Abstract
Salivary gland tumors represent a diverse group of neoplasms that occasionally pose a diagnostic challenge for pathologists, particularly with limited sampling. Gene fusions, which may reflect genetic drivers, are increasingly recognized in a subset of these neoplasms, and can be leveraged for diagnostic purposes. We performed a retrospective analysis on a cohort of 80 benign and malignant salivary gland tumors, enriched for subtypes known to harbor recurrent fusion events, to validate the diagnostic use of a targeted RNA sequencing assay to detect fusion transcripts. Testing identified fusion genes in 71% (24/34) of pleomorphic adenoma and carcinoma-ex-pleomorphic adenoma, with 56% of cases showing rearrangement of PLAG1 and 15% HMGA2. In addition to confirming known partners for these genes, novel PLAG1 fusion partners were identified, including DSTN, NTF3 and MEG3; CNOT2 was identified as a novel fusion partner for HMGA2. In adenoid cystic carcinoma, 95% of cases (19/20) were positive for a fusion event. MYB was rearranged in 60% (12/20), MYBL1 in 30% (6/20) and NFIB in 5% (1/20); two tumors exhibited novel fusion products, including NFIB-TBPL1 and MYBL1-VCPIP1. Fusion genes were identified in 64% (9/14) of cases of mucoepidermoid carcinoma; MAML2 was confirmed to partner with either CRTC1 (43%), or CRTC3 (21%). One salivary duct carcinoma was found to harbor a novel RAPGEF6-ACSL6 fusion gene. Finally, as anticipated, gene fusions were not detected in any of the five acinic cell carcinomas included in the cohort. In summary, targeted RNA sequencing represents a diagnostically useful ancillary technique for identifying a variety of existing, and novel, fusion transcripts in the classification of salivary gland neoplasms.
Keywords: salivary gland tumor, fusion, RNA sequencing
INTRODUCTION
Salivary gland tumors are uncommon and represent a diverse group of neoplasms with over 30 distinct entities recognized in the current edition of the World Health Organization (WHO) Classification of Head and Neck Tumors.1 These can present a diagnostic challenge to pathologists, particularly in the context of limited sampling, due to their rarity, morphologic heterogeneity, and overlapping cellular compositions and immunoprofiles. Indeed, differentiation between benign and malignant neoplasms can be challenging, particularly in the oral cavity where benign neoplasms, such as pleomorphic adenoma, may lack a delineating capsule. An ability to make this distinction is essential owing to differences in biologic potential and treatment.
Salivary gland tumors are increasingly recognized as frequently containing recurrent fusion genes, which has allowed diagnostic refinement and improved classification. To date, gene fusions have been identified in pleomorphic adenoma (PLAG1, HMGA2),2–9 mucoepidermoid carcinoma (CRTC1/CRTC3-MAML2),10–13 adenoid cystic carcinoma (MYB/MYBL1-NFIB),14–16 secretory carcinoma (ETV6-NTRK3/RET/MET/MAML3),17–19 hyalinizing clear cell carcinoma (EWSR1-ATF1/CREM),20,21 the cribriform variant of polymorphous adenocarcinoma (PRKD1/PRKD2/PRKD3),22 acinic cell carcinoma (NR4A3, HTN3-MSANTD3),23–25 intraductal carcinoma (RET-NCOA4/TRIM27/TRIM33/KIAA1217, TUT1-ETV5, STRN/EML4/MYO18A-ALK)26–33 and myoepithelial carcinoma (PLAG1).34,35 In addition, microsecretory adenocarcinoma, which has recently been proposed as a new entity, is characterized by a novel MEF2C-SS18 fusion gene.36 It should also be noted that other forms of recurrent genetic events have also been reported in salivary gland neoplasms. For example, a minority harbor point mutations, such as basal cell adenomas (CTNNB1),37,38 sialadenoma papilliferum (BRAF V600E)39 and the majority of the classical variant of polymorphous adenocarcinomas harbor a recurrent hostpot mutation (PRKD1 E710D).40
Conventional cytogenetics has traditionally guided the identification of translocations. This was supplanted with the introduction of fluorescence in situ hybridization (FISH) and reverse transcriptase-polymerase chain reaction (RT-PCR)-based assays. Due to its specificity and potential breadth in coverage, targeted RNA sequencing (RNA-Seq) would appear to offer a practical approach to fusion gene detection, particularly for entities in which a variety of variant gene fusions have been described. This technique is now routinely employed, for example, for fusion gene detection in mesenchymal neoplasms.41 In this study we examined a cohort of salivary gland neoplasms, enriched for those with gene fusions, with a commercially available targeted RNA-Seq assay to assess the potential of this technique as a diagnostic adjunct.
MATERIALS AND METHODS
Case Selection
Following institutional Research Ethics Board approval, a retrospective archive review was performed for benign and malignant salivary gland neoplasms (2015–2020), which included biopsy and resection specimens. The original slides were pulled and reviewed to confirm the diagnosis and select a representative block for RNA-Seq.
RNA Sequencing
Targeted RNA sequencing was performed on all cases. RNA was extracted from formalin-fixed, paraffin-embedded (FFPE) tissue scrolls (3 to 4 per case, cut at 10 μm) using the ExpressArt FFPE Clear RNA Ready kit (Amsbio, Cambridge, MA). RNA fragment length was assessed using the RNA 6000 chip on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA-seq libraries were prepared using an input of 20 to 100 ng total RNA with the TruSight RNA Fusion Panel (Illumina, San Diego, CA), an enrichment-based assay that targets 507 known fusion-associated genes. Each sample was sequenced with 76 base-pair paired-end reads on an Illumina MiSeq platform at 8 samples per flow cell (~3 million reads per sample). The results were analyzed using both the STAR and BOWTIE2 aligners, and Manta and JAFFA fusion callers, respectively.
Fluorescence in situ hybridization
The application of FISH in this study was two-fold: (1) It was used to independently validate the presence of fusion gene rearrangement in tumors containing novel fusion transcripts. (2) The entire MEC sub-cohort was examined to draw a comparison of the sensitivity between FISH and RNA-Seq.
Fluorescence in situ hybridization was performed as previously reported.42 Briefly, custom probes were made from bacterial artificial chromosome (BAC) clones flanking the specific genes of interest based on the UCSC genome browser (http://genome.ucsc.edu). They were obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, Ca: https://bacpacresources.org). The DNA from the BACs was isolated according to the manufacturer’s instructions and then labeled with fluorochromes (fluorescent-labeled dUTPs, Enzo Life Sciences, New York NY) by nick translation and subsequently validated on normal metaphase chromosomes. 4 μm-thick tissue sections were cut from the FFPE tissue blocks to prepare the slides, which were then deparaffinized, pretreated and hybridized with the denatured probes. After allowing for an overnight incubation, the slides were rinsed and stained with 4’,6-diamidino-2-phenylindole. The slides were then mounted with an anti-fade solution and examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) and using Isis 5 software (Metasystems). (Supplementary Table 1).
Immunohistochemistry
Previously constructed tissue microarrays (TMAs)43 containing 437 salivary gland tumours were investigated using immunohistochemistry (IHC) for MYB (clone: EP769Y; Abcam #ab45150); Pan-Trk (clone: EPR17341; Abcam #ab181560); and, HMGA2 (polyclonal; Biocheck, 59170AP). Tissue sections were cut at 4 μm thickness and stained using a Dako OMNIS autostainer (Alignet, Santa Clara, CA, USA) as per the manufacturer’s instructions. Any nuclear staining in the tumor cells was recorded as a positive result, while non-specific cytoplasmic staining was considered negative (Supplementary Table 2).
RESULTS
Cohort
A total of 83 tumors were identified. Three cases were excluded due to failure to meet minimum RNA quality control standards, including one case that had undergone decalcification due to bone involvement. The final cohort consisted of a total of 80 cases. There were 49 females and 31 males, with an average patient age of 53 years (range: 9–96) (Table 1). The cases included pleomorphic adenoma (PA; n = 27), carcinoma-ex pleomorphic adenoma (CA-ex-PA; n = 7), mucoepidermoid carcinoma (MEC; n = 14), adenoid cystic carcinoma (AdCC; n = 20), acinic cell carcinoma (AcCC; n = 5), hyalinizing clear cell carcinoma (HCCC; n = 3), secretory carcinoma (SC; n = 2), de novo salivary duct carcinoma (SDC; n = 1), and intraductal carcinoma (IC; n = 1). Of the CA-ex-PAs, the malignant component was not classified in two cases, the remainder included one each of: minimally invasive carcinoma, low-grade adenocarcinoma, intracapsular carcinoma, poorly differentiated carcinoma, and SDC.
Table 1.
Summary of clinical details. Abbreviations: AcCC: acinic cell carcinoma, AdCC: adenoid cystic carcinoma, CA-ex-PA: carcinoma ex pleomorphic adenoma, HCCC: hyalinizing clear cell carcinoma, F: female, IC: intraductal carcinoma, LN: lymph node, M: male, MEC: mucoepidermoid carcinoma, NOS: not otherwise specified, SC: secretory carcinoma, SDC: salivary duct carcinoma, SMG: submandibular gland.
| Case | Diagnosis | Age (y) | Sex | Location | Tumor Size (cm) |
|---|---|---|---|---|---|
| 1 | PA | 67 | M | Parotid gland | 4.6 |
| 2 | PA | 56 | F | Parotid gland | 3.6 |
| 3 | PA | 46 | F | Parotid gland | 2.1 |
| 4 | PA | 21 | M | SMG | 1.7 |
| 5 | PA | 43 | F | Parotid gland | 1.5 |
| 6 | PA | 53 | M | Parotid gland | 2.9 |
| 7 | PA | 38 | F | Parotid gland | 2.7 |
| 8 | PA | 59 | F | Parotid gland | 1.5 |
| 9 | PA | 26 | M | Parotid gland | 4.5 |
| 10 | PA | 31 | F | Parotid gland | 2.6 |
| 11 | PA | 77 | F | Parotid gland | 2.1 |
| 12 | PA | 36 | F | Parotid gland | 2.0 |
| 13 | PA | 52 | F | SMG | 1.2 |
| 14 | PA | 78 | F | Parotid gland | 3.1 |
| 15 | PA | 48 | F | Parotid gland | 2.1 |
| 16 | PA | 48 | F | Parotid gland | 1.6 |
| 17 | PA | 20 | M | SMG | 1.8 |
| 18 | PA | 9 | M | Oral cavity | 2.5 |
| 19 | PA | 17 | M | Oral cavity | Unknown |
| 20 | PA | 77 | F | SMG | Unknown |
| 21 | PA | 42 | M | Oral cavity | 1.6 |
| 22 | PA | 63 | M | Parotid gland | 3.2 |
| 23 | PA | 49 | M | Parotid gland | 4.0 |
| 24 | PA | 79 | F | Parotid gland | 2.8 |
| 25 | PA | 53 | M | Parotid gland | 3.0 |
| 26 | PA | 26 | F | Unknown | Unknown |
| 27 | PA | 33 | F | Oral Cavity | Unknown |
| 28 | CA-ex-PA | 62 | M | Parotid gland | 3.6 |
| 29 | CA-ex-PA | 68 | M | Parotid gland | 3.6 |
| 30 | CA-ex-PA | 58 | F | Parotid gland | 1.7 |
| 31 | CA-ex-PA | 69 | F | SMG | 4.5 |
| 32 | CA-ex-PA | 49 | M | Parotid gland | 4.5 |
| 33 | CA-ex-PA | 55 | F | Parotid gland | 5.9 |
| 34 | SDC-ex-PA | 65 | F | Parotid gland | 1.7 |
| 35 | AdCC | 28 | M | Nasal cavity | 3.5 |
| 36 | AdCC | 38 | F | Nasal cavity | Unknown |
| 37 | AdCC | 39 | F | Nasopharynx | Unknown |
| 38 | AdCC | 55 | F | Oral cavity | Unknown |
| 39 | AdCC | 70 | M | Ethmoid sinus | Unknown |
| 40 | AdCC | 72 | M | Orbit | Unknown |
| 41 | AdCC | 66 | F | Maxillary sinus | 1.7 |
| 42 | AdCC | 53 | F | Oral cavity | Unknown |
| 43 | AdCC | 51 | F | Oral cavity | 1.1 |
| 44 | AdCC | 71 | F | Oral cavity | 1.9 |
| 45 | AdCC | 56 | F | SMG | 1.8 |
| 46 | AdCC | 38 | M | Lacrimal gland | Unknown |
| 47 | AdCC | 59 | F | Larynx | Unknown |
| 48 | AdCC | 59 | M | Oral cavity | 5.9 |
| 49 | AdCC | 62 | M | Parotid gland | 3.9 |
| 50 | AdCC | 59 | M | Brain | Unknown |
| 51 | AdCC | 96 | F | Oral cavity | Unknown |
| 52 | AdCC | 74 | M | Nasal cavity | Unknown |
| 53 | AdCC | 53 | F | Vertebrae | Unknown |
| 54 | AdCC | 50 | M | Oral cavity | Unknown |
| 55 | MEC | 53 | M | Parotid gland | 2.3 |
| 56 | MEC | 33 | F | Parotid gland | 3.1 |
| 57 | MEC | 64 | F | Oral cavity | 3 |
| 58 | MEC | 84 | F | Oral cavity | Unknown |
| 59 | MEC | 57 | M | Oral cavity | Unknown |
| 60 | MEC | 25 | M | Parotid gland | 4.7 |
| 61 | MEC | 62 | F | Parotid gland | 3.5 |
| 62 | MEC | 65 | M | Oral cavity | 2.1 |
| 63 | MEC | 63 | F | Parotid gland | 1.2 |
| 64 | MEC | 44 | F | Parotid gland | 1.5 |
| 65 | MEC | 80 | F | Parotid gland | 0.6 |
| 66 | MEC | 61 | F | Parotid gland | 5.9 |
| 67 | MEC | 63 | F | Oral cavity | 0.8 |
| 68 | MEC | 35 | F | Neck, NOS | Unknown |
| 69 | AcCC | 20 | F | Parotid gland | 1.9 |
| 70 | AcCC | 68 | F | Parotid gland | 0.6 |
| 71 | AcCC | 59 | F | Parotid gland | 2 |
| 72 | AcCC | 69 | M | Parotid gland | 3.9 |
| 73 | AcCC | 46 | F | Parotid gland | 1.7 |
| 74 | HCCC | 42 | M | Base of tongue | 3.8 |
| 75 | HCCC | 54 | F | Oral cavity | 0.7 |
| 76 | HCCC | 71 | M | Cervical LN | Unknown |
| 77 | SC | 56 | F | Parotid gland | 1.8 |
| 78 | SC | 48 | F | Parotid gland | Unknown |
| 79 | SDC | 63 | F | Parotid gland | 1.9 |
| 80 | IC | 68 | M | Parotid gland | 1.5 |
The anatomic location of the tumors included the parotid gland (n = 42), oral cavity (n = 17), submandibular gland (n = 6), sinonasal tract (n = 5), metastases (n = 3; brain, vertebrae, cervical lymph node), lacrimal gland, nasopharynx, larynx, orbit, base of tongue, neck NOS and an unspecified site (n =1 each). In terms of the nature of specimens, 62 were resections or excisions and 18 were incisional or core biopsies. The mean tumor size was 2.8 cm for the PAs and CA-ex-PAs (range: 1.2 – 5.9 cm), 2.6 cm for the MECs (0.6 – 5.9 cm), 2.8 cm for the AdCCs (range: 1.1 – 5.9 cm) and 2.0 cm for AcCCs (range: 0.6 – 3.9 cm).
RNA Sequencing
In most cases RNA-Seq revealed fusion products that had previously been established in the literature, as well as several novel fusion products (Table 2; Figure 1).
Table 2:
Summary of molecular results. AcCC: acinic cell carcinoma, AdCC: adenoid cystic carcinoma, CA-ex-PA: carcinoma ex pleomorphic adenoma, HCCC: hyalinizing clear cell carcinoma, IC: intraductal carcinoma, MEC: mucoepidermoid carcinoma, N/A: not assessed, PA: pleomorphic adenoma, SC: secretory carcinoma, SDC: salivary duct carcinoma.
| Case | Diagnosis | RNA-Seq | 5’ Gene (NCBI Reference) |
3’ Gene (NCBI Reference) |
FISH |
|---|---|---|---|---|---|
| 1 | PA | PRB2-TAF15 * | - | - | TAF15− |
| 2 | PA | Negative | - | - | N/A |
| 3 | PA | NPM1-PRB2 * | 9 of 11 (NM_002520.6) | 3 of 4 (NM_006248.3) | Negative |
| 4 | PA | DSTN-PLAG1 | 1 of 4 (NM_006870.3) | 3 of 5 (NM_002655.2) | PLAG1− |
| 5 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 2 of 5 (NM_002655.2) | N/A |
| 6 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 3 of 5 (NM_002655.2) | N/A |
| 7 | PA | HMGA2-WIF1 | 3 of 5 (NM_003483.4) | 10 of 10 (NM_007191.4) | N/A |
| 8 | PA | HMGA2-WIF1 | 3 of 5 (NM_003483.4) | 10 of 10 (NM_007191.4) | N/A |
| 9 | PA | Negative | - | - | N/A |
| 10 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 3 of 5 (NM_002655.2) | N/A |
| 11 | PA | NTF3-PLAG1 | 1 of 2 (NM_001102654.1) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 12 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 3 of 5 (NM_002655.2) | N/A |
| 13 | PA | LIFR-PLAG1 | 1 of 20 (NM_002310.5) | 3 of 5 (NM_002655.2) | N/A |
| 14 | PA | Negative | - | - | N/A |
| 15 | PA | Negative | - | - | N/A |
| 16 | PA | Negative | - | - | N/A |
| 17 | PA | Negative | - | - | N/A |
| 18 | PA | ACTA2- PLAG1 | 1 of 9 (NM_001613.4) | 3 of 5 (NM_002655.3) | N/A |
| 19 | PA | NCALD- PLAG1 | 1 of 4 (NM_032041.2) | 3 of 5 (NM_002655.2) | N/A |
| 20 | PA | HMGA2-WIF1 | 3 of 5 (NM_003483.4) | 3 of 10 (NM_007191.4) | N/A |
| 21 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 3 of 5 (NM_002655.2) | N/A |
| 22 | PA | CHCHD7-PLAG1 | 3 of 5 (NM_001011667.2) | 3 of 5 (NM_002655.2) | N/A |
| 23 | PA | Negative | - | - | N/A |
| 24 | PA | NCALD-PLAG1 | 1 of 4 (NM_032041.2) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 25 | PA | CTNNB1-PLAG1 | 1 of 15 (NM_001904.3) | 3 of 5 (NM_002655.2) | N/A |
| 26 | PA | FBXO32-PLAG1 | 1 of 9 (NM_058229.3) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 27 | PA | NCALD- PLAG1 | 1 of 4 (NM_032041.2) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 28 | CA-ex-PA | FGFR1-PLAG1 | 2 of 18 (NM_023110.2) | 3 of 5 (NM_002655.2) | N/A |
| 29 | CA-ex-PA | FBXO32-PLAG1 | 1 of 9 (NM_058229.3) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 30 | CA-ex-PA | HMGA2-NFIB | 3 of 5 (NM_003483.4) | 9 of 11 (NM_001190737.1) | N/A |
| 31 | CA-ex-PA | MEG3-PLAG1 | 1 of 7 (NR_002766.2) | 3 of 5 (NM_002655.2) | PLAG1+ |
| 32 | CA-ex-PA | PLAG1-NFIB | 1 of 5 (NM_002655.2) | 3 of 11 (NM_001190737.1) | PLAG1+, NFIB+ |
| 33 | CA-ex-PA | HMGA2-CNOT2 | 2 of 5 (NM_003483.4) | 12 of 16 (NM_001199303.1) | HMGA2 amplification |
| 34 | SDC-ex-PA | Negative | - | - | N/A |
| 35 | AdCC | MYB-NFIB | 13 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 36 | AdCC | MYBL1-NFIB | 14 of 16 (NM_001080416.3) | 9 of 11 (NM_001190737.1) | N/A |
| 37 | AdCC | MYB-NFIB | 15 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 38 | AdCC | MYBL1-NFIB | 8 of 16 (NM_001080416.3) | 11 of 11 (NM_001190737.1) | N/A |
| 39 | AdCC | MYB-NFIB | 15 of 16 (NM_001130173.1) | 10 of 11 (NM_001282787.1) | N/A |
| 40 | AdCC | MYB-NFIB | 15 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 41 | AdCC | MYB-NFIB | 15 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 42 | AdCC | MYB-NFIB | 13 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 43 | AdCC | MYB-NFIB | 8 of 16 (NM_001130173.1) | 10 of 11 (NM_001282787.1) | N/A |
| 44 | AdCC | MYBL1-NFIB | 8 of 16 (NM_001080416.3) | 10 of 11 (NM_001282787.1) | N/A |
| 45 | AdCC | Negative | - | - | N/A |
| 46 | AdCC | MYB-NFIB | 8 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 47 | AdCC | MYB-NFIB | 15 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 48 | AdCC | MYB-NFIB | 8 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 49 | AdCC | MYB-NFIB | 9 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 50 | AdCC | MYBL1-NFIB | 8 of 16 (NM_001080416.3) | 9 of 11 (NM_001190737.1) | N/A |
| 51 | AdCC | NFIB-TBPL1 | 10 of 11 (NM_001190737.1) | 7 of 7 (NM_001253676.1) | NFIB+ |
| 52 | AdCC | MYBL1-NFIB | 12 of 16 (NM_001080416.3) | 2 of 11 (NM_001190737.1) | N/A |
| 53 | AdCC | MYB-NFIB | 13 of 16 (NM_001130173.1) | 11 of 11 (NM_001190737.1) | N/A |
| 54 | AdCC | MYBL1-VCPIP1 | 9 of 16 (NM_001080416.3) | 3 of 3 (NM_025054.4) | MYBL1+ |
| 55 | MEC | Negative | - | - | MAML2+ |
| 56 | MEC | CRTC1-MAML2 | 2 of 14 (NM_015321.2) | 1 of 5 (NM_032427.3) | MAML2+ |
| 57 | MEC | Negative | - | - | MAML2+ |
| 58 | MEC | CRTC3-MAML2 | 2 of 15 (NM_022769.4) | 2 of 5 (NM_032427.3) | MAML2+ |
| 59 | MEC | Negative | - | - | Negative |
| 60 | MEC | CRTC1-MAML2 | 2 of 14 (NM_015321.2) | 1 of 5 (NM_032427.3) | MAML2+ |
| 61 | MEC | CRTC1-MAML2 | 1 of 14 (NM_015321.2) | 2 of 5 (NM_032427.3) | MAML2+ |
| 62 | MEC | Negative | - | - | MAML2+ |
| 63 | MEC | CRTC1-MAML2 | 1 of 14 (NM_015321.2) | 2 of 5 (NM_032427.3) | MAML2+ |
| 64 | MEC | Negative | - | - | Negative |
| 65 | MEC | CRTC3-MAML2 | 2 of 15 (NM_022769.4) | 2 of 5 (NM_032427.3) | MAML2+ |
| 66 | MEC | CRTC1-MAML2 | 1 of 14 (NM_015321.2) | 2 of 5 (NM_032427.3) | MAML2+ |
| 67 | MEC | CRTC1-MAML2 | 1 of 14 (NM_015321.2) | 2 of 5 (NM_032427.3) | MAML2+ |
| 68 | MEC | CRTC3-MAML2 | 2 of 15 (NM_022769.4) | 2 of 5 (NM_032427.3) | Negative |
| 69 | AcCC | Negative | - | - | N/A |
| 70 | AcCC | Negative | - | - | N/A |
| 71 | AcCC | Negative | - | - | N/A |
| 72 | AcCC | Negative | - | - | N/A |
| 73 | AcCC | Negative | N/A | ||
| 74 | HCCC | EWSR1-ATF1 | 12 of 18 (NM_013986.3) | 3 of 7 (NM_005171.4) | N/A |
| 75 | HCCC | Negative | - | - | N/A |
| 76 | HCCC | EWSR1-ATF1 | 14 of 18 (NM_013986.3) | 5 of 7 (NM_005171.4) | N/A |
| 77 | SC | ETV6-NTRK3 | 5 of 8 (NM_001987.4) | 15 of 20 (NM_001012338.2) | N/A |
| 78 | SC | ETV6-NTRK3 | 5 of 8 (NM_001987.4) | 15 of 20 (NM_001012338.2) | N/A |
| 79 | SDC | RAPGEF6-ACSL6 | 1 of 29 (NM_001164386.1) | 2 of 21 (NM_015256.3) | N/A |
| 80 | IC | NCOA4-RET | 8 of 12 (NM_001145260.1) | 12 of 20 (NM_020975.5) | N/A |
Stochastic events highlighted by an asterisk (*).
Figure 1.
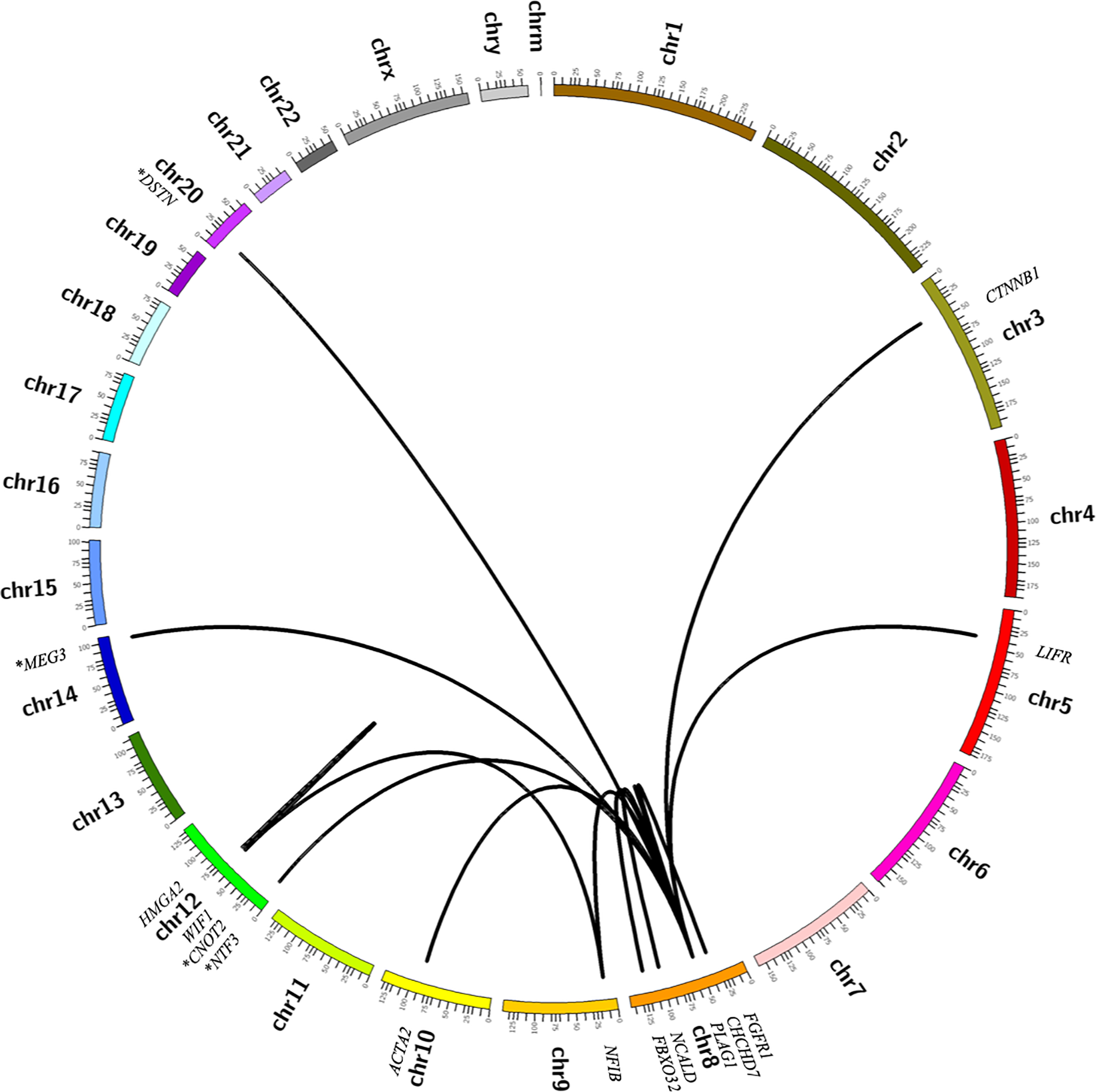
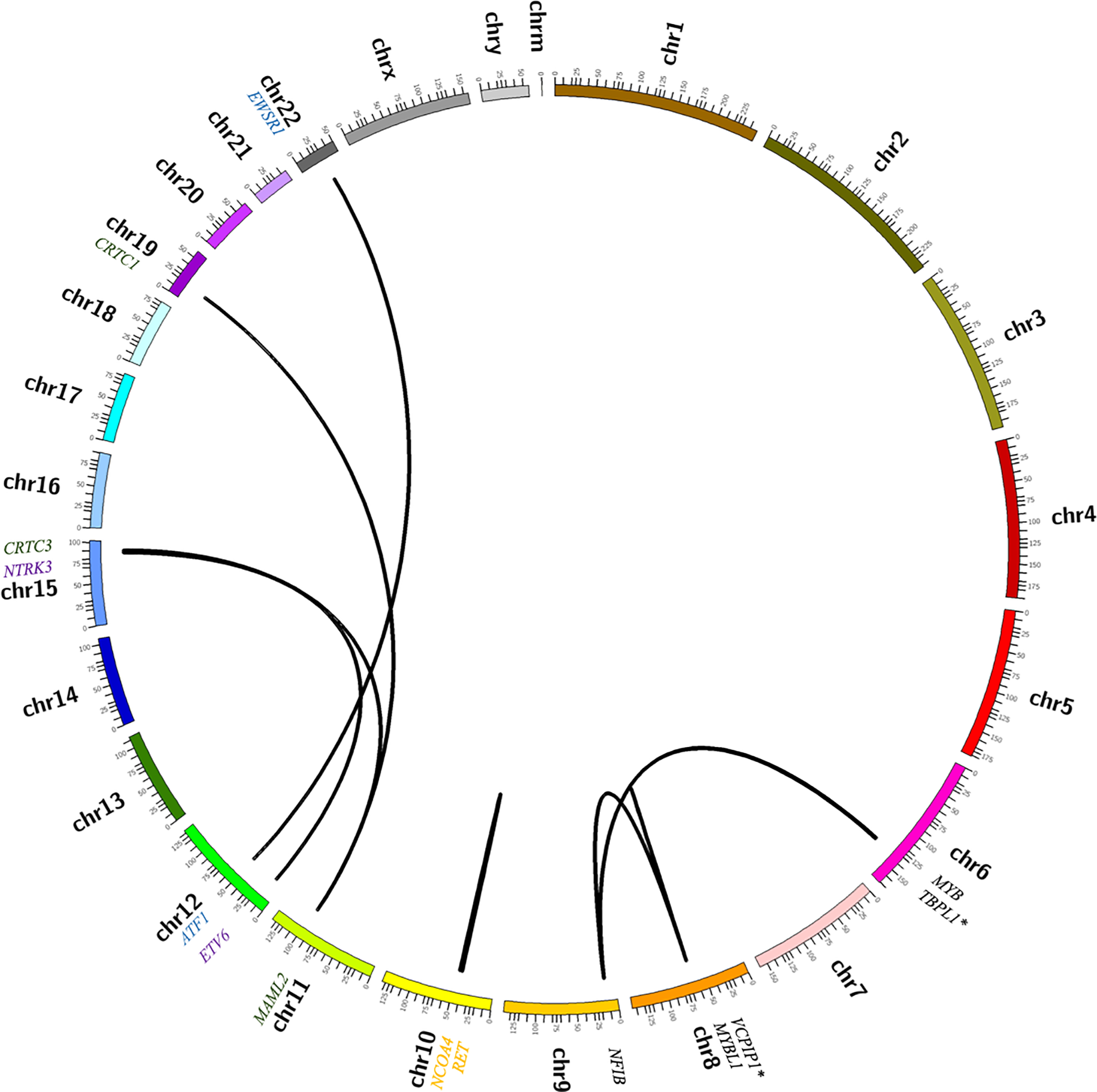
Circos plots summarizing molecular alterations detected by RNA sequencing in cohort of salivary gland tumors. (A) Pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. (B) Less common salivary gland neoplasms, including mucoepidermoid carcinoma (green), adenoid cystic carcinoma (black), hyalinizing clear cell carcinoma (blue), secretory carcinoma (purple) and intraductal carcinoma (orange). Novel fusion partners are indicated by an asterisk (*).
Overall, the prevalence of fusion transcripts in the PAs and CA-ex-PAs was 71% (24/34), with 56% (19/34) involving PLAG1 and 15% (5/34) involving HMGA2 (Figures 1 and 2). The number of supporting reads for PLAG1-rearranged tumors (range = 2–162; median = 19.5) was lower than those of HMGA2-rearranged cases (range = 2–759; median = 215.5). Of the benign PAs, 67% (18/27) were found to contain fusion genes, with a prevalence of 56% (15/27) and 11% (3/27) for PLAG1 and HMGA2 rearrangement, respectively. The most common PLAG1 fusion partner was CTNNB1 (n = 6), followed by NCALD (n = 3), LIFR, CHCHD7, ACTA2 and FBXO32 (n = 1, each). Novel PLAG1 fusion partners included DSTN and NTF3 (n = 1 each). The most common HMGA2 fusion partner was WIF1 (n = 3). Additionally, two PAs contained heretofore undescribed fusion transcripts (PRB2-TAF15 and NPM1-PRB2); however, based on a low number of supporting reads, and breakpoints involving partial exons, these were discounted as stochastic events. Of the seven CA-ex-PAs in our cohort, there was a prevalence of 57% (4/7) and 29% (2/7) of PLAG1 and HMGA2 rearrangements, respectively. Novel MEG3-PLAG1 and HMGA2-CNOT2 fusion transcripts were found in two cases.
Figure 2.
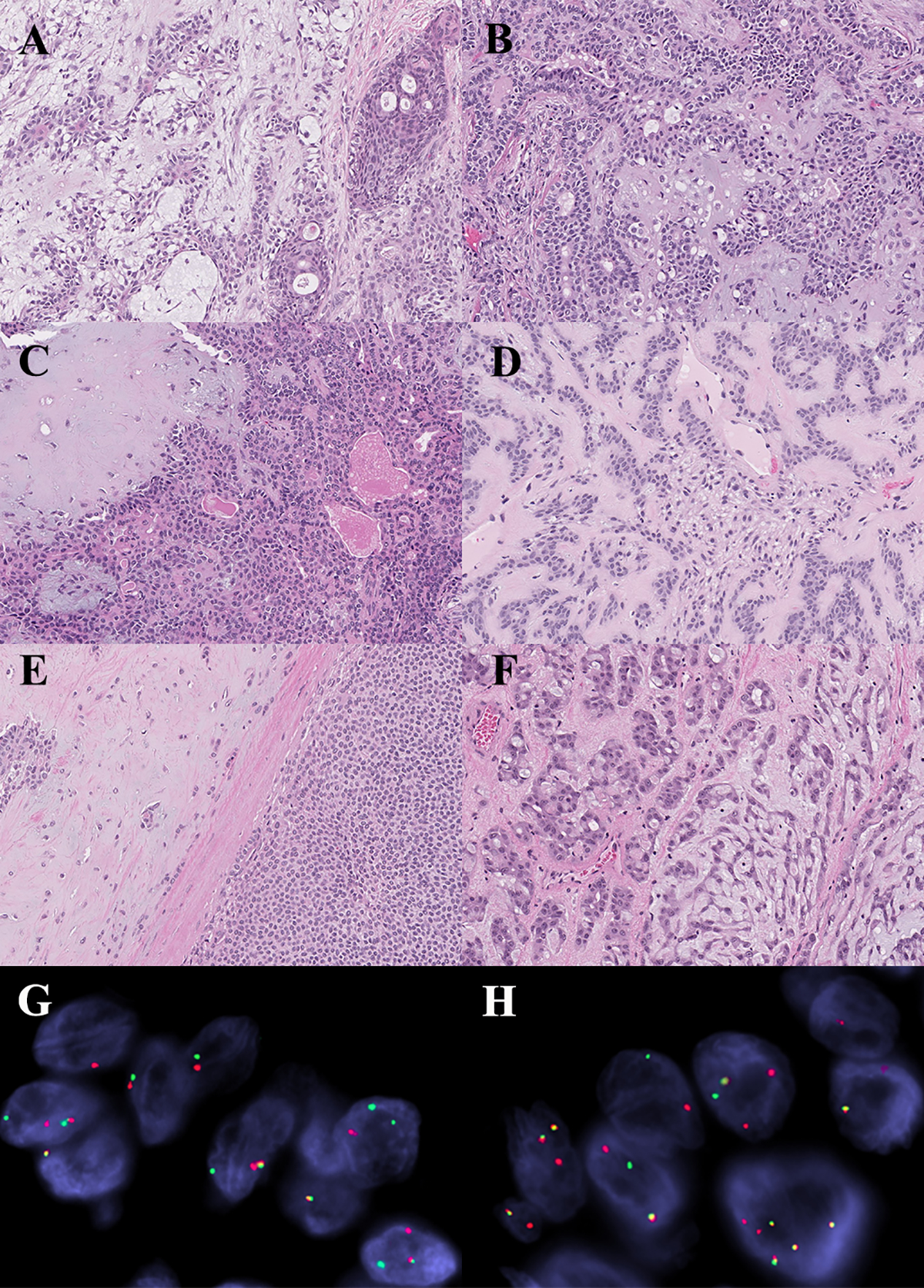
Histomorphologic and molecular correlates in pleomorphic adenoma. (A) Tumor with CTNNB1-PLAG1 fusion gene. (B) Tumor lacking fusion gene. (C) Tumor with novel DSTN-PLAG1 fusion product. (D) Tumor with novel NTF3-PLAG1 fusion product. Note: there is no significant morphologic differences amongst the tumors with known and novel fusions, or those that are fusion negative. (E) Carcinoma ex-pleomorphic adenoma with FBXO32-PLAG1 fusion gene. (F-H) Carcinoma ex-pleomorphic adenoma with PLAG1-NFIB fusion gene. (G) FISH demonstrating PLAG1 rearrangement. (H) FISH demonstrating NFIB rearrangement.
The prevalence of detectable fusion transcripts in AdCC was found to be 95% (19/20); 60% (12/20) contained the MYB-NFIB fusion, and 25% (5/20) the MYBL1-NFIB fusion (Figures 1 and 3). In addition, two cases were found to harbor novel fusion genes (NFIB-TBPL1 and MYBL1-VCPIP1). In general, the AdCCs exhibited a high number of supporting reads (range = 2–1336; median = 200).
Figure 3.
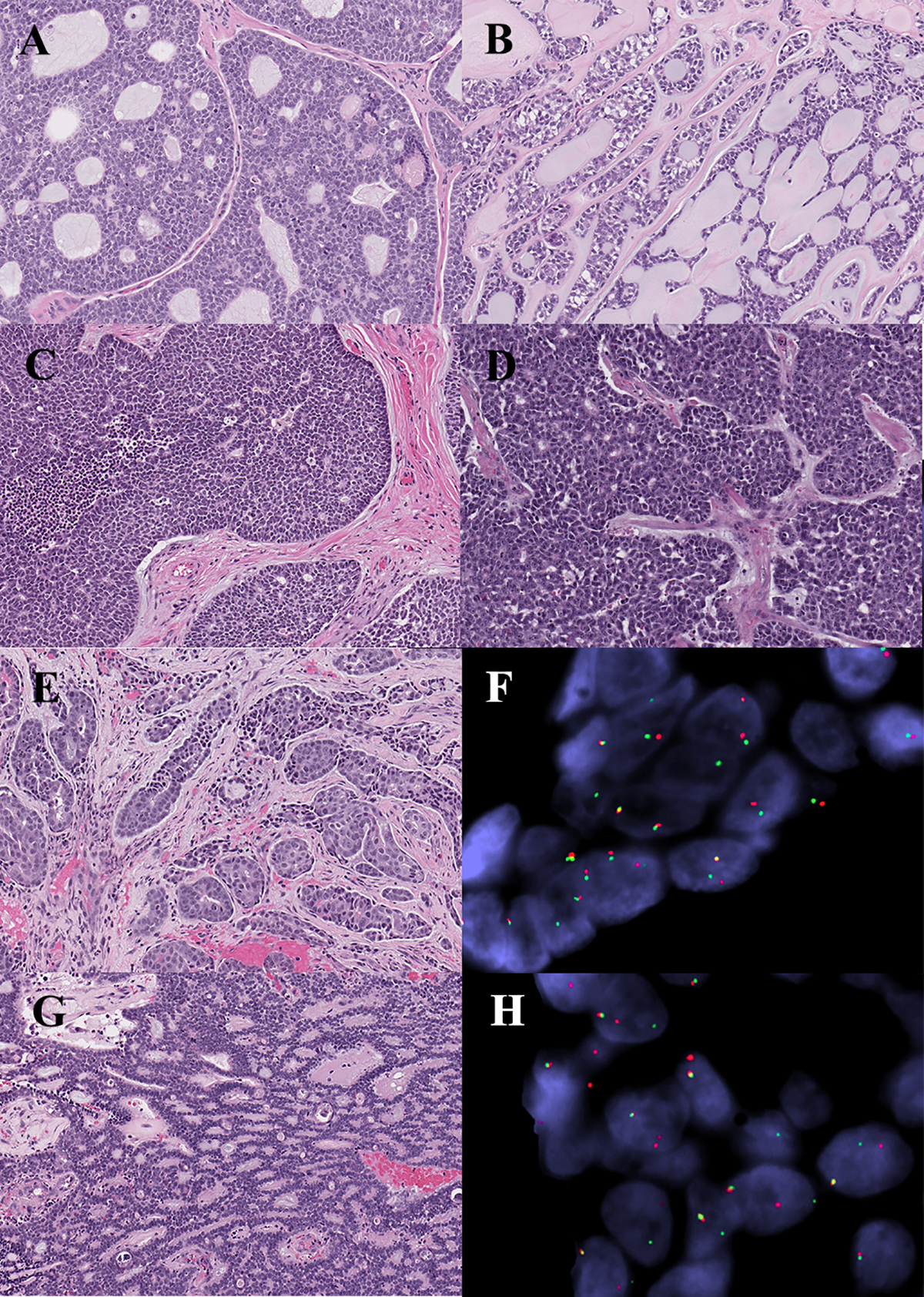
Histomorphologic and molecular correlates in adenoid cystic carcinoma. (A) Tumor with MYB-NFIB fusion product. (B) Tumor lacking identifiable fusion gene with prototypic cribriform morphology. (C) Tumor with MYB-NFIB fusion gene with solid growth. (D) Tumor with MYB-NFIB fusion gene and high-grade transformation. (E-F) Tumor with novel NFIB-TBPL1 fusion gene fusion, and tubular architecture. (F) FISH demonstrating NFIB rearrangement. (G-H) Tumor with novel MYBL1-VCPIP1 fusion gene, and features mimicking polymorphous adenocarcinoma. (H) FISH demonstrating MYBL1 rearrangement.
In MEC the prevalence of the CRTC1-MAML2 fusion gene was 43% (6/14), while 21% (3/14) contained the CRTC3-MAML2 fusion (Figures 1 and 4). Fusion transcripts were detected in 57% (4/7) of low-grade tumors, 67% (2/3) of intermediate-grade tumors, and none (0/1) of the high-grade tumors; the three remaining MECs in this sub-cohort included a clear cell variant, an oncocytic variant and a metastatic tumor which therefore did not receive a histologic grade. Overall, MECs contained a somewhat lower number of supporting reads by RNA-Seq (range = 1–11; median = 7) compared to other salivary carcinomas tested.
Figure 4.
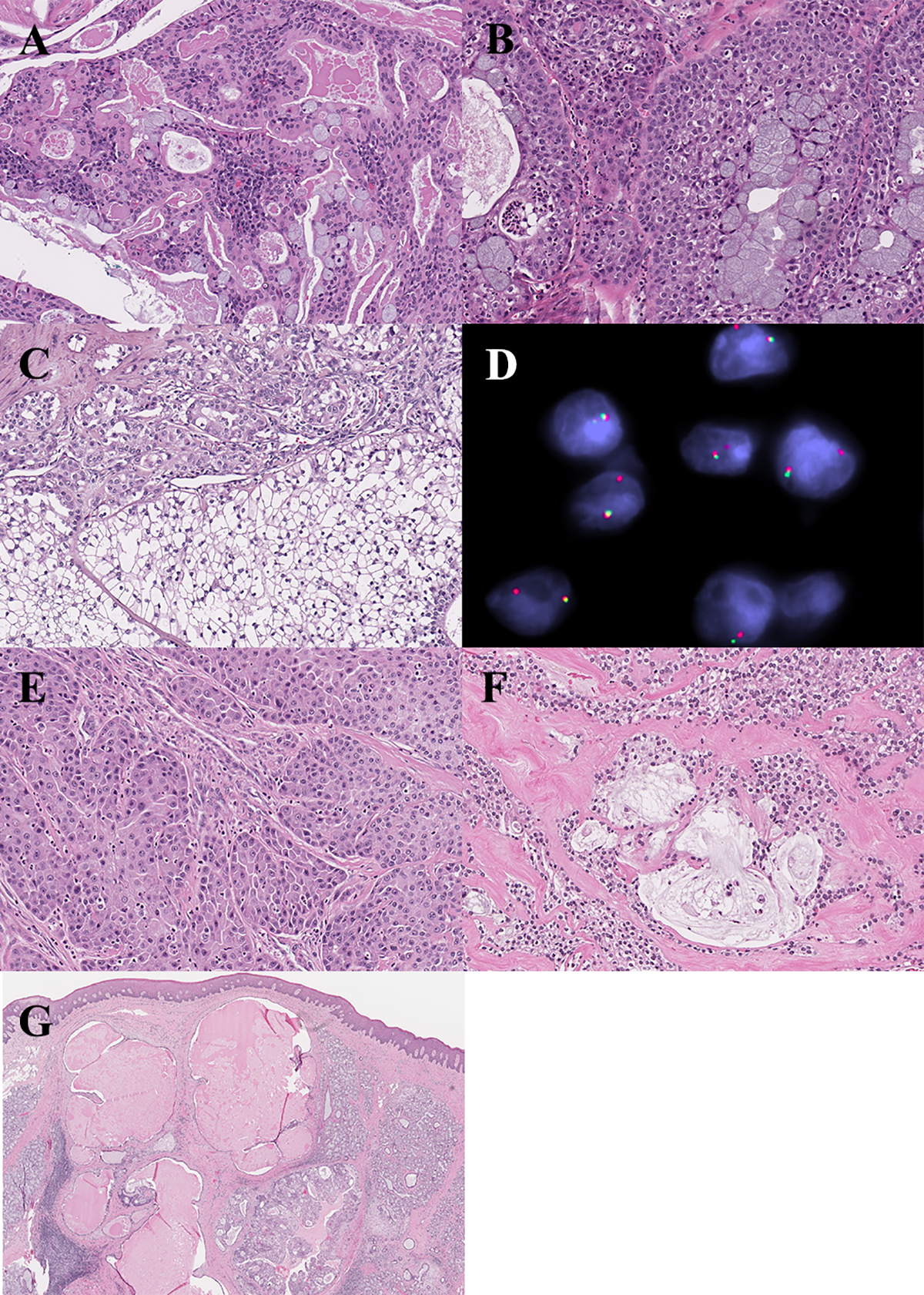
Mucoepidermoid carcinoma. (A) Tumor with CRTC1-MAML2 fusion gene. (B) Tumor lacking identifiable fusion, with classic morphology. (C-D) Clear cell variant with CRTC3-MAML2 fusion product. (D) FISH demonstrating MAML2 rerrangement. (E) Oncocytic variant with CRTC1-MAML2, and paucity of mucous cells. (F) Tumor with CRTC1-MAML2 fusion gene, and clear cells embedded in hyalinized stroma mimicking hyalinizing clear cell carcinoma. (G) Tumor with CRTC1-MAML2 fusion product, and predominantly cystic architecture, mimicking a benign cystic lesion on incisional biopsy. Classic morphology was observed only in the deep aspect of the resection specimen (inset).
The remainder of the cohort consisted of five AcCCs, three HCCCs, two SCs, one IC and one SDC. None of the AcCCs exhibited a fusion transcript, while 67% (2/3) of the HCCCs harbored an EWSR1-ATF1 fusion gene, both SCs harbored an ETV6-NTRK3 fusion and the one IC harbored a NCOA4-RET fusion (Figure 1). The de novo SDC was found to contain a novel RAPGEF6-ACSL6 fusion gene, which was in-frame; however, the significance of this finding is unknown and the possibility it may represent a secondary or stochastic event cannot be entirely excluded.
In terms of specimen type, 66% (41/62) of excision/resection specimens and 94% (17/18) of the incisional/core biopsy specimens were positive for fusion transcripts. The excision/resection specimens exhibited a range of 1–759 supporting reads (median = 26), while the incisional/core biopsies specimens had a range of 2–1336 supporting reads (median = 67). For 18% (14/80) of the tumors identified in our archival review, RNA-Seq had been employed as a diagnostic adjunct to assist in initial classification. This included PA (four cases) CA-ex-PA (one case), AdCCs (four cases), MEC (two cases), HCCC (two cases), and SC (one case).
Fluorescence in situ hybridization
FISH independently confirmed fusion gene rearrangement in all cases, apart from Case 4, which was negative for PLAG1 rearrangement, and Case 33, which was found to show HMGA2 amplification. Examination of the entire MEC sub-cohort by FISH showed MAML2 rearrangement in 80% (12/15) of cases (Table 2), including three cases with undetectable rearrangement by RNA-Seq and one case that was excluded based on insufficient quality RNA.
Immunohistochemistry
MYB IHC was found to have a sensitivity of 48.4% (45/93) and specificity of 93.6% (322/344) for AdCC. Interestingly, a distinctive abluminal or myoepithelial pattern was noted in three AdCCs with positive immunostaining for MYB. Nuclear Pan-Trk immunostaining demonstrated a sensitivity of 58.8% (10/17) and specificity of 89.5% (376/420) in the diagnosis of SC, with scattered positivity also seen in 17 mucoepidermoid carcinomas, 7 polymorphous adenocarcinomas, 5 squamous cell carcinomas, 3 adenoid cystic carcinomas, 2 intraductal carcinomas, 2 epithelial-myoepithelial carcinomas, 2 salivary duct carcinomas and 1 acinic cell carcinoma, amongst others. As the TMAs were largely composed of malignant salivary gland tumors, an accurate assessment of the sensitivity and specificity of the HMGA2 stain was not possible.
DISCUSSION:
Recurrent chromosomal translocations yielding fusion genes are commonplace in neoplasms of hematopoietic and mesenchymal origin; these are also increasingly recognized in tumors of epithelial origin, including salivary gland neoplasms.2–9,44 Next generation sequencing has contributed to an unparalleled rate of novel fusion gene discovery. While this offers insight into the molecular pathogenesis of these neoplasms, these genetic events can also be leveraged for diagnostic purposes. The purpose of this study was to validate a targeted RNA-Seq assay for fusion detection in salivary gland tumors.
The t(3;8)(p21;q12) translocation, resulting in a PLAG1-CTNNB1 fusion gene, is the most common fusion event observed in pleomorphic adenoma.3 Other reported PLAG1 fusion partners in this context include: LIFR, TCEA1, CHCHD7, FGFR1, FBXO32, C1orf116, and NFIB.2,4–6,45–47 A subset of PAs have 12q15 translocations resulting in HMGA2 rearrangement; this gene has been reported to partner with FHIT, NFIB and WIF1.7–9 Unsurprisingly, similar gene rearrangements have also been detected in carcinoma ex-pleomorphic adenomas, including salivary duct carcinoma (SDC) ex-PA and myoepithelial carcinoma ex-PA.48–52 Interestingly, PLAG1 rearrangements have likewise been documented in a subset of apparently de novo SDC and myoepithelial carcinomas.35,49,53
In this study PA / CA-ex-PA were found to have detectable fusion transcripts in 71% of cases; PLAG1 was rearranged in 56%, and HMGA2 in 15%. This appears similar, if not slightly better, to reports in the literature that suggest rearrangement of PLAG1 in approximately 58% (range = 24–85 %), and HMGA2 in about 7% (range = 2–13%) of cases.48–50,52–55 Novel PLAG1 fusion partners were also identified by RNA-Seq and included single cases with DSTN and MEG3 partners; and, one tumor was found to have a novel HMGA2-CNOT2 fusion transcript. One case was found to harbour NTF3-PLAG1 which has recently been identified in oncocytic myoepithelioma.47 In addition, NCALD-PLAG1 (n = 3) and ACTA2-PLAG1 (n = 1) gene products were identified in this sub-cohort; to date, the NCALD-PLAG1 fusion has only been reported in a myoepithelial carcinoma ex-PA, while the ACTA2-PLAG1 fusion has only been documented in a de novo myoepithelial carcinoma.53 These cases highlight the molecular overlap that exists between PA and myoepithelial neoplasms of salivary gland origin. Finally, one CA-ex-PA was found to contain a PLAG1-NFIB fusion transcript, which was only recently reported in a case of benign PA.46
Adenoid cystic carcinoma is characterized by a recurrent t(6;9)(q22–23;p23–24) translocation that results in a MYB-NFIB fusion gene.14,56 An alternative MYBL1-NFIB gene fusion has been reported in a subset of cases.15,16,57 Various other genes have been reported to substitute for MYB, MYBL1 and NFIB, including TGFRB3, RAD51B, YTHDF3, AIG1, XRCC4 and PTPRD, amongst others.15,16,58–60 In our cohort of 20 AdCCs, the MYB-NFIB fusion was identified in 60% and the MYBL1-NFIB fusion in 25%, while two cases harbored novel NFIB-TBPL1 and MYBL1-VCPIP1 fusions products. A review of the literature revealed a prevalence of 60% (range = 40–86%) for MYB rearrangement and 13% (8–24%) for MYBL1 rearrangement in AdCC.15,16,60–68
In our archival review, we identified four AdCCs in which RNA-Seq had been employed at the time of diagnosis to facilitate classification. The initial differential diagnosis for cases 36, 51 and 54 included AdCC, polymorphous adenocarcinoma, and adenocarcinoma, not otherwise specified. RNA-Seq identified MYBL1-NFIB, NFIB-TBPL1, and MYBL1-VCPIP1 fusion transcripts, respectively, supporting the diagnosis of AdCC in these three tumors. Case 37 presented as a nasopharyngeal mass in which the differential diagnosis included AdCC and HPV-related multiphenotypic sinonasal carcinoma with extension into the nasopharynx. Detection of the MYB-NFIB fusion transcript confirmed the diagnosis as AdCC. Additionally, two of the fusion-positive tumors in our cohort exhibited a solid growth pattern and high-grade transformation, respectively, highlighting how RNA-Seq may serve as a diagnostic adjunct in tumors lacking a prototypic morphology.
The majority of MECs are characterized by a t(11;19)(q21;p13) translocation, resulting in a CRTC1-MAML2 fusion gene;10–12 a subset are reported to harbor a CRTC3-MAML2 gene fusion.13 Detection of these fusion products are particularly helpful in the diagnosis of histologic variants of MEC. For example, in our cohort, both the clear cell variant (Case 65) and the oncocytic variant of MEC (Case 56) initially posed a diagnostic challenge. In both cases RNA-Seq had been used at the time of initial diagnosis, with the presence of MAML2 rearrangement supporting classification as MEC. RNA sequencing was helpful in resolving differential diagnoses in other cases as well. This is likewise the instance with Case 60, which had a variant morphology that mimicked HCCC. In contrast, Case 70 presented as a metastatic clear cell tumor in a cervical lymph node with a differential diagnosis that included the clear cell variant of MEC, HCCC and squamous cell carcinoma with clear cell change; the identification of an EWSR1-ATF1 fusion transcript enabled definitive classification as HCCC. And, Case 67 exhibited a predominantly cystic architecture with a bland epithelial lining, which mimicked a benign cystic lesion (i.e., mucous retention cyst) on the incisional biopsy. These cases reinforce how molecular testing can be successfully employed as a diagnostic adjunct in morphologically challenging variants of MEC.
Overall, fusion products were identified in 64% of MECs; MAML2 was partnered with CRTC1 in 43% and CRTC3 in 21% of cases. In comparison, a review of the literature suggests a prevalence of MAML2 rearrangement in 52% of MEC (range = 34–82%), with 5% (range = 2–6%) of cases exhibiting a CRTC3 fusion partner.13,69–78 The considerable variability in the reports of incidence of MAML2-rearrangment appears to be largely technique dependent. Noda et al (2013) previously noted the limitations of RT-PCR for fusion detection in these tumors due to comparatively low expression of fusion transcripts,75 suggesting FISH might offer a diagnostic advantage. We likewise noted a low number of supporting reads in this group by RNA-Seq and, for this reason, decided to examine this entire subcohort by FISH. This revealed MAML2 rearrangement in 80% (12/15). Three tumors that were negative for fusion products by RNA-Seq were found to have MAML2 rearrangement by FISH (Cases 55, 57 and 62); conversely, one tumor was negative for MAML2 rearrangement by FISH but positive by RNA-Seq (Case 68). In addition, one case that had been excluded based on insufficient quality RNA was examined by FISH and found to show MAML2 rearrangement. The raw data files for the aforementioned cases were subsequently re-examined and confirmed to lack any missed fusion calls. This confirms that, while RNA-seq can frequently identify fusions in MECs, FISH generally appears to show somewhat greater sensitivity.
Recurrent molecular alterations have only recently been reported in AcCC. Novel HTN3-MSANTD3 gene fusions have been described in a subset of AcCC, with 4.4–8% of cases showing MSANTD3 aberrations.23–25 A recurrent t(4;9)(q13;q31) translocation transferring the enhancer regions of the highly expressed SCPP gene cluster to a location upstream of the NR4A3 gene has also been reported.25,79 Consistent with the enhancer hijacking mechanism underlying this molecular finding, this rearrangement does not result in a chimeric gene fusion. Therefore, unsurprisingly none of the AcCC in our cohort were found to contain fusion transcripts by RNA-Seq.
While IHC offers a convenient and inexpensive alternative to molecular testing, these markers were found to have limited sensitivity in our hands compared to pre-existing reports in the literature. Application of IHC for MYB was found to have a sensitivity of 48.4% in the TMA for AdCC, compared to prior studies showing 64.9–82.4% sensitivity.61,62 Moreover, nuclear staining for Pan-Trk had a sensitivity of 58.8% for the diagnosis of SC, whereas the literature describes Pan-Trk IHC as having a sensitivity ranging from 64–74%.80–82 The decreased sensitivity of Pan-Trk may, in part, be attributable to the presence of non-NTRK3 fusion partners in a subset of cases. Additionally, while the EPR17341 Pan-Trk clone recognizes an amino acid sequence that conserved across all three Trk proteins, its sensitivity for NTRK3 fusion-positive tumors has been shown to be less than its sensitivity for detecting NTRK1 and NTRK2 fusion-positive tumors.83 Pan-Trk expression has also been described as focal and weak in a subset of NTRK3-positive tumors, with some cases exhibiting less than 5% of tumor cells staining,83 which might lead to false negative interpretation with the limited sampling inherent to TMA cores. Finally, for both stains, the reduced sensitivity in this study may be potentiated by the age of the tumors incorporated into the TMA, many of which being over 10 years old.
In summary, targeted RNA-Seq represents a useful diagnostic technique for fusion gene detection in salivary gland neoplasms. In addition to confirming the presence of known gene fusions, it also has the advantage of enabling identification of novel fusion partners, thereby also refining understanding of the molecular pathogenesis of these neoplasms. RNA-Seq was able to successfully detect fusion transcripts in both excision/resection specimens as well as incisional/core biopsy specimens. Cost and turnaround time notwithstanding, a potential limitation of this assay included an inability to detect certain molecular alterations such as the enhancer rearrangements seen in AcCCs, as well as missing fusions in a subset of cases with low copy expression (i.e., MEC). It is possible that with more comprehensive panels, and greater sequencing depth, the detection rate of these events will increase in the future. In the meantime, as this technology gains broader traction in clinical laboratories, RNA-Seq offers an important adjunct in the diagnosis of this diverse group of neoplasms.
Supplementary Material
Funding:
Panov 2 Research Fund
Footnotes
Conflicts of interest: none
Data Availability Statement:
Available from corresponding author upon reasonable request.
References:
- 1.El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P. WHO Classification of Head and Neck Tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Aström AK, Voz ML, Kas K, et al. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: identification of SII as a new fusion partner gene. Cancer Res. 1999;59(4):918–923. [PubMed] [Google Scholar]
- 3.Kas K, Voz ML, Röijer E, et al. Promoter swapping between the genes for a novel zinc finger protein and beta-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet. 1997;15(2):170–174. [DOI] [PubMed] [Google Scholar]
- 4.Voz ML, Aström AK, Kas K, Mark J, Stenman G, Van de Ven WJ. The recurrent translocation t(5;8)(p13;q12) in pleomorphic adenomas results in upregulation of PLAG1 gene expression under control of the LIFR promoter. Oncogene. 1998;16(11):1409–1416. [DOI] [PubMed] [Google Scholar]
- 5.Asp J, Persson F, Kost-Alimova M, Stenman G. CHCHD7-PLAG1 and TCEA1-PLAG1 gene fusions resulting from cryptic, intrachromosomal 8q rearrangements in pleomorphic salivary gland adenomas. Genes Chromosomes Cancer. 2006;45(9):820–828. [DOI] [PubMed] [Google Scholar]
- 6.Persson F, Winnes M, Andrén Y, et al. High-resolution array CGH analysis of salivary gland tumors reveals fusion and amplification of the FGFR1 and PLAG1 genes in ring chromosomes. Oncogene. 2008;27(21):3072–3080. [DOI] [PubMed] [Google Scholar]
- 7.Geurts JM, Schoenmakers EF, Röijer E, Stenman G, Van de Ven WJ. Expression of reciprocal hybrid transcripts of HMGIC and FHIT in a pleomorphic adenoma of the parotid gland. Cancer Res. 1997;57(1):13–17. [PubMed] [Google Scholar]
- 8.Geurts JM, Schoenmakers EF, Röijer E, Aström AK, Stenman G, van de Ven WJ. Identification of NFIB as recurrent translocation partner gene of HMGIC in pleomorphic adenomas. Oncogene. 1998;16(7):865–872. [DOI] [PubMed] [Google Scholar]
- 9.Queimado L, Lopes CS, Reis AM. WIF1, an inhibitor of the Wnt pathway, is rearranged in salivary gland tumors. Genes Chromosomes Cancer. 2007;46(3):215–225. [DOI] [PubMed] [Google Scholar]
- 10.Nordkvist A, Gustafsson H, Juberg-Ode M, Stenman G. Recurrent rearrangements of 11q14–22 in mucoepidermoid carcinoma. Cancer Genet Cytogenet. 1994;74(2):77–83. [DOI] [PubMed] [Google Scholar]
- 11.Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. [DOI] [PubMed] [Google Scholar]
- 12.Enlund F, Behboudi A, Andrén Y, et al. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp Cell Res. 2004;292(1):21–28. [DOI] [PubMed] [Google Scholar]
- 13.Fehr A, Röser K, Heidorn K, Hallas C, Löning T, Bullerdiek J. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes Chromosomes Cancer. 2008;47(3):203–206. [DOI] [PubMed] [Google Scholar]
- 14.Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106(44):18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitani Y, Liu B, Rao PH, et al. Novel MYBL1 Gene Rearrangements with Recurrent MYBL1-NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations. Clin Cancer Res. 2016;22(3):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor-Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer Discov. 2016;6(2):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. [DOI] [PubMed] [Google Scholar]
- 18.Skalova A, Vanecek T, Martinek P, et al. Molecular Profiling of Mammary Analog Secretory Carcinoma Revealed a Subset of Tumors Harboring a Novel ETV6-RET Translocation: Report of 10 Cases. Am J Surg Pathol. 2018;42(2):234–246. [DOI] [PubMed] [Google Scholar]
- 19.Guilmette J, Dias-Santagata D, Nosé V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–58. [DOI] [PubMed] [Google Scholar]
- 20.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. [DOI] [PubMed] [Google Scholar]
- 21.Chapman E, Skalova A, Ptakova N, et al. Molecular Profiling of Hyalinizing Clear Cell Carcinomas Revealed a Subset of Tumors Harboring a Novel EWSR1-CREM Fusion: Report of 3 Cases. Am J Surg Pathol. 2018;42(9):1182–1189. [DOI] [PubMed] [Google Scholar]
- 22.Weinreb I, Zhang L, Tirunagari LM, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–856. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen S, Varma S, Barasch N, et al. The HTN3-MSANTD3 Fusion Gene Defines a Subset of Acinic Cell Carcinoma of the Salivary Gland. Am J Surg Pathol. 2019;43(4):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barasch N, Gong X, Kwei KA, et al. Recurrent rearrangements of the Myb/SANT-like DNA-binding domain containing 3 gene (MSANTD3) in salivary gland acinic cell carcinoma. PLoS One. 2017;12(2):e0171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haller F, Bieg M, Will R, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skálová A, Vanecek T, Uro-Coste E, et al. Molecular Profiling of Salivary Gland Intraductal Carcinoma Revealed a Subset of Tumors Harboring NCOA4-RET and Novel TRIM27-RET Fusions: A Report of 17 cases. Am J Surg Pathol. 2018;42(11):1445–1455. [DOI] [PubMed] [Google Scholar]
- 27.Skálová A, Ptáková N, Santana T, et al. NCOA4-RET and TRIM27-RET Are Characteristic Gene Fusions in Salivary Intraductal Carcinoma, Including Invasive and Metastatic Tumors: Is “Intraductal” Correct? Am J Surg Pathol. 2019;43(10):1303–1313. [DOI] [PubMed] [Google Scholar]
- 28.Weinreb I, Bishop JA, Chiosea SI, et al. Recurrent RET Gene Rearrangements in Intraductal Carcinomas of Salivary Gland. Am J Surg Pathol. 2018;42(4):442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H, Graham RP, Seethala R, Chute D. Intraductal Carcinoma of Salivary Glands Harboring TRIM27-RET Fusion with Mixed Low Grade and Apocrine Types. Head Neck Pathol. 2020;14(1):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooper LM, Thompson LDR, Gagan J, Oliai BR, Weinreb I, Bishop JA. Salivary Intraductal Carcinoma Arising within Intraparotid Lymph Node: A Report of 4 Cases with Identification of a Novel STRN-ALK Fusion. Head Neck Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majewska H, Gorczyński A, Czapiewski P, et al. ALK alterations in salivary gland carcinomas. Virchows Arch. 2021;478(5):933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop JA, Nakaguro M, Whaley RD, et al. Oncocytic intraductal carcinoma of salivary glands: a distinct variant with TRIM33-RET fusions and BRAF V600E mutations. Histopathology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agaimy A, Baněčková M, Ihrler S, et al. ALK Rearrangements Characterize 2 Distinct Types of Salivary Gland Carcinomas: Clinicopathologic and Molecular Analysis of 4 Cases and Literature Review. Am J Surg Pathol. 2021. [DOI] [PubMed] [Google Scholar]
- 34.Skálová A, Weinreb I, Hyrcza M, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol. 2015;39(3):338–348. [DOI] [PubMed] [Google Scholar]
- 35.Skálová A, Agaimy A, Vanecek T, et al. Molecular Profiling of Clear Cell Myoepithelial Carcinoma of Salivary Glands With EWSR1 Rearrangement Identifies Frequent PLAG1 Gene Fusions But No EWSR1 Fusion Transcripts. Am J Surg Pathol. 2020. [DOI] [PubMed] [Google Scholar]
- 36.Bishop JA, Weinreb I, Swanson D, et al. Microsecretory Adenocarcinoma: A Novel Salivary Gland Tumor Characterized by a Recurrent MEF2C-SS18 Fusion. Am J Surg Pathol. 2019;43(8):1023–1032. [DOI] [PubMed] [Google Scholar]
- 37.Jo VY, Sholl LM, Krane JF. Distinctive Patterns of CTNNB1 (β-Catenin) Alterations in Salivary Gland Basal Cell Adenoma and Basal Cell Adenocarcinoma. Am J Surg Pathol. 2016;40(8):1143–1150. [DOI] [PubMed] [Google Scholar]
- 38.Lee YH, Huang WC, Hsieh MS. CTNNB1 mutations in basal cell adenoma of the salivary gland. J Formos Med Assoc. 2018;117(10):894–901. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh MS, Bishop JA, Wang YP, et al. Salivary Sialadenoma Papilliferum Consists of Two Morphologically, Immunophenotypically, and Genetically Distinct Subtypes. Head Neck Pathol. 2020;14(2):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinreb I, Piscuoglio S, Martelotto LG, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickson BC, Swanson D. Targeted RNA sequencing: A routine ancillary technique in the diagnosis of bone and soft tissue neoplasms. Genes Chromosomes Cancer. 2019;58(2):75–87. [DOI] [PubMed] [Google Scholar]
- 42.Kao YC, Sung YS, Zhang L, et al. EWSR1 Fusions With CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor With Predilection for Intracranial Location. Am J Surg Pathol. 2017;41(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop JA, Koduru P, Veremis BM, et al. SS18 Break-Apart Fluorescence In Situ Hybridization is a Practical and Effective Method for Diagnosing Microsecretory Adenocarcinoma of Salivary Glands. Head Neck Pathol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullerdiek J, Wobst G, Meyer-Bolte K, et al. Cytogenetic subtyping of 220 salivary gland pleomorphic adenomas: correlation to occurrence, histological subtype, and in vitro cellular behavior. Cancer Genet Cytogenet. 1993;65(1):27–31. [DOI] [PubMed] [Google Scholar]
- 45.Chen T, Wehrs R, Raslan S, et al. Identification of a Novel Fusion Transcript Involving F-box Protein 32 (FBXO32) and Pleomorphic Adenoma Gene 1 (PLAG1) in Pleomorphic Adenoma. Modern Pathology. 2018;31:474.29052596 [Google Scholar]
- 46.Kakay Afshari M, Fehr A, Tejera Nevado P, Andersson MK, Stenman G. Activation of PLAG1 and HMGA2 by gene fusions involving the transcriptional regulator gene NFIB. Genes Chromosomes Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 47.Baněčková M, Uro-Coste E, Ptáková N, et al. What is hiding behind S100 protein and SOX10 positive oncocytomas? Oncocytic pleomorphic adenoma and myoepithelioma with novel gene fusions in a subset of cases. Hum Pathol. 2020;103:52–62. [DOI] [PubMed] [Google Scholar]
- 48.Bahrami A, Dalton JD, Shivakumar B, Krane JF. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6(3):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katabi N, Ghossein R, Ho A, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asahina M, Saito T, Hayashi T, Fukumura Y, Mitani K, Yao T. Clinicopathological effect of PLAG1 fusion genes in pleomorphic adenoma and carcinoma ex pleomorphic adenoma with special emphasis on histological features. Histopathology. 2019;74(3):514–525. [DOI] [PubMed] [Google Scholar]
- 51.Persson F, Andrén Y, Winnes M, et al. High-resolution genomic profiling of adenomas and carcinomas of the salivary glands reveals amplification, rearrangement, and fusion of HMGA2. Genes Chromosomes Cancer. 2009;48(1):69–82. [DOI] [PubMed] [Google Scholar]
- 52.Andreasen S, von Holstein SL, Homøe P, Heegaard S. Recurrent rearrangements of the PLAG1 and HMGA2 genes in lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Acta Ophthalmol. 2018;96(7):e768–e771. [DOI] [PubMed] [Google Scholar]
- 53.Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017;8(1):1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18(8):1048–1055. [DOI] [PubMed] [Google Scholar]
- 55.Matsuyama A, Hisaoka M, Nagao Y, Hashimoto H. Aberrant PLAG1 expression in pleomorphic adenomas of the salivary gland: a molecular genetic and immunohistochemical study. Virchows Arch. 2011;458(5):583–592. [DOI] [PubMed] [Google Scholar]
- 56.Stenman G, Sandros J, Dahlenfors R, Juberg-Ode M, Mark J. 6q- and loss of the Y chromosome--two common deviations in malignant human salivary gland tumors. Cancer Genet Cytogenet. 1986;22(4):283–293. [DOI] [PubMed] [Google Scholar]
- 57.Šteiner P, Andreasen S, Grossmann P, et al. Prognostic significance of 1p36 locus deletion in adenoid cystic carcinoma of the salivary glands. Virchows Arch. 2018;473(4):471–480. [DOI] [PubMed] [Google Scholar]
- 58.Mitani Y, Rao PH, Futreal PA, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17(22):7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drier Y, Cotton MJ, Williamson KE, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rettig EM, Talbot CC, Sausen M, et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev Res (Phila). 2016;9(4):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brill LB, Kanner WA, Fehr A, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. [DOI] [PubMed] [Google Scholar]
- 62.West RB, Kong C, Clarke N, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Persson M, Andrén Y, Moskaluk CA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer. 2012;51(8):805–817. [DOI] [PubMed] [Google Scholar]
- 64.Ho AS, Kannan K, Roy DM, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rettig EM, Tan M, Ling S, et al. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope. 2015;125(9):E292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujii K, Murase T, Beppu S, et al. MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma. Histopathology. 2017;71(5):823–834. [DOI] [PubMed] [Google Scholar]
- 67.Frerich CA, Brayer KJ, Painter BM, et al. Transcriptomes define distinct subgroups of salivary gland adenoid cystic carcinoma with different driver mutations and outcomes. Oncotarget. 2018;9(7):7341–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Togashi Y, Dobashi A, Sakata S, et al. MYB and MYBL1 in adenoid cystic carcinoma: diversity in the mode of genomic rearrangement and transcripts. Mod Pathol. 2018;31(6):934–946. [DOI] [PubMed] [Google Scholar]
- 69.Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12(13):3902–3907. [DOI] [PubMed] [Google Scholar]
- 70.Miyabe S, Okabe M, Nagatsuka H, et al. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma: a molecular and clinicopathologic study of 101 cases. J Oral Maxillofac Surg. 2009;67(7):1432–1441. [DOI] [PubMed] [Google Scholar]
- 71.Nakayama T, Miyabe S, Okabe M, et al. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22(12):1575–1581. [DOI] [PubMed] [Google Scholar]
- 72.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. [DOI] [PubMed] [Google Scholar]
- 73.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122(8):1690–1694. [DOI] [PubMed] [Google Scholar]
- 74.Clauditz TS, Gontarewicz A, Wang CJ, et al. 11q21 rearrangement is a frequent and highly specific genetic alteration in mucoepidermoid carcinoma. Diagn Mol Pathol. 2012;21(3):134–137. [DOI] [PubMed] [Google Scholar]
- 75.Noda H, Okumura Y, Nakayama T, et al. Clinicopathological significance of MAML2 gene split in mucoepidermoid carcinoma. Cancer Sci. 2013;104(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luk PP, Wykes J, Selinger CI, et al. Diagnostic and prognostic utility of Mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):530–541. [DOI] [PubMed] [Google Scholar]
- 77.Saade RE, Bell D, Garcia J, Roberts D, Weber R. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(3):234–240. [DOI] [PubMed] [Google Scholar]
- 78.Birkeland AC, Foltin SK, Michmerhuizen NL, et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. 2017;68:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haller F, Skálová A, Ihrler S, et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am J Surg Pathol. 2019;43(9):1264–1272. [DOI] [PubMed] [Google Scholar]
- 80.Bell D, Ferrarotto R, Liang L, et al. Pan-Trk immunohistochemistry reliably identifies ETV6-NTRK3 fusion in secretory carcinoma of the salivary gland. Virchows Arch. 2020;476(2):295–305. [DOI] [PubMed] [Google Scholar]
- 81.Xu B, Haroon Al Rasheed MR, Antonescu CR, et al. Pan-Trk immunohistochemistry is a sensitive and specific ancillary tool for diagnosing secretory carcinoma of the salivary gland and detecting ETV6-NTRK3 fusion. Histiopathology. 2020;76(3):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung YP, Jo VY, Hornick JL. Immunohistochemistry with a pan-TRK antibody distinguishes secretory carcinoma of the salivary gland from acinic cell carcinoma. Histopathology. 2019;75(1):54–62. [DOI] [PubMed] [Google Scholar]
- 83.Solomon JP, Linkov I, Rosado A, et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available from corresponding author upon reasonable request.


