Key Points
Question
What proportion of SARS-CoV-2–positive youths tested in emergency departments (ED) experience severe outcomes (ie, intensive interventions, severe organ impairment, or death) within 14 days?
Findings
Among 3221 SARS-CoV-2–positive youths enrolled in a global prospective cohort study with outcome data, 3.3% had severe outcomes within 14 days. Across a subgroup of 2510 SARS-CoV-2–positive youths discharged home after testing, 0.5% had severe outcomes during the 2-week follow-up period.
Meaning
The findings of this study suggest that risk factors such as age, underlying chronic illness, and symptom duration may be useful for clinicians to consider when evaluating pediatric patients with SARS-CoV-2 infection.
This cohort study investigates the prevalence of severe outcomes among youths aged 17 years or younger within 14 days of testing positive for SARS-CoV-2 in emergency departments in 8 countries.
Abstract
Importance
Severe outcomes among youths with SARS-CoV-2 infections are poorly characterized.
Objective
To estimate the proportion of children with severe outcomes within 14 days of testing positive for SARS-CoV-2 in an emergency department (ED).
Design, Setting, and Participants
This prospective cohort study with 14-day follow-up enrolled participants between March 2020 and June 2021. Participants were youths aged younger than 18 years who were tested for SARS-CoV-2 infection at one of 41 EDs across 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States. Statistical analysis was performed from September to October 2021.
Exposures
Acute SARS-CoV-2 infection was determined by nucleic acid (eg, polymerase chain reaction) testing.
Main Outcomes and Measures
Severe outcomes, a composite measure defined as intensive interventions during hospitalization (eg, inotropic support, positive pressure ventilation), diagnoses indicating severe organ impairment, or death.
Results
Among 3222 enrolled youths who tested positive for SARS-CoV-2 infection, 3221 (>99.9%) had index visit outcome data available, 2007 (62.3%) were from the United States, 1694 (52.6%) were male, and 484 (15.0%) had a self-reported chronic illness; the median (IQR) age was 3 (0-10) years. After 14 days of follow-up, 735 children (22.8% [95% CI, 21.4%-24.3%]) were hospitalized, 107 (3.3% [95% CI, 2.7%-4.0%]) had severe outcomes, and 4 children (0.12% [95% CI, 0.03%-0.32%]) died. Characteristics associated with severe outcomes included being aged 5 to 18 years (age 5 to <10 years vs <1 year: odds ratio [OR], 1.60 [95% CI, 1.09-2.34]; age 10 to <18 years vs <1 year: OR, 2.39 [95% CI 1.38-4.14]), having a self-reported chronic illness (OR, 2.34 [95% CI, 1.59-3.44]), prior episode of pneumonia (OR, 3.15 [95% CI, 1.83-5.42]), symptoms starting 4 to 7 days prior to seeking ED care (vs starting 0-3 days before seeking care: OR, 2.22 [95% CI, 1.29-3.82]), and country (eg, Canada vs US: OR, 0.11 [95% CI, 0.05-0.23]; Costa Rica vs US: OR, 1.76 [95% CI, 1.05-2.96]; Spain vs US: OR, 0.51 [95% CI, 0.27-0.98]). Among a subgroup of 2510 participants discharged home from the ED after initial testing and who had complete follow-up, 50 (2.0%; 95% CI, 1.5%-2.6%) were eventually hospitalized and 12 (0.5%; 95% CI, 0.3%-0.8%) had severe outcomes. Compared with hospitalized SARS-CoV-2–negative youths, the risk of severe outcomes was higher among hospitalized SARS-CoV-2–positive youths (risk difference, 3.9%; 95% CI, 1.1%-6.9%).
Conclusions and Relevance
In this study, approximately 3% of SARS-CoV-2–positive youths tested in EDs experienced severe outcomes within 2 weeks of their ED visit. Among children discharged home from the ED, the risk was much lower. Risk factors such as age, underlying chronic illness, and symptom duration may be useful to consider when making clinical care decisions.
Introduction
During the early stages of the global COVID-19 pandemic, youths less than 18 years of age represented fewer than 5% of reported cases.1,2,3,4 These early estimates likely underreported the true number of children infected with SARS-CoV-2 because of testing capacity and the generally mild, or even asymptomatic, nature of the disease in children.5,6 However, the pandemic has evolved, and in the United States, youths now represent 25% of all new COVID-19 cases.7 Similarly, pediatric hospitalizations due to COVID-19, which increased 8-fold between May and November of 2020,8 have seen a further 5-fold increase between June and August 2021.9
Although COVID-19 is generally mild in children, severe outcomes and death do occur.10,11,12,13 The risk of severe outcomes among youths with SARS-CoV-2 infection is poorly understood with estimates varying considerably between study designs, settings, and regions.1,14,15 Studies generally include large administrative databases (ie, community based),16 hospitalized populations,17 and youths admitted to the intensive care unit (ICU).13 Consistently identified risk factors for severe COVID-19 in youths include young (ie, 1-3 months) or old (15-18 years) pediatric age group, male sex, and preexisting medical conditions.18,19,20,21 However, data from large prospective cohort studies which include youths with early or mild stages of disease seeking emergency department (ED) care are lacking.
We sought to quantify the frequency of and risk factors for severe outcomes in SARS-CoV-2–positive children enrolled in a prospective ED-based cohort study.
Methods
Setting, Design and Participants
The Pediatric Emergency Research Network (PERN)–COVID-19 prospective cohort study enrolled participants between March 7, 2020, and June 15, 2021, who were tested for SARS-CoV-2.22 Children and adolescents aged younger than 18 years who had a SARS-CoV-2 test performed because of suspected acute infection based on symptoms or exposure were eligible. Sites attempted to recruit the first 5 eligible youths each day, using a consecutive approach starting with the first test performed on chronological time. As this led to variations in the percentage of positive youths recruited across sites, the approach was revised to target the recruitment of the first 2 test-positive and 4 test-negative youths each day. For this study, we focused on youths with positive SARS-CoV-2 nucleic acid tests [eg, polymerase chain reaction (PCR)] enrolled in 38 of the PERN–COVID-19 participating EDs across 8 countries including Argentina, Canada, Costa Rica, Italy, Paraguay, Singapore, Spain, and the United States. We included those who were test-negative as a comparator group. All participants enrolled in 3 study sites in 2 countries were SARS-CoV-2 negative and were included in relevant analyses.
Enrolling sites had local institutional review board approval or established a reliance agreement with the Cincinnati Children’s Hospital Medical Center institutional review board. The legal guardians of all participants provided informed consent (written or verbal based on site) to participate in this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection
At the time of enrollment, the participant’s guardian provided data regarding demographic characteristics, epidemiological risk factors, and clinical symptoms. Two weeks after the index ED visit, guardians were contacted via telephone, text, or email to determine if there were subsequent health care visits, treatments, or interventions received. This information was supplemented by a medical record review performed a minimum of 30 days after the index ED visit to collect data related to medical care and interventions provided, disposition, and clinical outcomes. Participants were deemed lost-to-follow-up at 14 days if 5 attempts to contact the participant’s legal guardian were unsuccessful and a medical record review was not performed. Race and ethnicity data were only available for a subset of the study population and were not reported.
Definitions
Symptoms of SARS-CoV-2 Infection
Symptom duration was defined as time from reported onset of symptoms to time of ED evaluation and was grouped according to investigator consensus as 0 to 3 days, 4 to 7 days, and 8 or more days. Febrile or respiratory illness was defined as having any of the following symptoms: fever, cough, rhinorrhea, congestion, wheezing, difficulty breathing, sputum production, sore throat, or apnea.
SARS-CoV-2 Testing
Testing protocols varied by site and over time. Participants were classified as SARS-CoV-2 positive if they had a positive nucleic acid test (eg, PCR) during the qualifying ED encounter or in the subsequent 2 weeks in instances of repeat testing, on any of the following samples: nasal swab, nasopharyngeal swab, oropharyngeal/throat swab, and saliva. Dates of infection were grouped according to enrollment and severity trends (ie, waves).
Severe Outcomes
A priori, a severe outcome was defined by the occurrence of any of the following complications: cardiac or cardiovascular (cardiac arrest, cardiac ischemia, congestive heart failure, endocarditis, myocarditis, pericarditis, stroke), infectious (disseminated intravascular coagulation, mastoiditis, sepsis with bacteremia, septic shock, toxic shock syndrome), neurologic (encephalitis, meningitis), respiratory (acute respiratory distress syndrome, empyema, necrotizing or cryptogenic organizing pneumonia, pleural effusion or pneumothorax or pneumomediastinum requiring drainage, respiratory failure), and death. In the absence of documentation of one of the aforementioned events, performance of any of the following interventions was deemed to represent a severe outcome: chest drainage, extracorporeal membrane oxygenation, inotropic support, positive pressure ventilation (invasive or noninvasive), and renal replacement therapy. The diagnosis of multisystem inflammatory syndrome in children (MIS-C) and Kawasaki disease were reported as assigned by the clinical care teams and were considered severe if accompanied by one of the aforementioned diagnoses or interventions.
Demographics
Legal guardians of participants enrolled in the United States were provided the following race and ethnicity options from which to choose: American Indian or Alaska Native, Asian, Black or African American, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, White, and other. Responses were categorized for reporting purposes as Hispanic, non-Hispanic Black, non-Hispanic other (Native American, Pacific Islander, Asian, other), and non-Hispanic White.
Sample Size
The PERN–COVID-19 prospective cohort study originally planned to recruit up to 12 500 participants to obtain a subsample of test-positive cases that included at least 50 with severe outcomes, which would provide 93.9% power to detect when a predictive model discriminates severe from nonsevere outcomes in the larger population of test-positive youths, assuming a true C statistic of 0.70.22 These calculations used a variance inflation factor of 2.0 to account for model complexity as measured by degrees of freedom.23 As outcomes were classified in a delayed manner, we ended up having an excess number of severe outcomes prior to achieving our target sample size, which led to a termination of recruitment.
Statistical Analysis
Pearson χ2 or Fisher exact tests were used to compare the prevalence of participant characteristics across age groups. All youths with ED outcome (day 0) data were included in the primary analysis. Risk differences were calculated by subtracting the cumulative incidence between categories of the following predefined variables: sex, age, and chronic conditions. Median length of hospitalization and ICU stay were compared across dichotomous strata using the Mann-Whitney test and across the 5 age groups using the Kruskal-Wallis test. Outcomes among children discharged home from the ED on day 0 and who completed 14-day follow-up were summarized using similar methods.
A multivariable logistic regression model that included the country and time period of enrollment, and accounted for clustering by site, was used to identify factors associated with severe outcomes. The following variables were specified a priori for inclusion into the model: sex, age group, chronic underlying condition as reported by the guardian (excluding asthma), history of asthma, prior pneumonia, symptoms of febrile or respiratory illness, duration of symptoms, and date of infection. From this model, we derived additional models by removing nonsignificant variables (P > .05) in sequence and then computing the Akaike Information Criteria (AIC) weights for each model. The model with the lowest AIC was selected as the best fit model.24 To evaluate associations with race and ethnicity, a similar multivariable logistic regression analysis was performed for participants recruited in EDs in the United States alone. We compared the proportion of severe outcomes by time (each month) of study enrollment using a trend test that adjusted for cluster effects.
Baseline characteristics of enrolled youths who tested SARS-CoV-2 positive and negative were compared using the χ2 test and R × C contingency tables as appropriate. Severe outcomes among SARS-CoV-2–negative children were compared with SARS-CoV-2–positive children overall and based on index ED visit hospitalization status stratified by country using risk difference calculation methods.
No missing data approaches were used as only 0.8% of participants lacked data required to complete primary outcome analyses. All analyses were 2-sided and conducted using STATA version 16 (StataCorp) with statistical significance defined as P < .05. Statistical analysis was performed from September to October 2021.
Results
Among 10 382 study participants, 3222 (31%) tested positive for SARS-CoV-2 during the study period. Of those who tested positive, 3221 (>99.9%) had index ED visit outcomes data available for inclusion in the primary analysis, 1694 (52.6%) were male; the median (IQR) age was 3 (0-10) years. Most participants were recruited in the United States (n = 2007; 62.3%), and 484 participants (15.0%) participants reported having preexisting chronic underlying conditions (Table 1). Among SARS-CoV-2–positive symptomatic participants, respiratory symptoms were reported in 2325 (76.3%) while fever was reported in 2125 (69.7%); 172 SARS-CoV-2–positive participants (5.3%) were asymptomatic at the index visit.
Table 1. SARS-CoV-2 Positive Participant Demographic and Medical History Characteristics, Overall and by Symptom Status.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| All (n = 3222) | Symptomatic (n = 3048) | Asymptomatic (n = 172) | |
| Sex | |||
| Male | 1694 (52.6) | 1612 (52.9) | 80 (46.5) |
| Female | 1528 (47.4) | 1436 (47.1) | 92 (53.5) |
| Age group, y | |||
| <1 | 829 (25.7) | 815 (26.7) | 14 (8.1) |
| 1 to <2 | 425 (13.2) | 411 (13.5) | 14 (8.1) |
| 2 to <5 | 551 (17.1) | 508 (16.7) | 43 (25.0) |
| 5 to <10 | 576 (17.9)a | 525 (17.2) | 50 (29.1) |
| 10 to <18 | 841 (26.1)a | 789 (25.9) | 51 (29.7) |
| Country | |||
| Argentina | 28 (0.9) | 28 (0.9) | 0 |
| Canada | 532 (16.5)a | 521 (17.1) | 9 (1.1) |
| Costa Rica | 420 (13.0) | 393 (12.9) | 27 (15.7) |
| Italy | 18 (0.6) | 17 (0.6) | 1 (0.6) |
| Paraguay | 35 (1.1) | 33 (1.1) | 2 (1.2) |
| Singapore | 30 (0.9) | 17 (0.6) | 13 (7.6) |
| Spain | 152 (4.7) | 148 (4.9) | 4 (2.3) |
| United States | 2007 (62.3) | 1891 (62.0) | 116 (67.4) |
| Previous pneumoniab | 227 (7.1) | 221 (7.3) | 6 (3.5) |
| Asthmab | 423 (13.1) | 397 (13.0) | 26 (15.1) |
| Chronic conditionb,c | 484 (15.0) | 466 (15.3) | 18 (10.5) |
| Cardiac | 76 (2.4) | 74 (2.4) | 2 (1.2) |
| Developmental | 87 (2.7) | 84 (2.8) | 3 (1.7) |
| Diabetes | 23 (0.7) | 22 (0.7) | 1 (0.6) |
| Gastrointestinal | 13 (0.4) | 12 (0.4) | 1 (0.6) |
| Hematological | 67 (2.1) | 66 (2.2) | 1 (0.6) |
| Liver | 5 (0.2) | 5 (0.2) | 0 |
| Malignant neoplasm | 24 (0.7) | 23 (0.8) | 1 (0.6) |
| Neurological | 110 (3.4) | 108 (3.5) | 2 (1.2) |
| Pulmonary | 52 (1.6) | 49 (1.6) | 3 (1.7) |
| Renal | 47 (1.5) | 43 (1.4) | 4 (2.3) |
| Rheumatologic | 8 (0.3) | 7 (0.2) | 1 (0.6) |
| Other | 167 (5.2) | 164 (5.4) | 3 (1.7) |
| Clinical presentationd | |||
| Respiratory symptomse | 2325 (72.2) | 2325 (76.3) | NA |
| Fever | 2125 (66.0) | 2125 (69.7) | NA |
| Other systemic symptomsf | 1860 (57.7) | 1860 (61.0) | NA |
| Gastrointestinalg | 1338 (43.1) | 1338 (45.5) | NA |
| Neurologicalh | 967 (30.0) | 967 (31.7) | NA |
| Otheri | 617 (19.1) | 617 (20.2) | NA |
| Date of infection | |||
| Mar 7-May 31, 2020 | 235 (7.3) | 217 (7.1) | 18 (10.5) |
| Jun 1-Aug 31, 2020 | 851 (26.4)a | 790 (25.9) | 60 (34.9) |
| Sept 1-Nov 30, 2020 | 769 (23.9) | 728 (23.9) | 41 (23.8) |
| Dec 1, 2020-Feb 28, 2021 | 734 (22.8) | 705 (23.1) | 29 (16.9) |
| Mar 1-Jun 15, 2021 | 633 (19.7)a | 608 (20.0) | 24 (14.0) |
Abbreviation: NA, not applicable.
Symptom status was missing for 2 male Canadian youths, one between 5 and less than 10 years of age (enrolled between March and June 2021) and one between 10 and 18 years of age (enrolled between June and August of 2020).
Missing for 4 youths (2 youths without symptom status, and 2 other youths who were asymptomatic).
Youths may have more than one specific chronic condition.
Missing for 2 Canadian youths without symptom data.
Includes cough, rhinorrhea/congestion, difficulty breathing, sore throat, chest pain, wheeze, sputum production, apnea.
Includes myalgia, arthralgia, drowsiness or lethargy, anorexia, edema of extremities, irritability.
Includes vomiting, diarrhea, abdominal pain.
Includes headache, seizure, loss of smell or taste.
Includes conjunctivitis, skin rash, oral symptoms.
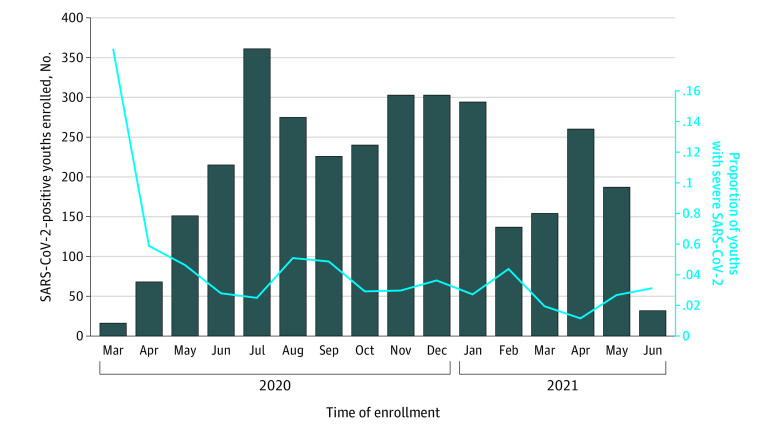
At the index ED visit (ie, day 0), 21.3% (n = 685) of SARS-CoV-2 positive youths were hospitalized, of whom 13.3% (91 of 685) were admitted to the ICU from the ED; (Figure 1). Within 14 days of testing positive for SARS-CoV-2 (ie, index ED visit plus 14-day follow-up period), 22.8% (95% CI, 21.4%-24.3%) of the youths (735 of 3221) had been hospitalized, 3.9% (95% CI, 3.2%-4.6%) (124 of 3221) had been admitted to ICUs, 3.3% (95% CI, 2.7%-4.0%) (107 of 3221) had severe outcomes, and 4 children (0.1%) died. Severe outcomes were most common among youths aged 10 years to less than 18 years (46 of 841; 5.5%; 95% CI, 4.0%-7.2%), and lowest among youths aged less than 1 year (14 of 828; 1.7%; 95% CI, 0.9-2.8) (Table 2). MIS-C or Kawasaki disease diagnoses were assigned to 50 of 3221 youths (1.6%; 95% CI, 1.2-2.0), 16 of whom met our severe outcome study definition. MIS-C or Kawasaki disease were most common in youths aged 5 to less than 10 years (23 of 551; 4.0%; 95% CI, 2.7-6.2); eTable 1 in Supplement 1. The proportion of youths with severe outcomes did not differ across the study enrollment period (Figure 2).
Figure 1. Flow of SARS-CoV-2–Positive Study Participants Including Outcomes and Follow-up.
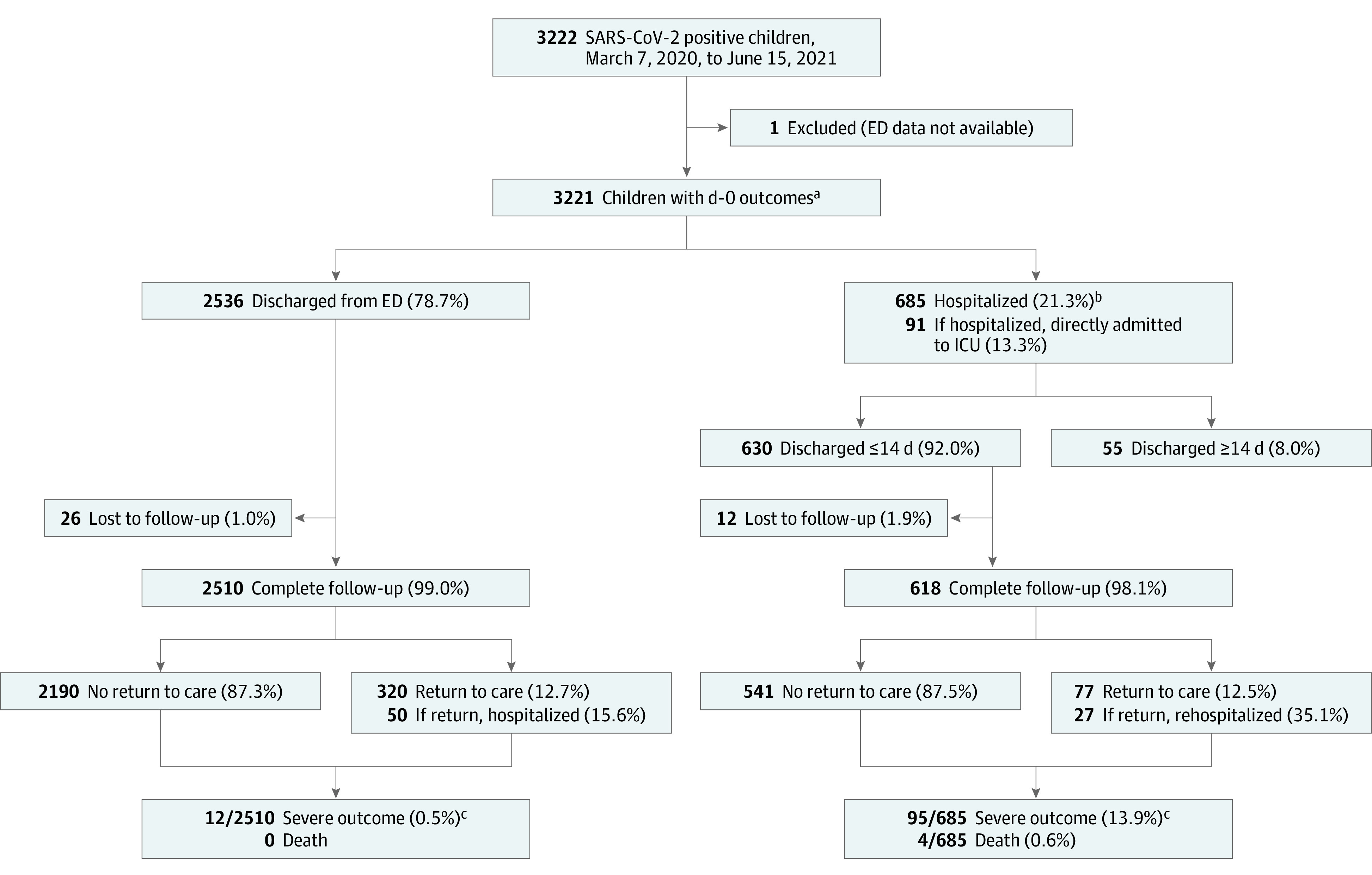
ED indicates emergency department; ICU, intensive care unit.
aFive of 3221 youths did not test positive on day 0, but did within 14 days.
bIncludes 8 youths transferred to other tertiary facilities.
cAs per analysis plan, considers only youths with full 14-day follow-up.
Table 2. Cumulative Outcomes of 3221 SARS-CoV-2–Positive Youths Within 14 Days of Index Emergency Department Visita,b.
| Outcome | Participants, No. (%) [95% CI] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All children (n = 3221) | Sex | Age group, y | Chronic conditionc | |||||||
| Male (n = 1693) | Female (n = 1528) | <1 (n = 828) | 1 to <2 (n = 425) | 2 to <5 (n = 551) | 5 to <10 (n = 576) | 10 to <18 (n = 841) | No (n = 2733) | Yes (n = 484) | ||
| Hospitalized | 735 (22.8) [21.4-24.3] | 406 (24.0) [22.0-26.1] | 329 (21.5) [19.5-23.7] | 189 (22.8) [20.0-25.8] | 70 (16.5) [13.1-20.3] | 120 (21.8) [18.4-25.5] | 128 (22.2) [18.9-25.8] | 228 (27.1) [24.1-30.3] | 539 (19.7) [18.2-21.3] | 195 (40.3) [35.9-44.8] |
| Stay, median (IQR), dd | 3 (1-5) | 3 (1-5) | 3 (1-6) | 2 (1-4) | 2 (1-4) | 3 (1-5) | 3 (1-7) | 3 (1-7) | 2 (1-5) | 3 (1-6) |
| ICU admission | 124 (3.9) [3.2-4.6] | 71 (4.2) [3.3-5.3] | 53 (3.5) [2.6-4.5] | 14 (1.7) [0.9-2.8] | 10 (2.4) [1.1-4.3] | 20 (3.6) [2.2-5.6] | 20 (3.5) [2.1-5.3] | 60 (7.1) [5.5-9.1] | 79 (2.9) [2.3-3.6] | 45 (9.3) [6.9-12.2] |
| Stay, median (IQR), de | 3 (1-5) | 2 (1-4) | 4 (1-7) | 2 (1-4) | 2.5 (0-4) | 1 (0-4.5) | 2 (0.5-5.5) | 3 (1-6) | 3 (1-5) | 2 (1-7) |
| Severe outcomef | 107 (3.3) [2.7-4.0] | 61 (3.6) [2.8-4.6] | 46 (3.0) [2.2-4.0] | 14 (1.7) [0.9-2.8] | 8 (1.9) [0.8-3.7] | 19 (3.5) [2.1-5.3] | 20 (3.5) [2.1-5.3] | 46 (5.5) [4.0-7.2] | 72 (2.6) [2.1-3.3] | 35 (7.2) [5.1-9.9] |
| Severe respiratory illness or ventilatory support | 73 (2.3) [1.8-2.8] | 39 (2.3) [1.6-3.1] | 34 (2.2) [1.5-3.1] | 9 (1.1) [0.5-2.1] | 5 (1.2) [0.4-2.7] | 13 (2.4) [1.3-4.0] | 12 (2.1) [1.1-3.6] | 34 (4.0) [2.8-5.6] | 43 (1.6) [1.1-2.1] | 30 (6.2) [4.2-8.7] |
| Inotropic support | 34 (1.1) [0.7-1.5] | 21 (1.2) [0.8-1.9] | 13 (0.9) [0.4-1.5] | 2 (0.2) [0-0.9] | 6 (1.4) [0.5-3.0] | 2 (0.4) [0-1.3] | 9 (1.6) [0.7-2.9] | 15 (1.8) [1.0-2.9] | 26 (1.0) [0.6-1.4] | 7 (1.4) [0.6-3.0] |
| Severe cardiac or cardiovascular illness | 9 (0.3) [0.1-0.5] | 9 (0.5) [0.2-1.0] | 0 [0-0.2]g | 0 [0-0.4]g | 1 (0.2) [0-1.3] | 0 [0-0.7]g | 2 (0.3) [0-1.2] | 6 (0.7) [0.3-1.5] | 7 (0.3) [0.1-0.5] | 2 (0.4) [0.1-1.5] |
| Other severe illness, intervention, death | 26 (0.8) [0.5-1.2] | 13 (0.8) [0.4-1.3] | 13 (0.9) [0.4-1.5] | 3 (0.4) [0.1-1.1] | 2 (0.5) [0.1-1.7] | 7 (1.3) [0.5-2.6] | 8 (1.4) [0.6-2.7] | 6 (0.7) [0.3-1.5] | 18 (0.7) [0.4-1.0] | 8 (1.7) [0.7-3.2] |
Abbreviation: ICU, intensive care unit.
No outcome information is known for the 1 youth without emergency department discharge/hospitalization information; and for 38 of 3221 youths, the, outcome data were missing after day 0 (26 youths lost to follow-up who were discharged at day 0) or at the time of initial hospitalization discharge (12 lost to follow-up youths discharged from hospital within 14 days).
May extend to 4 weeks for youths hospitalized within 14 days, whose hospitalization extends past the 14-day follow-up time point.
Chronic condition missing for 4 of 3221 youths.
Length of hospitalization missing for 16 youths.
Length of ICU stay missing for 1 youth.
A youth may have multiple diagnoses and/or interventions (categories are not mutually exclusive).
97.5% CI shown.
Figure 2. Severe Outcomes Among SARS-CoV-2–Positive Study Participants Displayed in Relation to Month of Enrollment.
Among the 2510 SARS-CoV-2–positive youths discharged home from the ED index visit, with complete 14-day follow-up, 320 (12.7%; 95% CI, 11.5%-14.1%) returned to a clinic or hospital for care within 14 days due to new, worsening, or persistent symptoms (Figure 1). Specific reasons were known for 47.2% (151 of 320), the most frequent were fever (47 of 151; 31.1%) and cough (42 of151; 27.8%) (eTable 2 in Supplement 1). Fifty of these 2510 (2.0%; 95% CI, 1.5%-2.6%) children were eventually hospitalized. Twelve children (0.5%; 95% CI, 0.3%-0.8%) had severe outcomes and none died (1-sided 97.5% CI, 0%-0.2%) (Figure 1 and eTable 3 in Supplement 1). Among children discharged home, severe outcomes were more common among those with self-reported chronic underlying conditions (risk difference, 1.7%; 95% CI, 0.6%-4.0%).
Multivariable logistic regression identified the following risk factors for severe outcomes: aged 5 to 18 years (age 5 to <10 years vs <1 year: odds ratio [OR], 1.60 [95% CI, 1.09-2.34]; age 10 to <18 years vs <1 year: OR, 2.39 [95% CI 1.38-4.14]), having a preexisting chronic illness (OR, 2.34 [95% CI, 1.59-3.44]), previous episode of pneumonia (OR, 3.15 [95% CI, 1.83-5.42]), and presenting to the hospital 4 to 7 days after symptom onset (vs starting 0-3 days before seeking care: OR, 2.22 [95% CI, 1.29-3.82]) (Table 3). Country also was associated with severe outcomes, with the United States as the referent group, the risk was lower in Canada and Spain and increased in Costa Rica. Among youths from the United States, similar risk factors were identified; and race and ethnicity were not associated with severe outcomes (eTable 4 in Supplement 1).
Table 3. Association of Demographic Factors and Medical History With Severe Outcomes Among the 3141 SARS-CoV-2–Positive Youths With Complete Covariate Dataa,b.
| Participants, No./Total No. | aOR (95% CI) | P value | |
|---|---|---|---|
| Countryc | |||
| Canada | 2/529 | 0.11 (0.05-0.23) | <.001 |
| Costa Rica | 19/420 | 1.76 (1.05-2.96) | .03 |
| Paraguay | 2/35 | 1.43 (0.78-2.61) | .25 |
| Spain | 3/152 | 0.51 (0.27-0.98) | .05 |
| United States | 81/2005 | [Reference] | [Reference] |
| Sex | |||
| Female | 46/1448 | [Reference] | [Reference] |
| Male | 61/1586 | 1.32 (0.83-2.12) | .24 |
| Age category, y | |||
| <1 | 14/806 | [Reference] | [Reference] |
| 1 to <2 | 8/416 | 1.00 (0.47-2.13) | >.99 |
| 2 to <5 | 19/537 | 1.66 (0.95-2.90) | .07 |
| 5 to <10 | 20/553 | 1.60 (1.09-2.34) | .02 |
| 10 to <18 | 46/829 | 2.39 (1.38-4.14) | .002 |
| Chronic condition | |||
| No | 72/2664 | [Reference] | [Reference] |
| Yes | 35/477 | 2.34 (1.59-3.44) | <.001 |
| Previous pneumonia | |||
| No | 84/2921 | [Reference] | [Reference] |
| Yes | 23/220 | 3.15 (1.83-5.42) | <.001 |
| Asthma | |||
| No | 89/2727 | [Reference] | [Reference] |
| Yes | 18/414 | 0.65 (0.39-1.08) | .10 |
| Symptom duration before testing, d | |||
| Asymptomatic | 9/156 | 2.31 (0.81-6.59) | .12 |
| 0-3 | 31/1369 | [Reference] | [Reference] |
| 4-7 | 35/702 | 2.22 (1.29-3.82) | .004 |
| ≥8 | 11/16 | 2.13 (0.86-5.28) | .10 |
| Unknown | 21/698 | 1.44 (0.84-2.45) | .18 |
| Date of index ED visit | |||
| Mar-May 2020 | 14/204 | 1.87 (0.63-5.51) | .26 |
| Jun-Aug 2020 | 29/790 | [Reference] | [Reference] |
| Sep-Nov 2020 | 27/733 | 0.90 (0.53-1.53) | .69 |
| Dec 2020-Feb 2021 | 25/701 | 1.02 (0.43-2.42) | .97 |
| Mar 2021-Jun 2021 | 12/606 | 0.75 (0.37-1.48) | .40 |
Abbreviations: aOR, adjusted odds ratio; ED, emergency department.
A total of 3145 youths with any outcome data were included from the 5 countries with any severe outcomes, and data were missing for 1 or more variables for 4 further youths (2 from Canada, 2 from the United States).
This table reflects the final parsimonious model.
Severe outcomes did not occur outside of these countries.
Outcome data were available for 7156 of 7160 SARS-CoV-2–negative youths enrolled in the cohort (eFigure in Supplement 1). SARS-CoV-2–negative and –positive youths differed by country, enrollment time period, age, presence of chronic underlying conditions, previous pneumonia episode, and index ED visit symptomatology (eTable 5 in Supplement 1). The proportion of SARS-CoV-2–negative youths who experienced severe outcomes within 14 days did not differ from that among the 3221 SARS-CoV-2–positive youths (risk difference, 0.6%; 95% CI, −0.1%-1.4%); eTable 6 in Supplement 1. The risk of a severe outcome was higher among hospitalized SARS-CoV-2 positive children (risk difference, 3.9%; 95% CI, 1.1%-6.9%) overall and within the United States specifically (risk difference, 6.0%; 95% CI, 2.1%-10.3%).
Discussion
In this global cohort study, after 14 days of prospective follow-up for 3221 youths who tested positive for SARS-CoV-2 infection, 23% had been hospitalized, 3% experienced severe outcomes, and 4 children died. We identified the following risk factors for severe outcomes: aged greater than 5 years, having a preexisting chronic illness, previous episode of pneumonia, and presenting to the hospital 4 to 7 days after symptom onset. Among 2510 SARS-CoV-2–positive youths discharged home, only 0.5% had severe outcomes during the follow-up period. Although the overall proportion of SARS-CoV-2–negative youths who experienced severe outcomes did not differ from that among test-positive youths, among hospitalized children, those who were SARS-CoV-2 positive were more likely to experience severe outcomes.
Retrospective multicenter and database studies of ambulatory and hospital-based pediatric cohorts have provided varying estimates of the risk of severe outcomes among youths infected by SARS-CoV-2. Although a recent international database study that included 242 158 children and adolescents with COVID-19 reported that only 1.3% of infected youths were hospitalized and that 30-day deaths were below reportable limits (ie, <5 per database),16 a US hospital ED and inpatient database analysis reported that among 43 465 youths with COVID-19, 10% were hospitalized and 3% had severe illness.25 Similarly, while 20% of youths hospitalized in a US-based retrospective cohort study had severe or very severe disease,17 in a prospective Canadian study of 150 hospitalized SARS-CoV-2–positive youths, 50% had severe or critical illness.26 Thus, an understanding of the study population is crucial to interpreting risk estimates. Our study population provides a risk estimate for youths brought for ED care. Our lower estimate of severe disease likely reflects our stringent definition which required the occurrence of complications or specific invasive interventions.
As with other pediatric COVID-19 studies, we identified that older age, and having a preexisting chronic condition, were risk factors for severe outcomes.18,19,21,25,26,27 In contrast with some other studies, we did not find that very young infants were at a higher risk for severe outcomes.18,21,27 In some studies where very young infants were identified as being at higher risk, the outcome of interest was hospitalization or ICU admission,21,27 whereas we required specific intensive care interventions or complications. As indications for ICU admission differ substantially by time and place, it is a strength of our study that we did not consider ICU admission in isolation to be an indicator of severe disease.
Although asthma has been suggested as a risk factor for severe illness in youths with COVID-19,18 our study, as well as a registry-based study in the United States, did not confirm this association.27 To the best of our knowledge, no other studies have identified symptom duration prior to hospital presentation as a risk factor for severe pediatric COVID-19. As effective therapeutics for youths with detected SARS-CoV-2 are infrequently administered, it is unlikely that this association indicates a beneficial effect of earlier presentation to care. Rather, this may reflect the natural history of infection in youths, with symptom progression appearing between 4 and 7 days being more likely to lead to both hospital presentation and severe outcomes. Previous studies have shown that compared with White non-Hispanic persons in the United States, Black race and Hispanic ethnicity are associated with increased test-positivity and hospitalizations,28,29,30,31 including among children.27 However, our and other analyses that adjust for age, comorbidities, and socioeconomic indicators, among both youths and adults, have not confirmed an increased risk of intensive care, severity, and mortality due to COVID-19 among these groups.29,32,33,34,35
Our findings from the prospective follow-up of the subgroup of SARS-CoV-2–positive youths who were discharged home from their index ED visits reflect the natural history of mild-to-moderate pediatric SARS-CoV-2 infection. Among these 2510 youths, approximately 13% returned to either a clinic or hospital for care within 14 days due to worsening, persistent or new symptoms, 2% were hospitalized, 0.5% had severe outcomes, and none died. These findings align with a recent retrospective cohort study that included 45 US-based children’s hospitals and 15 913 ED COVID-19 encounters which led to discharge among which 10% were associated with a repeat ED encounter within 30 days.17
Strengths and Limitations
This study had some strengths and limitations. A strength of our study was our ability to compare outcomes among SARS-CoV-2 test-positive children to similar, albeit not identical, test-negative children recruited prospectively, prior to the occurrence of outcomes. Although overall the risk of severe outcomes did not differ between those who were test-positive relative to those who were test-negative, the risk of severe outcomes was higher among hospitalized SARS-CoV-2 positive children when compared with those who were test-negative. This finding does not stand in isolation; in a large retrospective study that included 242 158 children with COVID-19 and 2 084 180 with influenza infection, hospitalization (5- to 13-fold), hypoxemia, and pneumonia were more frequent in those with COVID-19.16
As recruitment of study participants took place in EDs, our findings overestimate the risk of severe outcomes among SARS-CoV-2-positive youths and should not be interpreted to reflect the risk faced by community-based cases; rather, they are meant to provide an estimate of this risk among an ED-screened pediatric population. Sites were asked to enroll participants consecutively based on time of testing, however, the proportion of potentially eligible participants consenting to participate likely varied by site and period, which challenges the representativeness of our study population to reflect all youths tested for SARS-CoV-2 in pediatric EDs. Similarly, although participating EDs were given the same study protocol for recruitment, various factors including regional case definitions, screening criteria, and testing capacity, were not controlled by the study and could have differed by site and period. Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic—most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures. To account for this concern, our multivariable logistic regression model adjusted for country and period. Because participating EDs were located in academic pediatric institutions, we cannot generalize our results to all community EDs nor can we generalize to countries beyond those included in our analysis. Finally, as testing for variants of concern was not universal, we were unable to include circulating variants in our model.
Conclusions
The findings from this large global prospective cohort study support a growing body of literature on the risk of severe outcomes and factors associated with these events in SARS-CoV-2–infected youths. Among youths who sought care in the ED, these events occurred in approximately 3% of SARS-CoV-2–positive youths, with the risk varying by age, history of underlying conditions, symptom duration and country. Our findings suggest a low risk of severe outcomes among youths discharged to home. However, among hospitalized SARS-CoV-2–positive youths, the risk of severe outcomes exceeds that of test-negative youths.
eTable 1. Cumulative Outcomes, Up to 14 Days Following the Index ED Visit, Among 3221 SARS-CoV-2 Positive Children
eTable 2. Reasons for Return to Care Among 320 SARS-CoV-2 Positive Children Discharged on day 0 With Complete Follow-up
eTable 3. Cumulative Outcomes, Up to 14 Days Following the Index ED Visit, Among 2510 SARS-CoV-2 Positive Children Who Were Discharged to Home From the Index Emergency Department Visit
eTable 4. Association of Demographic Factors (Including Race and Ethnicity) and Medical History With Severe Outcomes Among the 2001 SARS-CoV-2 Positive Children From the United States With Complete Covariate Data
eTable 5. Comparison of Baseline Characteristics, SARS-CoV-2 Positive and SARS-CoV-2 Negative Participants Enrolled in the PERN-COVID-19 Cohort Study
eTable 6. Severe Outcomes in SARS-CoV-2 Negative and SARS-CoV-2 Positive Children, Stratified by Chronic Condition and Country of Enrollment
eFigure. Flow of SARS-CoV-2 Negative Study Participants Including Outcomes and Follow-up
Nonauthor Collaborators. Pediatric Emergency Research Network–COVID-19 Study Team
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344 [DOI] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team . Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426. doi: 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paquette D, Bell C, Roy M, et al. Laboratory-confirmed COVID-19 in children and youth in Canada, January 15-April 27, 2020. Can Commun Dis Rep. 2020;46(5):121-124. doi: 10.14745/ccdr.v46i05a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM; CMMID COVID-19 working group . Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. doi: 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 6.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882-889. doi: 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics and the Children’s Hospital Association . Children and COVID-19: state data report. Accessed October 13, 2021. https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%2010.7%20FINAL.pdf
- 8.Levin Z, Choyke K, Georgiou A, Sen S, Karaca-Mandic P. Trends in pediatric hospitalizations for coronavirus disease 2019. JAMA Pediatr. 2021;175(4):415-417. doi: 10.1001/jamapediatrics.2020.5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delahoy MJ, Ujamaa D, Whitaker M, et al. ; COVID-NET Surveillance Team; COVID-NET Surveillance Team . Hospitalizations associated with COVID-19 among children and adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1255-1260. doi: 10.15585/mmwr.mm7036e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. ; Spanish Pediatric Intensive Care Society working group on SARS-CoV-2 infection . Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020;24(1):666. doi: 10.1186/s13054-020-03332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin DA, Talj R, Howard LM, Halasa NB. Epidemiologic trends and characteristics of SARS-CoV-2 infections among children in the United States. Curr Opin Pediatr. 2021;33(1):114-121. doi: 10.1097/MOP.0000000000000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick DW, Richardson LC, Young PR, et al. ; Pediatric Mortality Investigation Team . Deaths in children and adolescents associated with COVID-19 and MIS-C in the United States. Pediatrics. 2021;148(5):e2021052273. doi: 10.1542/peds.2021-052273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868-873. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179(7):1029-1046. doi: 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idele P, Anthony D, You D, Luo C, Mofenson L. The evolving picture of SARS-CoV-2 and COVID-19 in children: critical knowledge gaps. BMJ Glob Health. 2020;5(9):e003454. doi: 10.1136/bmjgh-2020-003454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duarte-Salles T, Vizcaya D, Pistillo A, et al. Thirty-day outcomes of children and adolescents with COVID-19: an international experience. Pediatrics. 2021;148(3):e2020042929. doi: 10.1542/peds.2020-042929 [DOI] [PubMed] [Google Scholar]
- 17.Antoon JW, Grijalva CG, Thurm C, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16(10):603-610. doi: 10.12788/jhm.3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137-e145. doi: 10.1097/INF.0000000000003043 [DOI] [PubMed] [Google Scholar]
- 19.Fisler G, Izard SM, Shah S, et al. ; Northwell COVID-19 Research Consortium . Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10(1):171. doi: 10.1186/s13613-020-00790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariah P, Johnson CL, Halabi KC, et al. ; Columbia Pediatric COVID-19 Management Group . Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. doi: 10.1001/jamapediatrics.2020.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Götzinger F, Santiago-García B, Noguera-Julián A, et al. ; ptbnet COVID-19 Study Group . COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653-661. doi: 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk AL, Florin TA, Dalziel SR, et al. Prospective cohort study of children with suspected SARS-CoV-2 infection presenting to paediatric emergency departments: a Paediatric Emergency Research Networks (PERN) Study Protocol. BMJ Open. 2021;11(1):e042121. doi: 10.1136/bmjopen-2020-042121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh FY, Lavori PW, Cohen HJ, Feussner JR. An overview of variance inflation factors for sample-size calculation. Eval Health Prof. 2003;26(3):239-257. doi: 10.1177/0163278703255230 [DOI] [PubMed] [Google Scholar]
- 24.Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004;11(1):192-196. doi: 10.3758/BF03206482 [DOI] [PubMed] [Google Scholar]
- 25.Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4(6):e2111182-e2111182. doi: 10.1001/jamanetworkopen.2021.11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drouin O, Hepburn CM, Farrar DS, et al. ; Canadian Paediatric Surveillance Program COVID-19 Study Team . Characteristics of children admitted to hospital with acute SARS-CoV-2 infection in Canada in 2020. CMAJ. 2021;193(38):E1483-E1493. doi: 10.1503/cmaj.210053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176-184. doi: 10.1001/jamapediatrics.2020.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 Hospitalizations by Race/Ethnicity in 12 States. JAMA Intern Med. 2021;181(1):131-134. doi: 10.1001/jamainternmed.2020.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz-Price LS, Nattinger AB, Rivera F, et al. Racial Disparities in Incidence and Outcomes Among Patients With COVID-19. JAMA Netw Open. 2020;3(9):e2021892. doi: 10.1001/jamanetworkopen.2020.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikari S, Pantaleo NP, Feldman JM, Ogedegbe O, Thorpe L, Troxel AB. Assessment of community-level disparities in coronavirus disease 2019 (COVID-19) infections and deaths in large US metropolitan areas. JAMA Netw Open. 2020;3(7):e2016938. doi: 10.1001/jamanetworkopen.2020.16938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padalabalanarayanan S, Hanumanthu VS, Sen B. Association of state stay-at-home orders and state-level African American Population with COVID-19 case rates. JAMA Netw Open. 2020;3(10):e2026010. doi: 10.1001/jamanetworkopen.2020.26010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabarriti R, Brodin NP, Maron MI, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open. 2020;3(9):e2019795. doi: 10.1001/jamanetworkopen.2020.19795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8):e2018039. doi: 10.1001/jamanetworkopen.2020.18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes DM, Oliveira CR, Guerguis S, et al. ; Tri-State Pediatric COVID-19 Research Consortium . Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2021;230:23-31.e10. doi: 10.1016/j.jpeds.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cumulative Outcomes, Up to 14 Days Following the Index ED Visit, Among 3221 SARS-CoV-2 Positive Children
eTable 2. Reasons for Return to Care Among 320 SARS-CoV-2 Positive Children Discharged on day 0 With Complete Follow-up
eTable 3. Cumulative Outcomes, Up to 14 Days Following the Index ED Visit, Among 2510 SARS-CoV-2 Positive Children Who Were Discharged to Home From the Index Emergency Department Visit
eTable 4. Association of Demographic Factors (Including Race and Ethnicity) and Medical History With Severe Outcomes Among the 2001 SARS-CoV-2 Positive Children From the United States With Complete Covariate Data
eTable 5. Comparison of Baseline Characteristics, SARS-CoV-2 Positive and SARS-CoV-2 Negative Participants Enrolled in the PERN-COVID-19 Cohort Study
eTable 6. Severe Outcomes in SARS-CoV-2 Negative and SARS-CoV-2 Positive Children, Stratified by Chronic Condition and Country of Enrollment
eFigure. Flow of SARS-CoV-2 Negative Study Participants Including Outcomes and Follow-up
Nonauthor Collaborators. Pediatric Emergency Research Network–COVID-19 Study Team