Abstract
Background
If acute diarrhea in children is not treated promptly and effectively, it can lead to severe dehydration and serious sequelae. Due to the imbalance of intestinal bacteria in children with acute diarrhea, the supplementation with probiotics is important, which can improve the intestinal microenvironment, promote immunity, and enhance resistance. This meta-analysis provides further evidence and discussion of the therapeutic effect of probiotics on acute diarrhea in children.
Methods
MEDLINE, EMBASE, PubMed, and the Cochrane Library databases were searched by rapid matching. The input keywords were as follows: (probiotics/synbiotics) and (child/children) and (acute diarrhea/acute gastroenteritis). Articles reporting on randomized controlled trials (RCTs) of probiotics in treating acute diarrhea in children were retrieved. The studies were published from 2010 to 2020. After screening and quality evaluation, Stata 16.0 software was used for the analysis.
Results
Twelve articles with 744 patients were included in the study, and the overall quality of the articles was excellent. Meta-analysis showed that the duration of diarrhea in the probiotics group was shorter than that in the control group [standard mean difference (SMD) =−0.74, 95% CI: −1.11 to −0.37, Z=−3.935, P=0.000], the 2-day treatment efficacy for diarrhea in the probiotics group was greater than that in the control group [odds ratio (OR) =2.12, 95% CI: 1.47–3.05, Z=3.998, P=0.000], and the length of hospital stay in the probiotics group was shorter than that of the control group (SMD =−0.60, 95% CI: −0.74 to −0.47, Z=−8.781, P=0.000). In the subgroup analysis, combined probiotics shortened the duration of diarrhea compared with single probiotic use, and Lactobacillus reuteri and Saccharomyces boulardii had a better therapeutic effect than Lactobacillus rhamnosus or Lactobacillus acidophilus.
Discussion
In the treatment of acute diarrhea in children, the addition of probiotics can shorten the duration of diarrhea, increase treatment efficacy after 2 days of treatment, and shorten the length of hospital stay. However, because of possible publication bias in the current study, further high-quality RCT studies in clinical settings are needed to verify the current results and continue the exploration of this topic.
Keywords: Probiotics, children, acute diarrhea, meta-analysis
Introduction
Background
In children, acute diarrhea is the second most common disease after respiratory tract infection. The course of the disease is less than 2 weeks. It is caused by a variety of factors and a variety of pathogens. Diarrhea is characterized by increased stool frequency and changes in stool consistency and is often accompanied by fever, vomiting, and electrolyte and pH imbalances (1). The disease is prevalent worldwide, especially in developing countries. In some African countries, 15% of children under 5 years die of acute diarrhea (2). If acute diarrhea in children is not treated promptly and effectively, it can lead to severe dehydration and serious sequelae, such as hemolytic uremic syndrome, Guillain-Barre syndrome, malnutrition, and dysplasia, and ultimately can be life-threatening. Viruses (mainly rotavirus) and bacteria (pathogenic E. coli, Salmonella, Staphylococcus aureus, etc.) are the most common triggering factors for acute diarrhea and are related to poor local environmental sanitation, poor personal hygiene, and unsafe water supplies, other causative factors are the overuse of antibiotics resulting in bacterial intestinal disorders, allergy, inappropriate diet, poor air quality, and climatic factors (3).
Normal intestinal bacteria in the human body regulate immunity and promote nutrient absorption, play an essential role in protecting the ordinary function of the intestinal barrier, but the intestinal bacteria in children with diarrhea are in a state of imbalance and disorders of the composition of gut flora can be observed, of all cases rotavirus accounts for more than 80% , and the infection and colonization of rotavirus in the intestine can significantly decrease the amount of Lactobacillus, Bifidobacterium, and Enterococcus in the intestine (4). Probiotics are a kind of active microorganisms beneficial to the host by colonizing in the human body and changing the composition of flora in specific parts of the host (such as intestine), which could promoting the reproduction and growth of beneficial intestinal flora, enhancing the ability to resist external pathogenic bacteria, improving the intestinal microenvironment, and promoting increased immunity and resistance (5). We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/tp-21-511).
Purpose
Numerous randomized controlled trials (RCTs) have explored the therapeutic effect of probiotics on acute diarrhea in clinical practice. In a previous meta-analysis (6), the researchers included 20 RCT studies of 2,752 children. The utility of single probiotics and synbiotics (prebiotics and probiotics) was analyzed. The study reported that the addition of probiotics reduced the duration of diarrhea and stool frequency during treatment and accelerated improvements in vomiting, fever, and other symptoms. However, the literatures included in the study was older and generally of low quality, with no separate analysis of the efficacy of single probiotic use. In the current meta-analysis, 12 high-quality RCT articles were included to investigate the efficacy of probiotics and explore the source of heterogeneity in groups (by country and by species type) to provide more comprehensive evidence for the use of probiotics.
Methods
Inclusion criteria for studies
Study type
All studies were single-center or multi-center RCTs published in the past decade (January 2010 to September 2020). We only included English literatures because we believe that English is more logical and rigorous than other languages.
Participants
All participants were children over 6 months of age and under 10 years of age who had acute diarrhea. Children were included if the duration of diarrhea had lasted 3 days, the changes in stool frequency and consistency met the definition of acute diarrhea, there was thinning of stool consistency, and there were more than three watery stools within 24 hours. Children with malnutrition, bloody stools, meningitis, sepsis, or pneumonia infections were excluded, as well as those whose diarrhea was caused by antibiotic use or who had used antibiotics as the primary treatment.
Description of interventions
Studies were required to include a comparison of two groups of patients (experimental and control groups) where both groups of patients were given basic supportive treatment, such as oral rehydration solution and zinc supplementation, but the experimental group was additionally given probiotic additives, including single probiotics or combined probiotics and synbiotics.
Outcome indicators
Main indicators: these included the duration of diarrhea, the 2-day efficacy of treatment rate, and the length of hospital stay.
Secondary indicators: any adverse effects.
Indicators not analyzed: due to data inconsistency, some outcome indicators were not included in the final analysis, such as stool frequency after the intervention, stool characteristic scores (due to different scoring methods used in the studies), viral clearance rates, changes in serum IgA content, duration of vomiting, duration of fever, and recurrence rate.
Exclusion criteria for studies
All studies that focused on adults rather than children were excluded, as were reviews, individual cases, and meta-analyses that did not contain complete study protocols.
Search strategy
Computer search
The databases MEDLINE (January 2010 to September 2020), EMBASE (2010 to August 2021), PubMed (2010 to August 2021), and the Cochrane Library (August 2020) were searched using the following keywords:
(probiotics/synbiotics) and (child/children) and (acute diarrhea/acute gastroenteritis).
Search of other sources
The ClinicalTrials.gov website was searched for studies related to probiotics in the treatment of acute diarrhea in children.
Literature screening
Two researchers independently screened the selected studies by reading the titles and abstracts and excluded duplicate or unqualified articles. If there was a difference of opinion between the two researchers, a third researcher was consulted to resolve the disagreement.
Data extraction
Two researchers independently extracted the data and used Endnote Version X9 to assist in data storage and tracking. The extracted contents included:
The basic information of the studies, including title, author, mailing address, name of publication, and publication time.
The characteristics of the intervention, including grouping, number of groups, grouping method, number of samples in each group, and intervention method.
The characteristics of the participants, including age, gender ratio, mean age, duration of diarrhea before treatment, body temperature, abdominal pain, and vomiting symptoms.
The outcome assessments, including treatment time, observation time, stool characteristics assessment, primary outcome indicator data, and secondary outcome indicator data.
Literature bias
Two researchers assessed the risk of bias in the RCT studies based on the six dimensions defined in the Cochrane Handbook for Systematic Reviews of Interventions (7), where “high”, “unclear”, or “low” ratings indicated the risk of bias. The quality of the studies ranks as: Level A, all the six dimensions show low risk of bias; Level B, one or more dimentions show unclear risk of bias; Level C, one or more dimentions show high risk of bias.
Measurement of treatment efficacy
Binary variables (e.g., the treatment efficacy rate of diarrhea and the incidence of adverse reactions) were assessed using risk ratio (RR) and 95% confidence intervals (CI). Continuous variables (e.g., the duration of diarrhea and length of hospital stay) were assessed using the standard mean difference (SMD) and 95% CI.
Handling of data loss
If there were missing or unclear data in any of the studies, the original author was contacted to obtain the data. If the data could not be obtained, the study was excluded.
Measurement of heterogeneity
The I2 statistic and Q test were used to measure the degree of heterogeneity. An I2 value >50% or a P value <0.1 indicated statistically significant heterogeneity.
Publication bias analysis
Funnel plots were used to represent publication bias.
Statistical analysis
Stata 16.0 software (StataCorp, TX, USA) was used as the analysis tool for this study, with results presented as forest plots.
Heterogeneity investigation and grouping
Heterogeneity was investigated by analyzing subgroups to examine the effect of different geographical regions and probiotic intervention methods on the results. If the source of the heterogeneity was not identified, a general descriptive analysis was performed.
Sensitivity analysis
Sensitivity analyses were performed using the sensitivity analysis tool provided by Stata 16.0.
Results
Literature search results
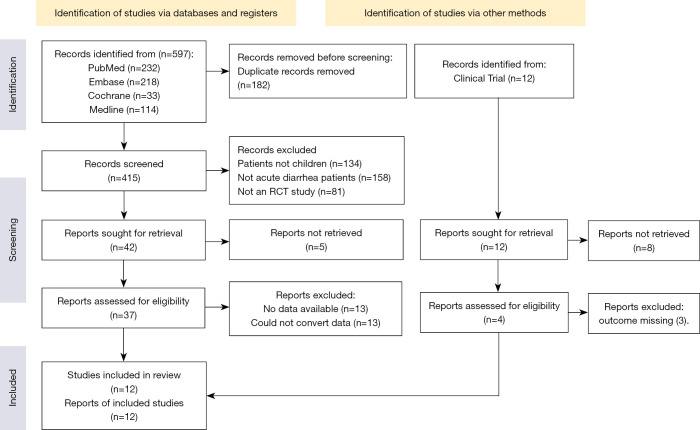
Figure 1 shows the results of the literature search and the screening process.
Figure 1.
Literature screening flow chart. RCT, randomized controlled trial.
Basic characteristics of the included RCTs
Twelve articles (8-19) with 744 patients were included in the current meta-analysis and were published between 2010 and 2020. Three studies (12,15,17) used combined probiotics for the treatment method, with 2–5 collective probiotic species. The remaining studies used a single probiotic, such as Lactobacillus reuteri, Lactobacillus rhamnosus, Lactobacillus casei, Saccharomyces cerevisiae, and Lactobacillus acidophilus, as shown in Table 1.
Table 1. Basic study characteristics, subjects, intervention methods, and outcome indicators of included RCTs.
| Author | Study type | Location | Age, years | Group | Number of subjects | Gender (M:F) | Intervention methods | Outcome indicators |
|---|---|---|---|---|---|---|---|---|
| Dinleyici EC 2015, (8) | Multicenter, randomized, single-blinded, case-control clinical trial | Turkey | 3–5 | E | 29 | 20:9 | (Lactobacillus reuteri DSM 17938) + ORS | (a)(b) |
| C | 31 | 22:9 | ORS | (a)(b) | ||||
| Maragkoudaki M 2018, (9) | Randomized, double-blind, placebo-controlled trial | Greece | 0.5–3 | E | 28 | 21:7 | (Lactobacillus reuteri DSM 17938) + ORS + Zinc | |
| C | 23 | 16:5 | ORS + Zinc | |||||
| Sindhu KN 2014, (10) | Randomized, double-blind, placebo-controlled trial | India | 0.5–5 | E | 65 | 42:23 | Lactobacillus rhamnosus GG | (a) |
| C | 59 | 34:25 | Placebo | |||||
| Lai HH 2019, (11) | Randomized, case-controlled study | Taiwan | 0.5–6 | E | 42 | 24:18 | Lactobacillus casei variety | (b) |
| C | 39 | 22:17 | Placebo | |||||
| Freedman SB 2018, (12) | Randomized, double-blind, placebo-controlled trial | Canada | 3–6 | E | 440 | 243:197 | L. rhamnosus R0011 and L. helveticus R0052 | (a)(c) |
| C | 437 | 252:185 | Placebo | |||||
| Mourey F 2020, (13) | Multicenter, randomized, single-blinded, case-control clinical trial | India | 0.5–6 | E | 49 | 23:26 | S. boulardii CNCM I-3799 | (a)(b)(c) |
| C | 51 | 23:28 | Placebo | |||||
| Hong Chau TT 2018, (14) | Randomized, double-blind, placebo-controlled trial | Vietnam | 0.5–5 | E | 150 | 101:49 | Lactobacillus acidophilus | (a)(d) |
| C | 150 | 98:52 | Placebo | |||||
| Dinleyici EC 2013, (15) | Single-blinded randomized study | Turkey | 0.5–10 | E | 113 | 70:43 | Synbiotic: Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Enterococcus faecium | (a)(d) |
| C | 96 | 46:50 | Placebo | |||||
| Riaz M 2012, (16) | Double-blind, randomized controlled trial | India | 0.5–5 | E | 54 | 32:22 | Saccharomyces boullardi | (a) |
| C | 54 | 30:24 | Placebo | |||||
| Chen K 2020, (17) | Multicenter, randomized, open-label, parallel-group, controlled | China | 1–3 | E | 96 | 45:51 | B. lactis Bi-07, L. rhamnosus HN001, and L. acidophilus NCFM | (a)(b)(d) |
| C | 98 | 51:47 | Placebo | |||||
| Corrêa NB 2011, (18) | Double-blind, placebo-controlled study | Brazil | 0.5–4 | E | 90 | 47:43 | S boulardii | (b) |
| C | 86 | 51:35 | Placebo | |||||
| Aggarwal SM 2014, (19) | Double-blind, randomized, placebo-controlled trial | India | 0.5–5 | E | 100 | 57:43 | Lactobacillus rhamnosus GG | (a)(b)(d) |
| C | 100 | 52:48 | Placebo |
Indicators: (a) duration of diarrhea; (b) 2-day treatment efficacy rate of diarrhea; (c) adverse events; (d) hospitalization days. RCT, randomized controlled trial; E, experiment; C, control; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen; ORS, oral rehydration solution; GG, first letters of the surnames of the founder Sherwood Gorbach and Barry Goldin.
Risk-of-bias assessment for included studies
The Cochrane Handbook for Systematic Reviews of Interventions was used to evaluate the included studies. All studies mentioned randomized sequence grouping. Only one study (15) failed to mention allocation concealment. All studies reported the blinding method (single-blind or double-blind) and whether blinding was used to evaluate results. The drop-out cases were described in detail. No selective report or other bias was found. The overall quality was excellent, as shown in Table 2.
Table 2. Risk of bias and quality assessment based on the Cochrane Handbook for Systematic Reviews of Interventions.
| Study | Random sequence generation | Classification hiding | Blind method | Data integrity | Optional reporting | Other bias | Quality |
|---|---|---|---|---|---|---|---|
| Dinleyici EC 2015, (8) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Maragkoudaki M 2018, (9) | Low | Low | Low (double blind) | Low | Low | Low | A |
| Sindhu KN 2014, (10) | Low | Low | Low (double blind) | Low | Low | Low | A |
| Lai HH 2019, (11) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Freedman SB 2018, (12) | Low | Low | Low (double blind) | Low | Low | Low | A |
| Mourey F 2020, (13) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Hong Chau TT 2018, (14) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Dinleyici EC 2013, (15) | Low | Unclear | Low (double blind) | Low | Low | Low | B |
| Riaz M 2012, (16) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Chen K 2020, (17) | Low | Low | Low (single-blind) | Low | Low | Low | A |
| Corrêa NB 2011, (18) | Low | Low | Low (double blind) | Low | Low | Low | A |
| Aggarwal SM 2014, (19) | Low | Low | Low (double blind) | Low | Low | Low | A |
Analysis of intervention effects
Duration of diarrhea (hours)
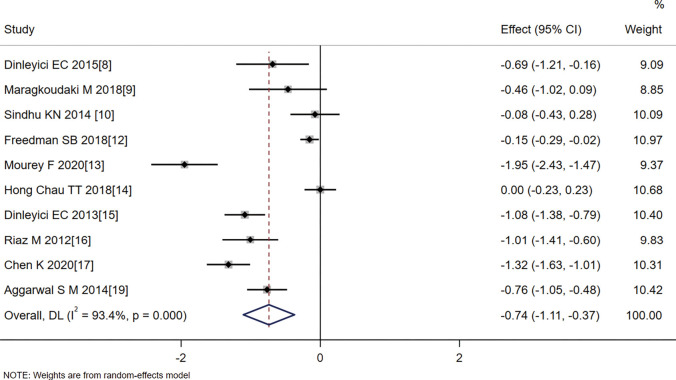
The meta-analysis showed that the duration of diarrhea in the probiotics group was shorter than that in the control group (SMD =−0.74, 95% CI: −1.11 to −0.37, Z=−3.935, P=0.000). As heterogeneity was identified amongst the articles (I2=93.4%, P=0.000), the analysis used a random effects model (Figure 2).
Figure 2.
Effect of probiotic treatment on duration of diarrhea.
The 2-day treatment efficacy rate for diarrhea (%)
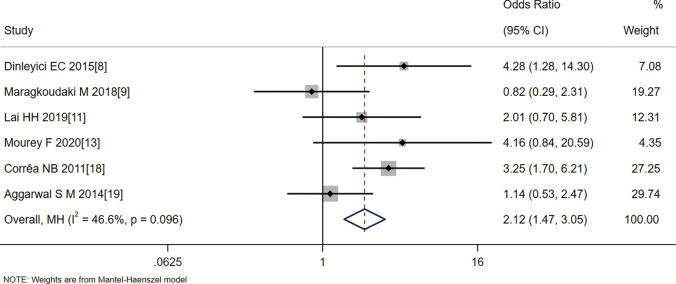
Six articles (8,9,11,13,18,19) reported changes in diarrhea in children 2 days after the treatment intervention, and the 2-day treatment efficacy rate for diarrhea in the probiotics group was greater than in the control group (OR =2.12, 95% CI: 1.47–3.05, Z=3.998, P=0.000). Statistical heterogeneity was evident between the articles (I2=46.6%, P=0.096) (Figure 3).
Figure 3.
Effect of probiotic treatment on the 2-day treatment efficacy rate for diarrhea.
Hospitalization (days)
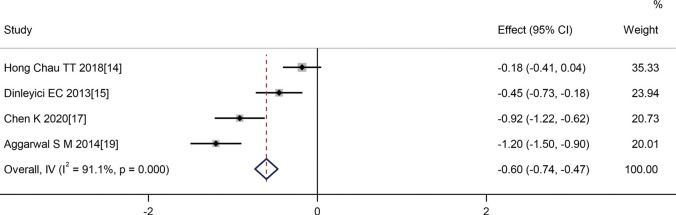
Four articles (14,15,17,19) reported comparisons in the length of hospital stay for children following treatment intervention. The length of hospital stay in the probiotics group was shorter than that of the control group (SMD =−0.60, 95% CI: −0.74 to −0.47, Z=−8.781, P=0.000). Statistical heterogeneity was evident between the studies (I2=91.1%, P=0.000) (Figure 4).
Figure 4.
Effect of probiotic therapy on the length of hospital stay.
Subgroup analysis
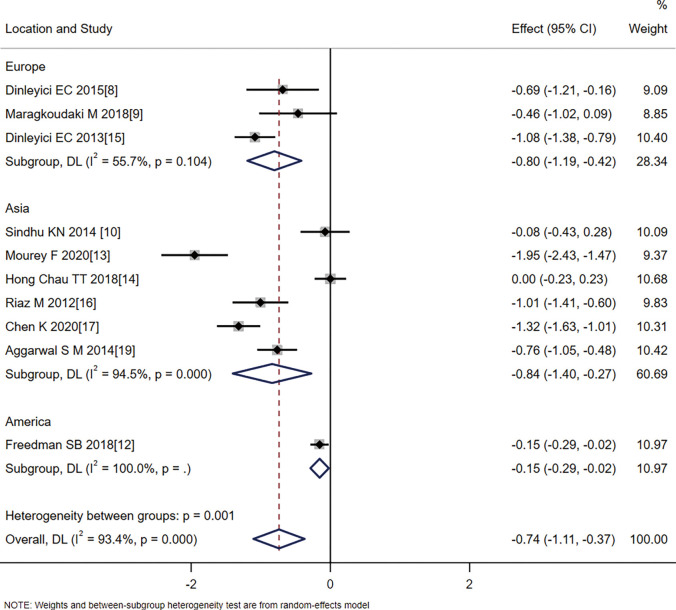
In the duration of diarrhea analysis, patients were further divided into three subgroups according to region: a European group (SMD =−0.80, 95% CI: −1.19 to −0.42, Z=−4.061, P=0.000), an Asian group (SMD =−0.84, 95% CI: −1.40 to −0.27, Z=−2.901, P=0.004), and an American group (SMD =−0.15, 95% CI: −0.29 to −0.02, Z=−2.284, P=0.022). The results from the three subgroups showed that probiotics significantly reduced the duration of diarrhea symptoms in children. However, heterogeneity within the subgroups also existed, which suggested that the source of heterogeneity was not related to the region where the study was conducted (Figure 5).
Figure 5.
Analysis of the effect of probiotics on the duration of diarrhea by location.
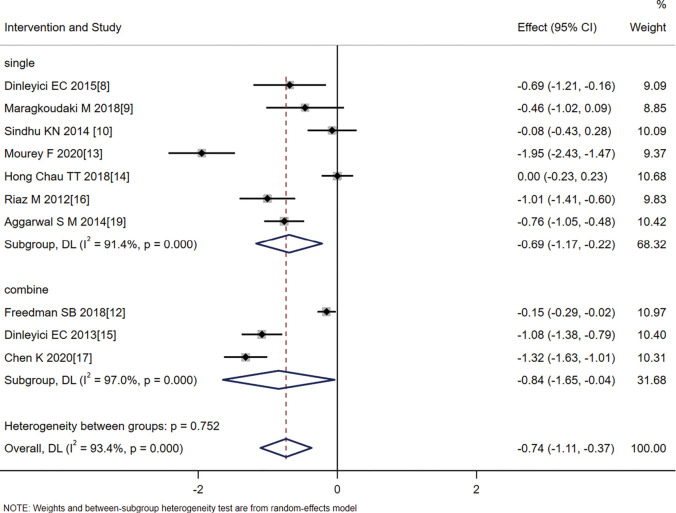
The patients were further divided into two subgroups: single probiotics and synbiotics. As shown in Figure 6, the effect size for single probiotics was SMD =−0.69, 95% CI: −1.17 to −0.220, Z=−2.867, P=0.006, while the effect size for combined probiotics was SMD =−0.84, 95% CI: −1.65 to −0.04, Z=−2.059, P=0.040. Heterogeneity remained in the two subgroups, suggesting that the intervention methods of combined probiotics or single probiotics were not the source of the heterogeneity; the effect size of the combined probiotics was less than that of probiotics alone, suggesting that the treatment effect of combined probiotics was superior, but this result will require further verification with larger sample sizes.
Figure 6.
Analysis of the effect of probiotics on the duration of diarrhea according to whether probiotics were combined.
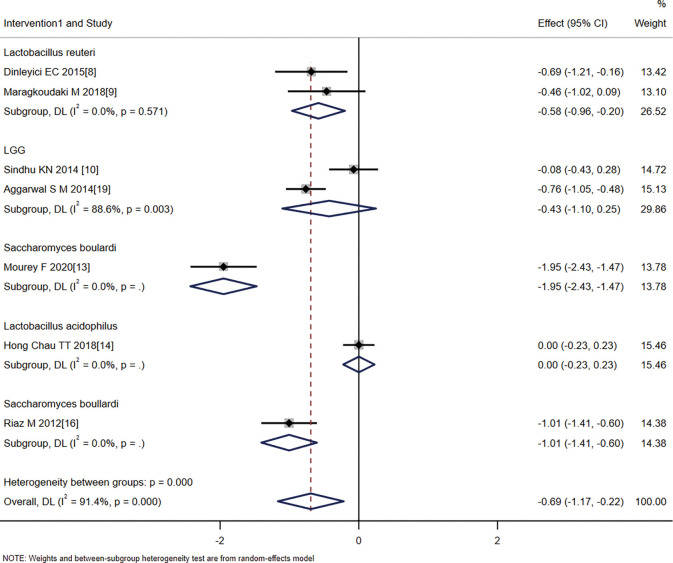
The single probiotics were further divided into four subgroups with the following effect sizes: Lactobacillus reuteri (SMD =−0.58, 95% CI: −0.96 to −0.20, Z=−2.995, P=0.003), Lactobacillus rhamnosus (LGG) (SMD =−0.43, 95% CI: −1.10 to 0.25, Z=−1.244, P=0.213), Saccharomyces boulardi (SMD =−1.28, 95% CI: −2.58 to −0.01, Z=−3.108, P=0.002), and Lactobacillus acidophilus (SMD =0, 95% CI: −0.23 to 0.23, Z=0.000, P=1.000). These results suggest that the treatment effect of L. rhamnosus and L. acidophilus was not statistically significant, while the treatment effect of L. reuteri and S. boulardii was statistically significant (Figure 7).
Figure 7.
Analysis of the effect of probiotics on the duration of diarrhea according to the probiotic species.
Safety analysis
None of the 12 included articles reported serious adverse effects that were significantly related to the intervention method.
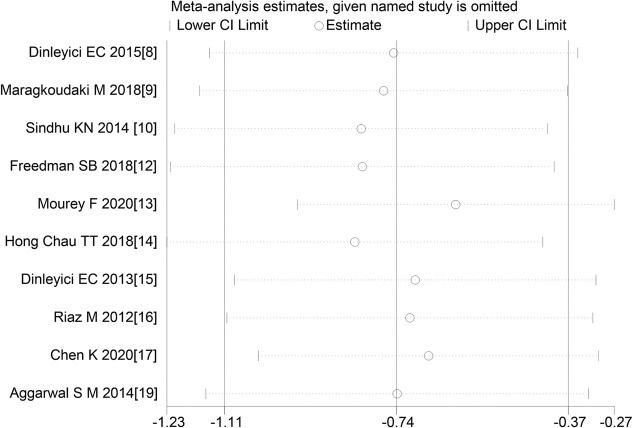
Sensitivity analysis
The sensitivity analysis showed that the study results of 10 articles had similar distributions on both sides and good stability, as shown in Figure 8.
Figure 8.
Sensitivity analysis.
Analysis of publication bias
The funnel plot of the analysis of diarrhea rate indicators after 2 days of treatment showed that the left and right distributions of the six articles were asymmetric, suggesting that there may be publication bias, as shown in Figure 9.
Figure 9.
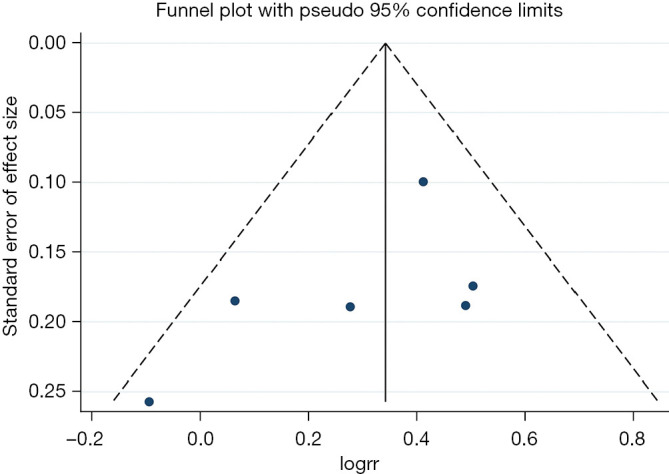
Funnel plot analysis of efficacy rate.
Discussion
The results of this meta-analysis showed that the addition of probiotics to the basic treatment of acute diarrhea in children shortened the duration of diarrhea and the length of hospital stay. These findings are consistent with the results of the study by Di and Gai (20). The novel finding of this study arose from our analysis contained the 2-day treatment effects, which showed that the severity of diarrhea in patients who had received probiotics was significantly reduced after 2 days of treatment. Our results revealed that probiotics can improve therapeutic efficacy and shorten the treatment time. One study (8) included in the current meta-analysis reported that after 5 days of treatment, diarrhea in both the experimental and control groups was 0%, indicating complete resolution of acute diarrhea in both groups. This suggests that the addition of probiotics did not enhance basic treatment (nutritional therapy, water, and electrolyte supplementation) in terms of the final overall clinical cure rate. However, probiotics indeed were shown to accelerate the rate of diarrhea recovery and shorten the treatment time, which remains an important factor in reducing children’s pain caused by diarrhea. In this study, the improvements in stool frequency and vomiting symptoms following treatment were not analyzed due to inconsistent reporting in the included RCTs. These two issues have been addressed in a previous meta-analysis (21). Improvements in abdominal pain symptoms following probiotic use should be the focus of future RCT studies to further clarify the role of probiotics in reducing children’s pain. A study by Phavichitr et al. (22) also pointed out that probiotics can shorten the length of hospital stay and thus reduce medical costs for children, but this issue was not addressed explicitly in the current study.
In this meta-analysis, the RCTs were grouped to explore the source of heterogeneity. However, statistically significant heterogeneity remained whenever the studies were grouped by region or by type of probiotic. The source of heterogeneity might be related to a mixture of multiple factors, such as patient ethnicity, age, gender (23), onset time, and severity of the disease. Although there was heterogeneity among the studies, the random-effects model analysis showed that probiotics have the same efficacy regardless of ethnicity or geographical location. After dividing the studies into two subgroups according to whether patients received single probiotics or synbiotics, the results showed that the effect size of combining probiotics to shorten the duration of diarrhea was smaller than that for single probiotic use, suggesting that the therapeutic effect of combining probiotics was superior. However, the efficacy of both needs to be verified by further RCT studies. After grouping the studies by individual probiotic species, the results showed that the treatment effects of L. rhamnosus and L. acidophilus were not statistically significant, while the treatment effects of L. reuteri and S. cerevisiae boulardii were statistically significant. One study (24) reported that the use of single Lactobacillus rhamnosus had no significant effect on the treatment of acute enteritis in children; another study (25) reported that Lactobacillus reuteri improved the length of hospital stay but had no significant improvement in diarrhea symptoms compared with placebo. This suggests that the use of single probiotics has limited efficacy, while the use of combined probiotics is more helpful for the recovery of the gut microbial environment. One RCT included in this study used a combination of Lactobacillus rhamnosus and Lactobacillus reuteri (12), another used the mixed probiotics Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, and Enterococcus faecium (15), and a further study used a combination of Bifidobacterium lactis, Lactobacillus rhamnosus, and Lactobacillus acidophilus (17), all of which achieved better efficacy than single probiotic use.
In this meta-analysis, 12 RCT studies were included, all published in the last decade [2010–2020]. We excluded studies with confounded treatment methods, such as combined treatment with probiotics and yogurt (26), or studies with ineligible study subjects, such as Ugandan children with severe malnutrition (27,28). In the risk-of-bias analysis, all studies described the random sequence generation and blinding method in detail, and only one study failed to describe the allocation concealment (15). All studies provided detailed descriptions of drop-out cases during follow-up, so the risk of bias was small, and the quality of all studies was high. The sensitivity analysis showed stable results, but the publication bias analysis showed an uneven distribution on both sides, suggesting possible publication bias. It should be noted that in a double-blind, randomized trial by Hegar et al. (29), probiotic food supplements did not shorten the duration of acute infectious diarrhea compared with oral rehydration and zinc. This result suggests that some studies may be biased in their reporting and may potentially exaggerate the efficacy of probiotics. Therefore, more RCT studies with well-matched baseline data for patients should be included in clinical practice to explore this topic further.
Conclusions
In summary, the addition of probiotics in the treatment of acute diarrhea in children can shorten the duration of diarrhea, increase the therapeutic effect after 2 days of treatment, and shorten the length of hospitalization. However, because of the possibility of publication bias in this meta-analysis, more high-quality RCT studies in clinical practice are needed to verify the current findings and continue the exploration of this topic.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-511
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-511). The authors have no conflicts of interest to declare.
(English Language Editor: D. Fitzgerald)
References
- 1.Sarker SA, Sultana S, Reuteler G, et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016;4:124-37. 10.1016/j.ebiom.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med 2011;364:105-15. 10.1056/NEJMoa0912254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valencia-Rodríguez A, Aquino-Matus J, Vera-Barajas A, et al. , Méndez-Sánchez N. New therapeutic options for bile acid malabsorption diarrhea. Ann Transl Med 2019;7:695. 10.21037/atm.2019.09.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz AB, Ali M, Basunia AH, et al. Impact of vaccination on the risk factors for acute rotavirus diarrhea: An analysis of the data of a cluster randomized trial conducted in a rural area of Bangladesh. Vaccine 2020;38:2190-7. 10.1016/j.vaccine.2020.01.041 [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeldt V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J 2002;21:411-6. 10.1097/00006454-200205000-00012 [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Lu P, Li MX, et al. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine (Baltimore) 2019;98:e16618. 10.1097/MD.0000000000016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J) 2015;91:392-6. 10.1016/j.jped.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 9.Maragkoudaki M, Chouliaras G, Moutafi A, et al. Efficacy of an Oral Rehydration Solution Enriched with Lactobacillus reuteri DSM 17938 and Zinc in the Management of Acute Diarrhoea in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018;10:1189. 10.3390/nu10091189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sindhu KN, Sowmyanarayanan TV, Paul A, et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2014;58:1107-15. 10.1093/cid/ciu065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai HH, Chiu CH, Kong MS, et al. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019;11:1150. 10.3390/nu11051150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman SB, Williamson-Urquhart S, Farion KJ, et al. Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. N Engl J Med 2018;379:2015-26. 10.1056/NEJMoa1802597 [DOI] [PubMed] [Google Scholar]
- 13.Mourey F, Sureja V, Kheni D, et al. A Multicenter, Randomized, Double-blind, Placebo-controlled Trial of Saccharomyces boulardii in Infants and Children With Acute Diarrhea. Pediatr Infect Dis J 2020;39:e347-51. 10.1097/INF.0000000000002849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Chau TT, Minh Chau NN, Hoang Le NT, et al. A Double-blind, Randomized, Placebo-controlled Trial of Lactobacillus acidophilus for the Treatment of Acute Watery Diarrhea in Vietnamese Children. Pediatr Infect Dis J 2018;37:35-42. 10.1097/INF.0000000000001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinleyici EC, Dalgic N, Guven S, et al. The effect of a multispecies synbiotic mixture on the duration of diarrhea and length of hospital stay in children with acute diarrhea in Turkey: single blinded randomized study. Eur J Pediatr 2013;172:459-64. 10.1007/s00431-012-1903-5 [DOI] [PubMed] [Google Scholar]
- 16.Riaz M, Alam S, Malik A, et al. Efficacy and safety of Saccharomyces boulardii in acute childhood diarrhea: a double blind randomised controlled trial. Indian J Pediatr 2012;79:478-82. 10.1007/s12098-011-0573-z [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Xin J, Zhang G, et al. A combination of three probiotic strains for treatment of acute diarrhoea in hospitalised children: an open label, randomised controlled trial. Benef Microbes 2020;11:339-46. 10.3920/BM2020.0046 [DOI] [PubMed] [Google Scholar]
- 18.Corrêa NB, Penna FJ, Lima FM, et al. Treatment of acute diarrhea with Saccharomyces boulardii in infants. J Pediatr Gastroenterol Nutr 2011;53:497-501. 10.1097/MPG.0b013e31822b7ab0 [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal S, Upadhyay A, Shah D, et al. Lactobacillus GG for treatment of acute childhood diarrhoea: an open labelled, randomized controlled trial. Indian J Med Res 2014;139:379-85. [PMC free article] [PubMed] [Google Scholar]
- 20.Di JB, Gai ZT. Protective efficacy of probiotics on the treatment of acute rotavirus diarrhea in children: an updated meta-analysis. Eur Rev Med Pharmacol Sci 2020;24:9675-83. [DOI] [PubMed] [Google Scholar]
- 21.Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010;(11):CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phavichitr N, Puwdee P, Tantibhaedhyangkul R. Cost-benefit analysis of the probiotic treatment of children hospitalized for acute diarrhea in Bangkok, Thailand. Southeast Asian J Trop Med Public Health 2013;44:1065-71. [PubMed] [Google Scholar]
- 23.Lönnermark E, Lappas G, Friman V, et al. Effects of probiotic intake and gender on nontyphoid Salmonella infection. J Clin Gastroenterol 2015;49:116-23. 10.1097/MCG.0000000000000120 [DOI] [PubMed] [Google Scholar]
- 24.Schnadower D, Sapien RE, Casper TC, et al. Association between Age, Weight, and Dose and Clinical Response to Probiotics in Children with Acute Gastroenteritis. J Nutr 2021;151:65-72. 10.1093/jn/nxaa313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szymański H, Szajewska H. Lack of Efficacy of Lactobacillus reuteri DSM 17938 for the Treatment of Acute Gastroenteritis: A Randomized Controlled Trial. Pediatr Infect Dis J 2019;38:e237-42. 10.1097/INF.0000000000002355 [DOI] [PubMed] [Google Scholar]
- 26.Eren M, Dinleyici EC, Vandenplas Y. Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non-bloody diarrhea in children: a randomized, controlled, open label study. Am J Trop Med Hyg 2010;82:488-91. 10.4269/ajtmh.2010.09-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grenov B, Namusoke H, Lanyero B, et al. Effect of Probiotics on Diarrhea in Children With Severe Acute Malnutrition: A Randomized Controlled Study in Uganda. J Pediatr Gastroenterol Nutr 2017;64:396-403. 10.1097/MPG.0000000000001515 [DOI] [PubMed] [Google Scholar]
- 28.Castro-Mejía JL, O'Ferrall S, Krych Ł, et al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 2020;11:855-67. 10.1080/19490976.2020.1712982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegar B, Waspada IM, Gunardi H, et al. A double blind randomized trial showing probiotics to be ineffective in acute diarrhea in Indonesian children. Indian J Pediatr 2015;82:410-4. 10.1007/s12098-014-1408-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as