Abstract
Purpose
The clinical significance of margin status in pancreatic head cancer is still controversial due to the nonstandardized definition of R status and pathologic reporting. This study aims to evaluate the impact of the margin status including location and the role of radiation therapy in pancreatic head cancer.
Methods
A total of 314 patients who underwent curative-intent surgery for pancreatic head cancer between 2010 and 2017 were analyzed. Demographics, survival, and local recurrences were compared according to 2 definitions: 0-mm R1 as direct involvement and 1-mm R1 as close resection margin less than 1 mm. The specific margins were divided into 4 groups according to the location around the pancreas: pancreas transection, anterior surface, posterior surface, and vessel (superior mesenteric artery/superior mesenteric vein) margin.
Results
The 0-mm R1-rate was 15.6%, and increased to 36.3% in 1-mm R1. The median overall survival rate of 0-mm R0 vs. R1 was 26 months vs. 16 months (P = 0.052) and that of 1-mm R0 vs. R1 was 27 months vs. 18 months, respectively (P = 0.016). In individual margins, posterior, anterior surface, and pancreas transection margin involvement were associated with poor outcome, and the 1 mm posterior surface involvement was an independent risk factor for disease-free survival (hazard ratio, 1.63). Adjuvant radiation therapy had oncologic benefits, especially in R1 patients (P = 0.011) compared to R0 patients (P = 0.088).
Conclusion
Margin status, especially 1-mm R1 status is an important predictive factor, and involved posterior surface has a clinical impact. Patients with positive margins should be considered adjuvant radiation therapy.
Keywords: Disease-free survival, Margins of excision, Pancreatic neoplasms, Pancreaticoduodenectomy, Prognosis
INTRODUCTION
Pancreatic cancer mostly presents as a systemic disease on diagnosis and curative resection can only be performed in a few patients. Even after curative resection, the median survival ranges from 15–20 months, and the 5-year overall survival (OS) ranges from 18% to 27% [1,2,3]. In spite of the development of novel chemotherapeutic agents, pancreatic cancer still has a high recurrence rate and poor survival outcome [4].
While achieving a clear resection margin is the main goal of pancreatic surgery, R1 resection is relatively common due to its aggressive growth patterns and the anatomical location of the pancreas surrounded by critical vascular structures in the pancreatic head.
Margin status is an important prognostic factor in pancreatic cancer and is used for establishing appropriate postoperative strategies [5,6]. However, there is a lack of an international agreement on the handling techniques of pancreaticoduodenectomy (PD) specimens, their nomenclature, indicating each margin or surface, and the definition of R0 resection [7,8].
In addition, 2 different definitions of margin clearance (R0 resection) are used according to the distance from the resected edge to the tumor. The 0-mm R1 is defined when there is direct cancer infiltration [9] and 1-mm R1 is defined when tumor cells are observed within 1 mm from the resection margin [10]. Therefore, a wide variation in the radical resection rate is reported and the clinical significance of each specific margin is still unclear [11,12]. Several studies have reported that margin status is an independent risk factor in pancreatic ductal adenocarcinoma (PDAC) [13,14]; however, conflicting results have been reported [15,16].
This study aims to evaluate the impact of margin status on survival and recurrence. The significance of each specific margin involvement was assessed using the 2 different R1 definitions. Clinical impact of adjuvant therapies was investigated according to resection margin status.
METHODS
Study design and patients
Pancreatic head cancer patients who underwent Whipple’s operation or pylorus-preserving pancreaticoduodenectomy (PPPD) between 2010 and 2017 in Seoul National University Hospital were enrolled. Among 381 PDAC patients managed during the study period, patients who received neoadjuvant treatment, R2 resection, or palliative surgery, and who had recurrent pancreatic cancer or a short survival (within 3 months) were excluded. Patients who received neoadjuvant treatment were excluded because neoadjuvant therapy is thought to alter the original tumor characteristics and progression, thereby affecting the margin status. Finally, 314 patients were included in the final study population. Clinical information including medical records, radiologic images, and pathologic reports were collected prospectively.
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. SNUH 2007-025-1139). The study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Pathological report and margin classification
PD specimens were examined in a standardized protocol using the axial slicing technique with 4–5 mm thickness, introduced by Verbeke [17]. Information including tumor size, histologic grade, margin status (distance and location), lymph node (LN) involvement, and the presence of angiolymphatic, venous, and perineural invasion were collected. Evaluated margins included the pancreas transection, bile duct margin, superior mesenteric vein (SMV) and superior mesenteric artery (SMA) groove margin, anterior and posterior surface. The margin clearance distance, tumor cells from individual surgical margins was reported in millimeters. Two pancreatobiliary tract specialized pathologists (Kyoung Bun Lee and Haeryoung Kim) were in charge of the entire PD specimen assessment and pathologic reporting.
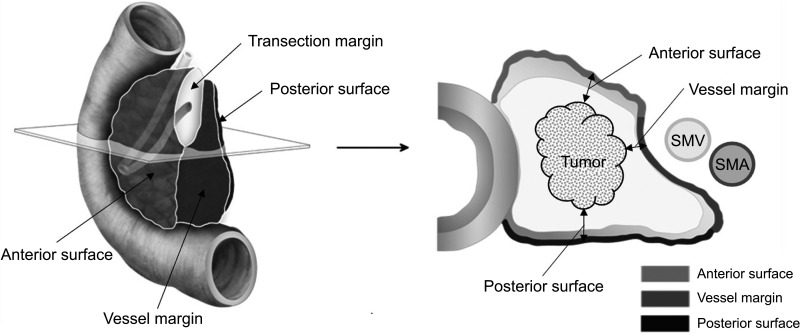
Two surgeons (Hee Ju Sohn and Hongbeom Kim) reviewed the preoperative radiologic images and specimen photographs and classified the margin results. Each margin status was reclassified by using 2 different definitions of R0/R1, 0 mm and 1 mm. Finally, the pancreatic margin was classified into 4 margins: pancreas transection, anterior surface, posterior, and vessel margin (SMV/SMA) (Fig. 1).
Fig. 1. Schematic anatomy of pancreaticoduodenectomy specimen and description of specific pancreatic margins. SMV, superior mesenteric vein; SMA, superior mesenteric artery.
Follow-up and statistical analyses
After surgical treatment, the patients underwent regular follow-ups. Abdominal CT or abdominal MRI was performed every 2–6 months and PET was performed annually or when clinically indicated. OS was calculated from the date of surgery to the date of death. Disease-free survival (DFS) was defined as the time interval between the date of operation and until the first event of recurrence or death from any cause. The time when local recurrence was first detected from the operation date, local recurrence-free survival (LRFS), was assessed. Recurrence patterns according to the margin status and location were analyzed for determining whether patterns were related to local recurrence or early recurrence within 6 months.
Comparative analysis was performed using the Student t-test, chi-square test, and Fisher exact test (if the total number of observations was less than 20%). Survival analysis was conducted using the Kaplan-Meier, method and the differences were tested using the log-rank test. The Cox regression analysis was used for the multivariate analysis for estimating the risk factors associated with the OS and DFS. All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
Patients and clinicopathologic data
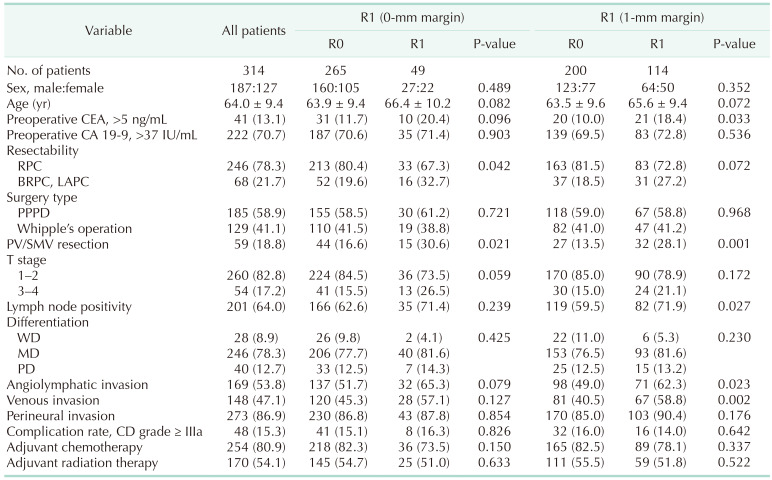
The demographic data of the study population according to R0/R1 status are presented in Table 1. The mean age was 64.0 years, and 58.9% and 41.1% of the patients underwent PPPD and PD, respectively. The combined resection rate of portal vein (PV) or SMV was 18.8%. Approximately 17.2% of the patients had T3 or T4 disease (8th edition of TNM classification), and 64.0% had positive LNs.
Table 1. Clinicopathological characteristics of 314 patients according to R0/R1 status.
Values are presented as number only, mean ± standard deviation, or number (%).
RPC, resectable pancreatic cancer; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer; PPPD, pylorus-preserving pancreaticodoudenectomy; PV, portal vein; SMV, superior mesenteric vein; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; CD, Clavien-Dindo classification.
A higher rate of combined resection of PV/SMV and a higher rate of borderline resectable pancreatic cancer and locally advanced pancreatic cancer (LAPC) patients were observed in the 0-mm R1 patients. No other significant difference in other clinicopathological data was seen between the 0-mm R0 and R1 patients. Meanwhile, higher rate of preoperative CEA level (>5 ng/mL), combined resection rate of PV/SMV, presence of positive LN, angiolymphatic and venous invasion rate were observed in 1-mm R1 patients (P < 0.05). There was no difference in men/women ratio, preoperative CA 19-9 level, surgery type, histologic grade, T stage 3–4 ratio, and postoperative treatment status.
Forty-nine patients had direct invasion and 114 patients had tumor cells within the resection margin (R1 rate: 0 mm, 15.6%; 1 mm, 36.3%). A subgroup analysis of each pancreatic margin was conducted (Supplementary Table 1). The direct invasion rate was 3.2% in the pancreas transection, 2.9% in the anterior surface, 2.5% in the posterior surface, and 8.3% in the vessel margin. Using <1 mm for defining a positive margin, vessel margin involvement increased to 20.4%, 8.0% in posterior surface, 10.8% in anterior surface, and 3.8% in pancreas transection margin (Fig. 2). The vessel margin was the most frequently involved margin location and increased the most with the change of the 0 mm definition to 1 mm.
Fig. 2. R1 rates in each margin according to 2 definitions.
Survival outcome according to R0/R1 status
The median follow-up time of the entire patient group was 24 months. The median OS of 0-mm R0 and R1 was 26 months and 16 months, respectively (P = 0.052) (Fig. 3A). The median OS of 1-mm R0 and R1 was 27 months and 18 months, respectively (P = 0.016) (Fig. 3B). In the DFS, there were significant differences in both the 0 mm (P = 0.045) and 1 mm definition (P = 0.009) (Fig. 3C, D). The 1 mm definition showed a significant difference between the R0 and R1 groups for both the OS and DFS. The 1 mm definition (R0/R1) seemed better in predicting prognosis.
Fig. 3. Kaplan-Meier curves for resection margin status and survival. Only tendency was seen in 0-mm R0/R1 status in overall survival (A) (median survival of 26 months vs. 16 months, P = 0.052) but significant difference was seen 1-mm R0/R1 status (B) (27 months vs. 18 months, P = 0.016). In disease-free survival and local recurrence-free survival, both 0-mm and 1-mm R status showed significant survival difference. (C) Median survival of 12 months vs. 6 months, P = 0.045; (D) 13 months vs. 8 months, P = 0.009; (E) median survival of 15 months vs. 7 months, P = 0.036; (F) median survival of 16 months vs. 9 months, P = 0.006.
The OS and DFS were analyzed according to the location of margin invasion. Posterior surface involvement in both the 0 mm/1 mm definitions showed poor OS results (P = 0.002 in 0 mm, P = 0.003 in 1 mm). All 25 patients who had tumor cells within 1 mm of the posterior surface did not survive for 5 years. In the DFS, involvement of the posterior surface in both definitions (P = 0.010 in 0 mm, P < 0.001 in 1 mm), 0 mm pancreas transection margin (P = 0.015), and 1 mm anterior surface (P = 0.003) involvement resulted in poor outcomes (Supplementary Table 2).
Recurrence pattern according to 2 definitions of R0/R1 status
During the follow-up period, 238 patients experienced recurrence; 72.3% of patients had systemic recurrence, and 27.7% of patients had local recurrence. The most common recurrence site was the liver. When local and systemic recurrences were detected simultaneously, it was regarded as systemic recurrence. The relationship between the recurrence site and margin status was investigated. However, there was no significant relationship between R1 status (including individual margins) and recurrence type (local or systemic).
By serial radiologic review, the time to the first event of local recurrence, LRFS was calculated. There was difference in LRFS depending on R0/R1 status; 0 mm definition (P = 0.036) and 1 mm definition (P = 0.006) (Fig. 3E, F). Patients who had event of early recurrence were evaluated and 97 patients (30.8%) took less than 6 months after surgery. Direct invasion (0-mm R1) or insufficient margin clearance (1-mm R1) were related to these cases.
Effect of adjuvant chemotherapy and radiation therapy
In our study population, 87.3% received adjuvant treatment; 75.2% received chemotherapy and 54.1% received radiation therapy. A total of 163 patients (64.2%) received gemcitabine-based chemotherapy and 87 patients (34.3%) received 5-fluorouracil (5-FU)–based chemotherapy. FOLFIRINOX (fluorouracil, leucovorin, irinotecan, oxaliplatin) regimen could not be applied as it is not yet approved for coverage by Korean insurance in the adjuvant setting. Majority of the patients received concurrent chemoradiation therapy (45–54 gray/25–28 fraction) in this cohort.
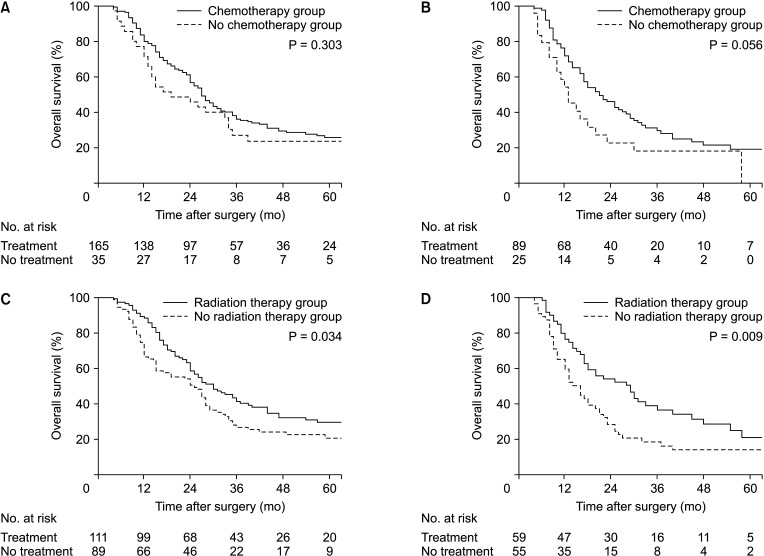
There were survival benefits in patients who received postoperative chemotherapy (P = 0.049) or radiation therapy (P = 0.001) (Supplementary Fig. 1). In the subgroup analysis of the 1-mm R0/R1 status, there was a survival improvement (Fig. 4C, D) with radiation therapy, though chemotherapy failed to show difference (Fig. 4A, B). DFS and LRFS also showed survival improvement with radiation therapy (P = 0.004 and P = 0.002, respectively). Radiation therapy appeared to be more effective in R1 patients (P = 0.011) compared to R0 patients (P = 0.088) for DFS. And for LRFS, all patients had survival benefits of radiation (Supplementary Fig. 2).
Fig. 4. Clinical impact of chemotherapy and radiation therapy on R0/R1 patients. Tendency of chemotherapy to survival difference in (A) R0 patients (27 months vs. 19 months, P = 0.303) and (B) R1 patients (21 months vs. 13 months, P = 0.056). Radiation therapy had survival benefit on both (C) R0 (30 months vs. 25 months, P = 0.034) and (D) R1 patients (29 months vs. 16 months, P = 0.009).
Risk factors for disease-free survival and overall survival
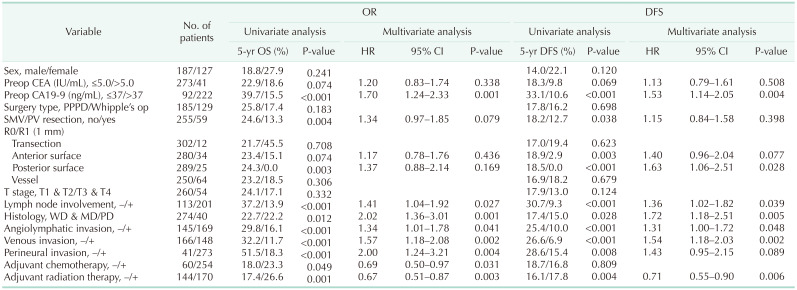
Univariate analysis and multivariable analysis of OS and DFS including both 0- and 1-mm R status was conducted. Risk factors for DFS included: high preoperative CA 19-9 level (>37 ng/mL), LN involvement, histologic grade, angiolymphatic, venous, perineural invasion, adjuvant chemotherapy, and radiation therapy. In addition to these factors, perineural invasion and adjuvant chemotherapy status turned out to be a prognostic factor for OS.
When including the 4 locations of margin, a poor prognosis was observed in patients with direct pancreas transection margin and posterior surface invasion; however, they were not independent risk factors. In the 1-mm margin, posterior surface invasion turned out to be an independent risk factor for the DFS, and the hazard ratio was 1.63 (Table 2).
Table 2. Risk factors for overall survival (OS) and disease-free survival (DFS), including location of margin status.
HR, hazard ratio; CI, confidence interval; Preop, preoperative; op, operation; PPPD, pylorus-preserving pancreaticodoudenectomy; SMV, superior mesenteric vein; PV, portal vein; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
DISCUSSION
There are still controversies concerning what constitutes the margin of the complex PD specimen. The circumferential margin in the pancreatic head is the dissected tissue that is surrounded by the duodenum and adjacent major vessels, including the inferior vena cava, SMV, and SMA. Twenty-eight different terms were used in previous PDAC reports for the different PD specimen margins [18]. Still, the clinical significance of the specific peripancreatic margin status is unclear. When a tumor is close to the anterior surface, which is a free space around and the posterior surface and SMA margin, it is out of the surgeon’s control, and further resection is not possible [7].
Accordingly, clinical interpretation of individual margins was reported heterogeneously. Jamieson et al. [19] introduced a novel term, “mobilization margin” which includes the anterior surface, posterior surface, and duodenal serosa, but each margin involvement did not have a prognostic significance. Delpero et al. [20] classified the retroperitoneal margin into the SMV, SMA, and posterior surface and found that SMV and SMA margins were related to poor outcome. Ghaneh et al. [13] found that the medial margin, posterior and anterior surface, and the number of involved margins in direct invasion were related to both the OS and DFS. In our study, unfavorable prognoses were observed in patients who had anterior surface, pancreas transection, and posterior surface invasion which are separated from major vessels.
Owing to the dispersed growth pattern of pancreatic cancer [21], the Royal College of Pathologists first suggested that a 1-mm margin clearance should be acquired in pancreatic cancer, and the American Joint Committee on Cancer, TNM 8th edition adopted this definition recently. Our study showed that the margin status still has a clinical impact on the prognosis of PDAC, and the 1 mm definition had a greater predictive value. Higher rates of LN positivity, angiolymphatic, venous, and perineural invasion in R1 patients according to the 1 mm definition were observed in our study, which implies that “R1 status” is an indicator of the tumor’s aggressive biologic nature [8]. Several studies reported a better prognosis [5,22] when the safety margin distances were increased to 1.5 mm or 2 mm; however, this was not observed in our study population.
In the multivariate analysis of OS and DFS, margin status (0 mm and 1 mm) was not an independent risk factor, probably because of stronger predictors such as high preoperative CA 19-9 level, LN positivity, histologic grade, tumor biology, and postoperative radiation therapy [16,23]. Among the peripancreatic margins in the pancreatic head, the posterior surface turned out to be an independent risk factor for the DFS. If tumor cells are seen less than 1 mm in the posterior surface after surgery, the chance of recurrence is higher. In cases of tumors extending the posterior surface, there is no tissue that can be further resected, and it seems that some tumor cells may remain even radical resection is performed. Therefore, clinicians should consider more aggressive postoperative treatments or neoadjuvant therapy first, rather than upfront surgery in these cases. Neoadjuvant chemoradiation, which has the advantage of increased R0 rates, is widely performed recently when it is deemed difficult to obtain sufficient surgical margin [24].
Even after curative intended surgery, a high probability of recurrence with 35%–60% of local recurrence and 80%–90% of systemic recurrence have been reported early after surgery [25]. Other studies reported that medial margin [13] and posterior margin [26] invasion were related to local recurrence. In this study group, most patients had systemic recurrence first, but eventually, R1 patients had a higher rate of local recurrence and a shorter time to local recurrence compared to R0 patients. The relationship between the individual margin status and local recurrence was not shown probably due to small number of cases in this study. These results support the concept that pancreatic cancer is a systemic disease that is likely to have micrometastases at the time of diagnosis.
A majority of patients received adjuvant chemotherapy after surgery except for some patients with poor general condition, and radiation therapy was performed in about half of all patients who were either margin positive or LN positive. The effect of adjuvant chemotherapy seemed to be relatively lower than that of radiation therapy. This is because most of the patients in this study received gemcitabine or 5-FU–based adjuvant chemotherapy, but if we had adopted FOLFIRINOX, which has recently shown groundbreaking results, the treatment effect might have increased [27].
The role of radiation therapy postoperative setting in is still an ongoing issue. While the European Study for Pancreatic Cancer (ESPAC-1) trial [28], failed to find survival benefit of chemoradiation, Takahashi et al. [29] reported an improved survival in R1/LN negative patients who received adjuvant chemoradiation therapy compared to those who received only chemotherapy. A large retrospective study using the Surveillance, Epidemiology, and End Results (SEER) database [30] found survival benefit in patients who received an addition of radiation therapy in LAPC. Kim et al. [6] reported that adding radiation therapy to chemotherapy had survival benefits in pancreatic head cancer patients with a close resection margin under 2 mm. In this current study, patients who received adjuvant radiation therapy had improved survival, especially in R1 (<1 mm) resected patients, as well.
There are several limitations in this study. Although all pancreatic cancer patients were subjected to postoperative adjuvant treatment, some patients could not receive treatment owing to their poor general conditions. Because the adjuvant treatment protocol and chemotherapeutic regimen were not unified during the entire study period, the effect of chemotherapy might have been underestimated. Lastly, to focus on the margin location and distance, patients who received neoadjuvant treatment were excluded. Nowadays, since the advent of effective chemotherapeutic agents, the proportion of patients who receive neoadjuvant chemoradiation is gradually increasing.
Each patient has different personal demographics, tumor characteristics, and receives different treatment modalities. Nevertheless, margin status still has an impact on prognosis, and the 1 mm definition is more efficient in predicting patients’ postoperative outcomes. Active adjuvant treatment is suggested in patients with positive surgical margins, especially when the posterior surface is involved. Unlike other solid organ malignancies, sufficient surgical margins for pancreatic head cancer may not always be feasible due to its unique anatomical structure. Therefore, combined radiation therapy for local control is essential in cases with insufficient surgical margins.
Footnotes
Fund/Grant Support: This study was supported by a grant from the National Research Foundation funded by the Ministry of Science and ICT, Republic of Korea (NRF-2017M3C9A5031591).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Hongbeom Kim, JYJ.
- Formal Analysis: HJS, SJK, YH, JML.
- Investigation: HJS, JSK, YH.
- Methodology: Haeryoung Kim, KBL, EKC.
- Project Administration: Hongbeom Kim, WK, JJY.
- Writing — Original draft: HJS, Hongbeom Kim.
- Writing — Review & Editing: All authors.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1, 2 and Supplementary Figs. 1, 2 can be found via https://doi.org/10.4174/astr.2022.102.1.10.
Overall survival of each margin according to R0/R1 status in 2 definitions (0 mm, 1 mm)
Disease-free survival of each margin according to R0/R1 status in 2 definitions (0 mm, 1 mm)
Impact of adjuvant chemotherapy (A) and adjuvant radiation therapy (B) in entire study population.
Disease-free survival and local recurrence-free survival of adjuvant radiation therapy status in 1-mm R0/R1 patients.
References
- 1.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol. 2015;21:262–268. doi: 10.3748/wjg.v21.i1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 6.Kim BH, Kim K, Jang JY, Kwon W, Kim H, Lee KH, et al. Survival benefit of adjuvant chemoradiotherapy for positive or close resection margin after curative resection of pancreatic adenocarcinoma. Eur J Surg Oncol. 2020;46:2122–2130. doi: 10.1016/j.ejso.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Adsay NV, Basturk O, Saka B, Bagci P, Ozdemir D, Balci S, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol. 2014;38:480–493. doi: 10.1097/PAS.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol. 2016;113:283–288. doi: 10.1002/jso.24092. [DOI] [PubMed] [Google Scholar]
- 9.Hruban RH, Pitman MB, Klimstra D. Tumors of the pancreas. AFIP Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 10.Campbell F, Bennett M, Foulis AJ. Minimum dataset for histopathological reporting of pancreatic, ampulla of vater and bile duct carcinoma. London: Royal College of Pathologists; 2002. [Google Scholar]
- 11.Chandrasegaram MD, Goldstein D, Simes J, Gebski V, Kench JG, Gill AJ, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102:1459–1472. doi: 10.1002/bjs.9892. [DOI] [PubMed] [Google Scholar]
- 12.Schlitter AM, Esposito I. Definition of microscopic tumor clearance (r0) in pancreatic cancer resections. Cancers (Basel) 2010;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg. 2019;269:520–529. doi: 10.1097/SLA.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 14.Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265:565–573. doi: 10.1097/SLA.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 15.Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- 16.Tummers WS, Groen JV, Sibinga Mulder BG, Farina-Sarasqueta A, Morreau J, Putter H, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg. 2019;106:1055–1065. doi: 10.1002/bjs.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 18.Gill AJ, Johns AL, Eckstein R, Samra JS, Kaufman A, Chang DK, et al. Synoptic reporting improves histopathological assessment of pancreatic resection specimens. Pathology. 2009;41:161–167. doi: 10.1080/00313020802337329. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251:1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 20.Delpero JR, Bachellier P, Regenet N, Le Treut YP, Paye F, Carrere N, et al. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB (Oxford) 2014;16:20–33. doi: 10.1111/hpb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology. 2011;59:1111–1121. doi: 10.1111/j.1365-2559.2011.04056.x. [DOI] [PubMed] [Google Scholar]
- 22.Osipov A, Nissen N, Rutgers J, Dhall D, Naziri J, Chopra S, et al. Redefining the positive margin in pancreatic cancer: impact on patterns of failure, long-term survival and adjuvant therapy. Ann Surg Oncol. 2017;24:3674–3682. doi: 10.1245/s10434-017-6076-z. [DOI] [PubMed] [Google Scholar]
- 23.Kimbrough CW, St Hill CR, Martin RC, McMasters KM, Scoggins CR. Tumor-positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol. 2013;107:602–607. doi: 10.1002/jso.23299. [DOI] [PubMed] [Google Scholar]
- 24.de Geus SW, Kasumova GG, Sachs TE, Ng SC, Kent TS, Moser AJ, et al. Neoadjuvant therapy affects margins and margins affect all: perioperative and survival outcomes in resected pancreatic adenocarcinoma. HPB (Oxford) 2018;20:573–581. doi: 10.1016/j.hpb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Castellanos JA, Merchant NB. Intensity of follow-up after pancreatic cancer resection. Ann Surg Oncol. 2014;21:747–751. doi: 10.1245/s10434-013-3289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147:753–760. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 27.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 28.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi C, Shridhar R, Harris CL, Lee J, Patel AJ, Brown RH, et al. Adjuvant therapy for margin positive pancreatic cancer. J Clin Oncol. 2018;36(4_suppl):390 [Google Scholar]
- 30.Sajjad M, Batra S, Hoffe S, Kim R, Springett G, Mahipal A. Use of radiation therapy in locally advanced pancreatic cancer improves survival: a SEER database analysis. Am J Clin Oncol. 2018;41:236–241. doi: 10.1097/COC.0000000000000261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall survival of each margin according to R0/R1 status in 2 definitions (0 mm, 1 mm)
Disease-free survival of each margin according to R0/R1 status in 2 definitions (0 mm, 1 mm)
Impact of adjuvant chemotherapy (A) and adjuvant radiation therapy (B) in entire study population.
Disease-free survival and local recurrence-free survival of adjuvant radiation therapy status in 1-mm R0/R1 patients.