Abstract
Conduct disorder (CD), characterized by youth antisocial behavior, is associated with a variety of neurocognitive impairments. However, questions remain regarding the neural underpinnings of these impairments. To investigate novel neural mechanisms that may support these neurocognitive abnormalities, the present study applied a graph analysis to resting-state functional magnetic resonance imaging (fMRI) data collected from a national sample of 4,781 youth, ages 9–10, who participated in the baseline session of the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®). Analyses were then conducted to examine the relationships among levels of CD symptomatology, metrics of global topology, node-level metrics for subcortical structures, and performance on neurocognitive assessments. Youth higher on CD displayed higher global clustering (β = .039, 95% CIcorrected [.0027 .0771]), but lower Degreesubcortical (β = −.052, 95% CIcorrected [−.0916 −.0152]). Youth higher on CD had worse performance on a general neurocognitive assessment (β = −.104, 95% CI [−.1328 −.0763]) and an emotion recognition memory assessment (β = −.061, 95% CI [−.0919 −.0290]). Finally, global clustering mediated the relationship between CD and general neurocognitive functioning (indirect β = −.002, 95% CI [−.0044 −.0002]), and Degreesubcortical mediated the relationship between CD and emotion recognition memory performance (indirect β = −.002, 95% CI [−.0046 −.0005]). CD appears associated with neuro-topological abnormalities and these abnormalities may represent neural mechanisms supporting CD-related neurocognitive disruptions.
Keywords: conduct disorder, graph analysis, neural topology, neurocognitive functioning, subcortical structures
Conduct disorder (CD) is a developmental disorder associated with engagement in aggressive, rule-breaking, destructive, and deceitful behaviors during childhood and/or adolescence. CD is associated with poor academic achievement, employment outcomes, and peer and family relationships (Brown, Jaffe, Silverstein, & Magee, 1991; Moffitt, 1993; Offord & Bennett, 1994; Woolfenden, Williams, & Peat, 2002). One well-studied factor related to the poorer psychosocial outcomes in youth with CD is the presence of neurocognitive difficulties (Blair, Leibenluft, & Pine, 2014; Moffitt, 1993; Nigg & Huang-Pollock, 2003; Ogilvie, Stewart, Chan, & Shum, 2011; Teichner & Golden, 2000; Viding & Jones, 2008).
Across many empirical studies, review papers, and meta-analyses, CD symptomatology reliably associates with neurocognitive difficulties in executive functioning (e.g., response inhibition, working memory, etc.; Frick, 2012; Moffitt, 1993; Morgan & Lilienfeld, 2000; Ogilvie et al., 2011), decision-making (see Blair et al., 2014 for review), verbal abilities, and general intellect (see Moffitt, 1993 for review). For example, within the domain of executive functioning, studies show that youth with CD struggle to inhibit prepotent responses, particularly in potentially rewarding contexts (Estrada, Tillem, Stuppy-Sullivan, & Baskin-Sommers, 2019; Fairchild et al., 2009; Hobson, Scott, & Rubia, 2011; Schoorl, van Rijn, de Wied, Van Goozen, & Swaab, 2018). As another example, research across decision-making paradigms demonstrates that youth with CD exhibit deficits in the prediction-error response, failures in contingency learning, and increased rates of risky decision-making (see Blair et al., 2014; Estrada et al., 2019 for reviews). Given the multifaceted neurocognitive abnormalities documented in youth with CD, some researchers suggest that broad, domain-general, neurocognitive disruptions present in youth with CD may play a role in the maintenance of their antisocial behavior and result in psychosocial problems that persist throughout development (Moffitt, 1993). Regardless of whether researchers focus on domain-specific neurocognitive dysfunction in CD or on domain-general neurocognitive dysfunction in CD, there is consensus that these CD-related neurocognitive deficits are rooted in aberrant neural structure, functioning, and/or connectivity (Blair et al., 2014).
Neuroimaging research demonstrates that youth with CD display several functional and structural abnormalities. CD correlates with functional and structural differences in the amygdala, hippocampus, caudate, orbital frontal cortex, anterior cingulate cortex, superior temporal gyrus, prefrontal cortex, insula, and fusiform gyrus (Baker, Clanton, Rogers, & De Brito, 2015; Noordermeer, Luman, & Oosterlaan, 2016; Rogers & De Brito, 2016; Waller et al., 2020). Beyond these region-specific differences, CD relates to atypical neural communication between various neural structures. For example, in resting-state functional magnetic resonance imaging (rs-fMRI) studies, youth with CD show reduced cortical–subcortical functional connectivity (e.g., between the amygdala and prefrontal cortex; Finger et al., 2012) and reduced functional connectivity between structures within the default network (e.g., between the precuneus and temporalparietal junction; Broulidakis et al., 2016; Lu et al., 2015; Zhou et al., 2016), but increased internetwork connectivity to frontoparietal network structures (e.g., between the frontoparietal network and the inferior frontal gyri; Aghajani et al., 2016; Cohn et al., 2015). Similarly, in diffusion tensor imaging (DTI) studies, youth with CD display abnormalities in the microstructural integrity of white matter tracks connecting cortical and subcortical structures (Passamonti et al., 2012), frontal lobe and temporal lobe structures (Haney-Caron, Caprihan, & Stevens, 2014; Sarkar et al., 2013), and cortical hemispheres (Menks et al., 2017; Zhang et al., 2014). The neural abnormalities documented in youth with CD are clearly complex and widespread, impacting multiple levels of functioning throughout the entire brain, often in mixed directions (i.e., decreased connectivity between some structures but increased connectivity between others). This type of complexity presents challenges when attempting to unpack how these various neural abnormalities may interact with each other to ultimately produce the aberrant neurocognitive profile associated with CD.
Graph theory: global metrics
Recent advances in the application of graph theory provide a new avenue for researchers to disentangle these types of complex, widespread disruptions (Bullmore & Sporns, 2009; Stam & Reijneveld, 2007). Rather than looking at the structure or responsivity of specific neural structures, or even the strength of neural connectivity between pairs of neural structures, graph analysis allows for a higher-level examination of the overall organization, or topology, of entire neural networks or even the brain as a whole. More specifically, graph analysis takes connectivity data from every single possible set of connections throughout an entire network, or throughout the entire brain, and utilizes that data to generate a “graph.” These graphs consist of a series of “nodes” (i.e., parts of the graph which represent a specific neural structure or set of structures averaged together) connected by various “edges” (i.e., lines directly linking different nodes meant to represent “true” connections present in the network/brain) and can act as a visual and mathematical representation of the topology of neural information flow throughout the entire network/brain. Moreover, researchers can extract quantifiable metrics from these graphs which reflect different global properties of neural information processing occurring within that network, as a whole, such as the global efficiency and/or robustness to disruption of the neural communication occurring within that network. These types of global graph network properties are thought to play a critical role in effective neurocognitive functioning, with more optimal (e.g., more efficient and/or robust) network topologies supporting more optimal neurocognitive functioning (e.g., better executive functioning and/or higher general intellect; Bullmore & Sporns, 2009; Hadley et al., 2016; Langer et al., 2012; Liu et al., 2008; Neubauer & Fink, 2009; Schoonheim et al., 2014; Shu et al., 2016; Stam & Reijneveld, 2007; Suprano et al., 2019; Tewarie et al., 2014; van den Heuvel, Mandl, Stam, Kahn, & Hulshoff Pol, 2010).
To date, however, only two studies utilize graph analytic approaches to examine neural topologies in CD. Jiang et al. (2016) find that CD is associated with less efficiently organized structural topology at a whole-brain level. By contrast, Lu, Zhou, Zhang, Wang, and Yuan (2017) report that CD is not significantly related to global abnormalities in the functional topology of the brain. While both of these studies provide invaluable insight into how CD may be related to alterations in the global topology of the brain, as a whole, they also have several limitations that impede their ability to fully explore the relationships among CD, global topology, and neurocognitive functioning.
First, and most critically, neither study examines behavioral metrics of neurocognitive functioning (e.g., executive functioning task or general intellect assessment performance). Accordingly, neither study can fully and specifically explore the relationships among CD, global neural topology, and direct behavioral measures of neurocognitive functioning, leaving any potential explanations for how CD-related alterations in global neural topology relate to neurocognitive functioning in CD purely speculative. Second, the sample size in Lu et al. (2017) is small (n = 18 adolescents with CD), raising the possibility that the reported null effect reflects a lack of statistical power. Third, Jiang et al. (2016) use an indirect measure of structural connectivity (gray matter thickness correlations), which means that the actual relationship between CD and alterations in the structural topology of white matter tracks (e.g., as measured by DTI) is unmeasured. Finally, since these studies are limited to examining adolescent samples (e.g., ages 13–17), the relationship between CD and global neural topology earlier in childhood (e.g., prior to age 13) remains unexamined.
Given these limitations in the prior research, the first set of aims for the current study are to use rs-fMRI data taken from a large national sample of children (ages 9–10) to: (a) assess whether CD is associated with global abnormalities in neural topology at a whole-brain level and (b) directly evaluate whether global abnormalities relate to neurocognitive impairments associated with CD. Given that CD is related to neurocognitive disruptions across multiple broad domains of neurocognitive functioning, including general intellect (Blair et al., 2014; Moffitt, 1993; Nigg & Huang-Pollock, 2003; Ogilvie et al., 2011; Teichner & Golden, 2000; Viding & Jones, 2008), and it remains unclear which specific domain of neurocognitive functioning, if any, may be central to CD, the current study evaluates whether CD-related alterations in whole-brain neural topology may mediate the relationship between CD and a domain-general measure of neurocognitive functioning.
Graph theory: node metrics
Graph analyses are not limited to these types of global analyses evaluating higher-level properties of a network or brain as a whole. Graph analyses also can examine how specific neural structures, or nodes, differentially influence the flow of information throughout an entire network/brain. These types of node-level analyses allow researchers to evaluate whether specific parts of the brain (i.e., specific nodes) may have different characteristics within a network across individuals or groups. For example, there may be individual- or group-level differences in the hubness of a node (i.e., the number of direct connections between a specific node and other nodes), the centrality of a node (i.e., how centrally located a specific node is in the global flow of information throughout a network/brain), and/or the local efficiency of a node (i.e., how quickly and effectively a specific node may be able to communicate with other nodes in its immediate “neighborhood”). Moreover, depending upon the specific node in question, and the neurocognitive functions that node is believed to support, alterations in these node-level properties may have serious implications for neurocognitive functioning. For instance, it has been theorized that, if subcortical structures (e.g., the amygdala, hippocampus, caudate, etc.) are less centrally located in the flow of information throughout the brain, then neurocognitive processes which rely on those structures (e.g., affective responding, memory, and reward and punishment processing; Baas, Aleman, & Kahn, 2004; Eichenbaum, 2001; Knutson & Cooper, 2005) may be less able to influence various aspects of cognition (e.g., decision-making; see Tillem et al., 2019).
To date, research examining node-level analyses in CD is limited. However, two findings suggest that node-level alterations in the hubness or centrality of subcortical structures, in particular, may be relevant for CD. First, while Lu et al. (2017) reported that CD is not associated with node-level differences, they also report that traits relevant to CD (e.g., trait impulsivity) are related to differences in the hubness of subcortical structures. More specifically, they show that in youth higher on trait impulsivity, the amygdala, a subcortical structure critical for affective responding (Baas et al., 2004), is less directly connected to other parts of the brain, and, therefore, may act as less of a “hub” of information flow in these youth. Second, Lindner et al. (2018) posited that the hippocampus, a subcortical structure critical for memory encoding and retrieval (Eichenbaum, 2001), may play a less central role in global information processing in older adolescent and young adult females higher on “social deviance” (defined by trait impulsivity and chronic, lifetime engagement in antisocial behavior, including CD symptomatology). These findings, combined with extensive prior work showing abnormalities in youth with CD in subcortical functioning, subcortical structure, cortical–subcortical functional connectivity, and cortical–subcortical structural connectivity (Baker et al., 2015; Dotterer et al., 2020; Finger et al., 2012; Noordermeer et al., 2016; Passamonti et al., 2012; Rogers & De Brito, 2016; Waller et al., 2020), highlight subcortical structures as regions of interest in youth with CD.
Moreover, prior theoretical accounts of violent antisocial behavior, in both youth and adult populations, focus on the potential role of cortical–subcortical communication. For example, the violence inhibition model (VIM; Blair, 1995)1 posits that neurotypical individuals have difficulty engaging in aggressive and antisocial behavior against other agents due to an inhibitory mechanism, mediated by amygdala–prefrontal circuitry, which is automatically activated in response to another agent’s distress cues (e.g., another agent’s fearful facial expression, crying, etc.). For individuals who engage in aggressive and antisocial behavior, such as youth with CD, it is possible that this amygdala–prefrontal communication is disrupted, impairing this inhibitory mechanism, and allowing these individuals to more easily engage in violent antisocial behavior even in the face of another agent’s distress cues.
Despite work highlighting subcortical functioning and connectivity as a potential mechanistic factor in youth CD, the link between subcortical node-level abnormalities and disruptions in relevant neurocognitive functions (e.g., emotion, memory, etc.) has not been investigated directly in either neurotypical or CD samples. Accordingly, the second set of aims for the current study are to: (a) examine the relationship between CD and node-level abnormalities in subcortical structures, and (b) evaluate whether node-level abnormalities relate to specific neurocognitive functions believed to rely on subcortical structures. Since abnormalities in encoding, decoding, and/or responding to other agent’s distress cues are relevant for antisocial behavior (VIM; Blair, 1995) and subcortical structures, such as the amygdala and hippocampus, play central roles in affective responding and memory, the current study examines whether any CD-related node-level abnormalities for subcortical structures mediate the relationship between CD and performance on an emotion recognition memory task (Baas et al., 2004; Eichenbaum, 2001; Keightley, Chiew, Anderson, & Grady, 2011).
The present study
In the present study, we conducted an unweighted, undirected graph analysis on rs-fMRI connectivity metrics included in the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®) baseline data (release 2.0.1; DOI 10.15154/1504041), extracting both global and node-level graph theory metrics. Then, we conducted two sets of analyses to examine the relationships among CD symptomatology, graph theory metrics of neural topology (global and node-level metrics), and behavioral measures of neurocognitive functioning (domain-general neurocognitive assessment and emotion recognition memory task). Together, these analyses allowed us to determine whether CD was linked to abnormalities in the topology of neural communication, and evaluate if these potential abnormalities mediated the relationship between CD and neurocognitive functioning.
Method and Materials
Participants
Participants were children, ages 9–10 years old, who completed the baseline session of the multisite ABCD Study. Details regarding the sampling strategy, sample norms, and sample composition previously have been described elsewhere (Garavan et al., 2018). All study procedures were approved by a centralized institutional review board at the University of California San Diego and/or by each site’s institutional review board (Clark et al., 2018). Parents provided signed informed consent and children provided written assent prior to the study. For the present analyses, participants were included if they: (a) had symptom-level CD data available from their baseline session, (b) were not missing any demographic data (e.g., age, sex, race, or data collection site), and (c) had valid rs-fMRI data released from their baseline session that also passed the ABCD Study overall MRI quality checks described by Hagler et al. (2019). Based on these inclusion criteria, the initial sample size was n = 5,615. Given the large number of ABCD Study families with multiple children and/or twins that participated in the study, siblings were overrepresented in the sample (Iacono et al., 2018). To help control for any family-related effects, only one, randomly selected, child per family was used in the current analyses, yielding a sample of n = 4,781 (see Table 1 for demographics summary).
Table 1.
Sample characteristics and zero-order correlations (N = 4781)
| Variable | N | Mean | Std. Dev. | Min | Max | Correlations | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | 3b | 4 | ||||||
| 1. Age | 4,781 | 9.51 | .51 | 8.00 | 11.00 | – | .01 | −.04* | −.05* |
| 2. Sexa | 4,781 | – | −.02 | .08* | |||||
| Male | 2,343 | ||||||||
| Female | 2,438 | ||||||||
| 3. Raceb | 4,781 | – | .09* | ||||||
| White | 2,483 | ||||||||
| Black | 713 | ||||||||
| Hispanic | 999 | ||||||||
| Other | 586 | ||||||||
| 4. CD symptomatology | 4,781 | .30 | .80 | .00 | 10.00 | – | |||
| CD diagnosis | 219 | ||||||||
| No CD diagnosis | 4,562 | ||||||||
Spearman correlations were used to examine the effect of Sex (dichotomously-coded).
Spearman correlations were used to examine the effect of Race (dichotomously-coded, white versus non-white).
95% Bootstrapped CI does not contain 0.
CD symptomatology
Kiddie schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL; Kaufman et al., 2013)
The K-SADS-PL is an evaluation of symptoms and diagnoses related to Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5 CD) criteria in children and adolescents between the ages of 6 and 18. In the ABCD Study protocol, the K-SADS-PL is computerized and self-administered by the parents or primary caregivers of youth participants. Approximately 4.6% (n = 219) of the current sample met criteria for a CD diagnosis, which is consistent with the national prevalence rate of approximately 4.4% reported for young adolescents in the United States (Merikangas et al., 2010). The current study used CD symptom counts as a continuous measure of CD.
Neurocognitive measures
National Institutes of Health (NIH) Toolbox cognition battery (Gershon, Wagster, et al., 2013)
The NIH Toolbox cognition battery is a battery of seven different neurocognitive tasks, including: a list sorting task assessing working memory (Tulsky et al., 2014), a picture vocabulary task assessing language and verbal abilities (Gershon et al., 2014), a Flanker task assessing cognitive control and attention (Fan, McCandliss, Sommer, Raz, & Posner, 2002), a dimensional change card sorting task assessing cognitive flexibility (Zelazo, 2006), a pattern comparison task assessing visual processing speed (Carlozzi, Beaumont, Tulsky, & Gershon, 2015; Carlozzi, Tulsky, Kail, & Beaumont, 2013; Carlozzi et al., 2014), a picture sequence task assessing episodic memory and visuospatial sequencing (Bauer et al., 2013; Dikmen et al., 2014), and an oral reading task assessing reading ability (Gershon, Slotkin, et al., 2013). The corrected, total cognition summary T-score for this assessment battery was used as our domain-general metric of neurocognitive functioning as this score reflects a standardized measure of neurocognitive functioning across components of cognition2,3 (Akshoomoff et al., 2013).
Emotion recognition memory
After the MRI session, participants were presented with a total of 96 pictures of faces (i.e., happy faces, neutral faces, and fearful faces) and pictures of places (Barch et al., 2013; Casey et al., 2018). Forty-eight of these pictures were the same stimuli used during an emotional n-back task completed earlier in the session (for details see Casey et al., 2018; Cohen et al., 2016) and 48 pictures were novel. Picture-type was evenly balanced (i.e., 12 pictures per type) across old and new pictures. Participants were asked to rate whether each picture was “old” or “new.” Given our interest in a behavioral assessment of both emotion and memory related processing, we used a sensitivity measure (d’; calculated as: z(hit rate) − z(false alarms)), which accounts for false alarm rates, for each of the different face-types presented in the task as our metrics of emotion recognition memory.4
Additional details regarding all neurocognitive measures administered during the baseline study session can be found in previously published papers (see Barch et al., 2018; Casey et al., 2018; and Luciana et al., 2018 for full details on all assessment tools).
Imaging procedures and processing
For each participant, 15–20 min of rs-fMRI data was acquired. Data acquisition occurred across 3–4 separate rs-fMRI sequences, each of which was 5-minutes in duration. During each rs-fMRI sequence participants were instructed to stay still and gaze at a central fixation cross. Imaging parameters were harmonized across all 21 data collection sites and scanner models. Details on image acquisition, rs-fMRI image preprocessing, quality control, motion correction/censoring, and connectivity analysis can be found elsewhere (see Casey et al., 2018; Hagler et al., 2019).
Rs-fMRI connectivity analysis and node identification
Rs-fMRI connectivity metrics were calculated by the ABCD Study Data Analysis, Informatics and Resources Center (DAIRC) using methods detailed in Hagler et al. (2019). Briefly, Hagler et al. (2019) performed a region of interest (ROI) to ROI connectivity analysis between all parcels defined by the Gordon et al. (2016) atlas. Following this initial connectivity analysis, Hagler et al. (2019) averaged the connectivity measures across each ROI within each major neural network (i.e., averaging connectivity measures across ROIs within the: default, dorsal attention, frontoparietal, salience, ventral attention, cingulo-opercular, cingulo-parietal, visual, auditory, retrosplenial-temporal, sensorimotorhand, sensorimotormouth, and “other” networks), producing a single connectivity metric for each potential between-network connection (e.g., default to dorsal attention, default to frontoparietal, dorsal attention to frontoparietal, etc.), for each participant.
In addition to this cortical network connectivity analysis, Hagler et al. (2019) preformed a connectivity analysis examining cortical network connectivity to various subcortical structures (specifically, the right and left amygdala, hippocampus, caudate, putamen, pallidum, thalamus, ventral diencephalon, nucleus accumbens, cerebellum, and brainstem). Similar to the cortical–cortical connectivity analysis, Hagler et al. (2019) then averaged connectivity measures across ROIs within each cortical network, and examined how each cortical network, as a whole, was connected with each of the subcortical ROIs (e.g., default to right amygdala, default to left amygdala, default to right hippocampus, etc.). These averaged, network-level measures (for both the cortical–cortical and cortical–subcortical connectivity analyses) were provided in the ABCD Study 2.0.1 data release.
In the current study, we used the available connectivity metrics from the cortical–cortical connectivity analysis to generate an initial 13 × 13 connectivity matrix where each matrix vector (or node) represented a cortical network. In addition, given our interest in cortical–subcortical communication, we averaged the connectivity data across each of the subcortical structures (see also Tillem et al., 2019), producing a single cortical–subcortical connectivity measure for each cortical network (e.g., default to subcortical, dorsal attention to subcortical, frontoparietal to subcortical, etc.). This allowed us to generate a 14 × 14 connectivity matrix for each participant, which, in turn, allowed us to generate graphs with 14 nodes in the subsequent graph analyses (i.e., graphs with one node for each of the 13 cortical networks, and a subcortical node).
Graph analysis
All graph analyses were completed in Matlab (version 2018b), using a combination of the Brain Connectivity Toolbox (Rubinov & Sporns, 2010), the MIT graph toolbox (http://strategic.mit.edu/downloads.php?page=matlab_networks), and native Matlab functions. To ensure all graphs were fully connected, a minimum spanning tree analysis using the Kruskal algorithm (Kruskal, 1956) was implemented to generate an initial fully connected subgraph for each participant. Following this initial subgraph generation, connections were added to each subgraph at proportional thresholds of .01 to .40 (i.e., from the strongest 1% of all possible connections being included in the graph, to the strongest 40% of all possible connections being included in the graph) at .01 step intervals, to generate 40 unweighted, undirected graphs of differing levels of sparsity per participant. Our various graph metrics of interest (see Table 2) were then extracted from each of these thresholded graphs for each participant. To help ensure that our graph metrics accurately reflected neural topology across different levels of sparsity, the area under the curve (AUC) was calculated for each graph metric across sparsity levels (Ginestet, Nichols, Bullmore, & Simmons, 2011; Hosseini, Hoeft, & Kesler, 2012), producing one AUC value, per metric, per participant. Following recent research in graph analysis, all AUC graph metrics were then natural log transformed prior to data analysis (Gonzalez et al., 2016; Smit, de Geus, Boersma, Boomsma, & Stam, 2016; Tillem et al., 2019).
Table 2.
Descriptions of graph metrics
| Metric | Definition | Description: Global analysis | Description: Node-level analysis |
|---|---|---|---|
| Degree | Number of connections to a single node. | Graphs with higher Degreemax have larger largest “hubs” (i.e., largest hubs with a greater number of connections), and thus may be able to more effectively integrate information between nodes. | Nodes with higher Degree have more connections and, therefore, may act as more of a hub in the global flow of information. |
| Betweenness centrality (BC) | Number of shortest paths passing through a specific node. | Graphs with higher BCmax have more information traveling through a single, centrally located hub, allowing for both efficient communication and effective information integration, but also potentially leaving the graph vulnerable if this central hub was damaged or overloaded. | Nodes with higher BC have more information passing through them (i.e., are more central) in the global flow of information. |
| Efficiency | Metrics related to either the average inverse shortest path length across an entire graph (efficiencyglobal) or the inverse shortest path length of a specific node within a smaller neighborhood (efficiencylocal). | Graphs with higher efficiencyglobal may require information to travel through fewer connections to get from any node to any other node in the network, allowing for more efficient neural communication. | Nodes with higher efficiencylocal may require information to travel through fewer connections to get to other nodes in that neighborhood, allowing that node to communicate more efficiently within that area of the graph. |
| Clustering coefficient | The fraction of nodes in a graph which form triangular connections (i.e., the fraction of nodes in a graph whose neighbors are also interconnected with each other). | Graphs with higher clustering coefficients tend to exhibit higher degrees of functional segregation and are more robust to disruptions. | – |
Data analysis
Global analysis
Separate linear regressions were run for each dependent variable of interest (e.g., each of the global graph metrics’ AUCs [see Table 2] and the NIH Toolbox cognition battery total cognition score) with CD symptomatology (z-scored) as the primary predictor of interest, controlling for sex (dichotomously coded, male vs. female), race (dichotomously coded, white vs. non-white), age (z-scored), and data collection site. Following these linear regression models, a mediation analysis was run (Hayes, 2013).
Since our primary predictor of interest (CD symptomatology) was not normally distributed (Shapiro–Wilk test for normality, p < .001), all effects were evaluated using confidence intervals (CIs) derived from nonparametric resampling procedures (bootstrapping) with 5,000 samples, which do not assume a normal distribution. To correct for multiple comparisons in our analyses, the CIs for the linear regression models were adjusted to match Bonferroni-corrected p values (i.e., 1−[.05/number of comparisons] = CI range). Specifically, for the four regression analyses examining global graph theory metrics, 98.75% bootstrapped CIs were examined (1−[.05/4] = .9875).
Node-level analysis
Separate linear regressions were run for each of the node-level metrics (see Table 2) for the subcortical node with CD symptomatology (z-scored) as the primary predictor of interest, controlling for sex (dichotomously coded, male vs. female), race (dichotomously coded, white vs. non-white), age (z-scored), and data collection site. Then, a three-level (neutral faces, happy faces, and fearful faces) repeated-level general linear model was performed with CD symptomatology (z-scored) included as a continuous, between-subject factor of interest, controlling for sex (dichotomously coded, male vs. female), race (dichotomously coded, white vs. non-white), age (z-scored), and data collection site. Finally, a mediation analysis was run (Hayes, 2013). As with the global analysis, all effects were assessed via CI derived from nonparametric resampling procedures (bootstrapping) with 5,000 samples. For the three regression analyses examining node-level graph theory metrics, 98.33% CIs were examined to match Bonferroni-corrected p values (1−[.05/3] = .9833).
Results
Global analysis
Global graph analysis
Higher CD symptomatology was related to higher clustering coefficients in the global graph analysis, F(5, 4,775) = 13.544, p < .001, β = .039, 98.75% CI [.0027 .0771], suggesting neural information processing has greater overall functional segregation in youth higher on CD symptomatology (see Figure 1). CD symptomatology was not related to any other global graph analysis metrics (Degreemax, F(5, 4,775) = 7.393, p < .001, β = .020, 98.75% CI [−.0169 .0595]; BCmax, F(5, 4,775) = 10.696, p < .001, β = .025, 98.75% CI [−.0081 .0624]; global efficiency, F(5, 4,775) = 6.763, p < .001, β = −.023, 98.75% CI [−.0582 .0125]).
Figure 1.
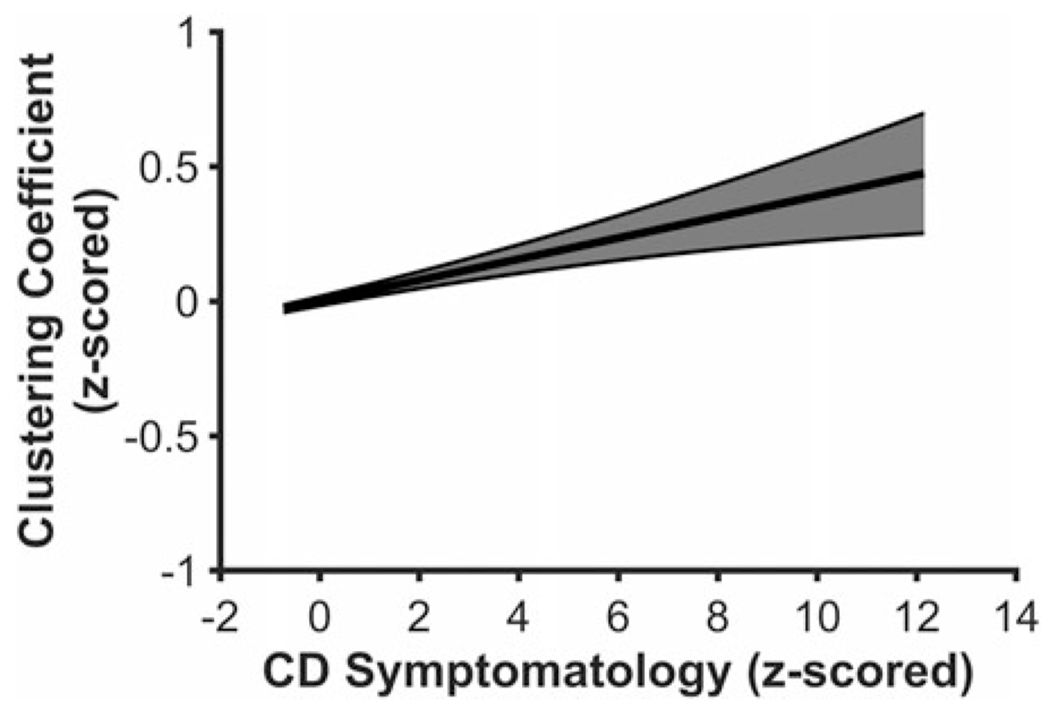
Youth higher on conduct disorder (CD) symptomatology exhibited higher clustering coefficients in the global analysis. Figure 1 displays a regression line depicting clustering coefficient from the global analysis as a function of CD symptomatology, controlling for age, sex, race, and data collection site. Error band represents one standard error.
Neurocognitive functioning
Consistent with prior research (Moffitt, 1993; Nigg & Huang-Pollock, 2003; Teichner & Golden, 2000), higher CD symptomatology was related to lower overall performance on the NIH Toolbox cognition battery, as measured by the corrected cognition total composite T-score, F(5, 4,369) = 16.644, p < .001, β = −.104, 95% CI [−.1328 −.0763], suggesting youth higher on CD symptomatology may exhibit a domain-general impairment in neurocognitive functioning.
Mediation analysis
CD symptomatology was specified as the independent variable, the NIH Toolbox total cognition score as the dependent variable, and clustering coefficient as the mediator. Clustering coefficient had a small but meaningful mediation effect on the relationship between CD symptomatology and overall neurocognitive functioning (indirect effect: β = −.002, 95% CI [−.0044 −.0002], proportion mediated = 0.017; see Figure 2 for path coefficients).
Figure 2.
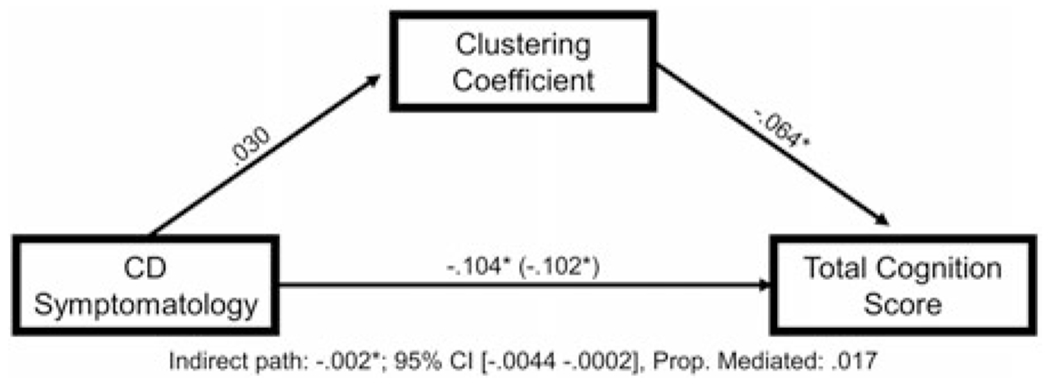
Clustering coefficient partially mediates the relationship between conduct disorder (CD) symptomatology and impairments in neurocognitive functioning. Figure 2 displays the mediation model testing the relationships among CD symptomatology, global clustering coefficient, and total cognition score on the National Institutes of Health (NIH) Toolbox cognition battery, controlling for age, sex, race, and data collection site.
Node-level analysis
Node-level metrics: subcortical
Higher CD symptomatology was related to lower Degreesubcortical, F(5, 4,775) = 28.804, p < .001, β = −.052, 98.33% CI [−.0916 −.0152], suggesting that subcortical structures, collectively, exhibit meaningfully fewer direct cortical connections, and therefore, may act as less of a hub for information integration in youth with higher CD symptomatology5 (see Figure 3). CD symptomatology was not related to either BCsubcortical, F(5, 4,775) = 2.871, p = .014, β = .036, 98.33% CI [−.0051 .0879] or local efficiency for subcortical structures, F(5, 4,775) = 4.725, p < .001, β = .039, 98.33% CI [−.0012 .0909].
Figure 3.
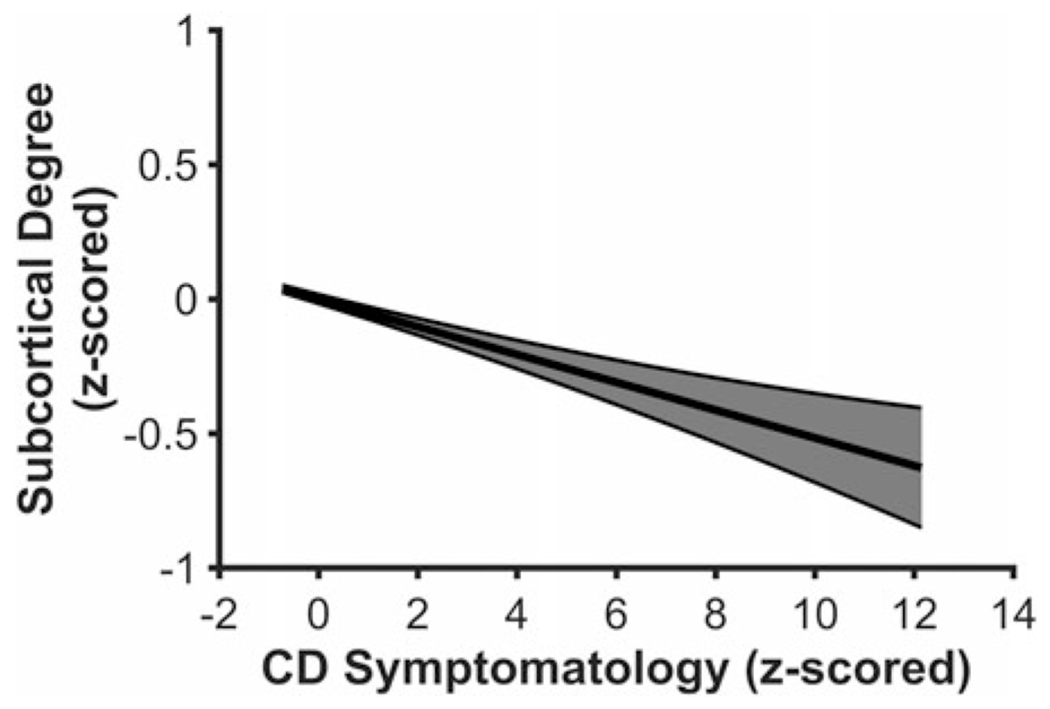
Youth higher on conduct disorder (CD) symptomatology exhibit lower Degreesubcortical in the node-level analysis. Figure 3 displays a regression line depicting Degreesubcortical as a function of CD symptomatology, controlling for age, sex, race, and data collection site. Error band represents one standard error.
Emotion recognition memory
There was a main effect of CD symptomatology on task performance, F(1, 3,688) = 12.672, p < .001, β = −.061, 95% CI [−.0919 −.0290] such that youth with higher CD symptomatology displayed worse performance on the emotion recognition memory task across all face stimuli, regardless of the specific expression being displayed in the stimulus (i.e., whether the face displayed a happy, fearful, or affectively neutral expression). CD symptomatology did not meaningfully interact with facial expression, F(2, 7,376) = 1.457, p = .233. Given these findings, an average of performance across all three face stimuli types were used in the subsequent mediation analysis.
Mediation analysis
For the mediation analysis, CD symptomatology was set as the independent variable, face recognition memory performance as the dependent variable, and Degreesubcortical as the mediator. There was a small, but meaningful, indirect effect through Degreesubcortical, β = −.002, 95% CI [−.0046 −.0005], proportion mediated = .032, indicating that Degreesubcortical partially mediated the relationship between CD symptomatology and emotion recognition memory performance6 (see Figure 4 for path coefficients).
Figure 4.
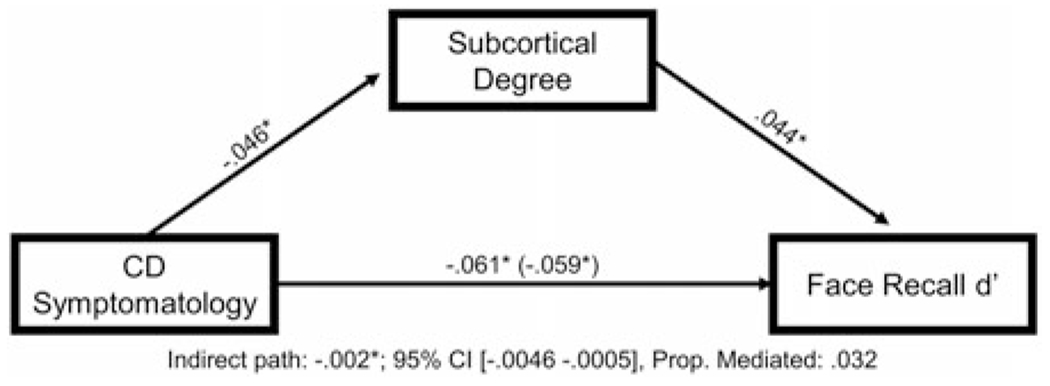
Degreesubcortical partially mediates the relationship between conduct disorder (CD) symptomatology and impairments in emotion recognition memory. Figure 4 displays the mediation model testing the relationships among CD symptomatology, Degreesubcortical, and performance on the emotion recognition memory task, controlling for age, sex, race, and data collection site.
Supplemental analyses
Additional supplemental analyses were conducted to further explore these findings (full details of which can be found in the Supplemental Materials). Here, we briefly summarize these analyses and findings.
First, an exploratory analysis was conducted to evaluate whether CD symptomatology was related to node-level differences for any of the cortical network nodes. However, this analysis yielded all null results (see Supplemental Materials: Supplemental Analyses and Results: Cortical Network Nodes, and Supplemental Materials, Table S4).
Second, a grouped analysis was conducted to determine if the current regression findings replicated at a diagnostic level (i.e., CD diagnosis vs. controls). This analysis revealed that all the current findings replicated at a diagnostic level (i.e., youth with CD compared to controls showed the same effects as reported above in youth higher on CD symptomatology); however, the relationship between Degreesubcortical and CD diagnosis was only meaningful prior to correcting for multiple comparisons (likely due to the dramatic drop in power when going from the full sample, n = 4,781, to the subsample, n = 507, used in the grouped analysis; see Supplemental Materials: Supplemental Analyses and Results: CD Diagnosis).
Third, an exploratory analysis was conducted to evaluate whether the effects of CD were moderated by participant’s sex. The analysis demonstrated that the relationship between CD and lower overall performance on the NIH Toolbox cognition battery was moderated by sex, such that, while the effect was present in both male and female participants, it was stronger in male participants. No other meaningful moderation effects were found (see Supplemental Materials: Supplemental Analyses and Results: Demographic Interactions with CD: CD Symptomatology × Sex Interactions).
Fourth, an exploratory analysis was conducted to evaluate whether the effects of CD were moderated by race. This analysis revealed a CD × Race interaction for local efficiencysubcortical, where CD was associated with increased local efficiency for subcortical structures, but only in non-white participants. No other moderation effects were detected (see Supplemental Materials: Supplemental Analyses and Results: Demographic Interactions with CD: CD Symptomatology × Race Interactions).
Fifth, to evaluate the uniqueness of the current findings to CD (over and above externalizing psychopathologies more generally), exploratory analyses were conducted examining the relationships between oppositional defiance disorder (ODD) and attention deficit/hyperactivity disorder (ADHD) symptomatology, respectively, and our dependent variables of interest (e.g., global graph theory metrics, global neurocognitive functioning, node-level metrics for subcortical structures, and emotion recognition memory task performance). These analyses revealed that neither ODD nor ADHD symptomatology were related to the graph theory metrics relevant to CD (i.e., global clustering or Degreesubcortical). However, the neurocognitive effects (i.e., worse performance on the NIH Toolbox cognition battery and the emotion recognition memory task) were shared across all three externalizing psychopathologies (see Supplemental Materials: Supplemental Analyses and Results: Uniqueness to CD).
Sixth, to help ensure that comorbidities among externalizing psychopathologies were not confounding the current regression findings, regression models for the four meaningful effects reported above (i.e., the effect of CD on global clustering, NIH Toolbox performance, Degreesubcortical, and emotion recognition memory task performance) were rerun with ODD and ADHD symptomatology as additional covariates. Including ODD and ADHD symptomatology in these models did not meaningfully alter the results, suggesting that the current findings are unlikely to be confounded by comorbid externalizing psychopathologies (see Supplemental Materials: Supplemental Analyses and Results: Comorbidities).
Finally, to help ensure the current regression findings were not confounded by socioeconomic status (SES), regression models for the four meaningful effects from the main analyses were rerun with an SES measure as an additional covariate of non-interest. Accounting for SES in these models did not meaningfully change the findings, suggesting that it is unlikely that the current findings are confounded by SES (see Supplemental Materials: Supplemental Analyses and Results: Socioeconomic Status).
Discussion
The present study is the first to use a graph theory approach to examine the relationships among CD symptomatology, metrics of neural topology, and behavioral assessments of neurocognitive functioning. Using this approach, we demonstrate that CD-related differences in neural topology partially mediate the relationship between CD symptomatology and neurocognitive functioning. More specifically, in the global analysis, we show that higher CD symptomatology is associated with increased clustering, which partially mediates the relationship between CD symptomatology and impairments in general neurocognitive functioning. In addition, in the node-level analysis, we show that higher CD symptomatology relates to the hubness of subcortical structures, which partially mediates the relationship between CD symptomatology and decreased ability to initially encode, and/or subsequently recognize previously seen, facial stimuli. These findings provide evidence that CD symptomatology is related to fundamental shifts in the topology of neural communication throughout the brain. Moreover, the present findings help elucidate some of the relationships between topological shifts and neurocognitive functioning.
Graph theory: global metrics
The present study shows that youth higher on CD symptomatology exhibit higher levels of clustering in their global neural communication. Interestingly, despite the association between CD and impairments in neurocognitive functioning, higher global clustering is typically associated with more optimal neural network topology, and, by extension, enhanced neurocognitive functioning (Achard, Salvador, Whitcher, Suckling, & Bullmore, 2006; Liao, Vasilakos, & He, 2017; Suprano et al., 2019; Wang et al., 2009). Higher clustering is thought to allow for greater functional segregation and to help facilitate robust and efficient local neural communication (i.e., communication between sets of nodes in a directly connected “neighborhood”). In addition, when paired with high global efficiency, networks with high global clustering coefficients are categorized as “small-world” networks, a type of network topology thought to be ideal for robust and effective information processing for human cognition (Achard et al., 2006; Liao et al., 2017; Wang et al., 2009). However, while the effect sizes are small, the results from the current study suggest that, for youth higher on CD symptomatology, this heightened global clustering actually may help support impairments in general neurocognitive functioning.
Though speculative, one possible explanation of this effect is that the CD-related increase in global clustering may increase functional segregation to such a degree that, without a corresponding increase in global efficiency, flexible neural communication and information integration between more distal nodes or networks may actually become inhibited. This, in turn, could unbalance global neural communication and impair broader neurocognitive functions that rely on this type of flexible and dynamic integration of information between distal networks (e.g., impair decision-making or general intellect).
Another potential explanation for the effect in the present study is that neurocognitive functioning and small-world characteristics (e.g., clustering or efficiency) may not be linearly related. Research in both neurotypical and antisocial adult populations suggests that some neural factors (e.g., small-world network characteristics) and neurocognitive functioning may have a “U-shaped” relationship, where any deviation from the “optimal” level of these metrics (e.g., either hyper- or hypo-clustering) negatively relates to neurocognitive abilities (Freches et al., 2020; Tillem, van Dongen, Brazil, & Baskin-Sommers, 2018). Accordingly, hyper-clustering associated with CD may place youth higher on CD symptomatology slightly outside the “optimal” clustering range for maximally effective neurocognitive functioning, explaining the small, but meaningful, mediation effects found in the current study.
Graph theory: node metrics
At a node-level, youth higher on CD symptomatology display lower Degree in subcortical structures, indicating fewer direct connections to these structures in these youth. While these findings are consistent with prior connectivity research in CD (Finger et al., 2012; Passamonti et al., 2012), these node-level effects are statistically (see Footnotes 5 and 6) and conceptually distinct from basic connectivity effects. More specifically, prior work using traditional rs-fMRI and DTI connectivity methods show that youth with CD display reduced functional and aberrant structural connectivity between discrete pairs of cortical and subcortical structures (e.g., reduced prefrontal-amygdala connectivity or aberrant microstructural integrity in the uncinate fasciculus; Finger et al., 2012; Passamonti et al., 2012). The current findings, however, suggest that youth higher on CD symptomatology exhibit fundamentally fewer direct connections between cortical and subcortical structures, in general. Accordingly, not only may certain specific cortical–subcortical connections be weakened in these youth, multiple direct cortical–subcortical connections actually may be absent.
The reduction in the number of direct cortical–subcortical connections, in turn, could result in subcortical structures acting as less of an integrative hub for neural communication in youth higher on CD symptomatology and subcortical structures being less able to communicate with various cortical networks directly and efficiently in these youth. As a result, any neurocognitive processes that utilize, or potentially require, direct cortical inputs to/from subcortical structures (e.g., affective responding, memory, etc.; Baas et al., 2004; Eichenbaum, 2001; Keightley et al., 2011) might be delayed or impaired in youth higher on CD. While somewhat speculative, such an interpretation is highly consistent with both the current mediation findings linking CD-related reductions in the number of direct cortical–subcortical connections to CD-related impairments during an emotion recognition memory task, and prior theoretical accounts of socioaffective disruptions in antisocial populations (e.g., VIM; Blair, 1995).
Limitations
The current findings provide strong evidence that youth with higher CD symptomatology display neuro-topological abnormalities, and that these topological abnormalities contribute to neurocognitive dysfunctions in CD. However, they must be considered in light of several key limitations.
First, since we used the curated data from the ABCD Study 2.0.1 release, we were limited to conducting our graph analysis at a network level (with entire cortical networks and subcortical structures, collectively, acting as the nodes of our graphs). As a result, we were unable to take a more fine-grained approach to look at how CD symptomatology may relate to node-level differences in specific regions of cortex or structures (e.g., the amygdala). Future research taking a more fine-grained approach would be needed to both replicate our current findings and identify which specific structures, if any, may be driving the results.
Second, consistent with previous national community samples (Merikangas et al., 2010), the prevalence of CD is low in the current sample, particularly in comparison to samples enriched for CD (e.g., justice-involved samples). Replication of the findings reported here in other samples more enriched for CD is warranted.
Third, the cross-sectional nature of the current data limited our ability to determine at what age these neurocognitive and/or neuro-topological abnormalities develop, gain additional insight into any age-specific neurodevelopmental mechanisms that may support their emergence, and assess the directionality and/or causality of the link between CD and neurocognitive disruptions (e.g., does CD onset first and lead to neurocognitive disruptions or vice versa?). Evaluating causal relationships longitudinally in future waves of the ABCD Study will be instrumental in addressing these concerns.
Fourth, prior research has subtyped youth with CD by age of onset (e.g., symptom onset during childhood vs. adolescent onset; Moffitt, 2006); however, the relatively young and narrow age range of the current sample limited our ability to subtype CD in this way. Future research with longitudinal data or older samples is warranted to assess any potential impact of age of onset on the current findings.
Fifth, the presence of CU traits may moderate the effects of CD on neurocognitive functioning (Dotterer et al., 2020; Graziano et al., 2019; Viding et al., 2012). However, a reliable CU trait measure was not available for the current study (but see Hawes et al. (2020) for an adapted CU score using items from various ABCD measures and future releases of the ABCD data with a Social Development substudy that will include the Inventory of Callous-Unemotional Traits).
Sixth, all the effects reported in the current paper have small effect sizes. While this does suggest that independent replication of these small effects is needed to ensure that they are present and stable across samples, it is worth noting that studies with large samples, such as the current ABCD Study, are well suited to reliably and precisely detect small, but potentially meaningful, effects (see Dick et al., 2020 for a discussion of the meaningfulness of small effects in the ABCD Study).
Finally, some participants in the current study were missing data for the emotion recognition memory task (n = 1,070, ~22% of the current sample), and it is possible that systematic differences in those who were missing that data may be biasing our findings for analyses using data from the emotion recognition memory task. While this possibility is unlikely since the subsample missing these data did not systematically differ on CD symptomatology (see Footnote 4 and Supplemental Materials: Supplemental Analyses and Results: Missing Subject Analysis), future research replicating these findings in samples with less missing data will help address any potential biasing of these results.
Conclusions
In sum, the present study reports that youth higher on CD symptomatology display abnormal neural topologies at multiple levels of analysis, and that these topological abnormalities mediate the relationship between CD symptomatology and different aspects of neurocognitive functioning. The topological abnormalities identified in the present study represent candidate neural mechanisms contributing to the well-documented neurocognitive deficits associated with CD. The application of graph analysis may serve to advance our understanding of the neural underpinnings of human cognition across clinical populations.
Supplementary Material
Acknowledgments.
Data used for the analyses reported in this study were obtained from the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®; http://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD Study is a multisite, longitudinal study tracking children starting at 9–10 years old throughout adolescence at 21 different sites across the United States and is supported by the National Institutes of Health (and additional federal partners). More information about the study, including a list of study investigators and participating study sites can be found at https://abcdstudy.org/study-sites/. The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1504041. In addition, we would like to thank Alexander Young for his assistance with this project.
Funding Statement.
ABCD Study award numbers include: U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025.
Footnotes
Supplementary Material. The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000237.
Conflicts of Interest. None.
While much of the work stemming from VIM and related models has focused on psychopathy and/or psychopathic traits, the original model addressed aggression and antisociality more broadly.
406 participants from the current sample were missing data for the fully corrected, total cognition summary T-score from the NIH Toolbox cognition battery.
Descriptive statistics and correlations for NIH Toolbox cognition battery individual task and composite scores can be found in the Supplemental Materials (see Tables S1–S3).
1,070 participants from the current sample were missing data for the emotion recognition memory task. However, CD symptomatology levels did not systematically differ between participants missing emotion recognition memory task data and those who completed the task (see Supplemental Materials for full details).
Since Degree is conceptually related to more traditional measures of functional connectivity (i.e., estimated number of direct connections vs. estimated strength of possible connections) this analysis was rerun including an overall subcortical–cortical connectivity measure as a covariate in the model; however, this did not meaningfully change the results, Degreesubcortical β = −.050, 98.33% CI [−.0802 −.0214].
To ensure the Degreesubcortical mediation effect was not being driven by CD-related differences in the strength of subcortical–cortical connectivity, the mediation analysis was rerun with a basic subcortical–cortical connectivity measure as a simultaneous mediator in the model; however, the results did not meaningfully change. The indirect path through Degreesubcortical remained meaningful, β= −.002, 95% CI [−.0058 −.0004], proportion mediated = .032, while neither the total indirect path nor the indirect path through subcortical–cortical connectivity were meaningful.
References
- Achard S, Salvador R, Whitcher B, Suckling J, & Bullmore ED (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal of Neuroscience, 26, 63–72. doi: 10.1523/jneurosci.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M, Colins OF, Klapwijk ET, Veer IM, Andershed H, Popma A, … Vermeiren RRJM (2016). Dissociable relations between amygdala subregional networks and psychopathy trait dimensions in conduct-disordered juvenile offenders. Human Brain Mapping, 37, 4017–4033. doi: 10.1002/hbm.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, … Zelazo PD (2013). VIII. NIH toolbox cognition battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monographs of the Society for Research in Child Development, 78, 119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Aleman A, & Kahn RS (2004). Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Research Reviews, 45, 96–103. [DOI] [PubMed] [Google Scholar]
- Baker RH, Clanton RL, Rogers JC, & De Brito SA (2015). Neuroimaging findings in disruptive behavior disorders. CNS Spectrums, 20, 369–381. doi: 10.1017/s1092852914000789. [DOI] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, … Yurgelun-Todd D (2018). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Developmental Cognitive Neuroscience, 32, 55–66. doi: 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, … Feldt C (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, & Beaumont JL (2013). III. NIH toolbox cognition battery (CB): Measuring episodic memory. Monographs of the Society for Research in Child Development, 78, 34–48. doi: 10.1111/mono.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J (1995). A cognitive developmental approach to morality: Investigating the psychopath. Cognition, 57, 1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair J, Leibenluft E, & Pine DS (2014). Conduct disorder and callous–unemotional traits in youth. New England Journal of Medicine, 371, 2207–2216. doi: 10.1056/nejmc1415936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broulidakis MJ, Fairchild G, Sully K, Blumensath T, Darekar A, & Sonuga-Barke EJS (2016). Reduced default mode connectivity in adolescents with conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 800–808. e801. doi: 10.1016/j.jaac.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Brown RT, Jaffe SL, Silverstein J, & Magee H (1991). Methylphenidate and hospitalized adolescents with conduct disorder: Dose effects on classroom behavior, academic performance, and impulsivity. Journal of Youth and Adolescence, 20, 501–518. doi: 10.1007/bf01540634. [DOI] [PubMed] [Google Scholar]
- Bullmore E, & Sporns O (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10, 186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, Beaumont JL, Tulsky DS, & Gershon RC (2015). The NIH toolbox pattern comparison processing speed test: Normative data. Archives of Clinical Neuropsychology, 30, 359–368. doi: 10.1093/arclin/acv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, & Gershon RC (2014). NIH toolbox cognitive battery (NIHTB-CB): The NIHTB pattern comparison processing speed test. Journal of the International Neuropsychological Society, 20, 630. doi: 10.1093/arclin/acv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Kail RV, & Beaumont JL (2013). VI. NIH toolbox cognition battery (CB): Measuring processing speed. Monographs of the Society for Research in Child Development, 78, 88–102. doi: 10.1111/mono.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, … Garavan H (2018). The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Fisher CB, Bookheimer S, Brown SA, Evans JH, Hopfer C, … Pfefferbaum A (2018). Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: The ABCD experience. Developmental Cognitive Neuroscience, 32, 143–154. doi: 10.1016/j.dcn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson K, … Heller AS (2016). When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Science, 27, 549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RR, … Popma A (2015). Differential relations between juvenile psychopathic traits and resting state network connectivity. Human Brain Mapping, 36, 2396–2405. doi: 10.1002/hbm.22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Watts AL, Heeringa SG, Lopez DA, Bartsch H, Fan CC, … Haist F (2020). Meaningful effects in the adolescent brain cognitive development study. bioRxiv. doi: 10.1101/2020.09.01.276451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Bauer PJ, Weintraub S, Mungas D, Slotkin J, Beaumont JL, … Heaton RK (2014). Measuring episodic memory across the lifespan: NIH toolbox picture sequence memory test. Journal of the International Neuropsychological Society, 20, 611. doi: 10.1017/s1355617714000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer HL, Waller R, Hein TC, Pardon A, Mitchell C, Lopez-Duran N, … Hyde LW (2020). Clarifying the link between amygdala functioning during emotion processing and antisocial behaviors versus callous-unemotional traits within a population-based community sample. Clinical Psychological Science, 20, 918–935. doi: 10.1177/2167702620922829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2001). The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behavioural Brain Research, 127, 199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Estrada S, Tillem S, Stuppy-Sullivan A, & Baskin-Sommers A (2019). Specifying the connection between reward processing and antisocial psychopathology across development: Review, integration, and future directions. In Gruber J (Ed.), Oxford handbook of positive emotion and psychopathology (pp. 312–332). New York, NY: Oxford University Press, doi: 10.1093/oxfordhb/9780190653200.013.21. [DOI] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Aitken MRF, Savage J, Moore SC, & Goodyer IM (2009). Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological Psychiatry, 66, 162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14, 340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, … Blair RJ (2012). Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research, 202, 239–244. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freches GB, Haak KV, Bryant KL, Schurz M, Beckmann CF, & Mars RB (2020). Principles of temporal association cortex organisation as revealed by connectivity gradients. Brain Structure and Function, 1–16. doi: 10.1007/s00429-020-02047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2012). Developmental pathways to conduct disorder: Implications for future directions in research, assessment, and treatment. Journal of Clinical Child & Adolescent Psychology, 41, 378–389. doi: 10.1080/15374416.2012.664815. [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, … Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Cook KF, Mungas D, Manly JJ, Slotkin J, Beaumont JL, & Weintraub S (2014). Language measures of the NIH toolbox cognition battery. Journal of the International Neuropsychological Society, 20, 642–651. doi: 10.1017/s1355617714000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Slotkin J, Manly JJ, Blitz DL, Beaumont JL, Schnipke D, … Hirsh-Pasek K (2013). IV. NIH toolbox cognition battery (CB): Measuring language (vocabulary comprehension and reading decoding). Monographs of the Society for Research in Child Development, 78, 49–69. doi: 10.1111/mono.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, & Nowinski CJ (2013). NIH toolbox for assessment of neurological and behavioral function. Neurology, 80, S2–S6. doi: 10.1212/wnl.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestet CE, Nichols TE, Bullmore T, & Simmons A (2011). Brain network analysis: Separating cost from topology using cost-integration. PLoS One, 6, doi: 10.1371/journal.pone.0021570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GF, Van der Molen MJ, Zaric G, Bonte M, Tijms J, Blomert L, … Van der Molen MW (2016). Graph analysis of EEG resting state functional networks in dyslexic readers. Clinical Neurophysiology, 127, 3165–3175. doi: 10.1016/j.clinph.2017.09.106. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral Cortex, 26, 288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Landis T, Maharaj A, Ros-Demarize R, Hart KC, & Garcia A (2019). Differentiating preschool children with conduct problems and callous-unemotional behaviors through emotion regulation and executive functioning. Journal of Clinical Child & Adolescent Psychology, 1–13. doi: 10.1080/15374416.2019.1666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JA, Kraguljac NV, White DM, Ver Hoef L, Tabora J, & Lahti AC (2016). Change in brain network topology as a function of treatment response in schizophrenia: A longitudinal resting-state fMRI study using graph theory. Schizophrenia, 2, 1–7. doi: 10.1038/npjschz.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick A, … Dale AM (2019). Image processing and analysis methods for the adolescent brain cognitive development study. NeuroImage, 202, 116091. doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney-Caron E, Caprihan A, & Stevens MC (2014). DTI-measured white matter abnormalities in adolescents with conduct disorder. Journal of Psychiatric Research, 48, 111–120. doi: 10.1016/j.jpsychires.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes SW, Waller R, Thompson WK, Hyde LW, Byrd AL, Burt SA, … Gonzalez R (2020). Assessing callous-unemotional traits: Development of a brief, reliable measure in a large and diverse sample of preadolescent youth. Psychological Medicine, 50, 456–464. doi: 10.1017/S0033291719000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). The PROCESS macro for SPSS and SAS (version 2.15) [Software]. Retrieved from http://www.processmacro.org/index.html
- Hobson CW, Scott S, & Rubia K (2011). Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry, 52, 1035–1043. doi: 10.1111/j.1469-7610.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Hosseini SMH, Hoeft F, & Kesler SR (2012). GAT: A graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One, 7, doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Heath AC, Hewitt JK, Neale MC, Banich MT, Luciana MM, … Bjork JM (2018). The utility of twins in developmental cognitive neuroscience research: How twins strengthen the ABCD research design. Developmental Cognitive Neuroscience, 32, 30–42. doi: 10.1016/j.dcn.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu W, Ming Q, Gao Y, Ma R, Zhang X, … Huang B (2016). Disrupted topological patterns of large-scale network in conduct disorder. Scientific Reports, 6, 37053. doi: 10.1038/srep37053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, & Ryan N (2013). Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version 2013 (K-SADS-PL). Pittsburgh, PA: Western Psychiatric Institute and Yale University. [Google Scholar]
- Keightley ML, Chiew KS, Anderson JAE, & Grady CL (2011). Neural correlates of recognition memory for emotional faces and scenes. Social Cognitive and Affective Neuroscience, 6, 24–37. doi: 10.1093/scan/nsq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, & Cooper JC (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18, 411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kruskal JB (1956). On the shortest spanning subtree of a graph and the traveling salesman problem. Proceedings of the American Mathematical Society, 7, 48–50. doi: 10.1090/s0002-9939-1956-0078686-7. [DOI] [Google Scholar]
- Langer N, Pedroni A, Gianotti LRR, Hänggi J, Knoch D, & Jäncke L (2012). Functional brain network efficiency predicts intelligence. Human Brain Mapping, 33, 1393–1406. doi: 10.1002/hbm.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Vasilakos AV, & He Y (2017). Small-world human brain networks: Perspectives and challenges. Neuroscience & Biobehavioral Reviews, 77, 286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Lindner P, Flodin P, Budhiraja M, Savic I, Jokinen J, Tiihonen J, & Hodgins S (2018). Associations of psychopathic traits with local and global brain network topology in young adult women. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 1003–1012. doi: 10.1016/j.bpsc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, … Jiang T (2008). Disrupted small-world networks in schizophrenia. Brain, 131, 945–961. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Lu FM, Zhou JS, Zhang J, Wang XP, & Yuan Z (2017). Disrupted small-world brain network topology in pure conduct disorder. Oncotarget, 8, 65506. doi: 10.18632/oncotarget.19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FM, Zhou JS, Zhang J, Xiang YT, Zhang J, Liu Q, … Yuan Z (2015). Functional connectivity estimated from resting-state fMRI reveals selective alterations in male adolescents with pure conduct disorder. PLoS One, 10, e0145668. doi: 10.1371/journal.pone.0145668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, & Banich MT (2018). Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience, 32, 67–79. doi: 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menks WM, Furger R, Lenz C, Fehlbaum LV, Stadler C, & Raschle NM (2017). Microstructural white matter alterations in the corpus callosum of girls with conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 258–265. doi: 10.1016/j.jaac.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in US adolescents: Results from the national comorbidity survey replication–adolescent supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49, 980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE (1993). The neuropsychology of conduct disorder. Development and Psychopathology, 5, 135–151. doi: 10.1017/S0954579400004302. [DOI] [Google Scholar]
- Moffitt TE (2006). Life-course-persistent versus adolescence-limited antisocial behavior. In Cicchetti D & Cohen DJ (Eds.), Developmental psychopathology: Risk, disorder, and adaptation (pp. 570–598). John Wiley & Sons, Inc. [Google Scholar]
- Morgan AB, & Lilienfeld SO (2000). A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review, 20, 113–136. doi: 10.1016/S0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, & Fink A (2009). Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews, 33, 1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Nigg JT, & Huang-Pollock CL (2003). An early-onset model of the role of executive functions and intelligence in conduct disorder/delinquency. In Lahey BB, Moffitt TE & Caspi A (Eds.), Causes of conduct disorder and juvenile delinquency (pp. 227–253). The Guildford Press. [Google Scholar]
- Noordermeer SDS, Luman M, & Oosterlaan J (2016). A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychology Review, 26, 44–72. doi: 10.1007/s11065-015-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord DR, & Bennett KJ (1994). Conduct disorder: Long-term outcomes and intervention effectiveness. Journal of the American Academy of Child & Adolescent Psychiatry, 33, 1069–1078. doi: 10.1097/00004583-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Ogilvie JM, Stewart AL, Chan RCK, & Shum DHK (2011). Neuropsychological measures of executive function and antisocial behavior: A meta-analysis. Criminology, 49, 1063–1107. doi: 10.1111/j.1745-9125.2011.00252.x. [DOI] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, & Calder AJ (2012). Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One, 7, e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JC, & De Brito SA (2016). Cortical and subcortical gray matter volume in youths with conduct problems: A meta-analysis. JAMA Psychiatry, 73, 64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell’Acqua F, Fahy T, Deeley Q, & Murphy DGM (2013). Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: A diffusion tensor imaging study. Psychological Medicine, 43, 401. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Schoonheim MM, Geurts JJG, Wiebenga OT, De Munck JC, Polman CH, Stam CJ, … Wink AM (2014). Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Multiple Sclerosis Journal, 20, 1058–1065. doi: 10.1177/1352458513516892. [DOI] [PubMed] [Google Scholar]
- Schoorl J, van Rijn S, de Wied M, Van Goozen SH, & Swaab H (2018). Boys with oppositional defiant disorder/conduct disorder show impaired adaptation during stress: An executive functioning study Child Psychiatry & Human Development, 49, 298–307. doi: 10.1007/s10578-017-0749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N, Duan Y, Xia M, Schoonheim MM, Huang J, Ren Z, … Shi FD (2016). Disrupted topological organization of structural and functional brain connectomes in clinically isolated syndrome and multiple sclerosis. Scientific Reports, 6, 1–11. doi: 10.1038/srep29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, de Geus EJ, Boersma M, Boomsma DI, & Stam CJ (2016). Life-span development of brain network integration assessed with phase lag index connectivity and minimum spanning tree graphs. Brain Connectivity, 6, 312–325. doi: 10.1089/brain.2015.0359. [DOI] [PubMed] [Google Scholar]
- Stam CJ, & Reijneveld JC (2007). Graph theoretical analysis of complex networks in the brain. Nonlinear Biomedical Physics, 1, 3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprano I, Delon-Martin C, Kocevar G, Stamile C, Hannoun S, Achard S, … Nusbaum F (2019). Topological modification of brain networks organization in children with high intelligence quotient: A resting-state fMRI study. Frontiers in Human Neuroscience, 13, 241. doi: 10.3389/fnhum.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichner G, & Golden CJ (2000). The relationship of neuropsychological impairment to conduct disorder in adolescence: A conceptual review. Aggression and Violent Behavior, 5, 509–528. doi: 10.1016/S1359-1789(98)00035-4. [DOI] [Google Scholar]
- Tewarie P, Hillebrand A, Schoonheim MM, van Dijk BW, Geurts J, Barkhof F, … Stam CJ (2014). Functional brain network analysis using minimum spanning trees in multiple sclerosis: An MEG source-space study. NeuroImage, 88, 308–318. doi: 10.1016/j.neuroimage.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Tillem S, Harenski K, Harenski C, Decety J, Kosson D, Kiehl KA, & Baskin-Sommers A (2019). Psychopathy is associated with shifts in the organization of neural networks in a large incarcerated male sample. NeuroImage: Clinical, 24, 102083. doi: 10.1016/j.nicl.2019.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillem S, van Dongen J, Brazil IA, & Baskin-Sommers A (2018). Psychopathic traits are differentially associated with efficiency of neural communication. Psychophysiology, 55, e13194. doi: 10.1111/psyp.13194. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi N, Chiaravalloti ND, Beaumont JL, Kisala PA, Mungas D, … Gershon RC (2014). NIH toolbox cognition battery (NIHTB-CB): The list sorting test to measure working memory. Journal of the International Neuropsychological Society, 20, 599. doi: 10.1017/S135561771400040X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, & Hulshoff Pol HE (2010). Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. Journal of Neuroscience, 30, 15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, & Jones AP (2008). Cognition to genes via the brain in the study of conduct disorder. Quarterly Journal of Experimental Psychology, 61, 171–181. doi: 10.1080/17470210701508889. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, & McCrory EJ (2012). Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry, 169, 1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Waller R, Hawes SW, Byrd AL, Dick AS, Sutherland MT, Riedel MC, … Gonzalez R (2020). Disruptive behavior problems, callous-unemotional traits, and regional gray matter volume in the ABCD study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5, 470–472. doi: 10.1016/j.bpsc.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, … He Y (2009). Parcellation-dependent small-world brain functional networks: A resting-state fMRI study. Human Brain Mapping, 30, 1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfenden SR, Williams K, & Peat JK (2002). Family and parenting interventions for conduct disorder and delinquency: A meta-analysis of randomised controlled trials. Archives of Disease in Childhood, 86, 251–256. doi: 10.1136/adc.86.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD (2006). The dimensional change card sort (DCCS): A method of assessing executive function in children. Nature Protocols, 1, 297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu X, Wang X, Gao J, Shi H, Huang B, … Yao S (2014). Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 466–475. e461. doi: 10.1016/j.jaac.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yao N, Fairchild G, Cao X, Zhang Y, Xiang YT, … Wang X (2016). Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging and Behavior, 10, 995–1003. doi: 10.1007/s11682-015-9465-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


