Abstract
Background: Sacroiliac (SI) joint subchondral resorption on high-resolution magnetic resonance imaging (MRI) may be an early sign of the development of sacroiliitis. At our institution, high-resolution intermediate-weighted (proton density) MRI sequences are used in the workup of suspected spondyloarthritis (SpA). Questions/Purpose: We sought to test the hypothesis that SI joint subchondral resorption might be a useful MRI feature in the diagnosis of sacroiliitis. Methods: We retrospectively reviewed the records of patients with suspected SpA from a single rheumatologist’s practice from January 1, 2010, to December 31, 2017. Patients had an MRI of the SI joints, using our institution’s specialized protocol, and underwent standard physical examination and laboratory evaluation. The sensitivity and specificity of SI joint subchondral resorption in the identification of sacroiliitis were estimated using the clinical diagnosis as the reference standard and from a Bayesian latent class model with conditional dependence. Results: SI joint subchondral resorption on SI joint MRI was highly correlated with a positive diagnosis in patients worked up for axial SpA. It demonstrated superior sensitivity when compared with other MRI features used in the MRI diagnosis of sacroiliitis, such as bone marrow edema pattern, erosion, and ankylosis. Interobserver reliability was high for subchondral resorption. Conclusion: This retrospective study found that subchondral resorption on MRI evaluation of the SI joints appeared to be a sensitive indicator of SpA, potentially of early disease. This imaging feature warrants evaluation in other cohorts of patients suspected of having axial SpA to validate diagnostic performance in diverse populations.
Keywords: MRI, diagnostic modalities, imaging, basic science, spine, inflammatory arthritis (nonrheumatoid)
Introduction
Spondyloarthritis (SpA) encompasses a spectrum of rheumatologic disorders, which includes ankylosing spondylitis, psoriatic arthritis, arthritis associated with inflammatory bowel disease, and reactive arthritis [12]. The Assessment of SpondyloArthritis international Society (ASAS) determined that a classification of axial SpA can be given to patients who report 3 or more months of back pain that started before the age of 45 years based on a combination of clinical, laboratory, and magnetic resonance imaging (MRI) features. There are 2 ASAS classification criteria for axial SpA, 1 that incorporates sacroiliitis on imaging and at least 1 SpA clinical feature, and the other combines human leukocyte antigen B27 (HLA-B27) positivity with at least 2 SpA clinical features [13]. Sacroiliitis on imaging is defined by the ASAS as “definite radiographic sacroiliitis according to the modified New York criteria” [13] or “active (acute) inflammation on MRI highly suggestive of sacroiliitis associated with SpA.” The latter has been characterized as at least 2 lesions of subarticular marrow edema pattern on 1 MRI section or 1 periarticular or subchondral bone marrow edema pattern (osteitis) lesion on at least 2 consecutive MRI sections [11,20].
Magnetic resonance imaging features can be 1 of the earliest objective manifestations of SpA [10], often occurring years before radiographic features [13]. Early identification has important clinical implications, as there are now effective biologic therapies, which, if used early, can prevent disease progression and prevent permanent damage [2,15,16,22].
Most sacroiliac (SI) joint MRI protocols rely on fluid-sensitive sequences, like short-TI inversion recovery (STIR), as well as T1-weighted sequences for structural evaluation. At our institution, we have successfully validated intermediate-weighted (proton density [PD]) fast/turbo spin echo sequences for other musculoskeletal imaging purposes evaluating hyaline (articular) cartilage [9]. Intermediate-weighted MRI sequences have many favorable properties, which include a high signal-to-noise ratio, high spatial resolution, and adequate contrast resolution to assess important structures in musculoskeletal imaging (hyaline cartilage, cortical bone, ligaments, and tendons). We have been using intermediate MRI technique, as part of the clinical diagnostic workup for patients with SpA, anecdotally noting that resorption of the hypointense subchondral plate without defined focal erosion is a feature that is observed in patients with SpA. This may be in isolation or combination with the traditional features used to diagnose sacroiliitis on MRI, such as bone marrow edema lesion, erosion, and ankylosis. The objective of this study was to evaluate retrospectively whether SI joint subchondral resorption was a useful MRI feature in the diagnosis of sacroiliitis.
Methods
All patients from the practice of a single expert rheumatologist were eligible for this study if they had a SI joint MRI for suspected SpA between January 1, 2010, and December 31, 2017. A diagnosis of SpA was established by the overall impression of the expert rheumatologist based on all available information (clinical, laboratory, and imaging).
Magnetic resonance imaging examinations were performed on 1.5T or 3.0T magnets with a phased array multichannel surface coil without the administration of intravenous contrast, using our institution’s specialized SI joint protocol. An initial localizer scan was obtained. Subsequently, a large field-of-view coronal inversion recovery and axial PD sequences followed by smaller field-of-view axial and coronal oblique PD, and oblique coronal inversion recovery sequences were performed, with their parameters delineated in Table 1. When an MRI sequence is set to produce a PD-weighted image, it is the tissues with the higher concentration or density of protons (hydrogen atoms) which produce the strongest signals and appear the brightest on the image. Proton density–weighted images are created by having a long repetition time and a short echo time. It is very useful for the detection of joint disease and injury. The total scan time was approximately 30 to 35 minutes.
Table 1.
MRI pulse sequence protocol.
| Sequence | TR (ms) | TE (ms) | Flip angle | ETL | NEX/NSA | Matrix | FOV (cm) | Slice thickness (mm) | Interslice gap (mm) | Bandwidth (kHz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Coronal inversion recovery (wide FOV) | 4000 | 17 | 180 | 7 | 2 | 256 × 192 | 34 | 5.0 | 0 | 31.25 |
| Axial PD (wide FOV) | 4000 | 30 | 180 | 7 | 2 | 512 × 256 | 36 | 5.0 | 0 | 31.25 |
| Axial oblique PD | 4000 | 30 | 180 | 7 | 2 | 512 × 320 | 20 | 3.0 | 0 | 31.25 |
| Coronal oblique PD | 4000 | 34 | 180 | 7 | 2 | 512 × 352 | 20 | 2.5 | 0 | 31.25 |
| Coronal oblique inversion recovery (small FOV) | 4000 | 17 | 180 | 7 | 2 | 256 × 192 | 22 | 3.0 | 0 | 31.25 |
MRI magnetic resonance imaging, TR repetition time, TE echo time, ETL echo train length, NEX number of excitations, NSA number of signal averages, FOV field of view, PD proton density.
The SI joint MRI examinations were scored by a single board-certified musculoskeletal radiologist with 26 years of experience. The radiologist was masked to each patient’s laboratory findings and clinical rheumatologic diagnosis. A second board-certified musculoskeletal radiologist with approximately 8 years of experience scored a random 50% subset of patients in our cohort to assess for interrater reliability. An additional 4 cases were reviewed, and the results from all 45 reviewed cases are reported.
The following definitions were employed for scoring assessment. Subchondral resorption is the loss of the hypointense (dark) subchondral plate without defined focal erosion (Fig. 1). Erosion is a full-thickness loss of the hypointense (dark) appearance of the iliac/sacral cortical bone of the (SI) joint colocated with subjacent structural/signal alteration in normal appearance of adjacent bone marrow representing a focal defect. Bone marrow edema lesion (pattern) is a region of hyperintense signal on fluid-sensitive sequences (increased high T2/STIR signal). Ankylosis is loss of the joint cavity with bridging fused bone.
Fig. 1.
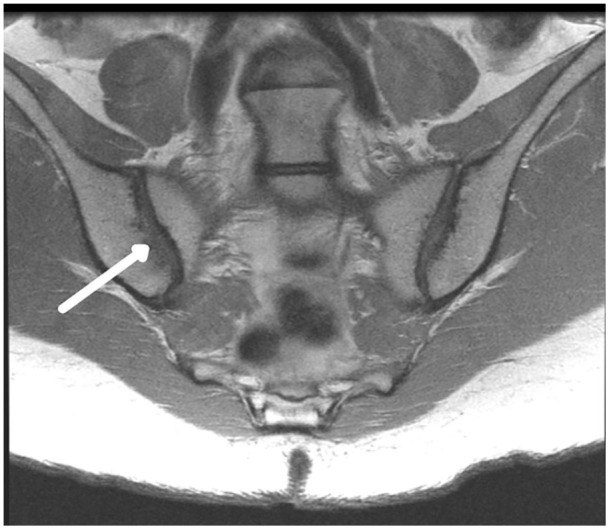
Subchondral resorption. Coronal oblique proton density intermediate-weighted image shows right sacroiliac joint subchondral resorption (arrow) demonstrating loss of the hypointense (dark) subchondral plate without defined erosion. Note that the left sacroiliac has a maintained subchondral plate (dark line).
Statistical Analysis
To evaluate the diagnostic utility of the presence of subchondral resorption, bone marrow edema pattern, erosion, and ankylosis individually, as well as the combination of edema, erosion, and ankylosis, 2 approaches were used. The conventional method assumed the rheumatologist’s clinical diagnosis as the reference standard. Sensitivity and specificity were estimated along with 95% Wilson confidence intervals.
Two potential issues of the conventional analysis that could bias estimates of accuracy arise due to using an imperfect reference and where sacroiliac joint MRIs may have been considered when the clinical diagnosis was made, resulting in dependency between the MR features and the rheumatologist’s diagnosis. The Bayesian latent class model (BLCM) following the framework of Dendukuri and Joseph [3] accounts for the fact that the true SpA status in our cohort is not directly observed as a result of our imperfect reference and allows for the dependency between the tests to be modeled. Thus, we specify a (1) conditionally dependent model with uniform (0, 1) priors for the covariance parameters between clinical diagnosis and MRI feature, (2) subjective priors beta (12.40, 3.72) for sensitivity and beta (21.38, 3.72) for specificity of clinical diagnosis, and (3) uninformative beta (1, 1) prior for the sensitivity and specificity of MR features and the prevalence of SpA. The subjective prior for the sensitivity and specificity of the clinical diagnosis was chosen based on a meta-analysis conducted by Sepriano et al [14] summarizing the evidence on ASAS performance, where the interquartile range of published sensitivity and specificity for ASAS axial and peripheral SpA criteria was estimated to be 71%–85% and 83%–92%, respectively. Spondyloarthritis prevalence, sensitivity, and specificity estimated from BLCM are presented as the posterior median with 95% credible intervals.
Gwet’s unweighted agreement coefficients (AC1) were calculated to evaluate the interobserver reliability between the radiologists in their MRI assessments in a subset of 45 sacroiliac joint MRIs. Strength of agreement was interpreted using the Landis and Koch [4] criteria: <0.00 = poor, 0.00 to 0.20 = slight, 0.21 to 0.40 = fair, 0.41 to 0.60 = moderate, 0.61 to 0.80 = substantial, and 0.81 to 1.00 = high.
All statistical analyses were performed with SAS Version 9.4 (SAS Institute Inc, Cary, North Carolina) and R version 3.4.4 (R Core Team, 2018) using the LearnBayes [1] package to estimate the beta parameters.
Results
Patient demographics of the cohort stratified by clinical diagnosis of SpA are listed in Table 2; 66.7% (54/81) of patients were clinically diagnosed with SpA.
Table 2.
Patient demographics stratified by clinical diagnosis of SpA.
| SpA− (n = 26) | SpA+ (n = 54) | Overall a (n = 81) | |
|---|---|---|---|
| Age (mean [SD]) | 60 (8) | 47 (19) | 51 (17) |
| Male (%) | 13 (50.0) | 19 (35.2) | 32 (39.5) |
| Inflammatory back pain (%) | 9 (45.0) | 41 (87.2) | 51 (75.0) |
| Enthesitis (heel) (%) | 1 (3.8) | 8 (15.4) | 9 (11.4) |
| Arthritis (%) | 23 (88.5) | 44 (86.3) | 68 (87.2) |
| Uveitis (%) | 2 (7.7) | 5 (9.4) | 7 (8.8) |
| Dactylitis (%) | 1 (3.8) | 2 (3.8) | 3 (3.8) |
| Psoriasis (%) | 9 (36.0) | 19 (36.5) | 28 (35.9) |
| IBD (%) | 4 (15.4) | 4 (7.7) | 8 (10.1) |
| Response to NSAIDs (%) | 11 (61.1) | 26 (74.3) | 37 (69.8) |
| Family history of SpA (%) | 0 (0.0) | 4 (8.2) | 4 (5.3) |
| Family history of psoriatic arthritis (%) | 3 (11.5) | 9 (18.4) | 12 (15.8) |
| Family history of rheumatoid arthritis (%) | 2 (7.7) | 4 (8.2) | 6 (7.9) |
| Family history of gout (%) | 1 (3.8) | 1 (2.0) | 2 (2.6) |
| Family history of scleroderma (%) | 0 (0.0) | 1 (2.0) | 1 (1.3) |
| Family history of ankylosing spondylitis (%) | 0 (0.0) | 3 (6.1) | 3 (3.9) |
| Family history of IBD (%) | 1 (3.8) | 4 (8.2) | 6 (7.9) |
| Elevated CRP or ESR (%) | 6 (26.1) | 11 (21.2) | 17 (22.4) |
| HLA-B27 (%) | 1 (5.3) | 11 (22.9) | 12 (17.6) |
SpA spondyloarthritis, IBD inflammatory bowel disease, NSAIDs nonsteroidal anti-inflammatory drugs, CRP C-reactive protein, ESR erythrocyte sedimentation rate, HLA human leukocyte antigen.
One case was indeterminate.
The presence of subchondral resorption, erosion, bone marrow edema pattern adjacent to subchondral resorption or erosion, bone marrow edema pattern elsewhere, ankylosis, and osteoarthritis are detailed in Table 3; 55.6% (45/81) had subchondral resorption (almost all in patients with SpA) and 23.5% (19/81) had subchondral resorption in isolation without colocated bone marrow edema pattern (all in patients with SpA).
Table 3.
MR findings stratified by clinical diagnosis of SpA.
| SpA− | SpA+ | Overall a | |
|---|---|---|---|
| Subchondral resorption (%) | 2 (7.7) | 42 (77.8) | 45 (55.6) |
| Bone marrow edema adjacent to subchondral resorption (%) | 0 (0.0) | 26 (48.1) | 26 (32.1) |
| Erosion (%) | 0 (0.0) | 33 (61.1) | 33 (40.7) |
| Bone marrow edema adjacent to erosion (%) | 0 (0.0) | 25 (46.3) | 25 (30.9) |
| Bone marrow edema elsewhere (%) | 1 (3.8) | 2 (3.7) | 3 (3.7) |
| Ankylosis (%) | 0 (0.0) | 3 (5.6) | 3 (3.7) |
| Osteoarthritis (%) | 16 (61.5) | 10 (18.5) | 26 (32.1) |
SpA spondyloarthritis, MR magnetic resonance.
One case was indeterminate.
The prevalence, sensitivities, specificities, and positive and negative predictive values for subchondral resorption, bone marrow edema pattern, erosion, ankylosis, and a fifth category, which included a combination of bone marrow edema, erosion, or ankylosis, are recorded in Tables 4 and 5.
Table 4.
Sensitivity and specificity of MR features with clinical diagnosis as reference standard.
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| Subchondral resorption | 78% (65, 87) | 92% (76, 98) |
| BME | 48% (35, 61) | 100% (87, 100) |
| Erosion | 61% (48, 73) | 100% (87, 100) |
| Ankylosis | 6% (2, 15) | 100% (87, 100) |
| BME, erosion, or ankylosis | 63% (50, 75) | 100% (87, 100) |
CI confidence interval, BME bone marrow edema, MR magnetic resonance.
Table 5.
Posterior medians of sensitivity and specificity of clinical diagnosis and MR features estimated from BLCM.
| Sensitivity (95% CrI) | φSE | Specificity (95% CrI) | φSP | |
|---|---|---|---|---|
| Clinical diagnosis | 81% (65, 95) | 85% (68, 95) | ||
| Subchondral resorption | 67% (50, 88) | 0.09 (0.0, 0.17) | 86% (23, 99) | 0.02 (–0.05, 0.14) |
| BME | 41% (28, 61) | 0.06 (–0.01, 0.12) | 92% (32, 100) | 0.02 (–0.04, 0.14) |
| Erosion | 52% (37, 72) | 0.08 (0.00, 0.14) | 92% (32, 100) | 0.02 (–0.03, 0.14) |
| Ankylosis | 6% (1, 14) | 0.00 (–0.04, 0.02) | 92% (29, 100) | 0.02 (–0.04, 0.12) |
| BME, erosion, or ankylosis | 53% (38, 73) | 0.08 (0.01, 0.15) | 92% (34, 100) | 0.02 (–0.03, 0.14) |
BLCM Bayesian latent class model, CrI credible interval, φSE covariance between sensitivities of clinical diagnosis and MR feature, φSP covariance between specificities of clinical diagnosis and MR feature, MR magnetic resonance, BME bone marrow edema.
Subchondral resorption demonstrated reasonable diagnostic performance in this cohort of patients with a high clinical suspicion of SpA yielding superior sensitivity compared with other MRI features classically associated with the MRI diagnosis of sacroiliitis, such as bone marrow edema pattern, erosion, and ankylosis. By the BLCM, subchondral resorption was associated with a sensitivity of 67% (50%, 88%), as compared with a sensitivity of 41% (28%, 61%) with bone marrow edema pattern, 52% (37%, 72%) with erosion, and 6% (1%, 14%) with ankylosis. Subchondral resorption was also associated with a specificity of 86% (23%, 99%), as compared with a specificity of 92% (32%, 100%) with bone marrow edema pattern, 92% (32%, 100%) with erosion, and 92% (29%, 100%) with ankylosis.
The interobserver reliability was high for all demonstrated features: subchondral resorption (.87 ± .07), bone marrow edema pattern (.97 ± .03), erosion (.90 ± .06), and ankylosis (.98 ± .02).
Discussion
The results of this study suggest that subchondral resorption may be a useful MRI feature in the diagnosis of SpA. Subchondral resorption exhibited superior sensitivity when compared with bone marrow edema lesions, erosion, and ankylosis, which are features traditionally used to diagnose sacroiliitis on MRI.
There are several limitations to this retrospective study, particularly within the context of the study design. Selection bias may have existed in the form of sample bias, as our subjects may not adequately reflect the spectrum of characteristics in another target population. Also, a number of patients with SpA who did not receive an MRI were by default excluded. Reviewer bias was minimized as the radiologists who evaluated the MRI studies of our cohort were masked to patients’ laboratory findings and clinical rheumatologic diagnoses. There is an incorporation bias as the sacroiliac joint MRI examinations performed as standard of care, functioned as the index test, and contributed to the overall reference standard concurrently, although the rheumatologist assessment was the overarching standard. There was a slight positive correlation in sensitivity, suggesting the rheumatologist’s reliance on positive imaging findings. We attempted to correct for this limitation by using a second statistical analysis method, the BLCM, when calculating our results. The drawbacks of using the BLCM are that it is a probabilistic model and not directly interpretable and that choice of prior distribution has to be carefully chosen because this has considerable influence on the final results.
The results of our investigation suggest an extension of the diagnostic capability of MRI for detecting sacroiliitis beyond the features described in prior studies. As per the ASAS classification criteria for axial SpA, a diagnosis made via the MRI arm of the criteria must demonstrate osteitis as manifested by bone marrow edema pattern; however, bone marrow edema is not specific to SpA, and patients with SpA do not always exhibit active inflammation [8]. A diagnosis of SpA can also be made on MRI via the identification of structural lesions, such as erosions, which can be detected on MRI before their appearance on projection radiography [8,21] and can be seen in the absence of MRI signs of active sacroiliitis [8,19]. An additional structural feature of chronic sacroiliitis that can be recognized on MRI is fat metaplasia, in which lipid tissue (often identified as T1 signal hyperintensity) develops at sites of prior inflammation in the subchondral marrow, and may fill foci of erosion, in which case it has been referred to as “backfill” [7]. Finally, ankylosis of the sacroiliac joints may occur at the location of prior backfill and is defined as fatty marrow signal intensity traversing the sacroiliac joint space [8]. It has been suggested that evidence of structural lesions on sacroiliac joint MRI may be associated with a more severe subtype of SpA that has a higher predilection for future spinal involvement and may consequently advocate for early initiation of antitumor necrosis factor agents in these patients [8], perhaps making the detection of subchondral resorption more crucial.
In an effort to improve the accuracy and reproducibility of sacroiliac joint evaluation on MRI, a multitude of scoring systems have been developed, variably tested, and advocated for. The vast majority of these scoring systems incorporate subchondral bone marrow edema lesions, as this feature is believed to be the first indication of active inflammation detectable on MRI in patients with inflammatory back pain [17]. Others have expanded on this by using dynamic contrast-enhanced MRI to evaluate for active inflammation by assessing the rate and maximal uptake of gadolinium-based contrast agent in a region of interest as a marker of increased vascularization [5].
Swami et al [17] performed a systematic review of MRI-based sacroiliitis scoring systems and found fair evidence to recommend the Spondyloarthritis Research Consortium of Canada (SPARCC) MRI sacroiliac joint inflammation scoring system but could not make recommendations on the other scoring systems because of limited available evidence on their accuracy. The SPARCC inflammation score demonstrated good construct validity and reliability and was a good reflection of treatment response [17].
Magnetic resonance imaging–based scoring systems addressing structural lesions about the sacroiliac joints have also been developed. Swami et al [17] also found fair evidence to recommend the SPARCC structural scoring tool based on their systematic review but could not recommend the remaining scoring systems evaluated due to inadequate data regarding their accuracy. The SPARCC structural scoring system evaluates for erosion, fat metaplasia, backfill, and ankylosis. This structural based scoring system demonstrated good interobserver reliability and acceptable reliability for change [6]. When both inflammation and structural disease are considered important, subchondral resorption may be considered an early “pre-erosion” lesion.
Accurate and reproducible sacroiliac joint assessment remains somewhat elusive in part by the overlap of expression of similar MRI features in non-SpA patients and disorders. Weber et al [18] reported that low-grade acute and structural findings on the sacroiliac joints could be seen in up to 27% of healthy patients and those with nonspecific back pain. A further avenue of research would be the prevalence of subchondral resorption in normal and noninflammatory back pain cohorts. As with other MRI features, it is likely that the pretest probability will influence the performance of subchondral resorption as a potentially confounding feature.
Our study suggests that an additional feature of sacroiliitis demonstrated on high-resolution MRI, subchondral resorption, may fortify the ability of MRI to diagnose sacroiliitis. The implications are 2-fold. First, it underscores the utility of high-resolution intermediate-weighted (PD) MRI sequences similar to those used for orthopedic imaging for internal derangement, which allows for identification of resorption of the sacroiliac joint subchondral plate. Second, it suggests that subchondral resorption should be considered an additional feature of sacroiliitis to be evaluated when interpreting sacroiliac joint MRIs for SpA, especially early in the course.
In conclusion, we found that the identification of subchondral resorption on high-resolution intermediate-weighted MRI sequences demonstrated overall good diagnostic performance in patients with a high clinical suspicion of SpA. These results should be validated in other cohorts to better understand the diagnostic and therapeutic implications of subchondral resorption. Future directions of research should include a prospective longitudinal study, which may elucidate the natural history of subchondral resorption in SpA.
Acknowledgments
The research team would like to acknowledge the work of Dr. Hollis Potter in the Department of Radiology and Imaging at the Hospital for Special Surgery, New York, NY 10021.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jennifer L. Berkowitz, Alissa J. Burge, John A. Roberts IV, and Bin Lin declare no potential conflicts of interest. Lisa A. Mandl reports relationships with UpToDate, Regeneron Pharmaceuticals, and Annals of Internal Medicine. Sergio Schwartzman reports relationships with Abbvie and Novartis. John A. Carrino reports relationships with Pfizer, Covera, Simplify Medical, Image Analysis Group (IAG), and Image Biopsy Lab.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent: Informed consent was waived from all patients included in this study.
Level of Evidence: Level III: retrospective diagnostic study
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
References
- 1. Albert J. LearnBayes: functions for learning Bayesian inference. Available at: https://CRAN.R-project.org/package=LearnBayes. Published 2018. Accessed March 19, 2020.
- 2. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatol Oxf Engl. 2019;58:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dendukuri N, Joseph L. Bayesian approaches to modeling the conditional dependence between multiple diagnostic tests. Biometrics. 2001;57:158–167. [DOI] [PubMed] [Google Scholar]
- 4. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 5. Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:703–709. [DOI] [PubMed] [Google Scholar]
- 6. Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. 2015;42:79–86. [DOI] [PubMed] [Google Scholar]
- 7. Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol Hoboken NJ. 2014;66:2958–2967. [DOI] [PubMed] [Google Scholar]
- 8. Maksymowych WP, Wichuk S, Dougados M, et al. MRI evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther. 2017;19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80:1276–1284. [DOI] [PubMed] [Google Scholar]
- 10. Puhakka KB, Jurik AG, Egund N, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218–229. [DOI] [PubMed] [Google Scholar]
- 11. Rudwaleit M, Jurik AG, Hermann K-GA, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–1527. [DOI] [PubMed] [Google Scholar]
- 12. Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 13. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. [DOI] [PubMed] [Google Scholar]
- 14. Sepriano A, Rubio R, Ramiro S, Landewé R, van der Heijde D. Performance of the ASAS classification criteria for axial and peripheral spondyloarthritis: a systematic literature review and meta-analysis. Ann Rheum Dis. 2017;76:886–890. [DOI] [PubMed] [Google Scholar]
- 15. Sieper J, Landewé R, Magrey M, et al. Predictors of remission in patients with non-radiographic axial spondyloarthritis receiving open-label adalimumab in the ABILITY-3 study. RMD Open. 2019;5:e000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013;72:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swami VG, Jaremko JL, Rumsey DG, et al. Diagnostic accuracy of MRI-based sacroiliitis scoring systems: a systematic review. AJR Am J Roentgenol. 2019;212:1112–1125. [DOI] [PubMed] [Google Scholar]
- 18. Weber U, Lambert RGW, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–3058. [DOI] [PubMed] [Google Scholar]
- 19. Weber U, Lambert RGW, Pedersen SJ, Hodler J, Østergaard M, Maksymowych WP. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res. 2010;62:1763–1771. [DOI] [PubMed] [Google Scholar]
- 20. Weber U, Østergaard M, Lambert RGW, et al. Candidate lesion-based criteria for defining a positive sacroiliac joint MRI in two cohorts of patients with axial spondyloarthritis. Ann Rheum Dis. 2015;74:1976–1982. [DOI] [PubMed] [Google Scholar]
- 21. Weber U, Pedersen SJ, Østergaard M, Rufibach K, Lambert RGW, Maksymowych WP. Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis? A cross-sectional study. Arthritis Res Ther. 2012;14:R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wick MC, Weiss RJ, Jaschke W, Klauser AS. Erosions are the most relevant magnetic resonance imaging features in quantification of sacroiliac joints in ankylosing spondylitis. J Rheumatol. 2010;37:622–627. [DOI] [PubMed] [Google Scholar]


