Abstract
Ginsenoside Rb1 (Rb1), one of the most important ingredients in Panax ginseng Meyer, has been confirmed to have favorable activities, including reducing antioxidative stress, inhibiting inflammation, regulating cell autophagy and apoptosis, affecting sugar and lipid metabolism, and regulating various cytokines. This study reviewed the recent progress on the pharmacological effects and mechanisms of Rb1 against cardiovascular and nervous system diseases, diabetes, and their complications, especially those related to neurodegenerative diseases, myocardial ischemia, hypoxia injury, and traumatic brain injury. This review retrieved articles from PubMed and Web of Science that were published from 2015 to 2020. The molecular targets or pathways of the effects of Rb1 on these diseases are referring to HMGB1, GLUT4, 11β-HSD1, ERK, Akt, Notch, NF-κB, MAPK, PPAR-γ, TGF-β1/Smad pathway, PI3K/mTOR pathway, Nrf2/HO-1 pathway, Nrf2/ARE pathway, and MAPK/NF-κB pathway. The potential effects of Rb1 and its possible mechanisms against diseases were further predicted via Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and disease ontology semantic and enrichment (DOSE) analyses with the reported targets. This study provides insights into the therapeutic effects of Rb1 and its mechanisms against diseases, which is expected to help in promoting the drug development of Rb1 and its clinical applications.
Keywords: Ginsenoside Rb1, Pharmacological effect, Effect mechanism, Perspectives
Graphical abstract
1. Introduction
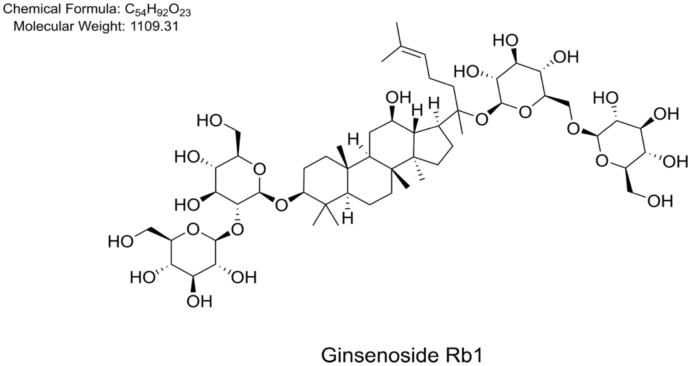
Panax ginseng Meyer, one of the most famous and valuable herbal medicines in East Asia, has been widely used as an “invigorating qi and strengthening yang” drug for the treatment of various critical illnesses in several countries in Asia for thousands of years [1]. P. ginseng belongs to the genus Panax of the Araliaceae family [2]. Plants of the same genus also include P. japonicus, P. notoginseng, P. quinquefolius, P. vietnamensis, and so on [3,4]. Ginsenosides were confirmed to be the main active components in P. ginseng. Recently, ginsenoside extracts derived from P. ginseng roots and rhizomes have been utilized as an adjuvant for the treatment of multiple diseases, including diabetes, cardiovascular diseases, and nervous system diseases, in China. Ginsenoside Rb1 (Rb1, Fig. 1), a dammarane triterpene saponin, is one of the most important components of ginseng and considered to have a large contribution to the therapeutic effects of this herb. Studies have reported that Rb1 has numerous beneficial effects on the human body, including the cardiovascular and central nervous system activities, antidiabetic and antitumor activities [[5], [6], [7]]. The ability of Rb1 to reduce antioxidative stress, suppress inflammation, regulate the process of cell autophagy, inhibit cell apoptosis, regulate sugar and lipid metabolism, and adjust various cytokines is due to its favorable properties. Rb1 plays many beneficial roles by regulating the expression of essential proteins such as HMGB1, GLUT4, 11β-HSD1, ERK, Akt, Notch, NF-κB, MAPK, PPAR-γ, with associated pathways involving TGF-β1/Smad pathway, PI3K/mTOR pathway, Nrf2/HO-1 pathway, Nrf2/ARE pathway, and MAPK/NF-κB pathway.
Fig. 1.
Chemical structure of ginsenoside Rb1.
The pharmacodynamics and action mechanisms of Rb1 have been investigated extensively. Several reviews have outlined the pharmacological activities of a single aspect of Rb1, such as its antidiabetic properties [8], neuroprotective effects [9], and therapeutic effects on myocardial ischemia–reperfusion injury (MIRI) [10]. However, summarizing and generalizing various drugs to a single system or disease is not the best option for exploring their therapeutic effects. The occurrence of a disease or the therapeutic effect of a drug is due to the co-interaction of various factors, including multiple genes, pathways, and even systems. Moreover, these reviews were published many years ago, and an updated outline of the latest research findings on Rb1 is lacking. Hence, an in depth review on the research progress about Rb1 in the last years is valuable and demanding. In this paper, studies published from 2015 to 2020 and archived in PubMed and Web of Science databases were searched using the keyword “Rb1”. The pharmacological effects, targets, and pathways of Rb1 were subsequently focused on. The possible regulatory pathways and targets of this active ingredient against neuronal diseases, cardiovascular diseases, diabetes, and their complications were summarized. The other potential effects and mechanisms of Rb1 against the said diseases were predicted via network pharmacology, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, and disease ontology semantic and enrichment (DOSE) analysis based on the reported targets. This study provides insights into the therapeutic effects of Rb1 and its mechanisms against diseases, which is expected to help in promoting the drug development of Rb1 and its clinical applications.
2. Nervous system
2.1. Effects of Rb1 on the brain
In general, acute ischemic stroke is caused by vascular occlusion and cerebral blood flow obstruction, leading to neurological dysfunction and brain infarction. Thus, this disease is a grave threat to human health and life [11,12]. Recent studies have indicated that Rb1 can promote functional recovery after cerebral ischemic injury, improve abnormal cerebral ischemic microenvironment, inhibit nervous cell apoptosis, release inflammatory factors after cerebral ischemia, and protect brain tissues (Table 1).
Table 1.
Summary of the protective effect and mechanism of ginsenoside Rb1 on cerebral ischemia-related injury.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Cerebral ischemia, pseudo-germ-free | MCAO, Intragastrical administration of neomycin sulfate combined with streptomycin | Sprague-Dawley rats | ↓IL-1β, IL-6, TNF-α | Regulation of Lactobacillus helveticus abundance and GABAA receptor | [16] |
| Cerebral ischemia | Middle cerebral artery occlusion (MCAO) | C57BL/6 J mice | ↓NADPH, ROS, NOX-1; ↑GSH | ↓ERK | [22] |
| Cerebral ischemia/reperfusion | MCAO | C57BL/6 mice | Axonal regeneration | ↑cAMP/PKA/CREB | [15] |
| Cerebral ischemia/reperfusion | MCAO | Sprague-Dawley rats | ↓Infarct size, Neurological deficit scores; Blood Brain Barrier (BBB) permeability | ↓Cx43, AQP4 (cerebral) | [13] |
| Traumatic Brain Injury | Craniotomy | Wistar rats | ↓Brain infarct volume, Brain edema, Neuronal deficit | ↓ERK1/2, Cx40 | [14] |
| Cerebral ischemia/reperfusion | MCAO | Wistar rats | ↓Infarct size, Caspase-3, Caspase-9, TNF-α, IL-6, NO, iNOS | ↓HMGB1, NF-κB | [23] |
| Abnormal hippocampal microenvironment | Microperfusion of L-Glu and Ca2+ in the rat hippocampus | Sprague-Dawley rats | ↓Glu, Cyt-C; Increased the regional cerebral blood flow and the stability of neuronal ultrastructure in the hippocampal CA1 region and improved the adaptability of neurons | ↑GLT-1, ↓NMDAR | [21] |
| Artificial Abnormal Hippocampal Microenvironment | Microperfusion of L-Glu and Ca2+ in the rat hippocampus | Sprague-Dawley rats | Alleviated Memory Deficit; Morphological Changes in Hippocampus | ↑P-Akt/P-mTOR; ↓P-PTEN | [20] |
Abbreviations are as shown in the literature. (↓), down-regulation or inhibition; (↑), up-regulation or activation.
2.1.1. Rb1 promotes functional recovery after cerebral ischemic injury
Aquaporin (AQP) is a kind of proteins with pores located on the cell membrane. AQP maintains the water balance and the homeostasis of the internal environment. AQP 4 (AQP4) influences ischemic cerebral edema. Rb1 can reduce the neurological deficit score of rats with focal cerebral ischemia-reperfusion, decrease infarction area, and downregulate the expression levels of connexin 43 and AQP4 [13]. Recent studies have demonstrated that Rb1 has a protective effect on traumatic brain injury in mouse models, and the mechanism is closely related to the downregulation of Cx40 expression and partially mediated by phosphorylation of the ERK1/2 signaling pathway [14]. Furthermore, Rb1 can promote the release of neurotransmitters in the middle cerebral artery occlusion (MCAO) animal models through the cAMP-dependent protein kinase A (PKA) pathway, which is related to axon regeneration [15,16].
2.1.2. Rb1 attenuates the abnormality in cerebral ischemia microenvironment
The abnormal microenvironment of central brain neurons under an ischemic state is the critical factor that causes cerebral damage. Mitochondrial stress caused by the increase in the local concentration of glutamate (Glu), calcium overload, and cytochrome C (Cyt-C) release is an essential cause of abnormal cerebral ischemic microenvironment [[17], [18], [19]]. Microperfusion of L-Glu and Ca2+ in the rat hippocampus can cause abnormalities of the brain microenvironment, which are closely related to cerebral ischemia. Rb1 increases the local cerebral blood flow in the hippocampal CA1 area and promotes the stability of neuron ultrastructure. In addition, Rb1 can inhibit Ca2+ overload, reduce the release of Cyt-C, alleviate neuron damage due to mitochondrial stress, and improve microenvironment abnormality, thereby effectively protecting the central neurons of ischemic injury. The mechanism might be related to the inhibition of NMDAR and p-PTEN protein expression, as well as to the activation of the p-AKT/p-mTOR signaling pathway [20,21].
2.1.3. Rb1 inhibits apoptosis and the release of inflammatory factors
During cerebral ischemia or stroke, the activation of extracellular signal-regulated kinase (ERK) may lead to early gene induction, triggering cell damage mechanisms, such as the production of cytokines, free radicals, or other inflammatory mediators. Rb1 treatment can substantially improve neurological deficits in the MCAO model with reduced infarct size of brain tissues. The mechanism of this protective effect is due to the blocking of oxidative stress and ERK signal activation [22]. High mobility group 1 (HMGB1) is released after focal cerebral ischemia/reperfusion (I/R), which aggravates brain injury. Liu et al. [23] adopted the MCAO model to investigate the protective effects of Rb1 on focal cerebral ischemia–reperfusion injury in rats and its mechanism. Their results showed that Rb1 could reduce the apoptosis induced by I/R by downregulating the levels of Caspase-3 and Caspase-9. Moreover, they observed that Rb1 inhibited the release of HMGB1 and decreased the levels of nuclear factor-κB (NF-κB), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and inducible nitric oxide synthase (NOS) and nitric oxide (NO) in MCAO rats. The mechanism of these effects is related to the inhibition of HMGB1 and inflammatory signal.
2.2. Rb1 increases resistance to spinal cord injury
Spinal cord injury (SCI) is a devastating neurological disorder. The neuropathology of SCI is very complicated and can be divided into primary injury and secondary injury. Primary injury includes mechanical compression caused by an external force that is mainly manifested as bleeding and changes in cell electrolyte. Secondary injury, which includes oxidative stress, inflammation, excitotoxicity, and apoptosis, can cause further damage to the spinal cord. Recent studies have shown that the neuroprotective effects of Rb1 on SCI could be related to inhibiting cell autophagy, antioxidative damage, reducing apoptosis after spinal cord ischemia–reperfusion, and decreasing intercellular edema (Table 2).
Table 2.
Summary of the protective effect and mechanism of ginsenoside Rb1 on spinal cord ischemic injury.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Spinal cord ischemia-reperfusion injury | Oxygen-glucose deprivation/Reoxygenation-induced | Primary astrocytes | ↑BDNF, NGF | ↑AQP4 (spinal cord) | [35] |
| Spinal cord ischemia-reperfusion injury | Abdominal aortic occlusion | Sprague-Dawley rats | ↓ Neural cell Apoptosis in the spinal cord, Improved hindlimb locomotor dysfunction | ↓ Bax/Bcl-2 ratio, Caspase-3 and p-Ask-1 | [30] |
| Spinal cord ischemia-reperfusion injury | Abdominal aortic occlusion | Sprague-Dawley rats | ↓Apoptosis; improves impaired nerve function | Restore the expression level of AQP4 in the spinal cord | [34] |
| Compressive Spinal Cord Injury | Laminectomy of the lower thoracic cord (Th12) vertebrae; | Wistar rats | Ameliorated Basso-Beattie Bresnahan score, Improved rearing activity and increased neural density | ↑Bcl-xL, VEGF | [32] |
| Oxidative stress injury in rat spinal cords | The T10 chest segment was exposed and injured with a heavy hammer | Sprague-Dawley rats | ↓MDA; ↑SOD, CAT, GSH | ↑eNOS/Nrf2/HO-1 | [51] |
| Spinal Cord Injury | Four-level T7-T10 laminectomy | Sprague-Dawley rats; PC12 | ↓Neuronal Apoptosis and autophagic | ↓Autophagy | [25] |
| Spinal cord ischemia-reperfusion injury | Artery occlusion | Sprague-Dawley rats | ↑SOD, Survivin protein; ↓Apoptosis, Oxidative stress, MDA | ↑SOD, Survivin protein | [31] |
| Experimental Autoimmune Encephalomyelitis | MBP68−82-Induced Acute EAE Model | C57BL/6 mice | Decreased behavioral impairment | Suppressing Th1 and Th17 Cells and Upregulating Regulatory T Cells | [26] |
2.2.1. Rb1 inhibits cellular autophagy
Autophagy is an intracellular degradation process in which misfolded proteins and damaged organelles are engulfed and degraded, which plays a crucial role in maintaining intracellular homeostasis [24]. Autophagy plays an essential role in spinal cord injury. Rb1 participates in neuroprotection by regulating autophagy. Rb1 treatment increased PC12 cell survival and inhibited apoptosis by suppressing excessive autophagy, whereas rapamycin-stimulated autophagy abolished the antiapoptotic effect of Rb1 [25]. In vivo, Rb1 treatment reduced motor neuron loss and promoted functional recovery in spinal cord injury models. Rb1 inhibited autophagy of neurons in SCI models and suppressed neuronal apoptosis and autophagic cell death. Taken together, the neuroprotective effect of Rb1 on SCI may be related to the inhibition of cellular autophagy. Moreover, Rb1 can reportedly alleviate experimentally induced autoimmune encephalomyelitis by inhibiting the differentiation of Th1 and Th17 cells and activating T cells [26]. These observations provide a basis to consider Rb1 a potential therapeutic agent for SCI, and the exact mechanism needs to be investigated in more depth.
2.2.2. Rb1 improves spinal cord ischemia-reperfusion injury
Spinal cord ischemia-reperfusion injury (SCII) is the process of restoring blood supply after spinal cord ischemia that leads to further damage by inflammatory factor release, cellular edema, and neuronal apoptosis [[27], [28], [29]]. Apoptosis is one of the main mechanisms of SCII. Rb1 can inhibit the apoptosis of spinal cord neurons in rats with abdominal aortic occlusion and improve the motor dysfunction of hind limbs. The mechanism might be due to the protective effect of Rb1 on the spinal neurons of SCII rats by downregulating the expression levels of Caspase-3, p-Ask-1, and Bax/Bcl-2 ratio [30]. Rb1 increases superoxide dismutase (SOD) activity, decreases malondialdehyde (MDA) content, increases survivin protein expression, and reduces neuronal apoptosis in serum and spinal cord tissues of artery occlusion model rats, indicating that Rb1 pretreatment can protect the rat spinal cord from ischemia-reperfusion injury by antioxidation, promoting survivin protein expression, and inhibiting cell apoptosis [31]. Furthermore, the administration of Rb1 by vein can alleviate the ischemic brain injury of rats by upregulating the antiapoptotic factor Bcl-xL. After spinal cord compression injury, Rb1 can improve BBB score and neuron density in the anterior horn of rats. Rb1 not only upregulates the expression of Bcl-xL in the spinal cord but also upregulates the expression of nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) [32].
2.2.3. Rb1 regulates aquaporins
Aquaporins are a family of water channel proteins that have a crucial role in regulating the water content of cells under pathological and physiological conditions. AQP4 is expressed explicitly in the brain and spinal cord and plays an essential role in maintaining the correct water balance in brain tissue [33]. Acute cytotoxic edema is a critical pathogenic condition of SCII. To avoid or reduce cell edema has been one of the main goals of SCII treatment. Rb1 remarkably improves the expression of NGF and brain-derived neurotrophic factor (BDNF) in OGD/R-induced rats’ primary astrocytes by increasing AQP4 expression in vitro, which is consistent with the in vivo experimental results that Rb1 inhibits neuronal apoptosis and damage, enhances spinal AQP4 expression and improves neurological deficits in rats with spinal cord ischemia-reperfusion injury [34,35]. These studies indicated that Rb1 could alleviate edema in spinal cord cells and improve neurological function by adjusting AQP4 expression.
2.3. Rb1 delays the development of neurodegenerative diseases
Alzheimer's disease (AD) and Parkinson's disease (PD) are common neurodegenerative diseases [36]. Rb1 has neuroprotective effects on neurodegenerative diseases and can delay the development of degenerative diseases. Its products mainly include inhibiting neuroinflammation and oxidative stress, improving cognitive and memory disorders, and improving motor dysfunction (Table 3).
Table 3.
Summary of the role and mechanism of ginsenoside Rb1 in anti-Alzheimer's disease, Parkinson's disease, etc.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| AD | Aβ | SH-SY5Y cells | ↓PARP-1, Bax | Predict CAP1, CAPZB, TOMM40, and DSTN proteins | [38] |
| Memory deficit | SAMP8 mice | SAMP8 mice | Attenuate memory deficits | Nervous system development and mitogen-activated protein kinase signaling pathway | [42] |
| AD | Hippocampal CA1 injection of soluble Aβ1-40 | Sprague-Dawley rats | ↑ Memory capability | ↓Bax, Caspase-3; ↑Bcl-2 | [39] |
| AD | Hippocampal injection of soluble Aβ1-40 | Sprague-Dawley rats | Increases the percentages of positive cells of neural astrocytes and neuronal | Promote the proliferation and differentiation of neural stem cells | [40] |
| AD | Aβ1-40-lesioned | Kun Ming mice | Restore cognitive function, Aβ accumulation | Regulate lecithin, amino acid, sphingolipid metabolism | [41] |
| Cognitive Dysfunction | Isoflurane surgery | C57BL/6 J mice | ↑ PSD-95; ↓ ROS, IL-6, TNF-α; Attenuated synapse dysfunction | Oxidative stress and neuroinflammation associated mechanisms | [43] |
| PD | α-synuclein | BE (2)-M17 cells | ↑Cell viability | Inhibits fibrillation and toxicity of α-synuclein and disaggregates preformed fibrils | [47] |
| PD | MPTP | C57BL/6 mice | Ameliorate motor deficits; Prevents DA neuron death; Suppresses α-synuclein expression and astrogliosis | Nuclear translocation of NF-κB, Promotion of glutamate transporters | [46] |
| Memory deficit/PD | MPTP | C57BL mice | Prevent memory deficits; ↑ Glutamate transporter GLT-1 | Transynaptic α-synuclein/PSD-95 pathway | [45] |
| Blood-Brain Barrier Damage | METH and HIV-1 Tat protein | Sprague-Dawley rats | Alleviate Blood–Brain Barrier Damage | ↓MDA; ↑ GSH, SOD Anti-oxidation | [44] |
2.3.1. Therapeutic effects of Rb1 on AD
AD is a hidden and progressive neurodegenerative disease that is difficult to cure completely. The main pathological changes in AD are due to the abnormal deposition of amyloid β-protein (Aβ) in the brain cell matrix [37]. Rb1 pretreatment prevented Aβ from causing PARP-1 cleavage and elevated Bax levels, thereby preventing Aβ-induced neurotoxicity in SH-SY5Y cells. Results of proteomics studies showed that the proteins CAP1, CAPZB, TOMM40, and DATN might be the potential molecular target proteins of Rb1 in the AD protection mechanism [38]. In vivo, injection of soluble Aβ1-40 into the rat hippocampus can cause learning and memory impairment. Rb1 can alleviate cognitive impairment in rats, substantially reduce the levels of Bax and cleaved-Caspase-3 in the hippocampus, and upregulate Bcl-2 levels [39]. Moreover, Rb1 might decrease the nuclear pyknosis and pyramidal cell defects in the rat hippocampus by increasing the expression of nestin, glial fibrillary acidic protein, and nucleotide sugar epimerase protein in AD rats’ model, as well as promoting the proliferation and differentiation of endogenous neural precursor cells in brain, thereby improving the cognitive function in AD rat model [40]. Metabolomic studies found that Rb1 exerted anti-AD effects through the regulation of lecithin and amino acid metabolism [41]. At the genetic level, Rb1 improves the learning ability of the SAMP8 mouse model from multiple aspects, such as nervous system development and mitogen-activated protein kinase signaling pathway [42]. Moreover, Rb1 mitigates the isoflurane/surgery-induced elevated levels of ROS, TNF-α, and IL-6 in the mice hippocampus and attenuates cognitive impairment and synapse dysfunction. Therefore, the mechanisms refer to inhibiting neuroinflammation and oxidative stress [43]. Another study showed that Rb1 protected the blood-brain barrier of rats from damage caused by HIV-1 Tat protein and methamphetamine by upregulating tight junction proteins in the rat brain, including Occludin, JAM-A, Claudin-5, and ZO-1, and increasing antioxidant levels. These Rb1 properties provide a potential treatment option for patients with HIV-related neurocognitive disorders or other neurodegenerative diseases [44].
2.3.2. Effects of Rb1 on PD
PD, also known as paralysis agitans, is a neurodegenerative disease of the extrapyramidal system. α-Synuclein is a soluble protein expressed in the presynaptic and perinuclear areas of the central nervous system, and it is closely related to the pathogenesis and related dysfunction of PD. In vivo, Rb1 prevents 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced memory defects and impaired glutamatergic transmission in mice, increases PSD-95 expression in an α-synaptic nucleoprotein-dependent manner, indicating that its protective mechanism for memory function is related to the regulation of the α-synuclein/PSD-95 pathway [45]. Glutamate excitotoxicity is considered an essential factor in the degeneration of DA neurons in the pathogenesis of PD. Dysfunction of glutamate transporter is the key to dyskinesia. Rb1 can increase the expression of glutamate transporter through nuclear translocation of NF-κB and inhibit the excitotoxicity of Glu. Rb1 can regulate the substantia nigra striatum and cortico-mesenchymal glutamatergic transmission pathways, protect dopaminergic neurons, and inhibit α-synuclein expression and astrocyte proliferation ameliorating motor deficits in rat model of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced PD [46].
In vitro, Rb1 was shown for inhibiting α-synuclein-induced fibrillation and toxicity in BE (2)-M17 human neuroblastoma cells and to be able to disaggregate preformed fibrils and block the α-synuclein seeded polymerization possibly by binding and stabilizing non-toxic α-synuclein oligomers without β-sheet content [47].
2.4. Other effects of Rb on the nervous system
Recent studies have shown that Rb1 has a notable relieving effect on neuronal damage, anxiety, and depression (Table 4).
Table 4.
Summary of the protective effects of ginsenoside Rb1 on nerve cells and its anxiolytic and depressive effects.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Oxidative stress-mediated neurotoxicity in neuronal model | Rotenone | SH-SY5Y cells | ↓ROS, TBARS, Caspase-3 and Bax; ↑Bcl-2, SOD | ↑Nrf2 | [50] |
| Acute immobilization stress | Immobilization stress | Sprague-Dawley rats | Reverse acute immobilization stress | ↑BDNF/TrkB; ↓CORT, ACTH | [54] |
| Post-traumatic stress disorder | Single prolonged stress | Sprague-Dawley rats | Ameliorate behavior of anxiety | ↑BDNF, Hypothalamic neuropeptide Y, Locus coeruleus tyrosine hydroxylase; ↓CORT | [55] |
| Brain injury, neuron injury | Pentylenetetrazol -induced, Mg2+ free-induced | Sprague-Dawley rats, Hippocampal neurons | ↑Nrf2, HO-1, Bcl-2; ↓ iNOS, LC3 | ↑Nrf2/ARE | [53] |
| Neuronal acute inflammatory nociception | Formalin | Sprague-Dawley rats | ↓Spinal c-Fos expression, p-ERK | ↑Nrf2; ↓NF-κB, ERK | [52] |
| Chronic unpredicted mild stress | 10 various stressors in random order | Wistar rats | ↑5-HT, 5-HIAA, NE, DA | Mediated by central neurotransmitters of serotonergic, noradrenergic and dopaminergic systems | [56] |
| Chronic unpredicted mild stress | 10 various stressors in random order | Institute of cancer research (ICR) mice | ↑5-HT, 5-HIAA, NE, DA, GABA; ↓Glutamate | Both monoaminergic and aminoacidergic receptors may be involved in the antidepressant-like effect | [57] |
2.4.1. Rb1 protects neurons against oxidative stress injury
The imbalance of the redox reaction in the body leads to reactive oxygen species (ROS) accumulation, causing oxidative stress-mediated damage. Owing to the particular physiological and biochemical properties of nerve tissues, they are sensitive to ROS-mediated injury and ROS damage, including increased permeability of cell membranes, DNA damage, and activation of corresponding apoptotic genes [48,49]. Nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase (HO-1) pathway is considered the essential antioxidant target. Activating this pathway can trigger the corresponding antioxidant enzymes, thereby enhancing the cell's ability to scavenge ROS, maintaining redox balance, and reducing oxidative damage. In vitro, Rb1 remarkably ameliorates the imbalance of redox state and mitochondrial dysfunction in SH-SY5Y cells induced by rotenone-induced oxidative stress by reducing ROS and thiobarbituric acid reactive substances levels, as well as elevating GSH levels and SOD activity. Rb1 can also decrease Caspase-3 and Bax, increase Bcl-2 expression, and reduce apoptosis in cells [50].
In vivo, Rb1 can activate the eNOS/Nrf2/HO-1 signaling pathway and has a remarkable protective effect on oxidative stress injury in rat spinal cord, including substantially improving the hindlimb function score of rats with SCI, reducing the content of MDA, increasing the level of superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), and upregulating the expression of eNOS/Nrf2 and NAD(P)H quinone dehydrogenase 1 (Nqo1) protein [51]. Coincidentally, Jang et al. [52] found that Rb1 could attenuate formalin-induced acute inflammatory nociception in rats by regulating the Nrf2 and NF-κB pathways. Rb1 has a protective effect on pentylenetetrazol-induced oxidative stress and Mg2+ free radical-induced neuronal damage by activating the Nrf2/ARE signaling pathway [53].
The above study confirmed the protective effect of Rb1 regarding the oxidative stress-induced neuronal injury. Rb1 can enhance the expression of Nrf2 and HO-1 in the hippocampus of rats in vivo and in vitro. Its primary action mechanism is to activate Nrf2/HO-1, Nrf2/ARE signaling pathway, increase SOD and CAT activity, upregulate endothelial nitric oxide synthase (eNOS), GSH, and HO-1 levels, reduce ROS and MDA content, thus improving intracellular redox status, reducing the expression of pro-apoptotic genes and inflammatory factor release in neuronal cells of rats, so as to alleviate damage of rat neuronal cells induced by oxidative stress.
2.4.2. Effects of Rb1 on acute stress, anxiety, and depression
Acute stress or prolonged exposure to stressful conditions can lead to a decrease in the expression of BDNF and tyrosine kinase B (TrkB) in hippocampal midbrain-derived neuromaturation. Changes in BDNF and TrkB affect the outgrowth, survival, differentiation, maintenance, and protection of functions in different neuronal populations. Rb1 pretreatment reverses the decrease in BDNF/TrkB expression in the hippocampus of acutely stressed rats and increases the levels of plasma corticosterone and adrenocortical hormone [54]. Post-traumatic stress disorder (PTSD) can cause changes in the hypothalamo–pituitary-adrenal axis. Rb1 can attenuate the anxiety-like responses in model mice produced by PTSD. Rb1 restores the level of BDNF mRNA in the hippocampus and increases the content of neuropeptide Y (NPY) mRNA. Rb1 attenuates anxiety-related behavior and neurochemical reactions by modulating the expression of NPY and the central norepinephrine system [55]. The mechanism of the anxiolytic effect of Rb1 may be related to the upregulation of the GABA receptor, the expression of NPY, and the regulation of central norepinephrine system [16].Depression, which is a depressive disorder with high incidence, disability, and recurrence rate, is characterized by chronic and lasting depression symptoms. Studies have shown that Rb1 can substantially increase the levels of serotonin, 5-HIAA, norepinephrine, and dopamine in the brain of chronic unpredictable mild stress rat model. The antidepressant mechanism of Rb1 is mainly mediated by the central neurotransmitters of serotoninergic, norepinephrine, and dopaminergic systems. Monoaminergic and amino acid receptors are involved in the antidepressant effect of Rb1 on the mouse hippocampus (CA3) and prefrontal cortex [56,57]. However, the mechanism of protein expression signaling pathway after receptor activation requires further study to determine.
3. Cardiovascular system
3.1. Protective effects of Rb1 on the heart
Recent studies demonstrated that the cardioprotective effects of Rb1 are mainly reflected in the protection of myocardial hypoxia/ischemia and ischemia-reperfusion injury, improvement of mitochondrial dysfunction, and anti-heart failure. The mechanism of this action mainly involves the regulation of the pathways in which p38αMAPK, RhoA, Rho/ROCK, PI3K/mTOR, and estrogen receptor participate (Table 5).
Table 5.
Summarized the effects and mechanisms of ginsenoside Rb1 on cardio protection, mainly including myocardial ischemia-reperfusion, Hypoxia/Ischemia, Hypoxia/Reoxygenation.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| I/R | Coronary artery ligation | Sprague-Dawley rats | ↓Infarct size, Cardiomyocyte injury, Apoptosis; ↑Blood flow, ATP | ↓RhoA/ROCK1 | [65] |
| I/R | Coronary artery ligation | Sprague-Dawley rats | ↓TNF-α, Caspase-3, Apoptosis, Myocardial infarction size | ↓p38αMAPK | [64] |
| I/R; Hypoxia/Reoxygenation | Langendorff technique; hypoxia (1 % O2) for 4 h, followed by 1 h reoxygenation | ICR-mice; Sprague-Dawley rats; Primary neonatal rat ventricular myocytes | ↓Succinate, Glycolysis, Mitochondrial dysfunction, Apoptosis | ↓HIF-1α, CPT1; ↑PDH | [67] |
| I/R; Hypoxia/Reoxygenation | Langendorff technique; 95 % N2 and 5 % CO2 for 20 min | Sprague-Dawley rats, Neonatal rat cardiomyocytes | ↓Infarct size, Cell viability, LDH, CK | ↑Akt, GSK-3β; ↓Mitochondrial permeability transition pore | [68] |
| I/R | Coronary artery ligation | Sprague-Dawley rats | ↓Myocardial enzymes (CK-MB and Trop l) and CtsB, Infarct size | ↑mTOR | [111] |
| Hypoxia | CoCl2 | Neonatal rat cardiomyocytes | ↑Cell viability, ↓Autophagy | ↓AMPK | [58] |
| Hypoxia/Reoxygenation | In sealed airtight culture bag | H9C2 | ↓LDH, ROS, Cleaved-Caspase-3 | Prevents the continuous opening of the mitochondrial permeability transition pore, Stabilizes the mitochondrial membrane potential | [66] |
| Hypoxia/Eoxygenation | In anaerobic glove box | H9C2 | ↑SOD, GSH-px, CAT; ↓MDA, ROS, LDH ↓Apoptosis | ↓Caspase-3, 8, 9; Estrogen receptor-dependent crosstalk among the Akt, JNK, and ERK 1/2 | [62] |
| Hypoxia/Ischemia | W-Zip package (Oxide Anaerobe Pouch System) | Neonatal rat cardiomyocytes | ↑Cell viability; ↓Autophagy | ↑miR-29a, miR-208; ↓miR-21, miR-320 | [59] |
| Hypoxia/Ischemia | Induced with the MGC AnaeroPack System in AnaeroPack jar | Neonatal rat cardiomyocytes | ↑Cell viability; ↓Apoptosis | ↑miR-208; ↓NLK | [60] |
3.1.1. Rb1 protects against myocardial hypoxia/ischemia and ischemia-reperfusion injury
Myocardial ischemic injury is caused by severe blocking of the coronary blood supply, leading to myocardial cell apoptosis and necrosis. Protecting cardiomyocytes from excessive autophagy under ischemic hypoxia has become a mainstream research. In vitro, Rb1 can considerably improve the vitality of CoCl2-induced hypoxic of neonatal rat cardiomyocytes and enhance hypoxic-induced transitional autophagy by regulating the AMP-activated protein kinase (AMPK) pathway [58]. miRNA is a potential biomarker for ischemic heart disease, and the imbalance of miRNA plays a crucial role in the process of ischemic heart disease. Rb1 substantially reduces mortality of neonatal rat cardiomyocytes induced by ischemia and hypoxia in a dose-dependent manner probably by upregulating mir-1, mir-29a, and mir-208 and downregulating mir-21 and mir-320 [59,60]. Besides, Rb1 can reduce the calcium overload of rabbit ventricular myocytes and prevent the occurrence of premature ventricular beats and ventricular tachycardia in ischemia-reperfusion injury [61]. A label-free quantitative proteomics study of H9c2 cardiomyocytes induced by hypoxia/reoxygenation (H/R) found 29 differential proteins, including estrogen receptor alpha and estrogen receptor beta (ERβ). This study reported that Rb1 provides myocardial protection by inducing an estrogen receptor-dependent crosstalk among the Akt, JNK, and ERK1/2 pathways to prevent injury and apoptosis induced by H/R to H9c2 cardiomyocytes [62].
After ischemia and hypoxia, blood reperfusion can lead to further damage of myocardial cells, namely, MIRI, including increasing myocardial infarct size, enhancing myocardial fibrosis, and aggravating cardiac dysfunction [63]. Apoptosis is an essential link in the pathogenesis of I/R. In vivo, Rb1 can reduce the area of myocardial infarction, reduce Caspase-3 activity and TNF-α level in coronary artery ligation rats, and exert an antiapoptotic effect by inhibiting p38α MAPK phosphorylation. Moreover, Rb1 can bind to RhoA in a dose-dependent manner, inhibit the activation of the RhoA signaling pathway during cardiac I/R, and restore ATP production [64,65]. These studies suggested that the protection provided by Rb1 against MIRI might involve the regulation of the pathways where p38α MAPK, RhoA, and estrogen receptors are located.
3.1.2. Rb1 inhibits mitochondria-mediated apoptosis
Mitochondria-mediated apoptosis plays a critical role in MIRI. When MIRI occurs, the continued opening of the mitochondrial permeability transition pore (MPTP) leads to mitochondrial damage and ultimately to apoptosis. Mitochondrial oxidation increases the vulnerability of cardiac I/R injury. In vitro, Rb1 can reduce MPTP by stabilizing mitochondrial membrane potential (MMP) and reducing reactive oxygen species (ROS) during HR. Importantly, Rb1 protects mitochondria by reducing the release of cytochrome c and the expression of cleaved-caspase-3 in the cytoplasm, and ultimately reduces H9C2 cell apoptosis induced by hypoxia-reoxygenation (HR) [66]. Besides, Rb1 reduces primary neonatal rat ventricular myocyte apoptosis during hypoxia/reoxygenation injury by increasing PDH activity, blocking succinate-related HIF-1α activation, preventing cardiac acidification, and improving mitochondrial dysfunction [67]. In addition, Rb1 can alter mitochondrial membrane permeability, inhibit the opening of mitochondrial MPTP, and exert protective effects against H/R injury in neonatal rat cardiomyocytes by reducing lactate dehydrogenase (LDH) and creatine kinase (CK) levels and inducing Akt and GSK-3β phosphorylation [68].
3.1.3. Rb1 improves heart failure
Heart failure (HF) refers to the failure of the heart's systolic and diastolic functions to fully discharge the venous blood back to the heart, resulting in stagnation of the venous system and insufficient blood perfusion in the arterial system. Rb1 can slow down rats' heart rate, improve heart functions, and reduce the histological changes caused by HF. Rb1 can attenuate myocardial hypertrophy and myocardial fibrosis by reducing the levels of atrial natriuretic factor, β-myosin heavy chain, periostin, collagen I, angiotensin II (Ang II), Ang-converting enzyme, and Ang II type 1 receptor [69]. Moreover, Rb1 can reduce the mitochondrial membrane potential and increase the translocation of GLUT4 to the plasma membrane possibly by inhibiting the TGF-β1/Smad and ERK pathways and activating the Akt pathway. Rb1 can remarkably increase Rho-associated protein kinase (ROCK), which plays a vital role in the regulation of autophagy in the myocardial tissue of an acute HF animal model. Rb1 can exert anti-HF functions by regulating the Rho/ROCK and PI3K/mTOR pathways and inhibiting myocardial transition autophagy in rats [70].
3.2. Rb1 protects blood vessels
Accumulating evidence shows that the protective effects of Rb1 on blood vessels mainly include anti-atherosclerosis, inhibition of vascular calcification, and resistance to vascular endothelial cell damage (Table 6).
Table 6.
Summary of the effects and mechanisms of ginsenoside Rb1 on different targets related to vascular protection, mainly including anti-angiogenesis, anti-atherosclerosis, inhibition of vascular endothelial cell oxidation.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Anti-angiogenesis | Pre-miR-33a | HUVECs | ↑PEDF | ↑PPAR-γ; ↓miR-33a | [76] |
| Vascular calcification | β-glycerophosphate | Vascular smooth muscle cell (VSMC) | ↓ Calcium deposition | ↑PPAR-γ, ↓Wnt/β-catenin axis | [74] |
| Vascular calcification | Inorganic phosphate | VSMC | ↓ Calcium deposition, Apoptosis | ↑Gas6/p-Akt | [75] |
| Atherosclerotic | Ox-LDL | ApoE−/−mice | ↑Atherosclerotic plaque stability, Macrophage Autophagy | ↑AMPK | [112] |
| Atherosclerosis | IL-4, IL-13 | Peritoneal macrophages | Promoting anti-inflammatory M2 macrophage polarization | ↑IL-4, IL-13, STAT6 | [72] |
| Atherosclerosis | Western diet | ApoE−/−mice | ↓Apoptosis; ↑Autophagy Decrease atherosclerotic plaque area | ↓TC, TG, LDL-C, IL-1β, IL-6, TNF-α; ↑HDL-C | [73] |
| Vascular endothelium senescence | Ox-LDL | HUVECs | ↓Senescence; ↑Autophagy | ↑SIRT1/Beclin-1/Autophagy axis | [71] |
| Oxidative injury | H2O2 | HUVECs | ↑Cell viability, Migration, Invasion | ↓BNIP3; ↑miR-210; Modulating NF-κB and mTOR | [78] |
| Hyperhomocysteinemia | Homocysteine | Endothelial progenitor cells | ↑Adhesive and migratory ability; | ↑VEGF/p38MAPK, SDF-1/CXCR4 | [79] |
| Oxidative Stress | Ritonavir | HUVECs | ↑eNOS; ↓ROS | ↑SOD, ER-β | [77] |
3.2.1. Rb1 regulates lipid metabolism and improves atherosclerosis
Atherosclerosis is a disease caused by abnormal lipid metabolism. Macrophages form cholesterol-rich foam cells by phagocytosing oxidized LDLs, which is the main factor for atherosclerotic lesions. In vitro, Rb1 can alleviate ox-LDL-induced vascular endothelium senescence via the SIRT1/Beclin-1/autophagy axis [71]. Macrophages can differentiate into two antagonistic subtypes: M1 and M2, of which pro-inflammatory M1 macrophages can lead to a more vulnerable plaque, whereas anti-inflammatory M2 macrophages have protective effects. Rb1 can promote anti-inflammatory M2 macrophage polarization to enhance the stability of atherosclerotic plaques by increasing IL-4 and IL-13 production and STAT6 phosphorylation [72]. In vivo, Rb1 can reduce the accumulation of lipids in macrophage foam cells and atherosclerotic plaques. It can also alter plaque composition by activating autophagy in vivo, regulating serum levels of lipids such as TC, TG, LDL-C, and HDL-C in ApoE−/− mice, thus promoting the stability of atherosclerotic plaques. In addition, various types of apoptosis caused by inflammation are the pathological changes of atherosclerosis, including apoptosis of endothelial cells, vascular smooth muscle cells, and even foam cells. Inhibiting cell apoptosis can slow the development of atherosclerosis. Rb1 treatment can reduce the expression levels of Bax, Caspase-3, and Caspase-9 and inhibit the expression of inflammatory cytokines in mice serum [73]. In conclusion, Rb1 can promote the stability of atherosclerotic plaques and exert a therapeutic effect. It can attenuate apoptosis related to anti-inflammatory activity and regulate cell autophagy by promoting lipid metabolism and inhibiting lipid accumulation.
3.2.2. Rb1 inhibits vascular calcification
Vascular calcification is a pathological process of cardiovascular disease. It refers to the presence of abnormal calcium deposits in the walls of blood vessels, resulting in the loss of vascular elasticity and manifesting as reduced vasodilation and contraction, leading to various cardiovascular diseases. In vitro, Rb1 can improve calcium deposition and vascular smooth muscle cells (VSMC) osteogenesis transformation both in vivo and in vitro. Rb1 can improve β-glycerophosphate-induced calcification of VSMC by activating peroxisome proliferator-activated receptor γ (PPAR-γ), thereby inhibiting the activation of the Wnt/β-catenin pathway [74]. In addition, Rb1 can substantially inhibit inorganic phosphate-induced VSMC calcification in a concentration-dependent manner by restoring the expression of growth arrest-specific gene six inhibited by inorganic phosphate [75]. Moreover, Rb1 treatment induces pigment epithelium-derived factor (PEDF) protein expression in human umbilical vein endothelial cells (HUVECs) in a concentration- and time-dependent manner, and its mechanism might be related to the regulation of miR-33-a and the activation of the PPAR-γ signaling pathway, suggesting its anti-hematopoietic effect [76].
3.2.3. Rb1 confers resistance to endothelial oxidative stress injury
Ritonavir (RTV), a highly active anti-retroviral therapy drug, can cause endothelial dysfunction through oxidative stress. In vitro, Rb1 can reverse the vascular dysfunction caused by oxidative stress associated with long-term ritonavir (RTV) use. The mechanism might be that Rb1 binds to the estrogen receptor ER-β, decrease the production of ROS and increase the expression of eNOS and SOD, thereby inhibiting RTV-induced oxidative damage to human endothelial cells [77]. Bcl-2/E1B-19 kDa interacting protein 3 (BNIP3) participates in oxidative damage by modulating the targets, namely, mTOR and NF-κB, whereas miR-210 can inhibit its expression. Rb1 inhibits H2O2-induced oxidative damage to human endothelial cell line (EA.hy926) by upregulating miR-210, negatively regulating BNIP3 expression, and inhibiting the activation of NF-κB [78]. Besides, Endothelial progenitor cells (EPCs), primarily derived from the bone marrow, help repair blood vessel damage and tissue ischemia. Stromal cell-derived factor-1 (SDF-1) enhances EPC functions, and the SDF-1/CXCR-4 axis plays a crucial role in modulating the mobilization of EPCs from bone marrow. Interestingly, Rb1 can prevent homocysteine-induced endothelial damage via activation of VEGF/p38MAPK and SDF-1/CXCR4 pathways [79].
4. Diabetes and its complications
Rb1 not only lowers blood glucose level, increases insulin sensitivity of adipocytes, and regulates lipid metabolism but also alleviates the occurrence of T2DM-related complications, including diabetic cardiomyopathy, diabetic retinopathy, diabetic encephalopathy, and obesity (Table 7).
Table 7.
Summarized effects and mechanisms of ginsenoside Rb1 on different targets related to diabetes and complications, including diabetic retinopathy, diabetic encephalopathy, diabetic cardiomyopathy, obesity.
| Model | Inducer/Method | Animal/Cell | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Diabetic retinopathy | Lipopolysaccharides | Mouse RAW264.7 cells, Human ARPE19 cells | ↓IL-1, TNF-α, CCL2 | ↓miR-155 | [94] |
| Diabetic cardiomyopathy | HFD and low-dose streptozotocin | C57BL/6 mice | ↓Cardiac dysfunction, Abnormal cardiomyocytes calcium signaling | ↓O-GlcNAcylation of calcium handling proteins, RyR2, OGT | [89] |
| Diabetic retinopathy | High Glucose-Induced | rat retinal capillary endothelial cells | ↑Cell viability, mtDNA copy number; ↓ROS, NOx, PARP | ↓NAD-PARP-SIRT | [93] |
| Diabetic retinopathy | Streptozotocin | Wistar rats | ↓MDA; ↑GSH | ↑Nrf2, GCLC, GCLM | [92] |
| T2D | HFD | C57BL/C mice | ↑Insulin sensitivity | ↓11β-HSD1 | [83] |
| Glucose metabolism | Pyruvate, glucagon | C57BL/6 J mice, Primary hepatocytes | ↓Gluconeogenesis | ↓cAMP, CREB, MPC1 | [85] |
| Glucose metabolism | Rb1 | C2C12 myoblasts | ↑Translocation of GLUT4 | ↑Leptin receptors, Phosphorylation of STAT3, PI3K and ERK2 | [87] |
| T2D | High glucose-Induced | 3T3-L1 adipocyte cells | ↓IL-1β, IL-6, ER stress; ↑Insulin sensitivity | Dephosphorylation of IRE1a and PERK; ↓TXNIP/NLRP3, IRS-1/PI3K/Akt | [81] |
| Diabetic encephalopathy | Methylglyoxal | SH-SY5Y | ↑Bcl-2/Bax ratio, SOD, CAT, GSH; ↓ROS, MDA, Cleaved Caspase-3 and Cleaved Caspase-9 | ↑PI3K/Akt | [96] |
| Glucose metabolism | Rb1 | C2C12 myoblasts | ↑Translocation of GLUT4 | ↑AdipoR1 and AdipoR2 | [86] |
| Obesity | Fed a high-saturated fat diet | C57Bl/6 J male mice | ↑BNDF, Leptin sensitivity | ↑Leptin-JAK2-STAT3 | [100] |
| Browning Effect | Rb1, Black ginseng | 3T3-L1, primary white adipocytes | ↑PPARγ, PGC-1α; ↓C/EBPα, SREBP-1c | ↑AMPK | [98] |
| Obesity | Free fatty acids-induced oxidative stress and inflammation | 3T3-L1 | ↑eNOS, NO, SOD; ↓ROS | ↓NF-κB | [97] |
| T2D | Diabetic mice | Diabetic db/db mice | ↓Liver fat accumulation, Circulating FFA levels, TNF-α; ↑Insulin sensitivity, Adiponectin | ↑Perilipin expression | [82] |
4.1. Rb1 increases insulin sensitivity
Insulin resistance is a major challenge in diabetes treatment. It is characterized by impaired insulin signal transduction [80]. In vitro, Rb1 can improve insulin signal transduction by inhibiting the activation of NLRP3 inflammatory bodies associated with endoplasmic reticulum stress and inhibiting the inflammatory response of adipose tissues [81]. The antidiabetic effects of Rb1 in humans require further research. In vivo, Rb1 upregulates perilipin expression in the adipose tissues of db/db obese mice, reduces hepatic fat accumulation, and inhibits adipocyte lipolysis [82]. Moreover, Rb1 increases insulin sensitivity by reducing 11β-hydroxysteroid dehydrogenase I (11β-HSD1) levels in the liver and adipose tissue of mice with high-fat diet (HFD)-induced type 2 diabetes (T2D), indicating that Rb1 may exert its anti-diabetic effect by inhibiting the expression of 11β-HSD1 [83]. However, after human resistance exercise, supplementation with low-dose Rb1 did not change glucose metabolism and insulin levels probably because of the continued increase in sympathetic nerve activity [84]. Inflammatory molecules induce insulin resistance by upregulating the phosphorylation of insulin receptor substrate-1 at serine residues and impairing insulin PI3K/Akt signaling, resulting in decreased glucose uptake by adipocytes.
4.2. Effects of Rb1 on glucose metabolism
Liver mitochondrial pyruvate carrier (MPC), which is a complex of MPC1 and MPC2 subunits, promotes gluconeogenesis by transporting pyruvate to the mitochondria. cAMP-responsive element binding protein (CREB) transcriptionally upregulates MPC1 to provide pyruvate for gluconeogenesis. Rb1 reduces hepatic cAMP formation in mice, thereby reducing CREB-mediated induction of MPC1. Rb1 might contribute to limiting pyruvate-dependent hepatic glucose production [85]. In vitro, Rb1 can promote GLUT4 translocation by upregulating AdipoR1 and AdipoR2 proteins and stimulating adiponectin signaling in C2C12 muscle cells [86]. Coincidentally, another study found that Rb1 could stimulate the mRNA of leptin receptors OBRa and OBRb and the protein expression and phosphorylation of STAT3, PI3K, and ERK2 in C2C12 muscle cells, indicating that Rb1 could promote the transport of GLUT4, thereby achieving the purpose of lowering blood glucose by upregulating the leptin receptors and activating the PI3K signaling pathway [87].
4.3. Rb1 improves diabetic cardiomyopathy
Diabetic cardiomyopathy is a recognized cause of cardiac insufficiency secondary to chronic hyperglycemia and myocardial lipotoxicity. This disease promotes cardiomyocyte hypertrophy, interstitial fibrosis, and a decrease in myocardial contractile performance [88]. Treatment with Rb1 remarkably improves cardiac dysfunction and abnormal cardiomyocyte calcium signaling caused by diabetes in mice likely because of the fact that it suppresses Ca2+ leakage caused by overactivated ryanodine receptor 2 (RyR2) and it increases Ca2+ uptake by sarcoplasmic reticulum Ca2+-ATPase 2a. Moreover, Rb1 not only enhances energy metabolism (like metformin) and eliminates O-GlcNAcylation of calcium handling proteins to regulate calcium signaling but also directly inhibits RyR2 activity from regulating calcium signaling, indicating that Rb1 could be a kind of adjunct therapeutic substance that is more effective in treating diabetic cardiomyopathy [89].
4.4. Rb1 relieves diabetic retinopathy
Diabetic retinopathy (DR) is one of the main complications of diabetes. It is mainly caused by the apoptosis of retinal capillary endothelial cells (RCECs) when retinal blood vessels are exposed to high glucose environment [90,91]. In vitro, Rb1 treatment notably increases cell viability and mtDNA copy number and inhibits ROS generation. Treatment with Rb1 increases the activities of SOD and CAT and reduces those of NOX and PARP. In vivo, Rb1 can reduce the content of MDA in the retina of streptozotocin-induced diabetic retinopathy rat model and increase the content of GSH. Furthermore, Rb1 treatment markedly increases the level of nuclear factor erythroid two related factor 2 (BA) in rat retinal nuclei and the expression of glutathione cysteine ligase catalytic subunit and glutathione cysteine ligase modulatory subunit, indicating that Rb1 can alleviate diabetic retinopathy by regulating the antioxidant function of the rat retina [92]. These findings suggested that Rb1 could attenuate high glucose-induced oxidative injury via the NAD-PARP-SIRT axis in RCECs [93]. Additionally, the combined application of Rb1 and ginsenoside Rd can inhibit the expression of pro-inflammatory genes induced by lipopolysaccharides in human retinal epithelial cells (ARPE19) and RAW264.7 cells. This combined treatment provides a new strategy for preventing and treating diabetic retinopathy [94].
4.5. Rb1 prevents and treats diabetic encephalopathy
Diabetic encephalopathy, a severe diabetic complication, is characterized by cognitive dysfunction and neuropsychiatric disorders [95]. Methylglyoxal (MGO), a highly reactive metabolite of hyperglycemia, plays a key role in diabetic complications. Accumulated MGO binds with DNA, proteins, and lipids, leading to their oxidative modification and disturbances in their molecular functions. The cytotoxicity of MGO is mainly mediated through oxidative stress, further leading to cell apoptosis. In vitro, Rb1 treatment alleviates MGO-induced apoptosis in SH-SY5Y cells by increasing the Bcl-2/Bax ratio and inhibiting the release of pro-apoptotic genes, including Caspase-3 and Caspase-9. Additionally, Rb1 can reduce mitochondrial damage and ROS production and decrease MDA level by increasing the activities of SOD, CAT, and total GSH [96].
4.6. Rb1 improve obesity and obesity-related diseases
Obesity is a metabolic disorder characterized by white adipose tissue hyperplasia and hypertrophy. It is associated with cardiovascular diseases, hypertension, stroke, diabetes, cancer, and other diseases. Most free fatty acids (FFAs) are derived from adipose tissues in the obese state. By activating the NF-κB pathway, FFAs stimulate fat cells to release pro-inflammatory cytokines and promote the development of inflammation and oxidative stress. Rb1 ameliorates FFA-induced ROS generation and NO reduction through the upregulation of SOD2 and eNOS expression. Moreover, Rb1 attenuates FFA-induced NF-κB phosphorylation, suggesting that Rb1 can suppress the pro-inflammatory and pro-oxidative effects of FFA on 3T3-L1 adipocytes, and its mechanism is related to blocking the NF-κB signaling pathway [97]. Furthermore, Rb1 can exert antiobesity effects by inducing browning in 3T3-L1 cells and primary white adipocytes through the activation of the AMPK-mediated pathway, indicating that Rb1 can act as a potential functional antiobesity food agent [98].
In vivo, intraperitoneal injection of Rb1 can reduce the food intake of normal rats and high fat-induced obese rats. The mechanism of this effect may be that Rb1 can exert its anorexia effect by enhancing the sensitivity of rats to satiety signals (such as CCK) and transmitting satiety signals to the hindbrain through the vagus afferent nerves, thereby reducing the amount of food consumed and achieving weight loss effect [99]. Obesity affects leptin-induced BDNF expression and the regulation of synapse formation, which is considered to be associated with neurodegenerative diseases, cognitive decline, and depression. Recent studies have shown that chronic Rb1 treatment improves central leptin sensitivity, leptin-JAK2-STAT3 signaling, and leptin-induced regulation of BDNF expression in the prefrontal cortex of high-fat diet-induced obese mice, suggesting that supplementation with Rb1 is beneficial to the treatment of obesity-related neurodegenerative diseases [100].
5. Other aspects in research on Rb1
Aside from the aforementioned pharmacological efficacy and action mechanisms of Rb1, recent studies have elucidated the other multiple roles and mechanisms about this ginsenoside. For example, Rb1 can mitigate the development of abdominal aortic aneurysms by inhibiting the JNK and p38 signaling pathways [101]. Moreover, Rb1 has therapeutic and ameliorative effects on hypertrophic scar remodeling [102], colitis [103], asthma [104], and tumor malignancy [105], as well as the ability to activate TMEM16A channels [106]. Also, Rb1 exerts protection on other tissues and organs through multiple targets and multiple pathways, which involve intestinal ischemia-reperfusion (II/R) injury, hepatotoxicity, liver fibrosis, skin aging, and osteoarthritis (See the details in Supplementary materials). Research on the pharmacological effects of Rb1 is still at a preliminary stage, and further exploration is required to reveal the role and mechanism of the ginsenoside.
6. Conclusion and perspectives
Ginseng, which has a long history of medicinal use and can treat a variety of diseases, has attracted extensive attention from researchers worldwide. The complex composition and unclear mechanism of action of ginseng have limited its widespread clinical use. Ginseng contains a variety of active ingredients, such as saponins, peptides, volatile oils, polysaccharides, etc. Among them, saponin has been considered as the main component responsible for its pharmacological activity. There are thousands of reports in the literature describing the beneficial effects of ginseng and its bioactive ginsenosides. Most of these studies have used cellular and animal models to describe the mechanistic effects of ginsenosides on oxidative stress, inflammation, apoptosis, tumors, cognition, and neurodegeneration. To provide researchers with a deeper understanding of ginseng and to expand its clinical applications, we present the review on the pharmacological effects and mechanisms of Rb1, one of the most prominent components of ginseng.
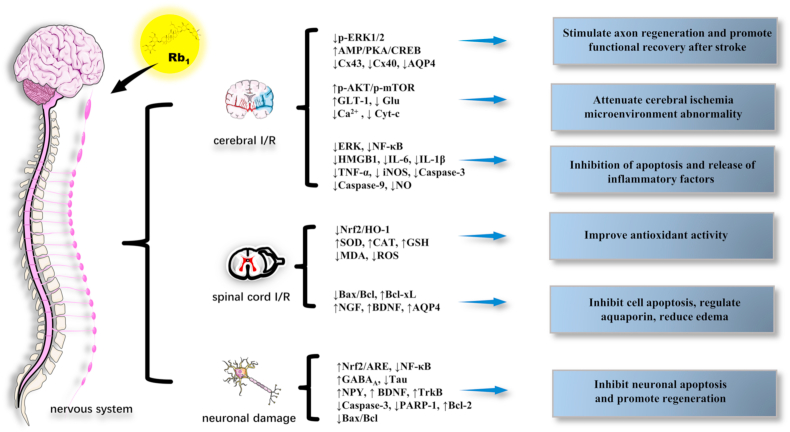
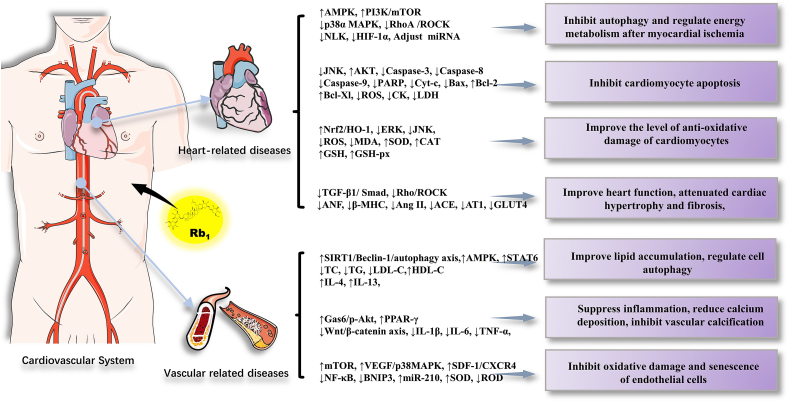
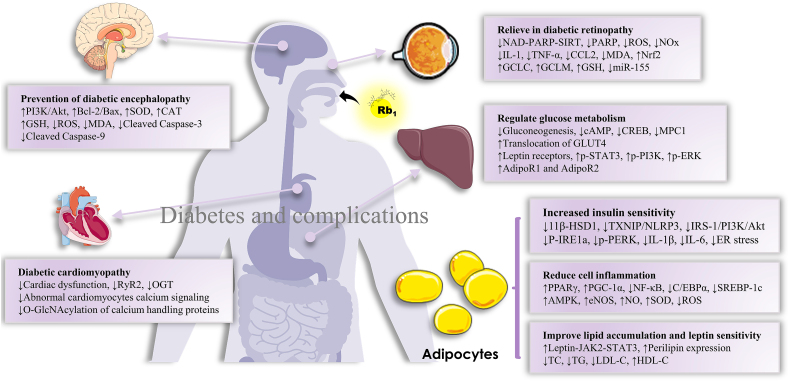
Rb1, a saponin obtained as a natural active ingredient from the extract of ginseng, has remarkable pharmacological effects, including favorable therapeutic effects on the central nervous system, cardiovascular system, diabetes, etc. In this review, we summarized the recently published reports (2015–2020) on Rb1. The articles reviewed indicated that Rb1 exerts the aforementioned protective effects and is involved in multiple signaling molecules and multiple pathways, presenting the characteristic of multiple effects, multiple targets, and multiple pathways (Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig. 2.
The main role and mechanism of Rb1 in the nervous system.
Fig. 3.
The main role and mechanism of Rb1 in the cardiovascular system.
Fig. 4.
The main role and mechanism of Rb1 in diabetes and complications.
Fig. 5.
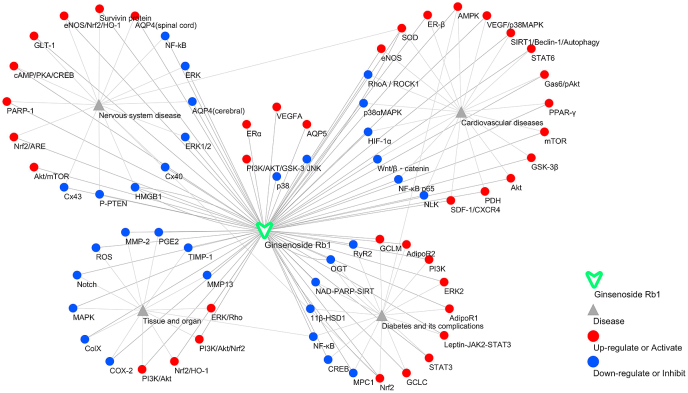
The main target and signal pathway network of ginsenoside Rb1 in the nervous system, cardiovascular disease, diabetes and its complications, and so on. The dots represent the primary target or pathway of ginsenoside Rb1 action. Among them, red indicates up-regulation or activation, and blue indicates down-regulation or inhibition.
Rb1 exhibits various pharmacological activities mainly via inhibition of the release of inflammatory factors, downregulation of the expression of pro-apoptotic genes, regulation of redox balance in the body, and regulation of cellular autophagy possibly through interactions with signaling networks and receptors, thereby exhibiting corresponding pharmacological effects on different tissues and organs and different pathophysiological environments. First, Rb1 can reduce inflammatory cytokines (TNF-α, COX-2, IL-6, IL-10, and IL-1β) and the inflammatory responses in adipose tissues and the liver probably through the regulation of HMGB1, NF-κB, MAPK/NF-κB, and PPAR-γ pathways. Second, in vivo and in vitro, Rb1 can increase the ability of antioxidants by upregulating the levels of antioxidants, such as SOD, GSH, eNOS, CAT, and Nrf2, and downregulating the levels of ROS, MDA, LDH, and MMP-2 possibly through the activation of the Nrf2/HO-1, Nrf2/ARE, and PI3K/Akt signal pathways. Third, Rb1 can downregulate pro-apoptotic factors, such as Bax, Caspase-3, and Caspase-9; upregulate the levels of antiapoptotic factors, including Bcl-2 and Bcl-xL; and exert antiapoptotic and modulatory autophagy effects by inducing estrogen receptor-dependent crosstalk and regulating the PI3K/mTOR and p38α MAPK signaling pathways. Finally, Rb1 could play a role in regulating glucose metabolism, lipid metabolism, and energy metabolism, which could be manifested in the upregulation of TC, TG, LDL-C, and HDL-C in serum, thereby increasing insulin sensitivity and improving glucose metabolism. The mechanisms of these effects are related to the regulation of the TGF-β1/Smad, ERK, and Akt signaling pathways and the regulation of 11β-HSD1 and GLUT4 levels.
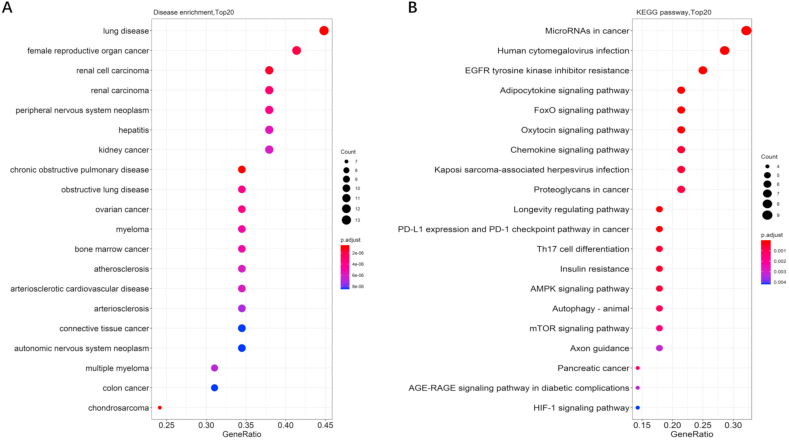
The potential effects of Rb1 on diseases and the underlying mechanisms were predicted via KEGG pathway enrichment and DOSE analyses of the reported targets by using R package [[107], [108], [109], [110]]. As shown in Fig. 6A, Rb1 not only has a certain protective effect on the aforementioned diseases but also could have effects on lung diseases, hepatitis, and many kinds of tumors, including chondrosarcoma, female reproductive organ cancer, renal cell carcinoma, myeloma, connective tissue cancer, and colon cancer. These potential effects could become the future research direction in the pharmacological effects of Rb1. Moreover, as shown in Fig. 6B, according to the KEGG analysis of the proteins collected in the literature, Rb1 can exert corresponding pharmacological effects mainly by influencing microRNAs in cancer, human cytomegalovirus infection, EGFR tyrosine kinase inhibitor resistance, adipocytokine signaling pathway, FoxO signaling pathway, oxytocin signaling pathway, chemokine signaling pathway, PD-L1 expression and PD-1 checkpoint pathway in cancer, AMPK signaling pathway, mTOR signaling pathway, and HIF-1 signaling pathway.
Fig. 6.
Dotplots of DO enrichment analysis (A) and KEGG analysis (B) of proteins screened in the literature.
On the basis of the results of previous studies reviewed herein and those of the present KEGG pathway and DO enrichment analyses, we offer the following ideas.
-
(1)
First, although all the reviewed targets reported in the literature have been proved to be related to the therapeutic effects of Rb1 on cardiovascular and nervous system diseases and diabetes, the results of DO enrichment analysis strongly supported the supposition that Rb1 could have an excellent anti-lung disease and anticancer effects, especially on obstructive pulmonary disease, female reproductive organ cancer, and renal cell carcinoma. Thus, these effects must be validated by in vitro and in vivo experiments because the results may provide a scientific basis for the development of Rb1 for the treatment of obstructive pulmonary disease, female reproductive organ cancer, and renal cell carcinoma.
-
(2)
Second, compared with those reported in the literature, the current KEGG enrichment analysis predicted more pathways, most of which, such as microRNAs in cancer, human cytomegalovirus infection, and EGFR tyrosine kinase inhibitor resistance, have been rarely mentioned. A comprehensive understanding of the therapeutic effects and mechanisms of Rb1 can be achieved by designing and conducting proteomics or genomics investigations both in vitro and in vivo. The unreported pathways can be validated and confirmed by other molecular techniques, such as Western blot and quantitative reverse transcription PCR.
-
(3)
Finally, molecular docking and target fishing should be performed to predict the possible target proteins. The prediction and identification of target proteins can then be further investigated via gene silencing, knockout experiments, or Rb1-target protein compound crystallization experiments to corroborate the protein targets of this ginseng ingredient.
In conclusion, these ideas may provide invaluable clues or perspectives to further research on the therapeutic effects and mechanisms of Rb1, so as to the promoting the drug development and clinical applications for this ingredient.
Declaration of competing interest
The author declares no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the financial supports of the National Natural Science Foundation of China of China (81973558), and the Joint Funds for the Innovation of Science and Technology, Fujian province (2017Y9123 and 2019Y9068).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.07.008.
Contributor Information
Peiying Shi, Email: peiyshi@126.com.
Hong Yao, Email: yauhung@126.com, hongyao@mail.fjmu.edu.cn.
Abbreviations
- Aβ
amyloid β-protein
- ACE
angiotensin converting enzyme
- ACTH
adrenocortical hormone
- AD
Alzheimer's disease
- AMPK
AMP-activated protein kinase
- ANF
atrial natriuretic factor
- Ang II
Angiotensin II
- AQP
aquaporin
- AQP4
aquaporin 4
- ARPE19
adult retinal pigment epithelial cell line-19
- AT1
Ang II type 1
- BNIP3
Bcl-2/E1B-19 kDa interacting protein 3
- β-MHC
β-myosin heavy chain
- CAT
catalase
- CK
creatine kinase
- CORT
corticosterone
- CREB
cAMP-responsive element binding protein
- Cx43
connexin 43
- Cyt-C
cytochrome C
- DA
dopaminergic
- DOSE
Disease Ontology Semantic and Enrichment
- DR
diabetic retinopathy
- EPCs
endothelial progenitor cells
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ERK
extracellular signal-regulated kinase
- FFAs
free fatty acids
- Gas6
growth arrest-specific gene 6
- GCLC
glutathione cysteine ligase catalytic subunit
- GCLM
glutathione cysteine ligase modulatory subunit
- GLT-1
glutamate transporter 1
- Glu
glutamate
- GSH
glutathione
- H/R
hypoxia/reoxygenation
- HF
heart failure
- HMGB1
high mobility group 1
- I/R
ischemia/reperfusion
- IL-6
interleukin-6
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MCAO
middle cerebral artery occlusion
- MDA
malondialdehyde
- MGO
methylglyoxal
- MIRI
myocardial ischemia-reperfusion injury
- MPC
mitochondrial pyruvate carrier
- MPTP
mitochondrial permeability transition pore
- NF-κB
nuclear factor kappa-B
- NGF
nerve growth factor
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- Nqo1
NAD(P)H quinone dehydrogenase 1
- Nrf2/HO-1
Nuclear factor erythroid 2-related factor 2/heme oxygenase
- PD
Parkinson's disease
- P. ginseng
Panax ginseng C. A. Meyer
- PPAR-γ
peroxisome proliferator-activated receptor γ
- PTSD
post-traumatic stress disorder
- PWATs
primary white adipocytes
- Rb1
Ginsenoside Rb1
- RCECs
retinal capillary endothelial cells
- ROCK
Rho-associated protein kinase
- ROS
reactive oxygen species
- RTV
ritonavir
- RyR2
ryanodine receptor 2
- SCI
spinal cord injury
- SCII
spinal cord ischemia-reperfusion injury
- SDF-1
stromal cell-derived factor-1
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
- TrkB
tyrosine kinase B
- VSMC
vascular smooth muscle cells
- 11β-HSD1
11β-hydroxysteroid dehydrogenase I
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yao L., Wang J., Sun J.C., He J.P., Paek K.Y., Park S.Y., Huang L.Q., Gao W.Y. A WRKY transcription factor, PgWRKY4X, positively regulates ginsenoside biosynthesis by activating squalene epoxidase transcription in Panax ginseng. Ind Crop Prod. 2020;154:112671. [Google Scholar]
- 2.Kim S.W., Gupta R., Lee S.H., Min C.W., Agrawal G.K., Rakwal R., Kim J.B., Jo I.H., Park S.Y., Kim J.K., et al. An integrated biochemical, proteomics, and metabolomics approach for supporting medicinal value of Panax ginseng fruits. Front Plant Sci. 2016;7:994. doi: 10.3389/fpls.2016.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.I., Park K.S., Cho I.H. Panax ginseng: a candidate herbal medicine for autoimmune disease. J Ginseng Res. 2019;43:342–348. doi: 10.1016/j.jgr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D., Liu T., Teng Y., Chen W., Zhao L., Li X. Ginsenoside Rb1 inhibits hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells by regulating microRNA-25. Exp Ther Med. 2017;14:2895–2902. doi: 10.3892/etm.2017.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou J., Kim S. Possible role of ginsenoside Rb1 in skin wound healing via regulating senescent skin dermal fibroblast. Biochem Biophys Res Commun. 2018;499:381–388. doi: 10.1016/j.bbrc.2018.03.170. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y., Li M., Lu Z., Wang Y., Yu X., Sui D., Fu L. Ginsenoside Rg3 induces ginsenoside Rb1-comparable cardioprotective effects independent of reducing blood pressure in spontaneously hypertensive rats. Exp Ther Med. 2017;14:4977–4985. doi: 10.3892/etm.2017.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front Pharmacol. 2018;9:423. doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed T., Raza S.H., Maryam A., Setzer W.N., Braidy N., Nabavi S.F., de Oliveira M.R., Nabavi S.M. Ginsenoside Rb1 as a neuroprotective agent: a review. Brain Res Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Q., Bao X.Y., Zhu P.C., Tong Q., Zheng G.Q., Wang Y. Ginsenoside Rb1 for myocardial Ischemia/Reperfusion injury: preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/6313625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers W.J. Acute ischemic stroke. N Engl J Med. 2020;383:252–260. doi: 10.1056/NEJMcp1917030. [DOI] [PubMed] [Google Scholar]
- 12.Thommessen B., Naess H., Logallo N., Kvistad C.E., Waje-Andreassen U., Ihle-Hansen H., Ihle-Hansen H., Thomassen L., Ronning O.M. Tenecteplase versus alteplase after acute ischemic stroke at high age. Int J Stroke. 2020 doi: 10.1177/1747493020938306. 1747493020938306. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Xu Q.Q., Shan C.S., Shi Y.H., Wang Y., Zheng G.Q. Combined use of emodin and ginsenoside Rb1 exerts synergistic neuroprotection in cerebral ischemia/reperfusion rats. Front Pharmacol. 2018;9:943. doi: 10.3389/fphar.2018.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W., Guo Y., Yang W., Zheng P., Zeng J., Tong W. Involvement of connexin 40 in the protective effects of ginsenoside Rb1 against traumatic brain injury. Cell Mol Neurobiol. 2016;36:1057–1065. doi: 10.1007/s10571-015-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X., Zhang X., Cui L., Chen R., Zhang C., Xue J., Zhang L., He W., Li J., Wei S., et al. Ginsenoside Rb1 promotes motor functional recovery and axonal regeneration in post-stroke mice through cAMP/PKA/CREB signaling pathway. Brain Res Bull. 2020;154:51–60. doi: 10.1016/j.brainresbull.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Shen J., Li H., Zheng X., Kang D., Xu Y., Chen C., Guo H., Xie L., Wang G., et al. Ginsenoside Rb1 exerts neuroprotective effects through regulation of lactobacillus helveticus abundance and GABAA receptor expression. J Ginseng Res. 2020;44:86–95. doi: 10.1016/j.jgr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi D.W., Rothman S.M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 18.Molz S., Decker H., Dal-Cim T., Cremonez C., Cordova F.M., Leal R.B., Tasca C.I. Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38 MAPK signaling. Neurochem Res. 2008;33:27–36. doi: 10.1007/s11064-007-9402-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J., Yu S., Zheng W., Feng G., Luo G., Wang L., Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y., Wang L.P., Li C., Xiong Y.X., Yan Y.T., Zhao L.Q., Li S.D., Sun J., Luo H.Y., Xian C.J. Effects of ginsenoside Rb1 on expressions of phosphorylation Akt/p-mTOR/p-PTEN in artificial abnormal hippocampal microenvironment in Rats. Neurochem Res. 2018;43:1927–1937. doi: 10.1007/s11064-018-2612-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang S., Li M., Guo Y., Li C., Wu L., Zhou X.F., Luo Y., An D., Li S., Luo H., et al. Effects of Panax notoginseng ginsenoside Rb1 on abnormal hippocampal microenvironment in rats. J Ethnopharmacol. 2017;202:138–146. doi: 10.1016/j.jep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Dong X., Zheng L., Lu S., Yang Y. Neuroprotective effects of pretreatment of ginsenoside Rb1 on severe cerebral ischemia-induced injuries in aged mice: involvement of anti-oxidant signaling. Geriatr Gerontol Int. 2017;17:338–345. doi: 10.1111/ggi.12699. [DOI] [PubMed] [Google Scholar]
- 23.Liu A., Zhu W., Sun L., Han G., Liu H., Chen Z., Zhuang L., Jiang W., Xue X. Ginsenoside Rb1 administration attenuates focal cerebral ischemic reperfusion injury through inhibition of HMGB1 and inflammation signals. Exp Ther Med. 2018;16:3020–3026. doi: 10.3892/etm.2018.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira P.I., Santos R.X., Zhu X., Lee H.G., Smith M.A., Casadesus G., Perry G. Autophagy in Alzheimer's disease. Expert Rev Neurother. 2010;10:1209–1218. doi: 10.1586/ern.10.84. [DOI] [PubMed] [Google Scholar]
- 25.Wang P., Lin C., Wu S., Huang K., Wang Y., Bao X., Zhang F., Huang Z., Teng H. Inhibition of autophagy is involved in the protective effects of ginsenoside Rb1 on spinal cord injury. Cell Mol Neurobiol. 2018;38:679–690. doi: 10.1007/s10571-017-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M.J., Jang M., Choi J., Chang B.S., Kim D.Y., Kim S.H., Kwak Y.S., Oh S., Lee J.H., Chang B.J., et al. Korean red ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 2016;53:1977–2002. doi: 10.1007/s12035-015-9131-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z.G., Li Y., Jiao J.H., Long H., Xin Z.Y., Yang X.Y. MicroRNA regulatory pattern in spinal cord ischemia-reperfusion injury. Neural Regen Res. 2020;15:2123–2130. doi: 10.4103/1673-5374.280323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu P., Li J.X., Fujino M., Zhuang J., Li X.K. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediat Inflamm. 2013;2013:701970. doi: 10.1155/2013/701970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning N., Dang X., Bai C., Zhang C., Wang K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol. 2012;139:504–512. doi: 10.1016/j.jep.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Zhao D., Zhang M., Yuan H., Meng C., Zhang B., Wu H. Ginsenoside Rb1 protects against spinal cord ischemia-reperfusion injury in rats by downregulating the Bax/Bcl-2 ratio and caspase-3 and p-Ask-1 levels. Exp Mol Pathol. 2018;105:229–235. doi: 10.1016/j.yexmp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Ye J.T., Li F.T., Huang S.L., Xue J.L., Aihaiti Y., Wu H., Liu R.X., Cheng B. Effects of ginsenoside Rb1 on spinal cord ischemia-reperfusion injury in rats. J Orthop Surg Res. 2019;14:259. doi: 10.1186/s13018-019-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu P., Hata R., Nakata K., Cao F., Samukawa K., Hiroko F., Sakanaka M. Intravenous infusion of ginsenoside Rb1 ameliorates compressive spinal cord injury through upregulation of Bcl-xL and VEGF. Int J Neurol Neurother. 2015;2:1017. [Google Scholar]
- 33.Li G., Liu X., Liu Z., Su Z. Interactions of connexin 43 and aquaporin-4 in the formation of glioma-induced brain edema. Mol Med Rep. 2015;11:1188–1194. doi: 10.3892/mmr.2014.2867. [DOI] [PubMed] [Google Scholar]
- 34.Huang F., Li Y.N., Yin F., Wu Y.T., Zhao D.X., Li Y., Zhang Y.F., Zhu Q.S. Ginsenoside Rb1 inhibits neuronal apoptosis and damage, enhances spinal aquaporin 4 expression and improves neurological deficits in rats with spinal cord ischemiareperfusion injury. Mol Med Rep. 2015;11:3565–3572. doi: 10.3892/mmr.2015.3162. [DOI] [PubMed] [Google Scholar]
- 35.Li Y.N., Gao Z.W., Li R., Zhang Y.F., Zhu Q.S., Huang F. Aquaporin 4 regulation by ginsenoside Rb1 intervenes with oxygen-glucose deprivation/reoxygenation-induced astrocyte injury. MEDICINE. 2019;98:e17591. doi: 10.1097/MD.0000000000017591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung A.W.K., Tzvetkov N.T., Georgieva M.G., Ognyanov I.V., Kordos K., Jozwik A., Kuhl T., Perry G., Petralia M.C., Mazzon E., et al. Reactive oxygen species and their impact in neurodegenerative diseases: literature landscape analysis. Antioxidants Redox Signal. 2021;34:402–420. doi: 10.1089/ars.2019.7952. [DOI] [PubMed] [Google Scholar]
- 37.Leissring M.A., Turner A.J. Regulation of distinct pools of amyloid beta-protein by multiple cellular proteases. Alzheimer's Res Ther. 2013;5:37. doi: 10.1186/alzrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang J.Y., Shim J.S., Song M.Y., Yim S.V., Lee S.E., Park K.S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in beta-amyloid-treated neuronal cells. J Ginseng Res. 2016;40:278–284. doi: 10.1016/j.jgr.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y.R., Li Y., Yang W.Y., Gao S.Y., Lin J.W., Wang T.Q., Zhou K.L., Hu H.Y. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer's disease induced by A beta(1-40) Am J Transl Res. 2018;10 796-+ [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J., Lu S., Yu H., Duan S., Zhao J. Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer's disease model rats. Brain Res. 2018;1678:187–194. doi: 10.1016/j.brainres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Li N., Zhou L., Li W., Liu Y., Wang J., He P. Protective effects of ginsenosides Rg1 and Rb1 on an Alzheimer's disease mouse model: a metabolomics study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:54–61. doi: 10.1016/j.jchromb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S., Zhu D., Li H., Zhang H., Feng C., Zhang W. Analyses of mRNA profiling through RNA sequencing on a SAMP8 mouse model in response to ginsenoside Rg1 and Rb1 treatment. Front Pharmacol. 2017;8:88. doi: 10.3389/fphar.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao H.H., Zhang Y., Ding G.N., Hong F.X., Dong P., Tian M. Ginsenoside Rb1 attenuates isoflurane/surgery-induced cognitive dysfunction via Inhibiting neuroinflammation and oxidative stress. Biomed Environ Sci. 2017;30:363–372. doi: 10.3967/bes2017.047. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Zeng B., Hu X., Li Z., Zhang D., Yang G., Dai J., Zeng X. Protective effects of ginsenoside Rb1 against blood-brain barrier damage induced by human immunodeficiency virus-1 Tat protein and methamphetamine in sprague-dawley rats. Am J Chin Med. 2018;46:551–566. doi: 10.1142/S0192415X18500283. [DOI] [PubMed] [Google Scholar]
- 45.Qu S.G., Meng X.J., Liu Y., Zhang X.P., Zhang Y.L. Ginsenoside Rb1 prevents MPTP-induced changes in hippocampal memory via regulation of the alpha-synuclein/PSD-95 pathway. Aging-US. 2019;11:1934–1964. doi: 10.18632/aging.101884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y.L., Liu Y., Kang X.P., Dou C.Y., Zhuo R.G., Huang S.Q., Peng L., Wen L. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson's disease. Neuropharmacology. 2018;131:223–237. doi: 10.1016/j.neuropharm.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Ardah M.T., Paleologou K.E., Lv G., Menon S.A., Abul Khair S.B., Lu J.H., Safieh-Garabedian B., Al-Hayani A.A., Eliezer D., Li M., et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89–101. doi: 10.1016/j.nbd.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Moriano C., Gonzalez-Burgos E., Iglesias I., Lozano R., Gomez-Serranillos M.P. Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS One. 2017:12. doi: 10.1371/journal.pone.0182933. 0182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X., Gu X., Yu M., Zi Y., Yu H., Wang Y., Xie Y., Xiang L. Effects of ginsenoside Rb1 on oxidative stress injury in rat spinal cords by regulating the eNOS/Nrf2/HO-1 signaling pathway. Exp Ther Med. 2018;16:1079–1086. doi: 10.3892/etm.2018.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang M., Lee M.J., Choi J.H., Kim E.J., Nah S.Y., Kim H.J., Lee S., Lee S.W., Kim Y.O., Cho I.H. Ginsenoside Rb1 attenuates acute inflammatory nociception by Inhibition of neuronal ERK phosphorylation by regulation of the Nrf2 and NF-kappaB pathways. J Pain. 2016;17:282–297. doi: 10.1016/j.jpain.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y., Miao W., Teng J., Zhang L. Ginsenoside Rb1 protects the brain from damage induced by epileptic seizure via Nrf2/ARE signaling. Cell Physiol Biochem. 2018;45:212–225. doi: 10.1159/000486768. [DOI] [PubMed] [Google Scholar]
- 54.Kang X., Hong W., Xie K., Tang H., Tang J., Luo S., Geng W., Jia D. Ginsenoside Rb1 pretreatment reverses hippocampal changes in BDNF/TrkB mRNA and protein in rats subjected to acute immobilization stress. Drug Des Dev Ther. 2019;13:2127–2134. doi: 10.2147/DDDT.S201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee B., Sur B., Cho S.G., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of post-traumatic stress disorder. J Nat Med. 2016;70:133–144. doi: 10.1007/s11418-015-0943-3. [DOI] [PubMed] [Google Scholar]
- 56.Wang G.L., He Z.M., Zhu H.Y., Gao Y.G., Zhao Y., Yang H., Zhang L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J Ethnopharmacol. 2017;204:118–124. doi: 10.1016/j.jep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Wang G.L., Wang Y.P., Zheng J.Y., Zhang L.X. Monoaminergic and aminoacidergic receptors are involved in the antidepressant-like effect of ginsenoside Rb1 in mouse hippocampus (CA3) and prefrontal cortex. Brain Res. 2018;1699:44–53. doi: 10.1016/j.brainres.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 58.Dai S.N., Hou A.J., Zhao S.M., Chen X.M., Huang H.T., Chen B.H., Kong H.L. Ginsenoside Rb1 ameliorates autophagy of hypoxia cardiomyocytes from neonatal rats via AMP-activated protein kinase pathway. Chin J Integr Med. 2019;25:521–528. doi: 10.1007/s11655-018-3018-y. [DOI] [PubMed] [Google Scholar]
- 59.Yan X., Xue J., Wu H., Wang S., Liu Y., Zheng S., Zhang C., Yang C. Ginsenoside Rb1 protects hypoxic- and ischemic-damaged cardiomyocytes by regulating expression of miRNAs. Evid Based Complement Alternat Med. 2015;2015:171306. doi: 10.1155/2015/171306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan X., Liu J., Wu H., Liu Y., Zheng S., Zhang C., Yang C. Impact of miR-208 and its target gene Nemo-Like Kinase on the protective effect of ginsenoside Rb1 in Hypoxia/Ischemia Injured cardiomyocytes. Cell Physiol Biochem. 2016;39:1187–1195. doi: 10.1159/000447825. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z., Song L., Zhang P., Cao Z., Hao J., Tian Y., Luo A., Zhang P., Ma J. Ginsenoside Rb1 exerts antiarrhythmic effects by inhibiting INa and ICaL in rabbit ventricular myocytes. Sci Rep. 2019;9:20425. doi: 10.1038/s41598-019-57010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ai Q.D., Sun G.B., Luo Y., Dong X., Hu R.F., Meng X.B., Sun X.B. Ginsenoside Rb1 prevents hypoxia-reoxygenation-induced apoptosis in H9c2 cardiomyocytes via an estrogen receptor-dependent crosstalk among the Akt, JNK, and ERK 1/2 pathways using a label-free quantitative proteomics analysis. RSC Adv. 2015;5:26346–26363. [Google Scholar]
- 63.Yang J., Zhang F., Shi H., Gao Y., Dong Z., Ma L., Sun X., Li X., Chang S., Wang Z., et al. Neutrophil-derived advanced glycation end products-Nepsilon-(carboxymethyl) lysine promotes RIP3-mediated myocardial necroptosis via RAGE and exacerbates myocardial ischemia/reperfusion injury. Faseb J. 2019;33:14410–14422. doi: 10.1096/fj.201900115RR. [DOI] [PubMed] [Google Scholar]
- 64.Li G., Qian W., Zhao C. Analyzing the anti-ischemia-reperfusion injury effects of ginsenoside Rb1 mediated through the inhibition of p38alpha MAPK. Can J Physiol Pharmacol. 2016;94:97–103. doi: 10.1139/cjpp-2014-0164. [DOI] [PubMed] [Google Scholar]
- 65.Cui Y.C., Pan C.S., Yan L., Li L., Hu B.H., Chang X., Liu Y.Y., Fan J.Y., Sun K., Li Q., et al. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci Rep. 2017;7:44579. doi: 10.1038/srep44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H., Wang X., Ma Y., Shi Y. The effect of ginsenoside Rb1, diazoxide, and 5-Hydroxydecanoate on hypoxia-reoxygenation Injury of H9C2 cardiomyocytes. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/6046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J., Yang Y.L., Li L.Z., Zhang L., Liu Q., Liu K., Li P., Liu B., Qi L.W. Succinate accumulation impairs cardiac pyruvate dehydrogenase activity through GRP91-dependent and independent signaling pathways: therapeutic effects of ginsenoside Rb1. Biochim Biophys Acta Mol Basis Dis. 1863;2017:2835–2847. doi: 10.1016/j.bbadis.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Li Y.H., Li Y.Y., Fan G.W., Yu J.H., Duan Z.Z., Wang L.Y., et al. Cardioprotection of ginsenoside Rb1 against ischemia/reperfusion injury is associated with mitochondrial permeability transition pore opening inhibition. Chin J Integr Med. 2016 doi: 10.1007/s11655-015-2433-6. [DOI] [PubMed] [Google Scholar]
- 69.Zheng X., Wang S., Zou X.M., Jing Y.T., Yang R.L., Li S.Q., Wang F.R. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp Anim. 2017;66:217–228. doi: 10.1538/expanim.16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang T., Miao Y., Zhang T., Mu N., Ruan L., Duan J., Zhu Y., Zhang R. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J Pharm Pharmacol. 2018;70:830–838. doi: 10.1111/jphp.12900. [DOI] [PubMed] [Google Scholar]
- 71.Shi G., Liu D., Zhou B., Liu Y., Hao B., Yu S., Wu L., Wang M., Song Z., Wu C., et al. Ginsenoside Rb1 alleviates oxidative low-density lipoprotein-induced vascular endothelium senescence via the SIRT1/Beclin-1/Autophagy Axis. J Cardiovasc Pharmacol. 2020;75:155–167. doi: 10.1097/FJC.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Liu M.H., Qiao L., Zhang X.Y., Liu X.L., Dong M., Dai H.Y., Ni M., Luan X.R., Guan J., et al. Ginsenoside Rb1 enhances atherosclerotic plaque stability by skewing macrophages to the M2 phenotype. J Cell Mol Med. 2018;22:409–416. doi: 10.1111/jcmm.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou P., Xie W.J., Luo Y., Lu S., Dai Z.R., Wang R.Y., Zhang X.L., Li G., Sun G.B., Sun X.B. Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE-/- mice via Inhibition of apoptosis and enhancing autophagy. Molecules. 2018;23:15. doi: 10.3390/molecules23112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou P., Zhang X., Guo M., Guo R., Wang L., Zhang Z., Lin Z., Dong M., Dai H., Ji X., et al. Ginsenoside Rb1 ameliorates CKD-associated vascular calcification by inhibiting the Wnt/beta-catenin pathway. J Cell Mol Med. 2019;23:7088–7098. doi: 10.1111/jcmm.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanao-Hamai M., Son B.K., Komuro A., Asari Y., Hashizume T., Takayama K.I., Ogawa S., Akishita M. Ginsenoside Rb1 inhibits vascular calcification as a selective androgen receptor modulator. Eur J Pharmacol. 2019;859:172546. doi: 10.1016/j.ejphar.2019.172546. [DOI] [PubMed] [Google Scholar]
- 76.Lu H., Zhou X., Kwok H.H., Dong M., Liu Z., Poon P.Y., Luan X. Ngok-Shun Wong R. Ginsenoside Rb1-mediated anti-angiogenesis via regulating PEDF and miR-33a through the activation of PPAR-gamma pathway. Front Pharmacol. 2017;8:783. doi: 10.3389/fphar.2017.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu J.M., Jiang J., Jamaluddin M.S., Liang Z., Yao Q., Chen C. Ginsenoside Rb1 blocks ritonavir-induced oxidative stress and eNOS downregulation through activation of estrogen receptor-beta and upregulation of SOD in human endothelial cells. Int J Mol Sci. 2019;20:17. doi: 10.3390/ijms20020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jia F., Mou L., Ge H. Protective effects of ginsenoside Rb1 on H2O2-induced oxidative injury in human endothelial cell line (EA.hy926) via miR-210. Int J Immunopathol Pharmacol. 2019;33:1–11. doi: 10.1177/2058738419866021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Lan T.H., Xu D.P., Huang M.T., Song J.X., Wu H.L., Li M. Ginsenoside Rb1 prevents homocysteine-induced EPC dysfunction via VEGF/p38MAPK and SDF-1/CXCR4 activation. Sci Rep. 2017;7:13061. doi: 10.1038/s41598-017-13436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith U. Impaired ('diabetic') insulin signaling and action occur in fat cells long before glucose intolerance–is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26:897–904. doi: 10.1038/sj.ijo.0802028. [DOI] [PubMed] [Google Scholar]
- 81.Chen W., Wang J., Luo Y., Wang T., Li X., Li A., Li J., Liu K., Liu B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2016;40:351–358. doi: 10.1016/j.jgr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu X., Ye L., Zhang H., Zhao J., Wang G., Guo C., Shang W. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res. 2015;39:199–205. doi: 10.1016/j.jgr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song B., Ding L., Zhang H., Chu Y., Liu X. Ginsenoside Rb1 increases insulin sensitivity through suppressing 11β-hydroxysteroid dehydrogenase type I. Am J Transl Res. 2017;9:1049–1057. [PMC free article] [PubMed] [Google Scholar]
- 84.Chang W.H., Tsai Y.L., Huang C.Y., Hsieh C.C., Chaunchaiyakul R., Fang Y., Lee S.D., Kuo C.H. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J Int Soc Sports Nutr. 2015;12:34. doi: 10.1186/s12970-015-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lou M.D., Li J., Cheng Y., Xiao N., Ma G., Li P., Liu B., Liu Q., Qi L.W. Glucagon up-regulates hepatic mitochondrial pyruvate carrier 1 through cAMP-responsive element-binding protein; inhibition of hepatic gluconeogenesis by ginsenoside Rb1. Br J Pharmacol. 2019;176:2962–2976. doi: 10.1111/bph.14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tabandeh M.R., Jafari H., Hosseini S.A., Hashemitabar M. Ginsenoside Rb1 stimulates adiponectin signaling in C2C12 muscle cells through up-regulation of AdipoR1 and AdipoR2 proteins. Pharm Biol. 2015;53:125–132. doi: 10.3109/13880209.2014.912237. [DOI] [PubMed] [Google Scholar]
- 87.Tabandeh M.R., Hosseini S.A., Hosseini M. Ginsenoside Rb1 exerts antidiabetic action on C2C12 muscle cells by leptin receptor signaling pathway. J Recept Signal Transduct Res. 2017;37:370–378. doi: 10.1080/10799893.2017.1286676. [DOI] [PubMed] [Google Scholar]
- 88.Bastin M., Andreelli F. The gut microbiota and diabetic cardiomyopathy in humans. Diabetes Metab. 2020;46:197–202. doi: 10.1016/j.diabet.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Qin L., Wang J., Zhao R., Zhang X., Mei Y. Ginsenoside-Rb1 improved diabetic cardiomyopathy through regulating calcium signaling by alleviating protein O-GlcNAcylation. J Agric Food Chem. 2019;67:14074–14085. doi: 10.1021/acs.jafc.9b05706. [DOI] [PubMed] [Google Scholar]
- 90.Tao D., Ni N., Zhang T., Li C., Sun Q., Wang L., Mei Y. Accumulation of advanced glycation end products potentiate human retinal capillary endothelial cells mediated diabetic retinopathy. Mol Med Rep. 2019;20:3719–3727. doi: 10.3892/mmr.2019.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen N.H., Kim D., Roy S. High glucose increases binding of lysyl oxidase to extracellular matrix proteins: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61:40. doi: 10.1167/iovs.61.4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong C., Liu P., Wang H., Dong M., Li G., Li Y. Ginsenoside Rb1 attenuates diabetic retinopathy in streptozotocin-induced diabetic rats 1. Acta Cir Bras. 2019;34:e201900201. doi: 10.1590/s0102-8650201900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan C., Ma Q., Xu M., Qiao Y., Zhang Y., Li P., Bi Y., Tang M. Ginsenoside Rb1 attenuates high glucose-induced oxidative injury via the NAD-PARP-SIRT Axis in rat retinal capillary endothelial cells. Int J Mol Sci. 2019;20:4396. doi: 10.3390/ijms20194936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bian M., Du X., Wang P., Cui J., Xu J., Gu J., Zhang T., Chen Y. Combination of ginsenoside Rb1 and Rd protects the retina against bright light-induced degeneration. Sci Rep. 2017;7:6015. doi: 10.1038/s41598-017-06471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y., Li M., Zhang Z., Ye Y., Zhou J. Role of microglia-neuron interactions in diabetic encephalopathy. Ageing Res Rev. 2018;42:28–39. doi: 10.1016/j.arr.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Nan F., Sun G., Xie W., Ye T., Sun X., Zhou P., Dong X., Sun J., Sun X., Zhang M. Ginsenoside Rb1 mitigates oxidative stress and apoptosis induced by methylglyoxal in SH-SY5Y cells via the PI3K/Akt pathway. Mol Cell Probes. 2019;48:101469. doi: 10.1016/j.mcp.2019.101469. [DOI] [PubMed] [Google Scholar]
- 97.Wang M., Chen Y., Xiong Z., Yu S., Zhou B., Ling Y., Zheng Z., Shi G., Wu Y., Qian X. Ginsenoside Rb1 inhibits free fatty acidsinduced oxidative stress and inflammation in 3T3L1 adipocytes. Mol Med Rep. 2017;16:9165–9172. doi: 10.3892/mmr.2017.7710. [DOI] [PubMed] [Google Scholar]
- 98.Park S.J., Park M., Sharma A., Kim K., Lee H.J. Black ginseng and ginsenoside Rb1 promote browning by inducing UCP1 expression in 3T3-L1 and primary white adipocytes. Nutrients. 2019;11:2747. doi: 10.3390/nu11112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen L., Wang D.Q., Lo C.C., Arnold M., Tso P., Woods S.C., Liu M. Gut vagal afferents are necessary for the eating-suppressive effect of intraperitoneally administered ginsenoside Rb1 in rats. Physiol Behav. 2015;152:62–67. doi: 10.1016/j.physbeh.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Y., Huang X.F., Bell C., Yu Y. Ginsenoside Rb1 improves leptin sensitivity in the prefrontal cortex in obese mice. CNS Neurosci Ther. 2018;24:98–107. doi: 10.1111/cns.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X.J., He C., Tian K., Li P., Su H., Wan J.B. Ginsenoside Rb1 attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of the JNK and p38 signaling pathways. Vascul Pharmacol. 2015;73:86–95. doi: 10.1016/j.vph.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Wang R.X., He R.L., Jiao H.X., Dai M., Mu Y.P., Hu Y., Wu Z.J., Sham J.S., Lin M.J. Ginsenoside Rb1 attenuates agonist-induced contractile response via inhibition of store-operated calcium entry in pulmonary arteries of normal and pulmonary hypertensive rats. Cell Physiol Biochem. 2015;35:1467–1481. doi: 10.1159/000373966. [DOI] [PubMed] [Google Scholar]
- 103.Toyokawa Y., Takagi T., Uchiyama K., Mizushima K., Inoue K., Ushiroda C., Kashiwagi S., Nakano T., Hotta Y., Tanaka M., et al. Ginsenoside Rb1 promotes intestinal epithelial wound healing through extracellular signal-regulated kinase and Rho signaling. J Gastroenterol Hepatol. 2019;34:1193–1200. doi: 10.1111/jgh.14532. [DOI] [PubMed] [Google Scholar]
- 104.Chen T., Xiao L., Zhu L., Ma S., Yan T., Ji H. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38:1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 105.Lu S., Zhang Y., Li H., Zhang J., Ci Y., Han M. Ginsenoside Rb1 can ameliorate the key inflammatory cytokines TNF-alpha and IL-6 in a cancer cachexia mouse model. BMC Complement Med Ther. 2020;20:11. doi: 10.1186/s12906-019-2797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo S., Chen Y., Pang C., Wang X., Qi J., Mo L., Zhang H., An H., Zhan Y. Ginsenoside Rb1, a novel activator of the TMEM16A chloride channel, augments the contraction of Guinea pig ileum. Pflügers Archiv. 2017;469:681–692. doi: 10.1007/s00424-017-1934-x. [DOI] [PubMed] [Google Scholar]
- 107.Yao H., Huang X., Xie Y., Huang X., Ruan Y., Lin X., Huang L., Shi P. Identification of pharmacokinetic markers for guanxin danshen drop pills in rats by combination of pharmacokinetics, systems pharmacology, and pharmacodynamic assays. Front Pharmacol. 2018;9:1493. doi: 10.3389/fphar.2018.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi P.Y., Xie Y.J., Xie R.F., Lin Z., Yao H., Wu S. An integrated pharmacokinetic study of an acanthopanax senticosus extract preparation by combination of virtual screening, systems pharmacology, and multi-component pharmacokinetics in rats. Front Pharmacol. 2020;11:1295. doi: 10.3389/fphar.2020.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang P.P., Huang H., Chen B., Su Y., Shi P.Y., Yao H. Systems pharmacology dissection of mechanisms of dengzhan xixin injection against cardiovascular diseases. Chem Pharm Bull. 2020;68:837–847. doi: 10.1248/cpb.c20-00122. [DOI] [PubMed] [Google Scholar]
- 110.Xie R.F., Liu Z.Z., Lin Z., Shi P.Y., Chen B., Li S.G., Li G.W., Huang L.Y., Lin X.H., Yao H. Potential mechanism of action of Ixeris sonchifolia extract injection against cardiovascular diseases revealed by combination of HPLC-Q-TOF-MS, virtual screening and systems pharmacology approach. RSC Adv. 2020;10:38497–38504. doi: 10.1039/d0ra07038f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li C.Y., Yang P., Jiang Y.L., Lin Z., Pu Y.W., Xie L.Q., et al. Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia reperfusion injury through mTOR signal pathway. Biomed Pharmacother. 2020;125:109913. doi: 10.1016/j.biopha.2020.109913. [DOI] [PubMed] [Google Scholar]
- 112.Qiao L., Zhang X., Liu M., Liu X., Dong M., Cheng J., et al. Ginsenoside Rb1 enhances atherosclerotic plaque stability by improving autophagy and lipid metabolism in macrophage foam cells. Front Pharmacol. 2017;8:727. doi: 10.3389/fphar.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.