Abstract
The matrix (M) protein of avian pneumovirus (APV) was evaluated for its antigenicity and reliability in an enzyme-linked immunosorbent assay (ELISA) for diagnosis of APV infection, a newly emergent disease of turkeys in United States. Sera from APV-infected turkeys consistently contained antibodies to a 30-kDa protein (M protein). An ELISA based on recombinant M protein generated in Escherichia coli was compared with the routine APV ELISA that utilizes inactivated virus as antigen. Of 34 experimentally infected turkeys, 33 (97.1%) were positive by M protein ELISA whereas only 18 (52.9%) were positive by routine APV ELISA 28 days after infection. None of the serum samples from 41 uninfected experimental turkeys were positive by M protein ELISA. Of 184 field sera from turkey flocks suspected of having APV infection, 133 (72.3%) were positive by M protein ELISA whereas only 99 (53.8%) were positive by routine APV ELISA. Twelve serum samples, which were negative by M protein ELISA but positive by routine APV ELISA, were not reactive with either recombinant M protein or denatured purified APV proteins by Western analysis. This indicates that the samples had given false-positive results by routine APV ELISA. The M protein ELISA was over six times more sensitive than virus isolation (11.5%) in detecting infections from samples obtained from birds showing clinical signs of APV infection. Taken together, these results show that ELISA based on recombinant M protein is a highly sensitive and specific test for detecting antibodies to APV.
Avian pneumovirus (APV) is a member of the genus Metapneumovirus in the family Paramyxoviridae (19). The virus causes turkey rhinotracheitis, an acute upper respiratory tract infection of turkeys characterized by coughing, nasal discharge, tracheal rales, foamy conjunctivitis, and sinusitis in young poults. In laying birds, there is a transient drop in egg production along with mild respiratory tract illness (12). Uncomplicated cases of APV infection have low mortality (2 to 5%), but infections accompanied by secondary bacterial and/or viral infections can result in up to 25% mortality (reviewed in reference 12). After it was detected in South Africa in 1978, APV infection was diagnosed in the United Kingdom, France, Spain, Germany, Italy, Netherlands, Israel, and countries in Asia (1, 12). The United States was free of APV infection until 1996, when the disease was reported in Colorado (14, 17, 23). Subsequently, APV infection was found in turkeys in Minnesota, from where it is spreading to neighboring states (14, 15). In 1999, 37% of the turkey flocks in Minnesota were positive for APV antibodies, causing economic losses of approximately $15 million.
APV infection is diagnosed by the demonstration of virus particles or nucleic acid in infected tissues or by the detection of anti-APV antibodies in convalescent-phase sera. Virus isolation can be performed in tracheal organ cultures, chicken embryo fibroblasts, or Vero cells (9), but it is time-consuming and often unsuccessful. APV RNA can be detected for a short period (2 to 10 days postinfection) by reverse transcriptase PCR (RT-PCR) in tracheal and choanal swabs (12, 25). Antibodies to APV are detectable for many weeks by enzyme-linked immunosorbent assay (ELISA), which is more rapid and economical than virus isolation or RT-PCR (4, 7, 10). During the first few months of the APV outbreak in the United States, it was not possible to detect the virus serologically using test reagents based on European APV isolates because of the lack of cross-reactivity. An ELISA based on the lysate from APV-infected cells as antigen was later developed at the National Veterinary Service Laboratories, Animal and Plant Health Inspection Service, U.S. Department of Agriculture, using inactivated purified Colorado isolate of APV (APV/CO) as an antigen. This test was later modified for routine detection of APV antibodies in turkeys in Minnesota (5). Unfortunately, this routine ELISA produces inconsistent results that depend on the infectivity of the virus isolate used for antigen preparation (5).
The APV genome is a linear molecule of negative-sense, nonsegmented single-stranded RNA of 13.3 kb that contains eight genes in the order 3′-N-P-M-F-M2-SH-G-L-5′. The NS1 and NS2 genes present in mammalian pneumoviruses are not found in APV (19, 20). Antigenic diversity among APV isolates is well documented among European isolates, in which two serologically distinct subgroups (A and B) have been described (20). These variations are mainly in the F (fusion) and G (attachment) proteins (24). Serological and molecular studies have indicated that APV isolates in the United States are distinct from those of subgroup A and B APV isolates in Europe and South Africa (15, 17, 23). Therefore, an ELISA based on a protein that is relatively conserved among different isolates of APV from the United States would be appropriate for use as a diagnostic test. Sequence analysis and predicted amino acid sequence of the matrix (M) gene from European and U.S. isolates of APV have indicated that it is relatively conserved (21, 23, 24). For example, the M gene has 98% nucleotide similarity among three U.S. isolates, with only one nonsynonymous change, and 73% sequence similarity between European subgroups A and B (21, 24). To determine the antigenicity of the M protein and its utility as an ELISA antigen in APV infections, we expressed the M protein of APV in E. coli and developed an M protein ELISA, which was found to be more sensitive and specific than the ELISA currently used for serological diagnosis of APV.
MATERIALS AND METHODS
Production and purification of recombinant APV M protein.
M gene cDNA clones of Minnesota 2A (APV/MN2A) and Colorado (APV/CO) strains of APV were isolated from the pCR-XL-TOPO vector (Invitrogen, Carlsbad, Calif.) (23) by PCR using a 5′ primer with a BamHI restriction site and a 3′ primer with a KpnI restriction site. Using these unique restriction sites, the APV genes were subcloned into a pQE-30 vector (Qiagen Inc., Valencia, Calif.) as specified by the manufacturer. The cDNA clones in expression plasmids were sequenced (both strands), using fluorescently labeled dideoxynucleotides, with an automated sequencer (26). Both clones contained sequences identified as the APV M gene (23). The pQE-30 vector has arginine-glycine-serine (RGS) and six consecutive histidine (His6 tag) coding sequences 5′ to the cloning region for purification and detection. Proteins were expressed in Escherichia coli strain M15 containing the repressor pREP4 plasmid, which constitutively expresses Lac repressor protein encoded by the lacI gene. Expression of M protein was induced by inactivating the Lac repressor protein by adding isopropyl-β-d-thiogalactoside, which enabled the E. coli RNA polymerase to transcribe downstream of the phage T5 promoter (cloning region). Proteins were purified using nickel-nitrilotriacetic acid metal affinity chromatography matrices which bind the His6 tag. The purity of the proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and confirmed by Western analysis using antibodies against the RGS-histidine tag. To eliminate contaminating E. coli proteins eluted from the nickel-nitrilotriacetic acid matrices, the M proteins were gel purified by electroelution followed by dialysis in phosphate-buffered saline.
Purification of APV.
APV/MN1A- or APV/MN2A-infected Vero cells were harvested 72 h postinfection, lysed with 0.5% IGEPAL CA-630, and clarified at 10,000 × g for 20 min at 4°C. The virus was pelleted through a 30% (wt/wt) sucrose cushion at 25,000 rpm for 5 h in an SW28 rotor. The pellet was resuspended in 10 mM Tris HCl (pH 7.4), overlaid on a CsCl density gradient (density between 1.2 and 1.6), and centrifuged in an SW41 rotor at 25,000 rpm overnight at 4°C. An opalescent band at a specific density between 1.31 and 1.37 was collected and dialyzed in phosphate-buffered saline. An APV preparation passed through the sucrose gradient but not through the CsCl gradient was used as partially purified virus.
Western blot analysis.
Purified APV proteins or recombinant APV M protein were separated by SDS-PAGE using a 15% polyacrylamide gel under reducing conditions and transferred to a polyvinylidene difluoride membrane by electroblotting (11, 27). The membrane was blocked for 1 h with 10% nonfat dry milk at room temperature before being incubated with a 1:40 dilution of turkey serum samples for 1 h. The membrane was washed three times with Tris-borate buffer containing 0.05% Tween 20, incubated with horseradish peroxidase-conjugated goat anti-turkey immunoglobulin G (IgG) (heavy and light chains) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and detected by chemiluminescence using high-performance films (Amersham International, Little Chalfont, England).
Virus isolation.
We attempted to isolate virus from samples collected from turkeys showing clinical signs of APV infection. Nasal turbinate tissues or swabs from exposed asymptomatic birds (in barns where other birds were showing overt clinical signs of APV) or from birds with early signs (rales, mild turbinate swellings) were processed for APV isolation. The samples were homogenized and cultured in chicken embryo fibroblasts for five blind passages (each passage was incubated for 48 to 72 h) and then passaged in Vero cells. In Vero cells, cytopathic changes could be observed as early as in the second passage. In the absence of detectable cytopathic changes, passaging was continued five more times and RT-PCR was performed (25). An isolate was confirmed by immunohistochemistry using rabbit polyclonal anti-APV antibody.
Serum specimens.
Turkey sera were submitted to the Minnesota Veterinary Diagnostic Laboratory for the detection of APV antibodies. A total of 184 turkey sera from different farms that reported clinical signs of APV disease in 1999 were used in this study. In addition, 34 sera from experimentally infected turkeys 28 days postinfection were analyzed for APV antibodies using M protein ELISA, and 41 sera from sham-inoculated controls were also analyzed. Turkeys for the experimental infections were obtained from the areas in Wisconsin that were free from APV disease.
Routine APV ELISA.
The routine APV ELISA was performed as described previously (5). A series of experiments were conducted that compared ELISA results based on APV/CO or two Minnesota isolates, APV/MN1A and APV/MN2A, and determined that the virus strains gave similar results in ELISA (5). Therefore, the routine ELISA was optimized using the APV/CO strain. Briefly, Vero cells infected with the APV/CO strain were lysed in 0.01 M phosphate-buffered saline plus 0.5% Nonidet P-40 and the lysate was clarified by centrifugation at 3,000 × g for 10 min. The clarified cell lysates were used as positive antigen. Cell lysates from mock-infected Vero cells were used as negative control antigens. Alternate rows of an ELISA plate (Immulon 1B; Dynatech, Chantilly, Va.) were coated overnight at 4°C with a 1:320 dilution of the infected cell lysate and noninfected Vero cells in 0.05 M carbonate buffer (pH 9.6). Test sera diluted 1:40 and anti-turkey IgG horseradish peroxidase conjugate (1:1500) diluted in ELISA blocking solution (Kirkegaard & Perry Laboratories) were each incubated for 1 h at room temperature in a 50-μl volume. The substrate chromogen solution consisted of 0.05 M citrate-phosphate buffer (pH 5.0), 0.04% (wt/vol) o-phenylenediamine, and freshly added 0.04% (vol/vol) H2O2. The results were expressed as the difference between the absorbance at 490 nm (A490) of APV antigen-coated wells and that of control lysate-coated wells of each serum sample. A sample with an A490 value of more than 0.2 was considered positive.
M protein ELISA.
For M protein-based ELISA, the purified recombinant M protein was used as a positive antigen whereas bovine serum albumin or recombinant Theiler's murine encephalomyelitis virus 2C protein generated in the same E. coli system as the APV M protein (3) was used as the negative control antigen. The optimum concentration of M protein to coat the ELISA plate was chosen in such a manner that maximum binding could be obtained as determined in a checkerboard titration. The plates were coated overnight at 4°C with 125 ng of M protein per well containing 100 μl of 0.05 M carbonate buffer (pH 9.6). All other steps were identical to those for the routine APV ELISA.
RESULTS
Production of recombinant M protein.
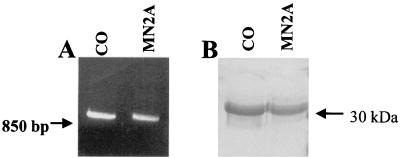
The cloned M genes of APV/CO and APV/MN2A (from the ATG start sequence at position 14 to position 860) were expressed in an E. coli system yielding the APV M protein of 282 amino acids, which gave a band of approximately 30 kDa by SDS-PAGE (Fig. 1). The expressed M proteins were purified on a His6 tag-based nickel-nitrilotriacetic acid column, gel purified further, and electroeluted. The purity of the proteins was confirmed by single banding in SDS-PAGE (Fig. 1) and Western blot analysis using anti-His tag and anti-APV antibodies (data not shown).
FIG. 1.
Isolation of the APV M gene and production of recombinant protein. The M gene was isolated from pCR-XL-TOPO by PCR and subcloned into the pQE-30 expression plasmid. A two-step process was used to purify M proteins, a nickel-nitriolotriacetic acid column that binds the His6, tag located at the NH2 terminus of the protein and gel purification. (A) PCR product of APV/CO (lane 1) and APV/MN2A (lane 2) in an agarose gel. (B) Recombinant M protein from APV/CO (lane 1) and APV/MN2A (lane 2) in an SDS-polyacrylamide gel stained with Coomassie blue.
Antigenicity of M protein in APV infection.
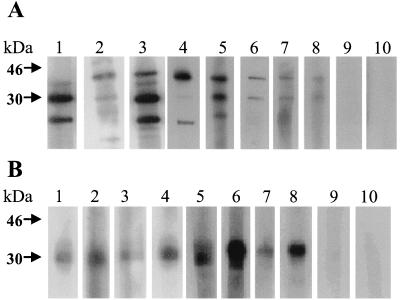
To determine if M protein is a major antigen during natural APV infections, turkey sera from APV-infected flocks were reacted with M protein and purified APV and analyzed by Western blotting. All positive sera at a 1:40 dilution consistently reacted with a protein of approximately 30 kDa by immunoblotting (Fig. 2B, lanes 1 to 8), whereas none of the negative sera had any reactivity at a 1:40 dilution (lanes 9 and 10). Sera from turkeys experimentally infected with APV/MN2A reacted with M proteins from both APV/MN2A and APV/CO, indicating that the two proteins had antigenic homology (data not shown). Using purified APV, two proteins of approximately 30 kDa (M protein) and 45 kDa (nucleocapsid protein) were consistently detected by Western blot analysis with sera positive for APV antibodies (Fig. 2A).
FIG. 2.
Western blot analysis of APV-positive and -negative turkey sera with APV proteins and recombinant M proteins. The partially purified APV proteins and the recombinant M protein were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Each lane of the membrane was incubated with a 1:40 dilution of turkey serum followed by horseradish peroxidase-conjugated anti-turkey IgG (1:20,000 dilution) and detected by chemiluminescence. (A) Reactivity of the sera with APV proteins; (B) reactivity with recombinant M protein. Lanes 1 to 8 show the reactivity with positive turkey sera, and lanes 9 and 10 show the reactivity with negative sera.
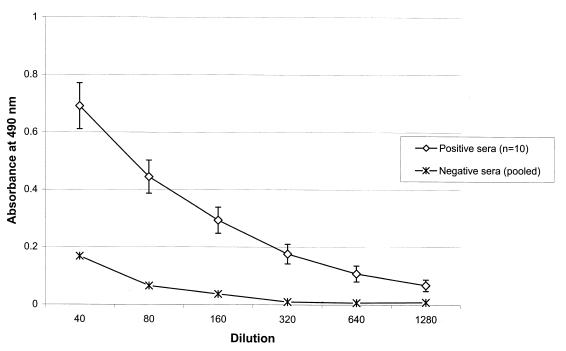
ELISA performed on serially diluted field sera from APV-infected flocks produced typical ELISA curves with an end point (A490 below 0.2) between 1:320 and 1:1280 dilutions (Fig. 3). Since all positive and negative sera gave distinct results at 1:40 dilutions, we used this dilution to analyze serum samples and compare M protein ELISA with routine APV ELISA.
FIG. 3.
Presence of APV-specific IgG in sera of APV-infected turkeys detected by M protein ELISA. Twofold serial dilutions of known positive turkey sera (n = 10) were tested in plates coated with recombinant M protein. Pooled sera from known APV-negative flocks were used as negative control. The results are expressed as mean and standard error.
Specificity of recombinant M protein ELISA.
Sera negative for APV were tested by M protein ELISA. The mean (± standard deviation) A490 of all negative sera (n = 41) by M protein ELISA was 0.088 (± 0.039). Using the mean ± 3 standard deviations as the cutoff value (A490 = 0.2), all the samples were negative by M protein ELISA and the relative specificity of the M protein ELISA was 100%.
Comparison of recombinant M protein ELISA and routine APV ELISA.
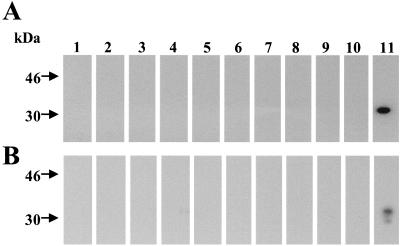
We tested 184 serum samples from turkeys suspected of having APV infection, using both routine APV ELISA and M protein ELISA. A total of 133 samples (72.3%) were positive by M protein ELISA, whereas only 99 (53.8%) were positive by routine APV ELISA (Table 1). Twelve samples were negative by M protein ELISA but positive by routine APV ELISA. To determine if these 12 samples had given false-positive results by routine APV ELISA, Western blot analysis was performed using either recombinant M protein or purified APV protein, and none of the 12 samples reacted with recombinant M protein or APV proteins in this analysis (Fig. 4). In contrast, all samples that were positive by M protein ELISA were positive by Western blot analysis using M protein or APV antigen. From the Western blotting results, we concluded that the sensitivity of the routine APV ELISA was only 74% compared to the newly developed M protein ELISA.
TABLE 1.
Comparison of routine APV and M protein ELISAs for detection of APV antibodies in turkey seraa
| M protein ELISA result | No. of samples analyzed by routine APV ELISA
|
||
|---|---|---|---|
| APV positive | APV negative | Total | |
| Positive | 87 | 46 | 133 |
| Negative | 12 | 39 | 51 |
| Total | 99 | 85 | 184 |
Field turkey sera suspected of APV infection.
FIG. 4.
Western blot analysis of turkey sera that were negative by M protein ELISA but positive by routine APV ELISA. Partially purified APV or recombinant M protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Each lane of the membrane was incubated with a sample of turkey serum (1:40) followed by horseradish peroxidase-conjugated anti-turkey IgG (1:20,000) before being subjected to chemiluminescence. (A) Reactivity with purified APV proteins; (B) reactivity with recombinant M protein. All 12 samples negative by M protein ELISA were not reactive with M protein or APV proteins, as shown for 10 representative samples (lanes 1 to 10). A sample positive by M protein ELISA (lane 11) was used as a positive control and reacted with the 30-kDa M protein in both antigen preparations.
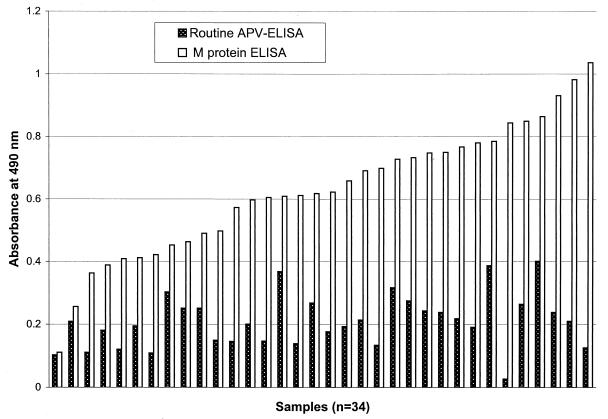
The M protein ELISA gave consistently higher A490 readings with the convalescent-phase sera (n = 34) collected from experimentally infected turkeys 28 days postinfection (Fig. 5) (P < 0.001, paired t test). Routine APV ELISA detected only 18 positive samples (52.9%), whereas M protein ELISA detected 33 positive samples (97.1%). This indicates that the M protein ELISA is more sensitive than the routine APV ELISA.
FIG. 5.
Comparison of the sensitivity of M protein ELISA with routine APV ELISA for detection of APV-specific IgGs from experimentally infected turkeys. Turkeys (n = 34) were experimentally inoculated with live APV (APV/MN1A), and serum was collected 4 weeks later. A 1:40 dilution of each sample was applied to plates coated with recombinant M protein or APV-infected cell lysate and detected using horseradish peroxidase-conjugated goat anti-turkey IgGs. The A490 values from M protein-coated plates were consistently higher than those from cell lysate-coated plates (P < 0.001).
Sensitivity of virus isolation.
To determine the sensitivity of APV isolation as a diagnostic test, we collected 52 nasal turbinate samples over a 3-month period from turkey flocks showing overt clinical signs of APV disease. These samples were tested by RT-PCR for detection of APV, and virus isolation was attempted by using chicken embryo fibroblasts and Vero cells as described in Materials and Methods. Of 52 samples, 46 (88.4%) were positive by M gene-based RT-PCR performed as described previously (25). However, only 6 (11.5%) of the 52 samples yielded virus (APV/MN6 to APV/MN11).
DISCUSSION
The antigenicity of the nucleocapsid (N), fusion (F), and attachment (G) proteins in respiratory syncytial viruses of bovines and humans has been demonstrated, and ELISA based on these proteins have been developed (2, 22). However, M protein has not been investigated for its antigenicity and applicability as a diagnostic antigen. Sequence data for APV isolates in Europe and the United States have shown that the M gene is highly conserved, with over 98% nucleotide sequence similarity among APV strains in the same subgroup and 73% similarity between strains of the European A and B subgroups (14, 21, 23, 24). In contrast, the G protein has a similar (97 to 98%) level of identity among strains of the same subgroup but only 38% similarity between strains of the A and B subgroups (14). Among three U.S. isolates that have been sequenced (APV/CO, APV/MN1B, and APV/MN2A), the M gene has 98% sequence similarity, with only one nonsynonymous nucleotide change in one isolate (24). Therefore, our objective was to determine the antigenicity of the M protein in APV infections and develop an ELISA, based on this protein, that was capable of detecting a wide variety of related APV strains that may be involved in the current U.S. outbreaks. The need for a more sensitive diagnostic test for APV infections was emphasized by the facts that routine ELISA using the APV-infected Vero cell lysate as antigen yielded inconsistent results depending on the method of antigen preparation (5, 6, 8).
Our data clearly demonstrate that the M protein is antigenic during an APV infection, because antibodies to M protein were observed consistently among field sera from infected birds. Based on these findings, M proteins from two U.S. isolates of APV were expressed in E. coli and purified to a high degree by a combination of metal affinity column chromatography and gel purification. The proteins were antigenically similar to the natural APV M protein, as evidenced by the presence of the same sized protein (30 kDa) in purified APV proteins, specific reactivity with sera from infected birds, and reactivity of the recombinant M protein with polyclonal anti-APV antibodies. As expected, the recombinant M protein from the APV/CO strain of APV was as effective in ELISA as was the M protein from a Minnesota isolate, APV/MN2A, suggesting that the test may be able to detect different strains of APV in the United States. The M protein ELISA was highly sensitive, because we detected 97.1% of experimentally infected turkeys in contrast to only 54.5% when using the routine ELISA. Compared to virus isolation, the M protein ELISA appears six times more sensitive in samples from birds in the field showing overt APV disease. The M protein ELISA was specific, as evidenced by the fact that all samples positive by the M protein ELISA were also positive by Western blot analysis whereas all samples negative by M protein ELISA were also negative by Western blot analysis. In contrast 12 samples positive by routine APV ELISA were negative by Western blot analysis. The sensitivity and specificity of M protein ELISA for APV are comparable to those of similar tests developed for bovine respiratory syncitial virus using recombinant N or F proteins of these viruses (18, 22) and for human respiratory syncytial virus using recombinant N and G proteins (2). In conclusion, we have demonstrated that antibodies to the APV M protein are consistently present in sera from turkeys naturally or experimentally infected with APV. The M protein ELISA developed here is a sensitive, specific, and reliable test for detecting serum antibodies to APV.
ACKNOWLEDGMENTS
The Minnesota Turkey Growers Association (grant 99-07) and the University of Minnesota Graduate School supported this research. B.S.S. is supported by ARS, USDA CRIS project 6612-32000-015-00D-085.
We thank Anwar M. Sheikh, Allison Heath, Evelyn Townsend, and H. J. Shin for their technical assistance.
REFERENCES
- 1.Alexander D J. Newcastle disease and other Paramyxoviridae infections. In: Barnes B W, Beard H J, McDougald C W, Saif L Jr, editors. Diseases of poultry. 10th ed. Iowa State University Press; Ames; 1997. pp. 541–569. [Google Scholar]
- 2.Buraphacheep W, Brit W J, Sullender W M. Detection of antibodies to respiratory syncytial virus attachment and nucleocapsid proteins with recombinant baculovirus-expressed antigens. J Clin Microbiol. 1997;35:354–357. doi: 10.1128/jcm.35.2.354-357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron, K. T., X. Zhang, B. Seal, M. Rodriguez, and M. Kariuki Njenga. Antigens to viral capsid and non-capsid proteins are present in brain tissues, and antibodies in sera, of Theiler's virus-infected mice. J. Virol. Methods, in press. [DOI] [PubMed]
- 4.Chettle N J, Wyeth P J. Turkey rhinotracheitis: detection of antibodies using an ELISA test. Br Vet J. 1988;144:282–287. doi: 10.1016/0007-1935(88)90115-7. [DOI] [PubMed] [Google Scholar]
- 5.Chiang S J, Dar A M, Goyal S M, Sheikh M A, Pendersen J C, Panigrahy B, Senne D, Halvorson D A, Nagaraja K V, Kapur V. A modified enzyme-linked immunosorbent assay for the detection of avian pneumovirus antibodies. J Vet Diagn Investig. 2000;12:381–384. doi: 10.1177/104063870001200417. [DOI] [PubMed] [Google Scholar]
- 6.Eterradossi N, Toquin D, Guittet M, Bennejean G. Evaluation of different turkey rhinotracheitis viruses as antigens for serological testing following live vaccination and challenge. J Vet Med. 1995;42:175–186. doi: 10.1111/j.1439-0450.1995.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 7.Eterradossi N, Toquin D, Guittet M, Bennejean G. Discrepancies in turkey rhinotracheitis ELISA results using different antigens. Vet Rec. 1992;131:563–564. [PubMed] [Google Scholar]
- 8.Gilette K G. Enzyme-linked immunosorbent assay for serum antibody to bovine respiratory syncytial virus: comparison with complement-fixation and neutralization tests. Am J Vet Res. 1983;44:2251–2255. [PubMed] [Google Scholar]
- 9.Goyal S M, Chiang S, Dar A M, Nagaraja K V, Halvorson D A, Kapur V. Isolation of avian pneumovirus from an outbreak of respiratory illness in Minnesota turkeys. J Vet Diagn Investig. 1999;12:166–168. doi: 10.1177/104063870001200214. [DOI] [PubMed] [Google Scholar]
- 10.Grant M, Baxter-Jones C, Wilding G P. An enzyme-linked immunosorbent assay for the serodiagnosis of turkey rhinotracheitis infection. Vet Rec. 1987;120:279–280. doi: 10.1136/vr.120.12.279. [DOI] [PubMed] [Google Scholar]
- 11.Hames B D. An introduction to polyacrylamide gel electrophoresis. In: Hames B D, Rickwood D, editors. Gel electrophoresis of proteins: a practical approach. Oxford, United Kingdom: IRL Press; 1981. pp. 1–91. [Google Scholar]
- 12.Jing L, Cook J K A, Brown T D K, Shaw K, Cavanagh D. Detection of turkey rhinotracheitis virus in turkeys using the polymerase chain reaction. Avian Pathol. 1993;22:771–783. doi: 10.1080/03079459308418963. [DOI] [PubMed] [Google Scholar]
- 13.Jones R C. Avian pneumovirus infection: questions still unanswered. Avian Pathol. 1996;25:639–648. doi: 10.1080/03079459608419171. [DOI] [PubMed] [Google Scholar]
- 14.Juhasz K, Easton A J. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: evidence for two distinct subgroups. J Gen Virol. 1994;75:2873–2880. doi: 10.1099/0022-1317-75-11-2873. [DOI] [PubMed] [Google Scholar]
- 15.Kleven S H. Proceedings of the U.S. Animal Health Association 101st Annual Meeting. Richmond, Va: Pat Campbell and Associates and Spectrum Press; 1997. Report of the Committee. Transmissible diseases of poultry and other avian species; pp. 486–491. [Google Scholar]
- 16.Lauer D C. Minnesota Poultry Testing Laboratory Monthly Report. Wilmar, Minn: Minnesota Board of Animal Health; 1999. Incidence of avian pneumovirus; pp. 1–3. [Google Scholar]
- 17.Panigrahy B, Senne D A, Pendersen J C, Gidlewski T, Edson R K. Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys. Avian Dis. 2000;44:17–22. [PubMed] [Google Scholar]
- 18.Pastey M K, Samal S K. Baculovirus expression of the fusion protein gene of bovine respiratory syncytial virus and utility of the recombinant protein in a diagnostic enzyme immunoassay. J Clin Microbiol. 1998;36:1105–1108. doi: 10.1128/jcm.36.4.1105-1108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pringle C R. Virus taxonomy 1996. A bulletin from the Xth International Congress of Virology in Jerusalem. Arch Virol. 1996;141:2251–2256. doi: 10.1007/BF01718231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randhawa J S, Marriott A C, Pringle C R, Easton A J. Rescue of synthetic minireplicons establishes the absence of the NS1 and NS2 genes from avian pneumovirus. J Virol. 1997;71:9849–9854. doi: 10.1128/jvi.71.12.9849-9854.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randhawa J S, Pringle C R, Easton A J. Nucleotide sequence of the matrix protein gene of a subgroup B avian pneumovirus. Virus Genes. 1996;12:179–183. doi: 10.1007/BF00572956. [DOI] [PubMed] [Google Scholar]
- 22.Samal S K, Pastey M K, McPhillips T, Carmel D K, Mohanty S B. Reliable confirmation of antibodies to bovine respiratory syncytial virus (BRSV) by enzyme-linked immunosorbent assay using BRSV nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1993;31:3147–3152. doi: 10.1128/jcm.31.12.3147-3152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seal B S. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first U.S. avian pneumovirus isolate is distinct from European strains. Virus Res. 1998;58:45–52. doi: 10.1016/s0168-1702(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 24.Seal B S, Sellers H S, Meinersmann R J. Fusion protein predicted amino acid sequence of the first U.S. avian pneumovirus isolate and lack of heterogeneity among other U.S. isolates. Virus Res. 2000;66:139–147. doi: 10.1016/s0168-1702(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 25.Shin H J, Rajashekara G, Jirjis F F, Shaw D P, Goyal S M, Halvorson D A, Nagaraja K V. Specific detection of avian pneumovirus U.S. isolates by RT-PCR. Arch Virol. 2000;145:1239–1246. doi: 10.1007/s007050070123. [DOI] [PubMed] [Google Scholar]
- 26.Smith L M, Sanders J Z, Kaiser R J, Hughs P, Dodd C, Connell C R, Heines C, Kent S B H, Hood L E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986;321:673–681. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]