This case-control study evaluates the association of an elevated platelet count with risk of cancer by cancer type and time from a complete blood count test to cancer diagnosis.
Key Points
Question
Is a high platelet count associated with an increased risk of cancer?
Findings
In this nested case-control study of 8 917 187 Ontario residents who had 1 or more routine complete blood count tests performed, an elevated platelet count was associated with a diagnosis of cancer within 10 years after the blood test. The magnitude of the association varied by cancer type and time elapsed since the blood test.
Meaning
The findings suggest that a high platelet count is associated with increased cancer risk.
Abstract
Importance
Individuals with cancer often have an elevated platelet count at the time of diagnosis. The extent to which an elevated platelet count is an indicator of cancer is unclear.
Objective
To evaluate the association of an elevated platelet count with a cancer diagnosis.
Design, Setting, and Participants
This nested case-control study included Ontario residents enrolled in the provincial health insurance plan who had 1 or more routine complete blood count (CBC) tests performed between January 1, 2007, and December 31, 2017, with follow-up through December 31, 2018. Case patients were individuals with a new cancer diagnosis during the observation period. Eligible control individuals were cancer free before the date of diagnosis for a case patient to whom they were matched. One case patient was matched to 3 controls based on sex, age, and health care use patterns. Data were analyzed from September 24, 2020, to July 13, 2021.
Exposures
Case patients and controls were assigned to 1 of 5 exposure groups based on age- and sex-specific platelet count distributions in the control population: very low (≤10th percentile), low (>10th to 25th percentile), medium (>25th to <75th percentile), high (75th to <90th percentile), and very high (≥90th percentile).
Main Outcomes and Measures
Odds ratios (ORs) were estimated for specific cancer sites for each category of platelet count at intervals up to 10 years after a blood test.
Results
Of the 8 917 187 eligible Ontario residents with a routine CBC record available, 4 971 578 (55.8%) were women; the median age at the first CBC was 46.4 years (IQR, 32.5-59.5 years). Among individuals with a routine CBC record available, 495 341 (5.6%) received a diagnosis of first primary cancer during the 10-year observation period. The OR for a solid tumor diagnosis associated with a very high platelet count vs a medium platelet count in the 6-month period before the diagnosis was 2.32 (95% CI, 2.28-2.35). A very high platelet count was associated with colon (OR, 4.38; 95% CI, 4.22-4.54), lung (OR, 4.37; 95% CI, 4.22-4.53), ovarian (OR, 4.62; 95% CI, 4.19-5.09), and stomach (OR, 4.27; 95% CI, 3.91-4.66) cancers. Odds ratios attenuated with increasing time from CBC test to cancer diagnosis.
Conclusions and Relevance
In this nested case-control study, an elevated platelet count was associated with increased risk of cancer at several sites. Our findings suggest that an elevated platelet count could potentially serve as a marker for the presence of some cancer types.
Introduction
Patients with cancer often have an abnormally high platelet count at the time of diagnosis (thrombocytosis), defined as a platelet count greater than 450 × 109/L (to convert to ×103 per microliter, divide by 1.0).1 A normal platelet count falls between 150 and 450 × 109/L and varies with the age and sex of the individual.1,2 Several conditions that commonly cause an elevated platelet count include acute blood loss, infection, and inflammation.3 Solid tumor cancers can sometimes lead to an elevated platelet count to the extent that an undiagnosed cancer is often considered in the diagnostic workup of a patient with thrombocytosis.3 Cancer is believed to induce platelet formation through the release of interleukin 6, a proinflammatory cytokine that stimulates the production of thrombopoietin hormone.4 Elevated levels of thrombopoietin have a direct effect on increased platelet production. Excess levels of thrombopoietin in the blood stimulate megakaryocyte cell division in the bone marrow, which in turn leads to platelet formation.5
An elevated platelet count has been shown to be associated with short-term risk of cancer in the general population.6,7,8 Prospective studies evaluating platelet count and survival among patients with newly diagnosed cancer have also noted a high proportion of patients who presented with thrombocytosis.9,10,11,12 The excess risk associated with an elevated platelet count varies by cancer site but has been most studied for lung, colon, and gastric cancers. The full range of cancers associated with a high platelet count and whether risks associated with platelet counts within the high-normal range exist remain unclear. Furthermore, it is unclear whether the association between a high platelet count and cancer is transient or prolonged. Based on results of previous studies,6,7,8 a high platelet count may be a risk factor for developing cancer or, alternatively, a marker indicative of an undetected cancer. It is also not clear whether an increasing platelet count is a better indicator of a new cancer than is a high but steady platelet count.
We identified a cohort of adult residents in Ontario, Canada, who had 1 or more routine blood tests performed for a complete blood count (CBC) including platelet counts and subsequently received a diagnosis of cancer to assess the range of cancers associated with a high platelet count. We also examined whether an increasing platelet count is associated with an increased cancer risk.
Methods
Study Design, Population, and Data
Ontario is the most populous province in Canada, with a population of 14.5 million. Ontario residents are covered under the universal health insurance program, which includes coverage for primary care services, emergency visits, hospitalizations, and (among older adults) medication. This nested case-control study used data from ICES, a nonprofit organization that provides researchers with deidentified data that can be used for research purposes. ICES is a prescribed entity under §45 of Ontario’s Personal Health Information Protection Act, which allows for research conduct without a research ethics board review and without the need for informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for case-control studies.
ICES data include results of laboratory tests conducted in Ontario from January 2007 to present. The Ontario Laboratory Information System data set includes more than 85 million CBC test records, including those for 9.5 million (of the 14.5 million) Ontario residents. The CBC records include the date of laboratory analysis, the platelet count, and other standard blood parameters.
Incident cancers in Ontario are recorded in the Ontario Cancer Registry, which was started in January 1964. This study also used data on physician billing (Ontario Health Insurance Plan claims database), emergency department visits (National Ambulatory Care and Reporting System database), acute care hospitalizations (Discharge Abstract Database), and dispensed medications among adults aged 65 years or older (Ontario Drug Benefit Claims database). These datasets were linked using unique encoded identifiers and analyzed at ICES.
Construction of the Cohort
The nesting cohort consisted of 8 917 187 Ontario residents who had at least 1 routine CBC test ordered by a practicing physician in a community health setting from January 1, 2007, through December 31, 2017. Cohort entry date was the date of the first eligible CBC test. Patients with cancer before the cohort entry date were excluded. Patients were observed from the date of their first routine blood test to the first date of any cancer diagnosis, death from any cause, end of Ontario Health Insurance Plan eligibility, or the end of the observation period (December 31, 2018) (eFigure 1 in the Supplement). Details on the inclusion criteria, exclusion criteria, and the study cohort are available in eTables 1-3 in the Supplement.
Baseline Variables
Baseline information was obtained and updated at the time of each CBC test. We included information on general demographic characteristics, health services use, comorbidities and chronic conditions, medication use (among individuals aged ≥66 years), and additional CBC test results. The Johns Hopkins ACG System software, version 10,13 was used to obtain aggregate diagnosis groups and resource utilization bands.
Case Patients
Case patients were defined as individuals who received a cancer diagnosis after the date of cohort entry. Data on first primary cancer diagnoses during the observation period were captured from the Ontario Cancer Registry. The Ontario Cancer Registry is a validated cancer registry that provides information on the date of diagnosis, cancer site, and tumor-specific data, such as morphologic features, stage, grade, lymph node involvement, and for certain cancers, hormone receptor status.14 We restricted the interpretation of our findings to solid cancers other than liver cancer because both liver and hematologic cancers may have a direct effect on platelet count; thrombopoietin production occurs in the liver, and megakaryocyte production occurs in the bone marrow. For case patients, the index date was defined as the date of diagnosis of cancer.
Matching
We hard-matched 3 control individuals to each case patient. Each matched control was alive and cancer free on the date of diagnosis of the case patient (eFigure 1 in the Supplement). Case patients and controls were matched based on sex, calendar date of CBC test (±30 days), age (±2 years), years of coverage by the Ontario Health Insurance Plan (±2 years), and the patient’s resource utilization band. Incidence density sampling was used such that case patients could serve as potential controls at prior time points.
Exposure
We assigned a categorical value to each platelet count based on the percentile distribution for the cancer-free controls. Five mutually exclusive categories were created: very low (≤10th percentile), low (>10th to 25th percentile), medium (>25th to <75th percentile), high (75th to <90th percentile), and very high (≥90th percentile). To account for variation in platelet count by sex and age, we defined categories of platelet count using reference distributions that were standardized according to age and sex from the pool of control patients (eFigure 2 in the Supplement).
Statistical Analysis
Primary Analysis
We performed a series of (nested) matched case-control analyses to measure the association of platelet count with risk of cancer at various time intervals before the index date. Each matched quadruplet of case patients and controls (3:1) was assessed at 7 time intervals before the index date: 0 to 6 months, 6 to 12 months, 12 to 18 months, 18 to 24 months, 2 to 3 years, 3 to 5 years, and 5 to 10 years. Each case patient could contribute to up to 7 observations (1 for each time interval). If multiple routine CBC tests were performed within a given period, 1 was chosen at random. For each cancer site and for each time point, an odds ratio (OR) was estimated using conditional logistic regression. At each time point, the medium platelet count category was used as the reference group and ORs were estimated for very high, high, low, and very low counts compared with the reference group. Information on tumor stage was available for several sites. A subanalysis was conducted after stratifying cancers by stage for these sites. In a thrombocytosis sensitivity analysis, we assigned platelet levels using clinical cutoffs: thrombocytopenia (<150 ×109 platelets/L), normal level (150 to 450 ×109 platelets/L), and thrombocytosis (>450 ×109 platelets/L).
Secondary Analysis
As a secondary objective, we sought to assess whether a change in platelet count over time was associated with a diagnosis of cancer. To study this, we selected individuals who had 2 routine CBC tests recorded 9 to 15 months apart. If multiple CBC records were available in the 9- to 15-month period, 1 was selected at random. A second iteration of matching (using the same matching criteria) was done using this subset of individuals (n = 4 372 288 [49% of the primary cohort]). A difference in platelet count was measured by subtracting the first platelet count from the second platelet count. Sex- and age-standardized reference distributions for change in platelet count were created (eFigure 3 in the Supplement). Five categories were created to classify the change in platelet count: large decrease (≤10th percentile), small decrease (>10th to 25th percentile), no substantial change (>25 to <75th percentile [reference group]), small increase (75th to <90th percentile), and large increase (≥90th percentile). All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc). Data were analyzed from September 24, 2020, to July 13, 2021.
Results
Of the 8 917 187 eligible individuals with 1 or more routine CBC tests identified in Ontario during the accrual period, 4 971 578 (55.8%) were women; the median age at the first CBC was 46.4 years (IQR, 32.5-59.5 years) (Table 1). Of the entire cohort, 495 341 individuals (5.6%) received a cancer diagnosis during the observation period. We successfully matched 491 779 case patients with cancer (99.3%) to 3 controls in 1 or more predefined time intervals. Case patients were similar to controls with respect to their demographic information, health services use, medication use, and comorbidity variables (eTable 4 in the Supplement).
Table 1. Characteristics of the Study Cohort at the First Eligible Routine CBC Test.
| Description | Individuals (N = 8 917 187)a |
|---|---|
| Sex | |
| Female | 4 971 578 (55.8) |
| Male | 3 945 609 (44.2) |
| Age, y | |
| Mean (SD) | 47.0 (17.8) |
| Median (IQR) | 46.4 (32.5-59.5) |
| Residence location | |
| Urban | 8 084 848 (90.7) |
| Rural | 820 419 (9.2) |
| Missing | 11 920 (0.1) |
| Core primary care visits to general practitioner or family practitioner in previous 2 y, No. | |
| Mean (SD) | 2.8 (3.4) |
| Median (IQR) | 2 (1-3) |
| Rostered to family physician | 6 940 867 (77.8) |
| Comorbidities and chronic conditions | |
| Asthma | 796 810 (8.9) |
| Congestive heart failure | 172 576 (1.9) |
| COPD | 192 482 (2.2) |
| Hypertension | 2 139 804 (24.0) |
| Diabetes | 666 477 (7.5) |
| Kidney disease | 88 520 (1.0) |
| Chronic coronary syndrome | 321 278 (3.6) |
| Hemoglobin concentration, g/L | |
| Mean (SD) | 139.5 (14.9) |
| Median (IQR) | 140 (130-150) |
| Platelet count, 109/L | |
| Mean (SD) | 247.2 (64.5) |
| Median (IQR) | 241 (205-282) |
| Observation time, yb | |
| Mean (SD) | 6.8 (3.0) |
| Median (IQR) | 7.3 (4.4-9.3) |
| Routine CBC tests in observation period, No. | |
| Mean (SD) | 4.3 (6.3) |
| Median (IQR) | 3 (1-6) |
| Cancer diagnosisb,c | |
| Any | 492 691 (5.5) |
| Solid tumor | 429 222 (4.8) |
| Colon | 51 521 (0.6) |
| Lung | 56 724 (0.6) |
| Breastd | 65 721 (1.3) |
| Ovaryd | 7661 (0.2) |
| Cervicald | 3494 (0.1) |
| Endometriald | 17 101 (0.3) |
| Prostatee | 62 946 (1.6) |
| Thyroid | 21 478 (0.2) |
| Pancreas | 12 021 (0.1) |
| Stomach | 9195 (0.1) |
| Kidney | 14 063 (0.2) |
| Bladder | 23 344 (0.3) |
| Liver | 7696 (0.1) |
| Esophagus | 4712 (0.1) |
| Other gastrointestinal tract | 5255 (0.1) |
| Brain | 5724 (0.1) |
| Melanoma | 20 192 (0.2) |
| Head and neck | 13 363 (0.1) |
| Other | 27 011 (0.3) |
| Hematologic tumor | 63 469 (0.7) |
| Leukemia | 5154 (0.1) |
| Lymphoma | 33 827 (0.4) |
| Multiple myeloma | 8274 (0.1) |
| Other | 16 214 (0.2) |
Abbreviations: CBC, complete blood count; COPD, chronic obstructive pulmonary disease; OHIP, Ontario Health Insurance Plan.
SI conversion factors: To convert hemoglobin concentration to grams per deciliter, divide by 10.0; platelet count to 103 per microliter, divide by 1.0.
Data are presented as number (percentage) of individuals unless otherwise indicated.
Period of observation was from the first eligible CBC test to the earliest date of death, end of OHIP eligibility, or end of the observation period (December 31, 2018).
Data are from the Ontario Cancer Registry.
Women only.
Men only.
The mean platelet count at the most recent blood test was higher among case patients with cancer than among matched controls (245.7 × 109/L vs 237.0 × 109/L). Case patients diagnosed with a solid tumor were more likely to have a recent platelet count in the highest category compared with cancer-free controls (44 344 [19.5%] vs 65 626 [9.6%]). For blood samples obtained during the 6 months before a cancer diagnosis, the OR for any solid cancer associated with a very high platelet count (≥90th percentile) vs a medium platelet count (reference, >25th to <75th percentile) was 2.32 (95% CI, 2.28-2.35) (Table 2). The OR for this association attenuated with increasing time from blood test to cancer diagnosis (Table 2); the ORs for the very high platelet category were 1.41 (95% CI, 1.39-1.44) for 6 to less than 12 months before diagnosis, 1.20 (95% CI, 1.18-1.22) for 12 to less than 24 months before diagnosis, 1.15 (95% CI, 1.13-1.17) for 24 to less than 60 months before diagnosis, and 1.13 (95% CI, 1.10-1.15) for 60 to 120 months before diagnosis.
Table 2. Odds Ratios of Any Solid Tumor Diagnosis by Platelet Count Category and Time From Complete Blood Count Test to Cancer Diagnosisa.
| Platelet count percentile category by time to cancer diagnosisb | No. (%) | Odds ratio (95% CI)c | |
|---|---|---|---|
| Case patients | Control individuals | ||
| <6 mo | |||
| Very low | 19 161 (8.4) | 74 891 (11.0) | 0.87 (0.86-0.89) |
| Low | 27 308 (12.0) | 106 707 (15.6) | 0.87 (0.86-0.89) |
| Medium | 98 480 (43.3) | 336 232 (49.2) | 1 [Reference] |
| High | 38 372 (16.9) | 99 539 (14.6) | 1.32 (1.30-1.34) |
| Very high | 44 344 (19.5) | 65 626 (9.6) | 2.32 (2.28-2.35) |
| 6 to <12 mo | |||
| Very low | 14 870 (10.1) | 48 700 (11.0) | 0.95 (0.93-0.97) |
| Low | 20 716 (14.0) | 69 561 (15.7) | 0.93 (0.91-0.95) |
| Medium | 70 078 (47.4) | 219 039 (49.4) | 1 [Reference] |
| High | 23 209 (15.7) | 64 288 (14.5) | 1.13 (1.11-1.15) |
| Very high | 19 038 (12.9) | 42 145 (9.5) | 1.41 (1.39-1.44) |
| 12 to <24 mo | |||
| Very low | 22 086 (10.2) | 70 743 (10.9) | 0.95 (0.93-0.96) |
| Low | 32 018 (14.7) | 103 336 (15.9) | 0.94 (0.93-0.95) |
| Medium | 106 239 (48.9) | 322 348 (49.5) | 1 [Reference] |
| High | 32 952 (15.2) | 94 872 (14.6) | 1.05 (1.04-1.07) |
| Very high | 23 942 (11.0) | 60 412 (9.3) | 1.20 (1.18-1.22) |
| 24 to <60 mo | |||
| Very low | 27 116 (10.1) | 87 869 (10.9) | 0.93 (0.92-0.95) |
| Low | 40 852 (15.2) | 127 941 (15.9) | 0.97 (0.95-0.98) |
| Medium | 132 330 (49.4) | 400 393 (49.8) | 1 [Reference] |
| High | 40 363 (15.1) | 115 881 (14.4) | 1.05 (1.04-1.07) |
| Very high | 27 481 (10.2) | 72 342 (9.0) | 1.15 (1.13-1.17) |
| 60-120 mo | |||
| Very low | 15 744 (10.1) | 49 761 (10.7) | 0.96 (0.94-0.98) |
| Low | 23 750 (15.3) | 74 880 (16.1) | 0.96 (0.94-0.97) |
| Medium | 76 963 (49.5) | 232 828 (49.9) | 1 [Reference] |
| High | 23 340 (15.0) | 66 787 (14.3) | 1.06 (1.04-1.08) |
| Very high | 15 655 (10.1) | 42 100 (9.0) | 1.13 (1.10-1.15) |
Liver cancer was excluded.
Platelet count percentile categories were defined as follows: very low (≤10th percentile), low (>10th to 25th percentile), medium (>25th to <75th percentile), high (75th to <90th percentile), and very high (≥90th percentile).
P < .001 for all.
The ORs for the association of a high platelet count with a cancer diagnosis were greatest for patients with cancers of the colon, lung, ovary, and stomach (Figure 1). During the 0- to 6-month period before a cancer diagnosis, the ORs for the very high platelet count category were 4.38 (95% CI, 4.22-4.54) for colon cancer, 4.37 (95% CI, 4.22-4.53) for lung cancer, 4.62 (95% CI, 4.19-5.09) for ovarian cancer, and 4.27 (95% CI, 3.91-4.66) for stomach cancer (Figure 1 and eTable 5 in the Supplement). Significant associations were also observed for esophageal cancer (OR, 3.18; 95% CI, 2.81-3.60), other gastrointestinal tract cancers (OR, 3.10; 95% CI, 2.75-3.49), and kidney cancer (OR, 2.55; 95% CI, 2.38-2.74) (eFigure 4 in the Supplement). The associations attenuated with increasing time to diagnosis to varying degrees.
Figure 1. Odds Ratios of Cancer by Platelet Count Category and Time From Complete Blood Count Test to Cancer Diagnosis.
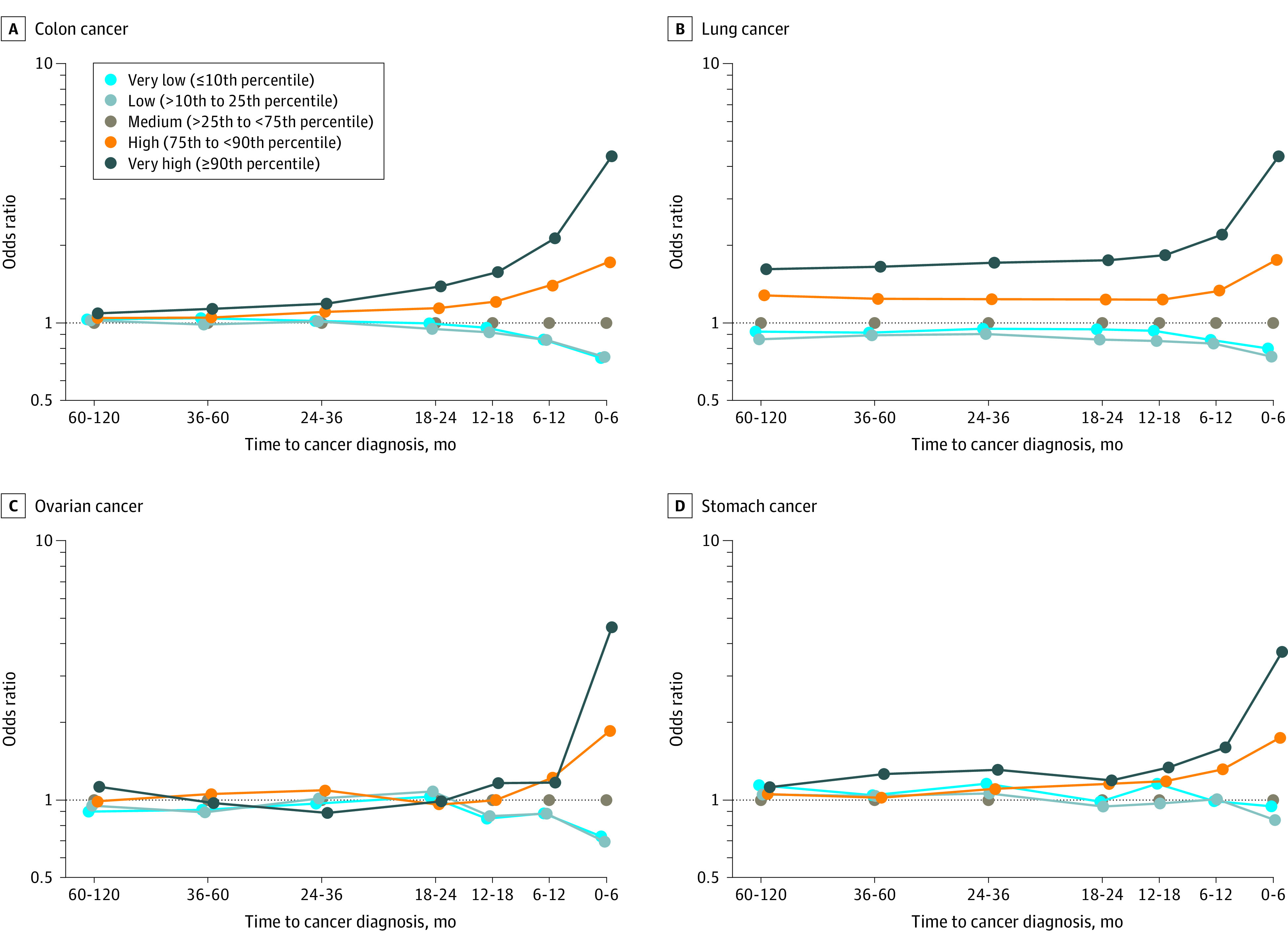
In addition, high platelet count was associated with risk of breast cancer (OR, 1.05; 95% CI, 1.01-1.10) and prostate cancer (OR, 1.24; 95% CI, 1.19-1.29) but was not associated with risk of melanoma (OR, 1.06; 95% CI, 0.97-1.15) or thyroid cancer (OR, 1.01; 95% CI, 0.94-1.09) (eFigure 4 in the Supplement). A low platelet count and a very low platelet count were associated with a decreased risk of breast and prostate cancer. In a sensitivity analysis, the ORs for the association of thrombocytosis and increased cancer risk were greatest for colon cancer, lung cancer, ovarian cancer, stomach cancer, esophageal cancer, and kidney cancer (eFigure 7 in the Supplement).
We also studied the associations between a high platelet count and risk of solid tumors by stage at diagnosis (when data were available). There was a significant association across all stages of colon cancer, but the OR for the association was greatest for metastatic disease (stage IV) (OR, 7.96; 95% CI, 7.26-8.72) (Figure 2). Data for the other cancer sites by stage are presented in eFigure 6 in the Supplement.
Figure 2. Odds Ratios of Colon Cancer by Platelet Count Category and Time From Complete Blood Count Test to Cancer Diagnosis by Cancer Stage.

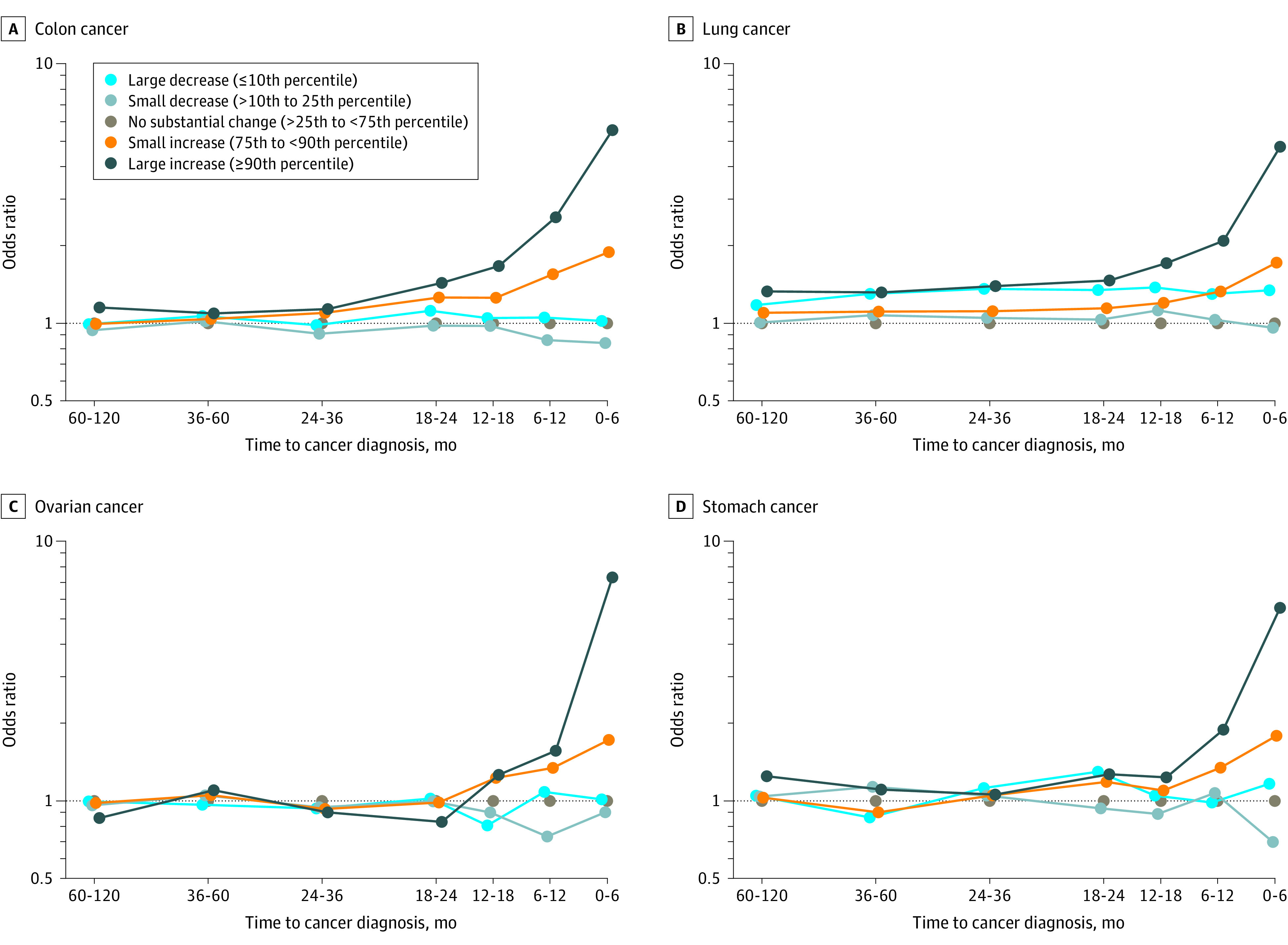
We also examined whether a substantial increase in platelet count (compared with a platelet count measured in the previous 9 to 15 months) was associated with an risk of cancer. Case patients diagnosed with a solid tumor were more likely to have a recent increase in platelet count (≥90th percentile) than were cancer-free controls (19 750[21.8%] vs 27 530 [10.2%]) (eTable 6 in the Supplement). A recent increase in the platelet count was associated with risk of colon cancer (OR, 5.52; 95% CI, 5.21-5.86), lung cancer (OR, 4.77; 95% CI, 4.51-5.04), ovarian cancer (OR, 7.23; 95% CI, 6.12-8.53), and stomach cancer (OR, 5.51; 95% CI, 4.82-6.29) (Figure 3 and eTable 7 in the Supplement). No associations were observed between a recent increase in the platelet count and breast cancer (OR, 1.01; 95% CI, 0.94-1.09), melanoma (OR, 1.01; 95% CI, 0.89-1.15), or thyroid cancer (OR, 0.97; 95% CI, 0.86-1.09) (eFigure 5 in the Supplement).
Figure 3. Odds Ratios of Cancer by Change in Platelet Count Category and Time From Complete Blood Count Test to Diagnosis.
Discussion
In this large, nested case-control study, we found that an elevated platelet count identified during a routine blood examination was associated with an increased risk of developing a range of solid tumors. The OR for the association was greatest for a diagnosis of cancer within 6 months of a blood test. For several cancer sites (lung, colon, stomach, esophagus, and kidney), a high platelet count was associated with a cancer diagnosis in the following 3 or more years. For lung cancer, a significant association was present 10 years before diagnosis. Long-term associations were also seen for kidney cancer and esophageal cancer. In contrast, for ovarian cancer, there was an association only in the 6 months before a diagnosis.
Overall, given the transient nature of the association with platelet count, our findings suggest that an elevated platelet count detected through routine blood examination may be a consequence of the presence of cancer rather than being a risk factor for the disease. The physiologic basis for the association is not clear but may be multifactorial. Platelets are produced in the bone marrow in response to thrombopoietin, which is upregulated by interleukin 6, primarily produced in the liver.15 It is possible that the increase in platelet count is a response to circulating factors produced by the cancer cells or is a local response to inflammation induced by the cancer cell mass. Various mechanisms have been proposed to explain the association between high platelet count and cancer, including the aggregation of cancer cells by platelets, increased extravasation or enhanced permeability of the basement membrane, and shielding cancer cells from immune attack in the bloodstream.16,17,18 Other possible mechanisms include iron deficiency, bleeding (among patients with colon cancer),19 abnormal platelet counts, and the infiltration of disseminated cancer cells in the bone marrow.20
Several studies have demonstrated an association between an elevated platelet count (thrombocytosis) and cancer risk. In general, these studies have either measured platelet counts at the time of diagnosis or had a short follow-up period subsequent to a CBC test.6,7,8 Pharmacoepidemiologic studies have further shown a lower incidence of certain cancer types among patients receiving platelet-inhibiting medications. For example, there is an established relationship between aspirin use (an antiplatelet drug) and decreased incidence of colon cancer.21,22,23 A protective effect of low-dose aspirin against ovarian cancer has also been suggested.24 Although antiplatelet medications inhibit platelet function as opposed to lowering the platelet count, the decreased incidence of cancer associated with aspirin use suggests the potential role functional platelets have in cancer risk.
Our study findings suggest that individuals with a high platelet count might be candidates for investigation for the presence of an occult cancer after other nonmalignant causes of an elevated platelet count have been ruled out. Of individuals who had a cancer diagnosed within 6 months after the blood test, 19.5% had a very high platelet count (top 10 percentile). In a sensitivity analysis, we observed similar findings, with an association with some cancers among individuals with thrombocytosis. Giannakeas and Narod25 recently reported an association between thrombocytosis and incident cancers using the same data from the present study. The findings of the present study suggest that platelet counts might be useful as a cancer screening tool alone or in combination with other cancer screening modalities, in particular spiral computed tomography for lung cancer, colonoscopy for colon cancer, and a cancer antigen 125 test or transvaginal ultrasonography for ovarian cancer. Novel screening tests that incorporate cell-free DNA and methylation signatures have shown promising results in identifying site-specific cancers.26 Platelet count could potentially be used as an affordable screening test to improve the predictive value of other screening modalities. Particular attention should be given to individuals who have an increasing platelet count (Figure 3). A relative increase in platelet count that exceeded 1.5 SDs (ie, ≥90th percentile) was associated with risk for many cancer types.
The associations found in this study were based on a single marker (platelet count) as a 1-time measurement and as a change over time. In future studies, we plan to investigate the clinical utility of platelet count testing as a screening test. We will incorporate additional blood count elements in combination with platelet count in a model to maximize predictive ability.
Limitations
This study has limitations. The extent to which unmeasured confounders influenced the association of platelet count with cancer diagnosis is unclear. For lung cancer, we observed a prolonged association with elevated platelet count throughout the 10-year observation period (Figure 1). Smoking status was not available through administrative data sources. Platelet counts have been shown to differ among smokers and nonsmokers.27 Body mass index was also not available and has been shown to be associated with platelet counts among women.28 Additional variables that are likely to influence platelet count include alcohol consumption, family history, and genetics. These variables were not attainable in our study because of the limitations of administrative health data. However, given the transient association observed between platelet count and a cancer diagnosis in this study, it is unlikely that prolonged exposures would be attributed to these variables. Moreover, the secondary analysis of the change in platelet count in an individual (in whom confounders were presumed to be fixed) over time revealed findings comparable to those seen in the primary analysis.
Conclusions
In this nested case-control study, an elevated platelet count was associated with increased risk of cancer at several sites. The association was transient and attenuated with increasing time from CBC test to the date of the cancer diagnosis. Odds ratios were greatest for colon, lung, ovary, gastroesophageal, and kidney cancers. Our findings suggest that an elevated platelet count could potentially serve as a marker for the presence of some cancer types.
eTable 1. Inclusion Table for CBC Tests
eTable 2. Exclusion Table for Study Cohort
eTable 3. Detailed Descriptive Table of Eligible Subjects, Measured at First Eligible Routine CBC Test (Cohort Entry Date)
eTable 4. Descriptive Table of Matched Subjects (Primary Analysis), Variables Measured on Index CBC
eTable 5. Odds Ratio of Cancer by Platelet Count Category and Time From Diagnosis. Select Cancer Sites
eTable 6. Odds Ratio of Any Solid Tumour Diagnosis (Excluding Liver) by Change in Platelet Count Category and Time From Cancer Diagnosis
eTable 7. Odds Ratio of Cancer by Change in Platelet Count Category and Time From Diagnosis. Select Cancer Sites
eFigure 1. Study Design Criteria Among Matched Individuals
eFigure 2. Age- and Sex-Specific Platelet Count Reference Distributions for Exposure Definition
eFigure 3. Age- and Sex-Specific Platelet Count Reference Distributions for Secondary Exposure Definition
eFigure 4. Odds Ratio of Cancer by Platelet Count Category and Time From Cancer Diagnosis. Additional Cancer Sites
eFigure 5. Odds Ratio of Cancer by Change in Platelet Count Category and Time From Cancer Diagnosis. Additional Cancer Sites
eFigure 6. Odds Ratio of Cancer by Platelet Count Category and Time From Diagnosis. Select Cancer Sites by Cancer Stage
eFigure 7. Odds Ratio of Cancer by Platelet Count Category (Clinical Definition) and Time From Diagnosis
References
- 1.Tefferi A. Approach to the patient with thrombocytosis. UpToDate. 2020. Accessed May 19, 2021. https://www.uptodate.com/contents/approach-to-the-patient-with-thrombocytosis
- 2.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16(2):123-130. doi: 10.1016/j.annepidem.2005.06.052 [DOI] [PubMed] [Google Scholar]
- 3.Harrison CN, Bareford D, Butt N, et al. ; British Committee for Standards in Haematology . Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol. 2010;149(3):352-375. doi: 10.1111/j.1365-2141.2010.08122.x [DOI] [PubMed] [Google Scholar]
- 4.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610-618. doi: 10.1056/NEJMoa1110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer AI. Thrombocytosis. N Engl J Med. 2004;350(12):1211-1219. doi: 10.1056/NEJMra035363 [DOI] [PubMed] [Google Scholar]
- 6.Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017;67(659):e405-e413. doi: 10.3399/bjgp17X691109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankus E, Price SJ, Ukoumunne OC, Hamilton W, Bailey SER. Cancer incidence in patients with a high normal platelet count: a cohort study using primary care data. Fam Pract. 2018;35(6):671-675. doi: 10.1093/fampra/cmy018 [DOI] [PubMed] [Google Scholar]
- 8.Mounce LT, Hamilton W, Bailey SE. Cancer incidence following a high-normal platelet count: cohort study using electronic healthcare records from English primary care. Br J Gen Pract. 2020;70(698):e622-e628. doi: 10.3399/bjgp20X710957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taucher S, Salat A, Gnant M, et al. ; Austrian Breast and Colorectal Cancer Study Group . Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost. 2003;89(6):1098-1106. doi: 10.1055/s-0037-1613413 [DOI] [PubMed] [Google Scholar]
- 10.Bensalah K, Leray E, Fergelot P, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175(3, pt 1):859-863. doi: 10.1016/S0022-5347(05)00526-4 [DOI] [PubMed] [Google Scholar]
- 11.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9(9):1826-1830. doi: 10.1183/09031936.96.09091826 [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106(7):887-891. doi: 10.1002/jso.23163 [DOI] [PubMed] [Google Scholar]
- 13.The Johns Hopkins ACG System, version 10.0. Technical reference guide. Accessed September 24 2020. https://www.hopkinsacg.org/document/acg-system-version-10-0-technical-reference-guide/
- 14.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495-501. doi: 10.1016/0895-4356(88)90052-2 [DOI] [PubMed] [Google Scholar]
- 15.Kaser A, Brandacher G, Steered W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720-2725. doi: 10.1182/blood.V98.9.2720 [DOI] [PubMed] [Google Scholar]
- 16.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123-134. doi: 10.1038/nrc3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231-269. doi: 10.1007/s10555-014-9498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylman JL, Mitrugno A, Tormoen GW, Wagner TH, Mallick P, McCarty OJT. Platelet count as a predictor of metastasis and venous thromboembolism in patients with cancer. Converg Sci Phys Oncol. 2017;3(2):023001. doi: 10.1088/2057-1739/aa6c05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MR, O’Leary DP, Flashman K, Asiimwe A, Ellis BG, Senapati A. Clinical assessment to determine the risk of bowel cancer using Symptoms, Age, Mass and Iron deficiency anaemia (SAMI). Br J Surg. 2017;104(10):1393-1404. doi: 10.1002/bjs.10573 [DOI] [PubMed] [Google Scholar]
- 20.George JN. Systemic malignancies as a cause of unexpected microangiopathic hemolytic anemia and thrombocytopenia. Oncology (Williston Park). 2011;25(10):908-914. [PubMed] [Google Scholar]
- 21.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77-85. doi: 10.7326/0003-4819-159-2-201307160-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burn J, Sheth H, Elliott F, et al. ; CAPP2 Investigators . Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855-1863. doi: 10.1016/S0140-6736(20)30366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HD, Vora P, Soriano-Gabarró M, Chan KA. Association between low-dose aspirin use and colorectal cancer incidence in Taiwan. JAMA Netw Open. 2020;3(11):e2026494. doi: 10.1001/jamanetworkopen.2020.26494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard ME, Poole EM, Curhan GC, et al. Association of analgesic use with risk of ovarian cancer in the Nurses’ Health Studies. JAMA Oncol. 2018;4(12):1675-1682. doi: 10.1001/jamaoncol.2018.4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannakeas V, Narod SA. Incidence of cancer among adults with thrombocytosis in Ontario, Canada. JAMA Netw Open. 2021;4(8):e2120633. doi: 10.1001/jamanetworkopen.2021.20633 [DOI] [PubMed] [Google Scholar]
- 26.Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-1177. doi: 10.1016/j.annonc.2021.05.806 [DOI] [PubMed] [Google Scholar]
- 27.Green MS, Peled I, Najenson T. Gender differences in platelet count and its association with cigarette smoking in a large cohort in Israel. J Clin Epidemiol. 1992;45(1):77-84. doi: 10.1016/0895-4356(92)90191-O [DOI] [PubMed] [Google Scholar]
- 28.Samocha-Bonet D, Justo D, Rogowski O, et al. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008;2008:834153. doi: 10.1155/2008/834153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion Table for CBC Tests
eTable 2. Exclusion Table for Study Cohort
eTable 3. Detailed Descriptive Table of Eligible Subjects, Measured at First Eligible Routine CBC Test (Cohort Entry Date)
eTable 4. Descriptive Table of Matched Subjects (Primary Analysis), Variables Measured on Index CBC
eTable 5. Odds Ratio of Cancer by Platelet Count Category and Time From Diagnosis. Select Cancer Sites
eTable 6. Odds Ratio of Any Solid Tumour Diagnosis (Excluding Liver) by Change in Platelet Count Category and Time From Cancer Diagnosis
eTable 7. Odds Ratio of Cancer by Change in Platelet Count Category and Time From Diagnosis. Select Cancer Sites
eFigure 1. Study Design Criteria Among Matched Individuals
eFigure 2. Age- and Sex-Specific Platelet Count Reference Distributions for Exposure Definition
eFigure 3. Age- and Sex-Specific Platelet Count Reference Distributions for Secondary Exposure Definition
eFigure 4. Odds Ratio of Cancer by Platelet Count Category and Time From Cancer Diagnosis. Additional Cancer Sites
eFigure 5. Odds Ratio of Cancer by Change in Platelet Count Category and Time From Cancer Diagnosis. Additional Cancer Sites
eFigure 6. Odds Ratio of Cancer by Platelet Count Category and Time From Diagnosis. Select Cancer Sites by Cancer Stage
eFigure 7. Odds Ratio of Cancer by Platelet Count Category (Clinical Definition) and Time From Diagnosis


