Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases ('Animal Health Law’). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for glanders. In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radius of the protection and surveillance zone, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere. Considering the epidemiology and distribution of glanders, it was foreseen that three different situations could lead to a suspicion of the disease. Sampling procedures were defined for each of the three different suspicion types, which can also be applied in most of the other scenarios assessed. The monitoring period (6 months) was assessed as effective in all scenarios. The AHAW Panel of experts considered the minimum radius and duration of the existing protection and surveillance zone, set at the establishment level, effective. Recommendations provided for each of the scenarios assessed aim to support the European Commission in the drafting of further pieces of legislation, as well as for plausible ad hoc requests in relation to glanders.
Keywords: disease control measures, glanders, Burkholderia mallei, sampling procedures, monitoring period, protection zone, surveillance zone
Summary
This opinion is part of a series of opinions, in which the three first Terms of Reference (ToR) of a mandate received from the European Commission have been considered. The background and specific details of this mandate can be found in the opinion. The ToRs in this mandate request an assessment of the effectiveness of:
the clinical and laboratory examination in their capacity to detect disease (or estimate the disease prevalence within an establishment), either in suspect or confirmed animals in a single establishment, or in establishments within restriction zones (ToR 1);
the effectiveness of the duration of the monitoring period (for different scenarios) in the control of suspected and confirmed outbreaks (ToR 2);
the size and duration of the restriction zones, in their capacity for mitigating disease spread (ToR 3).
In order to harmonise the approach to these assessments, the methodology used in this series of opinions, covering all Category A diseases, was agreed on, and published in a separate technical report.
Specific laboratory and clinical procedures for detecting glanders have not been found in European legislation; therefore, some specific procedures have been provided for some scenarios in ToR1. As glanders have only rarely been detected in the EU in recent decades, it is recommended that all samples with non‐negative results (or where there is a strong suspicion of disease) are sent to the EURL for confirmatory tests. When the disease is confirmed, all other animals of listed species in the affected establishment should be tested each month throughout the monitoring period (6 months). As the restriction zone is only foreseen to be at the level of the affected establishment, several scenarios of ToR1 regarding non‐affected establishments have not been assessed and considered as not relevant in case of glanders. The long incubation period, poor sensitivity and specificity of the available diagnostic tests and possible presence of clinically healthy but infected animals do not allow a derogation for the movement off the establishment during the monitoring period.
For ToR2, on assessing the length of measures in place during the monitoring period, an extensive literature search (ELS) was carried out. This ELS aimed to assess the average, shortest and longest period between the earliest point off infection of listed animals with glanders and the time of reporting of a suspicion by the competent authority. The average time to the reporting of a suspicion was then used to assess the effectiveness of the length of monitoring periods. For the relevant scenarios, the existing length of the monitoring period for glanders (6 months) was considered effective. Recommendations have been made for certain scenarios.
For ToR3, no restriction zones are recommended beyond the extent of the establishment. Maintaining restrictions on listed species for the length of the monitoring period (6 months) at the establishment level was considered effective to prevent disease spread.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’), hereinafter referred to as AHL, requires the Commission to lay down detailed rules on the disease control measures against listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). The Commission is empowered to adopt delegated acts supplementing the rules laid down in Part III of Regulation (EU) 2016/429 on transmissible animal diseases (Animal Health Law) on disease control measures for listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). Therefore, the Commission has developed and adopted a Delegated Regulation laying down rules for the prevention and control of certain diseases (‘the Delegated Regulation’). The rules laid down in the Delegated Regulation are in respect of terrestrial animals largely replicating the rules currently in force concerning the disease control measures in the event of animal diseases with serious effects on the livestock as they have proven to be effective in preventing the spread of those diseases within the Union. Consequently, many animal disease control measures laid down in existing Directives will be, to the extent that not already done by the Animal Health Law, replaced by the rules provided in the Delegated Regulation. At the same time, these rules have been aligned with the international standards from the World Organisation for Animal Health (OIE), wherever these existed. However, certain disease control measures proposed in the Delegated Regulation, in particular in its Annexes, were considered as outdated i.e. possibly not based on most recent scientific evidence at the time of development. Their review is considered as necessary. Moreover, for those category A diseases for which rules were not established before or were not detailed enough, certain disease control and risk mitigating measures are, due to the lack of scientific basis, extrapolated from other diseases, for which rules existed in the past. Finally, for some other diseases the evidence and scientific knowledge, was not available to the Commission and to the Member States at the time of developing the Delegated Regulation due to the time constraints. The following diseases are examples of the later: infection with Rift Valley fever (RVF), infection with Mycoplasma mycoides subsp. Mycoides SC (Contagious bovine pleuropneumonia) (CBPP), Contagious caprine pleuropneumonia (CCPP), Sheep pox and goat pox, infection with peste des petits ruminants virus (PPR), African horse sickness (AHS), glanders. In this regard, the existing rules will cease to apply as from the date of application of the Animal Health Law and its complementing legislation including the Delegated Regulation, i.e. from 21 April 2021. Certain of the proposed measures for the prevention and control of category A diseases of terrestrial animals should therefore be assessed in order to ensure that they are effective and updated based on the latest scientific knowledge in this new set of legislation. This is particularly important in the case of those diseases that are less common or have been never reported in the Union.
1.1.1. ToR 1: Sampling of animals and establishments for the detection of category A diseases in terrestrial animals
Based on available scientific information, assess the effectiveness of existing sampling procedures to detect or rule out the presence of each category A disease of terrestrial animals and, in case of absence of effective procedures, develop them, in order to complete the rules provided for in Annex I to the Delegated Regulation. In particular, provide for disease‐specific procedures for the sampling of:
ToR 1.1 Animals for clinical examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 13(3)(c), 14(1) and 26(2) of the Delegated Regulation.
ToR 1.2 Animals for laboratory examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 12(3), 13(3)(c), 14(1), 26(2) of the Delegated Regulation.
ToR 1.3 Establishments to ensure the detection of the relevant category A disease for the performance of visits in establishments located in protection zones larger than 3 km and establishments located in the surveillance zone in accordance with Articles 26(5) and 41 of the Delegated Regulation.
ToR 1.4 Animals for clinical and laboratory examinations to ensure the detection of the relevant category A disease for the movement of animals from restricted zones in accordance with Articles 28(5), 43(5), 56(1)(c) of the Delegated Regulation.
ToR 1.5 Animals for laboratory examinations to ensure the detection of the relevant category A disease before and after being introduced in the affected establishment for repopulation, in accordance with Article 59(2), (3) and (9) of the Delegated Regulation.
1.1.2. ToR 2: Monitoring period
ToR 2.1 Assess the effectiveness of the length of the monitoring periods set out in Annex II of the Delegated Regulation for each category A disease of terrestrial animals. In this regard, it is important to take into consideration that the monitoring period was introduced as a management tool, which represents a time frame of reference assigned to each category A disease for the competent authority to apply certain control measures and to carry out investigations in the event of suspicion and confirmation of category A diseases in terrestrial animals.
This assessment should be carried out with respect to the following situations:
the records analysis carried out by the competent authority in the framework of the epidemiological enquiry referred to in Article 57 of Regulation (EU) 2016/429, in the event of suspicion of a category A disease (Article 8(4) of the Delegated Regulation);
the derogation from killing in the event of an outbreak of a category A disease in establishments keeping animals of listed species in two or more epidemiological units (Article 13(1) of the Delegated Regulation);
the tracing carried out by the competent authority to identify establishments and other locations epidemiologically linked to an establishment affected by a category A disease (Article 17(2) of the Delegated Regulation);
the exemption applied to certain products from the prohibitions laid down in Annex VI taking into account the date they were produced (Article 27(3)(c) of the Delegated Regulation);
the specific conditions for authorising movements of semen from approved germinal product establishments in the protection and surveillance zones (Article 32(c) and 48(c) of the Delegated Regulation);
the repopulation of establishments affected by a category A disease (Article 57(1)(b) and 59(4)(b) of the Delegated Regulation).
ToR 2.2 Propose the length of what should be the monitoring period in those diseases for which the time is assessed as not effective.
1.1.3. ToR 3: Minimum radius of restricted zones and duration of the disease control measures in restricted zones
ToR 3.1 Assess the effectiveness to control the spread of the disease of the minimum radius of the protection and surveillance zones set out in Annex V of the Delegated Regulation for each category A disease of terrestrial animals.
ToR 3.2 Assess the effectiveness to control the spread of the disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI for each category A disease of terrestrial animals.
1.1.4. ToR 4: Prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials
ToR 4.1 Assess the effectiveness to control the spread of disease of prohibitions set out in Annex VI of the Delegated Regulation with respect to the risk associated for each category A disease, to the listed activities and commodities.
ToR 4.2 Review the available scientific information on risk‐mitigating treatments that are effective to control the presence of category A disease agents in products of animal origin and other relevant materials. Based on this:
provide an opinion on the effectiveness of the risk‐mitigating treatments for products of animal origin and other materials produced or processed in the restricted zone set out in Annex VII and VIII, and
if relevant, suggest new treatments or procedures that can be effective to mitigate or to eliminate such risk
1.2. Interpretation of the Terms of Reference
To address the ToRs of the mandate, EFSA proposed and agreed with the European Commission the following:
-
a
The publication of 14 individual opinions, one per each of the diseases included in the list of category A diseases for terrestrial animals, with each of these opinions providing the answer to ToRs 1, 2 and 3. The current manuscript is one of the 14 opinions covering ToRs 1, 2 and 3 for glanders.
-
b
The publication of a unique opinion covering ToR 4 for all diseases listed (i.e. ToR 4 is not covered in this opinion).
-
c
To address ToR 1 (effectiveness of sampling procedures), EFSA agreed with the European Commission on 21 scenarios based on different articles of the Delegated Regulation (EC) 2020/687 (hereinafter referred to as Delegated Regulation), for which the effectiveness of the sampling procedures will be assessed (Annex B). Although these scenarios will be assessed independently, some of these scenarios may be merged if the assessment processes are the same.
-
d
To address ToR 2 (effectiveness of the monitoring period), seven scenarios previously agreed with the contractor were defined (Annex D). The assessment of the effectiveness of the monitoring period will be done by assessing its ability to ensure that specific actions can be carried out without posing a risk of disease spread, if the monitoring period is calculated backwards or forwards from a specific date. If the length of the monitoring period estimated by EFSA is longer than the existing monitoring periods, the existing monitoring period will be considered non‐effective. If the length of the monitoring period estimated by EFSA is shorter than the existing monitoring period, this existing monitoring period will be considered effective from a disease control point of view. No assessment of the plausible unnecessary economic burden that may be placed on the stakeholders as a result of an excessive length of the monitoring periods will be done by EFSA.
-
e
The assessment of the minimum duration and the length of the radius of the protection and surveillance zones (ToR 3) will be done independently. The setting of these two zones (protection and surveillance zones) surrounding an affected establishment and the control measures implemented in each one of the zones are based on the general principle that the probability of disease spread is larger the closer the establishment is to an affected establishment. The validity of this statement will not be assessed in this manuscript; nonetheless, the limitations that this assumption may have in the control of certain diseases will, when relevant, be discussed.
-
f
The following scenarios of the ToR1 of the Annex B are not relevant for glanders, and therefore not included in the assessment of the current Opinion:
scenarios 6, 7, 8, 9, 12, 13, 14, 15 and 18 because both the protection and surveillance zones are limited to the affected establishment. The assessment and the reasoning behind maintaining the protection and surveillance zones at the establishment level are described in Section 4.3.1,
scenarios 10, 11, 16 and 17 because they are referring to poultry.
-
g
The duration of the monitoring period for glanders as described in Annex II of the Delegated Regulation is 6 months.
-
h
The protection zone (PZ) and surveillance zone (SZ) for glanders as described in Annex V of the Delegated Regulation is set at an establishment level.
-
i
The minimum duration of the measures in the PZ for glanders as described in Annex X of the Delegated Regulation is 6 months (not applicable for the SZ).
2. Epidemiological and clinical characteristics of glanders
2.1. Epidemiology
Glanders is a zoonotic bacterial disease affecting mainly domestic equids. The causative agent is Burkholderia mallei, a gram‐negative bacillus of the family Burkholderiaceae; it is genetically closely related to the agent of melioidosis, Burkholderia pseudomallei. Burkholderia mallei is an obligate animal and human pathogen with a limited capacity to survive outside its mammalian host (< 2 weeks in most conditions) (Van der Lugt and Bishop, 2004). Although this organism is inactivated by heat and sunlight, its survival is prolonged in wet and humid environments. It is destroyed by exposure to direct sunlight within 24 h and is killed by most common disinfectants (including 1% sodium hypochlorite, 70% ethanol, 2% glutaraldehyde, iodine, benzalkonium chloride, mercuric chloride in alcohol and potassium permanganate). It is less susceptible to phenolic disinfectants. This organism can be destroyed by heating to 55°C for 10 min, or exposure to ultraviolet irradiation (Spickler, 2018). Nevertheless, in moist environments, it can retain its vitality for 3–5 weeks and in decaying material for 20–30 days. It can survive for 20–30 days in clean water (up to 100 days for one early report – Spickler (2018)) and for about 6 weeks in contaminated stables (Van der Lugt and Bishop, 2004; OIE, 2021). Under most conditions, however, it is not likely to survive in the environment for more than 2 weeks.
Burkholderia mallei as well as B. pseudomallei are classified in many countries as potential bioweapons because of their high infectivity, the degree of incapacitation they cause and their resistance to antimicrobial treatment (Kettle and Wernery, 2016). Consequently, any handling of B. mallei or B. pseudomallei strains or of known or potentially infected samples from suspect animals or fomites for diagnostic purpose should be performed in biosafety level III containment laboratories. Likewise, strict precautions, including appropriate personal protective equipment with surgical masks and face shields, should be taken during clinical and necropsy examinations.
Glanders is a severe and usually fatal contagious disease of domestic equids: donkeys and mules are the most susceptible and typically develop an acute and subacute form, respectively. Horses are more resistant and rather develop a chronic or a subclinical form, and asymptomatic carriers (latent 1 or chronic infection, where the horses do not show clinical signs, although they are potentially infectious) are not rare (Lefèvre and Blancou, 2010; Kettle and Wernery, 2016). Camelids and small ruminants can be infected if kept in close contact with glanderous equids. According to some authors, sheep could be susceptible to glanders but are considered less susceptible than goats (Loeffler, 1886; Hu et al., 1958). Some rodents (e.g. field mice and voles) and bears may also be affected. Carnivores (wild felids, cats, dogs, wolves, jackals and hyenas) can be infected after ingestion of B. mallei‐infected meat (glanderous meat) with an often fatal outcome. Pigs, cattle and birds are resistant (OIE, 2018, 2020; Spickler, 2018). Underfed animals and animals kept in unhygienic environments are more susceptible than healthy, well‐cared‐for animals. In humans, who can be infected by diseased animals, glanders can provoke an acute and, if not treated, fatal disease, or a chronic form. However, infection in man is infrequent even during outbreaks of disease in horses (Khan et al., 2013; Kettle and Wernery, 2016; OIE, 2018; Spickler, 2018).
Known since antiquity, glanders was eradicated in the early 20th century in Australia, Europe, Japan, North America, and some other countries. The disease has never been reported in New Zealand. Nowadays, outbreaks or cases occur sporadically in parts of Asia and the Middle East, Northern Africa, and Central and South America. The disease is regarded as endemic in India, Iraq, Mongolia, Pakistan and regions of Brazil. However, over the last 25 years, an apparent increase in outbreaks or cases has been observed, which has led to the disease being considered re‐emergent (Kettle and Wernery, 2016; OIE, 2018, 2020; Spickler, 2018). It is worth considering that the disease is probably often misdiagnosed (possible confusion with melioidosis) and certainly underreported in many countries.
In equids, the mode of infection is unclear, but it generally appears to result from contaminated feed or water. Indeed, outbreaks are usually favoured where several animals are kept together and share feeding places and water troughs. The occurrence of aerosol infection through inhalation of dried infected particles remains controversial (Khan et al., 2013; Spickler, 2018); however, the disease has been reproduced by intranasal and intratracheal inoculation of the organism. Direct contact (including through skin following injury and ingestion) with secretions or exudates from infected animals (nasal discharge, cough, skin lesions and abscesses) is another route of transmission.
Furthermore, the importance of asymptomatic carriers, and the transmission of the infection from them to naive animals, should be emphasised (Van der Lugt and Bishop, 2004).
Indirect transmission occurs through contact with fomites such as blankets, halters, harnesses, saddles, grooming and hoof trimming material or veterinary equipment (Khan et al., 2013).
Other routes consisting of venereal transmission by an infected stallion, vertical transmission from mare to foal and mechanical transmission by insects (e.g. houseflies) have been reported as possible (OIE, 2018, 2020; Spickler, 2018).
In most cases, the infection remains confined to the establishment or group of animals reared together; it only spreads outside through the movement of infected animals or contaminated fomites. Given the absence of the disease in Europe for decades, it is extremely likely that its re‐emergence in the Union could only result from the introduction of infected animals or contaminated fomites from endemic areas.
Given the risk that glanders poses to equids, humans and other susceptible species, outbreak control relies in particular on the safe destruction (i.e. incineration) or burial of infected carcasses (and any contaminated material), decomposition of manure and (cleaning and) disinfection of premises. Additionally, meat from suspect or confirmed cases should not be fed to other animals or used for human consumption. Control measures in free countries against the introduction of glanders include the screening of imported horses, which should remain in quarantine until testing is completed and for at least 14 days in order to allow a period of observation during which any potentially inapparent infection may become active, because of the stress imposed by travel, and may thus become apparent. Other measures include passive clinical surveillance and laboratory testing of suspect cases, euthanasia of confirmed cases, quarantine and disinfection of infected premises. In endemic countries, testing and culling of infected equids can reduce the incidence; antibiotic treatment can reduce mortality, but contributes to the maintenance of chronical carriers. No vaccines are currently available for use in horses or humans (Spickler, 2018; OIE, 2020, 2021). Potentially efficient antibiotics are limited in range and treatment of infected humans must be prolonged but is frequently ineffective, with a mortality rate of up to 40% (Kettle and Wernery, 2016).
Clinical Signs and Diagnosis
Clinical glanders generally takes an acute form in donkeys and sometimes in mules, rarely in horses, with high fever (41–42°C), congestion of ocular mucosa, severe respiratory signs and death within a few days. In horses, the infection is generally chronic or even subclinical, with periods of exacerbation; infected animals may survive for several years, remaining infectious carriers, although many eventually die from the condition. In camelids, clinical signs as well as gross pathologic and microscopic lesions are similar to those seen in equids (Wernery et al., 2011).
The incubation period in equids is usually 2–6 weeks but varies from 6 days to several months (minimum 3 days for fever and 1 week for clinical signs in experimental infection). OIE considers the maximum incubation period in equids as 6 months (OIE, 2021). Less is known about the incubation period of glanders in other listed species (Van der Lugt and Bishop, 2004; Lefèvre and Blancou, 2010; Khan et al., 2013; OIE, 2018, 2021; Spickler, 2018).
Glanders is traditionally categorised into nasal, pulmonary and cutaneous forms, based on the main affected sites. In the nasal form, the first signs are fever, cough and difficult breathing followed by nasal discharge, initially watery but becoming mucopurulent (yellowish‐green) and sometimes haemorrhagic, crusts on the nostrils, nasal ulcers and unilateral or bilateral enlargement of submaxillary lymph nodes, which become indurated and may suppurate and drain. This form usually evolves to a pulmonary form causing nodules and abscesses in the lung and sometimes bronchopneumonia. Respiratory signs can be mild to severe and are accompanied with fever and sometimes diarrhoea and polyuria. A progressive loss of condition is usually observed. The cutaneous form is known as farcy, and is characterised by fever, cough, oedema of the limbs, enlargement of the lymph nodes, lymphangitis and multiple skin lesions on the limbs and abdomen in the form of multiple nodules and ulcers producing a thick yellow exudate. Swelling of the joints and orchitis can occur. In farcy, the course of the disease may last several months, but animals eventually die (Lefèvre and Blancou, 2010; Khan et al., 2013; OIE, 2018, 2020; Spickler, 2018). This form occurs as a result of infection of the skin following injury, or from metastases of pulmonary origin (Van der Lugt and Bishop, 2004). Nasal secretions and skin exudates from glanderous animals do contain a considerable number of bacteria (Khan et al., 2013).
In inapparent or subacute cases, most common in horses, lesions may occur sporadically in the lungs and other internal organs. The clinical signs are usually minimal and most often consist only of intermittent low fever, nasal discharge and/or occasional laboured breathing. Clinical signs of melioidosis in equids can be similar to those of glanders (OIE, 2018).
The characteristic clinical signs of glanders appear late in the course of the disease and a definite diagnosis based on clinical manifestation alone is rarely possible. Laboratory testing is therefore used to confirm suspect cases, based on clinical or pathological signs, in surveillance of horses in contact with confirmed cases or to screen horses before import/export. Again, given the zoonotic risk, sample collection and handling should be performed with biosecurity measures as mentioned above.
Direct diagnosis by identification of the agent (culture and morphology) and of genetic material (conventional or real‐time PCR) can be performed from nasal or respiratory exudates, smears from lesions or tissue samples from lesions. The current method for diagnosis is the isolation and identification of B. mallei from clinical samples. The bacteria can be grown on routine culture media, with viscid, smooth and creamy colonies obtained after 48 h at 37°C. They can be grown in pure culture from fresh glanderous lesions, but culture is easily overgrown by the normal flora of the sample because of its slow‐growing nature. Glycerol and antimicrobial enrichment enhance growth (Kinoshita et al., 2019). Another limitation for the isolation is the low concentration of B. mallei in tissues (in particular in older lesions) of the infected equids (Kettle and Wernery, 2016). Molecular tests are a good alternative, with better detection of B. mallei using specific PCR systems. However, due to the rarity of the disease, many PCR systems have not yet been thoroughly evaluated with clinical samples (Spickler, 2018) and most of them are available only in specialised reference laboratories, the EURL for Equine diseases in particular.
Additional molecular tools initially developed for B. pseudomallei such as multilocus sequence typing (MLST) and multilocus variable number of tandem repeat analysis (MLVA) have been applied for molecular discrimination between B. mallei isolates. However, MLST schemes have failed to distinguish between B. mallei strains (Godoy et al., 2003; Losada et al., 2010), mainly due to the clonal origin of this species and its limited diversity; and the MLVA scheme still requires technical fine‐tuning and validation (U'Ren et al., 2007; Hornstra et al., 2009; Scholz et al., 2014). Access to genomic sequences of strains now allows for fine‐grained phylogenetic analysis and identification of single‐nucleotide polymorphisms (SNPs) that have recently been used for rapid characterisation of B. mallei strains (Girault et al., 2018). Isolates from the Middle East, India/Pakistan and Brazil tested so far with these new markers cluster in distinct groups (Girault et al., 2018; Laroucau et al., 2021; Singha et al., 2021).
Indirect diagnostic tests are early and first‐line screening tools as detectable antibody titres develop within 7–14 days after infection (Miessner, 1909; Rice et al., 1951). Serological tests are nowadays preferred to the mallein test (only available at Institute Pasteur Romania). This test, consisting of localised delayed hypersensitivity reaction after intradermo‐palpebral injection of mallein, an antigen hardly available today, may induce conjunctivitis, sometimes purulent, fever and pain, and is therefore questionable in terms of animal welfare. It may also induce transient (permanent, if the test is repeated) false‐positive serological reactions and give inconclusive results in acute glanders or late stages of chronic disease.
The most commonly used serological test in equids is the complement fixation test (CFT), which has a good sensitivity as a screening test and is able to detect chronically infected carriers. It is the serological test prescribed by the World Organisation for Animal Health (OIE) for international trade of equines. It is also valid for mules and camels, while its use in donkeys needs a particular care to avoid misdiagnosis (OIE, 2018). However, this CFT method remains difficult to standardise and its reliability depends on the choice of protocol and antigen, still a crude whole‐cell preparation (Khan et al., 2014; Malik, 2016). Hence, sensitivity and specificity of the CFT may vary and yet the sensitivity of this test may range from 62.5% to 100% according to the antigen used (Kettle and Wernery, 2016). Anticomplementary activity of equid sera and CFT false‐positive/negative results generate additional difficulties. False‐negative results usually occur in old, pregnant and emaciated animals (Neubauer et al., 2005). It has been observed that the specificity of CFT may vary from glanders‐endemic to non‐endemic areas. Thus, two commercial antigens, for instance, were reported to present specificities of 75.71–77.45% on sera from endemic areas and 93.75–94.79% on sera from non‐endemic areas (Khan et al., 2011). It is therefore advised to combine the CFT with a more specific and complement‐independent test in series to increase the positive predictive value of the diagnosis (Khan et al., 2013).
Alternative serological tests based on B. mallei protein extracts or recombinant proteins have been developed and recent comparison studies concluded that sensitivity and specificity of evaluated tests were comparable with those obtained with the complement fixation test, opening the possibility of replacing it by more easily standardisable methods (Elschner et al., 2017, 2021). Nevertheless, one of the current constraints for ELISAs is their availability in a commercial format. Among the commercialised tests, one has recently been validated (Elschner et al., 2021). The specificity (99.8%) and sensitivity (96.5%) values obtained allow its use as a confirmatory test and as a realistic alternative to equine serological testing for trade and movement. Thus, ELISAs are currently considered to be the most accurate and reliable assays in equids.
An immunoblot assay has been developed, validated and found to be useful as confirmatory test for CFT‐positive results (Elschner et al., 2011, 2021). However, the test is difficult to perform outside of well‐equipped specialised laboratories. A Rose Bengal plate agglutination test, usable in equids as well as in other susceptible species, has been employed in Russia and reported in Pakistan as showing a 90% sensitivity and a 100% specificity (OIE, 2018).
According to OIE (2018), supporting evidence of infection may be provided by a positive result in, e.g. CFT, which should be confirmed by a second test with equal or higher sensitivity and higher specificity.
Most serological tests cannot distinguish antibodies due to B. mallei or B. pseudomallei (Khan et al., 2013; OIE, 2018, 2020, 2021; Spickler, 2018). Therefore, even the most specific tests to glanders at the laboratory level could lead to false‐positive results due to cross‐reaction with B. pseudomallei, the agent of melioidosis, which is endemic in the environment in some countries.
There is clearly a need for a highly sensitive and specific test, and its standardisation, or for a two‐tier approach to testing, for the accurate detection of B. mallei in inapparent infections (Kettle and Wernery, 2016). Development and standardisation of tests that could replace advantageously the CFT is all the more important as, on several occasions in recent years, certain reagents needed to perform CFT have proved to be unavailable on the market in some Member States, sometimes permanently.
2.1.1. Geographical distribution of glanders
As mentioned above, the most recent outbreaks or cases have occurred sporadically in parts of Asia and the Middle East, Africa and South America (Figure 1). A singleton case in 2014–2015 in Germany was notified to the OIE, with a diagnosis based on confirmed serological reactions in serial samples and a positive PCR within a skin lesion, but without isolation of the pathogen. Its origin has not been elucidated (Elschner et al., 2016).
Figure 1.
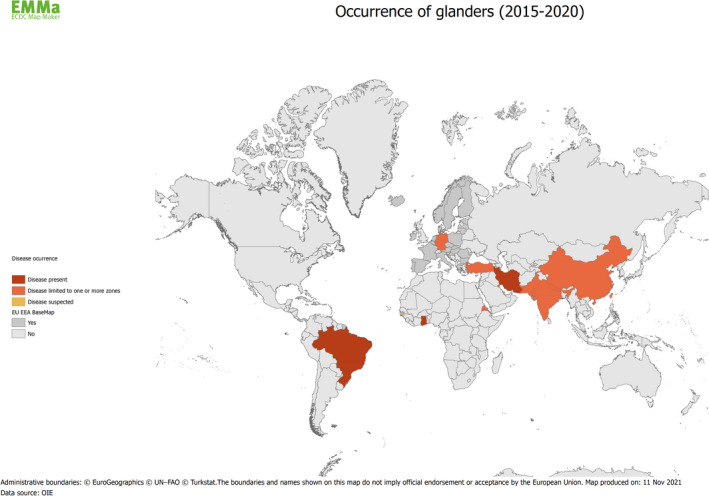
Map of countries with reported cases of glanders from 2015 to 2020 (Data sources: ADNS and OIE)
3. Data and methodologies
3.1. Methodology used in ToR 1
Although the general methodology applied to all opinions covering the assessment of control measures for the Category A diseases produced under this mandate has been published elsewhere (EFSA, 2020), specific details of the methodology related to the glanders opinion are presented below.
A PUBMED search using the terms (‘model’ AND ‘glanders’) did not identify any mathematical models for the transmission of B. mallei within or between establishments. Furthermore, there is a lack of suitable data from which to construct and parameterise such a mathematical model.
3.2. Methodology used in ToR 2
To estimate the time lag between infection and reporting of a glanders suspicion (ToR 2), an extensive literature search (ELS) was outsourced by EFSA (OC/EFSA/ALPHA/2020/02 – LOT 2). The aim of this ELS was to answer the epidemiological question of: ‘what is the average, shortest and longest period of time for an outbreak of glanders to be reported (measured as the number of days from the earliest point of infection with B. mallei to the time of declaration of a suspicion by the competent authority after the clinical investigation by an official veterinarian)?’. To answer this question, an ELS on case reports, papers describing outbreaks or epidemics of glanders and any other relevant grey literature or data was carried out. For the inclusion in the ELS, the earliest point of infection had to be estimated by carrying out an epidemiological investigation. Papers and other sources of data, where the earliest point of infection was determined purely by subtracting a known incubation period from the date of the suspicion of the outbreak, were excluded. The ELS was restricted to studies conducted in Europe or describing results obtained in Europe. If none or very few articles were retrieved (less or equal to 5) in the first search, the search was extended to the rest of the world. An ELS protocol similar to that shown in Annex 5 of the Methodology report (EFSA, 2020) was followed.
3.3. Methodology used in ToR 3
Methodology for assessing the effectiveness of the minimum radius of the protection and surveillance zones and their duration
As the current protection and surveillance zones are set at the establishment level, a qualitative assessment of this measure based on scientific evidence and expert opinion was performed.
3.4. Uncertainty
A description of the methodology followed to deal with uncertainty is provided in a Methodology report published by EFSA (EFSA, 2020). In this opinion, the sources of uncertainty are described qualitatively, although no quantification of these sources was carried out.
4. Assessment
4.1. Assessment of sampling procedures (ToR 1)
4.1.1. Assessment of sampling procedures in the event of suspicion or confirmation of glanders (Burkholderia mallei)
4.1.1.1. In the event of a suspicion of glanders in an establishment where animals of the listed species are kept
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect glanders in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). For further details, see Annex B.
1st Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 6(2) of the Delegated Regulation (EU) 2020/687
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns an event of suspicion of Burkholderia mallei/glanders in an establishment with kept animals of the listed species;
The listed species for glanders as provided in Commission Implemented Regulation 2018/1882 are those belonging to the Equidae, Capra ssp., Camelidae;
Subsequent to the suspicion, the competent authority shall immediately conduct an investigation to confirm or rule out the presence of the disease;
The official veterinarian must perform a clinical examination and collect samples for further laboratory examination (see Annex C for details on guidelines on how the clinical and laboratory examination must be carried out).
Summary of sampling procedures
While preventive and control measures have been recommended in several papers, and while existing guidelines for trade and freedom status certification have been prescribed by the OIE, no specific guidelines have been found either in the literature or in previous legislation. Nevertheless, OIE specifies (OIE, 2021) that:
‘The following defines the occurrence of infection with B. mallei:
B. mallei has been isolated from a sample from an equid; or
antigen or genetic material specific to B. mallei has been identified in a sample from an equid showing clinical or pathological signs consistent with glanders, or epidemiologically linked to a confirmed or suspected case of infection with B. mallei, or giving cause for suspicion of previous contact with B. mallei; or
antibodies specific to B. mallei have been detected by a testing regime appropriate to the species in a sample from an equid showing clinical or pathological signs consistent with glanders, or epidemiologically linked to a confirmed or suspected case of infection with B. mallei, or giving cause for suspicion of previous contact with B. mallei.
For the purposes of the Terrestrial Code, the infective period of B. mallei in equids is lifelong and the incubation period shall be six months’.
In the literature, the following recommendations have been found (Lefèvre and Blancou, 2010; Khan et al., 2013; Kettle and Wernery, 2016; Spickler, 2018):
Due to the shortcomings of serological tests and PCR, it is impossible to demonstrate the absence of disease in every equid in each case.
In holdings at risk, every equine, i.e. horse, mule, donkey and hinny, has to be tested because of the existence of clinically healthy shedders of B. mallei.
Strict veterinary regulations (OIE, 2021) including serological testing of animals prior to transport, can reduce the risk of importation of glanders to free areas.
Serological monitoring at defined intervals must be maintained for a certain period of time after the (apparent) eradication of the disease.
Paired sera may be taken for a more reliable CFT result interpretation.
Mallein testing of apparently healthy animals at an interval of 3 weeks has also been proposed.
Assessment
Given the absence of circulating disease in Europe for decades, it is extremely likely that a possible future re‐emergence in the Union would result from the introduction of an infected animal or contaminated fomites from enzootic areas.
A case of B. mallei infection can be suspected in three situations:
Situation 1: a positive result to a (pre‐movement) screening test corroborated by another positive result from an appropriate test on the same sample carried out by a national reference laboratory when possible, or with the support of the EURL if necessary and following OIE requirements.
Situation 2: observation of clinical or necropsy signs suggestive of glanders in a live or dead animal
Situation 3: existence of an epidemiological link with a confirmed outbreak/case.
The disease has a complex pathogenesis with three different forms (nasal, pulmonary and cutaneous form) and a long incubation period, which in equids can span from 6 days to several months. The course of disease, in addition, may be acute, subacute or chronic. Latently infected animals of susceptible species, horses particularly, can usually remain insidiously infected for months or years (Khan et al., 2013).
The above‐mentioned factors, along with the technical limitation of diagnostics (see Section 2.1), and with the scarce information regarding the serological status of subclinical animals, make unreliable any attempt to rule out the disease without the investigation of the suspicion in every animal of the establishment. Moreover, the mallein intradermo‐palpebral test, which could help to increase the diagnostic sensitivity or specificity when used in conjunction with currently available serological tests, cannot be recommended as an additional tool, especially in non‐endemic regions. Indeed, its low availability means that it should not be recommended in the case of a suspicion in these regions, given the seriousness of the disease and, even more, the consequences of its use in terms of welfare as mentioned above. Therefore, every animal of the listed species of a suspected establishment should be tested following the recommendations below, because of the potential existence of ‘clinically’ healthy shedders of B. mallei in infected establishments.
Considering (i) the sometimes insufficient standardisation of specific serological reagents, the variable reliability of tests and the lack of harmonisation of test performance at the international level, and (ii) the extremely low risk of introduction of glanders into the EU and, consequently, the possible lack of preparation of their veterinary structures and laboratories in some Member States, it may be appropriate to seek the expert opinion of the EURL when suspicions arise.
Development of new procedures
A decision tree based on the three identified situations is presented in Figure 2.
Figure 2.
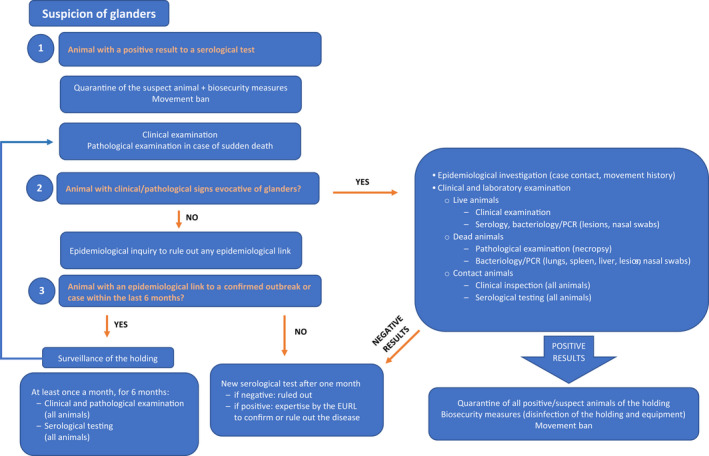
Decision tree in case of a glanders suspicion
All animals of the listed species in the establishment should undergo clinical and serological examination. If the suspect animals have been kept under high biosecurity conditions since the arrival to the establishment (no shared equipment, personnel, etc.), clinical and serological examination can be limited to the epidemiological unit where the suspect animal(s) are kept (particularly in large establishments). All suspicions should be notified to the EURL (EURL for Equine diseases other than AHS).
Situation 1: a positive result to a (pre‐movement) screening test (CFT) corroborated by another positive result using CFT and/or other complementary tests (ELISA, Immunoblot, etc.) on the same serum sample, carried out by a national reference laboratory (NRL) according to OIE requirements when possible, and with the support of the EURL if necessary, according to OIE requirements. This animal should be PCR tested and considered as a confirmed case if PCR results are positive. In the case of negative PCR results, the suspicion remains until ruled out by repeated negative testing and according to the epidemiological enquiry. In this event, if there is neither history of travel to or from an endemic area for the respective animal and for all other contact animals of the listed species in the establishment (same epidemiological unit), nor link to an outbreak of glanders, nor evocative clinical signs (nor pathological signs in case of sudden death), serological testing should be carried out on all animals of listed species. In the event of some epidemiological links being found, the procedures described for Situation 3 should be followed; if clinical signs are observed, the procedures described for Situation 2 should be followed. If any other animal in the establishment tests positive to a serological test, this should be supported by a complementary test as described above and a PCR if this complementary test is also positive.
Situation 2: observation of clinical signs or necropsy findings suggestive of glanders in a live or dead animal.
All animals of listed species in the establishment should be subjected to detailed clinical examination starting by animals without clinical signs and ending with the suspect animals, to prevent additional risk of transmission from animal to animal; serum samples should be taken from all animals for serological tests and specimens for bacteriology/PCR from any suspicious lesions and nasal swabs should be submitted to the NRL or EURL. Dead animals should be subjected to a detailed necropsy, including tissue collection (lungs, spleen, liver, lesions, nasal cavity) for bacteriology/PCR analysis (see Decision tree).
– If all tests are negative, a new serological test of all animals in the establishment should be carried out at least 1 month after the initial examination, even in the case the epidemiological investigation rules out an epidemiological link with a confirmed outbreak or case. In the case of positive serological results on at least one serum sample, expertise of the EURL should be requested to confirm or rule out the disease. Indeed, this constitutes a strong suspicion and, given the seriousness of the disease for both animal and public health, the EURL should be systematically involved in the investigations in cooperation with the respective NRL. In that case, additional sampling for complementary analyses, on request of the EURL, could help in identifying the source of the initial positive test result (cross‐reaction).
– In the event of negative results to a direct diagnostic test (bacteriology or PCR) but of positive serological results on at least one serum sample, expertise of the EURL should be required to confirm or rule out the disease. As above, a serological test of all animals in the establishment should be carried out 1 month after the initial examination, even in the case the epidemiological investigation rules out an epidemiological link with a confirmed outbreak or case. In the case of positive serological results on at least one serum sample, expertise of the EURL should be requested to confirm or rule out the disease. Again, additional sampling could help in identifying the source of the initial positive test result (cross‐reaction).
– In the event of a positive result to a direct diagnostic test, glanders should be considered as confirmed and appropriate samples (and, if relevant, the strain isolated) should be sent to the EURL for further investigation.
Situation 3: existence of an epidemiological link with a confirmed outbreak/case. Although no clinical signs have been reported, this is a follow‐up from a confirmed outbreak. In the event of imported listed animals or fomites, a clinical examination of all animals of the listed species (ending with those that have been moved) should be carried out every 3 weeks (over a 6‐month period). Samples should be taken at each clinical examination from all animals of listed species for serology.
– In the event of positive serological results on at least one serum sample, the procedure described in Situation 1 should be followed.
– In the event any clinical signs (or pathological sign in the event of a sudden death) evocative of glanders are found, samples should be collected from lesions and/or nasal secretions for being examined in bacteriology/PCR, even in the absence of positive serological result.
In any case, expertise of the EURL should be requested for further investigation to confirm or rule out the disease. Additional sampling could help in identifying the source of the positive serological test results (cross‐reaction).
4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an establishment affected and officially confirmed with Burkholderia mallei
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. For further details, see Annex B.
2nd Scenario of sampling procedures
ToR 1.2 in accordance with Mandate
Article 12(3) and the Art. 7 (4) (Preventive killing) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
The following elements of the scenario were taken into consideration for the assessment:
It concerns an affected establishment officially confirmed;
Kept animals of listed species found dead or before/when they are killed are sampled;
Competent authority collects samples for laboratory examination;
The purposes of the sampling are:
a) supporting the epidemiological enquiry to:
identify the likely origin of the disease;
calculate the likely length of time that the disease is present;
identify establishments where the animals could have contracted the disease and movements from the affected establishment that could have led to the spread of the disease; and
obtain information on the likely spread of the listed disease in the surrounding environment, including the presence and distribution of disease vectors
b) confirming/ruling out disease in the event of preventive killing.
Summary of sampling procedures
No existing guidelines.
Assessment
Length of infection
Because infection may be more or less acute depending on the infected animal species (see Section 2.1), it may be difficult to assess the length of time the bacterium has been present based on the age of lesions. Serological tests will not provide information on how long disease has been present, because of the presence of chronic infections and the cross reactivity with environmental B. pseudomallei. Molecular tests (e.g. SNP‐based typing methods) may be used to help in identifying the geographic origin of the bacterium.
Information collected from the infected animal having been introduced can be used to estimate the date and origin of entry. If no source can be identified but a strain can be isolated or genetic material is available, it might also be possible to trace the origin with backward/forward tracings.
Origin of the infection
Analysis of animal movements (introduction of new animals, participation in equestrian events…) within the establishment concerned, including fomites, should make possible to identify the source of contamination.
Development of new procedures
In the event of a confirmation, all animals of listed species present in the establishment should be serologically tested as described for Scenario 1.
If the epidemiological investigation suggests some links (either infected animals or contaminated fomites) with animals outside the establishment, clinical examination (or necropsy in case of sudden death) and serological sampling of all contact animals (outside the establishment) should be carried out at least once a month during a 6‐month period (as described in Scenario 1 Situation 3).
To help with the epidemiological investigation in a confirmed establishment (Situation 1, 2 or 3), post‐mortem sampling from all confirmed cases is recommended. In the event of a confirmation, all animals of listed species present in the establishment should be serologically tested as described for Scenario 1.
Genomic information about the B. mallei strain isolated in the affected holding can also be useful for determining possible links between different infected establishments and for determining the geographic area of origin of the contamination. Different molecular markers are available (see Section 2.1). This information can be obtained from the isolated strains and possibly from the infected tissues. If the epidemiological investigation suggests some links (either infected animals or contaminated fomites) with animals outside the establishment, clinical examination (or necropsy in case of sudden death) and serological sampling of all contact animals (outside the establishment) should be carried out as described in Scenario 1 Situation 3.
If the bacterium is isolated, genome sequencing could help in elucidating the origin of the disease.
To confirm/rule out of disease in an establishment, where preventive killing is carried out, sampling should be as described in Scenario 1. However, the preventive culling is unlikely, because glanders is a disease with no tendency to spread rapidly both within and outside the affected establishment, as long as it is quarantined and under active surveillance (Scenario 1).
4.1.1.3. For granting a specific derogation from killing animals of the categories described in article 13.2 of the Delegated Regulation in a glanders affected establishment
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. For further details, see Annex B.
3rd Scenario of sampling procedure
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 13(3)c of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration during for the assessment:
It concerns an affected establishment where infection is officially confirmed;
-
In the establishment where there are kept animals of listed species of the following specific categories animal categories based on article 13(2):
animals kept in a confined establishment
animals kept for scientific purposes or purposes related to conservation of protected or endangered species
animals officially registered in advance as rare breeds
animals with a duly justified high genetic, cultural or educational value
the competent authority may grant specific derogation from killing all the animals of listed species belonging to any of the above categories in an affected establishment, provided that specific conditions are fulfilled;
The animals should be subjected to clinical surveillance, including laboratory examinations;
Sampling procedures should ensure that the animals do not pose a risk of transmission of the category A disease if left alive
Summary of sampling procedures
No specific sampling procedures have been found in the literature.
Assessment
Given the low number of (suspected) outbreaks of glanders in Europe, knowing the sanitary consequences (zoonosis) and the long movement ban in the event of an outbreak, and knowing that there is no evidence that antibiotic treatment makes an animal less likely to transmit the disease throughout its life, confirmed animals should not be kept alive. The derogation to kill a test‐positive animal (to any test) in a confirmed establishment is not recommended.
Due to the possibility of latency (no clinical signs or antibodies), the consequences for humans (significant mortality in humans even after treatment, delays in treatments or access to treatments) and other animals, test‐negative contact animals being left alive should continue to be considered at risk. If despite this risk, animals are left alive, serological sampling of test‐negative contact animals as described in Scenario 1 Situation 1 should be carried out at least once a month for a 6‐month period after the last positive case.
Development of new procedures
See Section 4.1.1.1.
4.1.1.4. For the animals of non‐listed species kept in a glanders affected establishment
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the bacterium if present in these species. For further details, see Annex B.
4th scenario of sampling procedures.
ToR 1.1 and ToR 1.2 in accordance with Article 14(1) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns an affected establishment officially confirmed
In the affected establishment there are kept animals of non‐listed species of epidemiological relevance for the control of the disease
Animals of non‐listed species are those animals that are not listed in Commission Implementing Regulation (EU) 2018/1882 for each of the category A diseases
The animal species acting purely as mechanical carriers of the bacterium will not be covered
The competent authority is not obliged to carry out the sampling of non‐listed species, but they may establish it in addition to other measures
The purpose of the sampling procedures is to ensure detection of the bacterium in these species
Summary of sampling procedures
No specific sampling procedures have been found in the literature.
Assessment
The disease has been observed in wild felids and it has been reported that dogs can be infected. Members of the Felidae family seem to be particularly susceptible, with cases documented in domesticated cats, tigers, lions, leopards and other felids (Spickler, 2018). No information is available about the progression of the disease, the diagnostic approach and the outcome in these species. Although CFT and mallein tests have been used in the field, no immunological diagnostic tests have been validated in these species.
According to some authors, sheep could be susceptible to glanders but less susceptible than goats, although there are no validated immunological diagnostic tests for sheep.
If there are sudden deaths of sheep or carnivores, a post‐mortem examination and a careful bacteriological investigation are recommended. If clinical signs suggestive of B. mallei infection are found, a bacteriological investigation should be carried out.
Development of new procedures
In an establishment where a case has been confirmed, passive surveillance of carnivores and sheep in the establishment is recommended over 6 months after the cleaning and disinfection of the establishment. In the event any clinical sign (or pathological sign in the event of a sudden death) suggestive of glanders, samples should be collected from lesions and/or nasal secretions for being examined in bacteriology/PCR.
4.1.1.5. For wild animals of the listed species within a glanders affected establishment and its surroundings
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. For further details, see Annex B.
5th scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Article 14(1) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns a glanders affected establishment (officially confirmed)
It refers to wild animals of listed species within the establishment and in the surroundings of the establishment
As listed in Commission Implementing Regulation (EU) 2018/1882 for glanders; the wild animals of listed species animals are those of Equidae, Capra ssp., Camelidae species.
The competent authority may establish these sampling procedures in addition to other measures.
The purpose of the sampling procedures in wild animals of listed species is to ensure the detection of the bacterium, if the bacterium is present in these wild animals
Summary of sampling procedures
No specific sampling procedures have been found in the literature.
Assessment
In the European context, it is not expected that wild camelids would be found in the wild. However, such animals may be present within an establishment affected by glanders (e.g. zoos). Wild Equidae and Capra ssp. may be present within and in the surroundings of the affected establishment.
Development of new procedures
Passive surveillance of wild animals of the listed species within the glanders affected establishment and of wild Equidae and wild Capra ssp. in the surroundings of the affected establishment should be carried out, including a visual inspection of these animals from distance.
Dead animals (and animals with evocative clinical signs, if possible) should be investigated by bacteriology and of PCR over 6 months after cleaning and disinfection.
4.1.2. Assessment of sampling procedures for repopulation purposes
4.1.2.1. For the animals that are kept for the repopulation prior to their introduction
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. For further details, see Annex B.
19th scenario of sampling procedures
ToR 1.5 in accordance with article 59(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulation of a previous affected establishment
Animals intended to repopulation shall be sampled prior to their introduction into the establishment of destination
The samples shall be collected from a representative number of animals to be introduced of each consignment from each establishment or from a representative number of animals of each consignment (if animals are all to be introduced at different times or from different establishments of origin)
Laboratory examinations
The purpose sampling procedures is to rule out the presence of the disease
Summary of sampling procedures as described in the diagnostic manual
No specific sampling procedures were found.
Assessment
If the disease were to be present in Europe, it would be rare and sporadic, therefore no need for clinical or laboratory examination of the animals to be moved is necessary (animals used for repopulation should be from establishments free from the infection).
Development of new procedures
No need for new procedures.
4.1.2.2. In the event of unusual mortalities or clinical signs being notified during the repopulation
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. For further details, see Annex B.
20th scenario of sampling procedures
ToR 1.5 in accordance with article 59(9) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment
Unusual mortalities or clinical signs during the repopulation
The official veterinarians shall without delay collect samples for laboratory examination
The purpose of sampling procedures is to rule out the presence of the disease
Summary of sampling procedures as described in the diagnostic manual
No specific sampling procedures were found.
Assessment
See Scenario 1 Situation 2.
Development of new procedures
See Scenario 1 Situation 2.
4.1.2.3. For animals that have been repopulated
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. For further details, see Annex B.
21st scenario of sampling procedures
ToR 1.5 in accordance with article 59(5) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment
Animals that have been used for repopulation
Laboratory examinations
Sampling procedures to rule out the presence of the disease
Summary of sampling procedures as described in the diagnostic manual
No specific sampling procedures were found.
Assessment
Since the disease is sporadic and animals used for repopulation should be from establishments free from the infection, there is no need for clinical or laboratory examination of the animals.
Development of new procedures
Passive surveillance should be implemented for 6 months after the repopulation.
4.2. Assessment of the length of the monitoring period
The concept of the monitoring period was introduced as a management tool for the investigation and control of suspected and confirmed outbreaks of Category A diseases in terrestrial animals. This tool aimed to standardise the methodology by which relevant authorities responded to suspected and confirmed cases of these diseases. In this regard, a disease‐specific monitoring period was set for each of the 14 diseases included in the Category A list. Throughout the EU legislation, the monitoring period is used as an aid in the control of these diseases, although the specific purpose in which the monitoring period is used varies depending on the articles of the legislation.
The length of the monitoring period for each disease is set out in Annex II of the Commission Delegated Regulation (EU) 2020/687 supplementing the rules laid down in Part III of Regulation (EU) 2016/429 (Animal Health Law).
The table in Annex D in this manuscript describes the seven scenarios for which an assessment of the length of the monitoring period for glanders has been requested.
4.2.1. Results
A database search was carried out, identifying 121 unique references. As no references were available for outbreak data from the EU/EEA, the search was extended to data from the rest of the world and to simulation data. Among the 121 references, three were selected to be included in the qualitative review. The full selection process is displayed in Figure 3.
Figure 3.
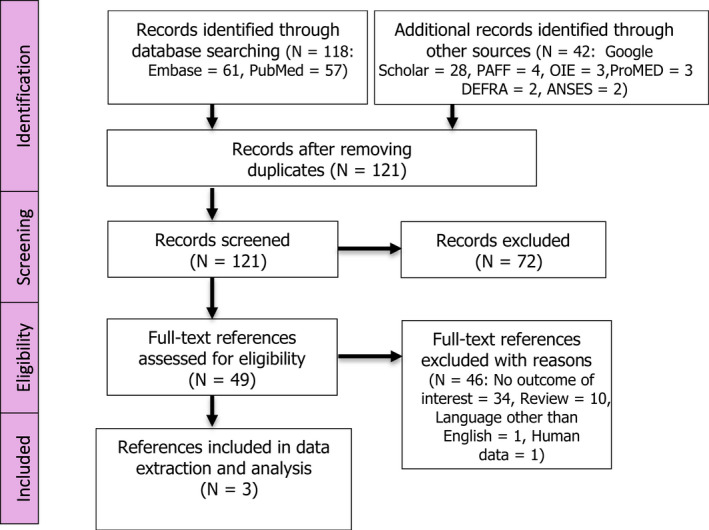
PRISMA diagram glanders Monitoring period ELR
One of the three references reported dates instead of periods, therefore, the dates were used to calculate the different periods of interest (as described in Section 2.1 – PICOS table).
Table 1 provides an overview of the data that were extracted for the main outcome of interest, i.e. the period between the earliest point of infection and the suspicion report, for which a single reference was retrieved:
Table 1.
Summary of the glanders extraction for the period between earliest point of infection and suspicion report: Outbreak data
| Reference | Country | Year | Species | Period (days) |
|---|---|---|---|---|
| ProMED ( 2004 ) | Emirate of Dubai | 2004 | Horse (Equus caballus) | 82 1 |
Secondary outbreak; Based on the arrival date of the index horses imported from another Middle Eastern country.
As described in Table 1, the only available period between the earliest point of infection and the suspicion report was 82 days. It was found in the context of an outbreak that took place in 2004 in the Emirate of Dubai. The index cases consisted of three horses that had been imported from another Middle Eastern country and were detected 2 days after their arrival in Dubai during routine post‐import checks. Glanders was then detected 82 days later in four local horses that had shared post‐import isolation premises with the three imported index cases.
A period of 14 days occurred between the arrival in 2006 in Germany of an infected horse imported from Brazil and the detection of the first unspecific clinical signs of glanders by the local veterinarian. Glanders was finally diagnosed after an undefined inefficient treatment period (Elschner et al., 2009).
Last, in 2010 in Bahrain, two horses were found positive to glanders without clinical signs 6 months after they were imported from Syria and Kuwait (ProMED, 2010).
As no data were available for the period between the first suspicion and suspicion report, we did not reconstruct the period between the earliest point of infection and the suspicion report for glanders.
Seroconversion in animals
To help with the assessment of Scenario 5, the literature regarding challenge studies with B. mallei was reviewed. The search revealed a lack of data on the time to seroconversion in glanderous animals. Based on agglutination, horses showed positive reactions 4–5 days post infection (dpi) with a maximum on day 11 pi (Miessner, 1909). Such antibody levels may decrease as the disease becomes chronic. Based on CFT after mallein injection, complement‐fixing activity was observed in horses 7 dpi (Rice et al., 1951). Also Ackerman et al. (1913) reported positive results in CFT starting from 7 to 10 dpi for the entire course of the disease. Based on this information and expert opinion, it is assumed that horses seroconvert 7–14 dpi.
There is no information available about the time of seroconversion in Capra ssp. and camelids.
4.2.1.1. Assessment
Considering the results presented above, an assessment of the effectiveness of the current monitoring period for glanders, depending on the purpose of that period in the different scenarios shown in Annex D, was carried out. For Burkholderia mallei (glanders), the length of the monitoring period as defined in Annex II of the Delegated Regulation is 6 months.
Scenarios 1, 2 and 3
1sr scenario of monitoring period
ToR 2 in accordance with article 8 and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion of a glanders outbreak
2nd scenario of monitoring period
ToR 2 in accordance with article 17(2) and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of a glanders outbreak
3rd scenario of monitoring period
ToR 2 in accordance with article 13(b) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a glanders outbreak in an epidemiological unit in which the disease has not been confirmed, in order to provide derogations from killing the animals in this unit, if this unit has been completely separated, and handled by different personnel during this monitoring period
For the first three scenarios, the main purpose of the use of the monitoring period is to be able to carry a full epidemiological investigation (i.e. in Scenarios 1 and 2, at the time of the suspicion and confirmation, respectively), or part of the epidemiological investigation (i.e. Scenario 3 where the aim is to identify any possible epidemiological links between the affected establishment and any separated non‐affected epidemiological units). The length of the monitoring period should then dictate how far back or forward the activities related to tracing (and other activities needed during an epidemiological investigation) should go (checks for production records, animal movement records, etc.). This monitoring period is the time where the infection could have been present unknowingly in an establishment, and due to the regular activities carried out in this establishment, could have spread to other epidemiological units. In the case of Scenario 3, if no epidemiological links between the establishment that has been confirmed positive and the other epidemiological units are found during the investigation (and only if other conditions described in the legislation are met), a derogation from killing the animals in the separated non‐affected epidemiological units could be granted.
The period of time when the disease could have been present, unknowingly, in an establishment, equates then to the time period between the entry of the B. mallei strain into the establishment, and the reporting of the suspicion. Once the suspicion has been officially reported, control measures are implemented, and further spread is in this way prevented.
Based on the very scarce data that were available in the literature, we conclude that the current monitoring period for glanders (6 months) is long enough to capture the period between the earliest point of infection and the suspicion report.
Scenario 4
4th scenario of monitoring period
ToR 2 in accordance with article 27(3)c and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the glanders outbreak in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements
The main purpose of the monitoring period in Scenario 4 is to ensure that certain products or materials, likely to spread the disease, that have been produced in a non‐affected establishment located in the protection zone of an affected establishment, can be moved safely and without posing a risk of disease spread. As the protection and surveillance zone is set at the establishment level, this scenario was not considered further.
Scenario 5
5th scenario of monitoring period
ToR 2 in accordance with article 32 (c), article 48(c) and Annex II of the Delegated Regulation (EU) 2020/687
The purpose of this section is to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period
In general, the aim of the monitoring period in this specific scenario is to ensure that semen from animals in a non‐affected establishment (located in a protection or surveillance zone) that has been collected and frozen after the earliest time of infection of the affected establishment that originated the protection zone, is safe to be moved without posing a risk of disease spread. For glanders, and due to the fact that the surveillance and protection zones are limited to the establishment, this scenario would only be relevant in case of an outbreak in a semen collection centre (or any establishment where these activities take place).
In the hypothetical event that an outbreak occurs in a semen collection centre, and due to the fact that the protection and surveillance zones for glanders are based at the establishment level, the assessment refers to semen that was collected prior to the confirmation of the suspicion and after the earliest point of infection, as determined by the epidemiological enquiry.
To assess the status of semen originated from infected horses, the semen itself would need to be tested. It is important to highlight that no information could be retrieved about testing semen for glanders. Nonetheless, venereal transmission of the bacterium from stallions to mares has been reported (Khan et al., 2013; OIE, 2020).
In regard to seronegative horses in the affected establishment, and the use of serological sampling as an indication of the safety of the semen, there is a considerable lack of data regarding the time to seroconversion in glanderous animals (as stated in Section 4.2.1). Nonetheless, and considering the recommendations made in this opinion, every animal of an affected establishment should be tested at least once a month over the length of the monitoring period, and infected animals should be killed (as described in Section 4.1.1). If these recommendations are followed, all the animals remaining in the establishment that had tested negative should not pose a risk for transmitting the disease after the length of the existing monitoring period (i.e. 6 months).
In general, in OIE (2021), a clinical examination of donor males for signs of orchitis and cutaneous lesions on penis or other parts of the body is recommended. Saqib (2009) reported orchitis in 45% of glanderous equines.
Scenarios 6 and 7
6th scenario of monitoring period
ToR 2 in accordance with article 57 (1) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date of the final cleaning and disinfection in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority (assuming relevant control of insects and rodents was carried out).
7th scenario of monitoring period
ToR 2 in accordance with article 59 (4) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date the first animal was introduced for the purpose of repopulation, during this monitoring period, all animals of the listed species intended for repopulation should be introduced.
In Scenarios 6 and 7, the monitoring period is used in the context of repopulation. In Scenario 6, the monitoring period is used to ensure that the repopulation process is not put at risk due to the disease still being present unknowingly in establishments within the surrounding area of the establishment to be repopulated (if an establishment tested positive to B. mallei within a distance equal or lower to the radius of the surveillance zone, the repopulation process could not take place). Repopulation can only take place after a number of days equal to the monitoring period has elapsed since the final cleaning, and disinfection of the affected establishment.
In this regard, the number of days of the monitoring period for glanders, counted from the day of the final cleaning and disinfection must ensure enough time for any potentially infected animal to be reported as a suspicion. Considering the results presented above, in case no animal in an affected establishment has been left alive, the period of 6 months is deemed to be long enough to ensure that the bacterium would not be able to survive in the environment, as its survival time is up to 6 weeks in contaminated stables and less than 2 weeks under most conditions. In the case negative‐tested animals of the affected establishment have been left alive, they have to be tested at least once a month over the length of the monitoring period, and infected animals are to be killed (as described in Section 4.1.1). Following these recommendations, the animals remaining in the establishment should not pose a risk for transmitting the disease to other animals after the length of the existing monitoring period. In case of glanders, introducing all animals for repopulation in a specified period, as foreseen in Scenario 7, is not relevant. To conclude, the existing length of the monitoring period was considered effective for both scenarios, for the restrictions can be lifted and animals can be introduced into the establishment after this period. Passive surveillance should nevertheless be implemented for 6 months after the repopulation.
4.3. Assessment of the minimum radius and time periods of the protection and surveillance zones set in place subsequent to a disease outbreak
4.3.1. Assessment of the minimum radius and the minimum period
Results
The purpose of this section is to assess the effectiveness to control the spread of glanders by implementing a protection and surveillance zones of a minimum radius, as set out in Annex V of the Delegated Regulation, surrounding the establishment where the disease has been confirmed. Based on this regulation, the minimum radius of the protection and surveillance zone for glanders should be the affected establishment (see Annex E).
Assessment
Glanders affects mainly domestic equids. The causative agent is an obligate animal and zoonotic pathogen with a limited capacity to survive outside the mammalian host. Under most conditions, it is likely to survive in the environment for less than 2 weeks. The transmission could be direct between infected and uninfected animals, or indirect by means of drinking water or through feed. Indirect transmission can also occur through contact with fomites. The risk from fomites relates to equipment used in close contact with horses, such as tack, surgical equipment, grooming equipment, rather than clothing or transport, while environmental contamination such as water run‐off or horse manure is unlikely to be a source of infection for nearby premises because there is relatively poor survival in the environment and there is no evidence of short distance spread through other means. Therefore, the disease generally spreads outside the establishment only through the movement of infected animals or the displacement of certain contaminated fomites. The protection and surveillance zones are planned to define the establishments where the disease should be investigated; therefore, these zones would not be applicable for glanders, as the spread pathways can be detected through the epidemiological investigation of movements, rather than related to proximity to the original case. Protection and surveillance zones are more applicable to epidemic diseases for which the probability of disease spread is depending on distance to the farm of origin. The existing protection and surveillance zones set at the establishment level are considered effective.
4.3.2. Uncertainty analysis
Although several sources of uncertainty were identified during the scientific assessment (see Annex F), their impact on the outputs of the assessment could not be quantified.
5. Conclusions and recommendations
| Sampling procedure | Laboratory guidelines | Conclusions | Recommendations |
|---|---|---|---|
| ToR 1: In the event of suspicion or confirmation | |||
|
1st scenario 4.1.1.1 In the event of a suspicion of glanders in an establishment where animals of the listed species are kept |
No specific guidelines on sampling procedures for clinical or laboratory examination in the event of a suspicion of glanders are available in the EU legislation. |
A case of B. mallei infection is suspected following a positive serological result in a pre‐movement screening test (Situation 1) or following the observation of clinical or necropsy signs suggestive of glanders in a live or dead animal (Situation 2), leading to investigations in farms with an epidemiological link to an outbreak or a confirmed case (Situation 3). The confirmation of a clinical suspicion is based on laboratory tests, mainly by confirming the presence of the bacteria by culture, of the nucleic acid (PCR) or of antibodies (CFT, ELISA). The collection of specimens for bacteriology or PCR testing can be performed either on dead or live animals, when clinical or pathological signs are apparent. CFT‐positive results should be corroborated by another positive result using CFT and/or other complementary tests (ELISA, Immunoblot, etc.) on the same serum sample, carried out by a national reference laboratory (NRL) when possible, and with the support of the EURL if necessary, according to OIE requirements. |
Considering the rarity and the severity of the disease for both listed species and humans, when the presence of B. mallei is confirmed in the national reference lab, samples should also be sent to the EURL for confirmatory testing and/or complementary investigation. Affected animals should be culled and all other animals of listed species should be tested to rule out the presence of clinically healthy infected individuals. |
|
2nd scenario 4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in a glanders officially confirmed establishment |
There are no sampling procedures defined for the purposes of the epidemiological enquiry in an establishment affected and officially confirmed with glanders. |
Information collected from the infected animal being introduced can be used to estimate the date and origin of entry. If no source can be identified but a strain can be isolated or genetic material is available, it might be possible to trace the origin with backward/forward tracings. |
Considering the rarity and the severity of the disease for both listed species and humans, it is important that the EURL has access to samples of infected animals and/or the isolated strain so that appropriate molecular epidemiology studies can be conducted to identify the source of infection. |
|
3rd scenario 4.1.1.3. For granting a specific derogation from killing animals of the categories of article 13.2 of the Delegated Regulation in a glanders affected establishment |
There are no sampling procedures to grant a derogation from killing of animals in a glanders affected establishment. |
Confirmed animals should not be kept alive. If, despite this risk, infected animals are left alive, these animals should be placed in lifelong quarantine or isolation with breeding restrictions. The negative in‐contact animals of listed species should be clinically examined and serologically sampled once a month for 6 months after the last positive case. |
The derogation to kill a positive animal is not recommended. |
|
4th scenario 4.1.1.4. For the animals of non‐listed species kept in a glanders affected establishment. |
There are no sampling procedures defined for non‐listed species kept in a glanders affected establishment | The listed species for glanders are Equidae, Capra ssp. and Camelidae. Where other susceptible domestic animals (sheep and carnivores such as cats) are also kept in the affected establishment, passive surveillance of such animals in the establishment should be carried out over 6 months after the cleaning and disinfection of the establishment. In the event any clinical sign (or pathological sign in the event of a sudden death) evocative of glanders, samples should be collected from lesions and/or nasal secretions for being examined in bacteriology/PCR. | No need for new procedures. |
|
5th scenario 4.1.1.5. For wild animals of the listed species within the glanders affected establishment and its surroundings. |
There are no sampling procedures defined for wild animals of the listed species within the glanders affected establishment and its surroundings | In the scenario where wild equids, camelids or wild goats are kept or living in the surrounding area of the affected establishment, they may acquire the infection by direct or indirect contact with affected animals if no or low biosecurity measures are in place to keep animal species separated. | The surveillance of wildlife around the affected establishment should include the visual inspection of these animals from distance and the testing of fallen stock and hunted animals by both bacteriology/PCR. |
| ToR 1: For repopulation purposes | |||
|
19th scenario 4.1.3.1 For the animals that are kept for the repopulation prior to their introduction |
There are no sampling procedures | If the disease were to be present in Europe, it would be rare and sporadic, therefore no clinical or laboratory examination of the animals to be moved is necessary (animals used for repopulation should be from establishments free from the infection). | No need for new procedures. |
|
20th scenario 4.1.3.2 In the event of unusual mortalities or clinical signs being notified during the repopulation |
There are no sampling procedures | Scenario 1 Situation 2 | All animals in the establishment should be subjected to detailed clinical examination and follow what suggested in Scenario 1 Situation 2 |
|
21st scenario 4.1.3.3 For animals that have been repopulated |
There are no sampling procedures | Since the disease is sporadic and animals used for repopulation should be from establishments free from the infection, there is no need for clinical or laboratory examination of the animals. | Passive surveillance for 6 months after repopulation |
| ToR 2 | ||
|---|---|---|
| Description | Conclusions | Recommendations |
|
4.2 Assessment of the length of the monitoring period of glanders |
Based on the very scarce data that were available in the literature, it would be concluded that the current Monitoring Period for glanders (6 months) is long enough to capture the period between the earliest point of infection and the suspicion report. Conversely, there is no data available to justify a reduction in the length of this period (recommended by OIE standards), especially since the incubation period could span, in equids in particular, from 6 days to several months. The existing length of the monitoring period was considered effective in all scenarios. |
The length of the monitoring period for glanders shall be 6 months. No further recommendations. |
| ToR 3 | ||
|---|---|---|
| Description | Conclusions | Recommendations |
|
4.3.1 Assessment of the minimum radius and the minimum length of time |
The disease generally spreads outside the establishment through the movement of infected animals or the displacement of contaminated fomites. PZ and SZ are planned to define the farms where the disease should be investigated; therefore, it seems not applicable for glanders for which the spread is not related to proximity to the original case. The existing protection and surveillance zone set at the establishment level is considered effective |
The minimum radius of the protection and surveillance zone for glanders should be the affected establishment. The length of the minimum period of the affected establishments is 6 months. |
Abbreviations
- ASF
African swine fever
- AHS
African horse sickness
- CSF
Classical swine fever
- CBPP
Contagious bovine pleuropneumonia
- CCPP
Contagious caprine pleuropneumonia
- dpi
days post inoculation
- ELISA
enzyme‐linked immunosorbent assay
- ELS
extensive literature search
- FMD
Foot and mouth disease
- FMDV
Foot and mouth disease virus
- HPAI
Highly Pathogenic Avian Influenza
- LSD
Lumpy skin disease virus
- NDV
Newcastle disease virus
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- PZ
protection zone
- RP
rinderpest virus
- RT‐PCR
reverse transcription polymerase chain reaction
- RVFV
Rift Valley fever virus
- SPGP
Sheep pox and goat pox
- SZ
surveillance zone
- ToR
Terms of Reference
Annex A – Definitions in EU legislation
1.
| Terms | Definitions |
|---|---|
| Clinical examination | The clinical examination comprises: (i) an initial general evaluation of the animal health status of the establishment which comprises all the animals of listed species kept in the establishment; and (ii) an individual examination of the animals included in the sample referred to in point (a). The sampling of animals for clinical examination is carried out in accordance with point A.1 of Annex I for terrestrial animals (Delegated Regulation article 3). |
| Confined establishment | Means any permanent, geographically limited establishment, created on a voluntary basis and approved for the purpose of movements, where the animals are: (a) kept or bred for the purposes of exhibitions, education, the conservation of species or research; (b) confined and separated from the surrounding environment; and (c) subject to animal health surveillance and biosecurity measures; (AHL: Regulation 2016/429 article 4(48)). |
| Epidemiological unit | Means a group of animals with the same likelihood of exposure to a disease agent (AHL: Regulation 2016/429 article 4(39)). |
| Establishment | Means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics (AHL: Regulation 2016/429 article 4(27)). |
| Health status | Means the disease status as regards the listed diseases relevant for a particular listed species with respect to: (a) an animal; (b) animals within: (i) an epidemiological unit; (ii) an establishment; (iii) a zone; (iv) a compartment; (v) a Member State; (vi) a third country or territory (AHL: Regulation 2016/429 article 4(34)). |
| Infected zone | Means a zone in which restrictions on the movements of kept and wild animals or products and other disease control and biosecurity measures may be applied with the view to preventing the spread of a category A disease in the event of official confirmation of the disease in wild animals (Delegated Regulation article 2(15)). |
| Kept animals | Means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; (AHL: Regulation 2016/429 article 4(5)). |
| Outbreak | Means the officially confirmed occurrence of a listed disease or an emerging disease in one or more animals in an establishment or other place where animals are kept or located (AHL: Regulation 2016/429 article 4 (40)). |
| Protection zone | Means a zone around and including the location of an outbreak, where disease control measures are applied in order to prevent the spread of the disease from that zone (AHL: Regulation 2016/429 article 4(42)). |
| Listed diseases |
Means diseases listed in accordance with Article 5(1); (AHL: Regulation 2016/429 article 4 (18)). List of the diseases (AHL: Regulation 2016/429, Annex II). |
| Listed species |
Means an animal species or group of animal species listed in accordance with Article 8(2), or, in the case of emerging diseases, an animal species or group of animal species which meets the criteria for listed species laid down in Article 8(2) (AHL: Regulation 2016/429 article 4(20)). List of species and groups of species (Commission Implemented Regulation 2018/1882). |
| Monitoring periods | It is appropriate to follow a single approach for the measures to apply in the event of a category A disease. However, the epidemiology of diseases should be taken into account to establish the appropriate moment for the competent authority to apply control measures and to carry out investigations if there is suspicion or confirmation of those diseases. Therefore, ‘monitoring periods’ should be provided, as reference time frames for each category A disease affecting terrestrial animals based on incubation periods and other relevant elements that may affect the spread of the disease (Delegated Regulation whereas 10). |
| Restricted zone | Means a zone in which restrictions on the movements of certain animals or products and other disease control measures are applied, with a view to preventing the spread of a particular disease into areas where no restrictions are applied; a restricted zone may, when relevant, include protection and surveillance zones (AHL: Regulation 2016/429 article 4(41)). |
| Surveillance zone | Means a zone which is established around the protection zone, and where disease control measures are applied in order to prevent the spread of the disease from the protection zone (AHL: Regulation 2016/429 article 4(43)). |
| Wild animals | Means animals which are not kept animals (AHL: Regulation 2016/429 article 4(8)). |
| Zone | Means: (a) for terrestrial animals, an area of a Member State, third country or territory with a precise geographical delimitation, containing an animal subpopulation with a distinct health status with respect to a specific disease or specific diseases subject to appropriate surveillance, disease control and biosecurity measures (AHL: Regulation 2016/429 article 4 (35)). |
Annex B –Scenarios of ToR 1
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenario |
|---|---|---|---|---|
| In the event of suspicion or confirmation | ||||
|
ToR 1.1 ToR 1.2 |
6(2) of the Delegated Regulation | 1st scenario | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
|
|
ToR 1.2 |
Art. 12(3), Art. 7 (4) (Preventive killing) of the Delegated Regulation, and Art. 57 Reg.2016/429 |
2nd scenario | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. |
|
|
ToR 1.1 ToR 1.2 |
Article 13(3)c of the Delegated Regulation | 3rd scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
|
|
ToR 1.1 ToR 1.2 |
Article 14(1) of the Delegated Regulation Art. 57 Reg.2016/429 |
4th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. |
|
|
ToR 1.1 ToR 1.2 |
Article 14(1) of the Delegated Regulation Art. 57 Reg.2016/429 |
5th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species |
|
|
ToR 1.1 ToR 1.2 |
Article 26(2) of the Delegated Regulation | 6th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. |
|
|
ToR 1.3 |
Article 26(5) of the Delegated Regulation point A.3 of Annex I |
7th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone |
|
| ToR 1.3 | Article 41 of the Delegated Regulation | 8th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone |
|
| Derogations to allow animal movements | ||||
| ToR 1.4 |
Article 28(5) of the Delegated Regulation Article 29 of the Delegated Regulation |
9th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29) |
|
| ToR 1.4 |
Article 28(5) and Article 30(1) of the Delegated Regulation |
10th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day‐old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day‐old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone |
|
| ToR 1.4 |
Article 28(5) and Article 30(2) of the Delegated Regulation |
11th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the protection zone to establishments located in the same MS and if possible within the restricted zone. |
|
| ToR 1.4 |
Article 28(5) and Article 37 of the Delegated Regulation |
12th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37) |
|
| ToR 1.4 |
Article 43(5) and Article 44 of the Delegated Regulation |
13th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
|
| ToR 1.4 |
Article 43(5) and Article 45(1) of the Delegated Regulation |
14th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone |
|
| ToR 1.4 |
Article 43(5) and Article 45(2) of the Delegated Regulation |
15th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter |
|
| ToR 1.4 |
Article 43(5) and Article 46(1) of the Delegated Regulation |
16th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day‐old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched |
|
| ToR 1.4 |
Article 43(5) and Article 46(2) of the Delegated Regulation |
17th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the surveillance zone to establishments located in the same MS. |
|
| ToR 1.4 | Article 56(1)c of the Delegated Regulation | 18th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI |
|
| Repopulation | ||||
| ToR 1.5 | Article 59(2),(3) of the Delegated Regulation | 19th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(9) of the Delegated Regulation | 20th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(5) of the Delegated Regulation | 21st scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. |
|
Annex C –Existing sampling procedures for glanders
1.
Sampling scenarios for glanders
| Scenario | Description of the Scenario | Clinical guidelines | Laboratory guidelines |
|---|---|---|---|
| 1st | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR1.1) and laboratory examination (TOR1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
No specific guidelines described in legislation Note: Council Directive 2009/156/EC: CHAPTER II: RULES FOR THE MOVEMENT OF EQUIDAE BETWEEN MEMBER STATES Article 3 Member States shall authorise the movement of registered equidae in their territory or send equidae to another Member State only where they satisfy the conditions laid down in Articles 4 and 5. … Article 4. … 5. In addition to the requirements laid down in Article 5, the equidae must not come from a holding which has been the subject of one of the following prohibition orders: (a) if all the animals of species susceptible to the disease located on the holding have not been slaughtered, the period of prohibition concerning the holding of origin must be at least: … (ii) six months in the case of glanders or equine encephalomyelitis, beginning on the day on which the equidae suffering from the disease in question are slaughtered; … CHAPTER III: RULES FOR IMPORTATION OF EQUIDAE FROM THIRD COUNTRIES Article 13 1. The equidae must come from third countries which: …(c) have been free for 6 months from dourine and glanders. OIE Terrestrial Code (OIE, 2021 ): Article 12.10.5. Recommendations for importation of equids from countries or zones not free from infection with B. mallei Veterinary Authorities should require the presentation of an international veterinary certificate attesting that the equid: 1) showed no clinical signs of infection with B. mallei on the day of shipment; 2) was kept for 6 months prior to shipment, or since birth, in an establishment where no case of infection with B. mallei was reported during the 12 months prior to shipment; 3) was isolated for at least 30 days prior to shipment, and during that time was subjected to a test for infection with B. mallei with negative result carried out on two samples taken 21–30 days apart. … Article 12.10.8. General principles of surveillance: Diagnosticians and those with regular contact with equids, including private veterinarians, veterinary paraprofessionals and animal handlers should report promptly any suspicion of infection with B. mallei. The reporting system efficacy should be enhanced by awareness programmes and animal identification of equids. The Veterinary Services should implement, when relevant and taking into account the results of previous surveillance, regular and frequent clinical inspections of equids and targeted serological surveys of high‐risk subpopulations or those neighbouring a country or zone infected with B. mallei. … Article 12.10.9. Surveillance strategies: … Clinical or pathological surveillance and laboratory testing are complementary diagnostic approaches that should always be applied in series to clarify the status of suspected cases. Agent identification should be carried out on any equid serologically positive or showing clinical signs consistent with glanders. Any suspected cases should be considered infected until contrary evidence is produced. 1. Clinical surveillance Clinical surveillance aims at detecting clinical signs by close physical examination of equids. However, systematic clinical surveillance is of limited use only, as asymptomatic carrier animals are the main reservoir of the disease. 2. Pathological surveillance Systematic pathological surveillance is an effective approach for the detection of infection with B. mallei and should be conducted on dead equids on farms, at slaughterhouses/abattoirs and facilities for the disposal of carcasses of equids. Pathological findings indicating possible infection with B. mallei should be confirmed by agent identification and any isolate should be characterised. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals ( OIE, 2018 ): B. Diagnostic technique 1. Identification of the agent. Cases for specific glanders investigation should be differentiated on clinical grounds from other chronic infections affecting the nasal mucous membranes, sinuses or the skin. Among these are strangles (Streptococcus equi), ulcerative lymphangitis (Corynebacterium pseudotuberculosis), pseudotuberculosis (Yersinia pseudotuberculosis) and sporotrichosis (Sporotrichium spp.). Glanders should be excluded from suspected cases of epizootic lymphangitis (Histoplasma farciminosum), with which it has many clinical similarities. In horses and humans in particular, glanders should be distinguished from melioidosis. Arrêté du 21 novembre 2011 sur la morve des équidés (Journal officiel de la République française, 2011) : Art. 2. − Dès la mise en évidence de: – tout équidé faisant l’objet d’une suspicion clinique ou d’un résultat positif à une épreuve diagnostique de la morve des équidés; – tout équidé importé en France à partir d’une zone où a été déclaré un cas de morve des équidés depuis moins de six mois après le départ de l’animal de cette zone, ou de tout équidé importé en France et ayant transité dans une zone où a été déclaré un cas de morve des équidés depuis moins de six mois après le passage de l’animal dans la zone, le préfet prend, sur proposition du directeur départemental en charge de la protection des populations, un arrêté préfectoral de mise sous surveillance de l’équidé concerné ou de l’établissement dans lequel il est détenu conformément aux instructions du ministre chargé de l’agriculture (direction générale de l’alimentation). Cet arrêté entraîne l’application des mesures de surveillance définies par instructions du ministre chargé de l’agriculture (direction générale de l’alimentation). L’arrêté préfectoral de mise sous surveillance est levé dès que tout risque d’infection de morve est écarté. Art. 3. − Au sens du présent arrêté, est considéré comme : 1° Equidé infecté: – équidé chez qui Burkholderia mallei a été isolée et identifiée; ou – équidé contaminé qui exprime un tableau clinique évocateur de morve des équidés, ou qui présente à l’autopsie un tableau nécropsique évocateur de morve des équidés, ou qui présente un résultat positif à une épreuve diagnostique de morve des équidés; 2° Equidé contaminé: équidé appartenant à un établissement infecté et ne répondant pas à la définition d’un équidé infecté; Avis de l’Afssa sur le diagnostic de la morve ( Afssa, 2018 ): p.2 : … Dans la forme chronique, les symptômes peuvent être d’apparition tardive et n’être ainsi observés qu’après plusieurs semaines voire plusieurs mois. La symptomatologie peut être assez fruste et la maladie passer inaperçue. Les animaux atteints des formes occultes chroniques ou sub‐cliniques constituent de dangereuses sources d’infection, et pourraient représenter jusqu’à 90% des cas d’infection. Dans les formes cliniques, l’incubation varie, chez les équidés, d’une à deux semaines à parfois plusieurs mois. Aux fins d’application des dispositions énoncées dans le Code sanitaire pour les animaux terrestres de l’OIE, la période maximale d’incubation est fixée à six mois. Foreign animal diseases (USAHA (United States Animal Health Association), 2008): Chap.24. GLANDERS. p.283: 6. CLINICAL SIGNS Classical descriptions of glanders distinguish between cutaneous, nasal, and pulmonary forms of the disease, but in most outbreaks these forms are not clearly distinct and may occur simultaneously in an animal. Chronic infections with slow progression of an insidious disease are more common than the acute form of glanders. The acute form (more common in donkeys and mules than in horses) typically progresses to death within about a week. p.284: 9. DIAGNOSIS a. Field diagnosis Typical nodules, ulcers, scars, and a debilitated condition can be sufficient to diagnose glanders. Unfortunately, many cases of glanders are latent and clinically inapparent. Therefore, systematic testing is essential to identify all infected animals in an outbreak. |
OIE Terrestrial Code (OIE, 2021 ): Article 12.10.1. The following defines the occurrence of infection with B. mallei: 1) B. mallei has been isolated from a sample from an equid; or 2) antigen or genetic material specific to B. mallei has been identified in a sample from an equid showing clinical or pathological signs consistent with glanders, or epidemiologically linked to a confirmed or suspected case of infection with B. mallei, or giving cause for suspicion of previous contact with B. mallei; or 3) antibodies specific to B. mallei have been detected by a testing regime appropriate to the species in a sample from an equid showing clinical or pathological signs consistent with glanders, or epidemiologically linked to a confirmed or suspected case of infection with B. mallei, or giving cause for suspicion of previous contact with B. mallei. Article 12.10.8. General principles of surveillance: An effective surveillance system is likely to identify suspected cases that require follow‐up investigation to confirm or exclude that the cause of the condition is infection with B. mallei. All suspected cases should be investigated as soon as possible and samples should be taken and submitted to a laboratory. This requires that sampling kits and other equipment be available to those responsible for the surveillance. Details of the occurrence of suspected cases and how they were investigated and dealt with should be documented. This should include the results of diagnostic testing and the control measures to which the equids concerned or affected establishments were subjected during the investigation (quarantine, movement control, euthanasia). Captive wild, feral and wild equine populations should be included in the surveillance. Article 12.10.9. Surveillance strategies: … The relatively high rate of occurrence of false positive reactions to tests for B. mallei should be considered and the rate at which these false positives are likely to occur should be calculated in advance. Every positive result should be investigated to determine whether it is indicative of infection or not. This involves supplementary tests, trace‐back and trace‐forward, and inspection of individual animals and herds for clinical signs. Clinical or pathological surveillance and laboratory testing are complementary diagnostic approaches that should always be applied in series to clarify the status of suspected cases. Agent identification should be carried out on any equid serologically positive or showing clinical signs consistent with glanders. Any suspected cases should be considered infected until contrary evidence is produced. Serological surveillance Serological surveillance for infection with B. mallei is the preferred strategy. Animal identification and repeated testing of the population are necessary to establish its infection status. Malleinisation Frequently used as a surveillance method, malleinisation demonstrates hypersensitivity to antigens of B. mallei. However, this method has shortcomings, such as low sensitivity, interference with other tests and animal welfare concerns. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (OIE, 2018 ): B. Diagnostic technique Table 1: Test methods available for the diagnosis of glanders and their purpose: purpose of “confirmation of clinical cases”: – Confirmation of the agent: PCR, Culture, – Detection of immune response: complement fixation, ELISA, Mallein skin test, Western blotting 1. Interpretation of tests for the diagnostic of glanders. Confirmation of a diagnosis of glanders should be based on the isolation and identification of Burkholderia mallei in a sample from an equid or a product derived from that equid; or the identification in such samples of antigen or genetic material specific to B. mallei. Supporting evidence may be provided by positive serological test results such as a titre of 1/5 in the complement fixation test (CFT), confirmed by a second test with equal or higher sensitivity and higher specificity, e.g. B. mallei‐specific lipopolysaccharide (LPS)‐western blot, I‐ELISA (indirect enzyme‐linked immunosorbent assay) (based on a recombinant protein from type VI secretion system) or C‐ELISA (competitive ELISA) (based on B. mallei‐specific monoclonal antibodies). … 4.1. The mallein test …The test is not generally recommended because of animal welfare concerns, however it can be useful in remote endemic areas where sample transport or proper cooling of samples is not possible. It depends on infected horses being hypersensitive to mallein. Advanced clinical cases in horses and acute cases in donkeys and mules may give inconclusive results requiring additional diagnostic methods. Avis de l’Afssa sur le diagnostic de la morve (Afssa, 2018 ): p.7 : … toute suspicion clinique devrait entraîner un contrôle sérologique et la mise en oeuvre d’un diagnostic bactériologique par culture pour isolement et caractérisation de B. mallei à partir des exsudats (incluant les sécrétions respiratoires) ou des lésions après mort ou abattage de l’animal. Toute suspicion nécropsique, avec mise en évidence de lésions (macroscopiques et microscopiques) évocatrices, après mort ou abattage de l’animal, devrait conduire à la même démarche. Une recherche par PCR spécifique peut être aussi envisagée, d’autant que dans les formes chroniques ou inapparentes, B. mallei est en faible quantité dans les lésions et difficile à isoler. pp.7–8: …compte tenu de la rareté de la morve et des conséquences sanitaires et économiques de la déclaration d’un cas de morve des équidés, le CES SA recommande que: ‐ Les épreuves sérologiques agréées, la recherche de B. mallei par culture et la PCR spécifique soient mises en oeuvre exclusivement au LNR (Afssa Maisons‐Alfort); ‐ Le résultat d’une épreuve sérologique agréée soit considéré comme positif lorsque ce résultat est obtenu sur deux échantillons de sérum prélevés à un mois d’intervalle; ‐ Tout sérum présentant une réaction positive à une épreuve sérologique agréée fasse l’objet d’une confirmation par le laboratoire de référence de l’OIE pour la morve (FLI, Iéna, Allemagne) 20. Foreign animal diseases (USAHA (United States Animal Health Association), 2008): Chap.24. GLANDERS. 9. DIAGNOSIS p.285: b. ii. Laboratory diagnosis: The causative organism may be cultured from fresh lesions or lymph nodes. It may also be demonstrated microscopically in films made from this material. … A variety of serologic tests for glanders has been developed. These are superior to mallein testing in sensitivity and specificity. The complement fixation test is widely used and is reported to have an overall accuracy of 95%. A counterimmunoelectrophoresis test has been described. Recently a dot enzyme‐linked immunosorbent assay has been developed and found to be superior to all previously described tests in its sensitivity. This test is inexpensive, rapid, and easy to perform and is not influenced by anticomplement activity. Cross‐reactions with B. pseudomallei, the cause of melioidosis, are features of all of the serological tests for glanders. Therefore, these tests will result in false positive reactions in animals from areas where melioidosis is endemic. Surveillance of glanders (Gonzalez‐Medina et al., 2015): The isolation of bacteria from samples collected from clinically affected animals is commonly unsuccessful, so a negative result from culture does not mean that the horse is free from infection as the presence of the bacteria in the various exudates changes over the course of disease. Post‐mortem examination is usually necessary to detect the organism by culture or PCR. Therefore, to rule out the possibility of glanders infection in live animals, serological tests are used for initial diagnosis. …. If, however, the suspicion of glanders cannot be ruled out, samples will have to be taken and submitted by the APHA to the UK’s National Reference Laboratory for glanders in Weybridge. Initially, these are blood samples for serology. In some circumstances further samples, such as nasopharyngeal or skin swabs, or a sample of the nasal or skin discharge, may be required. Most often, the suspicion of glanders arises during routine laboratory testing, if clinically healthy horses are serologically tested as part of pre‐export requirements. Although the standard serological test (the complement fixation test [CFT]) is an accurate test in general, non‐specific reactions occasionally happen which need to be differentiated from a true infection. In Great Britain, the APHA follows up all glanders test results that are not clearly negative by attending the premises, examining the horse, gathering epidemiological information, such as travel history, to assess the risk of the result potentially being a true positive. Further samples are also taken for confirmatory testing, where the CFT is repeated but other tests, such as immunoblot and, if appropriate, PCR, are used to reach a conclusion. |
| 2nd | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. | NA |
OIE Terrestrial Code (OIE, 2021 ): Article 12.10.9. Surveillance strategies: Clinical or pathological surveillance and laboratory testing are complementary diagnostic approaches that should always be applied in series to clarify the status of suspected cases. Agent identification should be carried out on any equid serologically positive or showing clinical signs consistent with glanders. Any suspected cases should be considered infected until contrary evidence is produced. … 2. Pathological surveillance Systematic pathological surveillance is an effective approach for the detection of infection with B. mallei and should be conducted on dead equids on farms, at slaughterhouses/abattoirs and facilities for the disposal of carcasses of equids. Pathological findings indicating possible infection with B. mallei should be confirmed by agent identification and any isolate should be characterised. 3. Serological surveillance Serological surveillance for infection with B. mallei is the preferred strategy. Animal identification and repeated testing of the population are necessary to establish its infection status. 4. Malleinisation Frequently used as a surveillance method, malleinisation demonstrates hypersensitivity to antigens of B. mallei. However, this method has shortcomings, such as low sensitivity, interference with other tests and animal welfare concerns. Arrêté du 21 novembre 2011 sur la morve des équidés (Journal officiel de la République française, 2011): Art. 5. ‐ Lorsque l’existence d’un ou plusieurs équidés infectés est confirmée, le préfet prend, sur proposition du directeur départemental chargé de la protection des populations, un arrêté portant déclaration d’infection de l’établissement infecté, entraînant l’application des mesures suivantes : – visite, recensement et contrôle de l’identification des équidés et des animaux d’autres espèces sensibles présents dans l’établissement; – une enquête épidémiologique destinée à détecter l’origine ou à prévenir la propagation de la maladie est mise en oeuvre et vise notamment à identifier tous les équidés ayant pu être en contact avec les équidés infectés dans les six mois précédant l’identification de l’infection. Ces équidés font l’objet de mesures de surveillance définies par instructions du ministre chargé de l’agriculture; … ‐ les équidés contaminés (équidé contaminé=équidé appartenant à un établissement infecté et ne répondant pas à la définition d’un équidé infecté) doivent faire l’objet, durant les six mois suivant l’élimination du dernier équidé infecté de morve, d’une épreuve diagnostique et d’une surveillance clinique mensuelles, ainsi que nécropsique pour les équidés décédés. |
| 3rd | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
No specific guidelines described in legislation Note: Arrêté du 21 novembre 2011 sur la morve des équidés (Journal officiel de la République française, 2011): Art. 5. − Lorsque l’existence d’un ou plusieurs équidés infectés est confirmée, le préfet prend, sur proposition du directeur départemental chargé de la protection des populations, un arrêté portant déclaration d’infection de l’établissement infecté, entraînant l’application des mesures suivantes: … – les équidés infectés de morve doivent être euthanasiés sans délai avec destruction du cadavre à l’équarrissage; |
No specific guidelines described in legislation |
| 4th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. |
No specific guidelines described in legislation Note: OIE Terrestrial Code (OIE, 2021 ): General provisions: Equids are the major hosts and reservoirs of glanders although scientific data are not available on the occurrence of infection in zebras. Camelids, goats and various carnivores including bears, canids and felids can also be infected but play no significant role in the epidemiology of the disease. Glanders in humans is a rare but potentially fatal disease. |
No specific guidelines described in legislation |
| 5th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. |
OIE Terrestrial Code (OIE, 2021 ): Article 12.10.8. General principles of surveillance: … Captive wild, feral and wild equine populations should be included in the surveillance. |
No specific guidelines described in legislation |
| 6th | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. | NA | NA |
| 7th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone. | NA | NA |
| 8th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone. | NA | NA |
| Derogations to allow animal movements | |||
| 9th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). | NA | NA |
| 10th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day‐old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day‐old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone. | NA | NA |
| 11th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the protection zone, to establishments located in the same Member State and if possible within the restricted zone. | NA | NA |
| 12th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37). | NA | NA |
| 13th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. | NA | NA |
| 14th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone. | NA | NA |
| 15th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow for them to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. | NA | NA |
| 16th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day‐old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched. | NA | NA |
| 17th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the surveillance zone to establishments located in the same Member State. | NA | NA |
| 18th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI. | NA | NA |
| Repopulation | |||
| 19th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. | NA | NA |
| 20th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. | NA | NA |
| 21st | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. | NA | NA |
Annex D –Scenarios of ToR 2
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenarios |
|---|---|---|---|---|
| ToR 2 |
Article 8 of the Delegated Regulation Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation |
1st scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion. |
|
| ToR 2 |
Article 17(2) and Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation |
2nd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of the disease. |
|
| ToR 2 |
Article 13(b) of the Delegated Regulation Annex II of the Delegated Regulation |
3rd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a category A disease in an establishment with kept animals of listed species, during which the epidemiological units in which the disease has not been confirmed were kept completely separated and handled by different personnel, in order to provide derogations from killing. |
|
| ToR 2 |
Article 27(3)c of the Delegated Regulation Annex II of the Delegated Regulation |
4th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the latest outbreak of a category A disease in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements. |
|
| ToR 2 |
Article 32(c) of the Delegated Regulation Article 48(c) of the Delegated Regulation Annex II of the Delegated Regulation |
5th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period. |
|
| ToR 2 |
Article 57(1)b of the Delegated Regulation Annex II of the Delegated Regulation |
6th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards from the date after the final cleaning and disinfection and when relevant control of insects and rodents was carried out in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority. |
|
| ToR 2 |
Article 59(4)b of the Delegated Regulation Annex II of the Delegated Regulation |
7th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards the date when the first animal was introduced, during which all the animals of listed species intended for repopulation should be introduced. |
|
Annex E –Minimum radius and minimum period of duration of protection and surveillance zones
1.
| Category A diseases | Minimum radius of Protection zone Annex V | Minimum radius of Surveillance zone Annex V | Minimum period of duration of measures in the protection zone (Article 39(1)) Annex X | Additional period of duration of surveillance measures in the protection zone (Article 39(3)) Annex X | Minimum period of duration of measures in the surveillance zone (as referred to in Articles 55 and 56 of this Regulation) Annex XI |
|---|---|---|---|---|---|
| Foot and mouth disease (FMD) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Infection with rinderpest virus (RP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with Rift Valley fever virus (RVFV) | 20 km | 50 km | 30 days | 15 days | 45 days |
| Infection with lumpy skin disease virus (LSD) | 20 km | 50 km | 28 days | 17 days | 45 days |
| Infection with Mycoplasma mycoides subsp. mycoides SC (Contagious bovine pleuropneumonia) (CBPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| Sheep pox and goat pox (SPGP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with peste des petits ruminant virus (PPR) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Contagious caprine pleuropneumonia (CCPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| African horse sickness (AHS) | 100 km | 150 km | 12 months | Not applicable | 12 months |
| Infection with Burkholderia mallei (Glanders) | Establishment | Establishment | 6 months | Not applicable | Not applicable |
| Classical swine fever (CSF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| African swine fever (ASF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Highly pathogenic avian influenza (HPAI) | 3 km | 10 km | 21 day | 9 days | 30 days |
| Infection with Newcastle disease virus (NDV) | 3 km | 10 km | 21 days | 9 days | 30 days |
Annex F –Uncertainty
1.
| Source or location of the uncertainty | # | Nature or cause of uncertainty as described by the experts | Impact of the uncertainty on the assessment |
|---|---|---|---|
| ToR 1 | 1 | Clinical presentation in horses may vary largely, and infections may be often chronic and even subclinical for a large period of time, thus complicating the identification of infected animals based on clinical signs. | The effectiveness of the proposed sampling strategies could be overestimated. |
| 2 | CFT, the most commonly used serological test for diagnosis of glanders, is difficult to standardise and its sensitivity and specificity may vary depending on the antigen used among other factors. | The effectiveness of the proposed sampling strategies could be overestimated. | |
| 3 | Culture‐based detection of B. mallei may be complicated due to its slow growth nature and the usually low concentration of the pathogen in tissue samples, and although PCRs may offer a higher sensitivity many PCR systems have not been fully validated and are only available in highly specialised laboratories. | The effectiveness of the proposed sampling strategies could be overestimated. | |
| ToR 2 | 4 | Very limited evidence on the time between infection and suspicion/reporting was available (only three references of which two originated from outside the EU). | The effectiveness of the proposed strategy could be over‐ or underestimated. |
| 5 | Very limited data was available on the time to seroconversion in horses, and no information was retrieved for other species (Capra ssp. and camelids). | The effectiveness of the proposed strategy could be over‐ or underestimated. | |
| ToR 3 | 6 | There is very limited knowledge on the possible risk associated with contaminated fomites for the spread of the disease to neighbouring farms. | The effectiveness of the proposed strategy could be overestimated. |
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Schmidt CG, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Laroucau K, Antoniou S‐E, Aznar I, Broglia A, Lima E, Van der Stede Y, Zancanaro G and Roberts HC, 2022. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: Burkholderia mallei (Glanders). EFSA Journal 2022;20(1):7069, 60 pp. 10.2903/j.efsa.2022.7069
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00802
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar Schmidt, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: P95, Evangelos Tsiamadis, Verena Oswaldi from EFSA and Mandy Elschner from the FLI.
Adopted: 15 December 2021
Notes
A latent infection is one that persists in an animal, in which there are no overt clinical signs. Based on the definition of latency in Thrusfield M, 2007. Veterinary epidemiology, 3rd Edition. Blackwell Science Ltd., Oxford. Available online: www.blackwellpublishing.com
References
- Ackerman EB, Eichhorn A, McGilvray CD, Cotton C, Kaene C and Reichel J, 1913. Report on detection of glanders. Proceedings of the Fiftieth Meeting American Veterinary Assn.: Annual Meeting, 50, 291–306. [Google Scholar]
- AFSSA , 2018. Avis de l’Agence française de sécurité sanitaire des aliments sur le diagnostic de la morve. Afssa, Saisine no 2008‐SA‐0326. Available online: https://www.anses.fr/fr/system/files/SANT2008sa0326 [Google Scholar]
- EFSA (European Food Safety Authority) , Alvarez J, Roberts HC, Stahl K, Viltrop A, Clercq KD, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Zancanaro G and Aznar I, 2020. Technical report on the methodological approach used for the assessment of the control measures for Category A diseases in the context of the new Animal Health Law. EFSA Supporting Publications 2020:EN-1988, 25 pp. 10.2903/sp.efsa.2020.EN-1988 [DOI] [Google Scholar]
- Elschner M, Klaus C, Liebler‐Tenorio E, Schmoock G, Wohlsein P, Tinschmann O, Lange E, Kaden V, Klopfleisch R and Melzer F, 2009. Burkholderia mallei infection in a horse imported from Brazil. Equine Veterinary Education, 21, 147–150. [Google Scholar]
- Elschner MC, Scholz HC, Melzer F, Saqib M, Marten P, Rassbach A, Dietzsch M, Schmoock G, de Assis Santana VL, de Souza MMA, Wernery R, Wernery U and Neubauer H, 2011. Use of a Western blot technique for the serodiagnosis of glanders. BMC Veterinary Research, 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elschner MC, Liebler‐Tenorio E, Brügmann M, Melzer F and Neubauer H, 2016. Re‐appearance of glanders into Western Europe: clinical, laboratory and pathological findings in a German horse. OIE Sci Tech Rev Bull, 1, 76–79. 10.20506/bull.2016.1.2502 [DOI] [Google Scholar]
- Elschner MC, Neubauer H and Sprague LD, 2017. The Resurrection of glanders in a new epidemiological scenario: a beneficiary of “global change”. Current Clinical Microbiology Reports, 4, 54–60. [Google Scholar]
- Elschner MC, Melzer F, Singha H, Muhammad S, Gardner I and Neubauer H, 2021. Validation of a Commercial Glanders ELISA as an Alternative to the CFT in International Trade of Equidae. Frontiers in Veterinary. Science, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault G, Wattiau P, Saqib M, Martin B, Vorimore F, Singha H, Engelsma M, Roest HJ, Spicic S, Grunow R, Vicari N, De Keersmaecker S, Roosens N, Fabbi M, Tripathi BN, Zientara S, Madani N and Laroucau K, 2018. High‐resolution melting PCR analysis for rapid genotyping of Burkholderia mallei. Infection, Genetics and Evolution, 63, 1–4. [DOI] [PubMed] [Google Scholar]
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R and Spratt BG, 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. Journal of Clinical Microbiology, 41, 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Medina S, Toth B and Mawhinney I, 2015. Surveillance focus: glanders. Veterinary Record, 177, 68–69. [DOI] [PubMed] [Google Scholar]
- Hornstra H, Pearson T, Georgia S, Liguori A, Dale J, Price E, O’Neill M, DeShazer D, Muhammad G, Saqib M, Naureen A and Keim P 2009. Molecular epidemiology of glanders, Pakistan. Emerging Infectious Diseases, 15, 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Kung C and Au B, 1958. Study on the experimental infection of cattle, sheep and goats with Glanders Bacilli. Chinese Journal of Animal and Veterinary Sciences. [Google Scholar]
- Journal officiel de la République française , 2011. Ministère de l’agriculture, de l’alimentation, de la pêche, de la ruralité et de l’aménagement du territoire. Arrêté du 21 novembre 2011 fixant certaines mesures techniques et administratives relatives à la police sanitaire de la morve des équidés NOR : AGRG1131605A. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000024893044/2015‐08‐11/
- Kettle AN and Wernery U, 2016. Glanders and the risk for its introduction through the international movement of horses. Equine Veterinary Journal, 48, 654–658. [DOI] [PubMed] [Google Scholar]
- Khan I, Wieler L, Melzer F, Gwida M, Santana VdA, de Souza M, Saqib M, Elschner M and Neubauer H, 2011. Comparative evaluation of three commercially available complement fixation test antigens for the diagnosis of glanders. Veterinary Record, 169, 495. [DOI] [PubMed] [Google Scholar]
- Khan I, Wieler L, Melzer F, Elschner M, Muhammad G, Ali S, Sprague L, Neubauer H and Saqib M, 2013. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transboundary and Emerging Diseases, 60, 204–221. 10.1111/j.1865-1682.2012.01342.x [DOI] [PubMed] [Google Scholar]
- Khan I, Wieler L, Saqib M, Melzer F, Santana V, Neubauer H and Elschner M, 2014. Effect of incubation temperature on the diagnostic sensitivity of the glanders complement fixation test. Revue Scientifique Et Technique, 33, 869–875. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Cloutier AK, Rozak DA, Khan MS, Niwa H, Uchida‐Fujii E, Katayama Y and Tuanyok A, 2019. A novel selective medium for the isolation of Burkholderia mallei from equine specimens. BMC Veterinary Research, 15, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroucau K, Aaziz R, Vorimore F, Varghese K, Deshayes T, Bertin C, Delannoy S, Sami AM, Al Batel M, El Shorbagy M, Almutawaa KAW, Alanezi SJ, Alazemi YSN, Guernier‐Cambert V and Wernery U, 2021. A genetic variant of Burkholderia mallei detected in Kuwait: consequences for the PCR diagnosis of glanders. Transboundary and Emerging Diseases, 68, 960–963. 10.1111/tbed.13777 [DOI] [PubMed] [Google Scholar]
- Lefèvre P and Blancou J, 2010. Glanders. In: Lefèvre P, Blancou J, Chermette R and Uilenberg G (eds.). Infectious and Parasitic Diseases of Livestock. CABI, Paris, France. [Google Scholar]
- Loeffler FAJ, 1886. Die Aetiologie der Rotzkrankheit, J. Springer. [Google Scholar]
- Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Stanley Kim H, Shabalina SA, Pearson TR, Brinkac L, Tan P, Nandi T, Crabtree J, Badger J, Beckstrom‐Sternberg S, Saqib M, Schutzer SE, Keim P and Nierman WC, 2010. Continuing evolution of Burkholderia mallei through genome reduction and large‐scale rearrangements. Genome Biology and Evolution, 2, 102–116. 10.1093/gbe/evq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik P, 2016. Harmonising diagnostic testing for glanders in equids. The Veterinary Record, 178, 630. [DOI] [PubMed] [Google Scholar]
- Miessner H, 1909. Die Schnellagglutination und ihre Verwendung bei der Serodiagnose des Rotzes. Centralblatt F. Bakteriologie, 48, 249–253. [Google Scholar]
- Neubauer H, Sprague L, Zacharia R, Tomaso H, Al Dahouk S, Wernery R, Wernery U and Scholz H, 2005. Serodiagnosis of Burkholderia mallei infections in horses: state‐of‐the‐art and perspectives. Journal of Veterinary Medicine, Series B, 52, 201–205. [DOI] [PubMed] [Google Scholar]
- OIE , 2018. OIE Terrestrial Manual, Chapter 3.5.11. Glanders and Melioidosis, OIE, Paris, France. pp. 1350–1362. Available online: https://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/3.05.11_GLANDERS.pdf [Google Scholar]
- OIE , 2020. Technical disease card, Glanders, OIE, Paris, France. Available online: https://www.oie.int/app/uploads/2021/03/glanders.pdf. [Google Scholar]
- OIE , 2021. Terrestrial Animal Health Code, Chapter 12.10. Infection with Burkholderia mallei (Glanders), OIE, Paris, France. Available online: https://www.oie.int/en/what‐we‐do/standards/codes‐and‐manuals/terrestrial‐code‐online‐access/?id=169&L=1&htmfile=chapitre_glanders.htm. [Google Scholar]
- ProMED , 2004. PRO/AH/EDR> Glanders, equine ‐ United Arab Emirates: OIE. Available online: https://promedmail.org/promed‐posts/
- ProMED , 2010. PRO/AH/EDR> Glanders, equine ‐ Bahrain. Available online: https://promedmail.org/promed‐posts/
- Rice CE, Konst H and Duthie R, 1951. Studies by complement‐fixation methods of malleins produced in broth and synthetic media. 1. relative immunizing activities in horses and rabbits. Canadian journal of comparative medicine and veterinary science, 15, 284. [PMC free article] [PubMed]
- Saqib M, 2009. Equine glanders: (I) Development and evalution of latex agglutination test, (II) Biochemical and molecular characterization of Burkholderia mallei isolates, and (III) Efficacy of experimental therapy. University of Agriculture, Faisalabad, Pakistan. [Google Scholar]
- Scholz HC, Pearson T, Hornstra H, Projahn M, Terzioglu R, Wernery R, Georgi E, Riehm JM, Wagner DM, Keim PS, Joseph M, Johnson B, Kinne J, Jose S, Hepp CM, Witte A and Wernery U, 2014. Genotyping of Burkholderia mallei from an outbreak of glanders in Bahrain suggests multiple introduction events. PLoS Neglected Tropical Diseases, 8, e3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha H, Vorimore F, Saini S, Deshayes T, Saqib M, Tripathi BN and Laroucau K, 2021. Molecular epidemiology of Burkholderia mallei isolates from India (2015–2016): new SNP markers for strain tracing. Infection, Genetics and Evolution, 95, 105059. 10.1016/j.meegid.2021.105059 [DOI] [PubMed] [Google Scholar]
- Spickler AR, 2018. Center for food security and public health (CFSPH). Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/glanders.pdf [Google Scholar]
- U'Ren JM, Schupp JM, Pearson T, Hornstra H, Friedman CLC, Smith KL, Daugherty RRL, Rhoton SD, Leadem B, Georgia S, Cardon M, Huynh LY, DeShazer D, Harvey SP, Robison R, Gal D, Mayo MJ, Wagner D, Currie BJ and Keim P, 2007. Tandem repeat regions within the Burkholderia pseudomallei genome and their application for high resolution genotyping. BMC Microbiology, 7, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USAHA (United States Animal Health Association) Committee on Foreign and Emerging D, 2008. Foreign animal diseases. Available online: https://www.usaha.org/upload/Disease%20Info/FAD.pdf
- Van der Lugt JJ and Bishop GC, 2004. Glanders. In: Coetzer JAW and Tustin RC (eds.). Infectious Diseases of Livestock. Oxford University Press, Cape Town. pp. 1500–1504. [Google Scholar]
- Wernery U, Wernery R, Joseph M, Al‐Salloom F, Johnson B, Kinne J, Jose S, Jose S, Tappendorf B and Hornstra H, 2011. Natural Burkholderia mallei infection in dromedary. Bahrain. Emerging Infectious Diseases, 17, 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]


