Abstract
Spinal cord injury (SCI) is defined as damage to the spinal cord that temporarily or permanently changes its function. There is no definite treatment established for neurological complete injury patients. This study investigated the effect of ginseng extract and ginsenosides on neurological recovery and antioxidant efficacies in rat models following SCI and explore the appropriate dosage. Searches were done on PubMed, Embase, and Chinese databases, and animal studies matches the inclusion criteria were selected. Pair-wise meta-analysis and subgroup analysis were performed. Ten studies were included, and the overall methodological qualities were low quality. The result showed ginseng extract and ginsenosides significantly improve neurological function, through the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (pooled MD = 4.40; 95% CI = 3.92 to 4.88; p < 0.00001), significantly decrease malondialdehyde (MDA) (n = 290; pooled MD = −2.19; 95% CI = −3.16 to −1.22; p < 0.0001) and increase superoxide dismutase (SOD) levels (n = 290; pooled MD = 2.14; 95% CI = 1.45 to 2.83; p < 0.00001). Both low (<25 mg/kg) and high dosage (≥25 mg/kg) showed significant improvement in the motor function recovery in SCI rats. Collectively, this review suggests ginseng extract and ginsenosides has a protective effect on SCI, with good safety and a clear mechanism of action and may be suitable for future clinical trials and applications.
Keywords: Ginseng extract, Ginsenosides, Meta-analysis, Spinal cord injury, Systematic review
Graphical abstract
1. Introduction
Spinal cord injury (SCI) is a disastrous condition that is defined as damage to the spinal cord that temporarily or permanently changes its function [1]. SCI can be caused by a traumatic event leading to immediate mechanical disruption and dislocation of the vertebral column, subsequently causing compression or transection of the spinal cord. Such injury damages the neurons and oligodendrocytes, disrupts the vasculature, and compromises the blood-spinal cord barrier [1]. These events eventually result in further spinal cord damage and severe neurological dysfunction due to a sustained cascade of secondary injuries, including cell dysfunction, cell death, apoptosis, and demyelination accompanied by severe hemorrhage due to blood vessel injuries, destruction of the microvascular supply of the spinal cord, neurotoxicity, or glial scarring. These conditions encourage cell permeabilization and activate pro-apoptotic signaling [[2], [3], [4]]. Subsequently, an influx of inflammatory cells (macrophages, neutrophils, and lymphocytes), cytokines (tumor necrosis factor (TNF) and interleukin (IL)-1β), and vasoactive peptides flood the spinal cord, contributing to the ischemic injury and neuroinflammation [5].
Patients with SCI may experience partial or complete loss of sensorimotor function, paralysis, or neurogenic bowel or bladder dysfunction; others experience fatal symptoms such as compromised respiratory function, disruption of the sympathetic nervous system, hypotension, and bradycardia [1]. The incidence of SCI varies worldwide. The estimated incidence of traumatic SCI (TSCI) increased from 133 to 226 thousand globally in 2007 to 3.6 to 195.4 cases per million in 2014 [6,7]. The incidence has decreased or has remained stable over time in developed countries due to the implementation of preventive measures based on the epidemiology of TSCI [8]. Previous studies in Japan and Spain reported that SCI affects younger patients due to road traffic accidents, but recent studies report that TSCI now affects mainly older adults due to falls [9,10].
Various meta-analyses have evaluated the potential efficacies of SCI treatments such as natural products [11,12], medical drugs [13], and transplantation of stem cells [14]. However, there is currently no established definitive treatment for patients with complete neurological injury in the clinical setting [15,16]. Currently available treatment methods include the maintenance of adequate spinal cord perfusion, intravenous administration of a large dosage of methylprednisolone sodium succinate, and decompressive surgery. Neurorehabilitation treatments such as body-weight supported locomotor training and physical training generally achieve only limited improvements in patients with SCI [17].
Ginsenosides are a group of natural steroid glycosides and triterpene saponins. Approximately 40 ginsenoside compounds have been identified [18], with the ginsenosides Rb1, Rg1, Rg3, Re, Rd, and Rh1 being commonly studied [[19], [20], [21]]. These compounds are found almost exclusively in the plant genus Panax, but are mainly derived from Panax ginseng roots and processed via purification of the column or high-performance liquid chromatography [22]. Ginsenosides possess the bioactivities of antioxidation [23], neuroprotection [24,25], promotion of neurite outgrowth [26], anti-inflammation [27], antitumor [28], and memory improvement [29]. In addition, various ginsenoside compounds improve the condition of patients with stroke and neuronal damage due to oxidative stress and neurotoxicity. Ginsenoside Rb 1 is beneficial in the treatment of cerebral ischemia and protects the blood-brain barrier under ischemic stroke condition [30,31]. Ginsenoside Rg1 prevents apoptosis of astrocytes by reducing brain edema and infarct volume in patients with middle cerebral artery occlusion [32], and protects the hippocampus from neuronal damage by reducing NADPH oxidase 2 -mediated reactive oxygen species (ROS) production [33]. Various studies have shown that ginsenosides Rb2, Rh2, Rg3, and Rd prevent neurotoxicity by reducing the level of neurotoxins such as trimethyltin or glutamate [[34], [35], [36]]. Ginsenosides also have a regulatory effect on the phosphatidylinositol 3-kinase (PI3K)/AKT and MAPK/ERK signaling pathways [37]. Furthermore, ginsenoside Rd promotes neural regeneration and axonal outgrowth by upregulating GAP-43 and increasing the expression of vascular endothelial growth factor and brain-derived neurotrophic factor in hypoxic PC 12 cells [26,38]. Therefore, with the vast array of ameliorative effects such as antioxidation, neuroprotection, promotion of neurite outgrowth, and anti-inflammation, P. ginseng and its major component, ginsenosides, could potentially reduce secondary complications in patients with SCI.
A systematic review aims to answer a particular research question by collecting empirical evidence that meets the eligibility criteria, while a meta-analysis is a subset of a systematic review. A meta-analysis systematically assesses the results of previous research by quantitatively deriving conclusions and assesses the strength of evidence regarding a specific disease and treatment [39]. The results of a meta-analysis can enhance the accuracy of estimates of effect, resolve controversies from current conflicting studies, and generate new hypotheses [39].
The present study aimed to investigate the effect of ginseng extract and ginsenosides on neurological recovery and their antioxidant efficacies in a rat model of SCI by assessing the results of previous related research, and to define the effectiveness of ginseng extract and ginsenosides in the treatment of SCI.
2. Methods
2.1. Study selection
The following electronic databases were searched from inception to April 1, 2019 to identify relevant animal studies without language restrictions: PubMed, Embase, China National Knowledge Infrastructure, SinoMed, VIP, and WanFang. The reference lists of the included studies were screened to identify any additional relevant studies. MeSH terms such as “spinal cord injury” “spinal cord diseases” “spinal cord contusion” “spinal cord laceration” “spinal cord transection” “spinal cord trauma” “ginsenosides” “ginsenoside Rb1” “ginsenoside Rg1” and “ginseng saponin” were used during the search process. Appendix 1 contains the PubMed database search strategy.
Two reviewers (K·S.S. and M.Y.) independently screened the abstracts and full texts of the retrieved studies. Any disagreements were resolved through discussion with a third reviewer (X.J.C.).
2.2. Eligibility criteria
2.2.1. Types of studies
All studies assessing the effect of ginseng extract and ginsenosides in rats with SCI were included. Clinical case reports or only in vitro studies were excluded.
2.2.2. Types of participants
There were no restrictions regarding the age, sex, or strain of laboratory rats. Rats that underwent contusion or compression to induce SCI were included. Rat models of SCI created using laceration, transection, nontraumatic ischemia-reperfusion, photochemical reaction, traumatic root avulsion, dorsal root entry zone damage, and genetic modification were excluded.
2.2.3. Types of intervention
Studies evaluating ginsenosides of any type compared with placebo controls were included. There were no restrictions on the dosage, formulation, administration route, and timing of ginsenosides. Placebo controls were physiological saline or no treatment.
2.3. Type of outcome measures
2.3.1. Functional evaluation
The rats' motor function was evaluated with the Basso, Bettie, Bresnahan (BBB) locomotor rating scale, inclined plane test, and open field test. The BBB locomotor rating scale assessment is widely used to evaluate the post-injury motor behavior of animals [40], and is validated and widely accepted [[41], [42], [43]]. Assessors observe the hindlimb movement of animals and assign a BBB scale score ranging from 0 (complete paralysis) to 21 (normal locomotion). The inclined plane test evaluates the rats' ability to maintain their position for 5 seconds at certain degrees of inclination to assess the motor behavior recovery after SCI [44]. The open field test is performed to evaluate locomotor deterioration in animal models of neuromuscular disorders [45]. The rats are placed in an open arena surrounded by walls to measure the open field activity, such as the total distance traveled through a grid of infrared light [[46], [47], [48]].
2.3.2. Biochemical analysis
During SCI, oxidative injury in the spinal cord produces lipid peroxidation (LPO), which contributes to cell damage. LPO is measured based on the levels of malondialdehyde (MDA) and superoxide dismutase (SOD). As ginsenosides stimulate the production of SOD and reduce the activity of MDA, both indicators are used to assess the efficacy of ginsenosides in animal models of SCI [49,50]. MDA is the most studied byproduct of the process of LPO of polyunsaturated fatty acids and is often used as a marker of LPO [51]. SOD is an enzyme produced during the inflammatory response that limits further damage caused by ROS elements [52]; increasing evidence has shown that pharmacological products [49] and antioxidant treatments [53] reduce the impact of secondary injury by limiting ROS. The MDA and SOD levels at 7 ± 3 days after SCI were analyzed in the present study.
2.3.3. Data extraction
The details of the included studies were independently extracted by 2 authors (K·S.S. and M.Y.). Extracted data included the name of the first author, publication year, animal strain, weight, and sex, number of animals in each group, method used to induce SCI, SCI levels, ginsenoside administration (including dosage, method, and time), and measured outcomes. The mean and standard deviation of each variable were extracted for comparisons. In accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, for studies with multiple intervention groups, the experimental group was combined to enable a single pair-wise comparison. The present review compared the effect of different dosages on the recovery from SCI, with 25 mg/kg set as the cutoff value to differentiate between high (≥25 mg/kg) and low (<25 kg/mg) dosages of ginsenosides. Intervention groups in the respective dosage groups were combined. If a study had 2 different dosage groups, the shared control group was divided approximately evenly among the comparisons. GetData Graph Digitizer 2.24 was used to interpret graph data. Disagreements were resolved via discussion with a third reviewer (X.J.C.).
2.3.4. Risk of bias assessment
The reporting quality and design of studies were assessed using the initial Stroke Therapy Academic Industry Roundtable (STAIR) guidelines [54]. In 2009, a 7-point checklist was released in accordance with the Recommendations for Ensuring Good Scientific Inquiry [55].
2.3.5. Statistical analysis
Data from all included studies were summarized, and data were analyzed using RevMan 5.3 software. Studies involving a direct comparison of the ginsenoside group versus the control group were analyzed using the pair-wise meta-analysis method. Mean differences (MD) were used for outcomes using the same unit, while standardized MD were used for outcomes using different units. Cochrane's I2 value was used to identify heterogeneity between the groups. Heterogeneity was presumed in the event that the p-value in the chi-squared test was less than 0.10, and was considered to be high when the I2 value was more than 50% [56]. Fixed-effect models were used for studies with homogenous clinical and statistical analyses, while random-effect models were used for studies with heterogeneous clinical and statistical analyses [56]. A subgroup meta-analysis was done of high (≥25 mg/kg) and low (<25 mg/kg) ginsenoside dosages. A pair-wise meta-analysis with subgroup analysis was done to evaluate the relative effects of each intervention with other effects. The effect of interventions was determined by obtaining the MD of postintervention values through a comparison of the ginsenoside and control groups [56]. A line graph was constructed by GraphPad Prism 8.0.2 software to highlight the BBB score improvements in both groups. The axes were the BBB score (points) against the time after SCI (days), with available data on day 1, 2, 3, 4, 7, 10, 14, 21, and 28 after SCI.
3. Results
3.1. Description of included studies
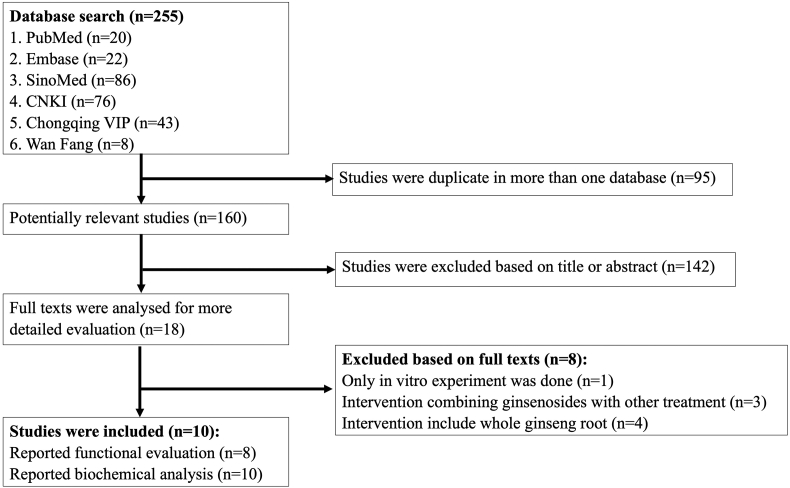
The study selection process is shown in Fig. 1. The search strategy identified 255 studies. Duplicate studies were eliminated, and further screening resulted in 18 studies being selected for full-text screening. Ten of these 18 studies were included; the 8 excluded studies were 1 in vitro experiment [57], 3 studies that reported intervention combining ginsenosides with other treatments [[58], [59], [60]], and 4 studies that reported intervention including the whole ginseng root [[61], [62], [63], [64]]. Among the 10 included studies, 6 were published in Chinese, while 4 were in English [[65], [66], [67], [68], [69], [70], [71], [72], [73], [74]].
Fig. 1.
Flow diagram of studies included in this review.
3.2. Characteristics of included studies
The characteristics of the included studies are summarized in Table 1. Of the 10 studies that met the inclusion criteria, 9 studies used Sprague-Dawley rats, while 1 study used Wistar rats. The sample size ranged from 24 to 108. A TSCI model was used in all included studies; 8 studies used a weight-drop impactor, and 2 studies used the aneurysm clip compression method. All studies established SCI from T7 to T12.
Table 1.
Description of the Characteristics of Studies Included in This Review
| Study | Animals | SCI | No. of Animals | Groups | Outcome | Motor Function Assessment Time |
|---|---|---|---|---|---|---|
| Sun JZ 201965 |
Male SD rats (210–230g) | T8 weight-drop impactor 250g/cm | 10/10/10 | A: Sham + Saline (10mg/kg i.p.) B: SCI + Saline (10mg/kg i.p.) C: SCI + Gs Rg1 (10mg/kg i.p.) for 7 days |
Biochemistry: MDA, SOD, IL-1β, IL-10 | - |
| Liu X 201866 |
Female/male SD rats (220-260g) | T10 weight-drop impactor 10g∗5cm | 10/10/10/10 | A: Sham B: SCI + Saline (10mg/kg i.p.) C: SCI + G-Rb1 (10mg/kg i.p.) D: SCI + G-Rb1 (10mg/kg i.p.) + inhibitor L-name (7 mg/kg i.p.) for 7 days |
Behavioral: BBBs Immunohistochemistry: HE Staining, Immunohistochemical Staining Biochemistry: SOD, MDA, glutathione, catalase, RT-qPCR analysis |
1d, 7d, 14d, 21d, 28d |
| Wang P 201767 |
Male SD rats (200-220g) | T7-T10 aneurysm clip injury 30g∗1 min | 6/6/6/6 | A: Sham + Saline (20mg/kg i.p.) B: SCI + Saline (20mg/kg i.p.) C: Sham + Rb1 (20mg/kg i.p.) D: SCI + Rb1 (20mg/kg i.p.) for 28 days |
Behavioral: BBBs Immunohistochemistry: HE and Nissl Staining, Double Immunofluorescence Staining Biochemistry: Cell Culture and Cell Viability Assessment, Scratch Test, Caspase-3, |
1d, 7d, 14d, 21d, 28d |
| Cong L 201668 |
Female SD rats (250–300g) | T8 weight-drop impactor 10g∗5cm | 18/18/18/18/18/18 | A: Sham B: SCI C: SCI + Gs Rd-L (12.5 mg/kg i.p.) D: SCI + GS Rd-M (25 mg/kg i.p.) E: SCI + GS Rd-H (50 mg/kg i.p.) F: SCI + DEX (1mg/Kg i.p) for 14 days |
Behavioral: BBBs Immunohistochemistry: HE Staining, TNF-a, IL-1b, IL-6 Biochemistry: MDA, GSH, SOD |
1d, 4d, 7d, 10d, 14d |
| Sun JZ 201569 |
Male SD rats (220–240g) | T7-T11 weight-drop impactor 25g/cm | 20/20/20 | A: Sham B: SCI + Saline C: SCI + Gs Rg1 (10 mg/kg i.p.) for 14 days |
Behavioral: BBBs Immunohistochemistry: SOD, MDA |
24h, 48h, 72h, 14d |
| Liu YL 201570 |
Female SD rats (220–260g) | T8-L1 weight-drop impactor 40g/cm | 15/15/15 | A: Sham B: SCI + Gs Rg1 (10mg/kg i.p.) C: SCI for 14 days |
Behavioral: BBBs Immunohistochemistry: HE Staining, Immunofluorescence Staining Biochemistry: SOD, MDA, Caspase-3, Bcl-2 |
1d, 3d, 7d, 14d |
| Li Q 201371 |
Female SD rats (200-250g) | T7-T8 weight-drop impactor 5g∗10cm | 18/18/18/18 | A: SCI + Rb1-L (20 mg/kg i.p.) B: SCI + Rb1-M (40 mg/kg i.p.) C: SCI + Rb1–H (80 mg/kg i.p.) D: SCI + Saline (40mg/kg i.p) for 28 days |
Behavioral: BBBs Biochemistry: SOD, MDA |
1d, 7d, 14d, 28d |
| Song YX 200972 |
Male SD rats (200–250g) | T8-T10 weight-drop impactor 5g∗10cm | 36/36/36 | A: Sham B: SCI C: SCI + Gs (5 mg/kg i.p.) for 14 days |
Behavioral: BBBs, Inclined Plane Test Biochemistry: Bcl-2, BaxPr, Capase-3 |
1d, 3d, 7d, 14d |
| Sakanaka M 200773 |
Male Wistar rats (250-300g) | T12 aneurysm clip injury 20g∗20 min | 8/8/8 | A: SCI + gRb1 (1.2μg/day i.p) B: SCI + gRb1 (6μg/day i.p) C: SCI + Saline (6μg/kg i.p) for 7 days |
Behavioral: Open Field Locomotor Score, BBBs, Brain Temperature, Water Maze Test Immunohistochemistry: TTC Staining Biochemistry: MAP2, VEGF, Bcl-xl |
1d, 2d, 4d, 7d |
| Guo DQ 200474 |
Female/male SD rats (250–320g) | Modified Allen's compression method | 6/18/18/18 | A: Sham B: SCI C: SCI + Gs (2.5mg/kg i.p.) D: SCI + MP (15mg/kg i.p.) for 14 days |
Immunohistochemistry: HE Staining Biochemistry: SOD, MDA, ET, NGF |
- |
SD, Sprague-Dawley; T, thoracic vertebrae; SCI, spinal cord injury; GS Rd, Ginsenoside Rd; i.p., intraperitoneal; BBBs, Basso-Beattie-Bresnahan locomotor rating scale; HE staining, hematoxylin-eosin staining; SOD, Superoxide Dismutase; MDA, malondialdehyde.
Among the studies, 8 reported both functional and biochemical outcomes, while 2 reported only biochemical outcomes. The BBB scale was used in 8 studies, while the inclined plane test, open field locomotor score, brain temperature, and water maze test were each used in 1 study. The duration of evaluation ranged from 24 hours to 28 days.
3.3. Risk of bias assessment
The risk of bias assessment for all included studies is shown in Table 2. Generally, the STAIR assessment reflected a relatively low methodological quality of the studies. None of the included studies reported the sample size calculation, potential conflicts of interest, and study funding. Five studies described the inclusion and exclusion criteria for the SCI model. Nine studies reported randomization, while no studies reported allocation concealment. The exclusion of animals from the analysis was reported in 2 studies. Blinded assessment of outcomes was reported in 4 studies.
Table 2.
Risk of Bias Summary
| Study | Sample-size calculation | Inclusion and exclusion criteria | Randomization | Allocation concealment | Reporting of animals excluded from analysis | Blinded assessment of outcome | Reporting potential conflicts of interest and study funding |
|---|---|---|---|---|---|---|---|
| Sun, J.Z. 201965 | Unclear | Unclear | Low | Unclear | Low | Unclear | Unclear |
| Liu, X. 201866 | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Unclear |
| Wang, P. 201767 | Unclear | Unclear | Low | Unclear | Unclear | Low | Unclear |
| Cong, L. 201668 | Unclear | Low | Low | Unclear | Unclear | Low | Unclear |
| Sun, J.Z. 201569 | Unclear | Unclear | Low | Unclear | Low | Unclear | Unclear |
| Liu, Y.L. 201570 | Unclear | Low | Low | Unclear | Unclear | Low | Unclear |
| Li, Q. 201371 | Unclear | Low | Low | Unclear | Unclear | Unclear | Unclear |
| Song, Y.X. 200972 | Unclear | Low | Low | Unclear | Unclear | Unclear | Unclear |
| Sakanaka, M. 200773 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Guo, D.Q. 200474 | Unclear | Low | Low | Unclear | Unclear | Low | Unclear |
SD, Sprague-Dawley; T, thoracic vertebrae, SCI, spinal cord injury; GS Rd, Ginsenoside Rd; i.p., intraperitoneal; BBBs, Basso-Beattie-Bresnahan locomotor rating scale; HE staining, hematoxylin-eosin staining; SOD, Superoxide Dismutase; MDA, malondialdehyde.
3.4. Ginsenoside efficacy in neurological recovery
3.4.1. Change in the BBB score after SCI
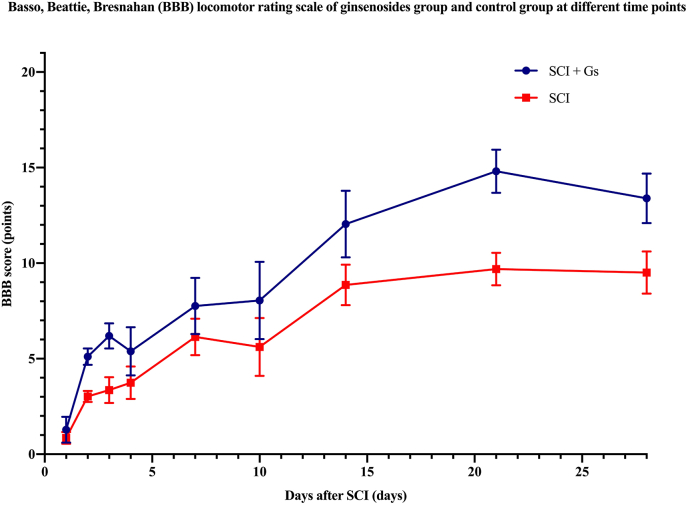
For the analysis of locomotor recovery, 8 studies reported the BBB score after treatment. The ginsenosides group showed better improvement in the BBB score than the control group, reaching a peak on the 21st day after SCI (BBB = 14.81 ± 1.1) (Fig. 2). The BBB scores in both the ginsenoside and control groups increased rapidly on day 1 and 2 after SCI. The increasing trend slowed down from the 3rd day (pooled MD = 1.72; 95% CI = 1.55 to 1.90; p < 0.00001) to the 10th day after SCI (pooled MD = 2.36; 95% CI = 1.70 to 3.02; p < 0.00001). The BBB score on the 28th day after SCI was significantly better in the ginsenoside group than the control group (pooled MD = 4.40; 95% CI = 3.92 to 4.88; p < 0.00001).
Fig. 2.
The Basso, Bettie, Bresnahan (BBB) score of the ginsenoside and control groups at different timepoints after spinal cord injury (SCI).
3.4.2. Subgroup analyses of the effect of ginsenosides
Subgroup analyses were performed based on the dose of ginsenosides and the method used to establish the SCI model. Day 14 after SCI was selected as the timepoint for analysis. Analyses of rat strains, injury location, and administration methods could not be carried out because these variables were consistent in the included studies.
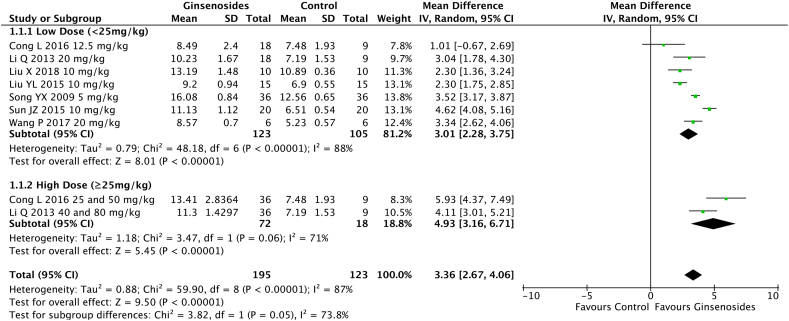
3.4.3. Effect of the ginsenoside dosage on the BBB score
The subgroup analysis included 7 studies. The administration of ginsenosides at a high dosage of ≥25 mg/kg (pooled MD = 4.93; 95% CI = 3.16 to 6.71; p < 0.00001) resulted in significantly better motor function recovery in rats with SCI compared with ginsenosides at a low dosage of <25 mg/kg (pooled MD = 3.01; 95% CI = 2.28 to 3.75; p < 0.00001) (p = 0.05) (Fig. 3).
Fig. 3.
Effect of different dosages of intraperitoneally injected ginsenosides on the Basso, Bettie, Bresnahan (BBB) score.
3.4.4. Effect of the ginsenoside dosage on the inclined plane test
Only 1 included study used the inclined plane test to evaluate the neurological recovery of rats with SCI after ginsenoside administration [72]. The administration of ginsenosides at a dosage of 5 mg/kg after SCI resulted in a significant improvement in motor function recovery in the intervention group compared with the control group (p < 0.01).
3.4.5. Effect of the ginsenoside dosage on the open field test
Only 1 included study used the open field test to evaluate the neurological recovery of rats with SCI after ginsenoside administration [73]. The administration of ginsenosides at a dosage of 6.0 μg/day resulted in significantly improved motor function recovery after SCI in the intervention group compared with the control group (p < 0.01).
3.4.6. Effect of the SCI establishment method on the BBB score
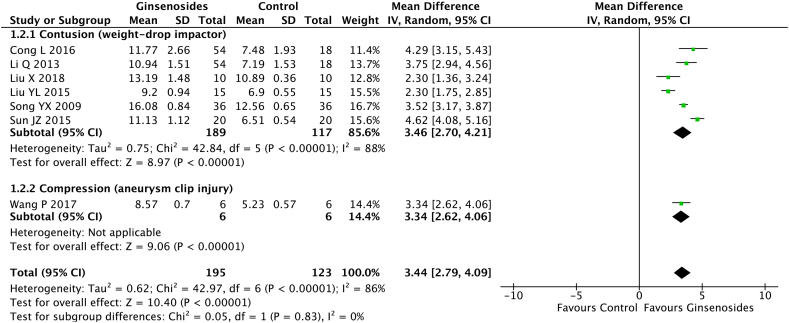
The contusion method of establishing SCI (pooled MD = 3.46; 95% CI = 2.70 to 4.21; p < 0.00001) and compression method of establishing SCI (pooled MD 3.34; 95% CI = 2.62 to 4.06; p < 0.00001) did not affect the rate of improvement of motor function recovery (p = 0.88) (Fig. 4).
Fig. 4.
Effect of the spinal cord injury (SCI) establishment method on the Basso, Bettie, Bresnahan (BBB) score.
3.4.7. Antioxidant effects of ginsenosides
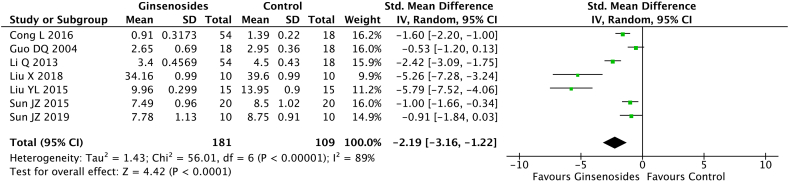
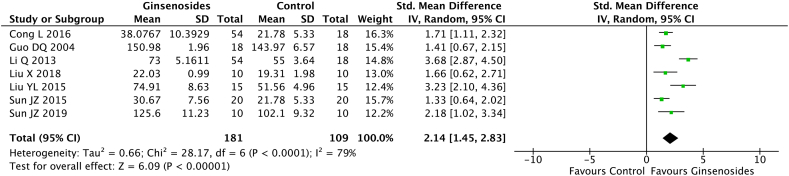
Seven studies (n = 290) evaluated the MDA and SOD levels; the levels were reported on day 7 in 2 studies, day 14 in 4 studies, and day 28 in 1 study. The random-effect model analysis showed that the ginsenoside group had a significant reduction in the MDA level (pooled MD = −2.19; 95% CI = −3.16 to −1.22; p < 0.0001) (Fig. 5) and a significant increase in the SOD level (pooled MD = 2.14; 95% CI = 1.45 to 2.83; p < 0.00001) (Fig. 6, Fig. 7) compared with the control group.
Fig. 5.
Malondialdehyde (MDA) levels in the ginsenoside and control groups.
Fig. 6.
Superoxide dismutase (SOD) levels in the ginsenoside and control groups.
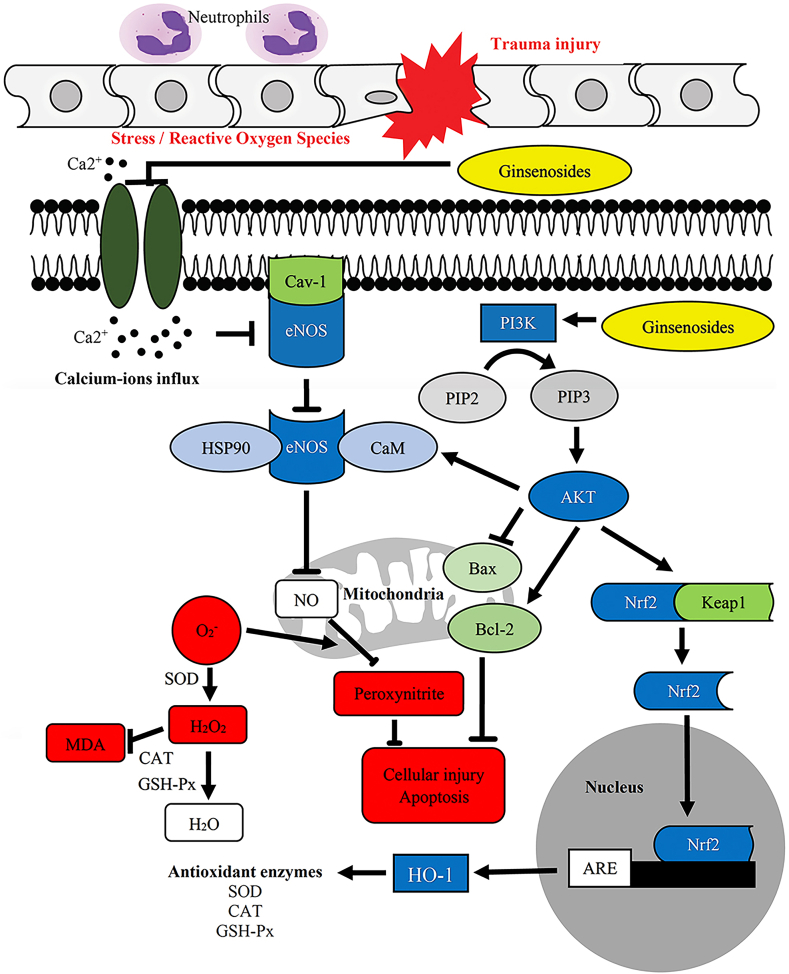
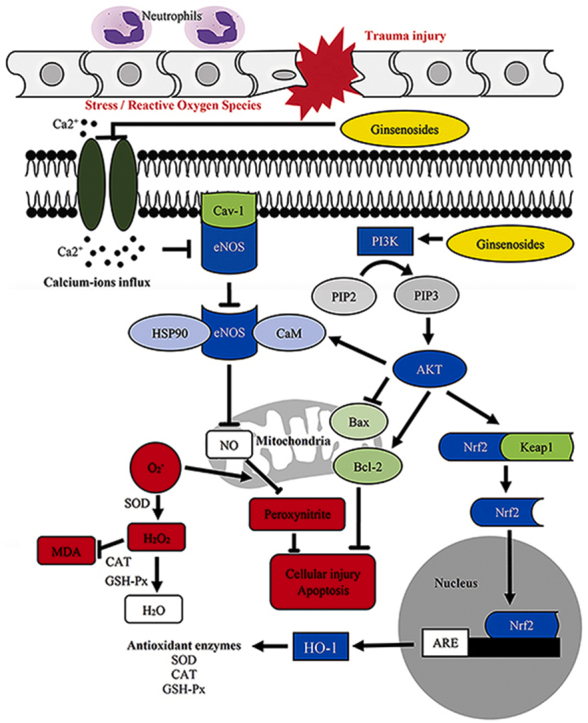
Fig. 7.
Potential mechanism of the effect of ginsenosides in treating spinal cord injury (SCI).
4. Discussion
4.1. Summary of evidence
To the best of our knowledge, no prior meta-analysis has quantitatively evaluated the efficacy of ginsenosides in treating SCI. The present meta-analysis demonstrated that compared with the control group, the group treated with ginsenosides achieved significant improvements in motor function that were directly correlated with time, with the most significant difference in improvement seen on day 21 after SCI. Although the motor function recovery remained stable from 21 days after SCI, the significant improvement in the ginsenoside group versus the control group shows that ginsenosides are effective in treating SCI. Both high and low dosages of ginsenosides improved the motor function compared with the control group. However, better improvement was seen after the administration of a high dosage of ginsenosides (≥25 mg/kg) compared with a low dosage (<25 mg/kg). The results of biochemical analysis, such as the MDA and SOD levels, showed that the antioxidant effect was significantly greater in the ginsenoside group than the control group.
4.2. Possible mechanism of ginsenosides in SCI
Ginseng is the root and rhizome of Ginseng Radix et Rhizoma, also known as Panax ginseng Meyer in the genus Panax of the Araliaceae family [75]. After the oral administration of ginseng, the gut microbiota converts ginsenoside into its metabolites. Generally, the metabolism of ginsenosides occurs in the gastrointestinal tract after oral administration [76]. Ginsenosides were first isolated in the 1960s and have been extensively studied because ginseng is widely used worldwide. Ginsenosides have a similar basic structure; almost all ginsenosides have 30 carbon atoms arranged in four rings of steroid nuclei [77]. Ginsenosides are divided into 4 types based on the amount and position of the sugar moiety: panaxadiol (Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2), panaxatriol (Re, Rg1, Rg2, Rh1), oleanolic acid (Ro), and ocotillol (Rs) [78,79].
The ginsenosides Rb1, Rg1, Rg3, Re, Rd, and Rh1 are commonly studied [[19], [20], [21]], and all studies included in this review evaluated at least one of these ginsenosides. Clinical research has shown that ginsenosides have distinct neuroprotective (Rb1, Rg1, Rg2, Rh1) [80,81], cognition-enhancing (Rg3, Rh2, Rg1) [[82], [83], [84]], anti-inflammatory (Rb1, Rg3, Rh2, Rc, Rh1) [80,85,86], antioxidative (Rb2, Rc, Rh1) [[87], [88], [89]], antitumor (Rb2, Rg3, Rh2) [[90], [91], [92]], and antiosteoporotic benefits (Rb2) [93].
Under normal circumstances, the human body produces byproducts such as ROS through numerous physiological and biochemical processes [94]. However, TSCI induces acute inflammatory responses triggered by the innate immune system, including systemic vasodilation, vascular leakage, and leukocyte emigration [95]. Secondary spinal cord injury then occurs due to the infiltration of leukocytes and glial cell activation [96]. The presence of polymorphonuclear neutrophils enhances the generation of ROS during inflammation and contributes to spinal cord swelling, which leads to further compression and worsens the injury [97]. The glial cell activation characterized by neuroinflammation is fueled by the vast infiltration of inflammatory cells and cytokines such as TNF- α, IL-1β, and IL-6 in the spinal cord [98]. Furthermore, as the spinal cord contains a large amount of unsaturated fatty acids, it has active cell metabolism and a low antioxidant capacity [99]. Therefore, SCI causes a severe oxidative stress response, as biomolecules are damaged due to the overproduction of free radicals [100].
MDA is a byproduct of the LPO process and is indicative of the level of LPO and cell damage by ROS. The mechanism by which ginsenosides relieve secondary spinal cord damage may be inhibition of the infiltration of calcium ions from the extracellular matrix into the damaged cells [101,102]. The infiltration of calcium ions triggers the activation of endothelial nitric oxide (NO) synthase, which contributes to the production of NO by binding with AKT. NO contributes to cell damage and possibly apoptosis by interacting with ROS. Ginsenosides also activate the PI3K/AKT pathway, which is crucial in regulating the Bax/Bcl-2 ratio and stimulating the production of SOD, glutathione peroxidase, and catalase through activation of the Nrf-2/HO-1 pathway. SOD is an important antioxidant enzyme that converts superoxide into hydrogen peroxide, while glutathione peroxidase and catalase convert hydrogen peroxide into water and prevent it from contributing to LPO [103]. Therefore, MDA and SOD are currently recognized as reliable indicators of oxygen free radical levels after SCI [104]. Hence, during the event of SCI, if the activity of SOD could be increased and the production of MDA could be reduced, this could potentially help to reduce secondary damage to the spinal cord.
The included studies showed that the ginsenosides Rg1 [65,69,70], Rb1 [66,71], Rd [68], and ginseng saponins [74] effectively reduced the production of MDA and increased the activity of SOD in rats with SCI. Furthermore, the ginsenosides Rg1 and Rb1, and ginseng saponin upregulated the Bcl-2/Bax ratio and downregulated the caspase-3 expression. The ginsenosides Rg1 and Rd also reduced the expression of TNF-α, IL-1β, IL-6, and IL-10. These findings are similar to the findings of a study investigating the effect of various ginsenosides in protecting against cerebral ischemic reperfusion injury [105]. The ginsenosides Rg1 [32], Rb1 [106], and Re [107] reduced MDA production and increased SOD activity, while the ginsenosides Rd [38] and Rg3 [108] upregulated the P13K/AKT and ERK1/2 pathways, and reduced caspase-3 expression.
Studies have also shown that both panaxadiol and panaxatriol ginsenosides inhibit the expression of TLR4 and MyD88, and activate SIRT1 to down regulate the expression of NF-κB [109]. This mechanism reduces the release of inflammatory factors such as TNF-α, IL-1β, and IL-6. Panaxadiol ginsenoside (Rb1 and Rg3) have a stronger effect than panaxatriol ginsenosides(Rg1 and Re) and reduce both cerebral ischemic injury and neurological deficits in ischemic rats [109]. Other studies have produced similar results, showing that both panaxadiol ginsenosides (Rb1, Rg3, Rh2) [[110], [111], [112]] and panaxatriol ginsenosides (Rg1, Re) reduce the release of inflammatory factors, while panaxatriol also reportedly regulates microglia and astrocyte activation [113,114]. In addition, studies have shown that both panaxadiol and panaxatriol ginsenosides inhibit inflammasome activation by suppressing the activation of NLRP3 and AIM2 inflammasomes during a macrophage-mediated inflammatory response in a dose-dependent manner [115]. In the regulation of oxidative stress, panaxatriol ginsenosides increase the amount of glutathione and reduce the production of MDA in cardiomyocytes with ischemic reperfusion [116]. Moreover, panaxadiol ginsenosides are superior to panaxatriol ginsenosides in lowering apoptosis rates [109,117,118], while both panaxadiol and panaxatriol ginsenosides are cardioprotective and protect against cerebral ischemic injury. However, both panaxadiol and panaxatriol ginsenosides show a diminished cardioprotective trend as the molecular weight and complexity of the compound increases [119]. This is evident by the fact that Rg3 has less cardioprotective capabilities than other panaxadiol ginsenosides such as Rh2, even though the ginsenosides share a similar chemical structure [120,121]. The ginsenosides Rh2 and Rg5 also provide a better protective effect than the ginsenosides Rb1, Rg2, and Rg3 in a cerebral ischemic injury model [109]. The cardioprotective properties tend to diminish in both groups with the increasing molecular weight and complexity of the compound. It is believed that a larger molecular weight inhibits the ability of the compound to cross the cell membrane to act on the mitochondrial permeability transition pore [119]. Furthermore, compounds with high polarity are poorly absorbed [122]. Hence, compounds with a large number of hydroxide groups (such as ginsenosides Rg2 and Rg3) are not cardioprotective [119].
There are currently several clinical trials related to SCI treatment, which can be categorized into physiological approaches, pharmacology, and stem cell treatment [123]. However, there are currently no clinical trials involving the administration of ginsenosides to patients with SCI, and clinical trials involving ginsenosides are scarce [124]. Clinical trials have evaluated ginsenoside intervention in patients with carcinoma in the liver, lung, and gastric system, acute ischemic stroke, rheumatoid arthritis, metabolic syndrome, cardiovascular disease, and blood pressure disorders [124]. Ginsenosides are mostly administered in oral capsule or tablet form, while some trials have evaluated ginsenosides administered through the intravenous infusion route to patients with acute ischemic stroke [125]. Trials have been performed to assess the effect of traditional Chinese and Korean herbal medicines on patients with cervical spondylotic myelopathy who present with symptoms of SCI. However, most such trials have involved a combination of non-surgical interventions such as herbal medicine, tuina or chuna (also known as massage therapy), electroacupuncture, acupotomy, cervical traction, exercise, and physiotherapy [[126], [127], [128], [129], [130], [131], [132]], while only one trial reported the use of herbal medicine alone [133].
Consistency in the chemical composition and pharmacological properties is essential in any trial evaluating the safety and effective administration of herbal drugs. However, ginseng and other herbal products generally fail to meet this standard [134]. This is most likely due to the poor extraction methods and lack of standardization regarding ginseng preparation [134]. Although chromatographic techniques and marker compounds are used to standardize the extraction of herbal products, this method does not guarantee consistent and stable pharmacological activity. Moreover, the production and quality of ginsenoside extracts is largely dependent on the extraction method, as ginsenosides become unstable at high temperatures [135]. Conventional extraction methods often cause thermal destruction of biologically active compounds due to long extraction times and large solvent volumes [136]. However, the supercritical extraction method utilizing CO2 extracts a richer extraction with an environmentally friendly technique due to easy removal of the solvent [137].
4.3. Safety of the consumption of ginseng and ginsenosides
Ginseng, which contains an abundant amount of ginsenosides, generally has a good safety profile [138]. However, there have been reports of adverse events after ginseng administration in randomized controlled trials, such as gastrointestinal discomfort [139,140], insomnia, headache, and chest discomfort [[141], [142], [143]]. Recently, the safety of ginsenosides has been investigated in preclinical studies. Many such studies have evaluated compound K (CK), which is a metabolite of the ginsenosides Rb1, Rb2, and Rc and is not present in ginseng itself [144]. One study showed that although no rats were killed during 26-week repeated-dosage toxicity testing of CK at dosages of 13 mg/kg, 40 mg/kg, and 120 mg/kg, rats in the 120 mg/kg group showed fur loss, weakness, underactive locomotor activity, and increased ALT and ALP levels, suggesting potential hepatotoxicity [145]. However, these adverse effects may be affected by the different sensibility and tolerance of test substances between different species [145]. Another study that performed 26-week repeated-dosage toxicity testing of ginsenoside Rg3 at dosages of 20 mg/kg, 60 mg/kg, and 180 mg/kg reported no rat deaths and no significant toxicological effects [146]. Furthermore, a clinical study of healthy Chinese volunteers showed that a single dose of CK (25–800 mg) or multiple doses of CK (100–400 mg) were well tolerated, without any significant adverse events [147].
4.4. Comparison of different ginsenoside doses
The oral administration of ginsenosides leads to differences in the administered dosage due to the poor absorption of orally administered ginsenosides [148]. Furthermore, the low oral bioavailability of ginsenosides leads to a low plasma concentration [149]. Therefore, all included studies chose to administer ginsenosides intraperitoneally rather than orally.
The included studies with groups that received various dosages of ginsenosides showed that groups that received higher dosages had higher SOD and lower MDA levels than the groups that received lower dosages. Both high and low dosages of ginsenosides resulted in significant improvements in the recovery of motor function after SCI in rats, with a higher dosage resulting in significantly better improvement than a lower dosage of ginsenosides. The ginsenoside Rb1 decreases ROS production [150] and recovers the disproportion of cellular redox enzymes [151]. Furthermore, oxidative stress is regulated by ginsenosides through the PI3K/AKT and Nrf2/HO-1 pathways [152].
4.5. Strengths and limitations of this review
To the best of our knowledge, no previous study has quantitatively analyzed the efficacies of ginseng extract and ginsenosides in SCI. The present meta-analysis included relevant studies retrieved from Chinese databases and analyzed the effect of different dosages of ginsenosides and the effect of different SCI establishment methods on the motor function recovery of rats with SCI.
The present review has several limitations. Although 10 studies were included, 6 were published in Chinese and 4 were in English. As ginseng is one of the most important and commonly used herbs in Asia, including China and Korea [153], these two countries would naturally contribute the most publications related to ginseng worldwide [154]. Therefore, it is inevitable that more than half of the studies included in this review were in published in Chinese, which may make it difficult for non-Chinese readers to access the se articles. Moreover, the STAIR assessment showed that the overall quality of the included studies was not high. None of the included studies achieved more than 3 out of the 7-point checklist. First, no studies mentioned the calculation of sample size. Some experiments had 36 rats per group, while other had as little as 6 rats per group. For ethical and financial reasons, it is crucial to design animal experiments well to ensure that the obtained data is reliable, especially regarding the sample size. The number of animals per group may be decided by referring to a table specifying the effect size and the respective number of samples [155]. Second, a moderate amount of heterogeneity was observed in the included studies. The heterogeneity among the included studies only involved different dosages of ginsenosides and different SCI establishment methods. In addition, no studies reported allocation concealment, which would potentially cause bias to occur during functional evaluation. Third, it is uncertain whether all relevant studies were identified. As there were limited studies on the effect of ginsenosides on rats with SCI, only few studies were included.
5. Conclusion
Our study suggests that ginseng extract and ginsenosides significantly improve functional rehabilitation in a rat model of SCI. The probable mechanism by which ginsenosides improve SCI is their antioxidant effects on cells. A higher dosage of ginseng extract and ginsenosides better promotes improvement in the motor function recovery after SCI than a lower dosage of ginseng extract and ginsenosides. However, most included studies were assessed as having low quality. Hence, more high-quality studies are needed to confirm and better explain the effect of ginseng extract and ginsenosides in treating SCI.
Declaration of competing interest
No competing financial interests exist.
Acknowledgements
We thank the National key R & D plan (No. 2018YFC1704302), National Natural Science Foundation of China (No. 81873317, 81930116, 82074454, 8217151915, 81929004) and Shanghai Natural Science Foundation (No. 20ZR1459000) for their financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.05.009.
Contributor Information
Min Yao, Email: yaomin19871223@126.com.
Xue-jun Cui, Email: 13917715524@139.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.LaPlaca M.C., Simon C.M., Prado G.R., Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- 3.Choo A.M., Liu J., Lam C.K., Dvorak M., Tetzlaff W., Oxland T.R. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6(3):255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- 4.Sandrow-Feinberg H.R., Houle J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12–21. doi: 10.1016/j.brainres.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pineau I., Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500(2):267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 6.Fitzharris M., Cripps R.A., Lee B.B. Estimating the global incidence of traumatic spinal cord injury. Spinal Cord. 2014;52(2):117–122. doi: 10.1038/sc.2013.135. [DOI] [PubMed] [Google Scholar]
- 7.Jazayeri S.B., Beygi S., Shokraneh F., Hagen E.M., Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24(5):905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee B.B., Cripps R.A., Fitzharris M., Wing P.C. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 9.Barbara-Bataller E., Mendez-Suarez J.L., Aleman-Sanchez C., Sanchez-Enriquez J., Sosa-Henriquez M. Change in the profile of traumatic spinal cord injury over 15 years in Spain. Scand J Trauma Resusc Emerg Med. 2018;26(1):27. doi: 10.1186/s13049-018-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo D., Miyakoshi N., Hongo M., Kasukawa Y., Ishikawa Y., Ishikawa N., et al. An epidemiological study of traumatic spinal cord injuries in the fastest aging area in Japan. Spinal Cord. 2019;57(6):509–515. doi: 10.1038/s41393-019-0255-7. [DOI] [PubMed] [Google Scholar]
- 11.Tian Z.R., Yao M., Zhou L.Y., Song Y.J., Ye J., Wang Y.J., et al. Effect of docosahexaenoic acid on the recovery of motor function in rats with spinal cord injury: a meta-analysis. Neural Regen Res. 2020;15(3):537–547. doi: 10.4103/1673-5374.266065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B.P., Yao M., Li Z.J., Tian Z.R., Ye J., Wang Y.J., et al. Neurological recovery and antioxidant effects of resveratrol in rats with spinal cord injury: a meta-analysis. Neural Regen Res. 2020;15(3):482–490. doi: 10.4103/1673-5374.266064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L.Y., Tian Z.R., Yao M., Chen X.Q., Song Y.J., Ye J., et al. Riluzole promotes neurological function recovery and inhibits damage extension in rats following spinal cord injury: a meta-analysis and systematic review. J Neurochem. 2019;150(1):6–27. doi: 10.1111/jnc.14686. [DOI] [PubMed] [Google Scholar]
- 14.Yousefifard M., Rahimi-Movaghar V., Nasirinezhad F., Baikpour M., Safari S., Saadat S., et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience. 2016;322:377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Nowrouzi B., Assan-Lebbe A., Sharma B., Casole J., Nowrouzi-Kia B. Spinal cord injury: a review of the most-cited publications. Eur Spine J. 2017;26(1):28–39. doi: 10.1007/s00586-016-4669-z. [DOI] [PubMed] [Google Scholar]
- 16.Hurlbert R.J., Hadley M.N., Walters B.C., Aarabi B., Dhall S.S., Gelb D.E., et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(Suppl 2):93–105. doi: 10.1227/NEU.0b013e31827765c6. [DOI] [PubMed] [Google Scholar]
- 17.Cote M.P., Murray M., Lemay M.A. Rehabilitation strategies after spinal cord injury: Inquiry into the mechanisms of success and failure. J Neurotrauma. 2017;34(10):1841–1857. doi: 10.1089/neu.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1–2):119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Wu W., Jiao C., Li H., Ma Y., Jiao L., Liu S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem Anal. 2018;29(4):331–340. doi: 10.1002/pca.2752. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z., Li Y., Li X., Ruan C.C., Wang L.J., Sun G.Z. The effects of dynamic changes of malonyl ginsenosides on evaluation and quality control of Panax ginseng C. A. Meyer. J Pharm Biomed Anal. 2012;64–65:56–63. doi: 10.1016/j.jpba.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q., Yang H., An J., Zhang R., Chen B., Hao D.J. Therapeutic effects of traditional Chinese medicine on spinal cord injury: a promising supplementary treatment in future. Evid Based Complement Alternat Med. 2016;2016:8958721. doi: 10.1155/2016/8958721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Liu Q., Xu Y., Zhang Y., Lv Y., Tan Y., et al. Ginsenoside Rg1 protects against oxidative stress-induced neuronal apoptosis through myosin IIA-actin related cytoskeletal reorganization. Int J Biol Sci. 2016;12(11):1341–1356. doi: 10.7150/ijbs.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kappaB activation. Neurochem Int. 2011;58(1):119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Hwang Y.P., Jeong H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol. 2010;242(1):18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Wu S.D., Xia F., Lin X.M., Duan K.L., Wang F., Lu Q.L., et al. Ginsenoside-rd promotes neurite outgrowth of PC12 cells through MAPK/ERK- and PI3K/AKT-Dependent pathways. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P., Xie W., Luo Y., Lu S., Dai Z., Wang R., et al. Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE-/- mice via inhibition of apoptosis and enhancing autophagy. Molecules. 2018;23(11) doi: 10.3390/molecules23112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang J.H., Song K.H., Woo J.K., Park M.H., Rhee M.H., Choi C., et al. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Hum Nutr. 2011;66(3):298–305. doi: 10.1007/s11130-011-0242-4. [DOI] [PubMed] [Google Scholar]
- 29.Geng J., Dong J., Ni H., Lee M.S., Wu T., Jiang K., et al. Ginseng for cognition. Cochrane Database Syst Rev. 2010;12:CD007769. doi: 10.1002/14651858.CD007769.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Dong X., Zheng L., Lu S., Yang Y. Neuroprotective effects of pretreatment of ginsenoside Rb1 on severe cerebral ischemia-induced injuries in aged mice: involvement of anti-oxidant signaling. Geriatr Gerontol Int. 2017;17(2):338–345. doi: 10.1111/ggi.12699. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Guo Y., Yang W., Zheng P., Zeng J., Tong W. Protective effect of ginsenoside Rb1 on integrity of blood-brain barrier following cerebral ischemia. Exp Brain Res. 2015;233(10):2823–2831. doi: 10.1007/s00221-015-4352-3. [DOI] [PubMed] [Google Scholar]
- 32.Sun C., Lai X., Huang X., Zeng Y. Protective effects of ginsenoside Rg1 on astrocytes and cerebral ischemic-reperfusion mice. Biol Pharm Bull. 2014;37(12):1891–1898. doi: 10.1248/bpb.b14-00394. [DOI] [PubMed] [Google Scholar]
- 33.Xu T.Z., Shen X.Y., Sun L.L., Chen Y.L., Zhang B.Q., Huang D.K., et al. Ginsenoside Rg1 protects against H2O2 induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int J Mol Med. 2019;43(2):717–726. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou J., Xue J., Wang Z., Li W. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother Res. 2018;32(12):2531–2540. doi: 10.1002/ptr.6193. [DOI] [PubMed] [Google Scholar]
- 35.Kim D.H., Kim D.W., Jung B.H., Lee J.H., Lee H., Hwang G.S., et al. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J Ginseng Res. 2019;43(2):326–334. doi: 10.1016/j.jgr.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X., Shi M., Bjoras M., Wang W., Zhang G., Han J., et al. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol. 2013;4:152. doi: 10.3389/fphar.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., et al. Panax ginseng components and the pathogenesis of Alzheimer's disease (Review) Mol Med Rep. 2019;19(4):2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X.Y., Zhou X.Y., Hou J.C., Zhu H., Wang Z., Liu J.X., et al. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin. 2015;36(4):421–428. doi: 10.1038/aps.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haidich A.B. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 40.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 41.Barros Filho T.E., Molina A.E. Analysis of the sensitivity and reproducibility of the Basso, Beattie, bresnahan (BBB) scale in wistar rats. Clinics (Sao Paulo). 2008;63(1):103–108. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borges P.A., Cristante A.F., Barros-Filho T.E.P., Natalino R.J.M., Santos G.B.D., Marcon R.M. Standardization of a spinal cord lesion model and neurologic evaluation using mice. Clinics (Sao Paulo). 2018;73:e293. doi: 10.6061/clinics/2018/e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheff S.W., Saucier D.A., Cain M.E. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19(10):1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- 44.Rivlin A.S., Tator C.H. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10(1):38–43. [PubMed] [Google Scholar]
- 45.Raben N., Nagaraju K., Lee E., Plotz P. Modulation of disease severity in mice with targeted disruption of the acid alpha-glucosidase gene. Neuromuscul Disord. 2000;10(4–5):283–291. doi: 10.1016/s0960-8966(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 46.Crawley J.N. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 47.Hamann M., Meisler M.H., Richter A. Motor disturbances in mice with deficiency of the sodium channel gene Scn8a show features of human dystonia. Experimental Neurology. 2003;184(2):830–838. doi: 10.1016/S0014-4886(03)00290-5. [DOI] [PubMed] [Google Scholar]
- 48.Nagaraju K., Raben N., Loeffler L., Parker T., Rochon P.J., Lee E., et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A. 2000;97(16):9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Liu T.E., Pan W., Chi H., Chen J., Yu Z., et al. Small molecule compounds alleviate anisomycin-induced oxidative stress injury in SH-SY5Y cells via downregulation of p66shc and Abeta1-42 expression. Exp Ther Med. 2016;11(2):593–600. doi: 10.3892/etm.2015.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu W., Sui D., Yu X., Gou D., Zhou Y., Xu H. Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells. Int J Clin Exp Med. 2015;8(11):19938–19947. [PMC free article] [PubMed] [Google Scholar]
- 51.Balci M., Namuslu M., Devrim E., Durak I. Effects of computer monitor-emitted radiation on oxidant/antioxidant balance in cornea and lens from rats. Mol Vis. 2009;15:2521–2525. [PMC free article] [PubMed] [Google Scholar]
- 52.Porfire A.S., Leucuta S.E., Kiss B., Loghin F., Parvu A.E. Investigation into the role of Cu/Zn-SOD delivery system on its antioxidant and antiinflammatory activity in rat model of peritonitis. Pharmacol Rep. 2014;66(4):670–676. doi: 10.1016/j.pharep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Poljsak B., Suput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroke Therapy Academic Industry R. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 55.Fisher M., Feuerstein G., Howells D.W., Hurn P.D., Kent T.A., Savitz S.I., et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higgins J., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for systematic reviews of interventions version 5.1.0. [Google Scholar]
- 57.Tang Y.Y., Guo W.X., Lu Z.F., Cheng M.H., Shen Y.X., Zhang Y.Z. Ginsenoside Rg1 promotes the migration of olfactory ensheathing cells via the PI3K/akt pathway to repair rat spinal cord injury. Biol Pharm Bull. 2017;40(10):1630–1637. doi: 10.1248/bpb.b16-00896. [DOI] [PubMed] [Google Scholar]
- 58.Guo W.X. Soochow University; Soochow, China: 2015. Experiment of ginsenoside Rg1 promoting migration of olfactory ensheathing cells and repairing spinal cord injury. [Google Scholar]
- 59.Wang W.Q., Li Y.F., Zhang D.W. The effect of ginseng and bone marrow mesenchymal stem cell transplantation on functional recovery of rats with spinal cord injury. Chin J TCM WM Crit Care. 2014;21(6):401–404. [Google Scholar]
- 60.Meng F.J., Li Y.F., Zhang D.W., Liu J.M. Effect of ginseng and rat amniotic epithelial cell transplantation on functional recovery in rats with spinal cord injury. Chin J TCM WM Crit Care. 2012;19(1):12–15. [Google Scholar]
- 61.Zhang Z.G., Li Y.F., Zhu D., Zhang D.W. Effect and significance of ginseng on the contents of MDA, SOD and NO in rats with spinal cord injury. Journal of Apoplexy and Nervous Diseases. 2014;31(12) [Google Scholar]
- 62.Kim Y.O., Kim Y., Lee K., Na S.W., Hong S.P., Valan Arasu M., et al. Panax ginseng improves functional recovery after contusive spinal cord injury by regulating the inflammatory response in rats: an in vivo study. Evid Based Complement Alternat Med. 2015;2015:817096. doi: 10.1155/2015/817096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Shen H., Xie J.J., Ling J., Lu H. Neuroprotective effect of ginseng against spinal cord injury induced oxidative stress and inflammatory responses. Int J Clin Exp Med. 2015;8(3):3514–3521. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu P., Samukawa K., Fujita H., Kato H., Sakanaka M. Oral administration of red ginseng extract promotes neurorestoration after compressive spinal cord injury in rats. Evid Based Complement Alternat Med. 2017;2017:1265464. doi: 10.1155/2017/1265464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun J.Z., Ma T., Ding R., Shi X., Huang J., Wu X., et al. Effects of ginsenoside Rg1 on serum MDA, SOD, IL-1β and IL-10 levels in SD rats with spinal cord injury. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2019;28(1):27–33. [Google Scholar]
- 66.Liu X., Gu X., Yu M., Zi Y., Yu H., Wang Y., et al. Effects of ginsenoside Rb1 on oxidative stress injury in rat spinal cords by regulating the eNOS/Nrf2/HO-1 signaling pathway. Exp Ther Med. 2018;16(2):1079–1086. doi: 10.3892/etm.2018.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang P., Lin C., Wu S., Huang K., Wang Y., Bao X., et al. Inhibition of autophagy is involved in the protective effects of ginsenoside Rb1 on spinal cord injury. Cell Mol Neurobiol. 2018;38(3):679–690. doi: 10.1007/s10571-017-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cong L., Chen W. Neuroprotective effect of ginsenoside rd in spinal cord injury rats. Basic Clin Pharmacol Toxicol. 2016;119(2):193–201. doi: 10.1111/bcpt.12562. [DOI] [PubMed] [Google Scholar]
- 69.Sun J.Z., Liu X.W., Guan H.P., Zhang P., Liu Q., Yang J., et al. Effect of ginsenoside Rg1 on transformation growth factor-beta and brain-derived neurotrophic factor expression in spinal cord injury rats. Chinese Journal of Tissue Engineering Research. 2015;19(18):2862–2866. [Google Scholar]
- 70.Liu Y.L. Soochow University; China: 2015. The protective effects of ginsenoside Rg1 in rats with spinal cord injury Soochow. [Google Scholar]
- 71.Li Q. Protective effects of ginsenoside Rb1 on acute spinal cord injury in rats. China Journal of Pharmaceutical Economics. 2013;7:74–75. [Google Scholar]
- 72.Song Y.X., Jin C.Y., Zeng Z.Y., Wang B., Zhang J.Q. Study on the mechanism underlying neuroprotective effect of ginsenosides in rats after spinal cord injury. Hainan Medical Journal. 2009;20(5):6–9. [Google Scholar]
- 73.Sakanaka M., Zhu P., Zhang B., Wen T.C., Cao F., Ma Y.J., et al. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL. J Neurotrauma. 2007;24(6):1037–1054. doi: 10.1089/neu.2006.0182. [DOI] [PubMed] [Google Scholar]
- 74.Guo D.Q., Yang L., Xiao J.D., Wang D.P., Xie W.J. Experimental effects of ginseng saponin in treatment of acute spinal cord injury of rat. China Journal of Modern Medicine. 2004;14(12) [Google Scholar]
- 75.Pharmacopoeia Committee of P. R. China . 10th ed. China Medical Science and Technology Press; Beijing: 2015. Pharmacopoeia of people’s Republic of China. [Google Scholar]
- 76.Kim D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42(3):255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo C.L., Cui X.M., Yang X.Y., Wu S. Advances in studies on biotransformation of ginsensides. China Journal of Chinese Materia Medica. 2014;39(20):3899–3904. [PubMed] [Google Scholar]
- 78.Shin K.C., Oh D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit Rev Biotechnol. 2016;36(6):1036–1049. doi: 10.3109/07388551.2015.1083942. [DOI] [PubMed] [Google Scholar]
- 79.Wang L., Zhao S.J., Liang Y.L., Sun Y., Cao H.J., Han Y. Identification of the protopanaxatriol synthase gene CYP6H for ginsenoside biosynthesis in Panax quinquefolius. Funct Integr Genomics. 2014;14(3):559–570. doi: 10.1007/s10142-014-0386-z. [DOI] [PubMed] [Google Scholar]
- 80.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41(4):435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L., Liu L.F., Liu J., Dou L., Wang G.Y., Liu X.Q., et al. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11(2):319–325. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen F., Eckman E.A., Eckman C.B. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20(8):1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 83.Qiu J., Li W., Feng S.H., Wang M., He Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet Mol Res. 2014;13(2):3586–3598. doi: 10.4238/2014.May.9.2. [DOI] [PubMed] [Google Scholar]
- 84.Nie L., Xia J., Li H., Zhang Z., Yang Y., Huang X., et al. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of alzheimer's disease. Oxid Med Cell Longev. 2017;2017:6473506. doi: 10.1155/2017/6473506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu T., Yang Y., Kwak Y.S., Song G.G., Kim M.Y., Rhee M.H., et al. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res. 2017;41(2):127–133. doi: 10.1016/j.jgr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee S.Y., Jeong J.J., Eun S.H., Kim D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. 2015;762:333–343. doi: 10.1016/j.ejphar.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30(4):724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 88.Kim D.H., Park C.H., Park D., Choi Y.J., Park M.H., Chung K.W., et al. Ginsenoside Rc modulates Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res. 2014;37(6):813–820. doi: 10.1007/s12272-013-0223-2. [DOI] [PubMed] [Google Scholar]
- 89.Jung J.S., Lee S.Y., Kim D.H., Kim H.S. Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant enzyme gene expression in rat primary astrocytes: involvement of MAP kinases and Nrf2/ARE signaling. Biomol Ther (Seoul) 2016;24(1):33–39. doi: 10.4062/biomolther.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato K., Mochizuki M., Saiki I., Yoo Y.C., Samukawa K., Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17(5):635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 91.Jiang J., Yuan Z., Sun Y., Bu Y., Li W., Fei Z. Ginsenoside Rg3 enhances the anti-proliferative activity of erlotinib in pancreatic cancer cell lines by downregulation of EGFR/PI3K/Akt signaling pathway. Biomed Pharmacother. 2017;96:619–625. doi: 10.1016/j.biopha.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 92.Kim M.J., Yun H., Kim D.H., Kang I., Choe W., Kim S.S., et al. AMP-activated protein kinase determines apoptotic sensitivity of cancer cells to ginsenoside-Rh2. J Ginseng Res. 2014;38(1):16–21. doi: 10.1016/j.jgr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Q., Gao B., Jie Q., Wei B.Y., Fan J., Zhang H.Y., et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone. 2014;66:306–314. doi: 10.1016/j.bone.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark R., Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125(4):629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 96.Okada S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm Regen. 2016;36:20. doi: 10.1186/s41232-016-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ren Y., Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia Z., Zhu H., Li J., Wang X., Misra H., Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50(4):264–274. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- 100.Hamann K., Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111(6):1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- 101.Lu C., Sun Z., Wang L. Inhibition of L-type Ca(2+) current by ginsenoside Rd in rat ventricular myocytes. J Ginseng Res. 2015;39(2):169–177. doi: 10.1016/j.jgr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li N., Liu B., Dluzen D.E., Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2007;111(3):458–463. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oh J.H., Hyun J.Y., Varshavsky A. Control of Hsp90 chaperone and its clients by N-terminal acetylation and the N-end rule pathway. Proc Natl Acad Sci U S A. 2017;114(22):E4370–E4379. doi: 10.1073/pnas.1705898114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng M., Xin Y., Li Y., Xu F., Xi X., Guo H., et al. Ginsenosides: a potential neuroprotective agent. Biomed Res Int. 2018;2018:8174345. doi: 10.1155/2018/8174345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu J., Jiang Y., Wu L., Lu T., Xu G., Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 107.Chen L.M., Zhou X.M., Cao Y.L., Hu W.X. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J Asian Nat Prod Res. 2008;10(5–6):439–445. doi: 10.1080/10286020801892292. [DOI] [PubMed] [Google Scholar]
- 108.He B., Chen P., Yang J., Yun Y., Zhang X., Yang R., et al. Neuroprotective effect of 20(R)-ginsenoside Rg(3) against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526(2):106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 109.Cheng Z., Zhang M., Ling C., Zhu Y., Ren H., Hong C., et al. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules. 2019;24(6) doi: 10.3390/molecules24061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miao H.H., Zhang Y., Ding G.N., Hong F.X., Dong P., Tian M. Ginsenoside Rb1 attenuates isoflurane/surgery-induced cognitive dysfunction via inhibiting neuroinflammation and oxidative stress. Biomed Environ Sci. 2017;30(5):363–372. doi: 10.3967/bes2017.047. [DOI] [PubMed] [Google Scholar]
- 111.Vinoth Kumar R., Oh T.W., Park Y.K. Anti-inflammatory effects of ginsenoside-Rh2 inhibits LPS-induced activation of microglia and overproduction of inflammatory mediators via modulation of TGF-beta1/smad pathway. Neurochem Res. 2016;41(5):951–957. doi: 10.1007/s11064-015-1804-x. [DOI] [PubMed] [Google Scholar]
- 112.Kang A., Xie T., Zhu D., Shan J., Di L., Zheng X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J Agric Food Chem. 2017;65(32):6861–6869. doi: 10.1021/acs.jafc.7b02386. [DOI] [PubMed] [Google Scholar]
- 113.Heng Y., Zhang Q.S., Mu Z., Hu J.F., Yuan Y.H., Chen N.H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol Lett. 2016;243:7–21. doi: 10.1016/j.toxlet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Cai M., Yang E.J. Ginsenoside Re attenuates neuroinflammation in a symptomatic ALS animal model. Am J Chin Med. 2016;44(2):401–413. doi: 10.1142/S0192415X16500233. [DOI] [PubMed] [Google Scholar]
- 115.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., et al. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158(1–2):143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 116.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48(6):1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 117.Kong L.T., Wang Q., Xiao B.X., Liao Y.H., He X.X., Ye L.H., et al. Different pharmacokinetics of the two structurally similar dammarane sapogenins, protopanaxatriol and protopanaxadiol, in rats. Fitoterapia. 2013;86:48–53. doi: 10.1016/j.fitote.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 118.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng R., Liu J., Wang Z., Zhang J., Cates C., Rousselle T., et al. The structure-activity relationship of ginsenosides on hypoxia-reoxygenation induced apoptosis of cardiomyocytes. Biochem Biophys Res Commun. 2017;494(3–4):556–568. doi: 10.1016/j.bbrc.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25(1):58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 121.Bae E.A., Han M.J., Kim E.J., Kim D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27(1):61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 122.Bowe C.L., Mokhtarzadeh L., Venkatesan P., Babu S., Axelrod H.R., Sofia M.J., et al. Design of compounds that increase the absorption of polar molecules. Proc Natl Acad Sci U S A. 1997;94(22):12218–12223. doi: 10.1073/pnas.94.22.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khalid S.I., Nunna R.S., Maasarani S., Kelly B.S.R., Sroussi H., Mehta A.I., et al. Pharmacologic and cellular therapies in the treatment of traumatic spinal cord injuries: a systematic review. J Clin Neurosci. 2020;79:12–20. doi: 10.1016/j.jocn.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 124.Fan S., Zhang Z., Su H., Xu P., Qi H., Zhao D., et al. Panax ginseng clinical trials: current status and future perspectives. Biomed Pharmacother. 2020;132:110832. doi: 10.1016/j.biopha.2020.110832. [DOI] [PubMed] [Google Scholar]
- 125.Liu X., Xia J., Wang L., Song Y., Yang J., Yan Y., et al. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol. 2009;16(5):569–575. doi: 10.1111/j.1468-1331.2009.02534.x. [DOI] [PubMed] [Google Scholar]
- 126.Chai S., Zhou B. Clinical observation of scalp acupuncture combined with abdominal acupuncture for cervical spondylotic myelopathy in early stage. Journal of New Chinese Medicine. 2018;50(4):166–168. [Google Scholar]
- 127.Zhang P., Wu X.P., Hu Z.D., Zhou C.J. Clinical observation on the treatment of cervical spondylotic myelopathy with needle-knife therapy. Hubei Journal of Traditional Chinese Medicine. 2014;36(9):62–63. [Google Scholar]
- 128.Li J.J., Zhao B.L., Bai G., Wang H.D., Cai J., Liu K., et al. Clinical study of comprehensive treatment by traditional Chinese medicine for cervical spondylotic myelopathy. China Medical Herald. 2014;11(32):77–82. [Google Scholar]
- 129.Gao Z.H. Small needle knife plus comprehensive therapy for cervical spondylotic myelopathy. Hubei Journal of Traditional Chinese Medicine. 2007;29(2):48. [Google Scholar]
- 130.Jiang H.T., Chen M. Curative effect observation of 60 cases of cervical spondylotic myelopathy treated by warming needle and moxibustion. Lishizhen Medicine and Materia Medica Research. 2014;25(2):389. [Google Scholar]
- 131.Lai C.B., Guo Q., Huang Y., Zie N.X., Wang Q., Liu C.Q. Traditional Chinese medicine comprehensive therapy in the conservative treatment of cervical spondylotic myelopathy for 18 cases. Chinese Medicine Modern Distance Education of China. 2018;16(4):129–130. [Google Scholar]
- 132.Guo J.B. Chengdu University of Traditional Chinese Medicine; Sichuan: 2011. Integrated Traditional Chinese medicine treatment of mild cervical spondylotic myelopathy clinical observation of 40 cases. [Google Scholar]
- 133.Gao Q.Y. Experience of treating cervical spondylotic myelopathy patients with Chinese traditional medicine doctor named Zhang Tianjian. The Journal of Traditional Chinese Orthopedics and Traumatology. 2006;18(2):64. [Google Scholar]
- 134.Wahid F., Khan T., Subhan F., Khan M.A., Kim Y.Y. Ginseng pharmacology: multiple molecular targets and recent clinical trials. Drugs of the Future. 2010;35(5):399–407. [Google Scholar]
- 135.Wood J., Bernards M.A., Wan W.K., Charpentier P.A. Extraction of ginsenosides from North American ginseng using modified supercritical carbon dioxide. J Supercrit Fluids. 2006;39:40–47. [Google Scholar]
- 136.Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–312. [Google Scholar]
- 137.Razgonova M., Zakharenko A., Shin T.S., Chung G., Golokhvast K. Supercritical CO2 extraction and identification of ginsenosides in Russian and north Korean ginseng by HPLC with tandem mass spectrometry. Molecules. 2020;25(6) doi: 10.3390/molecules25061407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee N.H., Son C.G. Systematic review of randomized controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Stud. 2011;4(2):85–97. doi: 10.1016/S2005-2901(11)60013-7. [DOI] [PubMed] [Google Scholar]
- 139.Engels H.J., Kolokouri I., Cieslak T.J., 2nd, Wirth J.C. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J Strength Cond Res. 2001;15(3):290–295. [PubMed] [Google Scholar]
- 140.Caron M.F., Hotsko A.L., Robertson S., Mandybur L., Kluger J., White C.M. Electrocardiographic and hemodynamic effects of Panax ginseng. Ann Pharmacother. 2002;36(5):758–763. doi: 10.1345/aph.1A411. [DOI] [PubMed] [Google Scholar]
- 141.Vuksan V., Sievenpiper J.L., Koo V.Y., Francis T., Beljan-Zdravkovic U., Xu Z., et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160(7):1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 142.Stavro P.M., Woo M., Leiter L.A., Heim T.F., Sievenpiper J.L., Vuksan V. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension. 2006;47(4):791–796. doi: 10.1161/01.HYP.0000205150.43169.2c. [DOI] [PubMed] [Google Scholar]
- 143.Seo J.C., Han S.W., Byun J.S., An H.D., Han I.D., Cho G.H., et al. The effects of ginseng and American ginseng on general symptoms in Koreans and Chinese: double-blind randomised controlled trials. J Ginseng Res. 2005;29(1):27–36. [Google Scholar]
- 144.Hou J., Xue J., Zhao X., Wang Z., Li W., Li X., et al. Octyl ester of ginsenoside compound K as novel anti-hepatoma compound: synthesis and evaluation on murine H22 cells in vitro and in vivo. Chem Biol Drug Des. 2018;91(4):951–956. doi: 10.1111/cbdd.13153. [DOI] [PubMed] [Google Scholar]
- 145.Gao Y., Wang T., Wang G., Li G., Sun C., Jiang Z., et al. Preclinical safety of ginsenoside compound K: acute, and 26-week oral toxicity studies in mice and rats. Food Chem Toxicol. 2019;131:110578. doi: 10.1016/j.fct.2019.110578. [DOI] [PubMed] [Google Scholar]
- 146.Li C., Wang Z., Li G., Wang Z., Yang J., Li Y., et al. Acute and repeated dose 26-week oral toxicity study of 20(S)-ginsenoside Rg3 in Kunming mice and Sprague-Dawley rats. J Ginseng Res. 2020;44(2):222–228. doi: 10.1016/j.jgr.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen L., Zhou L., Huang J., Wang Y., Yang G., Tan Z., et al. Single- and multiple-dose trials to determine the pharmacokinetics, safety, tolerability, and sex effect of oral ginsenoside compound K in healthy Chinese volunteers. Front Pharmacol. 2017;8:965. doi: 10.3389/fphar.2017.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X., Zhang D., Xu J., Gu J., Zhao Y. Determination of 25-OH-PPD in rat plasma by high-performance liquid chromatography-mass spectrometry and its application in rat pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858(1–2):65–70. doi: 10.1016/j.jchromb.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 149.Jin S., Jeon J.H., Lee S., Kang W.Y., Seong S.J., Yoon Y.R., et al. Detection of 13 ginsenosides (Rb1, Rb2, Rc, rd, Re, Rf, Rg1, Rg3, Rh2, F1, compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in human plasma and application of the analytical method to human pharmacokinetic studies following two week-repeated administration of red ginseng extract. Molecules. 2019;24(14) doi: 10.3390/molecules24142618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J., Shao Z.H., Xie J.T., Wang C.Z., Ramachandran S., Yin J.J., et al. The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch Pharm Res. 2012;35(7):1259–1267. doi: 10.1007/s12272-012-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen C., Jiang J., Lu J.M., Chai H., Wang X., Lin P.H., et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299(1):H193–H201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou P., Xie W., Sun Y., Dai Z., Li G., Sun G., et al. Ginsenoside Rb1 and mitochondria: a short review of the literature. Mol Cell Probes. 2019;43:1–5. doi: 10.1016/j.mcp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37(1):1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu W., Choi H.K., Huang L. State of panax ginseng research: a global analysis. Molecules. 2017;22(9) doi: 10.3390/molecules22091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Festing M.F., Altman D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43(4):244–258. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.