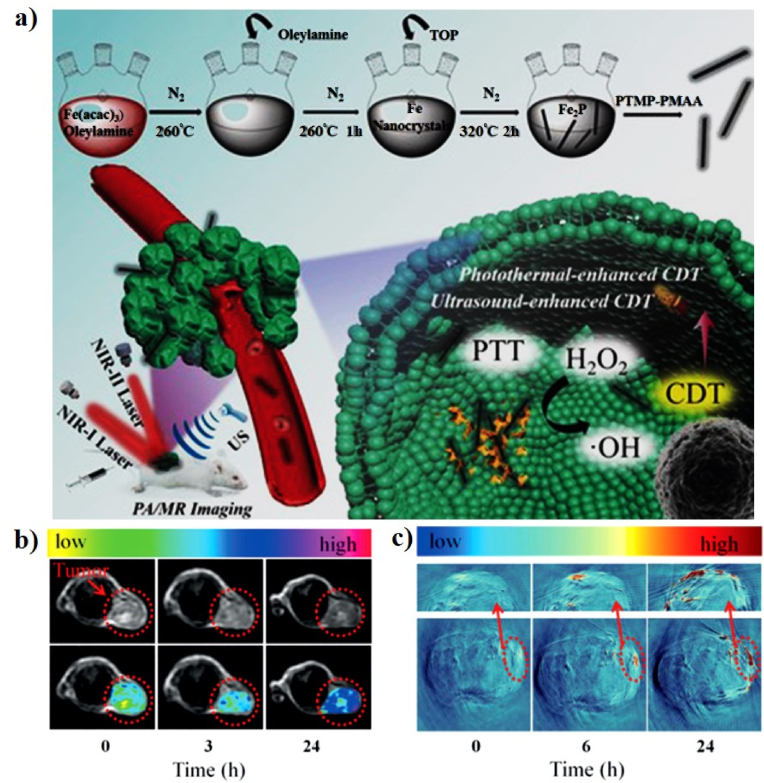
As a representative Fe-based Fenton nanocatalyst in CDT, Liu et al. developed a novel all-in-one ABTS-Fe/PVP (AFP) NP in which Fe3+ and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were dissolved in methanol, and the resulting solution mixed with polyvinylpyrrolidone (PVP) as a new CDT agent (AFP NP) for PAI-guided PT-enhanced CDT (Fig. 7b, c). ABTS is water-soluble horseradish peroxidase (HRP) substrate that can be catalyzed by HRP in the presence of H2O2 to generate its green oxidized form (oxABTS), which exhibits high absorption in the NIR region. PVP is a water-soluble polymer that coordinates to Fe3+ and Fe2+. The AFP NPs exhibit a sphere-like morphology with an average size of 242.3 nm and a zeta potential of −12.2 mV. The Fe3+ in the AFP NPs can produce •OH and deplete GSH in the presence of H2O2, demonstrating that AFP NPs can increase intracellular oxidative stress. The PA images and signals were observed at different time points post-injection of the AFP NPs and the PA signals gradually increase at the tumor site, demonstrating the rapid accumulation of AFP NPs at the tumor site through the enhanced permeability and retention (EPR) effect (Fig. 7d). To investigate the PT effect of AFP NPs, the tumor temperature of the AFP NPs was rapidly increased to 47.7 °C after laser irradiation, which was sufficient to kill the tumor, confirming that the AFP NPs show superior tumor-growth inhibition efficacy because of their efficient CDT through the simultaneous generation of •OH and depletion of GSH [118]. In a similar study, Shi et al. successfully prepared molybdenum (Mo)-based polyoxometalate (POM) cluster doped with Fe (Fe-POM) as an intelligent theranostic agent for PT-enhanced CDT in the NIR-II window (Fig. 8a, b). POM clusters were prepared via a facile synthesis procedure and FeCl3 and l-ascorbic acid were added to the POM solution to form Fe-POM. The Fe-POM had a highly uniform size and morphology with ultra-small diameters of 12.9 nm. The PCE (η) of Fe-POM was calculated to be 51.4%, suggesting the PT heating ability of Fe-POM in the NIR-II window. Fe2+ and Mo5+ can trigger the Fenton reaction, which can convert H2O2 into harmful •OH in the presence of Fe-POM and simultaneously deplete GSH for amplified CDT efficiency, confirming that Fe-POM can significantly improve the PT-catalytic efficacy. This PT effect can further improve the CDT effect, thereby achieving the synergistic effect of PT-enhanced CDT, which can effectively cause cancer cell death in vitro and fully inhibit the tumor in vivo. To obtain a more precise combination of PTT/CDT, PAI guidance will be beneficial for tracking the time-dependent tumor retention of Fe-POM. The PA signal observed at the tumor site gradually increases 2 h post-injection of Fe-POM, suggesting the rapid accumulation of Fe-POM in the tumor area [123].
Fig. 8.
A schematic illustration of the synthesis of Fe-POM (a) and (b) the synergistic mechanism of as-prepared Fe-POM applied in combination therapy. Reprinted with permission of Ref. [123]. Copyright 2020 Wiley.
In another example, Hu and co-workers successfully synthesized PB NCs and achieved the in situ reductions of FePt NPs, followed by coating with PEG and hyaluronic acid (HA) (PB@FePt-HA-g-PEG) for triple-modal imaging-guided PT-enhanced CDT (Fig. 9a). PB NCs have been used as new photo-absorbing agents for photothermal ablation therapy. FePt NPs can act as ferroptosis agents and have been used in MRI and CT imaging diagnoses. HA has been used as a specific targeting agent for cancer therapy. PEG is a synthetic polymer that can be used as a coating material on the surface of PB@FePt. The PB NCs have a nanocube structure with a hydraulic diameter of 150 nm. The PCE (η) of PB@FePt-HA-g-PEG was calculated to be 28.14%, implying that PB@FePt-HA-g-PEG possessed a good PT conversion ability. PB@FePt-HA-g-PEG shows a strong synergistic effect on cancer cell inhibition in vitro. PB@FePt-HA-g-PEG has been used in tri-model imaging diagnosis (MRI/CT/PT imaging) (Fig. 9b–d). Tumor-bearing mice were scanned using an MR, CT, and near-infrared (IR) thermal imaging system post-injection of PB@FePt-HA-g-PEG. The MR and CT signals of the tumor site increase significantly, suggesting that PB@FePt-HA-g-PEG has the potential to serve as an efficient CT/MR contrast agent for PT-enhanced CDT. The PT imaging ability of PB@FePt-HA-g-PEG was also investigated and the thermal signal of the tumor became dark red after laser irradiation, implying that the PB@FePt-HA-g-PEG can serve as a PT imaging agent. PB@FePt-HA-g-PEG can effectively catalyze the conversion of H2O2 in tumor-bearing mice into harmful •OH, which can effectively inhibit tumor growth under laser irradiation, implying that the PT-enhanced CDT effect of PB@FePt-HA-g-PEG effectively kills the cellular structure and promotes tumor ablation [124].
Fig. 9.
(a) A schematic illustration of the synthetic process used to construct PB@FePt-HA-g-PEG for PT-enhanced CDT. (b) In vivo MR images of tumor-bearing mice at various time points post-injection of PB@FePt-HA-g-PEG. (c) CT image of tumor-bearing mice injected with PB@FePt-HA-g-PEG. (d) IR thermal image of tumor-bearing mice injected with PB@FePt-HA-g-PEG. Reprinted with permission of Ref. [124]. Copyright 2020 Royal Society of Chemistry.
As a typical application, Chen et al. reported, for the first time, the use of biodegradable Fe-doped molybdenum oxide (MoOχ) ultra-fine nanowires (FMO) as Fenton reagents for PT-enhanced CDT. The FMO was synthesized using a single solvothermal technique and modified with PEG-4000. The ultra-fine FMO nanowires had a uniform thickness and lengths of ∼450 nm. The PCE (η) of FMO reached 48.5% under 808 nm laser irradiation, implying that FMO has outstanding PEC and thus strong potential as a PT agent for CDT. The in vitro synergistic therapeutic effect of FMO on tumor cells was evaluated. The Fe2+ and Mo5+ in the FMO can effectively catalyze the decomposition of H2O2 and generate •OH via the Fenton reaction, and subsequently, the intracellular GSH can also be depleted, which may further enhance tumor cell death. The FMO-treated cells were co-stained and observed under a fluorescence microscope. The FMO + H2O2 + laser showed a strong red fluorescence, indicating that the synergistic therapeutic effect of •OH and PT heat had an improved killing efficacy on tumor cells. The tumor-bearing mice were scanned before and after the intratumoral injection of FMO, and the MR signal was observed at the tumor site, indicating that the FMO can be applied as a potential contrast agent for T1-weighted MRI. The tumor temperature in mice injected with the FMO solution was increased up to 50 °C upon laser irradiation for 10 min and the treated tumors completely disappeared within 15 days, indicating that FMO has great potential for use in MRI-guided PT-enhanced CDT [125].
Recently, Zhao and co-workers reported the use of Fe3O4 magnetic NPs and bismuth trisulfide (Bi2S3) integrated virus-like Fe3O4@Bi2S3 nanocatalysts (F-BS NCs) modified with BSA (F-BSP NCs) for PT-enhanced CDT. Bi2S3 has been used as a PT agent for PTT because of its excellent in vivo biocompatibility, stability, and high PCE, and ease of synthesis. Fe3O4 magnetic NPs exhibit peroxidase-like activity. The Fe3O4 magnetic NPs were synthesized using a simple hydrothermal technique, which was dispersed in a thioacetamide (TAA) and Bi(NO3)3 solution under ultrasonication to form virus-like Fe3O4@Bi2S3 (F-BS NCs) and then modified with BSA (F-BSP NCs) to enhance their stability and in vivo biocompatibility. The Fe3O4 NPs have an average diameter of 80 nm. The zeta potentials of F-BS NCs and F-BSP NCs were −8.05 and −17 mV, respectively. The PCE (η) of the F-BS NCs was 23.46%. The Fe2+ ions in the F-BSP NCs react with H2O2 to produce highly toxic •OH via a mild PT effect and sequential Fenton reactions, which can further improve the CDT effect. The in vitro anticancer therapeutic efficacy of F-BSP NCs shows the highest apoptosis and necrosis after laser irradiation. The in vivo therapeutic ability and PAI performance of F-BSP NCs were further investigated in tumor-bearing mice. The tumor temperature of the F-BS NCs reached 59.1 °C upon laser irradiation and the treated tumor almost disappeared after 14 days of treatment, suggesting that the synergistic PT-enhanced CDT has an excellent therapeutic effect. The F-BS NCs also showed a clear PA signal in the tumor after intratumoral injection, confirming that the F-BS NCs can serve as ideal diagnostic PAI agents for diagnosis and treatment [128].
3.2. Copper-based nanomaterials for PT-enhanced CDT
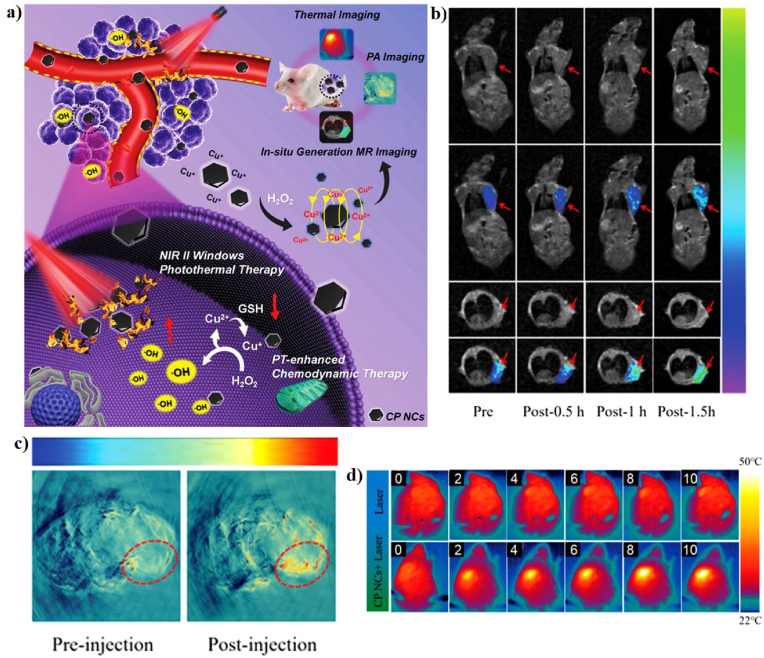
Recently, copper-based nanomaterials have found many applications in a variety of research fields in CDT as potential alternatives for iron-based nanomaterials with promising developments and progress [99]. Copper (Cu) ions can also drive the Fenton-like reactions that prompt the decomposition of H2O2 to produce •OH to induce cancer cell apoptosis [36,38]. More importantly, CDT triggered by copper ions can start under neutral and very mild acidic conditions, which accurately matches the TME [56]. Cu-based nanomaterials are currently explored as new PT agents due to their low biotoxicity, easy fabrication, and low cost [61]. For example, Liu et al. reported, for the first time, the use of trithiol-terminated poly-(methacrylic acid) (PTMP-PMAA)-modified copper(I) phosphide nanocrystals (CP NCs) for MRI-guided PT-enhanced CDT (Fig. 10a). Cu(I) phosphide was synthesized from Cu(II) chloride dehydrate (CuCl2·H2O) and triphenyl phosphite (TPOP) at high temperature to form Cu3P, which was then coated with PTMP-PMAA to form the CP NCs to enhance their hydrophilicity. The CP NCs exhibit a hexagonal morphology with an average particle size of ∼22 nm. The temperature of the CP NC aqueous solutions reached 51 °C after 10 min of laser irradiation, suggesting that the CP NCs have excellent PT properties. The PCE (η) of the CP NCs was determined to be 27%, which can further improve the Fenton-like reaction. The MR and PA imaging properties were investigated in vitro and in vivo. The T1-MR signal of the tumor region was significantly enhanced after injection of the CP NCs, indicating that CP NCs can be used as a new in situ self-generation MRI contrast agent (Fig. 10b). The PA signal in the tumor area was notably enhanced 12 h post-injection of the CP NCS, indicating the potential PA imaging ability of CP NCs (Fig. 10c). The Cu(I) on the surface of CP NCs is more effective in the weakly acidic TME with a high production of •OH and triggers the apoptosis of cancer cells, suggesting that CP NCs have excellent •OH generation capability with the help of 1064 nm laser irradiation. The in vivo synergistic therapeutic effect of CP NCs on U14 tumor-bearing mice was confirmed using IR thermal camera (Fig. 10d). The tumor temperature of mice injected with the CP NC solution was increased up to 48 °C upon 1064 nm laser irradiation and tumor growth was significantly inhibited on the 5th day. Furthermore, the excess GSH in the TME can further reduce the Cu(II) produced by the Fenton-like catalytic reaction to Cu(I), enhancing the production of •OH, indicating that the CP NCs have great potential for use in MRI/PAI-guided PT-enhanced CDT [130].
Fig. 10.
(a) A schematic illustration of the synthesis of CP NCs and their application in synergistic combination therapy. In vivo T1-MR (b) and PA (c) imaging of tumor-bearing mice. (d) In vivo IR thermal images of tumor-bearing mice. Reprinted with permission of Ref. [130]. Copyright 2019 Wiley.
Cu-based nanomaterials have become research hotspots in the field of biomedicine in recent decades because of their excellent photothermal conversion efficiency, biocompatibility, and Fenton-like properties [65]. Compared with other copper-based nanomaterials, copper selenide (Cu2Se) nanomaterials are particularly interesting due to their strong absorption in NIR window and Fenton-like properties, and Cu and selenium (Se) are considered as important trace elements in the human body [157,158]. For example, Wang and co-workers successfully synthesized PEG-coated copper selenide (Cu2Se) hollow nanocubes (HNCs) as a promising catalyst for PT-enhanced CDT (Fig. 11a, b). The HNCs were prepared via an anion exchange method using Cu2O nanocubes and then modified with thiolated PEG to enhance the stability and dispersibility (PEG-Cu2Se HNCs). The PEG-Cu2Se HNCs show excellent biocompatibility, high PCE, and strong Fenton-like catalytic properties. The Cu2Se HNCs have a regular cubic morphology with an average diameter of 86.89 ± 19.93 nm. The PCE (η) of the Cu2Se HNCs was 50.89% in the NIR-II window, which was significantly higher than that of commonly reported photothermal agents, such as Au–Cu9S5 NPs (37%) [159] and Cu3BiS3 nanorods (40.7%) [160]. In vitro synergistic treatment with PEG-Cu2Se HNCs + H2O2 + laser irradiation had a significant inhibitory effect on 4T1 cells. The strong NIR absorbance of the PEG-Cu2Se HNCs indicates their potential for use in PAI. A strong PA signal was observed at the tumor site after injection of PEG-Cu2Se HNCs, suggesting that the PEG-Cu2Se HNCs can serve as an outstanding agent for PAI. The PEG-Cu2Se HNCs exhibit a strong Fenton-like reaction rate in the presence of H2O2, which can produce a large amount of •OH and significantly improve the therapeutic efficacy of the Fenton-like catalyst to produce •OH, indicating that the mild PT effect with CDT can completely eradicate tumors [41].
Fig. 11.
(a) A schematic illustration of the preparation of PEG-Cu2Se HNCs. (b) Proposed mechanism of PEG-Cu2Se HNCs for PT-enhanced CDT in the NIR-II window. Reprinted with permission of Ref. [41]. Copyright 2019 American Chemical Society. A schematic illustration of the synthesis of PDA@Cu/ZIF-8 NPs (c) and its application in combination therapy (d). Reprinted with permission of Ref. [72]. Copyright 2020 Elsevier Ltd.
In another study, An et al. reported Cu2+-doped zeolitic imidazolate framework-8-coated polydopamine (PDA) NPs (PDA@Cu/ZIF-8 NPs) for GSH-triggered and PT-enhanced CDT (Fig. 11c, d). PDA NPs were synthesized via solution-phase oxidation and Cu/ZIF-8 was coated on the surface of PDA to achieve highly uniform PDA@Cu/ZIF-8. PDA acts as a PT agent that can produce heat under laser irradiation to generate •OH and deplete GSH, which significantly retards the growth of xenograft tumors. PDA NPs have an average diameter of 50 nm. The temperature of the PDA@Cu/ZIF-8 NP solutions increases to 70 °C after 10 min of laser irradiation, indicating their potential as a PT agent. The in vitro anticancer effect of PDA@Cu/ZIF-8 NPs resulted in increased cell death after laser irradiation, implying that the rationally designed PDA@Cu/ZIF-8 NPs for new PTT/CDT treatment can serve as a novel anticancer agent. The synthesized PDA@Cu/ZIF-8 NPs can react with intracellular GSH to simultaneously deplete GSH. In addition, Cu + decomposes H2O2 to produce a large amount of •OH via Fenton-like reactions, which can be further enhanced by the PT effect, causing GSH-controlled ROS generation and GSH depletion in the tumor area. The in vivo PT-enhanced CDT results suggest that PDA@Cu/ZIF-8 NPs exhibit excellent tumor-killing ability [72].
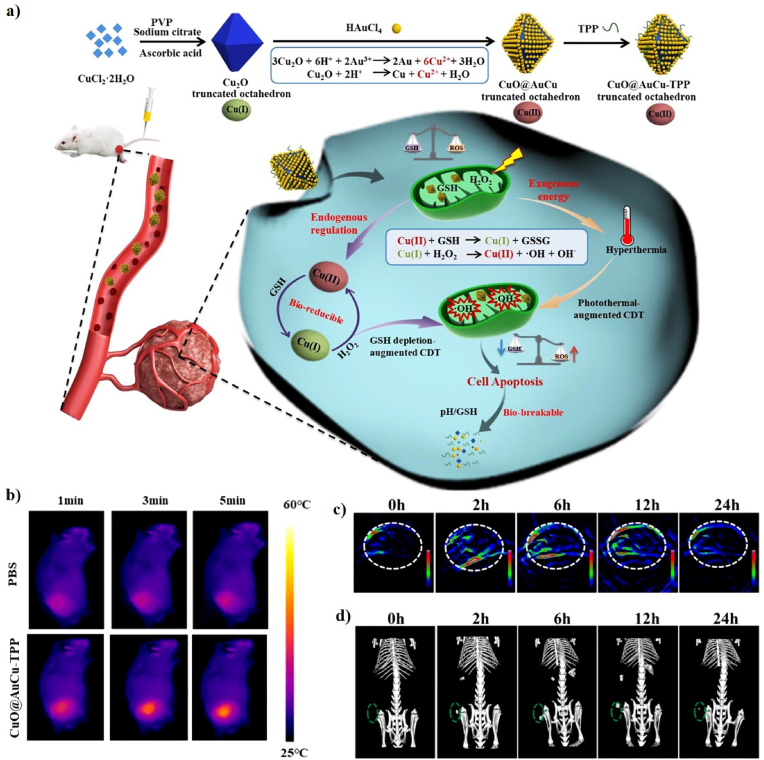
Currently, copper-oxide (Cu2O) nanocrystals have been reported to have a variety of morphologies, including spheres, nanocubes, triangular nanoplates, and octahedrons [[161], [162], [163]]. More interestingly, the truncated octahedron is considered an excellent candidate for the Fenton-like catalytic activity [132]. Similarly, Li et al. developed a triphenylphosphonium (TPP)-conjugated gold-coated copper(II)-based truncated octahedron (CuO@AuCu-TPP) as a Fenton-like catalyst for PA/CT imaging-guided PT-enhanced CDT (Fig. 12a). Cu2O was prepared by reducing a solution of copper citrate complexed with ascorbic acid and the Au NPs were then covered on the surface of the Cu2O via a disproportional reaction. Finally, the TPP as a targeting group was conjugated at the end through electrostatic interactions to form CuO@AuCu-TPP. The CuO@AuCu-TPP showed a truncated octahedral structure, with an average particle size of 255 nm and a zeta potential of −7 mV. The PCE (η) of CuO@AuCu-TPP with 808 nm laser irradiation was calculated to be 37.9%, indicating its outstanding PT properties. Cu(II) was reduced to Cu(I) because of the intracellular GSH, which simultaneously depletes GSH, and Cu (I) can react with H2O2 to disrupt the redox balance in the mitochondria via •OH generation and GSH consumption via Fenton-like reactions. The in vitro anticancer efficacy of CuO@AuCu-TPP significantly inhibited HeLa cell viability under laser irradiation, indicating that PT heat can effectively enhance the efficiency of •OH generation. The in vivo synergistic therapeutic efficacy of Au on the surface of CuO@AuCu-TPP showed an excellent PT effect upon laser irradiation, which causes the tumor temperature to increase and enhance the speed of the Fenton-like reaction, thus enabling a synergistic PT effect, Fenton-like reaction, and accurate mitochondrial-targeting ability to achieve strong therapeutic effects (Fig. 12b). In addition, PA and CT images were obtained in mice and strong PA and CT signals at the tumor site were observed 6 h post-injection, indicating that CuO@AuCu-TPP shows great promise for therapeutic application in cancer diagnostics (Fig. 12c, d) [132].
Fig. 12.
(a) A schematic illustration of the synthesis of CuO@AuCu-TPP and the therapeutic mechanism of CuO@AuCu-TPP. (b) IR thermal images of mice. In vivo PA (c) and CT (d) images post-injection of CuO@AuCu-TPP. Reprinted with permission of Ref. [132]. Copyright 2020 Elsevier Ltd.
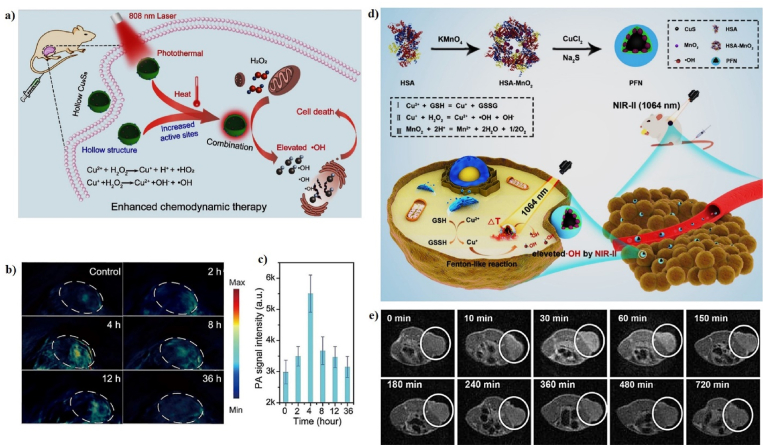
In addition, copper sulfide (CuS) NPs can be used as a theranostic agent because of their PT, PA, and Fenton-like properties [70]. For example, Wang et al. developed hollow and solid copper sulfide NPs (Cu9S8 NPs) as a Fenton-like agent for PT-enhanced CDT (Fig. 13a). The copper sulfide NPs have a hollow sphere structure with an average diameter of ∼18.05 nm and shell thickness of ∼3.50 nm. The hollow Cu9S8 NPs show excellent chemodynamic treatment effects for cancer, both in vitro and in vivo, due to the •OH generated from endogenous H2O2 under the catalytic conditions of copper ions. Subsequently, the mild temperature generated from the PT effects can effectively improve the CDT efficacy. 4T1 tumor-bearing mice were intravenously injected with Cu9S8 NPs at various time points and the PA images of the tumor site slowly changed from black to blue over 2–4 h (Fig. 13b, c), suggesting an increase in the signal strength, which can be used to provide useful information for guiding PT-enhanced CDT [64]. In another study, Sun et al. developed a NIR-II PT Fenton-like nanocatalyst (PFN), consisting of human serum albumin (HSA), manganese dioxide (MnO2), and copper sulfide (CuS) for MRI-guided PT-enhanced CDT (Fig. 13d). PFN was synthesized using a two-step technique. HAS-encapsulated MnO2 NPs were prepared using a redox reaction and the CuS NPs were deposited via a biomimetic mineralization process to form PFN. CuS NPs are promising Fenton-like nanocatalysts that react with H2O2 to generate •OH for PT-enhanced CDT. The MnO2 NPs decompose into Mn2+, which is a prominent imaging agent for T1-weighted MRI. The PFN generates local heat under 1064 nm laser irradiation, which accelerates the Fenton-like reactions. The particle size of PFN was 22 nm and its zeta potential was −18.7 mV because of the negative charge of CuS. The PCE (η) of PFN was 30.17%, implying that it is a good PT agent. The in vitro anticancer efficacy of PFN + H2O2 + laser irradiation showed the highest level of apoptotic cells, implying synergistic NIR-II PTT and CDT. The tumor temperature of mice injected with PFN gradually reached 55 °C after 5 min under laser irradiation and the treated tumor was completely inhibited without any reoccurrence, implying that the PFN shows superior PT-heating ability and potent antitumor efficiency in vivo. The MR signal in the tumor regions reached its brightest at 30 min post-injection of PFN, indicating the effective accumulation of PFN in tumors and demonstrating their, utility as an activatable MRI contrast agent for cancer therapy (Fig. 13e) [135].
Fig. 13.
(a) A schematic illustration of the fabrication of hollow Cu9S8 NPs for PT-enhanced CDT. In vivo PA images (b) and PA signal (c) of tumor-bearing mice before and after intravenous injection of hollow Cu9S8 NPs. Reprinted with permission of Ref. [64]. Copyright 2020 Elsevier Ltd. (d) A schematic illustration of PFN for PT-enhanced CDT. (e) In vivo T1-weight MR images at different time points. Reprinted with permission of Ref. [135]. Copyright 2020 American Chemical Society.
3.3. Gold-based nanomaterials for PT-enhanced CDT
Gold-based nanomaterials are the most well-studied nanomaterials for biomedical applications, such as biosensors, therapy, and multimodal imaging [[164], [165], [166], [167], [168], [169], [170]]. Many researchers are currently using gold-based nanomaterials as enzyme mimetics and have greatly expanded the number of new therapeutic applications derived from their enzyme-like functions [171,172]. Gold-based nanomaterials have been used in a variety of research fields in PT-enhanced CDT because of their strong NIR absorption and inherent catalytic properties [137,173]. For example, Fan et al. reported novel nanozymes with a yolk-shell structure in which the gold (Au) NP core was coated with a hollow carbon nanosphere with a porous shell (Au@HCNs) for PT-catalytic therapy. Au NPs have been one of the most widely studied nanozymes in recent years that generate ROS because of their intrinsic enzyme-like activities, and they are also good NIR-absorbing agents. AuNPs were synthesized according to a literature method and then coated with SiO2 using the Stӧber method, yielding Au@SiO2 NPs. PDA was polymerized on the surface of Au@SiO2, yielding Au@SiO2@PDA NPs, which were carbonized under a N2 atmosphere for 2 h at 800 °C and named as Au@HCNs. The Au@HCNs have an average size of 275 ± 0.355 nm and a PCE value (η) of 26.8%. The enzyme-like activity of Au@HCNs can produce ROS, such as •OH and superoxide radicals (•O2−), and PT heat can improve the generation of ROS to accelerate the enzymatic reactions. The in vitro anticancer effect of Au@HCNs significantly inhibits the growth of cancer cells after laser irradiation and generates ROS, inducing cell death. The temperature of Au@HCNs-treated tumors rapidly increases from 33 to 52.9 °C under laser irradiation for 10 min, leading to their complete disappearance without recurrence after treatment. Au@HCNs can react with H2O2 and O2 in the TME to produce toxic ROS, resulting in cell death, which may be enhanced by the mild PT effect [137].
3.4. Other metal oxide- and sulfide-based nanomaterials for PT-enhanced CDT
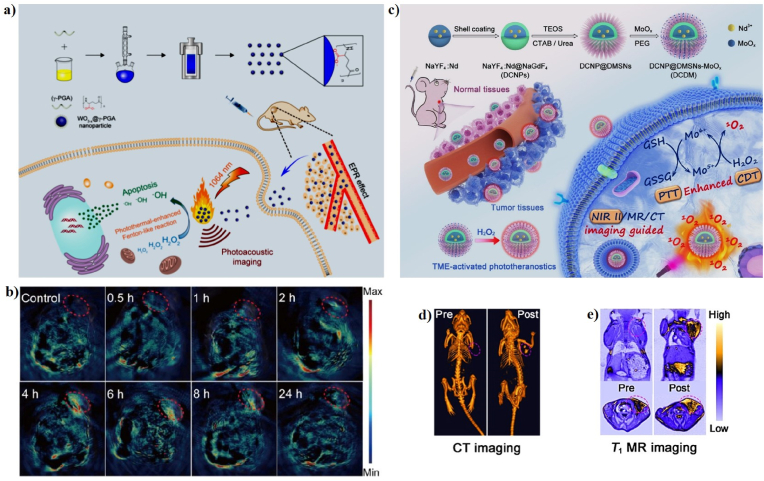
Several transition metals have also been used as Fenton-like agents for PT-enhanced CDT [107,174]. The tungsten trioxide (WO3−x) has been used as a PT material for PTT because of its strong absorption in the NIR region and has also been used as a Fenton-like agent for CDT [107,[175], [176], [177]]. In 2018, Liu et al. successfully prepared ultrasmall tungsten trioxide (WO3−x) NPs, which were then coated with γ-poly-l-glutamic acid (γ-PGA) for PAI and effective PT-enhanced CDT (Fig. 14a). The WO3−x@γ-PGA NPs were synthesized via a simple hydrothermal reaction using γ-PGA as a ligand and WCl6 as the tungsten source. The WO3−x@γ-PGA NPs have a monodispersed irregular morphology with an average particle size of 5.73 ± 0.93 nm. The 1064 nm laser PCE (η) of the WO3−x@γ-PGA NPs was 25.8%, as calculated from the heating and cooling curves. More importantly, the Fenton-like reaction occurred for WO3−x@γ-PGA NPs in the presence of H2O2, indicating that the WO3−x@γ-PGA NPs can react with H2O2 to generate a large amount of •OH; this Fenton-like reaction was promoted by the PT effect. Most of the cells were killed in the presence of WO3−x@γ-PGA NPs + H2O2 + laser irradiation, which can be attributed to the in vitro PT-enhanced CDT. The tumors of mice in the presence of WO3−x@γ-PGA NPs + laser irradiation gradually decrease and almost disappear after 18 days, indicating that WO3−x@γ-PGA NPs can cure tumors after laser irradiation. PAI was employed to detect the accumulation of nanomaterials in the tumor areas to guide PT-enhanced CDT. 4T1 tumor-bearing mice were intravenously injected with the WO3−x@γ-PGA NPs. PA images of the mice were scanned before and after injection at various time points (Fig. 14b). The strongest PA signal was observed at 4 h post-injection, suggesting that the WO3−x@γ-PGA NPs have great potential for use in PAI [24].
Fig. 14.
(a) A schematic illustration of the synthesis of WO3−x@γ-PGA NPs for PT-enhanced CDT. (b) In vivo PA imaging of tumor-bearing mice before and post-injection of WO3−x@γ-PGA NPs. Reprinted with permission of Ref. [24]. Copyright 2018 American Chemical Society. (c) A schematic illustration of the synthesis of DCDMs for PT-enhanced CDT. In vivo CT (d) and MR (e) images of tumor-bearing mice before and post-injection of DCDMs. Reprinted with permission of Ref. [139]. Copyright 2020 American Chemical Society.
Currently, molybdenum oxide NPs (MoOirst time, the use of biodegradable NPs) have aroused worldwide interest because of their excellent PT conversion efficiency and Fenton-like properties [178]. For instance, Dong et al. developed PEGylated down-conversion NPs (DCNPs) with dendritic mesoporous silica (DMSN) and then loaded ultra-small molybdenum oxide NPs (MoOirst time, the use of biodegradable NPs) as all-in-one nanotheranostic agents (PEG-DCNP@DMSN-MoOirst time, the use of biodegradable NPs) for multimodal imaging-guided PT-enhanced CDT (Fig. 14c). DCNP@DMSN had a uniform hexagonal structure with an average diameter of 123 nm. The PCE (η) of PEG-DCNP@DMSN-MoOirst time, the use of biodegradable NPs (DCDMs) was 51.5%, implying that the DCDMs possess excellent PT properties. Mo5+ can efficiently catalyze the decomposition of H2O2 to form a considerable amount of singlet oxygen (1O2) in the mildly acidic TME, leading to irreversible cell death. Subsequently, the generated Mo6+ can effectively decrease the levels of GSH in the tumor area, and the GSH depletion and PT effect can further accelerate the generation of 1O2 and enhance CDT. The synergetic PT-enhanced CDT of DCDMs effectively inhibits apoptosis in vitro and killed the tumors in vivo. Moreover, the CT and MR signals of the tumor area 12 h post-injection were obviously higher than those prior to injection, implying that the DCDMs are multimodal imaging contrast agents for clinical diagnosis (Fig. 14d, e) [139].
As a representative manganese dioxide (MnO2)-based Fenton nanocatalyst in CDT, Wang et al. designed a gold@manganese dioxide (Au@MnO2) core-shell nanostructure for PAI/MRI-guided PT-enhanced CDT and GSH-triggered CDT. Au@MnO2 has a uniform core-shell structure with an average size of ∼25 nm. The PCE (η) of Au@MnO2 after being triggered by GSH was 23.6%, demonstrating that Au@MnO2 had good PT properties. The GSH-triggered and PT-enhanced CDT of Au@MnO2 can efficiently catalyze the generation of •OH from H2O2 because of the high amount of Mn2+ produced upon the reduction of MnO2 by GSH and the PT effect can accelerate the production of •OH, indicating that Au@MnO2 has promising potential as a GSH-triggered smart theranostic agent for PAI/MRI-guided PT-enhanced CDT. The in vitro and in vivo therapeutic effects of Au@MnO2 with laser irradiation show the highest apoptosis and necrosis rates because of the synergistic effect of the GSH-triggered Au@MnO2 agents for PT-enhanced CDT. The complementary effects of MRI and PAI can efficiently enhance the diagnosis of cancer. PA and T1-weighted MR imaging of mice were performed at various time points post-injection of Au@MnO2. The T1-weighted MR signal was observed in the tumor area and the GSH-triggered PAI of Au@MnO2 was similar to that of MRI, indicating that Au@MnO2 has promising potential as a GSH-triggered smart theranostic agent for PAI/MRI-guided PT-enhanced CDT [43].
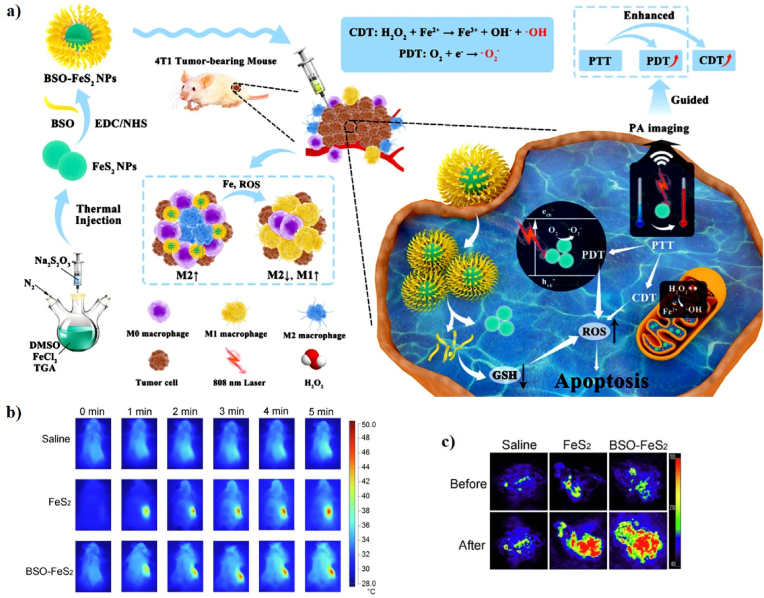
In another similar recent study, An et al. constructed PEG-decorated MnO2@HMCu2−xS nanocomposites (HMCMs), consisting of manganese dioxide (MnO2) and hollow mesoporous copper sulfide (HMCu2−xS), for PT-enhanced CDT (Fig. 15a). The particle size of MnO2 was 80 nm. The PCE (η) of HMCM was 22.3%, implying that HMCM has great potential as an ideal PT agent. MnO2 reacts with GSH, converting Mn(IV) to Mn(II) to enhance the Fenton-like reaction with additional •OH produced due to the increased generation of Mn2+ under laser irradiation. The in vitro therapeutic effect of HMCM with laser irradiation showed higher cytotoxicity to MCF-7 cells, which caused higher Mn2+ accumulation in the cells, allowing for the Fenton-like reaction to accelerate endogenous H2O2 for a high level of ROS production. The in vivo anticancer effects of HMCMs with laser irradiation efficiently eliminate tumor growth and showed a high tumor inhibition rate of 75%, demonstrating that HMCMs have superior anticancer efficiency [46]. In addition, Wang et al. developed biodegradable cobalt sulfide (CoS2) nanoclusters (CoS2 NCs) for the PT-enhanced CDT of cancer cells via Fenton-like catalytic activity (Fig. 15b). Cobalt chalcogenides have received a lot of attention in the field of biomedicine because of their high PT properties, biological safety, and catalytic properties. The CoS2 NCs were prepared via a simple one-pot solvothermal method using a cobalt and sulfur source. The CoS2 NCs have a spherical morphology with an average diameter of 19.79 ± 5.2 nm. The PCE (η) of the CoS2 NCs was 60.4%. The CoS2 NCs can generate •OH in the presence of H2O2 and PT heat accelerates the efficacy of the Fenton-like reaction. The in vitro PT-enhanced CDT based on CoS2 NCs was concentration-dependent, which can significantly improve their therapeutic effect on cancer. 4T1 tumor-bearing mice were injected with CoS2 NCs and laser-irradiated, resulting in complete inhibition and no reoccurrence. In addition, strong PA signals were observed at the tumor site after injection of CoS2 NCs, suggesting that CoS2 NCs have excellent PAI ability [42].
Fig. 15.
(a) A schematic illustration of the synthesis and working mechanisms of HMCMs. Reprinted with permission of Ref. [46]. Copyright 2019 American Chemical Society. (b) A schematic illustration of the fabrication of CoS2 NCs for PT-enhanced CDT. Reprinted with permission of Ref. [42]. Copyright 2020 Elsevier Ltd.
4. Combination therapy
Monotherapies such as PTT, CDT, CHT, SDT, PDT, IMT, and ST, have been extensively used in preclinical and clinical studies, and many researchers have reported that monotherapy is not as effective as expected because of the development of resistance and side effects over a long-term period of treatment [179]. Combination therapy commonly refers to the simultaneous co-delivery of drugs or combined-therapeutic strategies in various forms for the effective treatment of cancer [68]. Combination therapy has attracted an increasing amount of clinical attention because of its superior advantages in overcoming the intrinsic drawbacks of monotherapy [90]. The combination of PT-enhanced CDT with CHT, SDT, PDT, IMT, and ST has emerged as a potential alternative to monotherapies due to their synergistic therapeutic effects [99]. In addition, monomodal imaging (e.g., PAI, FLI, MRI, and CT alone) has been widely studied in clinical disease diagnosis, but there are still some short-comings and unsolvable problems that need to be overcome in multimodal imaging (PAI/FLI/MRI/CT multimodal diagnostic imaging) techniques to provide information for the effective diagnosis of disease [180]. Multimodal imaging (PAI/FLI/MRI/CT multimodal diagnostic imaging-guided combination cancer therapy) is also essential for the early stage of diagnosis, prognosis, and therapeutic effects of the tumor, which play a major role in accurate biomedicine [52,181].
4.1. PT-enhanced CDT with CHT
CHT is a widely accepted method and has been proven successful in cancer treatment in a clinical setting, which is the first-line treatment of patients for the advanced stage of tumors and metastatic tumors [182,183]. CHT has also recently achieved remarkable clinical success in extending the lives of millions of patients, which has several unique advantages such as high efficiency, reliability, reduce pain, and convenience [184]. The use of chemotherapeutic agents is based on the basic principle of highly toxic compounds to inhibit tumor growth and metastasis [185]. Cancer cell apoptosis is also one of the main mechanisms by which chemotherapeutic agents kill cells [186]. However, the major drawbacks of CHT include CHT-resistance, limited dosage, non-specific distribution, low drug levels in the tumor tissues, and severe systemic toxicity, which are responsible for the inefficiency of CHT in patients [[187], [188], [189]]. Hence, minimizing the side effects of CHT agents remains a challenge in the field of cancer CHT. PTT can effectively improve intracellular drug delivery, accelerate drug release, increase drug accumulation at the tumor site, and inhibit drug resistance [190,191]. ROS also plays an important role in the redox balance of biological processes [192]. The high amount of ROS in cancer cells can effectively increase the oxidative damage of cancer cells and enhance the delivery efficiency, thereby resulting in cell death [193]. Therefore, the integration of anticancer drugs and PT-enhanced Fenton-based nanocatalysts into one nanomaterial is highly desirable and PT-enhanced CDT with CHT has demonstrated to be have great potential to overcome the side effects, thus achieving synergistic cancer therapy [194].
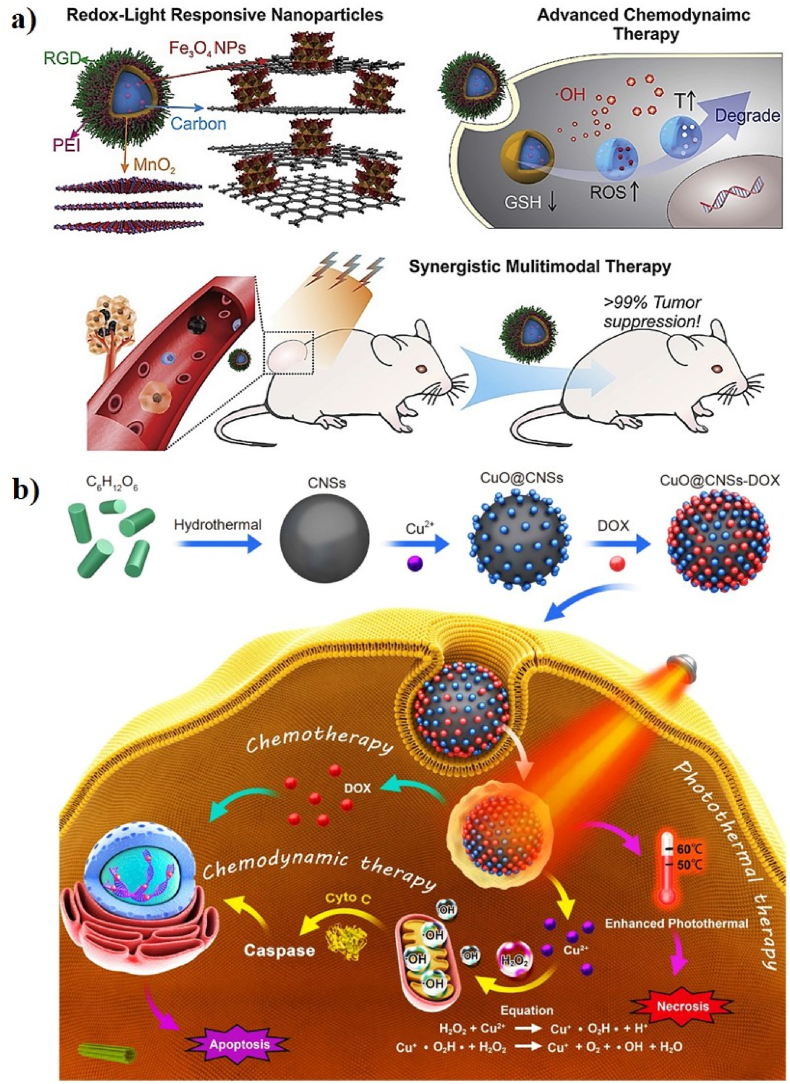
In a similar study, Qi et al. reported a multifunctional carbon dot (CD)-decorated silver (Ag)/gold (Au) bimetallic nanoshell (NS) as a smart nanozyme (CDs-Ag/Au NS, CAANS) for chemo-PTT. The CD-loaded Ag/Au NSs were proven conducive to generating toxic ROS to stimulate CHT. The CAANSs have an average size of 52 nm. Tryptophan (Trp) plays an important role in oxidative stress by inducing the formation of ROS such as superoxide radicals (•O2−), which are decomposed to produce H2O2 in cancerous cells under the PT catalytic process in the presence of CAANSs and effectively kill cancer cells. The apoptosis rate was 95%, suggesting that CAANS can serve as an effective catalytic nanomedicine for cancer therapy [136]. In another study, Wang et al. developed new redox and light-responsive (RLR) NPs with ultra-small iron oxide (Fe3O4) NP-engineered framework comprised of an amorphous hollow carbon matrix core (Fe3O4–C) with nanoflower-like MnO2 loaded with doxorubicin (DOX) for chemo-PT-CDT through a Fenton-like reaction, GSH depletion, and chemo-PT effect (Fig. 16a). The RLR NPs had an average diameter of 200 nm. The PCE (η) of the RLR NPs was 26.8%, which is the most efficient NIR of the PT agent. Ultra-small Fe3O4 NPs release Fe2+ and Fe3+ ions sequentially, which can self-activate the Fenton-like reactivity by catalyzing the conversion of H2O2 into a large number of toxic •OH radicals. This indicates that the acidic nature of the TME can accelerate the Fenton-like reactivity stimulated by the Fe3O4 NPs and enhance the therapeutic efficacy via the ferroptosis pathway. The RLR NPs were programmed to self-activate CDT by reducing the intracellular levels of the antioxidant GSH, accelerating ROS generation, spatiotemporal controllable PTT, and redox-triggered DOX release. The RLR NPs-treated cells have a significantly higher level of ROS after laser irradiation, suggesting the heat production from PTT can accelerate the RLR NPs-based CDT. In addition, the RLR NP-based PTT can also improve the accumulation of DOX in cancer cells for CHT. Tumor-bearing mice were intravenously injected with FITC-labeled RLR NPs and in vivo fluorescence signals were observed at the tumor site after 2 h. In addition, T1 MRI positive probes (Mn2+) were released, allowing in situ MRI and the MR signals were observed at 1 and 3 h. The in vivo synergistic therapeutic efficacy of the RLR NPs almost stopped tumor growth during the experimental periods, suggesting that the RLR NPs have great potential for use in MRI-guided chemo-PT-enhanced CDT [121].
Fig. 16.
(a) A schematic illustration of the fabrication of RLR NPs for chemo-PT-enhanced CDT. Reprinted with permission of Ref. [121]. Copyright 2019 Elsevier Ltd. (b) A schematic illustration of the synthesis of CuO@CNSs-DOX for chemo-PTT/CDT. Reprinted with permission of Ref. [133]. Copyright 2020 Springer.
In another effort, Jiang and co-workers designed multifunctional doxorubicin (DOX)-loaded copper oxide-decorated carbon nanospheres (CuO@CNSs) for chemo-PT-enhanced CDT (Fig. 16b). The CNSs were used as PT agents, which were synthesized via a hydrothermal reaction. CuO was adsorbed on the surface of the CNSs, which can serve as a Fenton-like agent and enhance the PCE of NPs. DOX was finally conjugated on the surface of the CuO@CNSs via electrostatic interactions. The CuO@CNSs have a uniform spherical morphology with an average size of 15 nm. The PCE (η) of the CuO@CNSs was 10.14%, indicating excellent PT properties. The temperature of the CuO@CNS aqueous solution reached up to 60.3 °C, indicating that CuO@CNSs have potential advantages as PT agents. Copper oxide (CuO) can release Cu2+ ions in the TME, which can produce •OH to induce cancer cell apoptosis via Fenton-like reactions. DOX is also released into the TME to kill cancer cells. The CuO@CNSs-DOX + NIR-treated 4T1 cells showed 29% cell apoptosis, indicating that the synergistic PT-enhanced CDT can enhance apoptosis and promote efficacy. The tumors treated with CuO@CNSs-DOX + NIR were completely inhibited, indicating that the synergistic PT-enhanced CDT had a good effect. PAI can provide a non-invasive diagnosis of diseases; the tumor region exhibits a strong PA signal after injection of CuO@CNS, indicating that the CuO@CNSs are ideal PA imaging contrast agents for PAI-guided cancer diagnosis. Multifunctional CuO@CNSs-DOX nanoplatforms have great potential for PAI-guided chemo-PT-enhanced CDT [133].
4.2. PT-enhanced CDT with SDT
SDT is a new non-invasive therapy that can eradicate solid tumors using ultrasound (US)-activated sonosensitizers to generate ROS, leading to tumor cell death [[195], [196], [197], [198], [199]]. Although its principle is similar to PDT, SDT is an emerging US-based cancer therapeutic method and is expected to be highly efficient because of its deeper penetration into the tumor site [[200], [201], [202]]. US can penetrate much deeper by several centimeters than PDT to trigger ROS generation and can therefore overcome the major problem of the deep penetration barrier of PDT [203,204]. PT-enhanced CDT with SDT has been used for high-efficiency cancer therapy [198]. For example, Liu et al. reported biocompatible PTMP-PMAA-modified ferrous phosphide (Fe2P) nanorods (FP NRs) as an “all-in-one” Fenton-like agent for ultrasound (US)- and PT-enhanced CDT (Fig. 17a). Fe2P was synthesized via a high-temperature method and PTMP-PMAA was coated onto the surface of Fe2P via a ligand exchange method to enhance their biocompatibility and hydrophilicity. The FP NRs have a rod-like morphology with an average size of 180 nm. The PCE (η) of the FP NRs was 56.6% upon irradiation at 1064 nm, indicating their outstanding PT conversion effect. The FP NRs generate heat under 1064 nm laser irradiation, which effectively enhances the production of •OH. The conversion of Fe3+ to Fe2+ was accelerated by US and the Fe2+ produced further reacts with H2O2 to generate more toxic •OH. Taken together, US and PT heat can effectively enhance the production of ROS by the FP NRs, allowing the application of FP NRs in PT-enhanced CDT with SDT in vitro and in vivo. HeLa cell-treated with FP NRs under the US and 1064 nm laser irradiation exhibit more dead cells, suggesting that the combination of PT-enhanced CDT and SDT shows outstanding anticancer effects. U14 tumor-bearing mice were intravenously injected with the FP NRs. The T2-weighted MRI (Fig. 17b) and PA signals (Fig. 17c) of the tumor were enhanced after 24 h, suggesting that the FP NRs can accumulate at the tumor site through the EPR effect. Tumors were fully inhibited after US and1064 nm laser irradiation, implying that the FP NRs have a good PT-enhanced CDT with SDT effect. Hence, FP NRs can serve as dual-modality MRI/PAI agents to accurately diagnose tumors and provide precise information for US- and PT-enhanced CDT. The synergistic ultrasound- and PT-enhanced CDT can cause cancer cell death in vitro and fully inhibit tumors in vivo, which indicates their remarkable anticancer activity [48].
Fig. 17.
(a) A schematic illustration of the synthesis of FP NRs for US/PT-enhanced CDT. In vivo MR (b) and PA (c) images of tumor-bearing mice. Reprinted with permission of Ref. [48]. Copyright 2019 Wiley.
4.3. PT-enhanced CDT with PDT
PDT has been developed over the last decade as an attractive alternative treatment modality, which uses photosensitizers to produce ROS under laser irradiation to induce cancer cell apoptosis [[205], [206], [207]]. PDT has been clinically approved for the treatment of various diseases [208,209]. It has the distinct advantages of high selectivity, reduced invasiveness, strong curative effect, and reduced side effects [206]. However, the clinical use of PDT is limited by its poor tissue penetration and tumor hypoxia [210]. To solve this problem, combination therapies using PTT, CDT, and PDT have been employed to achieve a remarkable inhibition of tumor growth by promoting multiple anticancer mechanisms, which can effectively improve the therapeutic effect of PDT [96,211]. For example, Xiao et al. prepared l-buthionine-sulfoximine (BSO)-modified FeS2 NPs (BSO–FeS2 NPs) as theranostic agents for PAI-guided PT-enhanced chemodynamic/photodynamic therapy (Fig. 18a). The FeS2 NPs were prepared using a simple synthesis of CdTe NPs and BSO was conjugated to TGA on the surface of FeS2 utilizing carbodiimide/N-hydroxysuccinimide (EDC/NHS) chemistry. FeS2 has been applied for PTT and PDT, and can also catalyze the decomposition of H2O2 to effectively produce •OH for CDT. BSO–FeS2 NPs have a uniform spherical morphology with an average particle size of 7.27 ± 1.43 nm. Moreover, they exhibited an outstanding PCE (η) of 49.5%. The FeS2 NPs can catalyze the decomposition of H2O2 to produce a large amount of •OH via a Fenton-like reaction in an acidic environment. The BSO–FeS2 NPs inhibit the synthesis of GSH, suggesting that BSO can considerably accelerate the accumulation of ROS to achieve excellent tumor treatment efficacy. 4T1 cells treated with BSO–FeS2 NPs show a higher rate of apoptosis (45.45%) after laser irradiation, indicating that BSO–FeS2 NPs with laser irradiation can successfully induce 4T1 cells apoptosis. Mice injected with BSO–FeS2 NPs exhibit an increase in the tumor temperature increase to 45 °C, which was sufficient to kill the tumor (Fig. 18b). The in vivo PA signals in tumor-bearing mice treated with BSO–FeS2 NPs were significantly high, indicating that the BSO–FeS2 NPs can be used as promising PA contrast agents for solid tumor monitoring (Fig. 18c). Therefore, BSO–FeS2 NPs have been used as an “all-in-one” theranostic agent for the PAI-guided PTT/CDT/PDT of cancer [120].
Fig. 18.
(a) A schematic illustration of the fabrication of BSO–FeS2 NPs for PT-enhanced PDT/CDT. (b) IR thermal images of tumor-bearing mice. (c) In vivo PA images tumor-bearing mice after intratumoral injection of BSO–FeS2 NPs. Reprinted with permission of Ref. [120]. Copyright 2020 Elsevier Ltd.
4.4. PT-enhanced CDT with IMT
Recently, IMT has emerged as a promising therapeutic modality that mobilizes the host's immunity against cancer [[212], [213], [214]]. However, the major drawbacks of IMT are its poor immunogenicity, high mutability, insufficient activation of the host immune system, and low immune cell infiltration in most cancers [215,216]. Recent reports have shown that combining IMT with PT-enhanced CDT may increase the response rates for non-inflamed tumors [217]. PT-induced IMT can activate innate and adaptive immune responses against cancer [218,219]. Therefore, the achievement of PTT/CDT mediated by nanomaterials conjugated with antigens and adjuvants has been developed over the past decade as a potential alternative for safer and more effective cancer IMT [220,221]. For example, Jiang et al. first reported the integration of semiconductor copper(II) oxide (CuO) and molybdenum disulfide (MoS2) nanoflowers to form MoS2–CuO heteronanocomposites, which were subsequently loaded with BSA and immunoadjuvant imiquimod (R837) for PTT/CDT/IMT (Fig. 19a). The MoS2 nanoflowers were prepared via a hydrothermal method and CuO was then loaded on the surface of the MoS2 nanoflowers, which were modified with BSA and R837 to enhance their immunogenicity and biocompatibility. The MoS2–CuO@BSA/R837 (MCBR) nanoplatforms significantly improved the antitumor efficiency of the synergetic PTT/CDT/IMT. MoS2 has a diameter of ∼130 nm and the PCE (η) of MoS2–CuO was calculated to be 24.6%. CuO can react with the overexpressed H2O2 present in the TME to produce a large amount of •OH via a Fenton-like reaction; this reaction can be further improved by the PT effect of MoS2. The MCBR can destroy the primary tumor and release tumor-associated antigens (TAAs), which combine with R837 as an immune-stimulating adjuvant to trigger a strong antitumor immunological response, which can effectively remove primary and metastatic tumors via synergetic PT-enhanced CDT/IMT. Multimodal imaging, such as MRI, CT, and infrared (IR) thermal imaging, can provide complementary information for the diagnosis and treatment of cancer. The T1-MR signal of the tumor site is significantly enhanced after the injection of MCBR, indicating that MCBR can serve as an ideal T1-MR contrast agent (Fig. 19b). A strong CT signal was observed in the tumor area, demonstrating the ideal CT imaging capability of MCBR (Fig. 19c). The temperature variations in the tumor area were also studied after post-injection of MCBR and laser irradiation (Fig. 19d), confirming that MCBR possesses the potential to act as a multifunctional contrast agent for MRI/IR/CT-guided PT-enhanced CDT/IMT [47].
Fig. 19.
(a) A schematic illustration of the synthesis of MoS2–CuO@BSA/R837 (MCBR) for PTT/CDT/IMT. In vivo MR (b), CT (c), and IR thermal (d) images of a tumor after intratumoral injection with MCBR. Reprinted with permission of Ref. [47]. Copyright 2020 Elsevier Ltd.
In another similar recent study, Wang et al. fabricated chlorin e6 (Ce6)-loaded PEG-coated Au2Pt (Au2Pt-PEG-Ce6) for collaborative phototherapy/CDT (Fig. 20a). The Au2Pt NPs have a spherical morphology with an average diameter of 42 ± 3 nm, and the zeta potential of Au2Pt-PEG-Ce6 was −24.2 mV. The PCE (η) of Au2Pt-PEG-Ce6 was 31.5%, implying that Au2Pt-PEG-Ce6 has an excellent PT conversion ability. Au2Pt-PEG-Ce6 possesses catalase- and peroxidase (POD)-like activities, which can effectively generate O2 and •OH for PT-enhanced PDT and CDT. In this reaction, H2O2 was decomposed by Au2Pt to produce •OH for 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation, which occurs in a pH- and temperature-dependent manner. The in vitro synergistic effect of Au2Pt-PEG-Ce6 against HeLa cells was further evaluated and the survival rate of the HeLa cells treated with Au2Pt-PEG-Ce6 was significantly reduced to 12% after 650 and 808 nm laser irradiation, indicating that the synergistic effect can enhance the therapy efficiency. Au2Pt-PEG-Ce6 showed significant tumor-suppressive therapeutic efficiency in intratumorally injected U14 tumor-bearing mice under 650 and 808 nm laser irradiation, implying the prominent synergistic effect of PT-enhanced PDT and CDT. In addition, the tumor temperature variations in the Au2Pt-PEG-Ce6 group were more prominent than those in the control group. Moreover, the temperature increased in the tumor area, implying that NIR laser irradiation was biosafe toward healthy tissues (Fig. 20b). The CT imaging ability of Au2Pt-PEG-Ce6 was evaluated in vitro and in vivo, and strong CT signals were observed in the tumor area, indicating that Au2Pt-PEG-Ce6 can serve as a good CT imaging agent (Fig. 20c). PA images and signals were observed after injection of Au2Pt-PEG-Ce6 (Fig. 20d, e). Thus, Au2Pt-PEG-Ce6 shows promising therapeutic potential for use in multimodal imaging-guided PT-enhanced PDT/CDT [138].
Fig. 20.
(a) A schematic illustration of the synthesis procedure of Au2Pt-PEG-Ce6 for collaborative phototherapy/CDT. (b) In vivo IR thermal images of tumor-bearing mice injected with saline and Au2Pt-PEG-Ce6. (c) In vivo CT images of tumor-bearing mice pre- and post-intratumoral injection of Au2Pt-PEG-Ce6. (d) In vivo PA images of tumor-bearing mice injected with saline and Au2Pt-PEG-Ce6. (e) In vivo PA images of hemoglobin (HB) and oxyhemoglobin (HBO2) in the tumor area post-injection of Au2Pt-PEG-Ce6. Reprinted with permission of Ref. [138]. Copyright 2020 Elsevier Ltd.
4.5. PT-enhanced CDT with ST
Tumor cells require a considerable amount of energy during tumor metabolism, growth, proliferation, and metastasis [222]. Based on the Warburg effect, the proliferation of tumor cells mainly produces sufficient energy through aerobic glycolysis, which makes tumor cells more sensitive to variations in the intracellular glucose concentration [223]. Therefore, glucose consumption in tumor cells can disrupt glucose metabolism in tumor cells, resulting in the ST of tumor cells [224]. Cancer ST is a new strategy that blocks the energy supply to tumor cells [225,226]. To date, various strategies have been developed to prevent tumor growth and metabolism via intratumoral glucose consumption [225]. Glucose oxidase (GOx or GOD) is an enzyme that can effectively catalyze the reaction between glucose and O2 to produce gluconic acid and H2O2, which can occur under acidic conditions, which are satisfactory reactants for CDT [227]. The use of glucose oxidation via the catalytic reaction of GOx can block the energy supply to prevent tumor growth [228]. In particular, GOx-based ST shows an extraordinary capability to inhibit tumor growth by catalyzing the oxidation of glucose, which has received a lot of attention from researchers in recent years [229]. However, GOx-based ST has limited therapeutic efficiency, such as low stability, immunogenicity, and fragile enzymatic activity, and has also been recently used as an auxiliary treatment [59,228]. Therefore, the combination of PT-enhanced CDT with ST can effectively produce a synergistic antitumor effect, which can significantly prevent the growth of tumors when compared with a single therapy (PTT, CDT, or ST) [230]. In particular, the large amount of generated/accumulated H2O2 and the production of gluconic acid in the TME is satisfactory for accelerating the Fenton or Fenton-like reactions to generate •OH and further improve the CDT efficacy [228,231]. In addition, the increased temperature in the TME can effectively accelerate the transformation of H2O2 and •OH, and therefore, the rational combination of PT-enhanced CDT with ST is expected to enhance its performance in the treatment of tumors [27].
As representative GOD-based nanocomposites in CDT, Feng et al. reported, for the first time, superparamagnetic Fe3O4-modified polypyrrole (PPy) NPs conjugated with glucose oxidase (GOD) for PT-enhanced CDT (Fig. 21a, b). PPy is a typical electroconductive polymer because of its high conductivity and biocompatibility. PPy NPs can serve as photoabsorbing agents for PTT. Fe3O4 can be used as an effective Fenton agent for CDT. However, the major disadvantage of Fe3O4 for CDT is the low intracellular levels of H2O2 in vivo and the unsatisfactory catalytic Fenton reaction in the mildly acidic TME. Therefore, PT-enhanced CDT with ST is urgently required to develop an efficient approach to successfully achieve synergistic-therapeutic efficiency. GOD is an enzyme with abundant amino groups, which are covalently bonded to the surface carboxyl groups of Fe3O4@PPy NPs to form Fe3O4@PPy@GOD nanocomposites (NCs). The Fe3O4@PPy@GOD NCs had uniform size, with an average diameter of 163.5 nm. The PCE (η) of Fe3O4@PPy@GOD NCs was 35.1 and 66.4% at 808 and 1064 nm, respectively. The intratumoral glucose was converted into gluconic acid and H2O2 by the conjugated GOD and the integrated Fe3O4 component catalyzes the conversion of the in situ generated H2O2 into highly toxic •OH via the well-established catalytic Fenton reactions, resulting in ST-enhanced CDT. Importantly, PPy NPs not only effectively generate heat, but also accelerate the intratumoral catalytic Fenton reaction to induce cancer cell apoptosis and necrosis, indicating the synergistic effect of PT-enhanced CDT with ST in combating cancer (Fig. 21c). 4T1 tumor-bearing mice treated with Fe3O4@PPy@GOD NCs and 808 or 1064 nm laser irradiation almost completely inhibit tumor growth during the experimental period, implying that Fe3O4@PPy@GOD NCs are potentially powerful therapeutic agents for combating cancer. Fe3O4@PPy@GOD NCs can serve as an efficient contrast agent for dual-modality PAI/MRI. The photoacoustic (PA) intensities of Fe3O4@PPy@GOD NCs at 808 or 1280 nm were evaluated in 4T1 tumor-bearing mice; the PA amplitude at 808 or 1280 nm in the tumor site increases gradually post-injection of the Fe3O4@PPy@GOD NCs, suggesting the time-dependent accumulation of Fe3O4@PPy@GOD NCs at the tumor site (Fig. 21d, e). The MR signaling properties of the Fe3O4@PPy@GOD NCs show a uniform signal distribution inside the entire tumor, suggesting that the Fe3O4@PPy@GOD Cs can reach the whole tumor site from blood circulation, leading to successful dual-mode imaging-guided PT-enhanced CDT/ST (Fig. 21f) [59].
Fig. 21.
A schematic illustration of the synthesis of Fe3O4@PPy@GOD NCs (a) and PT-enhanced CDT (b). (c) Proposed mechanism of Fe3O4@PPy@GOD NCs for PT-enhanced Fenton-based CDT. In vivo PAI and PA signals of the tumor area at various time points post-injection of Fe3O4@PPy@GOD NCs at 808 nm (d) and 1280 nm (e). (f) In vivo T2-weighted MR images of tumor-bearing mice before and after intratumoral and intravenous injection of Fe3O4@PPy@GOD NCs (the red dotted circles indicate the tumor areas). Reprinted with permission of Ref. [59]. Copyright 2018 Wiley.
In another study, Liu and co-workers synthesized glucose oxidase (GOx)-loaded polyethylenimine (PEI)-coated Fe3O4 that reacted with PEGylated multi-walled carbon nanotubes (MWNTs) to form PEG-MWNT-Fe3O4 (PMF) for PT-enhanced CDT. PEI is a highly charged cationic polymer that is widely used as a biocompatible coating material on the surface of NPs. MWNTs have been extensively used as effective photoablation agents in PTT. GOx is capable of converting glucose in the mildly acidic TME into gluconic acid and H2O2, which can act as an effective Fenton catalyst and boost the Fe3O4-based Fenton reaction to produce •OH to induce tumor cell death. Moreover, this Fenton reaction can be further enhanced upon laser irradiation via the PT effect from the MWNTs, which significantly enhances the CDT effect. Fe3O4 shows a spherical morphology with an average size of 12–20 nm. The temperature of the PMF-GOx (PMFG) aqueous solutions increases to 45 °C under 808 nm laser irradiation for 5 min, which was sufficient to improve CDT. The in vitro therapeutic effect of PMFG + NIR-treated on MCF-7 cells showed enhanced cell apoptosis, implying a highly effective •OH was generated under laser irradiation [25].
Transition metal chalcogenides, including, copper-molybdenum sulfide (Cu2MoS4) have received significant attention because of their strong absorption in the NIR region and great catalytic performance, which can combine with GOx to achieve ST and PT-enhanced CDT [232]. For example, Chang et al. reported, for the first time, the use of PEGylated hollow mesoporous copper-molybdenum sulfide (Cu2MoS4) (CMS) loaded with GOx (PEG-CMS@GOx) for synergetic cancer therapy via PT-enhanced photodynamic/chemodynamic/starvation/immunotherapy (Fig. 22). PEG-CMSs show plentiful pores with an average size of 5.88 nm, and a zeta potential of −13.7 mV. The 1064 nm laser irradiated CMS presents a high PCE (η = 63.27%), confirming that CMS has excellent PT stability and serves as a PT agent. The existence of multivalent metal ions, such as Cu+/Cu2+ and Mo4+/Mo6+ redox couples, in CMS results in a large amount of •OH being generated via Fenton-like, catalase-like, and glutathione peroxidase (GPx)-like reactions, which can significantly improve the CDT effect. CMS can react with endogenous H2O2 to produce O2, which can stimulate the conjugated GOx to produce gluconic acid and H2O2 within the tumors sites and boost the Fenton-like reaction to generate •OH. Meanwhile, CMS with 1064 nm laser irradiation shows an excellent tumor-killing capability by photo-based therapy because of its good PCE and toxic •O2− generation performance for PDT. In addition, CMS can elicit vaccine-like immune responses after primary tumor ablation, which can promote the conjugated anti-cytotoxic T-lymphocyte antigen-4 (CTLA4) and successfully inhibit tumor metastasis via IMT. The in vitro therapeutic effect of PEG-CMS@GOx was assessed in HeLa cells with various stimulations. PEG-CMS@GOx reacts with H2O2 in HeLa cells to produce a small amount of •OH and cause marginal cell death in CDT. Much more •OH is produced via GOx-enhanced CDT, indicating that the synergistic effect of CDT/ST can effectively inhibit tumors, resulting in more cell death than single CDT. Most importantly, 100% of HeLa cells were dead after 1064 nm laser irradiation because of the synergistic PT-enhanced CDT/PDT/ST. The antitumor effect of PEG-CMS@GOx can significantly induce in vitro dendritic cell (DC) maturation, suggesting that the comprehensive synergistic effect may elicit a powerful immune response. The in vivo synergistic therapeutic efficacy of PEG-CMS@GOx + anti-CTLA4 + 1064 nm laser irradiation almost completely inhibits tumor growth, indicating that PEG-CMS@GOx with anti-CTLA4 and laser irradiation can effectively eliminate primary and metastatic tumors via comprehensive synergistic cancer therapy [134].
Fig. 22.
A schematic illustration of the synthesis and working mechanisms of PEG-CMS@GOx. Reprinted with permission of Ref. [134]. Copyright 2019 Wiley.
By employing the characteristics of Fe2O3 NPs and GOx, Dong et al. proposed the introduction of novel ultra-small γ-Fe2O3 NPs and macromolecular GOx into dendritic mesoporous silica nanoparticles (DMSNs) as functional nanocatalysts (NCs) for PT-enhanced CDT into a single nanoplatform with superior T2-weighted MRI performance (Fig. 23a, b). DMSN has an average diameter of 130 nm and the ultra-small γ-Fe2O3 NPs were ∼5–6 nm in diameter. The PCE (η) of the γ-Fe2O3-GOx-DMSN NCs was 49.8%. The GOx release profile of the γ-Fe2O3-GOx-DMSN NCs exhibits temperature-dependent activity, reaching 79.24% after 10 h at 50 °C, causes a decrease in the pH value and an increase in the H2O2 levels in cancer cells, which further enhances the subsequent Fenton reaction. γ-Fe2O3 NPs can efficiently catalyze the Fenton reaction to produce •OH, inducing the apoptosis of the cancer cells. The in vivo T2-weighted MRI effect of the γ-Fe2O3-GOx-DMSN NCs was 68.013 m M−1 S−1 at pH 6.0, suggesting that more γ-Fe2O3-GOx-DMSN NCs are accumulated at the tumor site. Thus, they can be employed as multifunctional nanoagents for MRI-guided PT-enhanced CDT. The in vivo therapeutic effect of the γ-Fe2O3-GOx-DMSN NCs exhibits the most significant inhibition of tumor growth in mice after laser irradiation, indicating that the laser irradiation of γ-Fe2O3-GOx-DMSN NCs revealed a strong capacity to induce cytotoxic heat to ablate the cancer cells, which can significantly improve the therapeutic outcome of PT-enhanced CDT [119].
Fig. 23.
A schematic illustration of the (a) preparation of γ-Fe2O3-GOx-DMSN NCs and (b) PT-enhanced CDT using γ-Fe2O3-GOx-DMSN NCs. Reprinted with permission of Ref. [119]. Copyright 2020 American Chemical Society.
In another study, by employing the unique nature of GOX and molybdenum disulfide (MoS2)@manganese ferrite (MnFe2O4), Zheng et al. developed a multifunctional hybrid hydrogel (CFMG) by incorporating MoS2@MnFe2O4 in chitosan-grafted-dihydrocaffeic acid (CA-DA) and Pluronic F127 loaded with GOx for PT-enhanced CDT. The MnFe2O4 NPs were uniformly distributed on the ultra-thin MoS2 nanosheets, resulting in the formation of MoS2@ MnFe2O4 nanocomposite. The temperature variations in the CFMG hydrogel increase rapidly up to ∼49.5 °C with laser irradiation for 5 min, confirming its strong PT properties. GOx catalyzes the conversion of glucose into gluconic acid and H2O2, thereby inducing an effective reduction in the pH and production of H2O2. In addition, the low pH significantly accelerates the release of Fe2+ and Fe3+ from MnFe2O4 and enhances the reactivity of the subsequent Fenton reaction to generate •OH. Furthermore, the PT effect also accelerated the production of •OH, demonstrating that GOx and the PT effects were responsible for boosting •OH. The in vitro therapeutic effect of the CFMG hydrogel stimulated more cell death after laser irradiation with ∼97.8% of cancer cells being destroyed. The in vivo antitumor efficacy of the CFMG hydrogel with laser irradiation almost completely inhibited tumor growth after 15 days, demonstrating that CFMG has promising potential for use in PT-enhanced CDT [126]. In a similar study, An et al. reported a hypoxia-responsive-azo-BSA functionalized biomimetic nanoreactor (Fe(III)-GA/GOx@ZIF-Azo) encapsulating ferric-gallic acid NPs (Fe(III)-GA) and GOx into a zeolitic imidazolate framework (ZIF) for PT-enhanced CDT. The Fe(III)-GA NPs self-assembled via strong coordination bonds between Fe3+ and GA. The Fe(III)-GA and GOx were incorporated into the zeolitic imidazolate framework (ZIF) to form a highly effective •OH generating nanoplatform (Fe(III)-GA/GOx@ZIF). The 4,4ʹ-azobenzenecarboxylic acid moieties(Azo) can produce hypoxia-activated charge reversal from negative to positive, allowing for easier cellular uptake and tumor accumulation. Azo-BSA was prepared upon the reaction between the carboxyl and amino groups. Azo-BSA was functionalized on the surface of Fe(III)-GA/GOx@ZIF (FGGZ) to form Fe(III)-GA/GOx@ZIF-Azo (FGGZA). The Fe(III)-GA NPs have an average diameter of ∼3 nm, and the PCE (η) of Fe(III)-GA was 66.5%, implying that Fe(III)-GA exhibits outstanding PT performance. The PT conversion of Fe(III)-GA and the acidic pH caused by the gluconic acid and H2O2 produced by GOx, can efficiently improve the Fenton reaction, leading to the production of •OH for PT-enhanced CDT. The in vitro therapeutic effect of FGGZ + NIR toward MCF-7 cells shows a higher •OH generation efficacy, indicating that FGGZ can serve as a Fenton agent for highly effective ferroptosis therapy. The in vivo therapeutic efficiency of FGGZA + NIR exhibits a high tumor inhibition rate of 78%, implying that Fe(III)-GA/GOx@ZIF-Azo has great therapeutic potential as an effective ferroptosis-inducing nanoagent [129].
More recently, based on the same principles, Yang et al. developed GOx-functionalized ancient pigment (strontium copper tetrasilicate (SrCuSi4O10), SC) nanosheets (NSs) for NIR-II PT-enhanced CDT. The SC was prepared using a conventional solid-state reaction method and then covalently functionalized with GOx via EDC/NHS chemistry to form the GOx-loaded SC NSs (denoted as SC@G NSs). The particle size of the SC@G NSs was ∼60.94 nm and the calculated PCE (η) was 46.3% at 1064 nm, indicating that the SC@G NSs have great potential as PT agents for NIR-II PT-enhanced CDT. GOx was capable of converting glucose in the mildly acidic TME into gluconic acid and H2O2 to promote cytotoxic •OH, resulting in its enhanced CDT efficacy. The SC@G NSs sequentially release Cu2+ and Sr2+ ions, which can further catalyze the decomposition of the in situ generated H2O2 into cytotoxic •OH and depleted GSH. In addition, the Fenton-like reaction was significantly improved under laser irradiation, which confirmed the temperature-dependent GOx activity under laser irradiation. The in vitro synergistic PT- and ST-enhanced CDT effect of the SC@G NSs significantly inhibits the cellular viabilities under 808 and 1064 nm laser irradiation. 4T1 tumor-bearing mice were intravenously (i.v.) injected with the SC@G NSs to study their synergistic antitumor therapeutic efficacy. The tumor temperature of the SC@G NSs was elevated up to 47 °C under laser irradiation for 5 min, implying that the SC@G NSs can significantly suppress tumor growth. In addition, the SC@G NSs were intravenously injected into mice for in vivo PAI and the PA signals at the tumor site gradually increase post-injection, indicating that the SC@G NSs were accumulated in the tumor area. Furthermore, the PT effect can effectively improve the GOx activity and accelerate the intratumoral Fenton-like process, thereby achieving the synergistic effect of PAI-guided PT-enhanced CDT to combat cancer [131].
5. Conclusions and future perspectives
To date, the development of multifunctional nanomaterials for PT-enhanced Fenton-based nanocatalytic tumor therapy has aroused a lot of interest and has become promising toward the discovery of more effective cancer treatment. CDT has recently emerged as a new tumor-specific therapy based on Fenton or Fenton-like reactions, has gained a lot of interest from researchers because of its high specificity and sensitivity of the clinical transformation potential. However, major scientific progress is still required to improve therapeutic outcomes and reduce side effects. Numerous highly specific and selective nanomaterials have been prepared as CDT agents to promote in situ Fenton and Fenton-like reactions to destroy tumors, which have potential therapeutic applications for the treatment of cancer. This strategy utilizes pH-independent Fenton or Fenton-like reactions to effectively boost the generation of •OH for CDT, which is only efficient in a narrow pH range between 3.0 and 5.0, whereas ineffective Fenton or Fenton-like reactions occur under the neutral and mildly acidic pH conditions (pH 5.6–6.8) of the TME. However, the slow formation of •OH in the mildly acidic TME limits the therapeutic efficacy of CDT. Thus, enhancing the speed of •OH production in the mildly acidic TME will be critical for the further improvement of CDT. Inspired by the basic principle that an increase in the local temperature at the tumor site can significantly increase the therapeutic efficacy of the Fenton reactions with an increased yield of •OH, which provides promising integration potential for PTT with CDT. As previously reported, PTT commonly employs PT agents to convert light to heat for tumor ablation, significantly improving CDT in target tissues and controlling the release of therapeutic agents. Recently, synergistic PT-enhanced CDT has received an increasing amount of research interest for clinical application because of its high therapeutic efficacy, minimal side effects, and excellent biosafety when compared to monotherapy. It has been recognized that the integration of multimodal therapies such as PT-enhanced CDT with CHT, PDT, SDT, IMT, and ST and multimodal imaging techniques such as PAI, FLI, MRI, and CT into one nanoplatform will further improve the tumor suppression efficacy because of their synergistic effects. In this review, we have outlined the recent progress and development of multifunctional nanomaterials for PT-enhanced chemodynamic synergetic therapy. However, PT-enhanced CDT is still at an early stage of development and it is still highly challenging for researchers to achieve catalytic efficacy with biosafety. There are still many challenges that should be addressed before PT-enhanced CDT is ready for clinical application in the future (Fig. 24).
Fig. 24.
The summative scheme of the present status/development and future directions/prospects of PT-enhanced CDT.
First, the biosafety and biomedical effect of multifunctional nanomaterials with PT and chemodynamic properties are extremely important and urgent for its pre-clinical studies. The selectivity, biocompatibility, biodegradability, and biosafety of multifunctional nanomaterials are the most important factors affecting their clinical translation. Although the reported multifunctional nanomaterials have outstanding biosafety, selectivity, biocompatibility, and biodegradability in vivo, their long-term toxicity and side effects must be further investigated. Furthermore, a lot of multifunctional nanomaterials still have low toxicity and excellent biocompatibility but many additional factors, including metabolism, distribution, and immune response should be carefully considered. Additionally, most of the nanomaterials have demonstrated low degradability, raising concerns about long-term toxicity in vivo. Based on this, it is a very urgent need to synthesis ultra-small or biodegradable multifunctional nanomaterials to realize the rapid metabolism, degradation of nanomaterials, and remove them from the body while minimizing long-term toxicity concerns, which could be developed to improve biosafety. Therefore, it is of great importance to balance biosafety and PT-enhanced CDT therapeutic effect.
Second, the large-scale synthesis of high-quality multifunctional nanomaterials with PT and chemodynamic properties is also essential for clinical translation. Although the successful synthesis of multifunctional nanomaterials for mass production has been reported, these methods are still in the laboratory stage, and their industrialization is urgent. Furthermore, it is still a challenge to be industrialized due to the introduction of various functional components on the surface of nanomaterials to make them highly solubility, biocompatibility, and biodegradability.
Third, the therapeutic effect of PT-enhanced CDT can be further improved. To this aim, an in-depth investigation of the therapeutic pathway of PT-enhanced CDT using molecular and genetic approaches is essential for the further optimization of PT-enhanced CDT and its clinical applications. The actual mechanisms behind Fenton-based nanocatalytic tumor therapy and •OH generation in the TME are still unclear. To improve the efficacy of CDT, we much focus on their capabilities of Fenton-catalytic efficiency, including optimizing its physical and chemical properties as well as regulating the TME. The mechanisms of •OH generation based on CDT must be further investigated. Additionally, the mildly acidic TME (pH 5.6–6.8) has a significantly less effective role in the Fenton or Fenton-like reaction and there is also an urgent need to develop new Fenton agents with high catalytic efficiency under neutral and mildly acidic pH conditions.
Fourth, the lysosomal pH and H2O2 concentration in healthy cells are also low and induce •OH production, the biocompatibility and specificity of multifunctional nanomaterials for PT-enhanced CDT urgently need to be further improved to avoid potential damage to healthy cells. Therefore, the effective tumor targeting of nanomaterials for cancer therapy remains a challenge for researchers to improve the accumulation of drugs and nanomaterials in tumors. Furthermore, there is also an urgent need to accelerate the development of diagnostic methods for PT-enhanced CDT.
Fifth, a reasonable combination of various therapeutic modalities using multifunctional nanomaterials to achieve excellent tumor treatment efficacy is still a major challenge. Although combination cancer therapy showed significantly killing efficiency of tumors, the research on combination cancer therapy is still in its infancy, and many issues still need to be further improved. Therefore, there is an urgent need to develop nanomaterials with specified components to achieve strong therapeutic effects.
Finally, the current treatment of PT-enhanced CDT has limited to the mice or other small rodents subcutaneous transplantation tumor models. Up to now, there are no reports of clinical trials involving PT-enhanced CDT. In general, PT-enhanced CDT using multifunctional nanomaterials has made significant progress and achievement in recent years. Although the emerging PT-enhanced CDT faced several problems and challenges and further development of science and technology may promote its clinical translation of PT-enhanced CDT.
After addressing several challenges, multifunctional nanomaterials for PT-enhanced CDT may show great promise for more effective cancer treatment in future clinical applications and will pave the way for new concepts in cancer therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Basic Science Research Program (2016R1D1A3B02011756) through the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT & Future Planning and the Ministry of Science, ICT, and Future Planning (MSIP), Korea under the Information Technology Research Center (ITRC) support program (IITP-2016-H8601-16-1011) supervised by the Institute for Information & Communications Technology Promotion (IITP). This work was supported by the Global Research Laboratory Program (2014K1A1A2043032) through the National Research Foundation of Korea (NRF) grants funded by the Korean government Ministry of Science and ICT (MSIT). This work was also supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20142020200980 & 20182010600430). Prof. Eue-Soon Jang specially thanks a research grant from the Korean Ministry of Education, Science & Technology (2016R1D1A3B0201175615) and the Grand Information Technology Research Center Program (IITP-2021-2020-0-01612) through the Institute of Information & Communications Technology Planning & Evaluation (IITP) funded by the Ministry of Science and ICT (MSIT), Korea. Deanship of Scientific Research (RGP:1/275/1442) from King Khalid University, Abha, Saudi Arabia.
Contributor Information
Jungbae Kim, Email: jbkim3@korea.ac.kr.
Eue-Soon Jang, Email: euesoon@kumoh.ac.kr.
References
- 1.Cleary A.S., Leonard T.L., Gestl S.A., Gunther E.J. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508(7494):113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Counihan J.L., Grossman E.A., Nomura D.K. Cancer metabolism: current understanding and therapies. Chem. Rev. 2018;118(14):6893–6923. doi: 10.1021/acs.chemrev.7b00775. [DOI] [PubMed] [Google Scholar]
- 3.Anand P., Kunnumakara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach. Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 5.Moscow J.A., Fojo T., Schilsky R.L. The evidence framework for precision cancer medicine. Nat. Rev. Clin. Oncol. 2018;15(3):183–192. doi: 10.1038/nrclinonc.2017.186. [DOI] [PubMed] [Google Scholar]
- 6.Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019;119(8):4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Bragg L.M., Servos M.R., Liu J. Gold nanoparticles as dehydrogenase mimicking nanozymes for estradiol degradation. Chin. Chem. Lett. 2019;30(9):1655–1658. [Google Scholar]
- 8.Yang S., Zhou L., Su Y., Zhang R., Dong C.-M. One-pot photoreduction to prepare NIR-absorbing plasmonic gold nanoparticles tethered by amphiphilic polypeptide copolymer for synergistic photothermal-chemotherapy. Chin. Chem. Lett. 2019;30(1):187–191. [Google Scholar]
- 9.He H., Liu L., Morin E.E., Liu M., Schwendeman A. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Acc. Chem. Res. 2019;52(9):2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- 10.Sun H., Dong Y., Feijen J., Zhong Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Contr. Release. 2018;290:11–27. doi: 10.1016/j.jconrel.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milling L., Zhang Y., Irvine D.J. Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 2017;114:79–101. doi: 10.1016/j.addr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J., Rao L., Yu G., Cook T.R., Chen X., Huang F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021;50(4):2839–2891. doi: 10.1039/d0cs00011f. [DOI] [PubMed] [Google Scholar]
- 15.Ulbrich K., Hola K., Subr V., Bakandritsos A., Tucek J., Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016;116(9):5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 16.Stewart M.P., Sharei A., Ding X., Sahay G., Langer R., Jensen K.F. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 17.Sabri T., Pawelek P.D., Capobianco J.A. Dual activity of rose bengal functionalized to albumin-coated lanthanide-doped upconverting nanoparticles: targeting and photodynamic therapy. ACS Appl. Mater. Interfaces. 2018;10(32):26947–26953. doi: 10.1021/acsami.8b08919. [DOI] [PubMed] [Google Scholar]
- 18.Gu T., Cheng L., Gong F., Xu J., Li X., Han G., Liu Z. Upconversion composite nanoparticles for tumor hypoxia modulation and enhanced near-infrared-triggered photodynamic therapy. ACS Appl. Mater. Interfaces. 2018;10(18):15494–15503. doi: 10.1021/acsami.8b03238. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L., Wang C., Feng L., Yang K., Liu Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014;114(21):10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- 20.Pan X., Wang H., Wang S., Sun X., Wang L., Wang W., Shen H., Liu H. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci. China Life Sci. 2018;61(4):415–426. doi: 10.1007/s11427-017-9262-x. [DOI] [PubMed] [Google Scholar]
- 21.Dong X., Liang J., Yang A., Qian Z., Kong D., Lv F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials. 2019;209:111–125. doi: 10.1016/j.biomaterials.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Liang R., Li Y., Huo M., Lin H., Chen Y. Triggering sequential catalytic fenton reaction on 2D MXenes for hyperthermia-augmented synergistic nanocatalytic cancer therapy. ACS Appl. Mater. Interfaces. 2019;11(46):42917–42931. doi: 10.1021/acsami.9b13598. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q., Wang D., Yang G. Photoacoustic imaging guided photothermal and chemodynamic combined therapy for cancer using. J. Biomed. Nanotechnol. 2019;15(10):2090–2099. doi: 10.1166/jbn.2019.2832. [DOI] [PubMed] [Google Scholar]
- 24.Liu P., Wang Y., An L., Tian Q., Lin J., Yang S. Ultrasmall WO3–x@ γ-poly-l-glutamic acid nanoparticles as a photoacoustic imaging and effective photothermal-enhanced chemodynamic therapy agent for cancer. ACS Appl. Mater. Interfaces. 2018;10(45):38833–38844. doi: 10.1021/acsami.8b15678. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Liu Y., Wang J., Wei T., Dai Z. Mild hyperthermia-enhanced enzyme-mediated tumor cell chemodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11(26):23065–23071. doi: 10.1021/acsami.9b08257. [DOI] [PubMed] [Google Scholar]
- 26.Fan W., Yung B., Huang P., Chen X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017;117(22):13566–13638. doi: 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z., Zhang H., Liu Y., Ni D., Zhang H., Zhang J., Yao Z., He M., Shi J., Bu W. Antiferromagnetic pyrite as the tumor microenvironment-mediated nanoplatform for self-enhanced tumor imaging and therapy. Adv. Mater. 2017;29(47):1701683. doi: 10.1002/adma.201701683. [DOI] [PubMed] [Google Scholar]
- 28.Ni G., Yang G., He Y., Li X., Du T., Xu L., Zhou S. Uniformly sized hollow microspheres loaded with polydopamine nanoparticles and doxorubicin for local chemo-photothermal combination therapy. Chem. Eng. J. 2020;379:122317. [Google Scholar]
- 29.Zheng Z., Chen Q., Rong S., Dai R., Jia Z., Peng X., Zhang R. Two-stage activated nano-truck enhanced specific aggregation and deep delivery for synergistic tumor ablation. Nanoscale. 2020;12(29):15845–15856. doi: 10.1039/d0nr03661g. [DOI] [PubMed] [Google Scholar]
- 30.Lei P., An R., Zhang P., Yao S., Song S., Dong L., Xu X., Du K., Feng J., Zhang H. Ultrafast synthesis of ultrasmall poly (Vinylpyrrolidone)-protected Bismuth Nanodots as a multifunctional theranostic agent for in vivo dual-modal CT/Photothermal-imaging-guided photothermal therapy. Adv. Funct. Mater. 2017;27(35):1702018. [Google Scholar]
- 31.Wang X., Zhong X., Liu Z., Cheng L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today. 2020;35:100946. [Google Scholar]
- 32.Cheng Y., Yang F., Zhang K., Zhang Y., Cao Y., Liu C., Lu H., Dong H., Zhang X. Non-Fenton-type hydroxyl radical generation and photothermal effect by mitochondria-targeted WSSe/MnO2 nanocomposite loaded with isoniazid for synergistic anticancer treatment. Adv. Funct. Mater. 2019;29(45):1903850. [Google Scholar]
- 33.Lin L.S., Song J., Song L., Ke K., Liu Y., Zhou Z., Shen Z., Li J., Yang Z., Tang W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. 2018;130(18):4996–5000. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 34.Lin L., Wang S., Deng H., Yang W., Rao L., Tian R., Liu Y., Yu G., Zhou Z., Song J. Endogenous labile iron pool-mediated free radical generation for cancer chemodynamic therapy. J. Am. Chem. Soc. 2020;142(36):15320–15330. doi: 10.1021/jacs.0c05604. [DOI] [PubMed] [Google Scholar]
- 35.Yao J., Zheng F., Yao C., Xu X., Akakuru O.U., Chen T., Yang F., Wu A. Rational design of nanomedicine for photothermal-chemodynamic bimodal cancer therapy. WIRES Nanomed. Nanobi. 2021;13(3) doi: 10.1002/wnan.1682. [DOI] [PubMed] [Google Scholar]
- 36.Tang Z., Liu Y., He M., Bu W. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2019;58(4):946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 37.Yang B., Chen Y., Shi J. Nanocatalytic medicine. Adv. Mater. 2019;31(39):1901778. doi: 10.1002/adma.201901778. [DOI] [PubMed] [Google Scholar]
- 38.Lin L.-S., Huang T., Song J., Ou X.-Y., Wang Z., Deng H., Tian R., Liu Y., Wang J.-F., Liu Y. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019;141(25):9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Zhen X., Upputuri P.K., Jiang Y., Lau J., Pramanik M., Pu K., Xing B. Redox-activatable and acid-enhanced nanotheranostics for second near-infrared photoacoustic tomography and combined photothermal tumor therapy. ACS Nano. 2019;13(5):5816–5825. doi: 10.1021/acsnano.9b01411. [DOI] [PubMed] [Google Scholar]
- 40.Hao Y., Dong Z., Chen M., Chao Y., Liu Z., Feng L., Hao Y., Dong Z., Chen M., Chao Y. Near-infrared light and glucose dual-responsive cascading hydroxyl radical generation for in situ gelation and effective breast cancer treatment. Biomaterials. 2020;228:119568. doi: 10.1016/j.biomaterials.2019.119568. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Zhong X., Lei H., Geng Y., Zhao Q., Gong F., Yang Z., Dong Z., Liu Z., Cheng L. Hollow Cu2Se nanozymes for tumor photothermal-catalytic therapy. Chem. Mater. 2019;31(16):6174–6186. [Google Scholar]
- 42.Wang X., Zhong X., Zha Z., He G., Miao Z., Lei H., Luo Q., Zhang R., Liu Z., Cheng L. Biodegradable CoS2 nanoclusters for photothermal-enhanced chemodynamic therapy. Appl. Mater. Today. 2020;18:100464. [Google Scholar]
- 43.Wang H., An L., Tao C., Ling Z., Lin J., Tian Q., Yang S. A smart theranostic platform for photoacoustic and magnetic resonance dual-imaging-guided photothermal-enhanced chemodynamic therapy. Nanoscale. 2020;12(8):5139–5150. doi: 10.1039/c9nr10039c. [DOI] [PubMed] [Google Scholar]
- 44.Han R., Wu S., Yan Y., Chen W., Tang K. Construction of ferrocene modified and indocyanine green loaded multifunctional mesoporous silica nanoparticle for simultaneous chemodynamic/photothermal/photodynamic therapy. Mater. Today Commun. 2021;26:101842. [Google Scholar]
- 45.Wang X., Cheng L. Multifunctional Prussian blue-based nanomaterials: preparation, modification, and theranostic applications. Coord. Chem. Rev. 2020;419:213393. [Google Scholar]
- 46.An P., Gao Z., Sun K., Gu D., Wu H., You C., Li Y., Cheng K., Zhang Y., Wang Z. Photothermal-enhanced inactivation of glutathione peroxidase for ferroptosis sensitized by an autophagy promotor. ACS Appl. Mater. Interfaces. 2019;11(46):42988–42997. doi: 10.1021/acsami.9b16124. [DOI] [PubMed] [Google Scholar]
- 47.Jiang F., Ding B., Liang S., Zhao Y., Cheng Z., Xing B., Lin J. Intelligent MoS2–CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/immunotherapy. Biomaterials. 2020;268:120545. doi: 10.1016/j.biomaterials.2020.120545. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Zhen W., Wang Y., Liu J., Jin L., Zhang T., Zhang S., Zhao Y., Song S., Li C. One-dimensional Fe2P acts as a Fenton agent in response to NIR II light and ultrasound for deep tumor synergetic theranostics. Angew. Chem. 2019;131(8):2429–2434. doi: 10.1002/anie.201813702. [DOI] [PubMed] [Google Scholar]
- 49.Poinard B., Neo S.Z.Y., Yeo E.L.L., Heng H.P.S., Neoh K.G., Kah J.C.Y. Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10(25):21125–21136. doi: 10.1021/acsami.8b04799. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y., Meng X., Wang D., Zhang K., Dai W., Dong H., Zhang X. Intelligent MnO2/Cu2–x S for multimode imaging diagnostic and advanced single-laser irradiated photothermal/photodynamic therapy. ACS Appl. Mater. Interfaces. 2018;10(21):17732–17741. doi: 10.1021/acsami.8b05050. [DOI] [PubMed] [Google Scholar]
- 51.Lv R., Yang P., He F., Gai S., Li C., Dai Y., Yang G., Lin J. A yolk-like multifunctional platform for multimodal imaging and synergistic therapy triggered by a single near-infrared light. ACS Nano. 2015;9(2):1630–1647. doi: 10.1021/nn5063613. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., Bhattarai P., Dai Z., Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019;48(7):2053–2108. doi: 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X., Zhang W., Guan G., Song G., Zou R., Hu J. Design and functionalization of the NIR-responsive photothermal semiconductor nanomaterials for cancer theranostics. Acc. Chem. Res. 2017;50(10):2529–2538. doi: 10.1021/acs.accounts.7b00294. [DOI] [PubMed] [Google Scholar]
- 54.Abbas M., Zou Q., Li S., Yan X. Self-assembled peptide-and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv. Mater. 2017;29(12):1605021. doi: 10.1002/adma.201605021. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Zhen W., Jin L., Zhang S., Sun G., Zhang T., Xu X., Song S., Wang Y., Liu J. All-in-one theranostic nanoagent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication. ACS Nano. 2018;12(5):4886–4893. doi: 10.1021/acsnano.8b01893. [DOI] [PubMed] [Google Scholar]
- 56.Han Y., Gao S., Zhang Y., Ni Q., Li Z., Liang X.-J., Zhang J. Metal-based nanocatalyst for combined cancer therapeutics. Bioconjugate Chem. 2020;31(5):1247–1258. doi: 10.1021/acs.bioconjchem.0c00194. [DOI] [PubMed] [Google Scholar]
- 57.Chen H., Gu Z., An H., Chen C., Chen J., Cui R., Chen S., Chen W., Chen X., Chen X. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018;61(12):1503–1552. [Google Scholar]
- 58.Guan G., Wang X., Li B., Zhang W., Cui Z., Lu X., Zou R., Hu J. “Transformed” Fe 3 S 4 tetragonal nanosheets: a high-efficiency and body-clearable agent for magnetic resonance imaging guided photothermal and chemodynamic synergistic therapy. Nanoscale. 2018;10(37):17902–17911. doi: 10.1039/c8nr06507a. [DOI] [PubMed] [Google Scholar]
- 59.Feng W., Han X., Wang R., Gao X., Hu P., Yue W., Chen Y., Shi J. Nanocatalysts-augmented and photothermal-enhanced tumor-specific sequential nanocatalytic therapy in both NIR-I and NIR-II biowindows. Adv. Mater. 2019;31(5):1805919. doi: 10.1002/adma.201805919. [DOI] [PubMed] [Google Scholar]
- 60.Cui J., Jiang R., Guo C., Bai X., Xu S., Wang L. Fluorine grafted Cu7S4–Au heterodimers for multimodal imaging guided photothermal therapy with high penetration depth. J. Am. Chem. Soc. 2018;140(18):5890–5894. doi: 10.1021/jacs.8b00368. [DOI] [PubMed] [Google Scholar]
- 61.Liu S., Pan X., Liu H. Two-dimensional nanomaterials for photothermal therapy. Angew. Chem. 2020;132(15):5943–5953. doi: 10.1002/anie.201911477. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Shi Q., Zha Z., Zhu D., Zheng L., Shi L., Wei X., Lian L., Wu K., Cheng L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021;6(12):4389–4401. doi: 10.1016/j.bioactmat.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., Fan L., Cheng L., Sun Y., Wang X., Zhong X., Shi Q., Gong F., Yang Y., Ma Y. Biodegradable nickel disulfide nanozymes with GSH-depleting function for high-efficiency photothermal-catalytic antibacterial therapy. iScience. 2020;23(7):101281. doi: 10.1016/j.isci.2020.101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., An L., Lin J., Tian Q., Yang S. A hollow Cu9S8 theranostic nanoplatform based on a combination of increased active sites and photothermal performance in enhanced chemodynamic therapy. Chem. Eng. J. 2020;385:123925. [Google Scholar]
- 65.Wang X., Miao Z., Ma Y., Chen H., Qian H., Zha Z. One-pot solution synthesis of shape-controlled copper selenide nanostructures and their potential applications in photocatalysis and photothermal therapy. Nanoscale. 2017;9(38):14512–14519. doi: 10.1039/c7nr04851c. [DOI] [PubMed] [Google Scholar]
- 66.Jung H.S., Verwilst P., Sharma A., Shin J., Sessler J.L., Kim J.S. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem. Soc. Rev. 2018;47(7):2280–2297. doi: 10.1039/c7cs00522a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denkova A.G., de Kruijff R.M., Serra-Crespo P. Combined photo-therapies: nanocarrier-mediated photochemotherapy and photoradiotherapy. Adv. Health. Mater. 2018;7(8):1870033. doi: 10.1002/adhm.201701211. [DOI] [PubMed] [Google Scholar]
- 68.Gu Z., Zhu S., Yan L., Zhao F., Zhao Y. Graphene-based smart platforms for combined Cancer therapy. Adv. Mater. 2019;31(9):1800662. doi: 10.1002/adma.201800662. [DOI] [PubMed] [Google Scholar]
- 69.Yang X., Cheng X., Elzatahry A.A., Chen J., Alghamdi A., Deng Y. Recyclable Fenton-like catalyst based on zeolite Y supported ultrafine, highly-dispersed Fe2O3 nanoparticles for removal of organics under mild conditions. Chin. Chem. Lett. 2019;30(2):324–330. [Google Scholar]
- 70.Tian Q., Tang M., Sun Y., Zou R., Chen Z., Zhu M., Yang S., Wang J., Wang J., Hu J. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 2011;23(31):3542–3547. doi: 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- 71.Jiang F., Tian Q., Tang M., Chen Z., Yang J., Hu J. One-pot synthesis of large-scaled Janus Ag–Ag 2 S nanoparticles and their photocatalytic properties. CrystEngComm. 2011;13(24):7189–7193. [Google Scholar]
- 72.An P., Fan F., Gu D., Gao Z., Hossain A.M.S., Sun B. Photothermal-reinforced and glutathione-triggered in Situ cascaded nanocatalytic therapy. J. Contr. Release. 2020;321:734–743. doi: 10.1016/j.jconrel.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 73.He S., Song J., Qu J., Cheng Z. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018;47(12):4258–4278. doi: 10.1039/c8cs00234g. [DOI] [PubMed] [Google Scholar]
- 74.Qian X., Zhang J., Gu Z., Chen Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials. 2019;211:1–13. doi: 10.1016/j.biomaterials.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 75.Fan K., Cao C., Pan Y., Lu D., Yang D., Feng J., Song L., Liang M., Yan X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012;7(7):459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 76.Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., Wang T., Feng J., Yang D., Perrett S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 77.Shen Z., Song J., Yung B.C., Zhou Z., Wu A., Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv. Mater. 2018;30(12):1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saeed M., Ren W., Wu A. Therapeutic applications of iron oxide based nanoparticles in cancer: basic concepts and recent advances. Biomater. Sci. 2018;6(4):708–725. doi: 10.1039/c7bm00999b. [DOI] [PubMed] [Google Scholar]
- 79.Shen Z., Liu T., Li Y., Lau J., Yang Z., Fan W., Zhou Z., Shi C., Ke C., Bregadze V.I. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 80.Winterbourn C.C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 1995;82:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- 81.Liu M., Liu B., Liu Q., Du K., Wang Z., He N. Nanomaterial-induced ferroptosis for cancer specific therapy. Coord. Chem. Rev. 2019;382:160–180. [Google Scholar]
- 82.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 83.Fan J.X., Peng M.Y., Wang H., Zheng H.R., Liu Z.L., Li C.X., Wang X.N., Liu X.H., Cheng S.X., Zhang X.Z. Engineered bacterial bioreactor for tumor therapy via Fenton-like reaction with localized H2O2 generation. Adv. Mater. 2019;31(16):1808278. doi: 10.1002/adma.201808278. [DOI] [PubMed] [Google Scholar]
- 84.Yao X., Yang P., Jin Z., Jiang Q., Guo R., Xie R., He Q., Yang W. Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials. 2019;197:268–283. doi: 10.1016/j.biomaterials.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 85.Zhao P., Tang Z., Chen X., He Z., He X., Zhang M., Liu Y., Ren D., Zhao K., Bu W. Ferrous-cysteine–phosphotungstate nanoagent with neutral pH fenton reaction activity for enhanced cancer chemodynamic therapy. Mater. Horiz. 2019;6(2):369–374. [Google Scholar]
- 86.Ranji-Burachaloo H., Gurr P.A., Dunstan D.E., Qiao G.G. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano. 2018;12(12):11819–11837. doi: 10.1021/acsnano.8b07635. [DOI] [PubMed] [Google Scholar]
- 87.Zhang C., Bu W., Ni D., Zhang S., Li Q., Yao Z., Zhang J., Yao H., Wang Z., Shi J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew. Chem. Int. Ed. 2016;55(6):2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y., Zhu R., Xi Y., Zhu J., Zhu G., He H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: a review. Appl. Catal. B Environ. 2019;255:117739. [Google Scholar]
- 89.Wu W., Yu L., Jiang Q., Huo M., Lin H., Wang L., Chen Y., Shi J. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J. Am. Chem. Soc. 2019;141(29):11531–11539. doi: 10.1021/jacs.9b03503. [DOI] [PubMed] [Google Scholar]
- 90.Meng X., Zhang X., Liu M., Cai B., He N., Wang Z. Fenton reaction-based nanomedicine in cancer chemodynamic and synergistic therapy. Appl. Mater. Today. 2020;21:100864. [Google Scholar]
- 91.Anandan S., Miyauchi M. Ce-doped ZnO (Ce x Zn 1− x O) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu 2+) grafting. Phys. Chem. Chem. Phys. 2011;13(33):14937–14945. doi: 10.1039/c1cp21514k. [DOI] [PubMed] [Google Scholar]
- 92.Rae T., Schmidt P., Pufahl R., Culotta V., O'halloran T. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 93.Yao C., Wang W., Wang P., Zhao M., Li X., Zhang F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater. 2018;30(7):1704833. doi: 10.1002/adma.201704833. [DOI] [PubMed] [Google Scholar]
- 94.Umar M., Aziz H.A., Yusoff M.S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag. 2010;30(11):2113–2121. doi: 10.1016/j.wasman.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Lei Z., Zhang X., Zheng X., Liu S., Xie Z. Porphyrin–ferrocene conjugates for photodynamic and chemodynamic therapy. Org. Biomol. Chem. 2018;16(44):8613–8619. doi: 10.1039/c8ob02391c. [DOI] [PubMed] [Google Scholar]
- 96.Kim J., Cho H.R., Jeon H., Kim D., Song C., Lee N., Choi S.H., Hyeon T. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc. 2017;139(32):10992–10995. doi: 10.1021/jacs.7b05559. [DOI] [PubMed] [Google Scholar]
- 97.Wu X., Zhang H., Li Y., Zhang D., Li X. Factorial design analysis for COD removal from landfill leachate by photoassisted Fered-Fenton process. Environ. Sci. Pollut. Res. 2014;21(14):8595–8602. doi: 10.1007/s11356-014-2790-2. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Tu D., Zhu H., Chen X. Lanthanide-doped luminescent nanoprobes: controlled synthesis, optical spectroscopy, and bioapplications. Chem. Soc. Rev. 2013;42(16):6924–6958. doi: 10.1039/c3cs60060b. [DOI] [PubMed] [Google Scholar]
- 99.Hao Y.-N., Zhang W.-X., Gao Y.-R., Wei Y.-N., Shu Y., Wang J.-H. State-of-the-art advances of copper-based nanostructures in the enhancement of chemodynamic therapy. J. Mater. Chem. B. 2021;9(2):250–266. doi: 10.1039/d0tb02360d. [DOI] [PubMed] [Google Scholar]
- 100.Khan S., Sharifi M., Bloukh S.H., Edis Z., Siddique R., Falahati M. In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta. 2020;224:121805. doi: 10.1016/j.talanta.2020.121805. [DOI] [PubMed] [Google Scholar]
- 101.Lee K.-T., Lu Y.-J., Mi F.-L., Burnouf T., Wei Y.-T., Chiu S.-C., Chuang E.-Y., Lu S.-Y. Catalase-modulated heterogeneous Fenton reaction for selective cancer cell eradication: SnFe2O4 nanocrystals as an effective reagent for treating lung cancer cells. ACS Appl. Mater. Interfaces. 2017;9(2):1273–1279. doi: 10.1021/acsami.6b13529. [DOI] [PubMed] [Google Scholar]
- 102.Wang X., Zhong X., Cheng L. Titanium-based nanomaterials for cancer theranostics. Coord. Chem. Rev. 2021;430:213662. [Google Scholar]
- 103.Wang W., Jin Y., Xu Z., Liu X., Bajwa S.Z., Khan W.S., Yu H. Stimuli-activatable nanomedicines for chemodynamic therapy of cancer. WIRES Nanomed. Nanobi. 2020;12(4):e1614. doi: 10.1002/wnan.1614. [DOI] [PubMed] [Google Scholar]
- 104.Xue T., Xu C., Wang Y., Wang Y., Tian H., Zhang Y. Doxorubicin-loaded nanoscale metal–organic framework for tumor-targeting combined chemotherapy and chemodynamic therapy. Biomater. Sci. 2019;7(11):4615–4623. doi: 10.1039/c9bm01044k. [DOI] [PubMed] [Google Scholar]
- 105.Yang B., Chen Y., Shi J. Nanocatalytic medicine. Adv. Mater. 2019;31(39):1901778. doi: 10.1002/adma.201901778. [DOI] [PubMed] [Google Scholar]
- 106.Yang Z., Dai Y., Yin C., Fan Q., Zhang W., Song J., Yu G., Tang W., Fan W., Yung B.C. Activatable semiconducting theranostics: simultaneous generation and ratiometric photoacoustic imaging of reactive oxygen species in vivo. Adv. Mater. 2018;30(23):1707509. doi: 10.1002/adma.201707509. [DOI] [PubMed] [Google Scholar]
- 107.Bokare A.D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard Mater. 2014;275:121–135. doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 108.Neyens E., Baeyens J. A review of classic Fenton's peroxidation as an advanced oxidation technique. J. Hazard Mater. 2003;98(1–3):33–50. doi: 10.1016/s0304-3894(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 109.Chen Q., Luo Y., Du W., Liu Z., Zhang S., Yang J., Yao H., Liu T., Ma M., Chen H. Clearable theranostic platform with a pH-independent chemodynamic therapy enhancement strategy for synergetic photothermal tumor therapy. ACS Appl. Mater. Interfaces. 2019;11(20):18133–18144. doi: 10.1021/acsami.9b02905. [DOI] [PubMed] [Google Scholar]
- 110.Tian Q., An L., Lin J., Yang S. Ellagic acid-Fe@ BSA nanoparticles for endogenous H2S accelerated Fe (III)/Fe (II) conversion and photothermal synergistically enhanced chemodynamic therapy. Theranostics. 2020;10(9):4101–4115. doi: 10.7150/thno.41882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu F., Lin L., Zhang Y., Wang Y., Sheng S., Xu C., Tian H., Chen X. A tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy. Adv. Mater. 2019;31(40):1902885. doi: 10.1002/adma.201902885. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y., Zhao J., Chen Z., Zhang F., Wang Q., Guo W., Wang K., Lin H., Qu F. Construct of MoSe2/Bi2Se3 nanoheterostructure: multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials. 2019;217:119282. doi: 10.1016/j.biomaterials.2019.119282. [DOI] [PubMed] [Google Scholar]
- 113.Xie Z., Liang S., Cai X., Ding B., Huang S., Hou Z., Ma P.a., Cheng Z., Lin J. O2-Cu/ZIF-8@ Ce6/ZIF-8@ F127 composite as a tumor microenvironment-responsive nanoplatform with enhanced photo-/chemodynamic antitumor efficacy. ACS Appl. Mater. Interfaces. 2019;11(35):31671–31680. doi: 10.1021/acsami.9b10685. [DOI] [PubMed] [Google Scholar]
- 114.Zhou W., Guan H., Sun K., Xing Y., Zhang J. FeOOH/polypyrrole nanocomposites with an islands-in-sea structure toward combined photothermal/chemodynamic therapy. ACS Appl. Bio Mater. 2019;2(7):2708–2714. doi: 10.1021/acsabm.9b00435. [DOI] [PubMed] [Google Scholar]
- 115.Zhao G., Wu H., Feng R., Wang D., Xu P., Jiang P., Yang K., Wang H., Guo Z., Chen Q. Novel metal polyphenol framework for MR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10(4):3295–3304. doi: 10.1021/acsami.7b16222. [DOI] [PubMed] [Google Scholar]
- 116.Huo M., Wang L., Wang Y., Chen Y., Shi J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano. 2019;13(2):2643–2653. doi: 10.1021/acsnano.9b00457. [DOI] [PubMed] [Google Scholar]
- 117.Gao S., Lu X., Zhu P., Lin H., Yu L., Yao H., Wei C., Chen Y., Shi J. Self-evolved hydrogen peroxide boosts photothermal-promoted tumor-specific nanocatalytic therapy. J. Mater. Chem. B. 2019;7(22):3599–3609. [Google Scholar]
- 118.Liu F., Lin L., Sheng S., Xu C., Wang Y., Zhang Y., Wang D., Wu J., Li Y., Tian H. A glutathione-depleting chemodynamic therapy agent with photothermal and photoacoustic properties for tumor theranostics. Nanoscale. 2020;12(3):1349–1355. doi: 10.1039/c9nr09858e. [DOI] [PubMed] [Google Scholar]
- 119.Dong S., Dong Y., Jia T., Zhang F., Wang Z., Feng L., Sun Q., Gai S., Yang P. Sequential catalytic, magnetic targeting nanoplatform for synergistic photothermal and NIR-enhanced chemodynamic therapy. Chem. Mater. 2020;32(23):9868–9881. [Google Scholar]
- 120.Xiao S., Lu Y., Feng M., Dong M., Cao Z., Zhang X., Chen Y., Liu J. Multifunctional FeS2 theranostic nanoparticles for photothermal-enhanced chemodynamic/photodynamic cancer therapy and photoacoustic imaging. Chem. Eng. J. 2020;396:125294. [Google Scholar]
- 121.Wang S., Yang L., Cho H.-Y., Chueng S.-T.D., Zhang H., Zhang Q., Lee K.-B. Programmed degradation of a hierarchical nanoparticle with redox and light responsivity for self-activated photo-chemical enhanced chemodynamic therapy. Biomaterials. 2019;224:119498. doi: 10.1016/j.biomaterials.2019.119498. [DOI] [PubMed] [Google Scholar]
- 122.She D., Peng S., Liu L., Huang H., Zheng Y., Lu Y., Geng D., Yin B. Biomimic FeS2 nanodrug with hypothermal photothermal effect by clinical approved NIR-Ⅱ light for augmented chemodynamic therapy. Chem. Eng. J. 2020;400:125933. [Google Scholar]
- 123.Shi Y., Zhang J., Huang H., Cao C., Yin J., Xu W., Wang W., Song X., Zhang Y., Dong X. Fe-Doped polyoxometalate as acid-aggregated nanoplatform for NIR-II photothermal-enhanced chemodynamic therapy. Adv. Health. Mater. 2020;9(9):2000005. doi: 10.1002/adhm.202000005. [DOI] [PubMed] [Google Scholar]
- 124.Hu Z., Wang S., Dai Z., Zhang H., Zheng X. A novel theranostic nano-platform (PB@ FePt–HA-g-PEG) for tumor chemodynamic–photothermal co-therapy and triple-modal imaging (MR/CT/PI) diagnosis. J. Mater. Chem. B. 2020;8(24):5351–5360. doi: 10.1039/d0tb00708k. [DOI] [PubMed] [Google Scholar]
- 125.Chen Y., Gao M., Zhang L., Ha E., Hu X., Zou R., Yan L., Hu J. Tumor microenvironment responsive biodegradable Fe-doped MoOx nanowires for magnetic resonance imaging guided photothermal-enhanced chemodynamic synergistic antitumor therapy. Adv. Health. Mater. 2020;10:2001665. doi: 10.1002/adhm.202001665. [DOI] [PubMed] [Google Scholar]
- 126.Zheng H., Wang S., Zhou L., He X., Cheng Z., Cheng F., Liu Z., Wang X., Chen Y., Zhang Q. Injectable multi-responsive micelle/nanocomposite hybrid hydrogel for bioenzyme and photothermal augmented chemodynamic therapy of skin cancer and bacterial infection. Chem. Eng. J. 2020;404:126439. [Google Scholar]
- 127.Zhang S., Jin L., Liu J., Liu Y., Zhang T., Zhao Y., Yin N., Niu R., Li X., Xue D. Boosting chemodynamic therapy by the synergistic effect of Co-catalyze and photothermal effect triggered by the second near-infrared light. Nano-Micro Lett. 2020;12(1):1–13. doi: 10.1007/s40820-020-00516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao Y., Ding B., Xiao X., Jiang F., Wang M., Hou Z., Xing B., Teng B., Cheng Z., Ma P.a. Virus-like Fe3O4@ Bi2S3 nanozymes with resistance-free apoptotic hyperthermia-augmented nanozymitic activity for enhanced synergetic cancer therapy. ACS Appl. Mater. Interfaces. 2020;12(10):11320–11328. doi: 10.1021/acsami.9b20661. [DOI] [PubMed] [Google Scholar]
- 129.An P., Gu D., Gao Z., Fan F., Jiang Y., Sun B. Hypoxia-augmented and photothermally-enhanced ferroptotic therapy with high specificity and efficiency. J. Mater. Chem. B. 2020;8(1):78–87. doi: 10.1039/c9tb02268f. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Wu J., Jin Y., Zhen W., Wang Y., Liu J., Jin L., Zhang S., Zhao Y., Song S. Copper (I) phosphide nanocrystals for in situ self-generation magnetic resonance imaging-guided photothermal-enhanced chemodynamic synergetic therapy resisting deep-seated tumor. Adv. Funct. Mater. 2019;29(50):1904678. [Google Scholar]
- 131.Yang C., Younis M.R., Zhang J., Qu J., Lin J., Huang P. Programmable NIR-II photothermal-enhanced starvation-primed chemodynamic therapy using glucose oxidase-functionalized ancient pigment nanosheets. Small. 2020;16(25):2001518. doi: 10.1002/smll.202001518. [DOI] [PubMed] [Google Scholar]
- 132.Li W., Wang S., Ren C., Liu P., Lu Q., Yang L., Song Y., Xu M., Tan F., Yu M. Exo/endogenous dual-augmented chemodynamic therapy based on bio-reducible and bio-breakable copper (Ⅱ)-based truncated octahedron. Chem. Eng. J. 2020;396:125280. [Google Scholar]
- 133.Jiang F., Ding B., Zhao Y., Liang S., Cheng Z., Xing B., Teng B., Lin J. Biocompatible CuO-decorated carbon nanoplatforms for multiplexed imaging and enhanced antitumor efficacy via combined photothermal therapy/chemodynamic therapy/chemotherapy. Sci. China Mater. 2020;63(9):1818–1830. [Google Scholar]
- 134.Chang M., Wang M., Wang M., Shu M., Ding B., Li C., Pang M., Cui S., Hou Z., Lin J. A multifunctional Cascade bioreactor based on hollow-structured Cu2MoS4 for synergetic cancer chemo-dynamic therapy/starvation therapy/phototherapy/immunotherapy with remarkably enhanced efficacy. Adv. Mater. 2019;31(51):1905271. doi: 10.1002/adma.201905271. [DOI] [PubMed] [Google Scholar]
- 135.Sun H., Zhang Y., Chen S., Wang R., Chen Q., Li J., Luo Y., Wang X., Chen H. Photothermal fenton nanocatalysts for synergetic cancer therapy in the second near-infrared window. ACS Appl. Mater. Interfaces. 2020;12(27):30145–30154. doi: 10.1021/acsami.0c07013. [DOI] [PubMed] [Google Scholar]
- 136.Qi G., Zhang Y., Wang J., Wang D., Wang B., Li H., Jin Y. Smart plasmonic nanozyme enhances combined chemo-photothermal cancer therapy and reveals tryptophan metabolic apoptotic pathway. Anal. Chem. 2019;91(19):12203–12211. doi: 10.1021/acs.analchem.9b01655. [DOI] [PubMed] [Google Scholar]
- 137.Fan L., Xu X., Zhu C., Han J., Gao L., Xi J., Guo R. Tumor catalytic–photothermal therapy with yolk–shell gold@ carbon nanozymes. ACS Appl. Mater. Interfaces. 2018;10(5):4502–4511. doi: 10.1021/acsami.7b17916. [DOI] [PubMed] [Google Scholar]
- 138.Wang M., Chang M., Chen Q., Wang D., Li C., Hou Z., Lin J., Jin D., Xing B. Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomaterials. 2020;252:120093. doi: 10.1016/j.biomaterials.2020.120093. [DOI] [PubMed] [Google Scholar]
- 139.Dong Y., Dong S., Wang Z., Feng L., Sun Q., Chen G., He F., Liu S., Li W., Yang P. Multimode imaging-guided photothermal/chemodynamic synergistic therapy nanoagent with a tumor microenvironment responded effect. ACS Appl. Mater. Interfaces. 2020;12(47):52479–52491. doi: 10.1021/acsami.0c17923. [DOI] [PubMed] [Google Scholar]
- 140.Gao J., Gu H., Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc. Chem. Res. 2009;42(8):1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 141.Shubayev V.I., Pisanic T.R., II, Jin S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009;61(6):467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu A.H., Salabas E.e.L., Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007;46(8):1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 143.Laurent S., Forge D., Port M., Roch A., Robic C., Vander Elst L., Muller R.N. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108(6):2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 144.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 145.Xu H., Zhang X., Han R., Yang P., Ma H., Song Y., Lu Z., Yin W., Wu X., Wang H. Nanoparticles in sonodynamic therapy: state of the art review. RSC Adv. 2016;6(56):50697–50705. [Google Scholar]
- 146.Corot C., Robert P., Idée J.-M., Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 147.Klenk C., Gawande R., Uslu L., Khurana A., Qiu D., Quon A., Donig J., Rosenberg J., Luna-Fineman S., Moseley M. Ionising radiation-free whole-body MRI versus 18F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15(3):275–285. doi: 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 148.Ansari C., Tikhomirov G.A., Hong S.H., Falconer R.A., Loadman P.M., Gill J.H., Castaneda R., Hazard F.K., Tong L., Lenkov O.D. Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small. 2014;10(3):566–575. doi: 10.1002/smll.201301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fütterer S., Andrusenko I., Kolb U., Hofmeister W., Langguth P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD) J. Pharm. Biomed. Anal. 2013;86:151–160. doi: 10.1016/j.jpba.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Du B., Jiang X., Das A., Zhou Q., Yu M., Jin R., Zheng J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017;12(11):1096–1102. doi: 10.1038/nnano.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Du B., Yu M., Zheng J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 2018;3(10):358–374. [Google Scholar]
- 152.Guo G., Zhang H., Shen H., Zhu C., He R., Tang J., Wang Y., Jiang X., Wang J., Bu W. Space-selective chemodynamic therapy of CuFe5O8 nanocubes for implant-related infections. ACS Nano. 2020;14(10):13391–13405. doi: 10.1021/acsnano.0c05255. [DOI] [PubMed] [Google Scholar]
- 153.Liu T., Xiao B., Xiang F., Tan J., Chen Z., Zhang X., Wu C., Mao Z., Luo G., Chen X. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020;11(1):1–16. doi: 10.1038/s41467-020-16544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu Z.C., Li W.P., Luo C.H., Su C.H., Yeh C.S. Rattle-type Fe3O4@ CuS developed to conduct magnetically guided photoinduced hyperthermia at first and second NIR biological windows. Adv. Funct. Mater. 2015;25(41):6527–6537. [Google Scholar]
- 155.Huang C.-C., Chang P.-Y., Liu C.-L., Xu J.-P., Wu S.-P., Kuo W.-C. New insight on optical and magnetic Fe 3 O 4 nanoclusters promising for near infrared theranostic applications. Nanoscale. 2015;7(29):12689–12697. doi: 10.1039/c5nr03157e. [DOI] [PubMed] [Google Scholar]
- 156.Li S.L., Jiang P., Jiang F.L., Liu Y. Recent advances in nanomaterial-based nanoplatforms for chemodynamic cancer therapy. Adv. Funct. Mater. 2021;31(22):2100243. [Google Scholar]
- 157.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol. Aspect. Med. 2005;26(4–5):268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 158.Rayman M.P. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005;64(4):527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 159.Ding X., Liow C.H., Zhang M., Huang R., Li C., Shen H., Liu M., Zou Y., Gao N., Zhang Z. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J. Am. Chem. Soc. 2014;136(44):15684–15693. doi: 10.1021/ja508641z. [DOI] [PubMed] [Google Scholar]
- 160.Li A., Li X., Yu X., Li W., Zhao R., An X., Cui D., Chen X., Li W. Synergistic thermoradiotherapy based on PEGylated Cu3BiS3 ternary semiconductor nanorods with strong absorption in the second near-infrared window. Biomaterials. 2017;112:164–175. doi: 10.1016/j.biomaterials.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 161.Zhao X., Bao Z., Sun C., Xue D. Polymorphology formation of Cu2O: a microscopic understanding of single crystal growth from both thermodynamic and kinetic models. J. Cryst. Growth. 2009;311(3):711–715. [Google Scholar]
- 162.Zheng H., Qin L., Lin H., Nie M., Li Y., Li Q. Convenient route to well-dispersed Cu2O nanospheres and their use as photocatalysts. J. Nanosci. Nanotechnol. 2015;15(8):6063–6068. doi: 10.1166/jnn.2015.10270. [DOI] [PubMed] [Google Scholar]
- 163.Gou L., Murphy C.J. Controlling the size of Cu 2 O nanocubes from 200 to 25 nm. J. Mater. Chem. 2004;14(4):735–738. [Google Scholar]
- 164.Sharifi M., Hosseinali S.H., Yousefvand P., Salihi A., Shekha M.S., Aziz F.M., JouyaTalaei A., Hasan A., Falahati M. Gold nanozyme: biosensing and therapeutic activities. Mater. Sci. Eng. C. 2020;108:110422. doi: 10.1016/j.msec.2019.110422. [DOI] [PubMed] [Google Scholar]
- 165.Hu M., Chen J., Li Z.-Y., Au L., Hartland G.V., Li X., Marquez M., Xia Y. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006;35(11):1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 166.Huang Y., Huang P., Lin J. Plasmonic gold nanovesicles for biomedical applications. Small Methods. 2019;3(3):1800394. [Google Scholar]
- 167.Hernández-Sánchez D., Villabona-Leal G., Saucedo-Orozco I., Bracamonte V., Pérez E., Bittencourt C., Quintana M. Stable graphene oxide–gold nanoparticle platforms for biosensing applications. Phys. Chem. Chem. Phys. 2018;20(3):1685–1692. doi: 10.1039/c7cp04817c. [DOI] [PubMed] [Google Scholar]
- 168.Phiri M.M., Mulder D.W., Vorster B.C. Seedless gold nanostars with seed-like advantages for biosensing applications. R. Soc. Open Sci. 2019;6(2):181971. doi: 10.1098/rsos.181971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hasan A., Nurunnabi M., Morshed M., Paul A., Polini A., Kuila T., Al Hariri M., Lee Y.-k., Jaffa A.A. Recent advances in application of biosensors in tissue engineering. BioMed Res. Int. 2014;2014:1–18. doi: 10.1155/2014/307519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ashton J.R., Castle K.D., Qi Y., Kirsch D.G., West J.L., Badea C.T. Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics. 2018;8(7):1782–1797. doi: 10.7150/thno.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013;42(14):6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 172.Wu J., Li S., Wei H. Multifunctional nanozymes: enzyme-like catalytic activity combined with magnetism and surface plasmon resonance. Nanoscale Horizon. 2018;3(4):367–382. doi: 10.1039/c8nh00070k. [DOI] [PubMed] [Google Scholar]
- 173.Feng J., Huang P., Shi S., Deng K.-Y., Wu F.-Y. Colorimetric detection of glutathione in cells based on peroxidase-like activity of gold nanoclusters: a promising powerful tool for identifying cancer cells. Anal. Chim. Acta. 2017;967:64–69. doi: 10.1016/j.aca.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 174.Koo A.N., Min K.H., Lee H.J., Lee S.-U., Kim K., Kwon I.C., Cho S.H., Jeong S.Y., Lee S.C. Tumor accumulation and antitumor efficacy of docetaxel-loaded core-shell-corona micelles with shell-specific redox-responsive cross-links. Biomaterials. 2012;33(5):1489–1499. doi: 10.1016/j.biomaterials.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 175.Wen L., Chen L., Zheng S., Zeng J., Duan G., Wang Y., Wang G., Chai Z., Li Z., Gao M. Ultrasmall biocompatible WO3− x nanodots for multi-modality imaging and combined therapy of cancers. Adv. Mater. 2016;28(25):5072–5079. doi: 10.1002/adma.201506428. [DOI] [PubMed] [Google Scholar]
- 176.Zhou Z., Kong B., Yu C., Shi X., Wang M., Liu W., Sun Y., Zhang Y., Yang H., Yang S. Tungsten oxide nanorods: an efficient nanoplatform for tumor CT imaging and photothermal therapy. Sci. Rep. 2014;4(1):1–10. doi: 10.1038/srep03653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bu Y., Chen Z., Sun C. Highly efficient Z-Scheme Ag3PO4/Ag/WO3− x photocatalyst for its enhanced photocatalytic performance. Appl. Catal., B. 2015;179:363–371. [Google Scholar]
- 178.Gong F., Cheng L., Yang N., Jin Q., Tian L., Wang M., Li Y., Liu Z. Bimetallic oxide MnMoOX nanorods for in vivo photoacoustic imaging of GSH and tumor-specific photothermal therapy. Nano Lett. 2018;18(9):6037–6044. doi: 10.1021/acs.nanolett.8b02933. [DOI] [PubMed] [Google Scholar]
- 179.Gong L., Yan L., Zhou R., Xie J., Wu W., Gu Z. Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy. J. Mater. Chem. B. 2017;5(10):1873–1895. doi: 10.1039/c7tb00195a. [DOI] [PubMed] [Google Scholar]
- 180.Wang C., Fan W., Zhang Z., Wen Y., Xiong L., Chen X. Advanced nanotechnology leading the way to multimodal imaging-guided precision surgical therapy. Adv. Mater. 2019;31(49):1904329. doi: 10.1002/adma.201904329. [DOI] [PubMed] [Google Scholar]
- 181.Sun M., Xu L., Ma W., Wu X., Kuang H., Wang L., Xu C. Hierarchical plasmonic nanorods and upconversion core–satellite nanoassemblies for multimodal imaging-guided combination phototherapy. Adv. Mater. 2016;28(5):898–904. doi: 10.1002/adma.201505023. [DOI] [PubMed] [Google Scholar]
- 182.Pushpalatha R., Selvamuthukumar S., Kilimozhi D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018;45:45–53. [Google Scholar]
- 183.Wang S., Wang Z., Yu G., Zhou Z., Jacobson O., Liu Y., Ma Y., Zhang F., Chen Z.Y., Chen X. Tumor-specific drug release and reactive oxygen species generation for cancer chemo/chemodynamic combination therapy. Adv. Sci. 2019;6(5):1801986. doi: 10.1002/advs.201801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qin S.-Y., Zhang A.-Q., Cheng S.-X., Rong L., Zhang X.-Z. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234–247. doi: 10.1016/j.biomaterials.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 185.Lee W.-H., Loo C.-Y., Traini D., Young P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J. Pharm. Sci. 2015;10(6):481–489. [Google Scholar]
- 186.Wu Q., Yang Z., Nie Y., Shi Y., Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 187.Islam K., Anggondowati T., Deviany P., Ryan J., Fetrick A., Bagenda D., Copur M., Tolentino A., Vaziri I., McKean H. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer. 2019;19(1):1–9. doi: 10.1186/s12885-019-6054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mikoshiba T., Ozawa H., Saito S., Ikari Y., Nakahara N., Ito F., Watanabe Y., Sekimizu M., Imanishi Y., Ogawa K. Usefulness of hematological inflammatory markers in predicting severe side-effects from induction chemotherapy in head and neck cancer patients. Anticancer Res. 2019;39(6):3059–3065. doi: 10.21873/anticanres.13440. [DOI] [PubMed] [Google Scholar]
- 189.Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019;54(2):407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Su Y.Y., Teng Z., Yao H., Wang S.J., Tian Y., Zhang Y.L., Liu W.F., Tian W., Zheng L.J., Lu N. A multifunctional PB@ mSiO2–PEG/DOX nanoplatform for combined photothermal–chemotherapy of tumor. ACS Appl. Mater. Interfaces. 2016;8(27):17038–17046. doi: 10.1021/acsami.6b01147. [DOI] [PubMed] [Google Scholar]
- 191.Kim B., Lee E., Kim Y., Park S., Khang G., Lee D. Dual acid-responsive micelle-forming anticancer polymers as new anticancer therapeutics. Adv. Funct. Mater. 2013;23(40):5091–5097. [Google Scholar]
- 192.Kankala R.K., Liu C.-G., Chen A.-Z., Wang S.-B., Xu P.-Y., Mende L.K., Liu C.-L., Lee C.-H., Hu Y.-F. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactors. ACS Biomater. Sci. Eng. 2017;3(10):2431–2442. doi: 10.1021/acsbiomaterials.7b00569. [DOI] [PubMed] [Google Scholar]
- 193.Dai Y., Yang Z., Cheng S., Wang Z., Zhang R., Zhu G., Wang Z., Yung B.C., Tian R., Jacobson O. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv. Mater. 2018;30(8):1704877. doi: 10.1002/adma.201704877. [DOI] [PubMed] [Google Scholar]
- 194.Karyakin A.A. Advances of Prussian blue and its analogues in (bio) sensors. Curr. Opin. Electrochem. 2017;5(1):92–98. [Google Scholar]
- 195.Huang P., Qian X., Chen Y., Yu L., Lin H., Wang L., Zhu Y., Shi J. Metalloporphyrin-encapsulated biodegradable nanosystems for highly efficient magnetic resonance imaging-guided sonodynamic cancer therapy. J. Am. Chem. Soc. 2017;139(3):1275–1284. doi: 10.1021/jacs.6b11846. [DOI] [PubMed] [Google Scholar]
- 196.Wang X., Zhong X., Bai L., Xu J., Gong F., Dong Z., Yang Z., Zeng Z., Liu Z., Cheng L. Ultrafine titanium monoxide (TiO1+ x) nanorods for enhanced sonodynamic therapy. J. Am. Chem. Soc. 2020;142(14):6527–6537. doi: 10.1021/jacs.9b10228. [DOI] [PubMed] [Google Scholar]
- 197.Cui X., Han X., Yu L., Zhang B., Chen Y. Intrinsic chemistry and design principle of ultrasound-responsive nanomedicine. Nano Today. 2019;28:100773. [Google Scholar]
- 198.Lin K., Lin Z., Li Y., Zheng Y., Zhang D. Ultrasound-induced reactive oxygen species generation and mitochondria-specific damage by sonodynamic agent/metal ion-doped mesoporous silica. RSC Adv. 2019;9(68):39924–39931. doi: 10.1039/c9ra08142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rosenthal I., Sostaric J.Z., Riesz P. Sonodynamic therapy––a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004;11(6):349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 200.Zhao Y., Chen B.-Q., Kankala R.K., Wang S.-B., Chen A.-Z. Recent advances in combination of copper chalcogenide-based photothermal and reactive oxygen species-related therapies. ACS Biomater. Sci. Eng. 2020;6(9):4799–4815. doi: 10.1021/acsbiomaterials.0c00830. [DOI] [PubMed] [Google Scholar]
- 201.Cao Y., Wu T., Dai W., Dong H., Zhang X. TiO2 nanosheets with the Au nanocrystal-decorated edge for mitochondria-targeting enhanced sonodynamic therapy. Chem. Mater. 2019;31(21):9105–9114. [Google Scholar]
- 202.Yue W., Chen L., Yu L., Zhou B., Yin H., Ren W., Liu C., Guo L., Zhang Y., Sun L. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019;10(1):1–15. doi: 10.1038/s41467-019-09760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Costley D., Mc Ewan C., Fowley C., McHale A.P., Atchison J., Nomikou N., Callan J.F. Treating cancer with sonodynamic therapy: a review. Int. J. Hyperther. 2015;31(2):107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- 204.Dong X.-Y., Cheng J.-T., Zhang Y.-F., Li Z.-L., Zhan T.-Y., Chen J.-J., Wang F.-L., Yang N.-Y., Ye L., Gu Q.-S. Copper-catalyzed asymmetric radical 1, 2-carboalkynylation of alkenes with alkyl halides and terminal alkynes. J. Am. Chem. Soc. 2020;142(20):9501–9509. doi: 10.1021/jacs.0c03130. [DOI] [PubMed] [Google Scholar]
- 205.Lan G., Ni K., Veroneau S.S., Feng X., Nash G.T., Luo T., Xu Z., Lin W. Titanium-based nanoscale metal–organic framework for type I photodynamic therapy. J. Am. Chem. Soc. 2019;141(10):4204–4208. doi: 10.1021/jacs.8b13804. [DOI] [PubMed] [Google Scholar]
- 206.Nguyen V.N., Yim Y., Kim S., Ryu B., Swamy K., Kim G., Kwon N., Kim C.Y., Park S., Yoon J. Molecular design of highly efficient heavy-atom-free triplet BODIPY derivatives for photodynamic therapy and bioimaging. Angew. Chem. Int. Ed. 2020;59(23):8957–8962. doi: 10.1002/anie.202002843. [DOI] [PubMed] [Google Scholar]
- 207.Wang X., Zhong X., Li J., Liu Z., Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021;50(15):8669–8742. doi: 10.1039/d0cs00461h. [DOI] [PubMed] [Google Scholar]
- 208.Paszko E., Ehrhardt C., Senge M.O., Kelleher D.P., Reynolds J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011;8(1):14–29. doi: 10.1016/j.pdpdt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 209.Bechet D., Couleaud P., Frochot C., Viriot M.-L., Guillemin F., Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26(11):612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 210.Lan G., Ni K., Xu Z., Veroneau S.S., Song Y., Lin W. Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 2018;140(17):5670–5673. doi: 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yao C., Wang W., Wang P., Zhao M., Li X., Zhang F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater. 2018;30(7):1704833. doi: 10.1002/adma.201704833. [DOI] [PubMed] [Google Scholar]
- 212.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nam J., Son S., Park K.S., Zou W., Shea L.D., Moon J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019;4(6):398–414. [Google Scholar]
- 214.Rao L., Zhao S.K., Wen C., Tian R., Lin L., Cai B., Sun Y., Kang F., Yang Z., He L. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv. Mater. 2020;32(47):2004853. doi: 10.1002/adma.202004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fan Q., Chen Z., Wang C., Liu Z. Toward biomaterials for enhancing immune checkpoint blockade therapy. Adv. Funct. Mater. 2018;28(37):1802540. [Google Scholar]
- 216.Duan X., Chan C., Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. 2019;58(3):670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lu K., He C., Guo N., Chan C., Ni K., Weichselbaum R.R., Lin W. Chlorin-based nanoscale metal–organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc. 2016;138(38):12502–12510. doi: 10.1021/jacs.6b06663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu C., Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021 doi: 10.1039/d0cs00664e. [DOI] [PubMed] [Google Scholar]
- 219.Michot J., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., Berdelou A., Varga A., Bahleda R., Hollebecque A. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 220.Zhou Z., Song J., Nie L., Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016;45(23):6597–6626. doi: 10.1039/c6cs00271d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fu Q., Li Z., Ye J., Li Z., Fu F., Lin S.-L., Chang C.A., Yang H., Song J. Magnetic targeted near-infrared II PA/MR imaging guided photothermal therapy to trigger cancer immunotherapy. Theranostics. 2020;10(11):4997–5010. doi: 10.7150/thno.43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fu L.-H., Qi C., Lin J., Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018;47(17):6454–6472. doi: 10.1039/c7cs00891k. [DOI] [PubMed] [Google Scholar]
- 223.Marx J. A boost for tumor starvation. Science. 2003;301(5632):452–454. doi: 10.1126/science.301.5632.452. [DOI] [PubMed] [Google Scholar]
- 224.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8(9):705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 225.Zhu H., Li Y., Ming Z., Liu W. Glucose oxidase-mediated tumor starvation therapy combined with photothermal therapy for colon cancer. Biomater. Sci. 2021;9:5577–5587. doi: 10.1039/d1bm00869b. [DOI] [PubMed] [Google Scholar]
- 226.Li J., Li Y., Wang Y., Ke W., Chen W., Wang W., Ge Z. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett. 2017;17(11):6983–6990. doi: 10.1021/acs.nanolett.7b03531. [DOI] [PubMed] [Google Scholar]
- 227.He T., Xu H., Zhang Y., Yi S., Cui R., Xing S., Wei C., Lin J., Huang P. Glucose oxidase-instructed traceable self-oxygenation/hyperthermia dually enhanced cancer starvation therapy. Theranostics. 2020;10(4):1544–1554. doi: 10.7150/thno.40439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fan W., Lu N., Huang P., Liu Y., Yang Z., Wang S., Yu G., Liu Y., Hu J., He Q. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew. Chem. 2017;129(5):1249–1253. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 229.Zhang R., Zhang L., Ran H., Li P., Huang J., Tan M., Yang Y., Wang Z. A mitochondria-targeted anticancer nanoplatform with deep penetration for enhanced synergistic sonodynamic and starvation therapy. Biomater. Sci. 2020;8(16):4581–4594. doi: 10.1039/d0bm00408a. [DOI] [PubMed] [Google Scholar]
- 230.Li S.-Y., Cheng H., Xie B.-R., Qiu W.-X., Zeng J.-Y., Li C.-X., Wan S.-S., Zhang L., Liu W.-L., Zhang X.-Z. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–7018. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- 231.Zhang L., Wan S.-S., Li C.-X., Xu L., Cheng H., Zhang X.-Z. An adenosine triphosphate-responsive autocatalytic fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe (iii)/Fe (ii) conversion. Nano Lett. 2018;18(12):7609–7618. doi: 10.1021/acs.nanolett.8b03178. [DOI] [PubMed] [Google Scholar]
- 232.Chen J., Mohrhusen L., Ali G., Li S., Chung K.Y., Al-Shamery K., Lee P.S. Electrochemical mechanism investigation of Cu2MoS4 hollow nanospheres for fast and stable sodium ion storage. Adv. Funct. Mater. 2019;29(7):1807753. [Google Scholar]