Abstract
Objective
The benefits of a low-salt diet for patients with chronic kidney disease (CKD) are controversial. We conducted a systematic review and meta-analysis of the effect of a low-salt diet on major clinical outcomes.
Design
Systematic review and meta-analysis.
Data sources
MEDLINE by Ovid, EMBASE and the Cochrane Library databases.
Eligibility criteria for selecting studies
We included randomised controlled trials (RCTs) and cohort studies that assessed the effect of a low-salt diet on the renal composite outcomes (more than 50% decline in estimated glomerular filtration rate (eGFR) during follow-up, doubling of serum creatinine or end-stage renal disease), rate of eGFR decline, change in proteinuria, all-cause mortality events, cardiovascular (CV) events, and changes in systolic blood pressure and diastolic blood pressure.
Data extraction and synthesis
Two independent researchers extracted data and evaluated their quality. Relative risks (RRs) with 95% CIs were used for dichotomous data. Differences in means (MDs) or standardised mean differences (SMDs) with 95% CIs were used to pool continuous data. We used the Cochrane Collaboration risk-of-bias tool to evaluate the quality of RCTs, and Newcastle–Ottawa Scale to evaluate the quality of cohort studies.
Results
We found 9948 potential research records. After removing duplicates, we reviewed the titles and abstracts, and screened the full text of 230 publications. Thirty-three studies with 101 077 participants were included. A low-salt diet produced a 28% reduction in renal composite outcome events (RR: 0.72; 95% CI: 0.58 to 0.89). No significant effects were found in terms of changes in proteinuria (SMD: −0.71; 95% CI: −1.66 to 0.24), rate of eGFR (decline MD: 1.16; 95% CI: −2.02 to 4.33), risk of all-cause mortality (RR: 0.92; 95% CI: 0.58 to 1.46) and CV events (RR: 1.01; 95% CI: 0.46 to 2.22).
Conclusion
A low-salt diet seems to reduce the risk for renal composite outcome events in patients with CKD. However, no compelling evidence indicated that such a diet would reduce the eGFR decline rate, proteinuria, incidence of all-cause mortality and CV events. Further, more definitive studies are needed.
PROSPERO registration number
CRD42017072395.
Keywords: nephrology, chronic renal failure, health services administration & management
Strengths and limitations of this study.
This is the first meta-analysis of the effect of a low-salt diet on hard clinical outcomes, such as renal composite outcomes, all-cause mortality and cardiovascular events in patients with chronic kidney disease.
We included cohort studies and randomised controlled trials without language restriction, and screened reference lists from included articles, maximising the number of studies included in our review.
The existence of significant heterogeneity may restrict the explanation and clinical application of the results.
Confounding factors in the observational studies cannot be ignored, which might affect the results of the meta-analysis.
Introduction
Chronic kidney disease (CKD) has become an important global public health burden. Dietary interventions aimed at delaying the CKD progression and reducing complications play a crucial role in its management. Dietary sodium restriction can augment the antiproteinuric effects of ACE inhibitors (ACEIs)/angiotensin receptor blockers1 and decrease blood pressure (BP).2 In the 2021 Kidney disease improving global outcomes (KDIGO) guidelines for glomerulonephritis, reducing dietary sodium intake to <2 g/day is a primary tenet for controlling BP and oedema and improving urinary protein excretion independently of medications. However, it is unclear whether these effects translate into a significant total risk decrease in patients with CKD, including kidney failure events, cardiovascular (CV) events and all-cause mortality. In the Chronic Renal Insufficiency Cohort Study, which recruited patients with an estimated glomerular filtration rate (eGFR) 20–70 mL/min/1.73 m2 depending on age, in contrast to the lowest quartile of urinary sodium excretion (<116.8 mmol/24 hours), high urinary sodium excretion (>194.6 mmol/24 hours) was related to a 54% risk increase in end-stage renal disease (ESRD) or 50% decrease in eGFR and with a 43% risk increase in all-cause mortality.3 However, other cohort studies have suggested that sodium intake does not affect the progression of CKD to ESRD or eGFR decline.4 5 Furthermore, in one study, restricting salt intake to <6 g/day was related to an increased risk of all-cause mortality and CV mortality in haemodialysis patients, resulting in an L-shaped association curve.6 In the NHANES II Study, which recruited 7154 community elderly people in the USA, restriction of sodium intake to <2.3 g daily resulted in increases of 37% in CV mortality and 28% in all-cause mortality compared with individuals whose sodium intake was >2.3 g daily for more than 13.7 years of follow-up period.7 Evidence from randomised controlled trials (RCTs) of the effect of a low-salt diet in patients with CKD on kidney failure events, CV events and all-cause mortality is controversial. It is hard to make clinical strategy based on results from these studies, considering their small sizes, highly selected patients, short follow-up period and short assessment of hard clinical outcomes. Thus, the effect of dietary salt reduction on clinical endpoints in patients with CKD remains unclear. We performed this systematic review of relevant clinical studies and evaluated the effect of a low-salt diet on renal outcomes, all-cause mortality and CV events in patients with CKD.
Materials and methods
Data sources and retrieval strategy
We performed this systematic review following a prespecified protocol registered at the International Prospective Register of Systematic Reviews (online supplemental file 1),8 and reporting was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.9 A comprehensive search was conducted using the following databases: MEDLINE by Ovid (1946–July 2021), EMBASE (1966–July 2021) and Cochrane Central Register of Controlled Trials (no date limitation), with related keywords and medical subject headings that included all spellings of “CKD”, “RCT”, “Cohort Studies”, “Sodium Chloride”, “Sodium”, and “Sodium-Restricted” (see online supplemental file 2 for full search terms). Studies were considered without any language restriction. To ensure an overall literature search, we also screened reference lists from included studies. The ClinicalTrials.gov website was also searched for ongoing, but unpublished studies in this field.
bmjopen-2021-050843supp001.pdf (612.1KB, pdf)
bmjopen-2021-050843supp002.pdf (83.9KB, pdf)
Study screening and outcome evaluation
We included data from RCTs and cohort studies in which a low-salt diet was given to adults with CKD (as defined by the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of CKD) compared with usual therapy or different levels of salt intake. Salt reduction was defined as that recommended by guidelines: sodium <2.3 g/day (<100 mmol sodium, <6 g salt), or as defined by the authors of the study.
Predefined outcomes that contained analysable data were as follows: renal composite outcome events, defined as more than 50% decline in eGFR from baseline during follow-up,10 doubling of serum creatinine or ESRD; the rate of change in eGFR per year; changes in urinary protein or urinary albumin during follow-up, including urinary protein excretion, urinary albumin excretion and urinary albumin/creatinine ratio; all-cause mortality events; CV events, defined as a composite, including fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, coronary artery revascularisation, CV disease and CV death; and changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Data extraction and quality estimation
Two independent reviewers (HS and XS) extracted data and assessed their quality according to the prespecified protocol. Disagreements were resolved by a third reviewer (LW). Data from all eligible studies were extracted into a spreadsheet. The data sought included characteristics of studies (study type, randomisation method, follow-up time, withdrawals/dropouts), baseline patient traits (age, sex, baseline eGFR and baseline BP), intake of salt and outcome events.
We used Cochrane Collaboration risk-of-bias tool11 to assess all potential sources of bias for the included RCTs. Trials were assessed as being at low, some concerns or high risk, and the overall risk of bias generally corresponded to the worst risk of bias in any of the domains. However, if a study was judged to have some concerns about risk of bias for multiple domains, it might be judged as being at high risk for bias overall. We used the Newcastle–Ottawa Scale (NOS) checklist to assess the quality of cohort studies. The NOS contains eight items, categorised into three dimensions: selection of cohorts, comparability of cohorts and assessment of outcome.12 A star system is used for semiquantitative assessment of study quality, such that the highest quality studies are awarded a maximum of one star for each item, except the item related to comparability, which can be assigned two stars. The NOS ranges from zero to nine stars.
Data synthesis and analyses
When dichotomous outcome data from individual trials were analysed, relative risks (RRs) and 95% CIs were used. If the RR and 95% CI for an individual study were unavailable in the original article, we calculated them using event numbers extracted from each study before data pooling. In calculating the RR values, we used the total number of patients randomised in each group as the corresponding denominator. Continuous outcome data from individual trials were analysed using mean differences (MDs) with 95% CIs to pool eGFR, and standardised mean differences (SMDs) with 95% CIs were used to pool the proteinuria or albuminuria data. When continuous outcome data were analysed, the change in MD between baseline and end of treatment was used. If these data were not available in the studies, we calculated using correlations estimated from other included studies that had a similar follow-up and reported their results in considerable details according to the imputed formulation and its related interpretations in the Cochrane Handbook.13
Given the poor stability of the DerSimonian-Laird procedure for a small number of studies,14 we used the empirical Bayes procedure15 to estimate all outcomes. We also used DerSimonian-Laird random-effects model16 and restricted maximum likelihood approach17 to assess the summary effects as part of the sensitivity analyses. Considering the inevitable heterogeneity among studies, subgroup, meta-regression and sensitivity analyses were performed. Subgroup analyses were performed according to a prespecified protocol, including study type, baseline eGFR, baseline BP and comparator of control group. In addition, we performed sensitivity analyses excluding studies with a sample size of <50, studies with a follow-up of <12 months, studies of low quality (high risk for overall bias in RCTs, NOS <5 stars for cohort studies) and studies with extreme outliers. Heterogeneity among studies was evaluated using the I2 or τ2 statistic. I2 values of 25%, 50%, and 75%, respectively, represent low, moderate, and high heterogeneity. Publication bias was assessed using a funnel plot, Egger’s test or Begg’s test, and a p value of <0.05 indicated obvious publication bias. Stata V.15.0 (StataCorp, College Station, Texas, USA) was used for statistical analyses, and a two-sided p value of <0.05 was considered indicative of significance.
Patient and public involvement
No patient or member of the public was involved in the development of the research question, selection of the outcome measures, design and implementation of the study or interpretation of the results.
Results
Overview of included trials
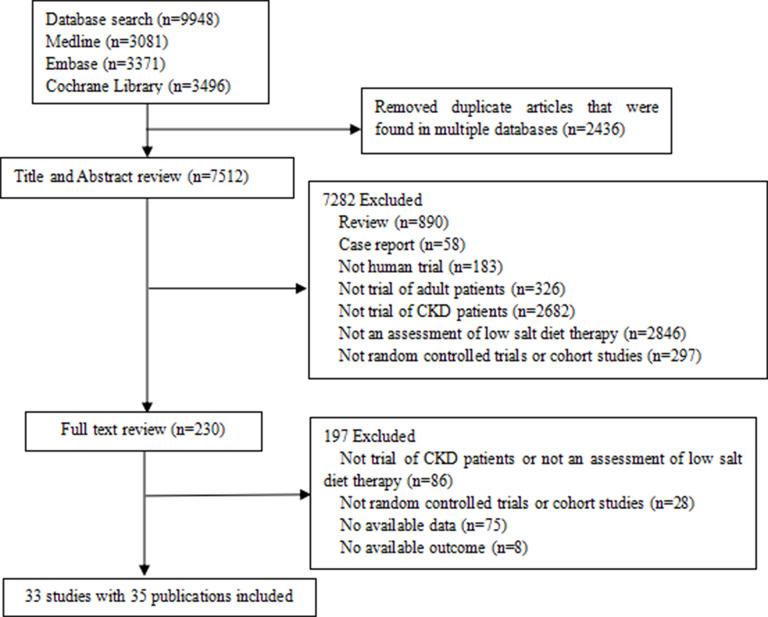
We identified 9948 potential relevant records. After removing duplicates, we screened the titles and abstracts, and the full text of 230 publications were reviewed. As shown in figure 1, online supplemental files 3 and 4, a total of 33 eligible studies3–6 18–48 including 13 RCTs18 20 25–27 31 32 35–40 45 and 19 cohort studies3–6 19 21–24 28 30 33 34 41–44 46–48 reported in 35 publications with 101 077 participants were included in our review. The median follow-up was 6 months (IQR 3–21 months). Participants were enrolled at an average age of 56 years, and male participants accounted for 60% of the total. The average eGFR of the participants was 47.45 mL/min/1.73 m2. Twelve studies were usual care or usual diet controlled, and 21 compared different levels of sodium intake.
Figure 1.
PRISMA flow chart of the included studies. CKD, chronic kidney disease; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
bmjopen-2021-050843supp003.pdf (131.3KB, pdf)
bmjopen-2021-050843supp004.pdf (60.7KB, pdf)
The risk of bias varied substantially across the RCTs. The results from the Cochrane Collaboration risk-of-bias tool are shown in online supplemental file 5. Bias arising from the randomisation process was considered to be low in 61.54% of the RCTs, and all studies had a low risk of bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported results. In terms of overall bias, 61.54% of the trials were considered to have low risk, and 38.46% had some concern of bias.
bmjopen-2021-050843supp005.pdf (94.6KB, pdf)
According to the NOS checklist, all included cohort studies were generally high quality and awarded six to eight stars (online supplemental file 6).
bmjopen-2021-050843supp006.pdf (99.2KB, pdf)
Effect of a low-salt diet on renal outcomes
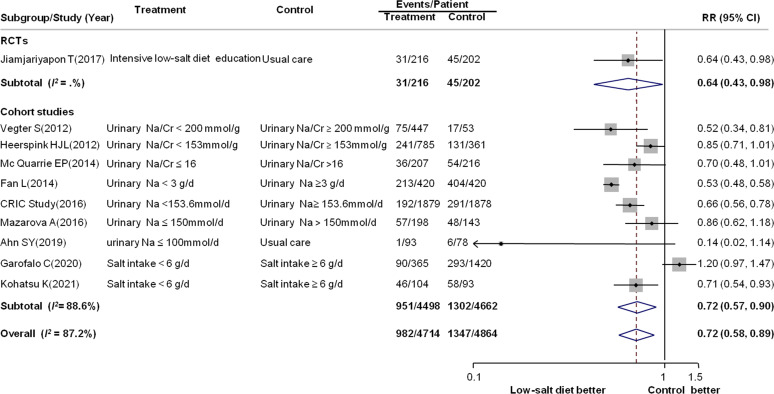
One RCT and nine observational studies including 9578 patients with 2329 events compared a low-salt diet with controls in terms of preventing renal composite outcome events. As shown in figure 2, compared with controls, a low-salt diet produced a 28% reduction in the risk for renal composite outcome events (RR: 0.72; 95% CI: 0.58 to 0.89), with substantial heterogeneity (I2=87.2%, p<0.001). Subgroup analyses and meta-regression were conducted to assess the source of heterogeneity. No significant difference between RCTs and observational studies was found, whereas the effect sizes were greater in studies that enrolled patients aged <58 years (p=0.03) (table 1). In univariate meta-regression, no clear relationship between 24-hour sodium in urine (p=0.80) or baseline eGFR (p=0.51) and the reduction in renal composite outcome events was observed (online supplemental files 7 and 8).
Figure 2.
Forest plot for renal composite outcome events. Renal composite outcome events were defined as more than 50% decline in eGFR from baseline during follow-up or a doubling of serum levels of Cr or ESRD. Cr, creatinine; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; Na, sodium; RCTs, randomised controlled trials; RR, relative risk.
Table 1.
Results of subgroup analysis of renal composite outcome events
| Subgroup | Number of trials | n | RR (95% CI) | P value for RR | I2 | P value for heterogeneity test* |
| Study type | ||||||
| RCT | 1 | 418 | 0.64 (0.43 to 0.98) | 0.04 | – | 0.76 |
| Cohort study | 9 | 9160 | 0.72 (0.57 to 0.90) | 0.005 | 88.6% | |
| Baseline eGFR (mL/min/1.73 m2) | ||||||
| 60–89 | 1 | 171 | 0.14 (0.02 to 1.14) | 0.07 | – | 0.48 |
| 30–59 | 7 | 8869 | 0.71 (0.55 to 0.92) | 0.009 | 90.8% | |
| 15–29 | 2 | 538 | 0.77 (0.63 to 0.94) | 0.01 | 0.0% | |
| Mean age (years) | ||||||
| <58 | 5 | 5691 | 0.58 (0.49 to 0.70) | <0.001 | 56.4% | 0.03 |
| ≥58 | 5 | 3887 | 0.85 (0.69 to 1.05) | 0.14 | 70.1% | |
| Baseline BP (mm Hg) | ||||||
| <140/90 | 6 | 5806 | 0.62 (0.52 to 0.73) | <0.001 | 58.5% | 0.32 |
| SBP ≥140 or DBP ≥90 | 2 | 1646 | 0.70 (0.44 to 1.11) | 0.13 | 74.6% | |
| Comparator of control group | ||||||
| Usual care or usual diet | 2 | 589 | 0.43 (0.11 to 1.64) | 0.21 | 50.2% | 0.12 |
| Different levels of sodium intake | 8 | 8989 | 0.73 (0.58 to 0.92) | 0.008 | 89.8% | |
- The bold P value indicates that there are statistical differences between subgroups.
*P value calculated by meta-regression.
BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; n, number of patients; RCT, randomised controlled trial; RR, relative risk; SBP, systolic blood pressure.
bmjopen-2021-050843supp007.pdf (539.9KB, pdf)
bmjopen-2021-050843supp008.pdf (519.5KB, pdf)
Five RCTs and seven observational studies with a total of 3287 individuals reported data regarding the effect of a low-salt diet on the rate of change in eGFR. The diet did not have a beneficial effect on the rate of change in eGFR (MD: 1.16; 95% CI: −2.02 to 4.33). There was evidence of significant heterogeneity across the included studies (I2=98.0%, p<0.001) (figure 3). Subgroup analyses showed that there was no statistical heterogeneity according to prespecified characteristics (online supplemental file 9).
Figure 3.
Forest plot of the rate of change in estimated glomerular filtration rate. Cr, creatinine; MD, mean difference; Na, sodium; RCTs, randomised controlled trials.
bmjopen-2021-050843supp009.pdf (127.9KB, pdf)
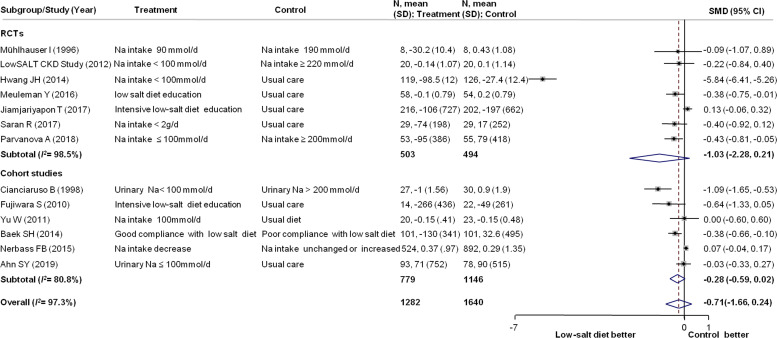
Data on the effect of a low-salt diet on proteinuria or albuminuria were available in 13 studies, with 2922 participants. The SMD for change in proteinuria or albuminuria was not statistically significant at −0.71 (95% CI: −1.66 to 0.24) compared with controls, with significant heterogeneity (I2=97.3%, p=0.009) (figure 4). No statistical heterogeneity was found in subgroup analyses of the effect of a low-salt diet on proteinuria or albuminuria (online supplemental file 9).
Figure 4.
Forest plot for the change in proteinuria or albuminuria. CKD, chronic kidney disease; Na, sodium; RCTs, randomised controlled trials; SMD, standard mean difference.
Effect of a low-salt diet on all-cause mortality and CV events
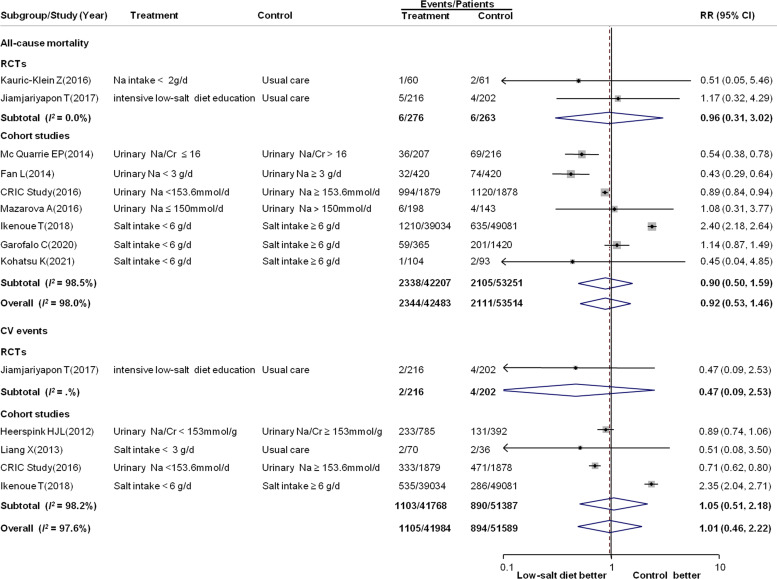
Data on the effect of low-salt diet on all-cause mortality and CV events were available for nine studies (two RCTs and seven observational studies) and five studies (one RCT and four observational studies), respectively. There was no effect of a low-salt diet on the risk of all-cause mortality (RR: 0.92; 95% CI: 0.53 to 1.46; 95 997 participants and 4455 events) or CV events (RR: 1.01; 95% CI: 0.46 to 2.22; 93 573 participants and 1989 events) compared with controls, with significant heterogeneity across the included studies (I2=98.0%, 97.6% for all-cause mortality and CV events, respectively, both p values for heterogeneity <0.001) (figure 5). A greater benefit was found in studies that included patients with an eGFR of 30–59 mL/min/1.73 m2 (p for subgroup heterogeneity=0.02) in the subgroup analyses of CV events (online supplemental file 9).
Figure 5.
Forest plot for all-cause mortality and cardiovascular (CV) events. CV events were defined as a composite of fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, coronary artery revascularisation, CV disease and CV death. Cr, creatinine; Na, sodium; RCTs, randomised controlled trials; RR, relative risk.
Effect of a low-salt diet on BP
Twenty studies reported the effect of a low-salt diet on BP, including 19 studies (3608 participants) for SBP and 18 studies (3608 participants) for DBP. The MD changes in SBP and DBP were statistically significant at −5.81 mm Hg (95% CI: −9.63 to −1.99) and −2.3 mm Hg (95% CI, −3.61 to −0.98), respectively (online supplemental files 10 and 11). Significant heterogeneity across the included studies was found (I2=87.1% and 68.2% for SBP and DBP, respectively; both p values for heterogeneity <0.05). Subgroup analyses showed that the association between the change in SBP or DBP and a low-salt diet was modified by the baseline BP. Patients with a baseline SBP >140 mm Hg or DBP >90 mm Hg had an average reduction in SBP and DBP of 10 and 5 mm Hg, respectively (online supplemental file 9).
bmjopen-2021-050843supp010.pdf (646.2KB, pdf)
bmjopen-2021-050843supp011.pdf (1.3MB, pdf)
Sensitivity analyses and publication bias
The results of all sensitivity analyses were robust (online supplemental file 12). The most notable exception was that the effect of a low-salt diet on the rate of change in eGFR became significant when studies with extreme outliers were omitted, and proteinuria or albuminuria became significant when the DerSimonian-Laird method was used to pool data. The risk of publication bias was significant for the rate of change in eGFR, proteinuria or albuminuria, all-cause mortality, CV events and BP (online supplemental files 13 and 14).
bmjopen-2021-050843supp012.pdf (80.7KB, pdf)
bmjopen-2021-050843supp013.pdf (1.3MB, pdf)
bmjopen-2021-050843supp014.pdf (76.3KB, pdf)
Discussion
Behavioural modifications can delay CKD progression to ESRD, and dietary salt restriction is one of the main concerns. Our meta-analysis showed that compared with the control groups, a low-salt diet reduced renal composite outcome events and lowered BP (SBP and DBP) in patients with CKD. No significant effects were observed on the eGFR decline rate, change in proteinuria, risk of all-cause mortality and CV events. The results were concordant across major subgroups and sensitivity analyses. However, the significant heterogeneity among the studies may restrict their explanation and clinical practice.
A prior review of 16 studies on salt consumption and kidney disease investigated whether variation in dietary sodium intake affects kidney outcomes in patients with CKD.49 Increased salt consumption was related to increased albuminuria and the possibility of a decrease in GFR. In 201550 and 2018,51 two meta-analyses of RCTs suggested that moderate dietary salt limitation obviously reduced BP and proteinuria. However, it is unclear whether these benefits convert to clinically obvious reductions in renal composite outcome events, all-cause mortality events and CV events. Our meta-analysis of 33 studies (13 RCTs and 20 observational studies), with more than 90 000 patients and 2329 renal composite outcome events, showed that compared with the control groups, a low-salt diet produced a significant 28% reduction in renal composite outcome events despite marked heterogeneity. Furthermore, the significant effect of a low-salt diet on BP (decreases in SBP and DBP of 5.8 and 2.3 mm Hg, respectively) was confirmed and is an important target for slowing the progression of CKD to ESRD. The benefit of proteinuria from low-salt diet became non-significant when the restricted maximum likelihood or empirical Bayes method was used to pool data. The standard DerSimonian-Laird procedure, which was applied in previous meta-analyses, can be unstable with small numbers of studies.14 52
Statistical heterogeneity was found in all outcomes, and clinical heterogeneity was an inevitable issue. Low-salt diets are not palatable for some patients.53 Therefore, it is hard to confirm persistent compliance with a salt restriction during long-term follow-up time. Sodium intake was quantified using different methods (such as, 24-hour urine collection, spot urine collection or dietary questionnaire). Because diuretics influence urine sodium excretion and eGFR, it would influence the accuracy of quantified salt intake. Ten studies had data on diuretic use, but included only the percentages of patients using diuretics in the intervention and control groups. The percentage of patients using diuretics was similar in most of the studies. There were no data on the type and dose of diuretics, or their associations with the eGFR. These inherent problems were reflected in paradoxically opposite findings, especially of observational studies with several confounding factors. Only three studies (one RCT and two observational studies) simultaneously reported the outcomes of eGFR and renal composite outcome events. Furthermore, the different follow-up periods (52 months of renal composite outcome events and 12 months of eGFR) should be noted. The inconsistent results of eGFR and renal composite outcome events may be explained in part by the heterogeneity, which likely limited the interpretation and generalisability of our results.
In patients with CKD, BP is typically sodium sensitive, and renin-angiotensin-aldosterone system (RAAS) blockers are considered the first-line therapy for hypertension and proteinuria. The function of RAAS blockers is weakened by high salt intake54–56; therefore, the significant effect of dietary sodium restriction on lowering BP and supporting the function of RAAS blockers was speculated to play a very important part in reducing the risk of renal failure. Interestingly, a BP-independent effect of dietary sodium on the kidney is substantial by data in healthy volunteers, in which dietary sodium restriction reduces albuminuria to within the normal range, without a detectable effect on BP.57 Similarly, proteinuria reduction by sodium restriction remained significant after adjustment for the decrease in BP.56 These results suggest an independent renoprotective function of a low-salt diet.
There was no convincing evidence that a low-salt diet was related to a lower incidence of all-cause mortality and CV events. Limited data on all-cause mortality (nine studies with 4455 events) and CV events (five studies with 1989 events) in the meta-analysis might introduce a risk of false-negative results because of low statistical power. A greater benefit for CV events was found in studies that included patients with an eGFR 30–59 mL/min/1.73 m2 in subgroup analyses, whereas the effects were not significant in patients with an eGFR <15 mL/min/1.73 m2. The substantial subgroup heterogeneity (p=0.02) could be attributable to more CV risk factors in patients with ESRD than in those with an eGFR 30–59 mL/min/1.73 m2. The benefit of a low-salt diet for CV events did not outweigh the increase in CV events caused by more risk factors (such as hyperuricemia, hyperphosphatemia and vascular calcification). Therefore, education on the importance of a low-salt diet should be emphasised in patients with early-stage CKD.
Observational data showing a J-curve between sodium intake and renal and CV outcomes have raised concern regarding the safety of rigorous sodium restriction.58–60 It is uncertain whether the presence of CKD modifies this association. In our meta-regression analysis, no clear relationship between 24-hour sodium in urine (p=0.80) or baseline eGFR (p=0.51) and the reduction in renal composite outcome events was observed. A combination of angiotensin-converting enzymes (ACEIs) and strict sodium control decreased BP, proteinuria and glomerular lesion but exacerbated tubule-interstitial injury in experimental renal disease.61 This could explain the reason of worse renal outcomes by very low salt intake. High or extremely low-dietary salt intake might be inappropriate for patients with CKD. KDIGO recommends a daily sodium intake of <2.0 g/day in patients with CKD without a clear lower limit. Therefore, an appropriate dietary salt intake should be discussed with patients with CKD. Further studies are necessary to identify the optimal dietary salt intake for renoprotection in patients with CKD.
This study had several potential limitations. First, the existence of statistical and clinical heterogeneity may raise concerns regarding validity, although we attempted to resolve these concerns by performing subgroup and sensitivity analyses. Heterogeneity in study populations hampers meaningful interpretation of the results. Because the CKD stages varied markedly, it is difficult to stratify the outcome analysis by CKD stage. Instead, we performed subgroup analyses according to baseline eGFR. Second, unlike RCTs, cohort studies are prone to selection bias. Therefore, pooling the results from RCTs and cohort studies will bias the results of the meta-analysis. We performed a subgroup analysis according to study design. There was no statistical heterogeneity in the outcomes according to study design. However, a low-salt diet did not exert a beneficial effect on the rate of change in eGFR in RCTs (MD: −0.75; 95% CI: −7.58 to 6.08) but did in eGFR in cohort studies (MD: 2.48; 95% CI: 0.32 to 4.64). Third, many of the included low-quality studies had small sample sizes and low incidences of events. Consequently, the uncertainty in the analyses was increased, resulting in wide CIs of effect measures. Fourth, confounding factors in the observational studies cannot be neglected, because they might affect the results. Therefore, the findings of this study should be viewed as hypothesis generating and need to be confirmed by further studies.
Conclusion
This meta-analysis suggests that a low-salt diet may reduce the risk of renal composite outcome events in patients with CKD. However, there was no compelling evidence that a low-salt diet reduces the rate of eGFR decline, proteinuria, incidence of all-cause mortality and CV events. The optimal dietary salt intake for patients with different CKD stages is unclear. Further well-designed RCTs targeting patients with different CKDs are required.
Supplementary Material
Acknowledgments
We thank Textcheck for proofreading and editing the English text of the manuscript.
Footnotes
Contributors: All authors take responsibility for the integrity of the data and the accuracy of data analysis. Study concept and design—LW, XS, HS and CL. Extraction, analysis and interpretation of data—HS and XS. Drafting of the manuscript—HS and LW. Critical revision of the manuscript—LW and XS. Statistical analysis—HS, CL and WG. Technical support—LW, XS and WG. All authors have read and agreed to the submission of this journal. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: Support was provided by the National Natural Science Foundation of China (no. 82000655) and the Science and Technology Project of Shanxi Province (no. 201801D121218).
Disclaimer: The funders had no role in the decision to publish or in preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data used to support the findings of this study are included within the article and supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not involve human participants.
References
- 1.Heeg JE, de Jong PE, van der Hem GK, et al. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int 1989;36:272–9. 10.1038/ki.1989.190 [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. 10.1056/NEJM200101043440101 [DOI] [PubMed] [Google Scholar]
- 3.He J, Mills KT, Appel LJ, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 2016;27:1202–12. 10.1681/ASN.2015010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan L, Tighiouart H, Levey AS, et al. Urinary sodium excretion and kidney failure in nondiabetic chronic kidney disease. Kidney Int 2014;86:582–8. 10.1038/ki.2014.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazarova A, Molnar AO, Akbari A, et al. The association of urinary sodium excretion and the need for renal replacement therapy in advanced chronic kidney disease: a cohort study. BMC Nephrol 2016;17:123. 10.1186/s12882-016-0338-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikenoue T, Koike K, Fukuma S, et al. Salt intake and all-cause mortality in hemodialysis patients. Am J Nephrol 2018;48:87–95. 10.1159/000492034 [DOI] [PubMed] [Google Scholar]
- 7.Cohen HW, Hailpern SM, Fang J, et al. Sodium intake and mortality in the NHANES II follow-up study. Am J Med 2006;119:275.e7–14. 10.1016/j.amjmed.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 8.et alLi C, Wang L, Su X. Effect of dietary sodium restriction on renal outcome in patients with chronic kidney disease. PROSPERO 2017 CRD42017072395. Available: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017072395
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–31. 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for systematic reviews of interventions. version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
- 14.Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med 2014;160:267–70. 10.7326/M13-2886 [DOI] [PubMed] [Google Scholar]
- 15.Friston KJ, Penny W, Phillips C, et al. Classical and Bayesian inference in neuroimaging: theory. Neuroimage 2002;16:465–83. 10.1006/nimg.2002.1090 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. J Am Stat Assoc 1977;72:320–38. 10.1080/01621459.1977.10480998 [DOI] [Google Scholar]
- 18.Mühlhauser I, Prange K, Sawicki PT, et al. Effects of dietary sodium on blood pressure in IDDM patients with nephropathy. Diabetologia 1996;39:212–9. 10.1007/BF00403965 [DOI] [PubMed] [Google Scholar]
- 19.Cianciaruso B, Bellizzi V, Minutolo R, et al. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab 1998;24:296–301. 10.1159/000057385 [DOI] [PubMed] [Google Scholar]
- 20.Keven K, Yalçin S, Canbakan B, et al. The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplant Proc 2006;38:1323–6. 10.1016/j.transproceed.2006.02.103 [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara S, Kotani K, Brantley PJ, et al. Dietary salt reduction in rural patients with albuminurea using family and community support: the Mima study. Asia Pac Fam Med 2010;9:6. 10.1186/1447-056X-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W, Luying S, Haiyan W, et al. Importance and benefits of dietary sodium restriction in the management of chronic kidney disease patients: experience from a single Chinese center. Int Urol Nephrol 2012;44:549–56. 10.1007/s11255-011-9986-x [DOI] [PubMed] [Google Scholar]
- 23.Vegter S, Perna A, Postma MJ, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 2012;23:165–73. 10.1681/ASN.2011040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambers Heerspink HJ, Holtkamp FA, Parving H-H, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 2012;82:330–7. 10.1038/ki.2012.74 [DOI] [PubMed] [Google Scholar]
- 25.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 2013;24:2096–103. 10.1681/ASN.2013030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell KL, Johnson DW, Bauer JD, et al. A randomized trial of sodium-restriction on kidney function, fluid volume and adipokines in CKD patients. BMC Nephrol 2014;15:57. 10.1186/1471-2369-15-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Brito-Ashurst I, Perry L, Sanders TAB, et al. The role of salt intake and salt sensitivity in the management of hypertension in South Asian people with chronic kidney disease: a randomised controlled trial. Heart 2013;99:1256–60. 10.1136/heartjnl-2013-303688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang X, Wang W, Li H. Water and sodium restriction on cardiovascular disease in young chronic hemodialysis patients. Chin Med J 2013;126:1667–72. [PubMed] [Google Scholar]
- 29.McQuarrie EP, Traynor JP, Taylor AH, et al. Association between urinary sodium, creatinine, albumin, and long-term survival in chronic kidney disease. Hypertension 2014;64:111–7. 10.1161/HYPERTENSIONAHA.113.03093 [DOI] [PubMed] [Google Scholar]
- 30.Baek SH, Kim S, Kim DK, et al. A low-salt diet increases the estimated net endogenous acid production in nondiabetic chronic kidney disease patients treated with angiotensin receptor blockade. Nephron Clin Pract 2014;128:407–13. 10.1159/000369558 [DOI] [PubMed] [Google Scholar]
- 31.Hwang JH, Chin HJ, Kim S, et al. Effects of intensive low-salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: a single-blinded randomized, controlled trial. Clin J Am Soc Nephrol 2014;9:2059–69. 10.2215/CJN.01310214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues Telini LS, de Carvalho Beduschi G, Caramori JCT, et al. Effect of dietary sodium restriction on body water, blood pressure, and inflammation in hemodialysis patients: a prospective randomized controlled study. Int Urol Nephrol 2014;46:91–7. 10.1007/s11255-013-0382-6 [DOI] [PubMed] [Google Scholar]
- 33.Nerbass FB, Pecoits-Filho R, McIntyre NJ, et al. Reduction in sodium intake is independently associated with improved blood pressure control in people with chronic kidney disease in primary care. Br J Nutr 2015;114:936–42. 10.1017/S0007114515002494 [DOI] [PubMed] [Google Scholar]
- 34.Mills KT, Chen J, Yang W, et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 2016;315:2200–10. 10.1001/jama.2016.4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauric-Klein Z, Peters RM, Yarandi HN. Self-Efficacy and blood pressure self-care behaviors in patients on chronic hemodialysis. West J Nurs Res 2017;39:886–905. 10.1177/0193945916661322 [DOI] [PubMed] [Google Scholar]
- 36.Meuleman Y, Hoekstra T, Dekker FW, et al. Sodium restriction in patients with CKD: a randomized controlled trial of self-management support. Am J Kidney Dis 2017;69:576–86. 10.1053/j.ajkd.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 37.Jiamjariyapon T, Ingsathit A, Pongpirul K, et al. Effectiveness of integrated care on delaying progression of stage 3-4 chronic kidney disease in rural communities of Thailand (escort study): a cluster randomized controlled trial. BMC Nephrol 2017;18:83. 10.1186/s12882-016-0414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saran R, Padilla RL, Gillespie BW, et al. A randomized crossover trial of dietary sodium restriction in stage 3-4 CKD. Clin J Am Soc Nephrol 2017;12:399–407. 10.2215/CJN.01120216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang B, Li Z, Wang Y, et al. Effectiveness of self-management support in maintenance haemodialysis patients with hypertension: a pilot cluster randomized controlled trial. Nephrology 2018;23:755–63. 10.1111/nep.13098 [DOI] [PubMed] [Google Scholar]
- 40.Parvanova A, Trillini M, Podestà MA, et al. Moderate salt restriction with or without paricalcitol in type 2 diabetes and losartan-resistant macroalbuminuria (proceed): a randomised, double-blind, placebo-controlled, crossover trial. Lancet Diabetes Endocrinol 2018;6:27–40. 10.1016/S2213-8587(17)30359-5 [DOI] [PubMed] [Google Scholar]
- 41.Martinez MG, Dos Santos Silva V, do Valle AP, et al. Association between sodium intake and urinary fractional albumin and immunoglobulin G excretion in chronic Nondialytic renal disease: a prospective longitudinal study. Nephron 2019;143:62–7. 10.1159/000500548 [DOI] [PubMed] [Google Scholar]
- 42.Nishimoto M, Ohtsu H, Marumo T, et al. Mineralocorticoid receptor blockade suppresses dietary salt-induced ACEI/ARB-resistant albuminuria in non-diabetic hypertension: a sub-analysis of evaluate study. Hypertens Res 2019;42:514–21. 10.1038/s41440-018-0201-7 [DOI] [PubMed] [Google Scholar]
- 43.Ahn SY, Kim DK, Park JH, et al. Long-Term effects of intensive low-salt diet education on deterioration of glomerular filtration rate among non-diabetic hypertensive patients with chronic kidney disease. Kidney Blood Press Res 2019;44:1101–14. 10.1159/000502354 [DOI] [PubMed] [Google Scholar]
- 44.Hu J, Hu L, Gong N, et al. [Effect of dietary sodium intake on residual renal function in patients undergoing peritoneal dialysis: a prospective study of 33 cases]. Nan Fang Yi Ke Da Xue Xue Bao 2019;39:657–64. 10.12122/j.issn.1673-4254.2019.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humalda JK, Klaassen G, de Vries H, et al. A self-management approach for dietary sodium restriction in patients with CKD: a randomized controlled trial. Am J Kidney Dis 2020;75:847–56. 10.1053/j.ajkd.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 46.Garofalo C, Provenzano M, Andreucci M, et al. Predictive effect of salt intake on patient and kidney survival in non-dialysis CKD: competing risk analysis in older versus younger patients under nephrology care. Nephrol Dial Transplant 2021;36:2232–40. 10.1093/ndt/gfaa252 [DOI] [PubMed] [Google Scholar]
- 47.Kinguchi S, Wakui H, Ito Y, et al. Relationship between basal sodium intake and the effects of dapagliflozin in albuminuric diabetic kidney disease. Sci Rep 2021;11:951. 10.1038/s41598-020-79687-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohatsu K, Shimizu S, Shibagaki Y, et al. Association between daily urinary sodium excretion, ratio of extracellular Water-to-Total body water ratio, and kidney outcome in patients with chronic kidney disease. Nutrients 2021;13:650. 10.3390/nu13020650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones-Burton C, Mishra SI, Fink JC, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol 2006;26:268–75. 10.1159/000093833 [DOI] [PubMed] [Google Scholar]
- 50.McMahon EJ, Campbell KL, Bauer JD, et al. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2015:CD010070. 10.1002/14651858.CD010070.pub2 [DOI] [PubMed] [Google Scholar]
- 51.Garofalo C, Borrelli S, Provenzano M, et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients 2018;10:732. 10.3390/nu10060732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55–79. 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welch JL, Bennett SJ, Delp RL, et al. Benefits of and barriers to dietary sodium adherence. West J Nurs Res 2006;28:162–80. 10.1177/0193945905282323 [DOI] [PubMed] [Google Scholar]
- 54.Navis G, de Jong PE, Donker AJ, et al. Moderate sodium restriction in hypertensive subjects: renal effects of ACE-inhibition. Kidney Int 1987;31:815–9. 10.1038/ki.1987.71 [DOI] [PubMed] [Google Scholar]
- 55.Vogt L, Waanders F, Boomsma F, et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008;19:999–1007. 10.1681/ASN.2007060693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slagman MCJ, Waanders F, Hemmelder MH, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 2011;343:d4366. 10.1136/bmj.d4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krikken JA, Lely AT, Bakker SJL, et al. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 2007;71:260–5. 10.1038/sj.ki.5002011 [DOI] [PubMed] [Google Scholar]
- 58.O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014;371:612–23. 10.1056/NEJMoa1311889 [DOI] [PubMed] [Google Scholar]
- 59.Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011;34:861–6. 10.2337/dc10-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011;34:703–9. 10.2337/dc10-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamming I, Navis G, Kocks MJA, et al. ACE inhibition has adverse renal effects during dietary sodium restriction in proteinuric and healthy rats. J Pathol 2006;209:129–39. 10.1002/path.1956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050843supp001.pdf (612.1KB, pdf)
bmjopen-2021-050843supp002.pdf (83.9KB, pdf)
bmjopen-2021-050843supp003.pdf (131.3KB, pdf)
bmjopen-2021-050843supp004.pdf (60.7KB, pdf)
bmjopen-2021-050843supp005.pdf (94.6KB, pdf)
bmjopen-2021-050843supp006.pdf (99.2KB, pdf)
bmjopen-2021-050843supp007.pdf (539.9KB, pdf)
bmjopen-2021-050843supp008.pdf (519.5KB, pdf)
bmjopen-2021-050843supp009.pdf (127.9KB, pdf)
bmjopen-2021-050843supp010.pdf (646.2KB, pdf)
bmjopen-2021-050843supp011.pdf (1.3MB, pdf)
bmjopen-2021-050843supp012.pdf (80.7KB, pdf)
bmjopen-2021-050843supp013.pdf (1.3MB, pdf)
bmjopen-2021-050843supp014.pdf (76.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data used to support the findings of this study are included within the article and supplemental information.