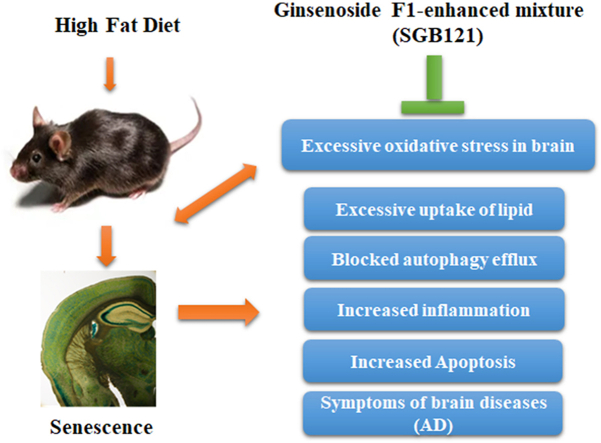
Abstracts
Background
Herbal medicines are popular approaches to capably prevent and treat obesity and its related diseases. Excessive exposure to dietary lipids causes oxidative stress and inflammation, which possibly induces cellular senescence and contribute the damaging effects in brain. The potential roles of selective enhanced ginsenoside in regulating high fat diet (HFD)-induced brain damage remain unknown.
Methods
The protection function of Ginsenoside F1-enhanced mixture (SGB121) was evaluated by in vivo and in vitro experiments. Human primary astrocytes and SH-SY5Y cells were treated with palmitic acid conjugated Bovine Serum Albumin, and the effects of SGB121 were determined by MTT and lipid uptake assays. For in vivo tests, C57BL/6J mice were fed with high fat diet for 3 months with or without SGB121 administration. Thereafter, immunohistochemistry, western blot, PCR and ELISA assays were conducted with brain tissues.
Results and conclusion
SGB121 selectively suppressed HFD-induced oxidative stress and cellular senescence in brain, and reduced subsequent inflammation responses manifested by abrogated secretion of IL-6, IL-1β and TNFα via NF-κB signaling pathway. Interestingly, SGB121 protects against HFD-induced damage by improving mitophagy and endoplasmic reticulum-stress associated autophagy flux and inhibiting apoptosis. In addition, SGB121 regulates lipid uptake and accumulation by FATP4 and PPARα. SGB121 significantly abates excessively phosphorylated tau protein in the cortex and GFAP activation in corpus callosum. Together, our results suggest that SGB121 is able to favor the resistance of brain to HFD-induced damage, therefore provide explicit evidence of the potential to be a functional food.
Keywords: Autophagy, Brain damage, SGB121, High fat diet, Senescence
Graphical abstract
Highlights
-
•
High fat diet induces oxidative stress and subsequent cellular senescence in mice brain.
-
•
High fat diet induces pathologies in cortex and GFAP activation in corpus callosum.
-
•
Ginsenoside F1-enhanced mixture ameliorates damaging effect by modulating autophagy flux and inflammation.
1. Introduction
Obesity is characterized as a chronic disease by the American Medical Association in 2013 [1]. In the United States, one-third of the adult population have a higher body mass index (BMI) than the normal of 30 kg/m2 [2]. Obesity is closely related to other emerging chronic diseases, such as insulin resistance [3], cardiovascular disease [4], metabolic syndrome [5], diabetes and diabetic retinopathy [6], and cancer [7]. Importantly, mortality associated with obesity-challenged disorders is increasing. However, the explicit explanations on the mechanism underlying obesity-related brain damaging effects are yet to be researched.
A key factor affording the prevalence of obesity is the style of diet. Existing researches revealed the vital role played by high-fat diets (HFD) in the development of dementia [8]. Factor found to be influencing the high fat diet linked brain damage have been proposed as oxidative stress [9] and inflammation [10]. Importantly, oxidative stress is the inducer of cellular senescence. Recent researches has demonstrated that accumulation of senescent cells in brain, possibly contributes to disease pathology [11]. Chronic activation of microglia with shortening telomeres, astrocytes with increased level of CDK p16INK4A and IL-6, the distinguished characteristics of senescent cells, are observed from Alzheimer’s disease (AD) and Parkinson’s disease (PD) patients manifested by synaptic impairment and neuronal death [12]. Moreover, a potential contributor to brain inflammation is the direct outcome of senescence associated secretory phonotype (SASP), likely occurring in replication-competent glial cells, which may exacerbate neurodegenerative processes or disrupt the structure and functional connection of neuron-glial [13]. Senescent endothelial cells afford the disruption of the blood-brain barrier (BBB), leading to the influx of peripheral inflammatory factors that could commit subsequent neuron loss [14]. Hence these evidence implicate senescence in the dysfunction of brain and provide senescent cells as a potential target for the novel therapeutic to counteract brain damage.
Preferred minor ginsenosides with less sugar moiety in the structure exert multiple biological functions superior to the enriched ones [15,16]. Despite there is accumulation of literature of ginsenoside on brain function improvement, especially in some aging-related neurodegeneration diseases, such as Alzheimer disease [17] and Parkinson’s disease [18], protective effect of ginsenoside on high fat diet induced damage mainly limited on liver injury and obesity [19]. Researches concerning the HFD impacts on brain still lacks evidence. Additionally, ginseng total extractions are principally used containing less selectively and pharmacologically effective minor ginsenosides. Herein we hypothesized that HFD induced brain impairment may elicit cellular senescence to maintain chronic inflammatory condition and progressive disease occurrence. Furthermore, we demonstrated that the protective effect of SGB121 exerts multiple aspects. These results highlight the potential benefits of SGB121 that targets HFD-induced brain dysfunction.
2. Material and methods
2.1. Chemicals and reagents
Palmitic acid (P0500), Bovine Serum Albumin Solution (A1595) and Triton ™ X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Formaldehyde (16%, 28908) was purchased from Thermo Fisher Scientific (Seoul, Korea Ltd.)
2.2. Preparation of ginsenoside SGB121
Korean ginseng extraction was purchased from Daedong Korean ginseng Co., Ltd (Rg1: 6.4 mg/g, Re: 9.0 mg/g, Rb1: 20.1 mg/g, Rc: 9.6 mg/g, Rb2:8.4 mg/g, Rb3: 2.1 mg/g, Rd: 4.1 mg/kg). The SGB121 was prepared with enzyme as our previously published method [20]. Briefly, total saponin extraction was diluted with distilled water and adjusted the pH value of 6, then subjected to enzyme embedded in bead (alginate and CaCl2) at 25 °C for 24 h. The samples were then freeze-dried to obtain the powder. HPLC analysis indicates that F1 (converted mainly by Rg1) consists of about 0.5 % percentage of total mixture (5 mg/g).
2.3. Animals test
Five-week-old female C57BL/6J mice were purchase from Central Laboratory Animal Inc. (Seoul, Korea) and housed in standard cages with free access to water and food in a room temperature (20 ± 2 °C) with a 12 h light/12 h dark cycle. Mice were accustomed to the laboratory environment for 1 week prior to random division of five groups (8 mice per group): (1) Normal diet-ND; (2) High fat diet-HFD; (3) HFD + Metformin (100 mg/kg); (4) HFD + SGB121 (100 mg/kg); (5) HFD + SGB121 (200 mg/kg). The normal Diet was the 2018S Teklad Global 18 % Protein Rodent Diet (Envigo, IN, USA) and HFD was the DIO Rodent Purified Diet w/60 % Energy from fat – Blue 58Y1(Test Diet, St. Louis, MO, USA). The mice were allowed free access to diets for a total of 12 weeks. SGB121 was administrated orally five times a week for the whole course. Body weights were examined every week. All experimental procedures have been approved by the Institutional Animal Care and Use Committee at Wonkwang University (WKU19-02).
2.4. Immunohistochemistry
Brain tissues from mice sacrificed at the indicated time point were fixed in 4% paraformaldehyde for 24 h and dehydrated with 30 % sucrose prior to OCT freezing. Frozen tissues were cut at 50 μm intervals by a Leica microtome cryostats (CM1850). Sections were permeabilized with 0.3 % Triton X-100 in PBS, saturated with 3 % goat serum (Thermo Fisher Scientific, Seoul, Korea), and incubated with phosphorylated tau, GFAP and Iba-1 antibodies (1:200, Cell Signaling Technology, Danvers, MA) at 4 °C overnight, followed by rinses with PBS and incubation with Alexa fluorescein-labeled secondary antibodies (Thermo Fisher Scientific, Seoul, Korea) for 1 h and mounting using Prolong anti-fade medium (Thermo Fisher Scientific, Seoul, Korea).
2.5. Biochemical analysis
Brain tissues from mice sacrificed at the indicated end point were snap-frozen in liquid nitrogen and lysed (RIPA buffer, Thermo Fisher Scientific, Seoul, Korea) before use. Homogenate was centrifuged at 15000 rpm for 30 min at 4 °C. The upper layer was transferred into new containers and subjected to protein assay (Bradford method, Life Science, CA). A total 400 μg of proteins were used in each individual target.
Analyses for Malondialdehyde (MDA, ab118970) and reduced glutathione (GSH, ab235670) from Abcam Company (Cambridge, UK) in tissues were performed according to manufacturer’s manuals.
Enzyme-linked immunosorbent assays (ELSIA) kits to detect TNF-α, IL-6 and IL-1β were purchased from R&D Systems (Minneapolis, MN) and explored according to the manufacture’s protocols. Signals were detected by Tecan plate reader (Männedorf, Zürich, Switzerland).
Antibody arrays kits to detect apoptosis and NF-κB activation purchased from R&D Systems (Minneapolis, MN) and cytokine secretion purchased from RayBiotech (Norcross, GA), were used according to the manufacture’s protocols. Brain lysates (200-500 μg) of mice were obtained from snap-frozen samples in liquid nitrogen and applied to array tests. The signals were detected with Odyssey-LC chemiluminsescent imaging system (LI-COR, Lincoln, NE). Signals were averaged and expressed as described in figure legend.
2.6. Telomere length assay
The hippocampi were dissected from brain and applied to genomic DNA extraction according to the kit’s protocol (Qiagen, Hilden, Germany). The telomere length was assayed with qPCR Assay kit (ScienCell, M8918, Carlsbad, CA) and qPCR master mix (Roche, #06402712001, Basel, Switzerland). Briefly, 2 ng genomic DNA template was well mixed with telomere (Tel) or single copy reference (SCR) primer and qPCR master mix in 20 μl reaction volume. The processing for qPCR program was as follows: Initial denaturation 95 °C for 10 min, denaturation 95 °C for 20 seconds, annealing 52 °C for 20 seconds, and extension 72 °C for 45 seconds. The total number of cycles was 32. Relative telomere length of the target sample to the reference sample was expressed by fold, which is equal to 2-(ΔCq Tel− ΔCq SCR).
2.7. SA-β-galactosidase staining
Brain sections were fixed with 0.5 % Glutaraldehyde, washed with 1mM MgCl2 pH 6.0 and stained with X-Gal staining solution (Cell Signaling Technology, Danvers, MA). Sections were incubated overnight at at 37°C. The images were obtained and analyses were performed with software from Nikon Ni2 microscopy (Melville, NY).
2.8. Palmitic acid preparation
Briefly, palmitic acid (PA) powder was dissolved with ethanol to a final concentration of 200 mM at 70 °C. After that, palmitic acid solution was diluted with 10 % fatty acid-free BSA to 5 mM at 55 °C for 20 min. The conjugated solution (PA-BSA) was cooled to room temperature, and further filtrated with 0.45-μm pore membrane filter. The solution was store at −80°C prior to use.
2.9. Cell culture and treatment
Human primary astrocytes (Lonza, CC-2565, Alpharetta, GA) were maintained with medium (Lonza, CC-3186, Alpharetta, GA) at 37 °C in a humidified 5 % CO2 atmosphere. Samples were collected from cells lower than 5 passages. SH-SY5Y cells (ATCC) were maintained with DMEM high glucose medium (Thermo Fisher Scientific, Seoul, Korea) with 10 % FBS, at 37 °C in a humidified 5 % CO2 atmosphere.
For PA-BSA treatment, cells were exposed to SGB121 (10 μg/mL) for 12 h, followed by 100 μM PA-BSA stimulation for extra 48 h. Control cells were treated with corresponding concentrations of BSA. Cell proliferation was checked using MTT (Promega, Madison, WI) method.
For intracellular lipid droplets assay, PA-provoked cells treated with or without SGB121 (10 μg/mL) were incubated with 5 μM LipidSpot Lipid Droplet Stain (Biotium, Fremont, CA) at 37 °C for 30 min. Then cells were imaged with Fluorescent Microscopy (Nikon, Ni2, Melville, NY). Lipid accumulation was evaluated and expressed as relative fold of fluorescence density.
2.10. Immunoblot analysis
Lysates from cells and tissues were subjected to 10 % SDS-PAGE (Bio-Rad, Pre-gel, MA); separated proteins were transferred onto Polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific, Seoul, Korea). Membranes were blocked with TBS-based blocking buffer (Li-COR Biosciences, Lincoln, NE) for 1h at room temperature, and incubated overnight at 4 °C with primary antibodies p16, SOD1,SOD2, ERK, p-ERK, CREB, p-CREB, p-elF2α, Beclin1, Bip, ATG12, LC3B, p62, BNIP3, PINK1, Parkin, PPARα, FATP4, Tau, P-tau, Beta-amyloid, GFAP, iba-1, beta-actin (1:1000, Cell Signaling Technology, Danvers, MA). Membranes were then washed with TBST three times for 10 min each and incubated with horseradish peroxidase-conjugated secondary antibodies (1:3000, Cell Signaling Technology, Danvers, MA) for 1 h at room temperature and washed again. Signals were detected by Odyssey-LC chemiluminsescent imaging system (LI-COR, Lincoln, NE). Signals were averaged and expressed as described in figure legend.
2.11. Statistical analysis
Data are presented as mean ± SEM for all data. All statistical analyses are performed using GraphPad Prism 7.01 and a P value < 0.05 was considered as significant. For group comparisons, one-way ANOVA with Tukey’s multiple comparison test was used.
3. Results
3.1. SGB121 mitigated high-fat-diet induced oxidative stress and cellular senescence in brain
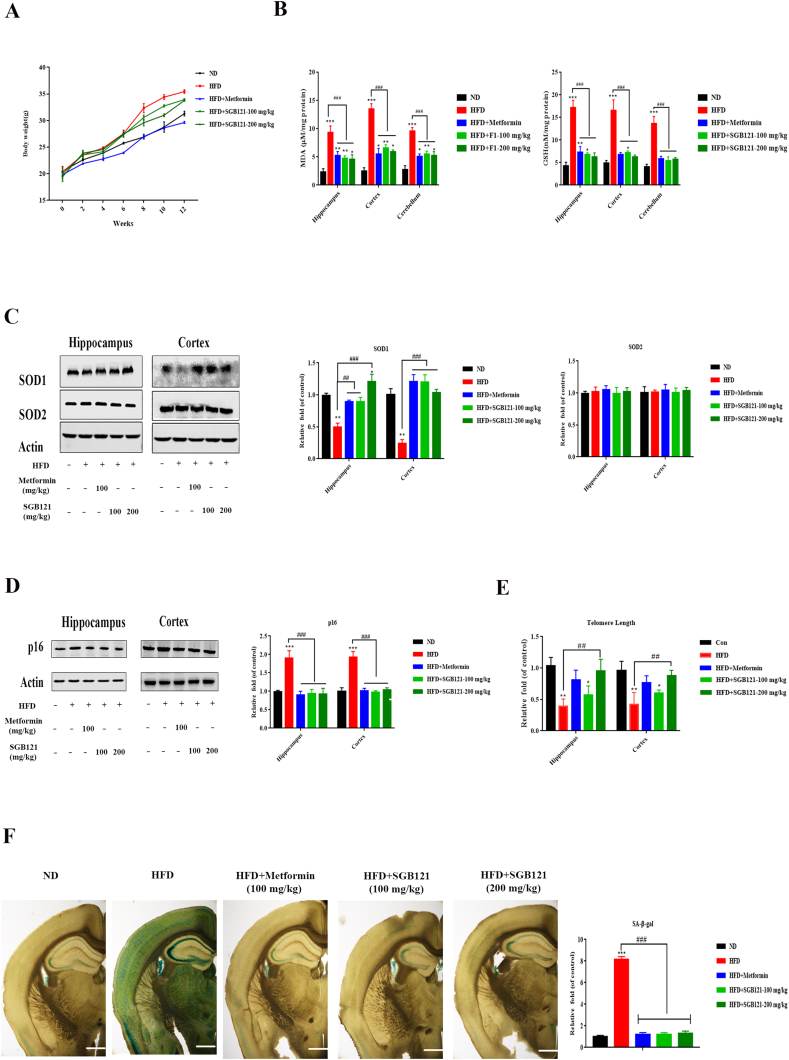
To evaluate the protective effect of SGB121 on high-fat-diet-fed mice, 5-week-old mice were challenged with a HFD (60 % kcal from fat) for 3 months. Interestingly, among all the groups, only positive control metformin (100 mg/kg body weight) statistically reduced the weight gain (Fig. 1A), suggesting SGB121 treatment did not improve the obesity in the present model. First, the oxidative marker MDA in brain was significantly increased in all indicated regions hippocampus, cortex and cerebellum, while these stimuli were profoundly suppressed by both doses of SGB121 (100, 200 mg/kg body weight) accompanied by restored reduced GSH content as seen in Fig. 1B. Next, the level of antioxidant enzymes such as SOD1 and SOD2 were analyzed by immunoblot method. Expectedly, the expression of SOD1 in hippocampus was suppressed by 0.4 fold in HFD compared with ND. In addition, both doses of SGB121 had increased level of SOD1, suggesting a protective action on antioxidant enzyme. Unchanged level of SOD2 were observed in the present study (Fig. 1C). Oxidative stress is proposed as one of the inducers for cellular senescence, which is causally linked to some tissue damages. We next checked whether HFD challenge induces cellular senescence in brain. Protein level of senescence marker p16 has similar patterns in both cortex and hippocampus with significant increase in HFD-fed mice. SGB121-treated mice have a dramatic reduction in comparison with HFD mice (Fig. 1D). Additionally, another typical marker of senescence, telomere length, was measured. On HFD, mice have a 0.6 fold shortening in telomere length, with a 0.4 fold shortening in 100 mg/kg SGB121. However, 200 mg/kg SGB121 have comparatively similar telomere length to ND mice (Fig. 1E). Similarly, on HFD, mice have 8-fold increase in SA-β-gal positive staining cells (Fig. 1F). Collectively, HFD induces oxidative stress and displays the hallmarks of cellular senescence in brain. This detrimental responses on HFD can be mitigated by SGB121 treatment.
Fig. 1.
SGB121 alleviated HFD-induced oxidative stress and subsequent cellular senescence in mouse brain. (A) Effects on Body weight gain. (B) MDA and reduced GSH content in brain lysates. (C) Protein level of SOD1 and SOD2. (D) Protein level of p16. (E) Effects on telomere length. (F) SA-β-gal staining and quantification. (40×). Actin was used as loading control. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD. Scale bar, 100 μm.
3.2. SGB121 suppressed high-fat-diet induced inflammation in brain
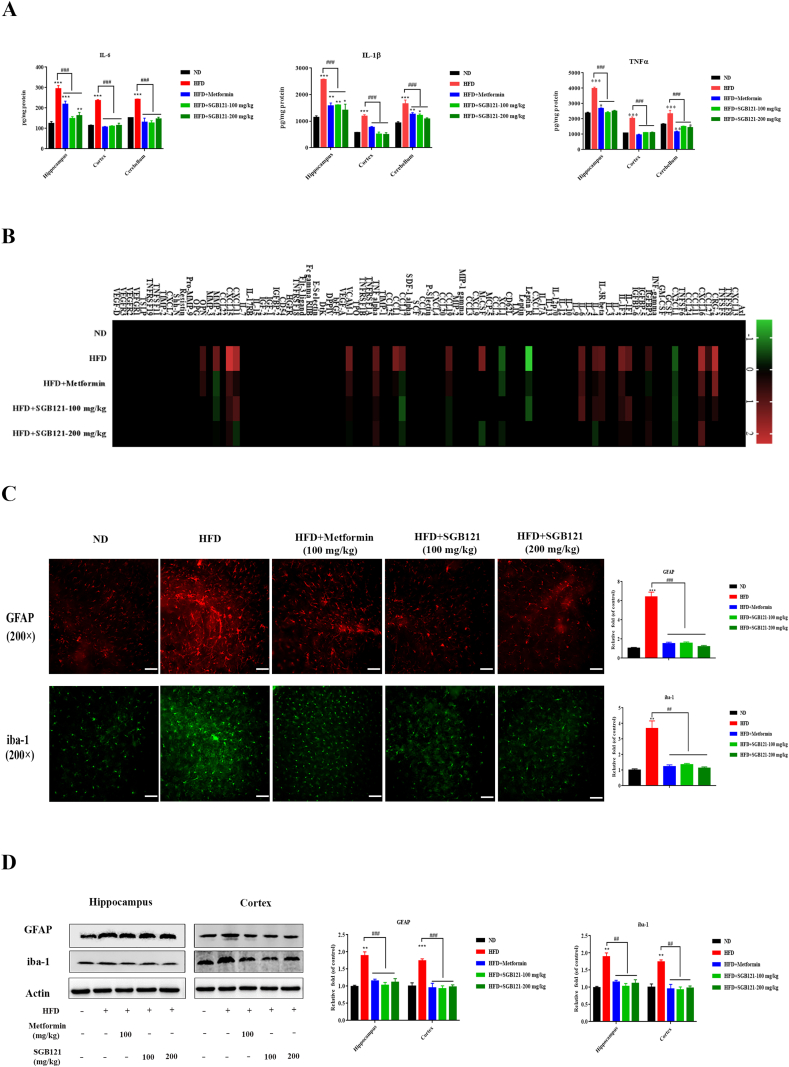
SASP contributes to brain inflammation. As expected, typical inflammatory cytokines such as IL-6, IL-1β and TNFα have significantly higher level in HFD mice than ND mice (Fig. 2A). This apparent inflammatory response can be partially inhibited by SGB121 treatment. Interestingly, response in hippocampus is much more robust than that in cortex. To investigate the other attributive cytokines in this response, hippocampus was further applied to antibody array with 96 mouse cytokines. On HFD, of factors detected by arrays and secreted at significant level are CRG-2, CXCL16, IL-1F2, CCL27, CXCL16, CX2CL1, IGFBP3, IL-1F1, IL-1F2, IL-3R beta, IL-4, IL-6, Leptin R, XCL1, M-CSF, CCL19, CCL17, CCL1, TNFα, VCAM-1, CXCL-11, CXCL-15, MMP-2 and OPN (Fig. 2B), which have been reported to be vigorously secreted by senescent, relative to proliferative or quiescent cells. SGB121 reduced the secretion of 24 factors, including several pro-inflammatory cytokines, chemokines and growth factors. Senescent glial cells contributes to inflammation in brain. On HFD mice, glial cells, as determined by strong GFAP and iba-1-positivity, demonstrated increased size and flat morphology, characteristics of senescent cells in hippocampus CA3 subregions (Fig. 2C). Concomitantly, western blot analysis also confirmed the glial cell activation in brain (Fig. 2D). Interestingly, SGB121 treatment partially suppressed the cytokine secretion and glial cell activation, and even rescued to ND level in certain factors.
Fig. 2.
SGB121 inhibited HFD-induced inflammation in mouse brain. (A) ELISA assay of IL-6, TNF-α and IL-1β in brain lysates. (B) Mouse cytokine array analysis. Levels of each protein in ND were arbitrary set to zero. Data shown represent log2-fold change in expression relative to ND. Signals higher than ND were shown in red; signals lower than ND were shown in green. (C) IHC staining for GFAP and iba-1. (200×) (D) Representative western blots of GFAP and iba-1. Actin was used as loading control. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD. Scale bar,100 μm.
3.3. SGB121 inhibited high-fat-diet induced apoptosis in brain
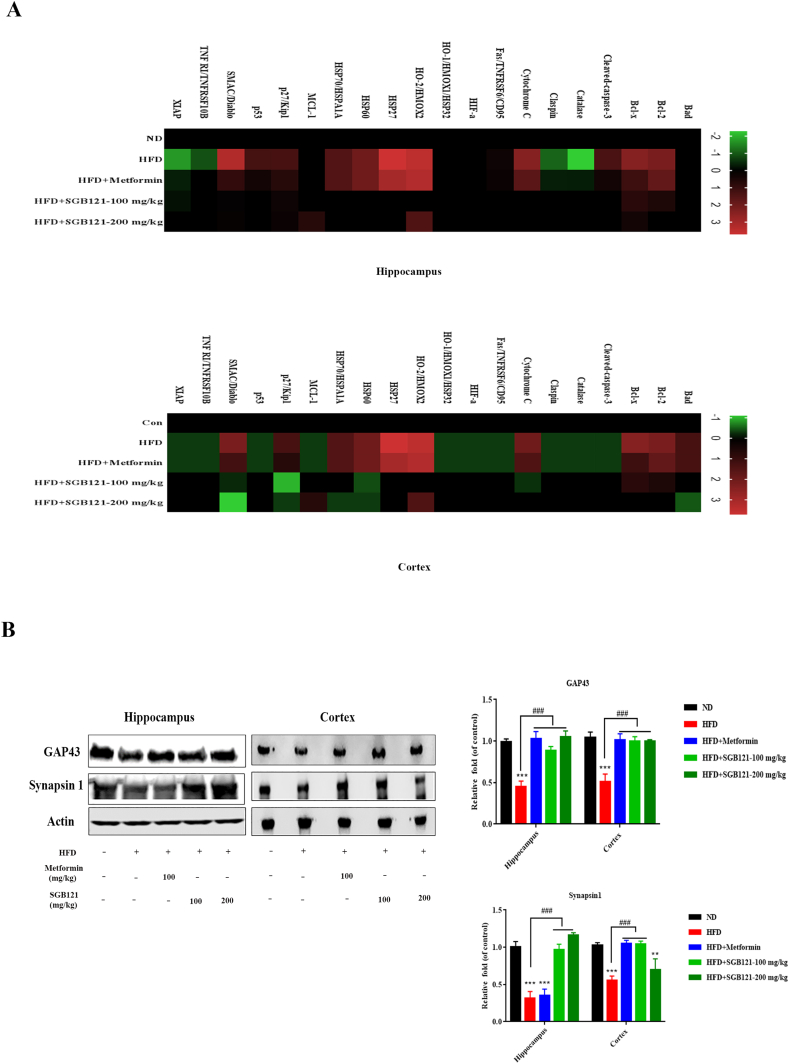
The apoptosis array was applied to understand which related factors were activated in apoptosis pathway. On HFD, most factors detected by arrays and expressed at significant level except Bad, Fas/TNFRSF6/CD95, HIFα, and HO-1 in hippocampus whereas all the factors altered in cortex (Fig. 3A). Interestingly, apart from the well-known apoptosis regulated proteins, heat shock proteins such as HSP27, HSP60 and HSP70 were noticeably increased about 3-5 fold in HFD mice, suggesting profound oxidative stress. Additionally, up-regulated expression of SMAC and Cytochrome C demonstrated typical mitochondrial damage. As expected, SGB121 treatments at both doses decreased these apoptosis associated factors, signifying a substantial protection effect in mitochondrial. Mitochondrial damage and subsequent apoptosis may be the direct cause of neurodegeneration in HFD mice, hence we examined neuron marker proteins GAP43 and synapsin 1. Correspondingly, reduced neuron markers in HFD mice were observed as evidenced by western blot analysis (Fig. 3B). Proteins responsible for axonal growth and neural network formation were up-regulated in SGB121-fed mice in comparison to HFD mice, and restored to ND levels. However, high dose of SGB121 showed more selectively protection of GAP43 other than synapsin 1.
Fig. 3.
SGB121 suppressed HFD-induced apoptosis in mouse brain. (A) Apoptosis array analysis. Levels of each protein in ND were arbitrary set to zero. Data shown represent log2-fold change in expression relative to ND. Signals higher than ND were shown in red; signals lower than ND were shown in green. (B) Brains were monitored for the GAP43 and Synapsin1 proteins by western blot. Actin was used as loading control. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD.
3.4. SGB121 alleviated high-fat-diet induced pathologies in brain
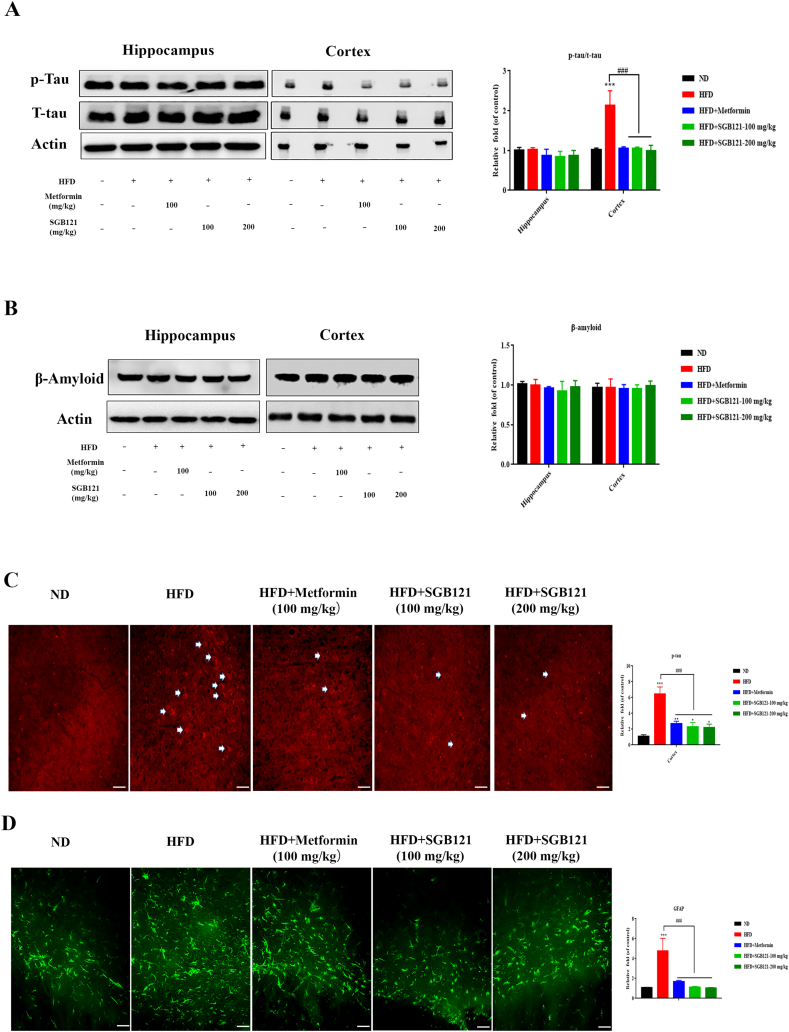
Neuronal apoptosis is frequently observed in neurodegeneration disease, thus we first checked the changes in hallmarks of Alzheimer’s disease (AD), tau and beta-amyloid proteins. SGB121 treatment appeared to powerfully suppress tau phosphorylation confirmed by western blot analysis (Fig. 4A). However, the protein levels of beta-amyloid showed little change in the present study (Fig. 4B). Similarly, phosphorylated tau protein was evidently increased in the cortex of mice fed on HFD manifested by immunohistochemistry analysis (Fig. 4C). Surprisingly, large accumulations agglomerations of astrocytic processes in while matter (corpus callosum) which is GFAP positive were observed in HFD mice, suggesting a potential detrimental condition of neuronal degeneration (demyelination) in brain. This change was partially ameliorated by SGB121 treatment (Fig. 4D).
Fig. 4.
SGB121 improved HFD-induced pathologies in mouse brain. (A) Western blots for total and phosphorylated tau protein. (B) Western blots for beta-amyloid protein. (C) HFD-induced changes on tau protein in mouse brain. Phosphorylated tau were analyzed by IHC method. (D) GFAP staining in white matter (corpus callosum) (200×). Actin was used as loading control. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD. Scale bar,100 μm.
3.5. SGB121 conferred resistance to HFD-induced detrimental effect by regulating multiple pathways
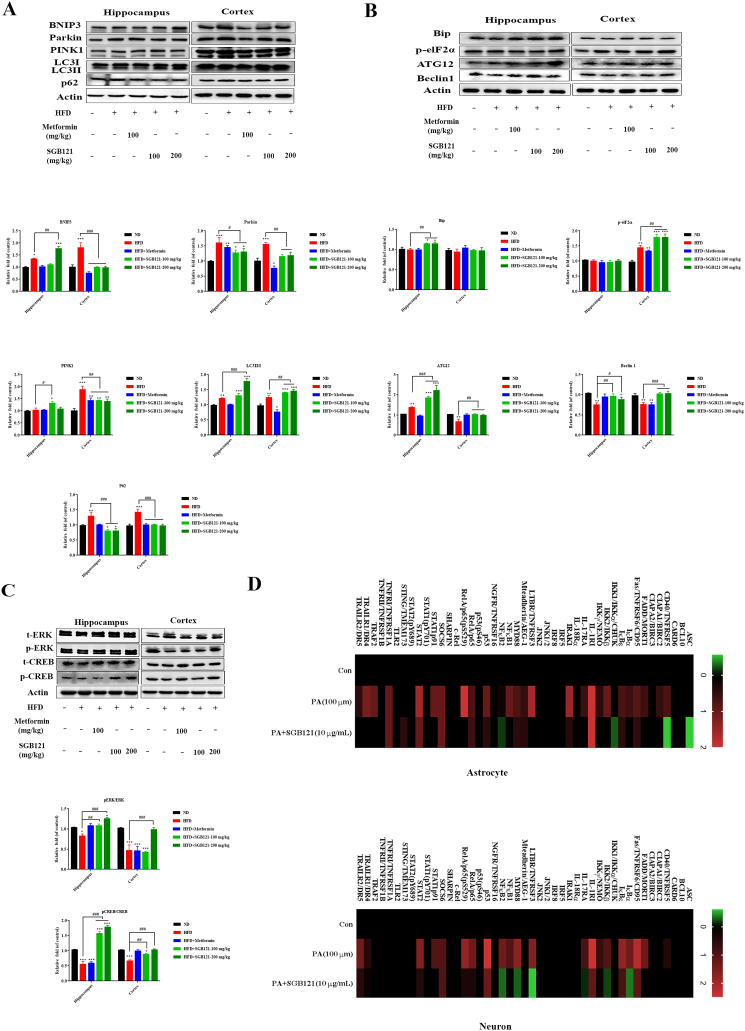
Autophagy may suppress cellular senescence by removing damaged macromolecules or organelles. In HFD mice, mitophagy promoting proteins BNIP3, PINK1 and Parkin were increased, suggesting severe mitochondrial damage. Similarly, protein phosphorylated elF2α was robust in all HFD mice, indicating increased endoplasmic reticulum (ER) stress. However, efficient autophagic flux was only observed in SGB121-fed mice, as an impaired one induced by HFD was evidenced by decreased level of ATG12 and Beclin1, less LC3 conversion and significant accumulation of p62 (Fig. 5A and B). Together, the results note that accumulation of damaged mitochondrial and ER, and disrupted autophagy flux were introduced in HFD mice, leading to the senescent cell accumulation in brain. Importantly, this damaging effect was efficiently improved by SGB121.
Fig. 5.
SGB121 regulating multiple pathways to suppress HFD-induced dark effects in mouse brain. (A) Western blot analysis for indicated proteins BNIP3, Pink1, Parkin, LC3, p62 in autophagy pathway. (B) Representative blots for Bip, phosphorylated elF2α, Beclin 1 and ATG12 in ER stress-associated autophagy pathway. (C) Blots for ERK and CREB protein. (D) NF-κB pathway array analysis. Levels of each protein in ND were arbitrary set to zero. Data shown represent log2-fold change in expression relative to ND. Signals higher than ND were shown in red; signals lower than ND were shown in green. Actin was used as loading control. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD.
Secondly, a transcription factor CREB in brain was further explored in HFD challenged mice. As expected, phosphorylation of CREB was powerfully suppressed on HFD, accompanied by reduced upstream regulator ERK proteins.SGB121 treatment fundamentally reversed these changes, which might prevents HFD induced apoptosis in brain (Fig. 5C).
Thirdly, NF-κB signaling pathway commonly modulates the inflammation and cytokine secretions in senescent cells. Thus we applied antibody array of NF-κB to confirm the involved proteins in the signaling pathway using primary human astrocytes and SH-SY5Y cells. In PA-BSA stimulated samples, highly expressed level of protein TNFRSF5, CIAPA1, FADD, TNFRSF6, IκBα, IκBε, IKKβ, IL-1RI, IL-17RA, IRAK1,TNFRSF3, AEG1, MYD88, NFκB1, p53, p65, p65(pS529), SOCS6, STAT1p91, STAT2, TNFRSF1A, TRAF2, TRAILR1 and TRAILR2 were detected. SGB121 more effectively inhibits these changes with less suppressions on IL-1RI, AEG1, MYD88, SOCS6 and TNFRSF1A in human astrocytes; For provoked neurons, TNFRSF5, FADD, CD95, IκBα, IκBε, IKK2, NEMO, IL-1RI, IL-17RA, TNFRSF3, MYD88, NFκB2, p53, p65, SOCS6, STAT1p91, STAT2, TRAILR1 and TRAILR2 were significantly increased, while SGB121 prominently suppressed the upregulation proteins, excluding SOCS6 (Fig. 5D).
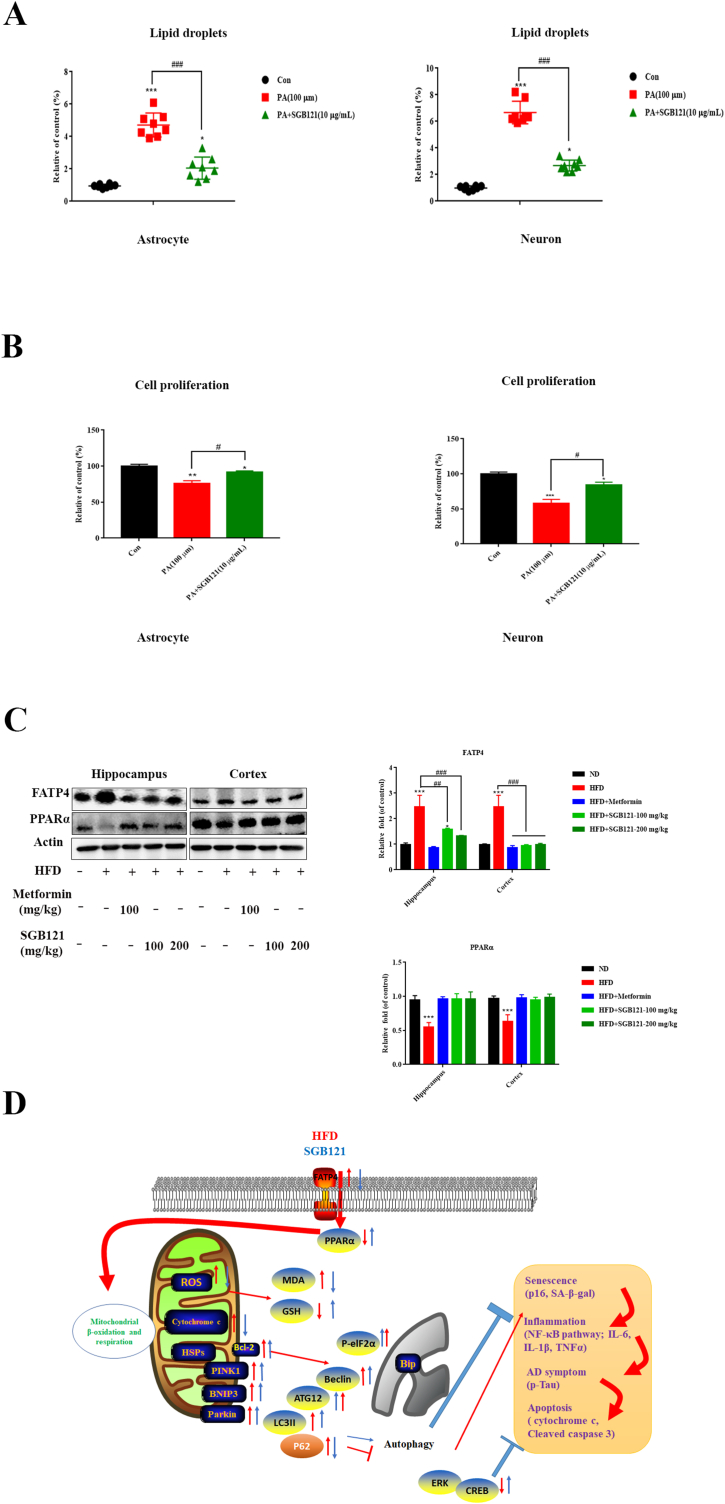
Lipotoxicity could be observed in neurodegenerative diseases and induces cellular damage and disrupt cellular homeostasis. Immensely accumulation of lipid droplets in human primary astrocyte and SH-SY5Y cells were observed, which was partially rescued by SGB121 pretreatment (Fig. 6A). Consequently, cellular senescence was induced confirmed by significant cell growth arrest (Fig. 6B). Fatty acid (FA) transporters play a key role in facilitating FA uptake into neurons and astrocytes. Significant increase in FATP4 protein was observed in hippocampus of HFD mice as expected. SGB121 treated mice had a similar level compared to ND mice in both cortex and hippocampus. Peroxisome proliferator-activated receptor α (PPARα), a transcription factor, which is reported to control the lipid catabolism, was remarkably suppressed by HFD (2.2 fold). Importantly, SGB121 treatment restored the expression level comparable to non-treated ones (Fig. 6C).
Fig. 6.
SGB121 prevented excessive lipid uptake and accumulation. (A) Lipid droplets staining in human primary astrocyte and neuron cells. (B) Cell proliferation assay by MTT (C) Western blots for FATP4 and PPARα protein. Actin was used as loading control. (D) Schematic explanation of protection effects of SGB121 in HFD mice brain. ∗ indicates p<0.05 versus ND, ∗∗∗ indicates p<0.001 versus ND, # indicates p<0.05 versus HFD, ## indicates p<0.01 versus HFD.
4. Discussion
Consuming high fat diet induces pernicious effects on brain and many herbals are reported to prevent this dark side, but the contributing factors and explicit mechanism by which natural product regulated HFD-caused overall changes in brain remain largely unknown. Importantly, few studies have documented the protective effect of ginseng (ginseng berry extraction and Re) on HFD-induced brain impairment via protecting the cholinergic and antioxidant system in mouse brain [21,22]. In this study, we first explored that SGB121 successfully ameliorated the detrimental actions induced by HFD, with suppressed oxidative stress, cellular senescence and subsequent inflammation. Surprisingly, we discovered that on HFD mice, such symptoms of Alzheimer’s disease, accumulation of phosphorylated tau protein was observed. In vitro tracking of lipid droplets demonstrated that astrocyte and neuron are vulnerable to overdose fatty acid. When challenged with SGB121, these mice exhibited less oxidative stress and lower inflammation level, showing reduced cellular senescence manifested by improved hallmarks SA-β-Galactosidase, telomere length and p16 level.
The catabolism of fatty acids links with peroxisomal β-oxidation and mitochondrial pathways [23]. Excess free fatty acids critically leads to reactive oxygen species (ROS) generation and consequent lipotoxicity associated with ER stress and mitochondrial dysfunction [24]. Oxidative stress was significantly generated in the brain of mice on HFD, which was remarkably suppressed by SGB121. As excess free fatty acid can result in mitochondrial ROS production and subsequent oxidative stress by inactivating the regeneration of reduced glutathione (GSH) from its oxidized form (GSSG) [25], in addition to the suppressed protein level of SOD1, we hypothesized that SGB121 may protect brain from HFD-induced oxidative stress partially by mediating the antioxidant system. Oxidative stress induces damaged mitochondria and ER stress that characterize cellular senescence, which is detrimental to cellular and tissue physiology [26]. It may also trigger telomere shortening and dysfunction, another hallmark for senescence [27], which is consistent with our results in the present study.
Autophagy is commonly believed to remove stressors and damaged organelles, promote cell survival and facilitating bioenergetics homeostasis [28]. Interestingly, autophagy can be activated by cellular senescence. However, in the case of cellular senescence, autophagy regulation may be different. One research group described the first instance of autophagic degradation for nuclear components and inhibition of autophagy attenuated oncogene-induced senescence [29]. Nonetheless, present results demonstrated disrupted autophagy in HFD mice, which was restored in SGB121-fed groups. This anti-senescence autophagy is rational and supported by recent researches. Parkin-mediated mitophagy, selective removed dysfunctional mitochondria, suppresses several characteristic of cellular senescence [30]. In addition, restoring autophagic activity under oxidative stress condition precisely reduces cell senesce in SH-SY5Y cells [31]. Hence, our mice model in HFD challenge supported that autophagy impairment induces senescence in brain and that is possibly a target for SGB121 protection.
Cellular senescence and SASP can alter the neighboring environment and maintain chronic inflammatory condition, thereby accelerating disease progress [32]. Accordingly, on HFD mice, typical cytokines including IL-6, TNF-α and IL-1β were highly secreted compared with ND mice as shown by ELISA analysis. Additionally, mouse cytokine arrays provided more detailed information. Of chemokines detected at high level are playing pivotal role in mediating the influx of inflammatory leukocytes into the CNS in a number of important diseases [33]; molecules such as VCAM-1 and MMP-2, which indicate inflammatory blood brain barrier pathology [34]; IGFBP-3 which is commonly released by astrocytes in the pathology of Alzheimer’s disease [35]; cytokines M-CSF, IL-1alpha, and IL-3R beta, which are pro-inflammatory cytokines released from microglia associated with inflammation in CNS [36]. Importantly, these secreted cytokines in the present study are reportedly contribute to SASP development and tissue functional decline [37], which are possibly released by activated and senescent glial cells. The SASP induced by high fat diet is greatly attenuated by SGB121. More importantly, SASP factors are also reportedly induced by NF-κB signaling [38,39]. In the present study, twenty-five proteins addressed in NF-κB signaling were identified at significantly elevated level in human astrocytes challenged by overloaded fatty acid, whereas 19 of those were reduced by SGB121. For neurons, 19 upregulated proteins were detected, with only SOCS6 unchanged by SGB121. Especially, these altered regulators have convincingly demonstrated that NF-κB signaling promotes the presence of SASP in senescence. Such as NEMO and p53 activation triggered by DNA damage response [40], some TLRs and TNFR receptors [41], and IL-1R [42]. Together, these results clearly indicated that SGB121 suppresses inflammation by repressing HFD-induced SASP in brain cells.
Prior studies have noted the importance of cell death in hippocampus, cortex and hypothalamus in HFD mice [[43], [44], [45]]. What surprising is that increase of heat shock proteins HSP27, HSP60 and HSP70 were found in HFD mice. These outcomes is contrary to those previous researches that HSPs is generally associated with increased neuronal resistance to cerebral injuries and better recovery [46,47]. However, this elevations are in agreement with other researches which found abnormal level of HSP60 expression was secreted by microglia, causing neuronal death [48] and increased HSP27 drives IL-6 and IL-1 mediated pro-inflammatory cell signaling [49,50]. Here, we infer that these changes are the initial responses of oxidative stress and inflammation, which may not closely associated with anti-apoptosis. Furthermore, we checked the changes of CREB and its upstream kinase ERK of which activation are linked neuroprotection in many experimental animal models [51]. Both phosphorylation of the proteins were suppressed in HFD mice, thereby preventing the cellular survival. Correspondingly, SGB121 potentially ameliorate apoptosis by targeting these factors.
With respect to chronic inflammation and apoptosis in brain tissue, we conjecture that HFD could cause neurodegeneration conditions. Thus we examined the hallmarks of Alzheimer’s disease, beta amyloid and tau proteins. Intriguingly, this study did not show any statistical changes in the expression level of beta amyloid, but did implicate significant accumulation of phosphorylated tau protein. SGB121 evidently alleviated the symptoms of Alzheimer’s disease, which also supported our previous work that F1 improves cognition in APP/PS1 transgenic mouse [52]. Another important finding was that hypertrophic GFAP-positive processes were observed in while matter (corpus callosum) in HFD mice, suggesting a potential myelin pathology in brain. However, further identifications are warranted for characterization of this white matter disorders.
The study further checked the uptake of free fatty acid in a model of astrocyte and neuron cells, and SGB121 significantly inhibited the lipid droplet accumulation. Accordingly, protein FATP4, which is responsible for lipid uptake and transport [53], remarkably increases in HFD mice, whereas similar level to ND mice was observed in SGB121-fed mice. Another important regulator for lipid metabolism, PPARα likewise reduced in HFD mice with mitigation in SGB121 treated ones. Thus, senescence is induced in astrocyte and neuron probably via FA uptake, and partially interfered with FATP4 and PPARα, which is another potential target of SGB121.
Robust senescence hallmarks are expressed in HFD-challenged brain, this could develop severe inflammation via NF-κB signaling, neurodegenerative disease and apoptosis. However, present study has not yet established a direct link for a relationship during this damaging effect. Targeted elimination of senescent cells is warranted option for future study.
These findings raise intriguing connections regarding the cellular senescence and HFD associated dark effects including neurodegeneration disease. The protection may be, in part, from multiple aspects encompassing limited lipid uptake and transport, reduced oxidative stress, improved autophagy flux, suppressed inflammation and repressed apoptosis (Fig. 6D). This study may shed light into understanding the underlying mechanism of HFD-induced brain impairment and propose a potential health food candidate.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Intelligent Synthetic Biology Center of the Global Frontier Project, funded by the Ministry of Education, Science and Technology (2011-0031955), Republic of Korea. Bio-Synergy Research Project (NRF-2021M3A9C4001028) of the Ministry of Science, ICT, Republic of Korea.
References
- 1.Seidell J.C. Obesity, insulin resistance and diabetes—a worldwide epidemic. Br J Nutr. 2000;83:S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 2.Flegal K.M. Body mass index of healthy men compared with healthy women in the United States. Int J Obes. 2006;30:374–379. doi: 10.1038/sj.ijo.0803117. [DOI] [PubMed] [Google Scholar]
- 3.Grundy S.M. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 4.Klein R., Klein B.E., Moss S.E. Is obesity related to microvascular and macrovascular complications in diabetes?: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Intern Med. 1997;157:650–656. [PubMed] [Google Scholar]
- 5.Kyle T.K., Dhurandhar E.J., Allison D.B. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin North Am. 2016;45:511–520. doi: 10.1016/j.ecl.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaven G., Abbasi F., McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–224. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 7.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 8.Cholerton B., Baker L.D., Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719:170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzoubi K.H., Khabour O.F., Salah H.A., Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiol Behav. 2013;119:72–78. doi: 10.1016/j.physbeh.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Dalvi P.S., Chalmers J.A., Luo V., Han D.Y., Wellhauser L., Liu Y., et al. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes. 2017;41:149–158. doi: 10.1038/ijo.2016.183. [DOI] [PubMed] [Google Scholar]
- 11.Baker D.J., Petersen R.C. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinta S.J., Woods G., Rane A., Demaria M., Campisi J., Andersen J.K. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiBattista A.M., Sierra F., Masliah E. NIA workshop on senescence in brain aging and Alzheimer’s disease and its related dementias. GeroScience. 2020;42:389–396. doi: 10.1007/s11357-020-00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves S.I., Baker D.J. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin Pharmacol Toxicol. 2020;127:102–110. doi: 10.1111/bcpt.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.E., Lee Wh, Yang Sm, Cho S.H., Baek M.C., Song G.Y., Bae J.S. Suppressive effects of rare ginsenosides, Rk1 and Rg5, on HMGB1-mediated septic responses. Food Chem Toxicol. 2019;124:45–53. doi: 10.1016/j.fct.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 16.Hou J.G., Yun Y.J., Xue J.J., Jeon B.M., Kim S.C. Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytother Res. 2020;34:1659–1669. doi: 10.1002/ptr.6636. [DOI] [PubMed] [Google Scholar]
- 17.Fang F., Chen Xc, Huang Tw, Lue L.F., Luddy J.S., Yan S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim Biophys Acta-Mol Basis Dis. 2012;1822:286–292. doi: 10.1016/j.bbadis.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L., Chen W.F., Wong M.S. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson’s disease through the IGF-I receptor signalling pathway. Br J Pharmacol. 2009;158:738–748. doi: 10.1111/j.1476-5381.2009.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z.P., Ji G.E. Ginseng and obesity. J Ginseng Res. 2018;42:1–8. doi: 10.1016/j.jgr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui C.H., Jeon B.M., Fu Yy, Im W., Kim S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl Microbiol Biotechnol. 2019;103:7003–7015. doi: 10.1007/s00253-019-09951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park C.H., Park S.K., Seung T.W., Jin D.E., Guo T.J., Heo H.J. Effect of ginseng (Panax ginseng) berry EtOAc fraction on cognitive impairment in C57BL/6 mice under high-fat diet inducement. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/316527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.M., Park C.H., Park S.K., Seung T.W., Kang J.Y., Ha J.S., et al. Ginsenoside re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J Agric Food Chem. 2017;65:2719–2729. doi: 10.1021/acs.jafc.7b00297. [DOI] [PubMed] [Google Scholar]
- 23.Wanders R.J.A., Ruiter J.P.N., Ijlst L., Waterham H.R., Houten S.M. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada L.S.D.R., Oliveira C.P., Stefano J.T., Nogueira M.A., da Silva I.D.C.G., Cordeiro F.B., et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH - proteomic and lipidomic insight. Clin Nutr. 2018;37:1474–1484. doi: 10.1016/j.clnu.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Pike L.S., Smift A.L., Croteau N.J., Ferrick D.A., Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta-Bioenergetics. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Burton G.J., Yung H.W., Murray A.J. Mitochondrial–endoplasmic reticulum interactions in the trophoblast: stress and senescence. Placenta. 2017;52:146–155. doi: 10.1016/j.placenta.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Wang Lh, Wang Zg, Liu J.P. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells. 2019;8:54. doi: 10.3390/cells8010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doherty J., Baehrecke E.H. Life, death and autophagy. Nat Cell Biol. 2018;20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou Zx, Ivanov A., Adams P.D., Berger S.L. Mammalian autophagy degrades nuclear constituents in response to tumorigenic stress. Autophagy. 2016;12:1416–1417. doi: 10.1080/15548627.2015.1127465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korolchuk V.I., Miwa S., Carroll B., Von Zglinicki T. Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nopparat C., Sinjanakhom P., Govitrapong P. Melatonin reverses H2O2-induced senescence in SH-SY 5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-κB. J Pineal Res. 2017;63 doi: 10.1111/jpi.12407. [DOI] [PubMed] [Google Scholar]
- 32.Davalos A.R., Coppe J.P., Campisi J., Desprez P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glabinski A.R., Tani M., Aras S., Stoler M.H., Tuohy V.K., Ransohoff R.M. Regulation and function of central nervous system chemokines. Int J Dev Neurosci. 1995;13:153–165. doi: 10.1016/0736-5748(95)00017-b. [DOI] [PubMed] [Google Scholar]
- 34.Ravindran J., Agrawal M., Gupta N., Rao P.L. Alteration of blood brain barrier permeability by T-2 toxin: role of MMP-9 and inflammatory cytokines. Toxicology. 2011;280:44–52. doi: 10.1016/j.tox.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Rensink A.A., Gellekink H., Otte-Höller I., Hans J., de Waal R.M., Verbeek M.M., Kremer B. Expression of the cytokine leukemia inhibitory factor and pro-apoptotic insulin-like growth factor binding protein-3 in Alzheimer’s disease. Acta Neuropathol. 2002;104:525–533. doi: 10.1007/s00401-002-0585-x. [DOI] [PubMed] [Google Scholar]
- 36.Merson T.D., Binder M.D., Kilpatrick T.J. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular Med. 2010;12:99–132. doi: 10.1007/s12017-010-8112-z. [DOI] [PubMed] [Google Scholar]
- 37.Stout M.B., Justice J.N., Nicklas B.J., Kirkland J.L. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32:9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Hou J.G., Yun Y.J., Xue J.J., Sun M.Q., Kim S.C. D-galactose induces astrocytic aging and contributes to astrocytoma progression and chemoresistance via cellular senescence. Mol Med Report. 2019;20:4111–4118. doi: 10.3892/mmr.2019.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maubach G., Schmädicke A.C., Naumann M. NEMO links nuclear factor-κB to human diseases. Trends Mol Med. 2017;23:1138–1155. doi: 10.1016/j.molmed.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Sun B., Dwivedi N., Bechtel T.J., Paulsen J.L., Muth A., Bawadekar M., et al. Citrullination of NF-kB p65 enhances its nuclear localization and TLR-induced expression of IL-1β and TNFα. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aal3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitley S.K., Balasubramani A., Zindl C.L., Sen R., Shibata Y., Crawford G.E., Weathington N.M., Hatton R.D., Weaver C.T. IL-1R signaling promotes STAT3 and NF-κB factor recruitment to distal cis-regulatory elements that regulate Il17a/f transcription. J Biol Chem. 2018;293:15790–15800. doi: 10.1074/jbc.RA118.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao S., Dey A., Yu X.L., Stranahan A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain, Behav, Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maciejczyk M., Żebrowska E., Zalewska A., Chabowski A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/6940515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Xc, Dong F., Ren J., Driscoll M.J., Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Franklin T.B., Krueger-Naug A.M., Clarke D.B., Arrigo A.P., Currie R.W. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005;21:379–392. doi: 10.1080/02656730500069955. [DOI] [PubMed] [Google Scholar]
- 47.Sharp F.R., Zhan X.H., Liu D.Z. Heat shock proteins in the brain: role of Hsp70, hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Yh, Zhang R., Hou Xl, Zhang Ym, Ding Fj, Li F., Yao Y., Wang Y. Microglia activation triggers oligodendrocyte precursor cells apoptosis via HSP60. Mol Med Report. 2017;16:603–608. doi: 10.3892/mmr.2017.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alford K.A., Glennie S., Turrell B.R., Rawlinson L., Saklatvala J., Dean J.L. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-β-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 50.Jin Ch, Cleveland J.C., Ao Lh, Li Jl, Zeng Qc, Fullerton D.A., Meng Xz. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F., Echeverry R., Wu J., An J., Haile W.B., Cooper D.S., Catano M., Yepes M. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK 1/2–CREB–ATF3 signaling pathway. Mol Cell Neurosci. 2013;52:9–19. doi: 10.1016/j.mcn.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Jh, Oh J.P., Yoo M., Cui C.H., Jeon B.M., Kim S.C., Han J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol Brain. 2019;12:1–8. doi: 10.1186/s13041-019-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stremmel W., Pohl J., Ring A., Herrmann T. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids. 2001;36:981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]