Abstract
This cross-sectional study evaluates changes in the rate of germline BRCA testing among patients with ovarian cancer between 2008 and 2018 and analyzes factors associated with testing rates.
Introduction
Approximately 15% of patients with ovarian cancer have a germline BRCA (gBRCA) variation,1 which has important implications, including increased sensitivity to platinum-based chemotherapy and poly(ADP-ribose) polymerase inhibitors and improved survival.2 Testing first-degree relatives is also cost-effective cancer prevention.3 Since 2010, guidelines have recommended universal testing in ovarian cancer. However, testing rates are reportedly between 10% and 30%,4 and few studies have examined commercially insured populations or identified patient-, physician-, and practice-level characteristics associated with testing rates.
Methods
Using data from a large national commercial insurer, this cross-sectional study included 12 989 patients with claims for ovarian, fallopian, or primary peritoneal cancers and a biopsy or surgery between 2008 and 2018. We restricted the cohort to patients with a biopsy or surgery and carboplatin or cisplatin within 6 months. We excluded those without surgery or outpatient visits, with less than 12 months of continuous insurance, with missing zip codes, or who were younger than 18 years (eFigure in the Supplement). We attributed patients to practices and physicians using outpatient evaluation and management claims with a diagnosis 6 months or less from the first outpatient claim for chemotherapy (eAppendix in the Supplement).5 The Harvard Medical School Committee on Human Studies deemed the study exempt from review and the requirement for informed consent because the study was a secondary analysis of previously collected data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The primary outcome was gBRCA testing using gene-specific and methodology-based procedure codes (eTable in the Supplement). Secondary outcomes included timeliness (ie, ≤6 months from biopsy/surgery) and median time from first chemotherapy claim to testing. We used χ2 tests and linear regression to assess patient, physician, and practice characteristics associated with outcomes. A 2-sided P ≤.05 was considered statistically significant. Analyses were performed using SAS statistical software version 14.1 (SAS Institute).
Results
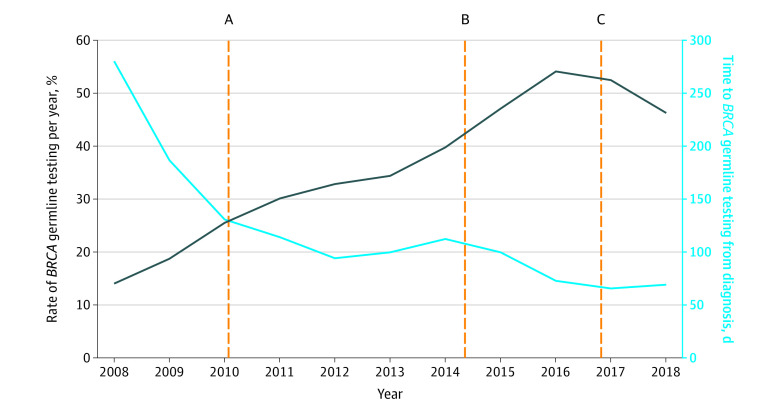
Among 3603 women with ovarian cancer (mean [SD] age, 57.0 [11.3] years), 1220 (33.9%) received gBRCA testing (Table). Testing rates increased from 14.7% (55 of 375 patients) in 2008 to 46.4% (96 of 207 patients) in 2018; the median time to testing decreased from 280.0 to 72.5 days (Figure). In adjusted analyses, testing was lower for older women (women ≥65 years vs <50 years: adjusted difference, −20.8 percentage points; 95% CI, −25.8 to −16.4 percentage points) and women with more comorbidities (Charlson Comorbidity Index score ≥2 vs 0: adjusted difference, −4.6 percentage points; 95% CI, −8.9 to −0.2 percentage points). Testing rates were similar among oncologists (medical vs gynecologic oncologist: adjusted difference, 1.5 percentage points; 95% CI, −1.8 to 4.7 percentage points) and lower in other physicians (other vs gynecologic oncologist: adjusted difference, −5.9 percentage points; 95% CI, −10.3 to −1.5 percentage points). Testing was higher at academic and NCI cancer centers compared with community practices (academic vs NCI: adjusted difference, 0.5 percentage points; 95% CI, −7.2 to 8.4 percentage points; community vs NCI: adjusted difference, −4.5 percentage points; 95% CI, −8.8 to −0.2 percentage points]). There was a statistically significant increase in testing over time (2018 vs 2008: adjusted difference, 32.0 percentage points; 95% CI, 24.4-39.7 percentage points), although rates remained below 50% for most years (Table). Results were similar for analyses of timeliness of gBRCA testing, which significantly improved from 2010 to 2018 (Table).
Table. Patient, Clinician, and Practice Characteristics Associated With Germline BRCA Testing Between 2008 and 2018.
| Characteristic | No. (%) (N = 3603) | BRCA testing | Time to testing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted No. (%) | Unadjusted P value | Adjusted % | Adjusted difference (95% CI), percentage point | Unadjusted No. (%) | Unadjusted P value | Adjusted % | Adjusted % point difference (95% CI) | ||
| All, % | 3603 (100) | 1220 (33.9) | NA | NA | NA | 867 (24.1) | NA | NA | NA |
| Patient | |||||||||
| Age, y | |||||||||
| <50 | 817 (22.7) | 332 (40.6) | <.001 | 42.6 | [Reference] | 241 (29.4) | <.001 | 30.9 | [Reference] |
| 50-59 | 1345 (37.6) | 515 (38.3) | 39.3 | −3.4 (−7.5 to 0.8) | 366 (27.2) | 27.6 | −3.3 (−7.1 to 0.4) | ||
| 60-64 | 656 (18.2) | 225 (34.3) | 36.0 | −6.6 (−11.4 to −1.7) | 159 (24.2) | 25.2 | −5.7 (−10.1 to −1.3) | ||
| ≥65 | 785 (21.8) | 148 (18.8) | 21.8 | −20.8 (−25.8 to −16.4) | 101 (12.9) | 14.4 | −16.6 (−20.5 to −12.6) | ||
| Charlson comorbidity score | |||||||||
| 0 | 2284 (63.5) | 800 (35.0) | .01 | 36.6 | [Reference] | 569 (24.9) | .12 | 25.9 | [Reference] |
| 1 | 845 (23.5) | 288 (34.1) | 36.2 | −0.4 (−4.0 to 3.2) | 201 (23.8) | 24.7 | −1.1 (−4.4 to 2.1) | ||
| ≥2 | 474 (13.2) | 132 (27.8) | 32.0 | −4.6 (−8.9 to −0.2) | 97 (20.5) | 22.9 | −2.9 (−6.9 to 1.0) | ||
| Education quartile, % HS or greatera | |||||||||
| First | 903 (25.1) | 270 (29.9) | .02 | 31.5 | [Reference] | 195 (21.6) | .06 | 22.2 | [Reference] |
| Second | 887 (24.4) | 303 (34.2) | 36.5 | 5.0 (0.2 to 9.7) | 203 (22.9) | 23.7 | 1.5 (−2.7 to 5.8) | ||
| Third | 919 (25.5) | 321 (34.9) | 34.6 | 3.1 (−2.5 to 8.6) | 231 (25.1) | 24.2 | 2.2 (−2.9 to 7.1) | ||
| Fourth | 894 (24.8) | 326 (36.5) | 37.1 | 5.6 (−0.8 to 11.9) | 238 (26.6) | 28.0 | 5.8 (0.1 to 11.6) | ||
| Income quartile, % <200% of the federal poverty levela | |||||||||
| First | 901 (25.0) | 323 (35.8) | .009 | 34.7 | [Reference] | 218 (24.2) | .006 | 21.8 | [Reference] |
| Second | 900 (25.0) | 334 (37.1) | 36.9 | 2.2 (−2.5 to 7.0) | 253 (28.1) | 27.6 | 5.8 (1.4 to 10.2) | ||
| Third | 901 (25.0) | 285 (31.6) | 33.4 | −1.3 (−6.9 to 4.2) | 200 (22.2) | 23.8 | 2.0 (−2.9 to 7.0) | ||
| Fourth | 901 (25.0) | 278 (30.9) | 34.7 | 0.0 (−6.7 to 6.6) | 196 (21.8) | 24.7 | 2.9 (−3.0 to 8.9) | ||
| Region | |||||||||
| West | 539 (15.0) | 191 (35.4) | .77 | 35.7 | [Reference] | 140 (26.0) | .69 | 25.1 | [Reference] |
| Midwest | 572 (15.9) | 187 (32.6) | 34.4 | −1.2 (−6.7 to 4.1) | 137 (24.0) | 24.9 | −0.2 (−5.1 to 4.8) | ||
| Northeast | 1004 (27.9) | 344 (34.3) | 34.3 | −1.3 (−6.5 to 3.7) | 242 (24.1) | 24.0 | −1.0 (−5.7 to 3.6) | ||
| South | 1488 (41.3) | 498 (33.5) | 35.2 | −0.5 (−5.1 to 4.1) | 348 (23.4) | 24.1 | −0.9 (−5.1 to 3.2) | ||
| Location | |||||||||
| Urban | 1716 (47.6) | 579 (33.7) | .37 | 35.7 | [Reference] | 419 (24.4) | .13 | 26.2 | [Reference] |
| Suburban | 1731 (48.0) | 596 (34.4) | 35.4 | −0.3 (−3.5 to 3.0) | 421 (24.3) | 25.6 | −0.6 (−3.6 to 2.3) | ||
| Rural | 156 (4.3) | 45 (28.8) | 33.8 | −1.9 (−9.1 to 5.3) | 27 (17.3) | 21.7 | −4.5 (−10.5 to 1.5) | ||
| Physician specialty | |||||||||
| Gynecologic oncologist | 1542 (42.8) | 543 (35.2) | .03 | 36.4 | [Reference] | 365 (23.6) | .08 | 24.1 | [Reference] |
| Medical oncologist | 1534 (42.6) | 524 (34.2) | 37.9 | 1.5 (−1.8 to 4.7) | 392 (25.6) | 27.9 | 3.9 (0.9 to 6.8) | ||
| Other physician | 527 (14.6) | 153 (29.0) | 30.5 | −5.9 (−10.3 to −1.5) | 110 (20.9) | 21.5 | −2.6 (−6.5 to 1.4) | ||
| Practice type | |||||||||
| NCI cancer center | 593 (16.5) | 236 (39.8) | .002 | 36.2 | [Reference] | 168 (28.3) | .03 | 25.5 | [Reference] |
| Academic | 177 (4.9) | 64 (36.2) | 36.8 | 0.5 (−7.2 to 8.4) | 44 (24.9) | 25.4 | 0.0 (−7.0 to 7.0) | ||
| Community | 2833 (78.6) | 920 (32.5) | 31.7 | −4.5 (−8.8 to −0.2) | 655 (23.1) | 22.6 | −2.8 (−6.8 to 1.1) | ||
| Year | |||||||||
| 2008 | 375 (10.4) | 55 (14.7) | <.001 | 12.6 | [Reference] | 23 (6.1) | <.001 | 3.7 | [Reference] |
| 2009 | 419 (11.6) | 81 (20.8) | 18.0 | 5.3 (0.2 to 10.5) | 39 (9.3) | 7.4 | 3.7 (0.0 to 7.5) | ||
| 2010 | 458 (12.7) | 119 (26.0) | 25.5 | 12.9 (7.6 to 18.1) | 78 (17.0) | 15.6 | 11.9 (7.7 to 16.2) | ||
| 2011 | 381 (10.6) | 116 (30.4) | 30.9 | 18.3 (12.5 to 24.1) | 87 (22.8) | 22.3 | 18.6 (13.8 to 23.5) | ||
| 2012 | 365 (10.1) | 121 (33.1) | 32.7 | 20.1 (14.2 to 26.0) | 92 (25.2) | 23.9 | 20.2 (15.2 to 25.3) | ||
| 2013 | 311 (8.6) | 108 (34.7) | 32.5 | 19.9 (13.6 to 26.3) | 74 (23.8) | 21.2 | 17.5 (12.2 to 22.9) | ||
| 2014 | 290 (8.0) | 116 (40.0) | 37.6 | 24.9 (18.3 to 31.6) | 77 (26.6) | 24.0 | 20.4 (14.7 to 26.0) | ||
| 2015 | 284 (7.9) | 134 (47.2) | 45.9 | 33.3 (26.5 to 40.0) | 101 (35.6) | 33.6 | 29.9 (23.9 to 35.9) | ||
| 2016 | 294 (8.2) | 159 (54.1) | 53.2 | 40.6 (33.8 to 47.3) | 116 (39.5) | 37.9 | 34.2 (28.1 to 40.3) | ||
| 2017 | 219 (6.1) | 115 (52.5) | 50.6 | 38.0 (30.5 to 45.4) | 98 (44.7) | 42.5 | 38.8 (31.8 to 45.8) | ||
| 2018 | 207 (5.7) | 96 (46.4) | 44.7 | 32.0 (24.4 to 39.7) | 82 (39.6) | 37.6 | 33.9 (26.8 to 41.0) | ||
Abbreviations: HS, high school; NA, not applicable; NCI, National Cancer Institute.
Census division and zip code–level measures used to identify proportion of residents who graduated from high school or above and the proportion of residents living in poverty.
Figure. Rate and Days to Testing From Diagnosis of Germline BRCA Testing Annually Between 2008 and 2018.
Vertical lines indicate landmark events related to germline BRCA testing. A, In 2010, National Comprehensive Cancer Network guidelines recommend universal genetic testing for all patients with ovarian cancer. B, In December 2014, the first poly(ADP-ribose) polymerase inhibitor receives US Food and Drug Administration approval with indication for treatment of recurrent disease in patients with germline BRCA who had received 3 or more prior lines of chemotherapy. C, On March 27, 2017, first poly(ADP-ribose) polymerase inhibitor receives US Food and Drug Administration approval for maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer (independent of BRCA).
Discussion
Despite unequivocal recommendations for universal genetic testing in ovarian cancer, only 33.9% of patients with commercial insurance were tested between 2008 and 2018—clear evidence it remains underused—and a minority received timely testing. In this study, medical and gynecologic oncologists had similar rates of testing, while other physicians tested less often, perhaps reflecting a lack of knowledge of guidelines. Nearly 80% of patients received care in community practices, where rates were statistically lower. Although independent practices often lack access to genetic counselors, women in this study had insurance coverage for in-person and telephonic counseling. Future studies should examine barriers to timely testing to identify scalable strategies for increasing testing, particularly for older women in community practices. Interventions targeting clinicians are essential because the absence of physician recommendations remains the largest barrier to testing.6 Study limitations include the use of biopsy/surgery for diagnosis date, limited sociodemographic characteristics, and the possibility that women received testing later.
eFigure 1. Flowchart of Cohort With Incident Diagnosis of Ovarian Cancer
eAppendix. Assigning Patients to Practice and Physicians
eTable. Codes Used for Cohort and Genetic Screening
References
- 1.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2(4):482-490. doi: 10.1001/jamaoncol.2015.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557-1565. doi: 10.1001/jama.2011.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80-87. doi: 10.1093/jnci/djn442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurian AW, Ward KC, Howlader N, et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol. 2019;37(15):1305-1315. doi: 10.1200/JCO.18.01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi S, Wright AA, Landrum MB, Zubizarreta J, Chernew ME, Keating NL. Multimodality cancer care and implications for episode-based payments in cancer. Am J Manag Care. 2019;25(11):537-538. [PubMed] [Google Scholar]
- 6.Armstrong J, Toscano M, Kotchko N, et al. Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT Study. JAMA Oncol. 2015;1(9):1251-1260. doi: 10.1001/jamaoncol.2015.3048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of Cohort With Incident Diagnosis of Ovarian Cancer
eAppendix. Assigning Patients to Practice and Physicians
eTable. Codes Used for Cohort and Genetic Screening