Key Points
Question
Among adults with overweight or obesity without diabetes, what is the effect of once-weekly subcutaneous semaglutide, 2.4 mg, vs once-daily subcutaneous liraglutide, 3.0 mg, on weight loss when each is added to counseling for diet and physical activity?
Findings
In this randomized clinical trial that included 338 participants, mean body weight change from baseline to 68 weeks was –15.8% with semaglutide vs –6.4% with liraglutide, a statistically significant difference.
Meaning
Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide, compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity resulted in significantly greater weight loss at 68 weeks.
Abstract
Importance
Phase 3 trials have not compared semaglutide and liraglutide, glucagon-like peptide-1 analogues available for weight management.
Objective
To compare the efficacy and adverse event profiles of once-weekly subcutaneous semaglutide, 2.4 mg, vs once-daily subcutaneous liraglutide, 3.0 mg (both with diet and physical activity), in people with overweight or obesity.
Design, Setting, and Participants
Randomized, open-label, 68-week, phase 3b trial conducted at 19 US sites from September 2019 (enrollment: September 11-November 26) to May 2021 (end of follow-up: May 11) in adults with body mass index of 30 or greater or 27 or greater with 1 or more weight-related comorbidities, without diabetes (N = 338).
Interventions
Participants were randomized (3:1:3:1) to receive once-weekly subcutaneous semaglutide, 2.4 mg (16-week escalation; n = 126), or matching placebo, or once-daily subcutaneous liraglutide, 3.0 mg (4-week escalation; n = 127), or matching placebo, plus diet and physical activity. Participants unable to tolerate 2.4 mg of semaglutide could receive 1.7 mg; participants unable to tolerate 3.0 mg of liraglutide discontinued treatment and could restart the 4-week titration. Placebo groups were pooled (n = 85).
Main Outcomes and Measures
The primary end point was percentage change in body weight, and confirmatory secondary end points were achievement of 10% or more, 15% or more, and 20% or more weight loss, assessed for semaglutide vs liraglutide at week 68. Semaglutide vs liraglutide comparisons were open-label, with active treatment groups double-blinded against matched placebo groups. Comparisons of active treatments vs pooled placebo were supportive secondary end points.
Results
Of 338 randomized participants (mean [SD] age, 49 [13] years; 265 women [78.4%]; mean [SD] body weight, 104.5 [23.8] kg; mean [SD] body mass index, 37.5 [6.8]), 319 (94.4%) completed the trial, and 271 (80.2%) completed treatment. The mean weight change from baseline was –15.8% with semaglutide vs –6.4% with liraglutide (difference, –9.4 percentage points [95% CI, –12.0 to –6.8]; P < .001); weight change with pooled placebo was –1.9%. Participants had significantly greater odds of achieving 10% or more, 15% or more, and 20% or more weight loss with semaglutide vs liraglutide (70.9% of participants vs 25.6% [odds ratio, 6.3 {95% CI, 3.5 to 11.2}], 55.6% vs 12.0% [odds ratio, 7.9 {95% CI, 4.1 to 15.4}], and 38.5% vs 6.0% [odds ratio, 8.2 {95% CI, 3.5 to 19.1}], respectively; all P < .001). Proportions of participants discontinuing treatment for any reason were 13.5% with semaglutide and 27.6% with liraglutide. Gastrointestinal adverse events were reported by 84.1% with semaglutide and 82.7% with liraglutide.
Conclusions and Relevance
Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity, resulted in significantly greater weight loss at 68 weeks.
Trial Registration
ClinicalTrials.gov Identifier: NCT04074161
This clinical trial compares the efficacy and adverse event profiles of once-weekly subcutaneous semaglutide, 2.4 mg, vs once-daily subcutaneous liraglutide, 3.0 mg (both with diet and physical activity), in people with overweight or obesity.
Introduction
Once-weekly subcutaneous semaglutide, 2.4 mg, a glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA), is available for weight management in people with obesity (or overweight and ≥1 weight-related comorbidities).1 It has demonstrated sustained, clinically meaningful reductions in body weight in people with overweight or obesity, with and without type 2 diabetes, in the ongoing global phase 3 Semaglutide Treatment Effect in People With Obesity (STEP) program.2,3,4,5 Semaglutide was the second GLP-1RA approved for weight management after once-daily subcutaneous liraglutide, 3.0 mg, which is available for chronic weight management in people with obesity (or overweight and ≥1 weight-related comorbidities).6
Semaglutide and liraglutide are modified, long-acting analogues of native GLP-1.7 Through addition of an albumin-binding C16 fatty acid side chain, liraglutide’s half-life is 13 to 15 hours. Semaglutide’s half-life is 165 hours, resulting from an amino acid replacement (preventing dipeptidyl peptidase 4 degradation) and a C18 fatty diacid addition.7,8
In a phase 2 trial, once-daily subcutaneous semaglutide, 0.4 mg (equivalent to 2.8 mg once weekly), significantly increased weight loss vs liraglutide, 3.0 mg.9 The STEP 8 trial directly compared once-weekly semaglutide, 2.4 mg, vs once-daily liraglutide, 3.0 mg, for weight management in adults with overweight or obesity to rigorously assess differences in efficacy and adverse event (AE) profiles.
Methods
Trial Design and Oversight
See Supplement 1 and Supplement 2 for the trial protocol and statistical analysis plan, respectively.
This phase 3, 68-week, randomized, open-label trial was conducted at 19 US sites from September 2019 (enrollment: September 11-November 26) to May 2021 (end of follow-up: May 11; eFigure 1 in Supplement 3). It complied with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol and amendments were approved by the institutional review board or independent ethics committee at each site; all participants provided written informed consent.
Participants
Adults (≥18 years old) with 1 or more self-reported unsuccessful dietary weight loss efforts and a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 30 or greater or 27 or greater with 1 or more weight-related comorbidities (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) were eligible (eAppendix 1 in Supplement 3). Key exclusion criteria included diabetes, hemoglobin A1c (HbA1c) level of 6.5% (48 mmol/mol) or greater, and self-reported body weight changes of more than 5 kg 90 days or less before screening. For regulatory requirements, race and ethnicity were recorded, determined by each participant according to fixed selection categories (including “other”).
Procedures
Participants were randomized (3:1:3:1) using a blocking schema (block size of 8) via an interactive web response system to receive once-weekly subcutaneous semaglutide, 2.4 mg, or matching placebo, or once-daily subcutaneous liraglutide, 3.0 mg, or matching placebo, for 68 weeks, with a 7-week follow-up.
Randomization to semaglutide or liraglutide was not masked (due to dosing differences), but active treatment groups were double-blinded against matching placebo groups to mitigate potential bias arising from open-label comparisons. The placebo groups also facilitated comparisons of semaglutide and liraglutide vs placebo (secondary trial objectives), allowing evaluation of trial results in the context of previous findings.
Semaglutide, initiated at 0.25 mg, was escalated to 2.4 mg (maintenance dose) over 16 weeks (eFigure 1 in Supplement 3). A 1.7-mg maintenance dose was permitted if 2.4 mg could not be tolerated; 1 or more attempts to reescalate was advised. Liraglutide was initiated at 0.6 mg and escalated to 3.0 mg over 4 weeks; escalation could be delayed by a week to aid tolerability. Commensurate with the prescribing information,6 treatment was discontinued if liraglutide, 3.0 mg, was not tolerated; treatment could be restarted, with reescalation over 4 weeks. Treatments were administered using a multidose pen injector; the semaglutide (and matched placebo) group switched to a single-dose pen injector for weeks 44 to 68.
All participants received counseling (from qualified health care professionals, every 4-6 weeks, via in-person visits or telephone) to adhere to diet (500-kcal/d deficit relative to baseline estimated energy expenditure) and physical activity recommendations (≥150 minutes/week).
Outcomes
The primary end point was percentage change from baseline in body weight at week 68. Confirmatory secondary end points (hierarchical testing order) were achievement of weight loss of 10% or more, 15% or more, and 20% or more by week 68. Primary and confirmatory secondary end points were assessed for semaglutide vs liraglutide; comparisons vs pooled placebo were supportive secondary end points. Other supportive secondary end points were changes from baseline in absolute body weight, waist circumference, blood pressure, fasting lipid concentrations, C-reactive protein, HbA1c, fasting plasma glucose, fasting serum insulin, and glycemic status, and permanent trial product discontinuations, all assessed to week 68 for semaglutide vs liraglutide (absolute body weight also assessed vs pooled placebo; eAppendix 2 in Supplement 3). Change in glycemic status data will be reported separately. Achievement of 5% or more weight loss was a prespecified exploratory end point. Separate placebo group body weight changes and changes in pulse were assessed post hoc. AEs were assessed at week 75.
Sample Size Calculation
The hypothesized superiority of semaglutide to liraglutide for the primary and confirmatory secondary end points was assessed using a predefined hierarchical gatekeeping approach (eTable 1 in Supplement 3),10 with a statistically superior result (2-sided at the 5% significance level) required for each end point before the next could be tested. Data from the 2 placebo groups were pooled to increase power for statistical analyses of active treatment vs placebo, while limiting the number of participants required.
The sample size calculations used a t test on the mean difference assuming equal variances for body weight changes, and a Pearson χ2 test for 2 independent proportions for categorical weight loss. The calculation included assumed differences between active treatment groups of 5.5 percentage points in body weight change and ratios of 1.6, 2.2, and 4.5 for the proportions achieving 10% or more, 15% or more, and 20% or more weight loss (eTable 2 in Supplement 3). These assumptions were based on previous trials.9,11 The assumed difference in weight loss was greater than the US Food and Drug Administration–recommended 5% or greater difference threshold.12 Under these assumptions, 126 participants in each active treatment group provided the desired power of more than 90%.
In the pooled placebo group, 84 participants (42 per separate group) gave more than 99% power for the semaglutide vs pooled placebo comparison, and 80% or more power for liraglutide vs pooled placebo, for the primary end point.
Statistical Analysis
Two estimands evaluated treatment efficacy from different perspectives and accounted for intercurrent events and missing data differently (eAppendix 3 in Supplement 3).13,14,15 Analyses in the statistical testing hierarchy addressed the treatment policy estimand (primary estimand) using data from all randomized participants from the in-trial period (time from randomization to last contact with trial site), regardless of treatment adherence or rescue intervention initiation (antiobesity medications or bariatric surgery).
Continuous end points were analyzed using analysis of covariance, with randomized treatment (semaglutide, liraglutide, or pooled placebo) as a factor and baseline value of the outcome measure of interest (eg, baseline body weight in kilograms for analysis of percentage change in body weight) as a covariate. Binary confirmatory secondary end points were analyzed using logistic regression, with the same factor, and baseline body weight as a covariate. Analyses included all randomized participants from all treatment groups.
A multiple imputation approach16 was used in which missing data were imputed by sampling from available measurements at week 68 from participants in the same treatment group and with the same treatment completion status. Imputation used a linear regression model with baseline value and last available observation of the outcome measure of interest from the on-treatment period as covariates. One thousand complete data sets were generated and analyzed, with results combined using Rubin’s rules17 to obtain overall estimates.
Sensitivity analyses of the primary end point included prespecified tipping-point and jump-to-reference analyses (eAppendix 4 in Supplement 3), and a post hoc mixed-effects regression analysis with site as a random effect (to account for the multicenter design).
The secondary estimand (the trial product estimand) evaluated the effect of taking the drug as intended. Analyses addressing this estimand used data from all randomized participants from the on-treatment period (receipt of any dose of treatment within the previous 2 weeks [49 days for safety-related analyses]) until first discontinuation or rescue intervention initiation. The statistical models for assessing this estimand (including the post hoc analysis of change in pulse at week 68) are described in eAppendix 5 in Supplement 3.
Efficacy and AE-related end points were assessed for the full analysis set (all randomized participants) and the safety analysis set (all randomized participants exposed to ≥1 doses of randomized treatment), respectively.
Only the primary and confirmatory secondary end points were controlled for multiplicity. Because of the potential for type I error due to multiple comparisons, findings for other secondary end points and analyses should be interpreted as exploratory. Two-sided 95% CIs and corresponding P values were calculated for all statistical analysis results. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). Efficacy results are reported for the treatment policy estimand only (unless stated otherwise). Data for permanent treatment discontinuations, achievement of 5% or more weight loss, AEs, and change in pulse at week 75 were summarized by descriptive statistics only.
Results
Participants
Overall, 387 participants were screened; 338 were enrolled and randomized to semaglutide (n = 126), liraglutide (n = 127), and placebo (n = 85) (Figure 1A). Demographics and baseline characteristics were similar between active treatment groups, whereas the placebo group had a slightly greater baseline body weight, greater proportions of participants in higher BMI groups, and a greater proportion of participants with 5 or more comorbidities (Table 1). Most participants were White (73.7%) and female (78.4%). Participants’ mean age was 49 years, mean body weight was 104.5 kg, mean BMI was 37.5, mean waist circumference was 113.3 cm, and 36.1% had prediabetes, per American Diabetes Association criteria.18 Most had 0 to 2 comorbidities at screening, with dyslipidemia and hypertension being the most prevalent.
Figure 1. Participant Disposition and Dosing During the Trial.
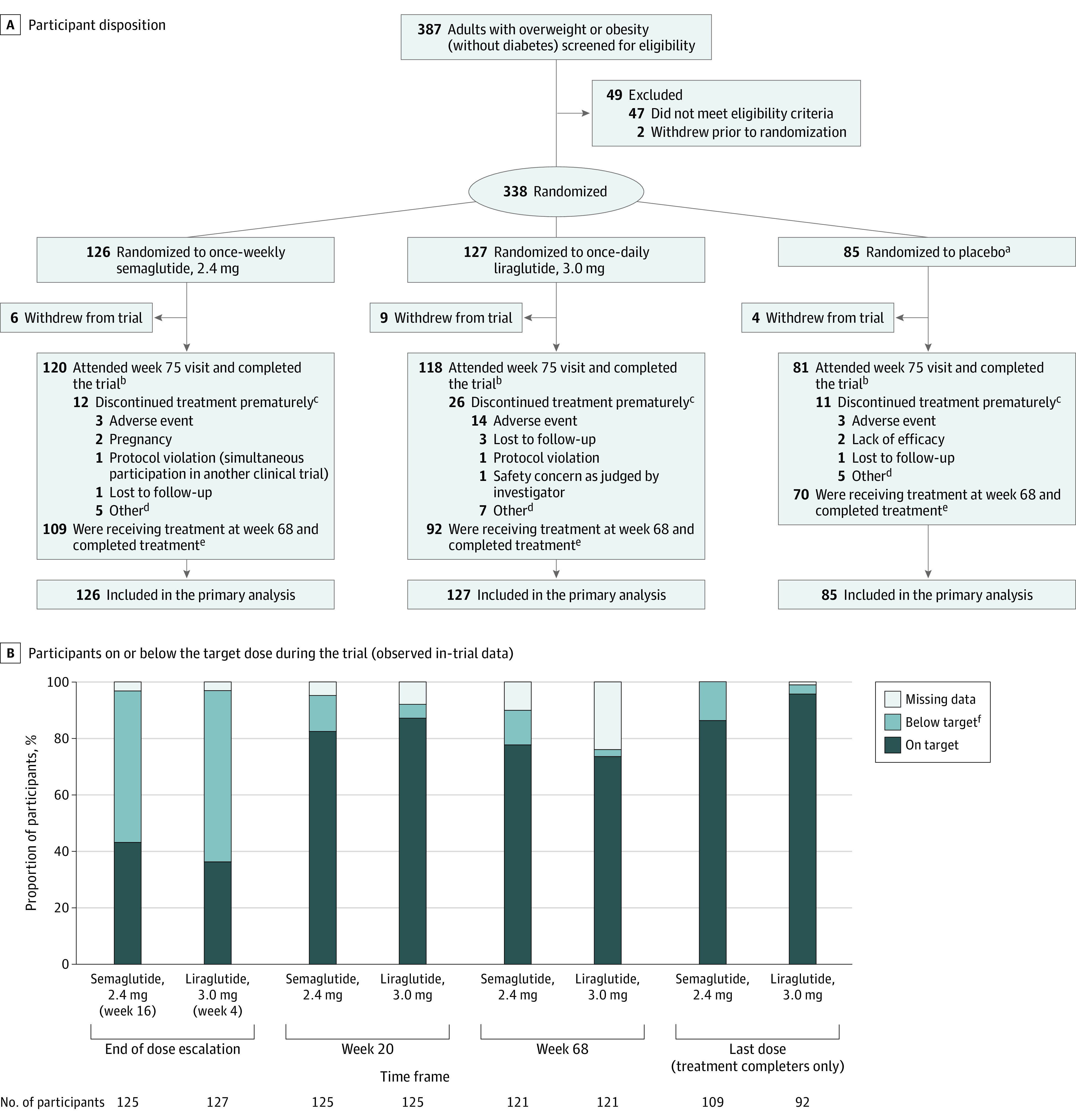
A, Flow of participants during the Semaglutide Treatment Effect in People With Obesity Trial. B, The proportions of participants on or below the target dose at the end of the dose escalation period (week 16 for semaglutide, week 4 for liraglutide), at weeks 20 and 68, and for the last dose. Data presented are observed (ie, as-measured) proportions during the in-trial period (the time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention), based on the numbers of participants remaining in the trial at each time point (except for the last dose, which is based on the number of treatment completers). On target indicates a dose of 2.4 mg for semaglutide and 3.0 mg for liraglutide; below target, a dose of >0.0 to <2.4 mg for semaglutide and >0.0 to <3.0 mg for liraglutide; missing data, no dosing information was provided at the visit; and treatment completers, participants who were receiving treatment at week 68. Data are only presented for the active treatment groups.
aPooled placebo data. Data from the 2 placebo groups were pooled to increase power for statistical analyses of active treatments vs placebo, while limiting the number of participants required.
bThese participants were trial completers. Participants were considered trial completers if they attended the week 75 end–of–follow-up visit, regardless of whether they completed treatment.
cThese participants were trial completers who did not complete treatment.
dOther reasons for premature treatment discontinuation are listed in eTable 6 in Supplement 3.
eThese participants were treatment completers: they were receiving treatment at week 68, regardless of whether they completed the trial. One participant in the semaglutide group completed treatment without completing the trial.
fThe numbers and proportions of participants who were below the target dose are shown in eTable 7 in Supplement 3 and are based on the total number of participants with dose data at each time point (ie, excluding those with missing data).
Table 1. Baseline Demographics and Clinical Characteristics (Full Analysis Set).
| Characteristic | No. (%) | ||
|---|---|---|---|
| Semaglutide, 2.4 mg (n = 126) | Liraglutide, 3.0 mg (n = 127) | Placebo (n = 85)a | |
| Age, mean (SD), y | 48 (14) | 49 (13) | 51 (12) |
| Sex | |||
| Female | 102 (81.0) | 97 (76.4) | 66 (77.6) |
| Male | 24 (19.0) | 30 (23.6) | 19 (22.4) |
| Raceb | |||
| Asian | 4 (3.2) | 6 (4.7) | 3 (3.5) |
| Black/African American | 25 (19.8) | 20 (15.7) | 19 (22.4) |
| White | 94 (74.6) | 95 (74.8) | 60 (70.6) |
| Otherc | 3 (2.4) | 6 (4.7) | 3 (3.5) |
| Hispanic/Latino ethnicityb | 15 (11.9) | 17 (13.4) | 7 (8.2) |
| Body weight, mean (SD), kg | 102.5 (25.3) | 103.7 (22.5) | 108.8 (23.1)d |
| BMI | |||
| Mean (SD) | 37.0 (7.4) | 37.2 (6.4) | 38.8 (6.5) |
| Distribution | |||
| <30 | 9 (7.1) | 11 (8.7) | 4 (4.7) |
| ≥30 to <35 | 51 (40.5) | 42 (33.1) | 20 (23.5) |
| ≥35 to <40 | 37 (29.4) | 38 (29.9) | 31 (36.5) |
| ≥40 | 29 (23.0) | 36 (28.3) | 30 (35.3) |
| Waist circumference, mean (SD), cm | 111.8 (16.3) | 113.5 (15.0) | 115.4 (15.1) |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 125 (14) | 126 (16) | 123 (14) |
| Diastolic | 81 (9) | 81 (10) | 79 (9) |
| Pulse, mean (SD), bpme | 71 (9) | 71 (10) | 72 (10) |
| Fasting lipid profile, geometric mean (CV) [No.f], mg/dL | |||
| Cholesterol level | |||
| Total | 184.9 (21.0) [125] | 188.6 (20.8) [124] | 182.2 (22.8) [84] |
| HDL | 51.9 (24.1) [125] | 53.7 (25.3) [124] | 50.7 (27.7) [84] |
| LDL | 106.4 (32.5) [125] | 108.1 (30.4) [124] | 105.2 (32.9) [84] |
| VLDL | 21.4 (47.2) [125] | 22.0 (48.1) [124] | 21.1 (49.2) [84] |
| Free fatty acids | 10.5 (72.0) [125] | 11.8 (60.5) [121] | 10.6 (56.5) [84] |
| Triglycerides | 110.1 (49.1) [125] | 113.1 (49.4) [124] | 108.2 (49.2) [84] |
| CRP, geometric mean (CV) [No.f], mg/L | 3.9 (124.1) [125] | 3.9 (144.8) [124] | 4.1 (187.1) [84] |
| HbA1c level, mean (SD), % | 5.5 (0.3) | 5.5 (0.3) | 5.6 (0.4) |
| Fasting plasma glucose, mean (SD) [No.f], mg/dL | 96.1 (10.2) [125] | 95.2 (8.5) [125] | 97.6 (12.2) [84] |
| Fasting serum insulin, geometric mean (CV) [No.f], μIU/mL | 12.4 (60.1) [125] | 11.5 (51.2) [121] | 12.1 (67.0) [84] |
| Prediabetesg | 43 (34.1) | 45 (35.4) | 34 (40.0) |
| eGFR, geometric mean (CV), mL/min/1.73 m2e,h | 96.1 (21.1) | 95.3 (19.0) | 92.4 (20.0) |
| Comorbidities at screeninge,i | |||
| Dyslipidemia | 60 (47.6) | 65 (51.2) | 36 (42.4) |
| Hypertension | 48 (38.1) | 55 (43.3) | 39 (45.9) |
| Knee osteoarthritis | 23 (18.3) | 17 (13.4) | 22 (25.9) |
| Obstructive sleep apnea | 24 (19.0) | 18 (14.2) | 19 (22.4) |
| Asthma/COPD | 18 (14.3) | 18 (14.2) | 13 (15.3) |
| Nonalcoholic fatty liver disease | 5 (4.0) | 12 (9.4) | 7 (8.2) |
| Polycystic ovary syndromej | 5 (4.9) | 6 (6.2) | 1 (1.5) |
| Coronary artery disease | 4 (3.2) | 3 (2.4) | 4 (4.7) |
| No. of comorbidities at screeninge,i | |||
| 0 | 32 (25.4) | 25 (19.7) | 16 (18.8) |
| 1 | 31 (24.6) | 29 (22.8) | 17 (20.0) |
| 2 | 25 (19.8) | 29 (22.8) | 21 (24.7) |
| 3 | 17 (13.5) | 24 (18.9) | 9 (10.6) |
| 4 | 10 (7.9) | 11 (8.7) | 9 (10.6) |
| ≥5 | 11 (8.7) | 9 (7.1) | 13 (15.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CV, coefficient of variation (in percentage); eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
SI conversion factors: To convert HDL-C, LDL-C, and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; free fatty acids to mmol/L, multiply by 0.0355; glucose to mmol/L, multiply by 0.055; and insulin to pmol/L, multiply by 6.945.
Pooled placebo data.
To meet regulatory requirements, race and ethnicity were recorded in this study and were determined by the participant according to fixed selection categories (with the option of answering “other”). Percentages may not total 100 due to rounding.
Native American, Alaska Native, Native Hawaiian, Other Pacific Islander, or other.
The mean (SD) baseline body weight values for the separate placebo groups were 110.4 (28.3) kg for the semaglutide-matched placebo group and 107.2 (16.4) kg for the liraglutide-matched placebo group.
Data are for the safety analysis set.
[No.] = number of participants analyzed (where different from the number in the full analysis set).
The presence of prediabetes was determined by investigators on the basis of available information (eg, medical records, concomitant medication, and blood glucose variables) and in accordance with American Diabetes Association criteria.18
Assessed at screening (week −1).
Comorbidities were reported at screening based on medical history. Selected comorbidities are presented and included: dyslipidemia, hypertension, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, impaired glucose metabolism, reproductive system disorders, liver disease, kidney disease, osteoarthritis, gout, and asthma/COPD. Percentages may not total 100 due to rounding.
Percentages are of female participants.
Of the randomized participants, 80.2% (n = 271) completed treatment (on-treatment at week 68) and 94.4% (n = 319) completed the trial (attended week 75 end–of–follow-up visit); see eTable 3 in Supplement 3 for completion rates by site. Among treatment completers, 86.2% received the 2.4-mg dose of semaglutide and 95.7% received the 3.0-mg dose of liraglutide (Figure 1B). Overall, 92.3% of participants (n = 312) had a week 68 body weight assessment; data were missing for 9 participants in the semaglutide group, 10 in the liraglutide group, and 7 in the placebo group. Six participants used rescue interventions: 1 had bariatric surgery (liraglutide) and 5 initiated other antiobesity medications, including GLP-1RAs used off-label (semaglutide: n = 1 [oral semaglutide]; liraglutide: n = 1 [semaglutide]; placebo: n = 3 [phentermine]).
Primary Outcome
At week 68, the estimated mean change in body weight was –15.8% with semaglutide and –6.4% with liraglutide (Table 2, Figure 2; see eFigure 2A in Supplement 3 for cumulative distribution plot). Weight loss with semaglutide was significantly greater vs with liraglutide (difference, –9.4 percentage points [95% CI, –12.0 to –6.8]; P < .001; Table 2). The prespecified and post hoc sensitivity analyses supported the robustness of the primary analysis (Table 2). The primary end point result could not be reversed in the tipping point analysis because of the small amount of missing data.
Table 2. Change in Efficacy Outcomes From Baseline to Week 68 (Treatment Policy Estimand; Full Analysis Set)a,b.
| Estimated mean change (95% CI) [No.] | Difference for semaglutide, 2.4 mg, vs liraglutide, 3.0 mg (95% CI)c | P value | ||
|---|---|---|---|---|
| Semaglutide, 2.4 mg (n = 126) | Liraglutide, 3.0 mg (n = 127) | |||
| Primary end point | ||||
| Body weight, % change | –15.8 (–17.6 to –13.9) [117] | –6.4 (–8.2 to –4.6) [117] | –9.4 (–12.0 to –6.8) | <.001 |
| Confirmatory secondary end points | ||||
| Weight loss at week 68, No. (%)d | ||||
| Participants with ≥10% | 83/117 (70.9) | 30/117 (25.6) | Odds ratio: 6.3 (3.5 to 11.2) | <.001 |
| Participants with ≥15% | 65/117 (55.6) | 14/117 (12.0) | Odds ratio: 7.9 (4.1 to 15.4) | <.001 |
| Participants with ≥20% | 45/117 (38.5) | 7/117 (6.0) | Odds ratio: 8.2 (3.5 to 19.1) | <.001 |
| Supportive secondary end points | ||||
| Body weight, kg | –15.3 (–17.3 to –13.4) [117] | –6.8 (–8.8 to –4.9) [117] | –8.5 (–11.2 to –5.7) | |
| Waist circumference, cm | –13.2 (–15.0 to –11.5) [114] | –6.6 (–8.3 to –4.9) [113] | –6.6 (–9.1 to –4.2) | |
| Blood pressure, mm Hg | ||||
| Systolic | –5.7 (–8.1 to –3.3) [114] | –2.9 (–5.3 to –0.5) [112] | –2.8 (–6.1 to 0.6) | |
| Diastolic | –5.0 (–7.0 to –3.1) [114] | –0.5 (–2.3 to 1.3) [112] | –4.5 (–7.1 to –1.9) | |
| Fasting lipid profile, % changee | ||||
| Cholesterol | ||||
| Total | –7.1 (–10.7 to –3.3) [113] | –0.1 (–3.3 to 3.2) [107] | –7.0 (–11.7 to –2.1) | |
| HDL | –0.3 (–3.6 to 3.0) [112] | 1.9 (–1.0 to 5.0) [107] | –2.2 (–6.5 to 2.2) | |
| LDL | –6.5 (–12.4 to –0.1) [112] | 0.9 (–4.4 to 6.5) [107] | –7.3 (–14.9 to 1.0) | |
| VLDL | –20.7 (–25.1 to –16.0) [112] | –10.9 (–16.7 to –4.8) [107] | –11.0 (–18.5 to –2.7) | |
| Free fatty acids | –12.6 (–22.1 to –2.0) [108] | –8.8 (–19.0 to 2.7) [110] | –4.2 (–18.8 to 13.1) | |
| Triglycerides | –20.7 (–25.6 to –15.6) [112] | –11.0 (–16.9 to –4.7) [107] | –11.0 (–18.9 to –2.2) | |
| CRP, % changee | –52.6 (–61.3 to –42.0) [113] | –24.5 (–36.1 to –10.9) [110] | –37.2 (–51.7 to –18.5) | |
| HbA1c, % | –0.2 (–0.3 to –0.2) [113] | –0.1 (–0.1 to 0.0) [107] | –0.2 (–0.2 to –0.1) | |
| Fasting plasma glucose, mg/dL | –8.3 (–10.4 to –6.1) [112] | –4.3 (–6.7 to –1.9) [106] | –3.9 (–7.2 to –0.7) | |
| Fasting serum insulin, % changee | –27.8 (–36.5 to –17.9) [108] | –15.4 (–23.1 to –7.0) [110] | –14.6 (–27.3 to 0.3) | |
| Exploratory end point | ||||
| Participants with ≥5% weight loss at week 68, No./total (%)d | 102/117 (87.2) | 68/117 (58.1) | NA | |
| Prespecified sensitivity analysis (J2R) | ||||
| Body weight, % changef | –15.3 (–17.0 to –13.6) [117] | –6.0 (–7.7 to –4.3) [117] | –9.2 (–11.6 to –6.8) | |
| Post hoc sensitivity analysis | ||||
| Body weight, % changeg | –15.8 (–17.7 to –13.8) [117] | –6.4 (–8.2 to –4.5) [117] | –9.4 (–12.0 to –6.7) | |
Abbreviations: CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; J2R, jump-to-reference; LDL, low-density lipoprotein; NA, not applicable; VLDL, very low-density lipoprotein.
Data are only presented for the active treatment groups. Data for the placebo groups are presented in eTable 5 in Supplement 3. Numbers of participants with an observation at week 68 are denoted by [No.] for each end point. The number of participants with imputed data can be calculated by subtracting No. from the number in the full analysis set, provided in the column headers.
The treatment policy estimand assessed the treatment effect at week 68, regardless of treatment discontinuation or rescue intervention use. The analyses were based on data from the in-trial observation period (the time from randomization to last contact with the trial site). Continuous end points were assessed using analysis of covariance, with randomized treatment as a factor and baseline value of the outcome measure of interest as a covariate, and a multiple imputation approach for missing data. Categorical end points were analyzed with logistic regression, with the same factor, and baseline body weight as a covariate. Analyses were not controlled for multiple comparisons, except for the primary and secondary confirmatory end points. Corresponding data for the trial product estimand (which assessed the treatment effect assuming participants continued taking randomized treatment for the planned study duration without rescue intervention) are shown in eTable 4 in Supplement 3.
Data are absolute differences between estimated mean changes unless stated otherwise. The differences between mean percentage changes in body weight and mean changes in HbA1c level are expressed in percentage points. P values are only shown for primary and confirmatory secondary end points.
Data are observed (ie, as-measured) numbers and proportions of participants at week 68 from the in-trial period (the time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention), and where applicable, estimated odds ratios for semaglutide vs liraglutide for the treatment policy estimand (achievement of ≥5% weight loss was an exploratory end point and not analyzed statistically).
These parameters were initially analyzed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups). For interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula: (estimated ratio – 1) × 100.
The sensitivity analysis was performed according to the primary analysis, but using jump-to-reference imputation (a technique in which missing data for participants in the active treatment groups were imputed by sampling from all available data [regardless of treatment completion status] from the pooled placebo group).
The post hoc analysis was performed using a mixed-effects regression analysis with study site as a random effect.
Figure 2. Percentage Change in Body Weight From Baseline to Week 68 (Observed In-Trial Data; Full Analysis Set).
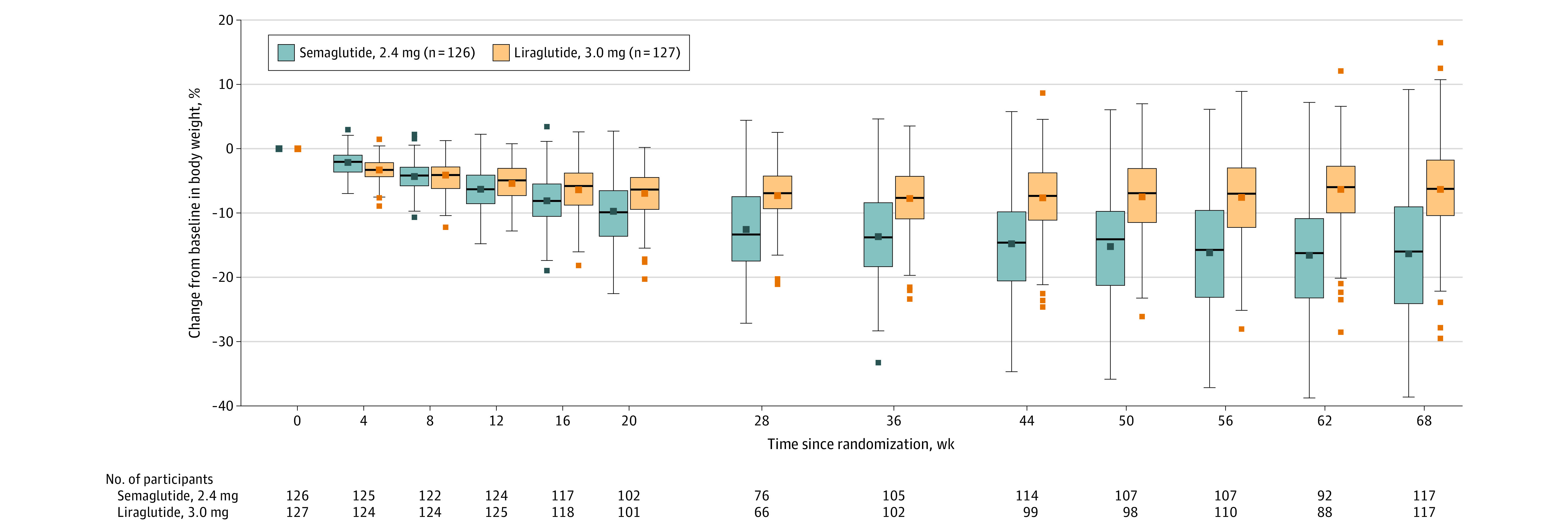
Data presented are observed (ie, as-measured) changes during the in-trial period (the time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention) for the full analysis set. Data for the on-treatment period are presented in eFigure 5 in Supplement 3. The middle lines within each box represent the median observed changes from baseline; the symbols in the boxes represent the mean observed percentage change; the box tops and bottoms represent the interquartile range; the whiskers extend to the most extreme observed values with 1.5 times the IQR of the nearer quartile; and the symbols beyond these points represent the observed values outside that range. More negative values indicate greater reductions. Numbers shown below the graph are the number of participants with observed data at each time point. Participant numbers in the legend are for the full analysis set. Data are only presented for the active treatment groups.
Results for the primary end point were similar for the trial product estimand (eTable 4 and eFigure 2B in Supplement 3).
Confirmatory Secondary Outcomes
The proportions of participants achieving 10% or more, 15% or more, and 20% or more weight loss were 70.9%, 55.6%, and 38.5% with semaglutide and 25.6%, 12.0%, and 6.0% with liraglutide, respectively (Table 2; eTable 4 and eFigure 3 in Supplement 3). The odds of achieving weight loss of 10% or more (odds ratio, 6.3 [95% CI, 3.5 to 11.2]), 15% or more (odds ratio, 7.9 [95% CI, 4.1 to 15.4]), and 20% or more (odds ratio, 8.2 [95% CI, 3.5 to 19.1]) were significantly greater with semaglutide vs with liraglutide (P < .001 for all).
Supportive Secondary Outcomes
Overall, 19.8% (n = 67) of participants permanently discontinued treatment (Figure 1); discontinuations were greatest with liraglutide (27.6%), followed by placebo (17.6%) and semaglutide (13.5%). Consistent with the shorter escalation period, time to first discontinuation and permanent discontinuation were shorter with liraglutide than semaglutide and placebo (eFigure 4 in Supplement 3).
At week 68, reductions in absolute body weight (difference, –8.5 kg [95% CI, –11.2 to –5.7]; see Figure 3 for changes in individual participants), waist circumference (–6.6 cm [95% CI, –9.1 to –4.2]), total cholesterol level (–7.0% [95% CI, –11.7% to –2.1%]), very low-density lipoprotein cholesterol level (–11.0% [95% CI, –18.5% to –2.7%]), triglyceride level (–11.0% [95% CI, –18.9% to –2.2%]), HbA1c level (–0.2 percentage points [95% CI, –0.2 to –0.1]), fasting plasma glucose level (–3.9 mg/dL [95% CI, –7.2 to –0.7]), and C-reactive protein level (–37.2% [95% CI, –51.7% to –18.5%]) were significantly greater with semaglutide vs with liraglutide (Table 2; eTable 4 and eFigures 5, 6A, and 7 in Supplement 3). Changes in other end points were not significantly different (Table 2; eTable 4 and eFigures 6B and 7 in Supplement 3). The reduction in diastolic blood pressure was significantly greater with semaglutide vs liraglutide (–4.5 mm Hg [95% CI, –7.1 to –1.9]) at week 68, but changes at all other time points were comparable (Table 2; eFigure 6C in Supplement 3). Systolic blood pressure increased with placebo (eTable 5 in Supplement 3).
Figure 3. Change in Absolute Body Weight From Baseline to Week 68 for Individual Participants (Observed In-Trial Data; Full Analysis Set).
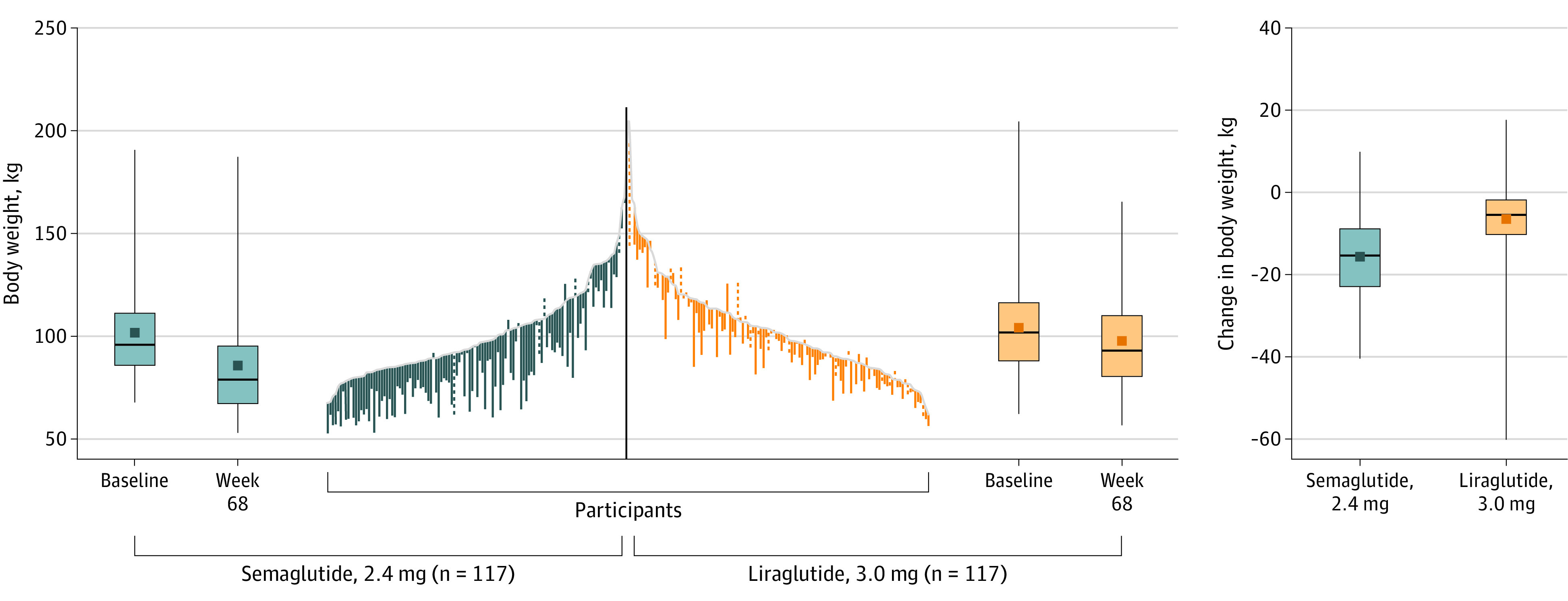
Data presented are observed (ie, as-measured) changes during the in-trial period (the time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention) for each individual participant in the full analysis set. Solid lines are for treatment completers (ie, participants who were receiving treatment at week 68), and dashed lines are for participants who prematurely discontinued treatment. A total of 117 participants in the semaglutide, 2.4 mg, group and 117 in the liraglutide, 3.0 mg, group had a week 68 assessment and so contribute to the data. Data are only presented for the active treatment groups. The middle lines within each box represent the median data; the symbols in the boxes represent the mean data; the box tops and bottoms represent the interquartile range; and the whiskers extend to the most extreme observed values with 1.5 times the IQR of the nearer quartile. The gray line indicates baseline.
At week 68, the estimated mean change in body weight was –1.9% for pooled placebo (eTable 5 in Supplement 3). Weight loss with semaglutide and liraglutide were significantly greater vs placebo (difference, –13.9 percentage points [95% CI, –16.7 to –11.0] and –4.5 percentage points [95% CI, –7.3 to –1.7]). The proportions of participants achieving 10% or more, 15% or more, and 20% or more weight loss with placebo were 15.4%, 6.4%, and 2.6% (eTable 5 in Supplement 3). Changes in other end points for the placebo group are in eTable 5 in Supplement 3.
Exploratory Outcomes
The proportions of participants achieving 5% or more weight loss were 87.2% with semaglutide, 58.1% with liraglutide, and 29.5% with placebo (Table 2; eTables 4 and 5 and eFigure 3 in Supplement 3).
Post Hoc Outcomes
The estimated mean body weight changes at week 68 for the separate placebo groups were –0.5% (95% CI, –3.1% to 2.2%) for semaglutide-placebo and –3.2% (95% CI, –5.9% to –0.5%) for liraglutide-placebo (eTable 5 in Supplement 3).
Adverse Events
AEs were reported by 95.2% of participants with semaglutide, 96.1% with liraglutide, and 95.3% with placebo (Table 3). Gastrointestinal disorders were the most frequent AEs with semaglutide and liraglutide, reported by 84.1% and 82.7% of participants, respectively (placebo: 55.3%); more events occurred with semaglutide than with liraglutide. Most gastrointestinal events were mild to moderate in severity (severe gastrointestinal events were reported by 3.2% [n = 4], 2.4% [n = 3], and 3.5% [n = 3] with semaglutide, liraglutide, and placebo, respectively), transient, and resolved without permanent treatment discontinuation (Table 3; eFigure 8 in Supplement 3). Reports of gastrointestinal AEs were greatest during, and shortly after, dose escalation, with mild events persisting throughout the trial (eFigure 8 in Supplement 3).
Table 3. Adverse Event and Tolerability Profile (Safety Analysis Set)a.
| Semaglutide, 2.4 mg (n = 126) | Liraglutide, 3.0 mg (n = 127) | Placebo (n = 85)b | ||||
|---|---|---|---|---|---|---|
| Participants, No. (%) | Events, No. | Participants, No. (%) | Events, No. | Participants, No. (%) | Events, No. | |
| Fatal AEsc | 0 | 0 | 0 | |||
| SAEs | 10 (7.9) | 14 | 14 (11.0) | 18 | 6 (7.1) | 9 |
| AEs leading to trial product discontinuation | 4 (3.2) | 4 | 16 (12.6) | 21 | 3 (3.5) | 3 |
| GI disorders | 1 (0.8) | 1 | 8 (6.3) | 10 | 1 (1.2) | 1 |
| Any AEs | 120 (95.2) | 904 | 122 (96.1) | 823 | 81 (95.3) | 522 |
| AEs in ≥10% of participants in any treatment group by MedDRA-preferred term | ||||||
| Nausea | 77 (61.1) | 130 | 75 (59.1) | 102 | 19 (22.4) | 24 |
| Constipation | 49 (38.9) | 80 | 40 (31.5) | 52 | 20 (23.5) | 24 |
| Diarrhea | 35 (27.8) | 51 | 23 (18.1) | 37 | 22 (25.9) | 26 |
| Vomiting | 32 (25.4) | 50 | 26 (20.5) | 34 | 5 (5.9) | 6 |
| Headache | 20 (15.9) | 46 | 18 (14.2) | 20 | 10 (11.8) | 12 |
| Eructation | 17 (13.5) | 20 | 5 (3.9) | 5 | 4 (4.7) | 4 |
| Decreased appetite | 15 (11.9) | 15 | 16 (12.6) | 18 | 3 (3.5) | 3 |
| Fatigue | 12 (9.5) | 12 | 14 (11.0) | 17 | 4 (4.7) | 4 |
| Dyspepsia | 11 (8.7) | 14 | 15 (11.8) | 16 | 5 (5.9) | 7 |
| Nasopharyngitis | 10 (7.9) | 10 | 11 (8.7) | 13 | 9 (10.6) | 11 |
| Upper respiratory tract infection | 9 (7.1) | 11 | 19 (15.0) | 26 | 18 (21.2) | 23 |
| Arthralgia | 8 (6.3) | 8 | 14 (11.0) | 15 | 7 (8.2) | 7 |
| Sinusitis | 8 (6.3) | 9 | 8 (6.3) | 8 | 13 (15.3) | 14 |
| Back pain | 6 (4.8) | 6 | 9 (7.1) | 10 | 9 (10.6) | 10 |
| Influenza | 5 (4.0) | 5 | 14 (11.0) | 14 | 6 (7.1) | 6 |
| Safety areas of interestd | ||||||
| GI disorders | 106 (84.1) | 440 | 105 (82.7) | 313 | 47 (55.3) | 130 |
| Cardiovascular disordersc | 16 (12.7) | 20 | 18 (14.2) | 21 | 9 (10.6) | 23 |
| Allergic reactions | 9 (7.1) | 13 | 11 (8.7) | 12 | 10 (11.8) | 13 |
| Psychiatric disorders | 7 (5.6) | 10 | 19 (15.0) | 27 | 9 (10.6) | 10 |
| Injection site reactions | 0 | 14 (11.0) | 16 | 5 (5.9) | 7 | |
| Malignant neoplasmsc | 3 (2.4) | 3 | 3 (2.4) | 3 | 1 (1.2) | 1 |
| Hepatic disorders | 2 (1.6) | 2 | 1 (0.8) | 1 | 3 (3.5) | 4 |
| Gallbladder-related disorders | 1 (0.8) | 2 | 4 (3.1) | 5 | 1 (1.2) | 1 |
| Cholelithiasis | 1 (0.8) | 1 | 2 (1.6) | 2 | 1 (1.2) | 1 |
| Hypoglycemia | 0 | 1 (0.8) | 1 | 0 | ||
| Acute pancreatitis | 0 | 1 (0.8) | 1 | 0 | ||
| Acute kidney failure | 1 (0.8) | 1 | 0 | 1 (1.2) | 1 | |
Abbreviations: AE, adverse event; GI, gastrointestinal; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Data are for the on-treatment period (the time during which treatment with any dose of trial intervention was given within the previous 49 days [after excluding any temporary interruptions in taking trial intervention]) unless indicated otherwise.
Pooled placebo data.
Data are for the in-trial period (the time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention).
Identified via MedDRA (version 23.1) searches.
Serious AEs were reported by 7.9% (n = 10) with semaglutide, 11.0% (n = 14) with liraglutide, and 7.1% (n = 6) with placebo (Table 3). Permanent treatment discontinuations because of AEs were more common with liraglutide (12.6% [n = 16]) vs semaglutide (3.2% [n = 4]) and placebo (3.5% [n = 3]); half of liraglutide discontinuations were gastrointestinal-related and tended to occur during dose escalation (Table 3; eFigure 9 in Supplement 3). There was no clustering of AEs leading to discontinuation by system organ class with semaglutide and placebo. No deaths occurred.
Gallbladder-related disorders (mostly cholelithiasis) were reported by 0.8% (n = 1) with semaglutide, 3.1% (n = 4) with liraglutide, and 1.2% (n = 1) with placebo. One participant (liraglutide group) reported subclinical pancreatitis that did not require treatment. Malignant neoplasms occurred in 2.4% with semaglutide (n = 3; basal cell carcinoma, clear cell renal cell carcinoma, and invasive ductal breast carcinoma), 2.4% with liraglutide (n = 3; basal cell carcinoma, invasive ductal breast carcinoma, and invasive lobular breast carcinoma), and 1.2% with placebo (n = 1; invasive ductal breast carcinoma). More participants reported psychiatric-related AEs with liraglutide than semaglutide or placebo (Table 3), driven by differences in insomnia events (semaglutide: n = 3 [2.4%]; liraglutide: n = 7 [5.5%]; placebo: n = 2 [2.4%]). Other AEs are reported in Table 3.
At week 68, the estimated mean change in pulse (assessed post hoc for the trial product estimand) was 5.4 beats/min (95% CI, 3.7 to 7.1) with semaglutide, 4.3/min (95% CI, 2.5 to 6.0) with liraglutide, and 1.2/min (95% CI, –0.9 to 3.2) with placebo. At week 75, observed mean (SD) changes from baseline were 2 (10)/min with semaglutide, 3 (9)/min with liraglutide, and 2 (10)/min with placebo.
Discussion
Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity, resulted in significantly greater weight loss at 68 weeks, accompanied by significantly greater improvements in several cardiometabolic risk factors.
Semaglutide and liraglutide induce weight loss by lowering energy intake.19,20,21,22 However, the reduction in caloric intake vs placebo appears to be larger with semaglutide (35%) than liraglutide (approximately 16%).19,20 Semaglutide has also been associated with reductions in food cravings, which is less evident with liraglutide, suggesting different mechanisms of energy intake regulation.20,21,22 Further research is needed to investigate whether structural differences affect these mechanisms, for example, by allowing semaglutide to target a wider range of neuronal GLP-1 receptors than liraglutide.
Obesity is a chronic disease associated with multiple complications, including type 2 diabetes, nonalcoholic fatty liver disease, and cardiovascular disease, which place significant burdens on individuals and health care systems.23,24,25,26 Treatment guidelines recommend 5% to 15% weight loss to improve these conditions,23,24,25 with associated health care expenditure savings.27 In this trial, the odds of achieving these clinically meaningful levels of weight loss were significantly greater with semaglutide vs liraglutide, accompanied by significant improvements in several cardiometabolic parameters. Whether semaglutide could be beneficial in preventing progression of cardiometabolic disease will be evaluated in the SELECT trial (NCT03574597).28
The rates of AEs, which were mostly gastrointestinal-related, were similar with semaglutide and liraglutide in this trial, consistent with previous trials.2,3,4,5,9,11,29,30,31,32 More insomnia events occurred with liraglutide than semaglutide. Liraglutide has previously been found to slightly increase rates of insomnia and suicidal ideation/behavior when assessed post hoc, but is not associated with increased depression or suicidality indicators when assessed prospectively.33
In contrast with previous trials,9,34 there were more discontinuations (AE-related and permanent discontinuations for any reason) with liraglutide than semaglutide in the present trial. There are several potential reasons for this. First, liraglutide has a shorter half-life (13-15 hours) than semaglutide (165 hours),1,6,8 potentially causing a more abrupt, and thus noticeable, return in hunger on pausing liraglutide vs semaglutide treatment. This could have negatively affected efficacy perceptions among the liraglutide group, leading some to permanently stop treatment. Second, the response to poor tolerance of the maintenance dose differed for semaglutide and liraglutide (see the Limitations subsection). Third, half of the AE-related discontinuations with liraglutide were gastrointestinal-related, potentially exacerbated by the weekly dose escalation schedule used, per the approved label.6 Fourth, liraglutide is dosed more frequently than semaglutide; dosing frequency is a key attribute for patients with type 2 diabetes when choosing a GLP-1RA treatment.35 However, treatment satisfaction, including perceived efficacy, was not assessed and so the effect on the discontinuation rate remains unclear. No differences in treatment satisfaction have been identified between semaglutide and liraglutide in patients with type 2 diabetes, potentially because of the satisfactory glycemic control provided by liraglutide.34 More research in the context of obesity is needed.
The results of this trial were generally consistent with previous semaglutide and liraglutide trials. The effect of semaglutide on body weight over liraglutide was similar to that in the phase 2 trial in obesity,9 while the placebo-adjusted weight loss and adverse effect profiles were similar to that in STEP 1 for semaglutide2 and in SCALE Obesity and Prediabetes for liraglutide.6,11
The COVID-19 pandemic occurred during this trial, with some visits conducted via telephone and not all planned body weight assessments obtained. However, 92% of participants in each treatment group had body weight assessments at week 68, suggesting no major effect on the efficacy findings.
This trial found weight loss with semaglutide was significantly greater than with liraglutide. However, the variability in treatment response means an individual’s tolerance and sensitivity to a specific treatment is important for obesity management.23,24,25,36 Therefore, having multiple antiobesity medications proven to lower body weight through different mechanisms, with different adverse effect profiles and dosing regimens, can only benefit clinicians and patients.
Limitations
This trial has several limitations. First, the response to poor tolerance of the maintenance dose differed; semaglutide was administered at a lower dose, whereas liraglutide was discontinued and had to be reescalated if restarted. This difference ensured the liraglutide regimen was consistent with the approved prescribing information,6 but could have led to more participants permanently discontinuing liraglutide after an AE than semaglutide. Furthermore, weight loss achievable with liraglutide could have been affected as participants may have continued with treatment for a shorter period of time, thus deriving less benefit, and potentially introducing bias into the treatment comparisons. A crossover trial with a washout period could clarify the reasons for, and effects of, the greater discontinuation rate with liraglutide.
Second, dosing differences meant participants knew which active treatment they could potentially receive. The potential bias in the treatment comparisons was mitigated by the matched double-blind placebo controls, but this could have been further improved with a double-dummy approach. This, however, would have necessitated a greater number of injections for participants (8 per week) and so was not chosen for this trial.
Third, missing data were handled through multiple imputation, which can potentially introduce bias because there may be differences between the participants for whom data are imputed and those used for the imputation. However, retention in this trial was high, so the number of participants with missing data that needed to be imputed was low. Furthermore, the sensitivity analyses confirmed the primary analysis was robust.
Conclusions
Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide, compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity, resulted in significantly greater weight loss at 68 weeks.
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Eligibility Criteria
eAppendix 2. Supportive Secondary, Exploratory, and Post Hoc Efficacy End Points
eAppendix 3. Estimands
eAppendix 4. Prespecified Sensitivity Analyses
eAppendix 5. Statistical Analysis for the Trial Product Estimand
eTable 1. Analysis and Imputation Methods to Address the Treatment Policy and Trial Product
Estimands for the Primary and Confirmatory Secondary End Points in the Statistical Testing Hierarchy
eTable 2. Assumptions and Marginal Power Used in the Sample Size Calculation
eTable 3. Participant Disposition by Trial Site
eTable 4. Change in Efficacy Outcomes from Baseline to Week 68a (Trial Product Estimand; Full Analysis Set)
eTable 5. Change in Efficacy Outcomes from Baseline to Week 68 for the Placebo Group (Treatment Policy and Trial Product Estimands; Full Analysis Set)
eTable 6. Other Reasons for Premature Treatment Discontinuation Among Trial Completers
eTable 7. Participants Who Were Below the Target Dose
eFigure 1. Trial Design
eFigure 2. Cumulative Distribution Plot of Percent Change in Body Weight from Baseline to Week 68 (Observed In-Trial and On-Treatment Data; Full Analysis Set)
eFigure 3. Proportions of Participants Achieving Weight Loss Thresholds at Week 68 (Observed In-Trial and On-Treatment Data; Full Analysis Set)
eFigure 4. Time to First Discontinuation and Time to Permanent Discontinuation of Trial Product (Observed Data; Full Analysis Set)
eFigure 5. Percent Change in Body Weight from Baseline to Week 68a (Observed On-Treatment Data; Full Analysis Set)
eFigure 6. Change in Selected Cardiovascular-Related Efficacy Outcomes from Baseline to Week 68 (Observed In-Trial Data; Full Analysis Set)
eFigure 7. Change in Fasting Lipid Profile from Baseline to Week 68 (Treatment Policy Estimand; Full Analysis Set)
eFigure 8. Prevalence of Gastrointestinal Events by Severity (Observed On-Treatment Data; Safety Analysis Set)
eFigure 9. Time to Onset of First Adverse Event Leading to Permanent Trial Product Discontinuation (Observed On-Treatment Data; Safety Analysis Set)
eReferences
Nonauthor Collaborator Spreadsheet. STEP 8 Investigators
Data Sharing Statement
References
- 1.US Food and Drug Administration . Wegovy prescribing information. Accessed June 11, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215256
- 2.Wilding JPH, Batterham RL, Calanna S, et al. ; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 3.Davies M, Færch L, Jeppesen OK, et al. ; STEP 2 Study Group . Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Bailey TS, Billings LK, et al. ; STEP 3 Investigators . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino D, Abrahamsson N, Davies M, et al. ; STEP 4 Investigators . Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425. doi: 10.1001/jama.2021.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . Saxenda prescribing information. Accessed May 19, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321
- 7.Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155. doi: 10.3389/fendo.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370-7380. doi: 10.1021/acs.jmedchem.5b00726 [DOI] [PubMed] [Google Scholar]
- 9.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-649. doi: 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 10.Yadav K, Lewis RJ. Gatekeeping strategies for avoiding false-positive results in clinical trials with many comparisons. JAMA. 2017;318(14):1385-1386. doi: 10.1001/jama.2017.13276 [DOI] [PubMed] [Google Scholar]
- 11.Pi-Sunyer X, Astrup A, Fujioka K, et al. ; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration . Guidance document: developing products for weight management, revision 1. Published February 2007. Accessed October 29, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/developing-products-weight-management-revision-1
- 13.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . Efficacy guidelines: E9, statistical principles for clinical trials. Accessed May 19, 2021. https://www.ich.org/page/efficacy-guidelines
- 14.US Food and Drug Administration . E9(R1) Statistical principles for clinical trials: addendum: estimands and sensitivity analysis in clinical trials. Published May 2021. Accessed May 19, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9r1-statistical-principles-clinical-trials-addendum-estimands-and-sensitivity-analysis-clinical
- 15.Wharton S, Astrup A, Endahl L, et al. Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data. Int J Obes (Lond). 2021;45(5):923-933. doi: 10.1038/s41366-020-00733-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy BW. Missing data in clinical trials for weight management. J Biopharm Stat. 2016;26(1):30-36. doi: 10.1080/10543406.2015.1094814 [DOI] [PubMed] [Google Scholar]
- 17.Little RJA, Rubin D B. Statistical Analysis With Missing Data. John Wiley & Sons; 1987. [Google Scholar]
- 18.American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24. doi: 10.2337/dc17-S005 [DOI] [PubMed] [Google Scholar]
- 19.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784-793. doi: 10.1038/ijo.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754-762. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi: 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao AM, Wadden TA, Walsh OA, et al. Effects of liraglutide and behavioral weight loss on food cravings, eating behaviors, and eating disorder psychopathology. Obesity (Silver Spring). 2019;27(12):2005-2010. doi: 10.1002/oby.22653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yumuk V, Tsigos C, Fried M, et al. ; Obesity Management Task Force of the European Association for the Study of Obesity . European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402-424. doi: 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvey WT, Mechanick JI, Brett EM, et al. ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 25.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875-E891. doi: 10.1503/cmaj.191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divino V, Ramasamy A, Anupindi VR, et al. Complication-specific direct medical costs by body mass index for 13 obesity-related complications: a retrospective database study. J Manag Care Spec Pharm. 2021;27(2):210-222. doi: 10.18553/jmcp.2020.20272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. Pharmacoeconomics. 2015;33(7):707-722. doi: 10.1007/s40273-014-0230-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan DH, Lingvay I, Colhoun HM, et al. Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design. Am Heart J. 2020;229:61-69. doi: 10.1016/j.ahj.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 29.Astrup A, Carraro R, Finer N, et al. ; NN8022-1807 Investigators . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843-854. doi: 10.1038/ijo.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadden TA, Hollander P, Klein S, et al. ; NN8022-1923 Investigators . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443-1451. doi: 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- 31.Davies MJ, Bergenstal R, Bode B, et al. ; NN8022-1922 Study Group . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314(7):687-699. doi: 10.1001/jama.2015.9676 [DOI] [PubMed] [Google Scholar]
- 32.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40(8):1310-1319. doi: 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neil PM, Aroda VR, Astrup A, et al. ; Satiety and Clinical Adiposity—Liraglutide Evidence in individuals with and without diabetes (SCALE) study groups . Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529-1536. doi: 10.1111/dom.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi: 10.1016/j.diabet.2019.101117 [DOI] [PubMed] [Google Scholar]
- 35.Hauber AB, Nguyen H, Posner J, Kalsekar I, Ruggles J. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin. 2016;32(2):251-262. doi: 10.1185/03007995.2015.1117433 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen SD, Manjoo P, Wharton S. Canadian adult obesity clinical practice guidelines: pharmacotherapy in obesity management. Accessed August 10, 2021. https://obesitycanada.ca/guidelines/pharmacotherapy/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Eligibility Criteria
eAppendix 2. Supportive Secondary, Exploratory, and Post Hoc Efficacy End Points
eAppendix 3. Estimands
eAppendix 4. Prespecified Sensitivity Analyses
eAppendix 5. Statistical Analysis for the Trial Product Estimand
eTable 1. Analysis and Imputation Methods to Address the Treatment Policy and Trial Product
Estimands for the Primary and Confirmatory Secondary End Points in the Statistical Testing Hierarchy
eTable 2. Assumptions and Marginal Power Used in the Sample Size Calculation
eTable 3. Participant Disposition by Trial Site
eTable 4. Change in Efficacy Outcomes from Baseline to Week 68a (Trial Product Estimand; Full Analysis Set)
eTable 5. Change in Efficacy Outcomes from Baseline to Week 68 for the Placebo Group (Treatment Policy and Trial Product Estimands; Full Analysis Set)
eTable 6. Other Reasons for Premature Treatment Discontinuation Among Trial Completers
eTable 7. Participants Who Were Below the Target Dose
eFigure 1. Trial Design
eFigure 2. Cumulative Distribution Plot of Percent Change in Body Weight from Baseline to Week 68 (Observed In-Trial and On-Treatment Data; Full Analysis Set)
eFigure 3. Proportions of Participants Achieving Weight Loss Thresholds at Week 68 (Observed In-Trial and On-Treatment Data; Full Analysis Set)
eFigure 4. Time to First Discontinuation and Time to Permanent Discontinuation of Trial Product (Observed Data; Full Analysis Set)
eFigure 5. Percent Change in Body Weight from Baseline to Week 68a (Observed On-Treatment Data; Full Analysis Set)
eFigure 6. Change in Selected Cardiovascular-Related Efficacy Outcomes from Baseline to Week 68 (Observed In-Trial Data; Full Analysis Set)
eFigure 7. Change in Fasting Lipid Profile from Baseline to Week 68 (Treatment Policy Estimand; Full Analysis Set)
eFigure 8. Prevalence of Gastrointestinal Events by Severity (Observed On-Treatment Data; Safety Analysis Set)
eFigure 9. Time to Onset of First Adverse Event Leading to Permanent Trial Product Discontinuation (Observed On-Treatment Data; Safety Analysis Set)
eReferences
Nonauthor Collaborator Spreadsheet. STEP 8 Investigators
Data Sharing Statement


