Abstract
With increasing prevalence of obesity, the substantial contribution of obesity hypoventilation syndrome (OHS) to morbidity and mortality is likely to increase. It is therefore crucial that the condition has a clear definition to allow timely identification of patients. OHS was first described as “Pickwickian syndrome” in the 1950s; in subsequent decades, case reports did not clearly delineate between patients suffering from OHS and those suffering from obstructive sleep apnoea. In 1999, the American Academy of Sleep Medicine published a guideline that delineated the cause of daytime hypercapnia as either predominantly upper airway or predominantly hypoventilation. This was the first formal definition of OHS as the presence of daytime alveolar hypoventilation (arterial carbon dioxide tension >45 mmHg) in patients with body mass index >30 kg·m−2 in the absence of other causes of hypoventilation. This definition is reflected in the most recent guidelines published on OHS. Recent developments in defining OHS include proposed classification systems of severity and demonstrating the value of using serum bicarbonate to exclude OHS in patients with a low index of suspicion.
Educational aims
To provide an overview of the historical basis of the definition of obesity hypoventilation syndrome.
To explain the rationale for the current definition of obesity hypoventilation syndrome.
To demonstrate areas that need further investigation in defining obesity hypoventilation syndrome.
Short abstract
Obesity hypoventilation syndrome is defined as daytime alveolar hypoventilation in obese patients in the absence of other causes of hypoventilation. Classifications of severity are now needed to target treatment at the most appropriate individuals. https://bit.ly/3yLuiL9
Introduction
Obesity hypoventilation syndrome (OHS) is the combination of obesity, sleep disordered breathing and daytime hypercapnia in the absence of a neuromuscular, mechanical or metabolic cause of hypoventilation. Although this broad definition is widely accepted, specific cut-off figures for each element remain debated. In this review, we explain why defining OHS is important. We then provide an historical overview of the definition of OHS and its association with the term “Pickwickian syndrome”, followed by a description of current definitions for the syndrome. The review ends with a discussion about the value of daytime serum bicarbonate in the diagnosis of OHS.
The problem with OHS
The prevalence of OHS is likely to rise with the continued worldwide increase in body mass index (BMI) [1–3]. This is significant because OHS is arguably the most severe manifestation of obesity-related respiratory disease. It is a progressive condition with a high mortality, despite treatment with positive airway pressure therapy. Outcome studies tend to report data comparing OHS patients with those with obstructive sleep apnoea (OSA) and/or the overlap syndrome. 3-year mortality despite treatment with ventilator therapy is 12–32% [4–7]. This is higher than OSA and overlap syndrome, despite the relatively younger age at diagnosis of OHS [4]. Patients with OHS suffer from more comorbidities than those with OSA or overlap syndrome [8] and, in particular, cardiovascular comorbidity is common and predictive of mortality [9]. Healthcare utilisation is high [10], with more frequent hospitalisations than for patients with OSA [11]. OHS also places a significant burden on health-related quality of life, which is worse than in patients with OSA [12] and worsens despite treatment with ventilator therapy [13].
Despite the clear burden that OHS places on individuals, resources and the healthcare system, the actual prevalence of this important condition remains unknown. Extrapolations from national BMI and OSA prevalence data suggest the prevalence of OHS is 0.4–0.6% of the population [14, 15]. This uncertainty in prevalence stems from two reasons: 1) estimates tend to be made from OSA populations, which inevitably ignore individuals with OHS without OSA; and 2) there is no clear single definition of the condition. The definition of OHS has evolved ever since it was first reported in the literature and there is uncertainty about true diagnoses in some of the earliest case studies. More recently, the definition and identification of hypoventilation has also been debated and adds to the uncertainty about defining OHS.
Historical descriptions of OHS
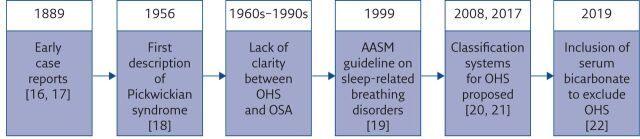
Although the term “Pickwickian syndrome” was first used in the 1950s, descriptions of overweight individuals with somnolence have been present in the medical literature since 1889 (figure 1). Richard Caton described a patient who complained of extreme daytime drowsiness that was associated with progressive weight gain. With significant reduction in weight, the drowsiness almost completely resolved [16]. Caton concluded with a request for support in diagnosing this case to his fellow members of the Clinical Society of London. While the society was unable to provide any useful insights, the president of the society made reference to the similarity between Caton's patient and the character Joe, in Dickens' book “The Posthumous Papers of the Pickwick Club” [23]. Another early report described a sleepy card player whose extreme daytime somnolence improved after weight loss [17]. The first use of the term “Pickwickian syndrome” was in the widely cited early report of OHS by Burwell et al. [18]. They described a 51-year-old gentleman progressively gaining weight and suffering from increased fatigue and somnolence. He was admitted for assessment following an episode of falling asleep while playing poker. They reported chronic hypoxia and hypercapnia and attributed this to nocturnal hypoventilation. Given the similarity with the sleepy card player from Dickens' book, they characterised this condition as the Pickwickian syndrome. Prior to this, Auchincloss et al. [24] had reported on a young overweight gentleman with significant symptomatic alveolar hypoventilation, which was attenuated after weight loss interventions. Estes et al. [25] described six patients suffering from a syndrome of obesity, somnolence, cyanosis, periodic breathing and congestive cardiac failure, which was reversed following significant weight loss. Although they did not specifically name this syndrome Pickwickian, they undoubtedly describe features of OHS.
Figure 1.
Evolution of the identification and definition of OHS. AASM: American Academy of Sleep Medicine.
The first electroencephalographic description of what was described as Pickwickian syndrome was published in German by Gerardy et al. [26]. They reported periodic breathing with short apnoeas triggered by the tongue rolling back and obstructing the airway. They ascribed the symptoms to hypercapnia and made little note of the effect of the tongue rolling back as a cause of intermittent apnoeas. Another contemporaneous report described an overweight female with daytime somnolence, who suffered from very frequent apnoeas during sleep [27], attributing this to the Pickwickian syndrome. Weight loss interventions resulted in an almost complete disappearance of daytime somnolence. Neither study noted the impact of upper airway obstruction as a cause of apnoeas. This association was established by Gastaut et al. [28], who performed detailed respiratory and upper airway characterisation of a patient with what was thought to be OHS. They demonstrated that the diurnal somnolence could not be explained by contemporaneous hypercapnia as arterial carbon dioxide tension (PaCO2) was normal. They observed that the tongue frequently rolled back due to muscle hypotonia during sleep causing an obstructive apnoea which then roused the patient. They then postulated that the insufficient nocturnal sleep was responsible for daytime somnolence [28]. Thus, they reported the first physiological description of OSA.
The following decades would see increased confusion in the literature between OHS and OSA, as the term “Pickwickian syndrome” was used for OSA-related hypersomnolence, irrespective of whether hypercapnia was present [29]. Rapoport et al. [30] evaluated eight patients diagnosed with the Pickwickian syndrome and described two mechanisms for the chronic hypercapnia in these patients: 1) a balance between daytime ventilation and hypoventilation due to apnoeas during sleep, corrected by treatment for OSA; and 2) sustained hypoventilation irrespective of any observed apnoeas, which did not improve with treatment. They reported that only the second group represented true Pickwickian syndrome [30]. This confusion was only clarified as recently as 1999, when the American Academy of Sleep Medicine (AASM) published a guideline on sleep-related breathing disorders [19]. This guideline stated that daytime hypercapnia can be explained either by predominant upper airway obstruction (OSA) or predominant hypoventilation (OHS). This was the first formal definition of OHS as the presence of daytime alveolar hypoventilation (PaCO2 >45 mmHg) in patients with BMI >30 kg·m−2 in the absence of other causes of hypoventilation. They also noted that both conditions are associated with obesity and there may be some overlap in the physiology between the two conditions; they noted that while most OHS patients suffered from concurrent OSA, approximately 10% do not demonstrate any evidence of OSA. They recommended the use of polysomnography to determine the pattern of nocturnal hypoventilation (obstructive or non-obstructive), in order to allow tailored treatment.
As an interesting historical side-note, the term “Pickwickian syndrome” is entirely a misnomer [31]. The term suggests that the syndrome is an eponymous name for Mr Pickwick. Although the central character in the book is Mr Pickwick, the overweight boy who has a tendency to fall asleep is “Joe”, a servant to Mr Pickwick's friend. Mr Pickwick himself is not described as having any sleeping difficulty or somnolence [32]; thus, OHS has been erroneously attributed to Mr Pickwick.
Current definitions
Although the AASM defined OHS in 1999, more than 20 years later the literature continues to demonstrate some discrepancy in defining OHS. The most recent published international guidelines and reviews are consistent in their cut-offs for BMI and mostly for the cut-off for daytime hypercapnia. The American Thoracic Society (ATS) clinical practice guideline [22], the European Respiratory Society (ERS) Task Force outcomes [20], and reviews by Masa et al. [15] and Piper and Grunstein [33] all state that the BMI cut-off for OHS is 30 kg·m−2. All except the ERS Task Force also stated that the PaCO2 cut-off for OHS is simply 45 mmHg [15, 22, 33]. The ERS Task Force adds colour to this discussion by introducing a staging system for hypoventilation in obesity, which incorporates progressive changes in the degree of hypercapnia (table 1) [20]. The widest discrepancy in these publications is in the inclusion of sleep disordered breathing as part of the diagnosis of OHS. While the ATS guideline [22] and Masa et al. [15] include sleep disordered breathing in their definition of OHS (albeit without any specific definition of sleep disordered breathing itself), the ERS Task Force does not make mention of it [20]. Piper and Grunstein [33] state that sleep disordered breathing is not part of the definition of OHS but do mention that breathing in patients with OHS does reflect polysomnographically defined phenotypes.
Table 1.
Staging of hypoventilation in obesity
| 0 | At risk | BMI >30 kg·m−2 | No hypercapnia |
| 1 | Obesity-associated sleep hypoventilation | BMI >30 kg·m−2 | Intermittent nocturnal hypercapnia; serum bicarbonate <27 mmol·L−1 |
| 2 | Obesity-associated sleep hypoventilation | BMI >30 kg·m−2 | Intermittent nocturnal hypercapnia; serum daytime bicarbonate ≥27 mmol·L−1 |
| 3 | Obesity hypoventilation | BMI >30 kg·m−2 | Sustained daytime hypercapnia (PaCO2 >45 mmHg) |
| 4 | Obesity hypoventilation syndrome | BMI >30 kg·m−2 | Sustained daytime hypercapnia with cardiometabolic abnormalities |
Reproduced and modified from [20] with permission.
While these guidelines and reviews appear to be mostly consistent in their definition of OHS, other publications continue to use other definitions. BMI cut-offs have been reported as >40 kg·m−2 [34, 35] and many observational studies focus on patients with BMI >40 kg·m−2. In doing so, they focus on the most severe patients with most severe morbidity, but potentially miss a larger and wider problem in those with BMI 30–40 kg·m−2.
Notwithstanding the proposed, although not widely adopted, staging of hypoventilation in obesity (table 1), there is no extant scale of severity in OHS. Cabrera Lacalzada and Díaz-Lobato [21] suggested categorising OHS into mild, moderate or severe based upon daytime PaCO2, daytime arterial oxygen tension (PaO2), BMI or apnoea/hypopnoea index (table 2). While this classification may hold some value, a single study has been identified that has used it to assess severity. In a cohort of newly diagnosed OHS patients, a retrospective analysis demonstrated that an increasing degree of hypercapnia was associated with increased BMI and bicarbonate level, and worse nocturnal hypoxaemia, pulmonary function tests and sleep architecture [36]. As prevalence of OHS increases with increasing obesity levels, in order to target healthcare resources, there will likely be an increasing call to classify OHS further according to associated complication and morbidity, and outcome. This may see the return of this proposed classification system or other novel forms.
Table 2.
Proposed classification system for obesity hypoventilation syndrome
| Mild | Moderate | Severe | |
| PaCO2 (mmHg) | 46–60 | 60–80 | >80 |
| PaO2 (mmHg) | >70 | 60–70 | <60 |
| BMI (kg·m−2) | 30–40 | 40–50 | >50 |
| Apnoea/hypopnoea index (events·h−1) | <5 | 5–15 | >15 |
| Comorbidities | No | No | Yes |
Reproduced from [21] with permission.
Serum bicarbonate
The recent ATS guideline made a recommendation that clinicians can use serum bicarbonate level <27 mmol·L−1 to exclude OHS in individuals where the suspicion is low [22]. This inclusion reflects increasing calls to incorporate serum bicarbonate into the definition of OHS [37]. The primary driver for this was the volatility of PaCO2 and the patient's ability to volitionally lower it through anxiety-induced hyperventilation when undergoing arterial blood gas sampling. In patients referred to a sleep disorders clinic, a serum bicarbonate threshold of 27 mmol·L−1 had a sensitivity of 92% and specificity of 50% for identifying OHS [3]. Macavei et al. [38] assessed the value of bicarbonate levels obtained from capillary blood gas samples. A threshold of 27 mmol·L−1 demonstrated 86% sensitivity and 90% specificity, with positive predictive value for OHS of 69% and negative predictive value of 96% [38]. A cohort of obese patients [39] were characterised with overnight respiratory polygraphy and categorised into three groups: 1) normal blood gas and acid–base balance, 2) isolated raised base excess (≥2 mmol·L−1), 3) awake hypercapnia. The group with isolated raised base excess represented an intermediate group in terms of respiratory physiology and sleep outcomes, but these data were closer to those with frank daytime hypercapnia than those with normal blood gas and acid–base balance. The mean serum bicarbonate was 27 mmol·L−1 in the intermediate group [39]. Another large cohort investigating predictors of obesity-related sleep hypoventilation (categorised according to the ERS Task Force report [20]) reported that capillary blood gas bicarbonate was 26 mmol·L−1 in individuals with nocturnal-only hypoventilation without daytime hypercapnia [40].
Clinical features of OHS vary depending upon ethnicity. Japanese individuals with OHS tend to be younger than OSA patients [41, 42], while in the Middle East, OHS patients appear to be older than OSA patients [43, 44]. East Asian [45] and Indian [46] populations tend to develop OHS at lower BMIs than non-Asian populations. Reflecting upon these ethnic differences, Saeseow et al. [47] investigated for predictors of OHS specifically in the Thai population and identified serum bicarbonate as an independent predictor.
These studies demonstrate that serum bicarbonate is clearly of relevance in the diagnosis of OHS. There is increasing evidence for the concept that bicarbonate may be a useful marker to screen for obese individuals without daytime hypercapnia but who may suffer from nocturnal hypoventilation. The data support the assertion that it is best used in the exclusion of OHS in individuals in whom the clinician has low suspicion. A serum bicarbonate level >27 mmol·L−1 therefore cannot define OHS but may serve as a useful screening tool and should warrant further investigation with arterial blood gas analysis. OHS studies report base excess, arterial gas bicarbonate and serum bicarbonate as potential screening biomarkers for OHS, almost interchangeably. It is worth noting here the methodological factors involved: arterial gas bicarbonate is a calculation a blood gas analyser makes, and the base excess is a further calculation from this. Results of both tests are susceptible to preanalytical considerations and, furthermore, are more challenging to obtain than simple venepuncture. Serum bicarbonate, therefore, is potentially the most useful marker of the three, for exclusion of low-suspicion OHS [48].
Future directions
There remain several key areas where further study is required. The place of sleep disordered breathing in the definition and diagnosis of OHS remains unclear and observational work is needed to determine whether it does indeed serve as a feature of OHS. There is no widely used severity scale for OHS. As prevalence increases and healthcare resource allocation is increasingly scrutinised, classifying OHS according to severity and potential outcomes would aid the provision of treatment to those who would most benefit. Finally, further work needs to be conducted to demonstrate the extent to which serum bicarbonate can be used as both a screening tool for OHS and as a biomarker to monitor the effectiveness of treatment.
Summary
OHS is an important cause of morbidity and mortality with potential to increase in significance as the prevalence of obesity rises worldwide. Despite this, the condition only received a formal diagnosis just over 20 years ago. The literature prior to the formal definition by the AASM does not clearly delineate between OSA and OHS; it is therefore difficult to extrapolate clinical and public health conclusions from the older studies. The ATS and ERS mostly appear to agree on their definition of OHS: the presence of daytime hypercapnia (PaCO2 >45 mmHg) in individuals with obesity (BMI >30 kg·m−2) with or without sleep disordered breathing. Other literature continues to use different definitions but with time and wider adoption of the ERS and ATS definition, some degree of consistency may be achieved. An important outstanding issue is defining a classification system for OHS based on severity of outcome. Existing classification systems have not been tested in large-scale prospective studies. With the potential for increasing prevalence of this condition with a heavy burden on health resources, it is crucial now that severity is defined and assessed against outcome, to allow resources to be targeted at the most appropriate individuals.
Key points
OHS is associated with significant morbidity and mortality, over and above other respiratory disorders associated with obesity, such as OSA. It is therefore imperative that we have a clear definition for this condition, to allow timely identification of patients.
OHS was formally defined in 1999 as the presence of daytime alveolar hypoventilation (PaCO2 >45 mmHg) in patients with BMI >30 kg·m−2 in the absence of other causes of hypoventilation. Prior to this, the literature does not clearly delineate between OHS and OSA.
Recent guidelines continue to demonstrate discrepancies in their exact definition of the condition.
There is an urgent need to conduct prospective studies evaluating the proposed classification system (that may incorporate serum bicarbonate) against outcomes, to allow resource allocation to be targeted at those individuals who will benefit the most.
Self-evaluation questions
- Which of the following criteria are most commonly included in definitions of OHS?
- a) Daytime hypercapnia
- b) Raised BMI
- c) Sleep disordered breathing
- d) Nocturnal hypoxia
An evidence-based classification system for OHS is currently in clinical use. True or false?
- When was the current definition of OHS adopted?
- a) 1888
- b) 1956
- c) 1999
- d) 2019
Suggested answers
a–c.
False.
c.
Footnotes
Conflict of interest: N.M. Shah has nothing to disclose.
Conflict of interest: S. Shrimanker has nothing to disclose.
Conflict of interest: G. Kaltsakas has nothing to disclose.
References
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019; 92: 6–10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Williams EP, Mesidor M, Winters K, et al. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 2015; 4: 363–370. doi: 10.1007/s13679-015-0169-4 [DOI] [PubMed] [Google Scholar]
- 3.Mokhlesi B, Tulaimat A, Faibussowitsch I, et al. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11: 117–124. doi: 10.1007/s11325-006-0092-8 [DOI] [PubMed] [Google Scholar]
- 4.Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One 2015; 10: e0117808. doi: 10.1371/journal.pone.0117808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik PE, Chen C. The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract 2016; 2: 40–47. doi: 10.1002/osp4.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest 2010; 138: 84–90. doi: 10.1378/chest.09-2472 [DOI] [PubMed] [Google Scholar]
- 7.Blankenburg T, Benthin C, Pohl S, et al. Survival of hypercapnic patients with COPD and obesity hypoventilation syndrome treated with high intensity non invasive ventilation in the daily routine care. Open Respir Med J 2017; 11: 31–40. doi: 10.2174/1874306401711010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacedonia D, Carpagnano GE, Patricelli G, et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin Respir J 2018; 12: 1905–1911. doi: 10.1111/crj.12754 [DOI] [PubMed] [Google Scholar]
- 9.Borel JC, Burel B, Tamisier R, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS One 2013; 8: e52006. doi: 10.1371/journal.pone.0052006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg G, Delaive K, Manfreda J, et al. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120: 377–383. doi: 10.1378/chest.120.2.377 [DOI] [PubMed] [Google Scholar]
- 11.Ojeda Castillejo E, de Lucas Ramos P, López Martin S, et al. Noninvasive mechanical ventilation in patients with obesity hypoventilation syndrome. Long-term outcome and prognostic factors. Arch Bronconeumol 2015; 51: 61–68. doi: 10.1016/j.arbres.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Hida W, Okabe S, Tatsumi K, et al. Nasal continuous positive airway pressure improves quality of life in obesity hypoventilation syndrome. Sleep Breath 2003; 7: 3–12. doi: 10.1007/s11325-003-0003-1 [DOI] [PubMed] [Google Scholar]
- 13.Budweiser S, Tratz F, Gfüllner F, et al. Long-term outcome with focus on pulmonary hypertension in obesity hypoventilation syndrome. Clin Respir J 2020; 14: 940–947. doi: 10.1111/crj.13225 [DOI] [PubMed] [Google Scholar]
- 14.Balachandran JS, Masa JF, Mokhlesi B. Obesity hypoventilation syndrome epidemiology and diagnosis. Sleep Med Clin 2014; 9: 341–347. doi: 10.1016/j.jsmc.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masa JF, Pépin JL, Borel JC, et al. Obesity hypoventilation syndrome. Eur Respir Rev 2019; 28: 180097. doi: 10.1183/16000617.0097-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caton R. A case of narcolepsy. Trans Clin Soc Lond 1889; 22: 133–137. [Google Scholar]
- 17.Morison A. Somnolence with cyanosis cured by massage. Practitioner 1889; 42: 277–281. [DOI] [PubMed] [Google Scholar]
- 18.Burwell CS, Robin ED, Whaley RD, et al. Extreme obesity associated with alveolar hypoventilation – a Pickwickian syndrome. Am J Med 1956; 21: 811–818. doi: 10.1016/0002-9343(56)90094-8 [DOI] [PubMed] [Google Scholar]
- 19.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22: 667–689. doi: 10.1093/sleep/22.5.667 [DOI] [PubMed] [Google Scholar]
- 20.Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017; 49: 1600959. doi: 10.1183/13993003.00959-2016 [DOI] [PubMed] [Google Scholar]
- 21.Cabrera Lacalzada C, Díaz-Lobato S. Grading obesity hypoventilation syndrome severity. Eur Respir J 2008; 32: 817–818. doi: 10.1183/09031936.00059508 [DOI] [PubMed] [Google Scholar]
- 22.Mokhlesi B, Masa JF, Brozek JL, et al. Evaluation and management of obesity hypoventilation syndrome. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e6–e24. doi: 10.1164/rccm.201905-1071ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavie P. Who was the first to use the term Pickwickian in connection with sleepy patients? History of sleep apnoea syndrome. Sleep Med Rev 2008; 12: 5–17. doi: 10.1016/j.smrv.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Auchincloss JH Jr, Cook E, Renzetti AD. Clinical and physiological aspects of a case of obesity, polycythemia and alveolar hypoventilation. J Clin Invest 1955; 34: 1537–1545. doi: 10.1172/JCI103206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estes EH Jr, Sieker HO, McIntosh HD, et al. Reversible cardiopulmonary syndrome with extreme obesity. Circulation 1957; 16: 179–187. doi: 10.1161/01.CIR.16.2.179 [DOI] [PubMed] [Google Scholar]
- 26.Gerardy W, Herberg D, Kuhn HM. [Comparative studies on pulmonary function and the electroancephalogram in 2 patients with Pickwick's syndrome]. Z Klin Med 1960; 156: 362–380. [PubMed] [Google Scholar]
- 27.Drachman DB, Gumnit RJ. Periodic alteration of consciousness in the “Pickwickian” syndrome. Arch Neurol 1962; 6: 471–477. doi: 10.1001/archneur.1962.00450240049006 [DOI] [PubMed] [Google Scholar]
- 28.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res 1966; 1: 167–186. doi: 10.1016/0006-8993(66)90117-X [DOI] [PubMed] [Google Scholar]
- 29.Peters BR, Guilleminault C. A short history of obstructive sleep apnea syndrome. In: Chokroverty S, Billiard M, eds. Sleep Medicine: A Comprehensive Guide to Its Development, Clinical Milestones, and Advances in Treatment. New York, Springer; , 2015; pp. 357–364. [Google Scholar]
- 30.Rapoport DM, Garay SM, Epstein H, et al. Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest 1986; 89: 627–635. doi: 10.1378/chest.89.5.627 [DOI] [PubMed] [Google Scholar]
- 31.Hassel B. Pickwickian syndrome, a misnomer. Hum Pathol 1997; 28: 1329–1330. doi: 10.1016/S0046-8177(97)90216-1 [DOI] [PubMed] [Google Scholar]
- 32.Bray GA. What's in a name? Mr. Dickens’ “Pickwickian” fat boy syndrome. Obes Res 1994; 2: 380–383. doi: 10.1002/j.1550-8528.1994.tb00079.x [DOI] [PubMed] [Google Scholar]
- 33.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med 2011; 183: 292–298. doi: 10.1164/rccm.201008-1280CI [DOI] [PubMed] [Google Scholar]
- 34.Shetty S, Parthasarathy S. Obesity hypoventilation syndrome. Curr Pulmonol Rep 2015; 4: 42–55. doi: 10.1007/s13665-015-0108-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokhlesi B. Chapter 120: Obesity-hypoventilation syndrome. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th Edn. Philadelphia, Elsevier; , 2017; pp. 1189–1199. [Google Scholar]
- 36.Damiani MF, Falcone VA, Carratù P, et al. Using PaCO 2 values to grade obesity-hypoventilation syndrome severity: a retrospective study. Multidiscip Respir Med 2017; 12: 14. doi: 10.1186/s40248-017-0093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart N, Mandal S, Manuel A, et al. Obesity hypoventilation syndrome: does the current definition need revisiting? Thorax 2014; 69: 83–84. doi: 10.1136/thoraxjnl-2013-204298 [DOI] [PubMed] [Google Scholar]
- 38.Macavei VM, Spurling KJ, Loft J, et al. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J Clin Sleep Med 2013; 9: 879–884. doi: 10.5664/jcsm.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manuel ARG, Hart N, Stradling JR. Is a raised bicarbonate, without hypercapnia, part of the physiologic spectrum of obesity-related hypoventilation? Chest 2015; 147: 362–368. doi: 10.1378/chest.14-1279 [DOI] [PubMed] [Google Scholar]
- 40.Sivam S, Yee B, Wong K, et al. Obesity hypoventilation syndrome: early detection of nocturnal-only hypercapnia in an obese population. J Clin Sleep Med 2018; 14: 1477–1484. doi: 10.5664/jcsm.7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akashiba T, Akahoshi T, Kawahara S, et al. Clinical characteristics of obesity-hypoventilation syndrome in Japan: a multi-center study. Intern Med 2006; 45: 1121–1125. 10.2169/internalmedicine.45.1747 [DOI] [PubMed] [Google Scholar]
- 42.Harada Y, Chihara Y, Azuma M, et al. Obesity hypoventilation syndrome in Japan and independent determinants of arterial carbon dioxide levels. Respirology 2014; 19: 1233–1240. 10.1111/resp.12367 [DOI] [PubMed] [Google Scholar]
- 43.Alzaabi A, Fizal S, Moilothkandy R, et al. Obesity hypoventilation syndrome in obstructive sleep apnea patients in the United Arab Emirates: a retrospective cross-sectional study. JRSM Short Rep 2013; 4: 2042533313510156. 10.1177/2042533313510156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BaHammam AS. Prevalence, clinical characteristics, and predictors of obesity hypoventilation syndrome in a large sample of Saudi patients with obstructive sleep apnea. Saudi Med J 2015; 36: 181–189. 10.15537/smj.2015.2.9991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Fujimoto K, Urushibata K, et al. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest 2003; 124: 212–218. 10.1378/chest.124.1.212 [DOI] [PubMed] [Google Scholar]
- 46.Patro M, Gothi D, Ojha UC, et al. Predictors of obesity hypoventilation syndrome among patients with sleep-disordered breathing in India. Lung India 2019; 36: 499–505. 10.4103/lungindia.lungindia_61_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeseow S, Chattakul P, Khamsai S, et al. Predictors for obesity hypoventilation syndrome in Thai population. Sleep Med Res 2019; 10: 13–16. doi: 10.17241/smr.2019.00318 [DOI] [Google Scholar]
- 48.Monneret D. Bicarbonate or base excess in early obesity hypoventilation syndrome: a methodologic viewpoint. Chest 2015; 147: e231. doi: 10.1378/chest.15-0189 [DOI] [PubMed] [Google Scholar]