Abstract
Background:
Perioperative chemotherapy is a recommended treatment approach for localised oesophago-gastric junction adenocarcinoma, but not all patients respond to neoadjuvant chemotherapy. Early identification of non-responders and treatment adaptation in the preoperative period could improve outcomes. GastroPET is a national, multicentre phase II trial evaluating a 18FDG-PET/CT-guided preoperative treatment strategy with the R0 resection rate as a primary endpoint. Here, we report on the accuracy of the methodology, the feasibility of the study design and patient safety data after enrolment of the first 63 patients.
Methods:
Patients with locally advanced oesophago-gastric junction adenocarcinoma (Siewert I – III) stage Ib–IIIc underwent baseline 18FDG-PET/CT scanning and re-evaluation after 14 days of oxaliplatinum-5FU-(docetaxel) chemotherapy. Responders were defined by a ⩾ 35% decrease in tumour FDG standardised uptake value (SUV)average from baseline. Responders continued with the same chemotherapy for 2 to 3 months prior to surgery. PET-non-responders switched to preoperative chemoradiotherapy [weekly carboplatin and paclitaxel with concurrent radiotherapy (45 Gy in 25 fractions)]. Here, we aim to confirm the feasibility of FDG-PET-based response assessment in a multicenter setting and to compare local versus central reading. In addition, we report on the feasibility of the study conduct and patient safety data.
Results:
A total of 64 patients received baseline and sequential 14-day 18FDG-PET/CT scanning. And, 63 were allocated to the respective treatment arm according to PET-response [35 (56%) responders and 28 (44%) non-responders]. The concordance of local versus central reading of SUV changes was 100%. Until the date of this analysis, 47 patients (28 responders and 19 non-responders) completed surgery. Postoperative complications of grade ⩾ 3 (Common Terminology Criteria for Adverse Events, CTCAE Version 5.0) were reported in five responders (18%; 95% CI: 7.9–36%) and two non-responders (11%; 95% CI: 2.9–31%), with no statistical difference (p = 0.685). One patient in each arm died after surgery, leading to a postoperative in-hospital mortality rate of 4.3% (2/47 patients; 95% CI: 1.2–14%).
Conclusion:
Local and central FDG-SUV quantification and PET-response assessment showed high concordance. This confirms the accuracy of a PET-response-guided treatment algorithm for locally advanced oesophago-gastric junction cancer in a multicenter setting. Preoperative treatment adaptation revealed feasible and safe for patients.
Keywords: localised oesophago-gastric junction adenocarcinoma, metabolic imaging, non-responders, PET/CT-guided preoperative treatment strategy
Introduction
Oesophageal cancer is the seventh most common malignancy worldwide and the sixth leading cause of cancer-related death. 1 In locally advanced oesophageal adenocarcinoma including adenocarcinoma of the oesophago-gastric junction (AEG), either perioperative chemotherapy or preoperative chemoradiotherapy is the recommended standard of care.2–6 As a downside, preoperative treatment postpones surgery and thereby can increase the risk of disease progression in non-responding patients. Several groups have investigated the early identification of non-responders and the adaptation of the preoperative treatment approach before. In 2006, Ott et al. evaluated the maximum standardised uptake value (SUV) of 2-deoxy-2-[18 F]fluoro-D-glucose PET (18FDG-PET) before and on day 14 of preoperative chemotherapy. They defined an early metabolic response based on SUV decrease and established a reduction of SUVmax ⩾ 35% as the best cut-off to predict major histopathologic response and improved progression-free survival. 7 In the consecutive MUNICON I and II studies, the same group of investigators demonstrated that an early metabolic response-guided treatment algorithm identifies non-responding patients and allows for the adjustment of the perioperative treatment strategy.8,9 Since then, several other groups developed response-guided treatment algorithms with some modifications in methodology, endpoints and trial designs10–13 but implementation into clinical practice is still lagging behind.
The goal of the national multicenter GastroPET study reported here was to explore whether early metabolic non-responders benefit from a switch from induction chemotherapy to chemoradiotherapy. This design was based on the reports of promising histopathological response rates following preoperative chemoradiotherapy 6 compared to the rate known from preoperative chemotherapy alone at the time when GastroPET was designed. Here, we present the data of the first 63 patients receiving both 18FDG-PET scans focusing on (1) the feasibility of a multicentre methodology, (2) interobserver variability (local versus central) of FDG-PET readings and (3) postoperative complications and mortality. This analysis was requested by the study steering board and the funding organisation during the study conduct as a consequence of slightly delayed accrual, to ensure the feasibility and safety of the FDG-PET-response tailored multicenter treatment approach for patients with localised oesophago-gastric junction cancer.
Materials and methods
Study design
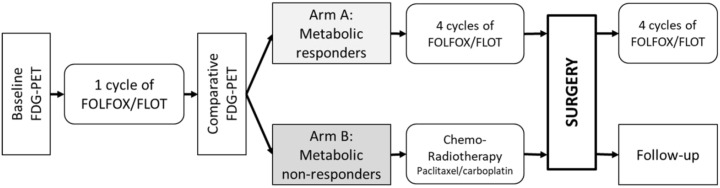
GastroPET is a phase II study evaluating sequential 18FDG-PET/CT scanning as an imaging biomarker for response to the standard treatment of locally advanced adenocarcinoma of the oesophago-gastric junction (AEG). In addition, blood and tumour tissues were collected for miRNA assessment which will be reported in a separate paper. Included patients were stratified by metabolic response to either of two arms (Figure 1). The primary objective, which is not reported here, is the R0 resection rate in non-responders.
Figure 1.
Study design diagram.
18FDG-PET scan, 2-deoxy-2-[18 F]fluoro-D-glucose PET; FOLFOX, oxaliplatin 85 mg/m2, leucovorin 200 mg/m2 and fluorouracil 2600 mg/m2 as a 48-h infusion on day 1 every 2 weeks; FLOT regimen, docetaxel 50 mg/m2 is added to FOLFOX, and fluorouracil 2600 mg/m2 as a 24-h infusion on day 1 every 2 weeks.
The study was designed as an academic investigator-initiated trial and was sponsored and coordinated by the Masaryk Memorial Cancer Institute (MMCI), Brno, Czech Republic. Patients from three institutions across the Czech Republic were recruited: MMCI, General University Hospital in Prague (GUH) and University Hospital in Olomouc (UHO). The trial was approved by the National regulatory agency (State Institute for Drug Control, 28 June 2017, ref. no.: sukls146974/2017), Local Ethics Committees (LEC MMCI, 25 July 2017, ref. no.: 2017/2123/MOU, MOU 174 875; LEC+MEC UHO and Faculty of Medicine of the Palacky University Olomouc, 14 October 2017, ref. no.: 144/17 MEK 22; LEC GUH Prague, 11 January 2017, ref. no.: 74/17 Grant), and assigned EudraCT number 2017-001264-38 in the European Clinical Trial Database (16 October 2017). The study is funded by the Ministry of Health, Czech Republic – grant no. 17-29389A, Conceptual Development of Research Organization (MMCI 00209805) and the Ministry of Education, Youth and Sports, MEYS-Czech Clinical Research Infrastructure (CZECRIN) LM2018128 and BBMRI-CZ LM2018125.
Patients
The total number of participants planned to be recruited is 120. Eligibility criteria included the presence of biopsy-proven locally advanced resectable AEG (Siewert I–III) stage Ib–IIIc. Staging procedures included endoscopy, endoscopic ultrasound and 18FDG-PET/CT scan of the chest and abdomen. Eligible patients had to be fit for oxaliplatin-fluoropyrimidine-(docetaxel) containing chemotherapy (FOLFOX or FLOT), and tumours were deemed R0 resectable after consultation of the institutional multidisciplinary tumour board.
Key exclusion criteria were age < 18 years, Eastern Cooperative Oncology Group (ECOG) score > 2, life expectancy < 3 months, uncontrolled tumour bleeding and previous chemotherapy, radiotherapy or endoscopic therapy for early stage cancer within the past 3 months. All participants provided written informed consent. Patients had to consent to additional diagnostic procedures. Compared to treatment standard blood samples, the second PET scan and oesophagogastroduodenoscopy with sampling for translation research were performed. The first patient was enrolled on 3 November 2017 at MMCI. Recruitment was ongoing at the time of this analysis.
18FDG-PET/CT
The baseline 18FDG-PET/CT was performed before the initiation of treatment to exclude metastatic disease and determine the baseline tumour FDG-SUV. The second comparative scan was performed 14 days after the start of the first cycle of neoadjuvant chemotherapy. 18FDG-PET/CT scans were carried out in specialised PET study centres working according to the study-protocol-defined standard operating procedure and the European Association of Nuclear Medicine (EANM) guidelines. 14 All patients underwent 18FDG-PET/CT on one of the following hybrid PET/CT scanners: Biograph mCT Flow, Siemens (in MMCI), Discovery 690, GE (in GUH) and Biograph 40, Siemens (in UHO). The identical scanner was used for the baseline and the comparative 18FDG-PET/CT scans for all patients. Data and records of the 18FDG-PET/CT scans were sent for the second (central) reading to the PET Center at MMCI. When PET scans were performed locally at MMCI, a different nuclear medicine physician was responsible for the central read.
Tracer uptake was assessed semiquantitatively as SUVaverage using a two-dimensional circular region of interest with a diameter of 1.5 cm (2D ROI) in axial slice using the TrueD software (Siemens Medical Solutions) or GE Advantage Workstation 4.5. In the comparative scan, the ROI was placed in the same anatomical position. 8 The percentage difference [ΔSUV = 100 × (SUVcomparative – SUVbaseline)/SUVbaseline] was calculated. 9 Patients whose tumour FDG-SUV decreased by ⩾ 35% (ΔSUV ⩽ –35%) were defined as metabolic responders.
Treatment
All eligible patients started preoperative treatment with chemotherapy (FOLFOX or FLOT). One of the two regimens was chosen according to the patient’s performance status and comorbidities. FOLFOX consists of oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, fluorouracil 400 mg/m2 as a bolus and fluorouracil 2600 mg/m2 as a 48-h infusion given every 2 weeks. In the FLOT regimen, docetaxel 50 mg/m2 is added to FOLFOX with continuous fluorouracil as a 24-h infusion on day 1.
Metabolic responders (Arm A) subsequently received additional four preoperative cycles (8 weeks) of FLOT or FOLFOX every 2 weeks. Surgery in Arm A was planned 4 to 6 weeks after day 1 of the last FLOT/FOLFOX administration. In addition, patients were planned to receive four postoperative cycles (8 weeks) of FLOT/FOLFOX starting within 12 weeks after surgery.
Metabolic non-responders (Arm B) switched to concurrent chemoradiotherapy consisting of five times weekly carboplatin at AUC = 2 mg/ml/min and paclitaxel at 50 mg/m2 together with concurrent radiotherapy (45 Gy in 25 fractions, 1.8 Gy per daily fraction, 5 days per week for 5 weeks with no additional boost).
Dose reductions in both arms were made according to common clinical practice. G-CSF was prescribed according to the investigator’s decision and was prioritised to chemotherapy dose reductions in case of haematological toxicities. In Arm B, G2 thrombocytopenia for more than 2 weeks was a reason for the discontinuation of chemotherapy but not radiotherapy.
The recommended surgical treatment was Ivor-Lewis oesophagectomy for Siewert type 1 and total gastrectomy with transhiatal extension if needed for Siewert type 3 tumours. For Siewert type 2 cancers, gastrectomy with distal oesophagectomy was recommended, but transthoracic oesophagectomy was also allowed. The main goal of the surgical approach was to obtain complete resection including adequate regional lymphadenectomy, with negative surgical margins.
Adverse events during neoadjuvant therapy and perioperative complications were documented and reported according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0
Histopathology
R0 resection was defined as margin-negative resection, in which no gross or microscopic tumour residues at resection margins exist (definition according to the College of American Pathologists). 15 The tumour regression grade (TRG) was determined according to the Mandard score. 16
Follow-up
Clinical follow-up visits were conducted every 3 months in the first 2 years and then every 6 months for 3 years. Endoscopy and chest/abdomen CTs were performed once a year from the first to the fifth year.
Endpoints and statistic methods
This study was designed as a two-arm trial stratified by the metabolic response. The primary endpoint is to achieve 85% R0 status in resected metabolic non-responders. This threshold was chosen based on previously published trial results.6,9 A total of 40 evaluable patients are required in Arm B to confirm an 85% R0 resection rate with a 95% confidence interval (74–96%). Patient and treatment characteristics were described using standard summary statistics, that is, median and interquartile range (IQR) for continuous variables and frequencies and proportions for categorical variables. Confidence intervals (CIs) for proportions were calculated using the Wilson method. Correlation of SUV decrease and metabolic response with histopathological response were evaluated using the Mann–Whitney and Fisher exact tests, respectively. Preplanned secondary endpoint analyses will assess disease-free survival (DFS) and overall survival (OS). All statistical analyses were performed employing R version 4.0.3, 17 and a significance level of 0.05. This analysis aims to assess the accuracy of the study methodology and patient safety data after 60 recruited patients.
Results
Patients
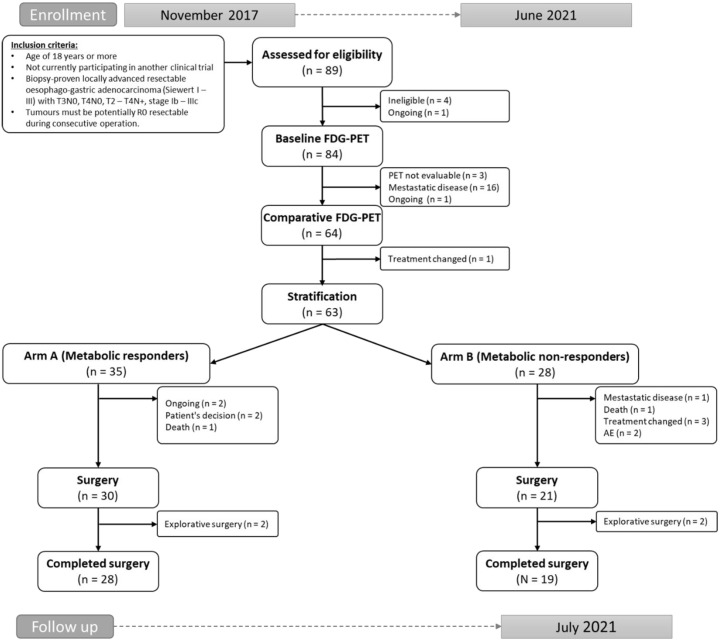
Between July 2017 and June 2021, 89 patients were screened. Patient enrolment is shown in the Consort diagram (Figure 2). After the first 18FDG-PET/CT scan, three patients (3.6%) were non-eligible due to low tumour FDG uptake, and 16 patients (19%) were diagnosed with metastatic disease. Sixty-three patients were eligible for this analysis.
Figure 2.
Flowchart of the study.
Fifty-three patients (84%) received FLOT, and all others were treated with FOLFOX. Patient characteristics of the study cohort reported here are shown in Table 1.
Table 1.
Patient baseline characteristics.
| N = 63 | Arm A PET-responders | Arm B PET-non-responders | |||
|---|---|---|---|---|---|
| All patients N = 35 |
Completed surgery N = 28 |
All patients N = 28 |
Completed surgery N = 19 |
||
| Age | |||||
| Median (IQR) | 65 (57–72) | 65 (58–70) | 65 (56–70) | 66 (57–74) | 63 (57–73) |
| Range | 30–81 | 30–75 | 44–74 | 51–81 | 52–78 |
| Sex | |||||
| Female | 8 (13%) | 6 (17%) | 5 (18%) | 2 (7%) | 1 (5%) |
| Male | 55 (87%) | 29 (83%) | 23 (82%) | 26 (93%) | 18 (95%) |
| T category | |||||
| 2 | 2 (3%) | 2 (6%) | 2 (8%) | 0 (0%) | 0 (0%) |
| 3 | 47 (81%) | 26 (81%) | 20 (77%) | 21 (81%) | 14 (78%) |
| 4 | 9 (16%) | 4 (12%) | 4 (15%) | 5 (19%) | 4 (22%) |
| X | 5 | 3 | 2 | 2 | 0 |
| N category | |||||
| 0 | 25 (41%) | 12 (35%) | 11 (41%) | 13 (48%) | 9 (47%) |
| 1 | 18 (30%) | 12 (35%) | 9 (33%) | 6 (22%) | 5 (26%) |
| 2 | 14 (23%) | 8 (24%) | 6 (22%) | 6 (22%) | 4 (21%) |
| 3 | 4 (7%) | 2 (6%) | 1 (4%) | 2 (7%) | 1 (5%) |
| Unknown | 2 | 1 | 1 | 1 | 0 |
| ECOG PS | |||||
| 0 | 27 (46%) | 17 (50%) | 15 (56%) | 10 (40%) | 5 (29%) |
| 1 | 32 (54%) | 17 (50%) | 12 (44%) | 15 (60%) | 12 (71%) |
| Unknown | 4 | 1 | 1 | 3 | 2 |
| Siewert type | |||||
| Siewert 1 | 22 (36%) | 11 (33%) | 9 (33%) | 11 (39%) | 8 (42%) |
| Siewert 2 | 31 (51%) | 18 (55%) | 15 (56%) | 13 (46%) | 8 (42%) |
| Siewert 3 | 8 (13%) | 4 (12%) | 3 (11%) | 4 (14%) | 3 (16%) |
| Unknown | 1 | 2 | 1 | 0 | 0 |
| Histological type | |||||
| Diffuse | 10 (17%) | 3 (10%) | 3 (12%) | 7 (26%) | 5 (26%) |
| Intestinal | 34 (59%) | 22 (71%) | 17 (68%) | 12 (44%) | 9 (47%) |
| Indeterminate | 12 (21%) | 5 (16%) | 4 (16%) | 7 (26%) | 5 (26%) |
| Other/mucinous | 2 (3%) | 1 (3%) | 1 (4%) | 1 (4%) | 0 (0%) |
| Unknown | 4 | 3 | 3 | 1 | 0 |
| Chemotherapy | |||||
| FLOT | 53 (84%) | 30 (86%) | 25 (89%) | 23 (82%) | 17 (89%) |
| FOLFOX | 10 (16%) | 5 (14%) | 3 (11%) | 5 (18%) | 2 (11%) |
ECOG PS, performance status according to the Eastern Cooperative Oncology Group; FLOT, docetaxel added to FOLFOX; FOLFOX, oxaliplatin, leucovorin and fluorouracil; IQR, interquartile range; PET, positron emission tomography.
Metabolic response
Baseline and comparative 18FDG-PET/CT scans were performed in 64 patients. The median radioactive dose administered was 342 MBq in the baseline PET (range: 144–520) and 328 MBq in the comparative PET (range: 148–499). The median time interval between FDG injection and the start of the emission scan was 61 min at baseline (range: 51–94) and 61 min at the comparative PET (range: 55–90). The median blood glucose level was 5.8 mmol/L before baseline (range: 4.1–9.4) and 5.8 mmol/L before the comparative PET (range: 4.3–11.7).
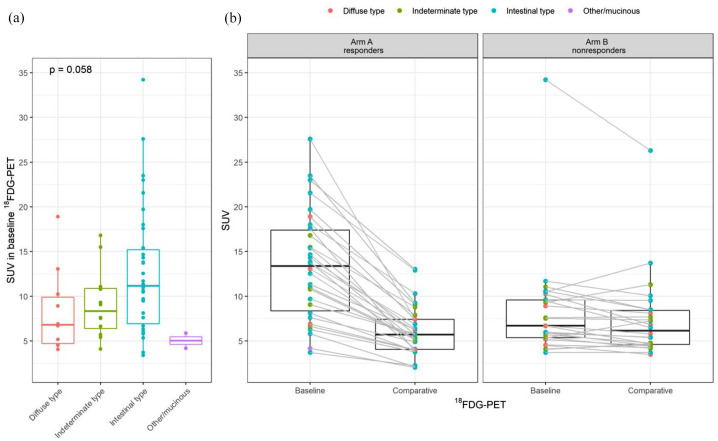
The 18FDG-SUV at baseline was numerically higher in the intestinal versus diffuse subtype cancers with borderline statistical significance (p = 0.058, Figure 3(a)), therefore an exploratory analysis of PET-response according to histology subtypes was performed. The SUV decrease in both arms per histological subtypes is shown in Figure 3(b).
Figure 3.
FDG-SUV in the study cohorts. (a) SUV at baseline according to histological type and (b) SUV changes for PET-responders and non-responders.
Thirty-five patients (56%) were PET-responders with a median SUV decrease of 50% (IQR 42–60%). For FLOT, the metabolic response rate was 57% compared to 50% with FOLFOX (p = 0.740). Twenty-eight patients (44%) were non-responders according to the study criteria. The ΔSUV range in this group was from −33% to +31%.
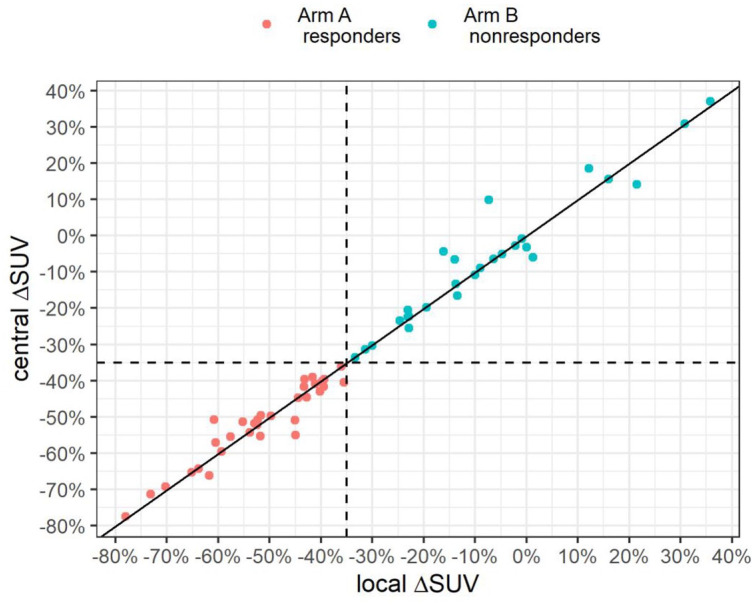
SUV quantification was compared between local and central reading (Supplementary Figure 1). The observed concordance of stratification by response was 100% (Figure 4).
Figure 4.
Comparison of local and central reading of PET-response based on FDG-SUV changes. The solid line represents the correlation between local and central reading; the horizontal and vertical dashed lines separate PET-responders and non-responders (SUV cut-off decrease of 35%).
Surgery
In metabolic responders, 30 (86%) patients proceeded to surgery, 28 patients underwent radical tumour resection and 2 ended the procedure after exploration because of inoperable disease. Two patients have not yet completed chemotherapy, two patients refused surgery and one patient died during chemotherapy due to pulmonary embolism.
In metabolic non-responders, 21 (75%) patients proceeded to surgery, 19 underwent radical tumour resection and 2 ended the procedure after exploration because of the diagnosis of inoperable disease. One patient died during chemotherapy due to cardiac arrest, one patient did not undergo surgery due to newly diagnosed metastases, two patients due to worsening of their performance status and comorbidities (ischemic cardiac disease), and three patients changed the treatment (one was not able to undergo radiotherapy, two refused the switch to chemoradiotherapy because of subjective improvement of symptoms during chemotherapy).
R0 resection was achieved in 25 responders (89.3%; 95% CI: 72.8–96.3%), of which 21 had been treated with FLOT (R0 resection rate 91.3% in this group; 95% CI: 73.2–97.6%), and in 17 non-responders (89.5%; 95% CI: 68.6–97.1%).
Histopathology
Histopathological response (TRG 1-3, according to Mandard) was found in 15 PET-responders post chemotherapy (54%) and in 15 PET-non-responders (83%) post chemoradiotherapy. The difference between both arms was not statistically significant (p = 0.058). Characteristics after neoadjuvant treatment and surgery according to PET-response are summarised in Table 2.
Table 2.
Characteristics after neoadjuvant treatment and surgery according to PET-response.
| Overall N = 47 |
Arm A PET-responders N = 28 |
Arm
B PET-non-responders N = 19 |
|
|---|---|---|---|
| ΔSUV | |||
| Median (IQR) | −40 (–52 to −16) | −50 (–60 to −42) | −12 (–22 to −1) |
| Range | −78 to 31 | −78 to −36 | −33 to 31 |
| Unknown | 2 | 1 | 1 |
| Grade | |||
| 1 | 5 (11%) | 3 (12 %) | 2 (11%) |
| 2 | 17 (39%) | 10 (40%) | 7 (37%) |
| 3 | 20 (45%) | 11 (44%) | 9 (47%) |
| 2–3 | 2 (5 %) | 1 (4 %) | 1 (5%) |
| Unknown | 3 | 3 | 0 |
| TRG | |||
| 1 | 6 (13%) | 3 (11%) | 3 (17%) |
| 2 | 15 (33%) | 10 (36%) | 5 (28%) |
| 3 | 9 (20%) | 2 (7%) | 7 (39%) |
| 4 | 11 (24%) | 8 (29%) | 3 (17%) |
| 5 | 5 (11%) | 5 (18%) | 0 (0%) |
| Unknown | 1 | 0 | 1 |
| Adequate regional lymphadenectomy |
45 (96%) | 27 (96%) | 18 (95%) |
| Residual disease | |||
| R0 | 42 (89%) | 25 (89%) | 17 (89%) |
| R1 | 4 (9%) | 2 (7%) | 2 (11%) |
| R2 | 1 (2%) | 1 (4%) | 0 (0%) |
IQR, interquartile range; PET, positron emission tomography; SUV, standardised uptake value; TRG, tumour regression grade (Mandard).
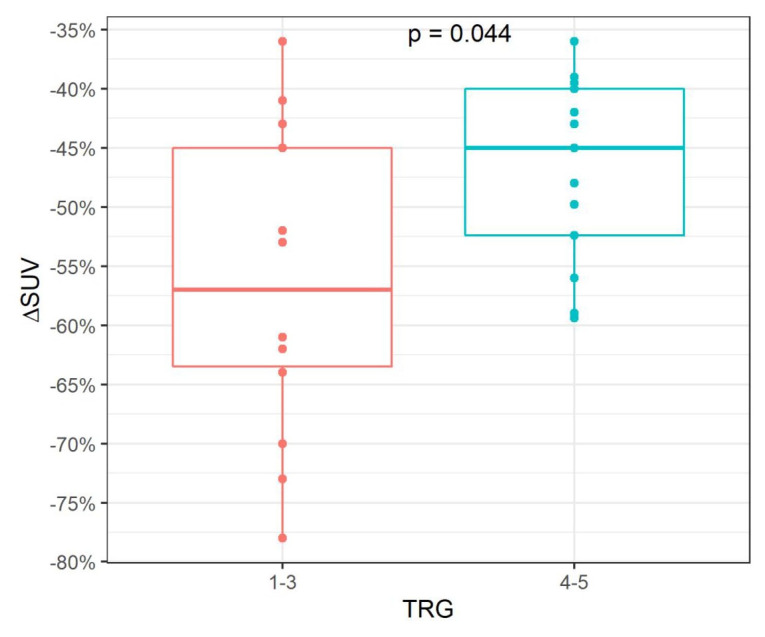
In PET-responders, the histopathological response was associated with a higher FDG-SUV decrease with a median of 57% versus 45% in patients without histopathological response (p = 0.044, Figure 5).
Figure 5.
Association of FDG-SUV decrease with histopathological response in metabolic responders.
Postoperative complications and mortality
The postoperative mortality rate (30 days and in-hospital mortality) was 4.3% (2/47 patients; 95% CI: 1.2–14%); one patient in each arm died. Postoperative complications of grade 3 (CTCAE 5.0) or more, including nonsurgical morbidity, were reported in five PET-responders (18%; 95% CI: 7.9–36%) and two non-responders (11%; 95% CI: 2.9–31%), with no statistical difference between both groups (p = 0.685). Postoperative adverse events according to study arms are summarised in Supplementary Table 1.
Discussion
The phase II academic GastroPET study aims to investigate the predictive value of 18FDG-PET-based response assessment and its impact on tailoring preoperative treatment according to the metabolic response. Contrary to the first trial using this approach (MUNICON-1), 8 GastroPET uses a multicenter design. Therefore, we planned central reading of PET/CT scans to verify the feasibility of the methodology. The main finding of this analysis was a reassuring concordance of local versus central assessment of PET-response, confirming the feasibility of the methodology in a multicenter setting. In addition, we can confirm the safety of the treatment approach in terms of perioperative complications and mortality.
Recently, FLOT was established as a novel standard of care for the perioperative treatment of gastric and oesophago-gastric junction cancers. 5 From a clinical perspective, not all patients are candidates for the multi-drug regimen FLOT. Therefore, we allowed for FOLFOX as an alternative regimen. Regarding PET-response, no significant difference between FLOT (57% response) and FOLFOX (50% response) was observed. Similarly, in the CALBG 80803 trial, no significant difference in the rate of PET-response was seen between FOLFOX and carboplatin/paclitaxel (64.9% versus 56.1%). 13 This observation confirms that PET-response is a sustainable predictor of tumour chemosensitivity and to a large extent independent from variations in platinum-based chemotherapy regimens. Accordingly, Ott et al. 7 reported that PET-response was the only independent factor predicting recurrence (p = 0.018) in a group of completely resected (R0) patients with oesophago-gastric cancer. Whether this holds true for novel combinations of chemotherapy plus targeted therapy (such as HER2-antibodies) or immune checkpoint inhibitors, which may soon become standard not only in stage IV, and also in the adjuvant setting after an incomplete response to preoperative chemoradiation and surgery, remains to be investigated. 18 In summary, PET-response indicates a favourable prognosis which is consistent among multiple prior studies.7,8,10,11
One might criticise that five cycles of preoperative FLOT as given in the GastroPET study is diverging from the initially published four preoperative cycles applied in the FLOT4 trial. 5 However, only around 50% of patients can finish the postoperative part of treatment. In terms of PET-proven chemosensitivity, we therefore decided to intensify the preoperative period by using five cycles of chemotherapy. Another issue of uncertainty is the optimal neoadjuvant radiotherapy dose. According to the guidelines, the standard dose of radiation in a preoperative setting is in the range of 41.4–50.4 Gy.11,19–21 In view of poor prognosis of metabolic non-responders to chemotherapy, the dosing schedule of 45 Gy in 25 fractions for 5 weeks was chosen which is slightly more intensive compared to the 41.4 Gy that was applied in the CROSS study. 6 In addition, compared to the CROSS trial where a 3D conformal radiation technique was used, we applied the volumetric modulated arc therapy (VMAT). Finally, the perioperative treatment landscape is expected to change in the near future with increasing implementation of adjuvant immunotherapy according to the results of Checkmate-57718 and potentially also following or in addition to preoperative chemotherapy if ongoing trials such as EORTC1707-VESTIGE will be positive. 22 However, we expect the recruitment of GastroPET to be finished until then.
Numerically, higher baseline FDG-SUVs were observed in intestinal versus diffuse subtype cancers. Therefore, an exploratory analysis according to histologic subtypes was performed. Although patient numbers were limited and cancers with diffuse subtypes were rare in our data set (17%), this analysis indicates that diffuse oesophago-gastric cancers are probably not the ideal subgroup for a PET-response tailored treatment approach. 23 The observation that diffuse type gastric cancers are less FDG-avid is not new and was previously described by several other investigators.24–27 Typically, diffuse subtype cancers are less common at the oesophago-gastric junction and more frequent in non-cardia gastric cancers. Patients with this tumour location were not included in the GastroPET study. Ott and coworkers identified patients with PET-non-avid tumours as a specific subgroup with histopathological response rates and survival rates close to PET-non-responders. 28
To the best of our knowledge, GastroPET is the first study to evaluate PET-response during neoadjuvant FLOT chemotherapy with a planned change to CROSS-type chemoradiotherapy as a salvage strategy for non-responders. Two practice-changing phase III trials contributed to establishing these two treatment regimens as the standard of care.5,6,29 The phase III randomised international Neo-AEGIS trial aimed to compare perioperative chemotherapy to preoperative CROSS chemoradiotherapy. 30 NeoAegis showed a higher R0 resection rate in the chemoradiotherapy arm (95% versus 82%); however, only 10% of the patients were treated with FLOT compared to 84% in our study. The important observation of NeoAegis is that neither a higher R0 resection rate nor a higher histopathologic complete remission (pCR) rate in the chemoradiotherapy arm led to improved overall survival. Two ongoing randomised phase III trials (Esopec 31 and TopGear 32 ) comparing perioperative chemotherapy versus CROSS may shed more light on the question of which pre-/perioperative treatment regimen should be preferred for localised oesophago-gastric junction adenocarcinoma. Recent data8,10,11 including the results from GastroPET suggest that PET-response assessment during neoadjuvant chemotherapy may allow for a personalised treatment selection.
The histopathological response was more frequently observed in the metabolic non-responder arm compared to the metabolic responder arm. This observation is in line with published data where the addition of radiation therapy led to a higher rate of histopathologic response.9,33
The relatively high dropout rate seen before and during neoadjuvant therapy deserves a critical appraisal. The specific biology of non-FDG-avid oesphago-gastric cancers is already discussed above, 28 and the higher than initially expected rate of newly diagnosed metastatic disease underscores the value of FDG-PET baseline staging to identify patients with clinically occult metastases. Detection of distant metastases complements the advantages of 18FDG-PET/CT scanning for oesophageal and oesophago-gastric junction cancers regarding the accuracy of clinical staging, response evaluation, radiation target volume definition and follow-ups. 34 Our dropout rate was still lower than that reported from the German MEMORI trial 35 where 85 patients (53%) could not be involved, that is, 40 (25%) because of previously undetectable metastases, 21 (13%) for too low FDG tumour uptake and 24 (15%) for other reasons.
The postoperative mortality rate (30 days and in-hospital mortality) was 4.3% which is acceptable but not ideal. However, this was almost similar to 4.0% reported in the CROSS trial, 6 where – like in GastroPET – patients were operated only in selected expert centres. Interestingly, we saw no difference in mortality between PET-responders who received chemotherapy alone and non-responders who received chemoradiotherapy (p = 0.682) confirming observations from the NeoAegis trial. 30
To conclude, our data confirm the feasibility of a PET-response-tailored treatment approach in a multicentre setting and are in concordance with recently published studies. However, the limitation of the phase II non-randomised design in general and a small number of patients with complete follow-up at this stage have to be admitted. The recruitment is ongoing and final results are planned to be published in 2023.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211065153 for PET/CT-tailored treatment of locally advanced oesophago-gastric junction adenocarcinoma: a report on the feasibility of the multicenter GastroPET study by Radka Obermannova, Iveta Selingerova, Zdenek Rehak, Vaclav Jedlicka, Marek Slavik, Pavel Fabian, Ivo Novotny, Milada Zemanova, Hana Studentova, Peter Grell, Lenka Zdrazilova Dubska, Regina Demlova, Tomas Harustiak, Renata Hejnova, Igor Kiss and Rostislav Vyzula in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to thank all patients for participating in the trial. Moreover, they thank all participating and recruiting physicians for their help with the collection of patient samples.
Footnotes
Author contributions: RO: conception, writing and revision of the manuscript. IS: analysis tools, writing and revision of manuscript. MS, PF, IN, PG, IK, RV: collected data, revision of the manuscript. RD, RH, TH, VJ, LZD: collected data, revision of the manuscrpit. MZ, HS: collected data, revision of the manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the Ministry of Health, Czech Republic – grant no. 17-29389A, the Conceptual Development of Research Organization (MMCI 00209805) and the Ministry of Education, Youth and Sports, MEYS-Czech Clinical Research Infrastructure (CZECRIN; LM2018128 and BBMRI-CZ LM2018125).
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The trial was approved by the National regulatory agency (State Institute for Drug Control, 28 June 2017, ref. no.: sukls146974/2017) and by Local Ethics Committees (LEC MMCI, 25 July 2017, ref. no.: 2017/2123/MOU, MOU 174 875; LEC+MEC UHO and Faculty of Medicine of the Palacky University Olomouc, 14 October 2017, ref. no.: 144/17 MEK 22; LEC GUH, 11 January 2017, ref. no.: 74/17 Grant). All patients provided written consent.
ORCID iDs: Radka Obermannova  https://orcid.org/0000-0001-7363-7879
https://orcid.org/0000-0001-7363-7879
Iveta Selingerova  https://orcid.org/0000-0003-3713-3504
https://orcid.org/0000-0003-3713-3504
Hana Studentova  https://orcid.org/0000-0003-2105-9258
https://orcid.org/0000-0003-2105-9258
Tomas Harustiak  https://orcid.org/0000-0003-1850-7638
https://orcid.org/0000-0003-1850-7638
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Radka Obermannova, Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, Zluty kopec 7, 656 53 Brno, Czech Republic; Department of Comprehensive Cancer Care, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Iveta Selingerova, Research Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic; Department of Pharmacology, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Zdenek Rehak, Department of Nuclear Medicine, Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Vaclav Jedlicka, Department of Surgery, Masaryk Memorial Cancer Institute, Brno, Czech Republic; Department of Surgery, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Marek Slavik, Department of Radiation Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Pavel Fabian, Department of Pathology, Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Ivo Novotny, Department of Gastroenterology, Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Milada Zemanova, Department of Oncology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague, Czech Republic.
Hana Studentova, Department of Oncology, University Hospital Olomouc, Olomouc, Czech Republic.
Peter Grell, Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, Brno, Czech Republic; Department of Comprehensive Cancer Care, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Lenka Zdrazilova Dubska, Department of Laboratory Medicine – Clinical Microbiology and Immunology, University Hospital Brno, Brno, Czech Republic.
Regina Demlova, Department of Pharmacology, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Tomas Harustiak, Third Department of Surgery, First Faculty of Medicine, Charles University, Prague, Czech Republic.
Renata Hejnova, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Igor Kiss, Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, Brno, Czech Republic; Department of Comprehensive Cancer Care, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Rostislav Vyzula, Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, Brno, Czech Republic; Department of Comprehensive Cancer Care, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v50–v57. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 4. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 5. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393: 1948–1957. [DOI] [PubMed] [Google Scholar]
- 6. van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 7. Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006; 24: 4692–4698. [DOI] [PubMed] [Google Scholar]
- 8. Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007; 8: 797–805. [DOI] [PubMed] [Google Scholar]
- 9. Zum Büschenfelde CM, Herrmann K, Schuster T, et al. 18F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011; 52: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 10. Goodman KA, Hall N, Bekaii-Saab TS, et al. Survival outcomes from CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol 2018; 36: 4012–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbour AP, Walpole ET, Mai GT, et al. Preoperative cisplatin, fluorouracil, and docetaxel with or without radiotherapy after poor early response to cisplatin and fluorouracil for resectable oesophageal adenocarcinoma (AGITG DOCTOR): results from a multicentre, randomised controlled phase II trial. Ann Oncol 2020; 31: 236–245. [DOI] [PubMed] [Google Scholar]
- 12. Harustiak T, Zemanova M, Fencl P, et al. [18 F]Fluorodeoxyglucose PET/CT and prediction of histopathological response to neoadjuvant chemotherapy for adenocarcinoma of the oesophagus and oesophagogastric junction. Br J Surg 2018; 105: 419–428. [DOI] [PubMed] [Google Scholar]
- 13. Goodman KA, Ou F-S, Hall NC, et al. Randomized phase II study of pet response–adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (Alliance) trial. J Clin Oncol 2021; 39: 2803–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010; 37: 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karstens KF, Izbicki JR, Reeh M. Does the margin matter in esophageal cancer. Dig Surg 2018; 35: 196–203. [DOI] [PubMed] [Google Scholar]
- 16. Mandard A-M, Dalibard F, Mandard J-C, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–2686. [DOI] [PubMed] [Google Scholar]
- 17. R Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2020, https://www.r-project.org [Google Scholar]
- 18. Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021; 384: 1191–1203. [DOI] [PubMed] [Google Scholar]
- 19. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001; 19: 305–313. [DOI] [PubMed] [Google Scholar]
- 21. Leichman LP, Goldman BH, Bohanes PO, et al. S0356: a phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma. J Clin Oncol 2011; 29: 4555–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smyth E, Knödler M, Giraut A, et al. VESTIGE: adjuvant immunotherapy in patients with resected esophageal, gastroesophageal junction and gastric cancer following preoperative chemotherapy with high risk for recurrence (N+ and/or R1): an open label randomized controlled phase-2-study. Front Oncol 2020; 9: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atay-Rosenthal S, Wahl RL, Fishman EK. PET/CT findings in gastric cancer: potential advantages and current limitations. Imaging Med 2012; 4: 241–250. [Google Scholar]
- 24. Mochiki E, Kuwano H, Katoh H, et al. Evaluation of 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography for gastric cancer. World J Surg 2004; 28: 247–253. [DOI] [PubMed] [Google Scholar]
- 25. De Potter T, Flamen P, Van Cutsem E, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging 2002; 29: 525–529. [DOI] [PubMed] [Google Scholar]
- 26. Stahl A, Ott K, Weber W, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003; 30: 288–295. [DOI] [PubMed] [Google Scholar]
- 27. Herrmann K, Ott K, Buck AK, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 2007; 48: 1945–1950. [DOI] [PubMed] [Google Scholar]
- 28. Ott K, Herrmann K, Lordick F, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 2008; 14: 2012–2018. [DOI] [PubMed] [Google Scholar]
- 29. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v38–v49. [DOI] [PubMed] [Google Scholar]
- 30. Reynolds JV, Preston SR, O’Neill B, et al. Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 2021; 39: 4004–4004.34672688 [Google Scholar]
- 31. Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016; 16: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017; 24: 2252–2258. [DOI] [PubMed] [Google Scholar]
- 33. Klevebro F, Alexandersson von Döbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016; 27: 660–667. [DOI] [PubMed] [Google Scholar]
- 34. Kwee RM, Marcus C, Sheikhbahaei S, et al. PET with fluorodeoxyglucose F 18/computed tomography in the clinical management and patient outcomes of esophageal cancer. PET Clin 2015; 10: 197–205. [DOI] [PubMed] [Google Scholar]
- 35. Lorenzen S, Quante M, Rauscher I, et al. PET-directed combined modality therapy for gastroesophageal junction cancer: first results of the prospective MEMORI trial. J Clin Oncol 2019; 37: 4018–4018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211065153 for PET/CT-tailored treatment of locally advanced oesophago-gastric junction adenocarcinoma: a report on the feasibility of the multicenter GastroPET study by Radka Obermannova, Iveta Selingerova, Zdenek Rehak, Vaclav Jedlicka, Marek Slavik, Pavel Fabian, Ivo Novotny, Milada Zemanova, Hana Studentova, Peter Grell, Lenka Zdrazilova Dubska, Regina Demlova, Tomas Harustiak, Renata Hejnova, Igor Kiss and Rostislav Vyzula in Therapeutic Advances in Medical Oncology