Abstract
Fifteen Borrelia burgdorferi sensu lato isolates from questing ticks and skin biopsy specimens from erythema migrans patients in three different areas of Spain were characterized. Four different genospecies were found (nine Borrelia garinii, including the two human isolates, three B. burgdorferi sensu stricto, two B. valaisiana, and one B. lusitaniae), showing a diverse spectrum of B. burgdorferi sensu lato species. B. garinii isolates were highly variable in terms of pulsed-field gel electrophoresis pattern and OspA serotype, with four of the seven serotypes described. One of the human isolates was OspA serotype 5, the same found in four of seven tick isolates. The second human isolate was OspA serotype 3, which was not present in ticks from the same area. Seven B. garinii isolates were able to disseminate through the skin of C3H/HeN mice and to cause severe inflammation of joints. One of the two B. valaisiana isolates also caused disease in mice. Only one B. burgdorferi sensu stricto isolate was recovered from the urinary bladder. One isolate each of B. valaisiana and B. lusitaniae were not able to disseminate through the skin of mice or to infect internal organs. In summary, there is substantial diversity in the species and in the pathogenicity of B. burgdorferi sensu lato in areas in northern Spain where Lyme disease is endemic.
Lyme borreliosis (LB) is considered the most prevalent tick-borne disease worldwide. In Europe, the causative agent, Borrelia burgdorferi sensu lato, is diverse and has been divided into several species or genomic groups, three of which (B. burgdorferi sensu stricto [29] [B. burgdorferi in this paper], B. garinii [7], and B. afzelii [13]) are pathogenic for humans. The pathogenicity of B. lusitaniae (35), also present in Europe, remains to be elucitated, since it has been isolated only from Ixodes ricinus. B. valaisiana (61), isolated for the first time in Switzerland (45), has been detected by PCR in skin lesions of erythema migrans (EM) patients (52), and there is some evidence of pathogenic potential in humans (53). There have also been descriptions of genotypic and phenotypic similarities of human European isolates to strain 25015 of B. bissettii (47, 58), but this strain has been isolated only from ticks and small mammals (50). In addition, Wang et al. (62) have suggested that apart from the established genospecies, there is another Borrelia genomic group with culture-confirmed pathogenic potential for causing human LB.
Investigations into the geographical distribution of B. burgdorferi sensu lato in Europe have revealed that B. garinii is the most frequently cultured species, followed by B. afzelii, B. burgdorferi, and B. valaisiana in that order (25, 54). B. valaisiana and B. lusitaniae have been isolated from or detected in I. ricinus in a few countries (25). A genospecies specificity has been proposed in Eurasia, with rodents as the main host for B. afzelii (19, 24, 26, 27, 38, 39), and birds as the main host for B. garinii (33, 41), where a migration restlessness-associated transient spirochetemia occurs (23). However, there are some descriptions of the existence of such cycles for B. garinii and B. valaisiana in different Eurasian countries (19, 26, 33, 38). Other authors argue against this, describing an even distribution of Borrelia species in local ticks and rodents (51), proposing a one-vector–one-reservoir system. In addition, B. garinii has been detected in small rodents in other studies (28, 32), and all three genospecies were detected in larval ticks feeding on birds (42).
In Spain, the first isolation of B. burgdorferi (strain Esp1) from I. ricinus was reported in 1992 (17). Previously, Oteo Revuelta et al. (44) had described spirochetes in the midgut of I. ricinus in a different area from the one where the strain Esp1 was isolated. Although LB has been reported in Spain since 1977 (60) and several series of cases have been studied (2, 20, 55), it was not until 1998 that the first isolation of B. garinii from an EM lesion was described (43), confirming the role of this strain as a human pathogen in Spain.
Since information about the prevalence of Borrelia spirochetes in tick populations and about the different genospecies is essential for our understanding of the epidemiology, diagnosis, and prevention of LB, we have conducted the first study involving the molecular and pathogenic characterization of B. burgdorferi sensu lato isolates from ticks from different areas of Spain known to harbor populations of I. ricinus (8, 16), as well as from skin biopsy specimens from patients with LB.
MATERIALS AND METHODS
Isolation of the spirochetes.
Questing I. ricinus ticks were collected by flagging at three regions in the northern half of Spain (Basque Country, La Rioja, and Castilla-León), in areas known to harbor dense populations of I. ricinus (8, 16). The ticks were disinfected by serial passages of 2 min in 2-propanol and 70% ethanol, serially washed in phosphate-buffered saline and Barbour-Stoenner-Kelly medium II (BSKII), and placed in fresh BSKII, where the specimens were broken up with two needles. The suspension was either filtered through a syringe filter (μStar 0.45-μm-pore-size filter; Corning Inc., Corning, N.Y.) and added to a 5-ml culture tube containing 4.5 ml of BSK supplemented with 6% rabbit serum (BSK-RS) (14) or directly added without previous filtration to a BSK-RS tube supplemented with 0.4 μg of ciprofloxacin per ml and 40 μg of rifampin per ml (BSK-CR) (11). The second type of medium used, to which unfiltered tick suspension was added, was composed of BSK-RS supplemented with 8 μg of kanamycin per ml and 230 μg of 5-fluorouracil per ml (BSK-K5) (30). Blind passages were done always at 24 to 48 h of inoculation to avoid possible toxicity of tick debris and to prevent any adverse effects of the antibiotics on the growth of the spirochetes (6). Cultures were examined by dark-field microscopy weekly for the first month and twice a month for the second and third months after inoculation. Spirochetes from positive cultures were frozen at −70°C in BSK supplemented with 10% dimethyl sulfoxide (Sigma-Aldrich Química S.A., Alcobendas, Madrid, Spain). When possible, only isolates from the first blind passage in antibiotic-free medium were used throughout all the study. Skin biopsy specimens from patients with EM were shipped to the laboratory in complete BSK medium. They were processed as described previously (43).
In addition to the spirochetes isolated in this study, a total of 30 B. burgdorferi sensu lato strains were used for comparison as shown in Table 1.
TABLE 1.
B. burgdorferi sensu lato strains used in this study and their characteristics
| Strain | Sourcea | Country of origin | PFGE pattern | OspA serotype | Genospecies | Reference |
|---|---|---|---|---|---|---|
| B31T | I. dammini | United States | MLb1 | 1 | B. burgdorferi | 65 |
| 272 | Skin | United States | NAb | 1 | B. burgdorferi | 65 |
| 297 | Human CSF | United States | MLb2 | 1 | B. burgdorferi | 65 |
| Esp1 | I. ricinus | Spain | MLb2 | 1 | B. burgdorferi | 17 |
| PBi | Human CSF | Germany | MLg2 | 4 | B. garinii | 65 |
| DK27 | Skin of EM patient | Denmark | NA | NA | B. garinii | 59 |
| R-IP90 | I. persulcatus | Russia | NA | NA | B. garinii | 36 |
| DK6 | Human CSF | Denmark | NA | 4 | B. garinii | 65 |
| WABSou | Skin | Austria | NA | 5 | B. garinii | 65 |
| PHei | CSF | Germany | NA | 5 | B. garinii | 65 |
| TN | I. ricinus | Germany | NA | 6 | B. garinii | 65 |
| PWudII | Skin of EM patient | Germany | MLg2 | 6 | B. garinii | 65 |
| TIs I | I. ricinus | Germany | NA | 6 | B. garinii | 65 |
| DK29 | Skin of EM patient | Denmark | NA | 6 | B. garinii | 65 |
| Gö2 | I. ricinus | Germany | NA | 6 | B. garinii | 65 |
| T25 | I. ricinus | Germany | MLg3 | 7 | B. garinii | 65 |
| PBr | CSF | Germany | MLg2 | 3 | B. garinii | 65 |
| PLa | CSF | Germany | NA | 8 | B. garinii | 65 |
| VS461T | I. ricinus | Switzerland | MLa1 | 2 | B. afzelii | 10 |
| DK1 | Skin of EM patient | Denmark | NA | 2 | B. afzelii | 65 |
| DK2 | Skin of ACA patient | Denmark | NA | 2 | B. afzelii | 65 |
| M49 | I. ricinus | The Netherlands | NA | NA | B. valaisiana | 62 |
| VS116T | I. ricinus | Switzerland | NA | NA | B. valaisana | 48 |
| POTIB1 | I. ricinus | Portugal | NA | NA | B. lusitaniae | 48 |
| POTIB2T | I. ricinus | Portugal | NA | NA | B. lusitaniae | 48 |
| H014T | I. ovatus | Japan | NA | NA | B. japonica | 48 |
| IKA2 | I. ovatus | Japan | NA | NA | B. japonica | 31 |
| 19857 | Rabbit | United States | NA | NA | B. andersonii | 48 |
| 21038T | I. dentatus | United States | NA | NA | B. andersonii | 37 |
| DN127T | I. pacificus | United States | MLb14 | NA | B. bissettii | 48 |
CSF, cerebrospinal fluid; ACA, acrodermatitis chronica atrophicans.
NA, not available.
Sequencing of the 16S rRNA gene and phylogenetic analysis.
Partial sequencing of the 16S rRNA gene was done by PCR with primers constructed as described previously (3). The primers used were based on the published sequences of the bacterial 16S rRNA (4, 18, 36, 56). For this study we used primer 16-1 (5′-CGAAGAGTTTGATCCTGGCTTAG-3′) as the forward primer and primer 16-3 (5′-GCGGCTGCTGGCACGTAATTAGC-3′) as the reverse primer. The amplified fragment was 519 bp long. Products were purified using the Qiaquick PCR purification columns (Qiagen Inc., Chatsworth, Calif.) as specified by the manufacturer and sequenced using the ABI PRISM Dye Terminator cycle-sequencing ready reaction kit (Perkin-Elmer Co.) on an ABI 377 DNA sequencer.
The DNASTAR package (DNASTAR, Inc., Madison, Wis.) and the Clustal method were used for sequence alignment and construction of the phylogenetic tree. 16S rRNA sequences from other B. burgdorferi strains were used in the analysis to construct the phylogenetic tree. These included B. burgdorferi 272 (GenBank accession number X85189), 297 (X85204), and Esp1 (U28501); B. garinii DK27 (X85193), R-IP9 (M89937), and Rio1 (U28500); B. afzelii DK1 (X85190) and DK2 (X85188); B. valaisiana M49 (U78155) and VS116T (X98232); B. lusitaniae POTIB1 (X98226) and POTIB2T (X98228); B. japonica H014T (L40597) and IKA2 (L40598); B. andersonii 19857 (L46688) and 21038T (L46701); and B. bissettii DN127T (L40596). The 16S rRNA sequence from Treponema pallidum (M88726) was used as well.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was done as described previously (10, 46). Two restriction endonucleases were used: MluI and SmaI (MBI Fermentas, Amherst, N.Y.). A contour-clamped homogeneous electric field pulsed-field apparatus (CHEF-DRII; Bio-Rad Laboratories, Richmond, Calif.) was used for all separations. For the separation of undigested genomic DNA, we used a pulse time ramped from 1 to 6 s for 24 h at 200 V; for the separation of digested DNA, pulse times were ramped from 3 to 40 s for 20 h. Lambda concatamers with a monomer size of 48.5 kbp (Boehringer, Mannheim, Germany) and a high-molecular-weight marker (Gibco-BRL Life Technologies, Inc., Gaithersburg, Md.) were used as standards. In describing MluI- and SmaI PFGE profiles, we followed the definition and nomenclature previously devised by Belfaiza et al. (10) and Picken et al (46), with the inclusion of the pulsotypes MLv1 and SMv1 for the pattern observed in the B. valaisiana isolates tested.
SDS-PAGE.
For protein analysis, whole-cell sonicates of cultured spirochetes were prepared from Borrelia isolates from ticks and EM patient biopsy specimens, as well as from B. burgdorferi (strain B31T and strain Esp1), B. garinii (strain PBiT), and B. afzelii (strain VS461T). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using Laemmli's discontinuous buffer system and 10% polyacrylamide gels (34). The gels were stained with Coomassie brilliant blue R-250 (Merk AG, Darmstadt, Germany). Protein molecular weight standards (GIBCO BRL, Life Technologies, Inc., Gaithersburg, Md.) were used to determine the relative molecular mass of major proteins by comparison. Monoclonal antibody 84C (15) was used to assess the expression of OspB.
Western blotting.
Sera from the mice inoculated with the different isolates were tested for reactivity to the respective homologous strain by Western blotting, following the previously described protocol (2) with the minor modification of using NuPAGE Bis-Tris System (Novex, San Diego, Calif.).
Partial sequencing of ospA genes.
Nested PCR was carried out as described elsewhere (22). Each PCR amplification product was purified using the Qiaquick PCR purification columns (Qiagen Inc.) as specified by the manufacturer and sequenced as above. The DNASTAR package and the Clustal method were used for sequence alignment and construction of the phylogenetic tree. For comparison of the sequences, a series of other strains were included in this study: Phei (GenBank accession number X80251), TN (X80252), PWudII (X80253), T25 (X80254), PBr (X80256), WABSou (X85441), TIs I (X85440), DK29 (X63412), Gö2 (X60300), DK6 (L38657), PBi (S48323), and PLa (X95355) (genospecies for these strains are indicated in Table 1).
Animal studies.
The C3H/He Lyme disease mouse model (9) was used to assess the pathogenicity of the strains from this study. A total of 20 mice were injected intradermally in the lower back with 104 spirochetes of each isolate. The percentage of mice that developed arthritis after injection was determined for each isolate by monitoring signs of inflammation of the tibiotarsal joints (TTJ) daily during the first week after injection, every other day during the second week, and twice a week until the end of the fourth week. The level of spirochete dissemination through the skin was determined on day 15 by culturing in BSK-RS 3-mm-diameter ear punch biopsy specimens (EPB) from two mice in each group that had shown signs of inflammation (in the groups where no mice showed signs of inflammation, the two mice were selected on the basis of the level of antibodies to the homologous strain). On day 30, the selected mice were euthanized in CO2 chambers and necropsy material from the liver, kidneys, heart, brain, spleen, and bladder was collected and cultured in BSK-RS. Citrated blood samples were also cultured for each mouse to ensure that the isolates were tissue and not blood associated.
A score for pathogenicity was constructed as explained in Table 2.
TABLE 2.
Pathogenicity scores in C3H mice
| Pathogenicity | TTJ inflammation | EPB culture | No. of organs colonized |
|---|---|---|---|
| Nonpathogenic | − | − | 0 |
| Low pathogenicitya | +/− | +/− | 0 or 1b |
| Pathogenic | + | + | 1–6 |
At least one of the three must be positive.
Bladder.
Level of IL-6 in serum.
Quantification of interleukin-6 (IL-6) in the sera of the same mice selected for culture was done using the InterTest-6X mouse IL-6 enzyme-linked immunosorbent assay ELISA kit (Genzyme Diagnostics, Cambridge, Mass.) as specified by the manufacturer.
Nucleotide sequence accession numbers.
Partial sequences of the ospA gene generated in this study were deposited in GenBank under the following accession numbers: AF227323 (Rio1), AF227319 (Rio2), AF227320 (Rio3), AF227321 (Rio4), AF227322 (Rio5), AF227316 (PV4), AF227317 (PV5), AF227318 (PV6), and AF227315 (CL1). Partial sequences of the 16S RNA generated in this study were deposited in GenBank under the following accession numbers: AF245110 (Rio1), AF245111 (Rio2), AF245097 (Rio3), AF245102 (Rio4), AF245108 (Rio5), AF245109 (Rio6), AF245103 (PV1), AF245105 (PV2), AF245106 (PV3), AF245098 (PV4), AF245107 (PV5), AF245100 (PV6), AF245099 (PV7), AF245101 (PV8), and AF245104 (CL1).
RESULTS
Four B. burgdorferi genospecies are present in Spain.
A total of 13 isolates were obtained from pooled I. ricinus. Eight isolates (PV1 to PV8) were derived from ticks collected in the Basque Country, one isolate (CL1) was derived from Castilla-León, and four isolates (Rio3 to Rio6) were derived from La Rioja. We also obtained one more isolate from a skin biopsy specimen of an EM patient in La Rioja (Rio2). The human strain Rio1, previously isolated in our laboratory (43), was also used in this study. For isolate PV8, only PCR-related tests were possible, due to its slow growth.
Sequencing of a fragment at the 3′ end of the 16S rRNA (519 bp long) and subsequent phylogenetic analysis grouped our isolates as follows (Fig. 1): Rio1, Rio2, Rio3, Rio4, Rio5, PV4, PV5, PV6, and CL1 grouped with other B. garinii strains; PV1, PV2, and PV3 grouped with the B. burgdorferi cluster; PV7 and Rio6 formed a branch group with the recently named B. valaisiana species; and PV8 grouped with the B. lusitaniae strains.
FIG. 1.
Phylogenetic tree of B. burgdorferi strains based on the sequence of the 16S rRNA gene as described in the text. The scale under the tree measures the distance between sequences.
There is genotypic and phenotypic variability in the isolated strains.
Figure 2A and Table 3 show the results of the restriction fragment length polymorphism patterns of MluI-digested total genomic DNA by PFGE. According to nomenclature previously described (10, 46), 3 of the 14 isolated strains were B. burgdorferi (PV1 is MLb13, and PV2 and PV3 are MLb2). All of them had the 135-kbp characteristic band. There were nine B. garinii strains (PV4, PV5, PV6, Rio3, Rio4, Rio5, and CL1 from ticks and Rio1 and Rio2 from skin biopsy specimens). These had the two B. garinii characteristic bands of 220 and 80 kbp, and all corresponded to pattern MLg2. PV7 and Rio6, which grouped with B. valaisiana in the phylogenetic analysis, shared an atypical pattern, with three fragments of 380, 320, and 90 kbp. This pattern was named MLv1 in this study. There was a total correlation of these results with the ones obtained by analyzing the 16S rRNA gene. Both analyses yielded the same result with respect to the genospecies of each isolate.
FIG. 2.
PFGE separation of MluI-digested (A) and SmaI-digested (B) genomic DNA. Lanes: L, DNA lambda concatemers (48.5 to 485 kb); 1, strain TI-1; 2, Esp1; 3, PV2; 4, PV1; 5, PBi; 6, Rio4; 7, PV6; 8, PV4; 9, Rio1; 10, Rio2; 11, Rio3; 12, Rio5; 13, PV7; 14, Rio6; 15, PV3; 16, PV5; 17, CL1. The arrowhead indicates the 135-kbp band characteristic of B. burgdorferi sensu stricto, and the arrows indicate the 220- and 80-kbp bands characteristic of B. garinii.
TABLE 3.
Summary of results
| Isolate | Speciesa | PFGE pattern
|
No. of plasmids
|
OspA serotype | % Arthritogenicityb | Recovery from:
|
IL-6 secretion | Pathogenicityd | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MluI | SmaI | 60–40 kb | 39–30 kb | 29–20 kb | <20 kb | EPB | Organsc | ||||||
| PV1 | S | MLb13 | SMb1 | 2 | 2 | 1 | 0 | 1 | 0 | − | B | + | LP |
| PV2 | S | MLb2 | SMb2 | 2 | 2 | 1 | 0 | 1 | 0 | − | − | − | NP |
| PV3 | S | MLb2 | SMb1 | 3 | 0 | 1 | 0 | 1 | 25 | − | − | − | LP |
| PV4 | G | MLg2 | SMg1 | 2 | 1 | 2 | 0 | 5 | 100 | + | B, S, H, Br | ++ | P |
| PV5 | G | MLg2 | SMg2 | 4 | 0 | 2 | 0 | 5 | 25 | − | B | − | LP |
| PV6 | G | MLg2 | SMg1 | 3 | 2 | 2 | 0 | 5 | 100 | + | B, S, H, Br, K, L | +++ | P |
| PV7 | V | MLv1 | SMv1 | 2 | 0 | 1 | 0 | NDe | 0 | − | − | − | NP |
| PV8 | L | ND | ND | ND | ND | ND | ND | ND | 0 | − | − | − | NP |
| CL1 | G | MLg2 | SMg3 | 3 | 0 | 2 | 0 | 8 | 100 | + | B | ++ | P |
| Rio1 | G | MLg2 | SMg1 | ND | ND | ND | ND | 5 | 25 | + | B, S | − | P |
| Rio2 | G | MLg2 | SMg3 | 2 | 1 | 2 | 0 | 3 | 100 | + | − | + | LP |
| Rio3 | G | MLg2 | SMg2 | 3 | 0 | 2 | 0 | 5 | 100 | + | S, H | ++ | P |
| Rio4 | G | MLg2 | SMg4 | 2 | 1 | 1 | 1 | 6 | 100 | + | B, H, K | + | P |
| Rio5 | G | MLg2 | SMg2 | 2 | 2 | 1 | 0 | 6 | 100 | + | B, K | +++ | P |
| Rio6 | V | MLv1 | SMv1 | 1 | 0 | 1 | 0 | ND | 25 | + | B, K | − | P |
S, B. burgdorferi sensu stricto; G, B. garinii; V, B. valaisiana; L, B. lusitaniae.
Percentage based on 20 mice.
B, urinary bladder; S, spleen; H, heart; Br, brain; K, kidney; L, liver.
NP, nonpathogenic; LP, low pathogenicity; P, pathogenic.
ND, not determined.
A higher variability was found when SmaI was used, since some isolates that had the same MluI pulsotype had different SmaI patterns. PV2 and PV3 (MLb2) showed different restriction bands (named types SMb2 and SMb1, respectively). PV1, which was MLb13, had the same pulsotype as PV3 (SMb1). For the nine B. garinii isolates, there were four different patterns (named SMg1 to SMg4). The two strains that grouped with B. valaisiana in the phylogenetic analysis had the same SmaI pattern (named SMv1). Figure 2B and Table 3 show the results of the LRFP patterns of the SmaI-digested total genome.
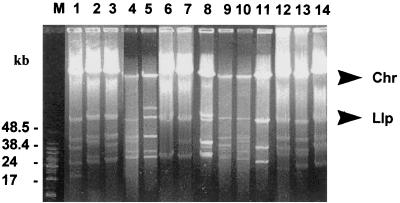
When the total genome (chromosome and plasmids) of the isolates was separated by PFGE, there was a variable number of plasmids, ranging between two and seven, per strain (Fig. 3, Table 3). All isolates contained a large plasmid in the 45- to 50-kbp range, which was identified as the linear ospAB-containing plasmid (12). The size of this plasmid varied, but the variation did not correlate with the different genospecies. The diffuse band of DNA immediately below the chromosome in same strains could correspond to a multimeric form of a small circular plasmid. The plasmid content of each strain, expressed as the number of bands seen in four size ranges (60 to 40, 39 to 30, 29 to 20, and <20 kb), is shown in Table 3.
FIG. 3.
PFGE separation of the total undigested genome of the B. burgdorferi sensu lato isolates and reference strains. Lanes: M, DNA molecular size markers of 8.3, 8.6, 10.1, 12.2, 15.0, 17.1, 19.4, 22.6, 24.8, 29.9, 33.5, 38.4, and 48.5 kb; 1, Rio4; 2, Rio3; 3, Rio5; 4, Rio2; 5, CL1; 6, Rio6; 7, PV7; 8, PV6; 9, PV4; 10, PV5; 11, Esp1; 12, PV2; 13, PV1; 14, PV3.
The phenotypic variability of the isolates was demonstrated by SDS-PAGE (Fig. 4). The protein profiles were compared with the profile of the reference strains, demonstrating a correlation with the results obtained in the genotypic analysis. All the isolates had a protein profile consistent with that for each B. burgdorferi sensu lato species. All had protein bands of various sizes ranging from 13 to 97 kDa. They were homogeneous with regard to the size and level of expression of their higher-molecular-mass proteins (>41 kDa) but heterogeneous in their lower-molecular-mass proteins (<41 kDa). The sizes of OspA and OspB of the B. burgdorferi isolates correlated with those previously described by Baranton et al. (7). Seven of the B. garinii isolates and the two B. valaisiana isolates expressed a protein with a molecular mass higher than that of the OspA protein, which was confirmed to be OspB (by reactivity with monoclonal antibody 84C [data not shown]). The level of expression of a protein in the appropriate size range for OspC (22 to 25 kDa) (64) varied highly among the genospecies (Fig. 4).
FIG. 4.
SDS-PAGE and Coomassie blue staining of B. burgdorferi sensu lato isolates. In each panel, the protein profiles of B31T (B. burgdorferi sensu stricto), PBi (B. garinii), and VS461T (B. afzelii) are also given. Lanes M contain molecular mass markers of the sizes shown. (A) Isolates from Basque Country. Lanes: 1, B31; 2, Esp1; 3, PV2; 4, PV1; 5, PV3; 6, PBi; 7, PV6; 8, PV4; 9, PV5; 10, VS461; 11, PV7. (B) Isolates from La Rioja. Lanes: 1, B31; 2, VS461; 3, Rio6; 4, PBi; 5, Rio4; 6, Rio2; 7, Rio3; 8, Rio5.
Partial sequencing of the ospA gene was performed, and a phylogenetic tree was constructed using representative strains of each serotype described for B. garinii (63, 64) (Fig. 5). Among the B. garinii isolates, PV4, PV5, PV6, Rio1, and Rio3 are serotype 5, Rio4 and Rio5 are serotype 6, Rio2 is serotype 3, and CL1 is serotype 8.
FIG. 5.
Phylogenetic tree of B. burgdorferi strains based on the sequence of the ospA gene as described in the text. The scale under the tree measures the distance between sequences. OspA serotypes of each strain are given in parentheses.
B. burgdorferi isolates have low pathogenicity in C3H mice.
Of the three B. burgdorferi isolates tested, PV2 was nonpathogenic (Table 3) for mice, PV1 did not induce inflammation of the TTJ but was recovered from urinary bladder, and PV3 induced inflammation of the TTJ in only 5 of 20 mice and was considered to be of low pathogenicity (Table 3). The sera of the mice inoculated with PV1 and PV3 showed a reactivity in Western blots (with the respective homologous strain) to the 41-kDa protein, OspA, and OspB (Fig. 6). The isolate that was recovered from urinary bladder also induced secretion of IL-6 at a low level.
FIG. 6.
Reactivity of sera from the B. burgdorferi PV1, PV3, PV7, and Rio6 isolates by Western blotting to the respective homologous strain. M, molecular mass markers of the sizes shown.
B. garinii isolates are pathogenic for mice.
Eight of nine B. garinii isolates disseminated through the skin and induced inflammation of the TTJ (in 25% of mice for two strains, PV5 and Rio1). The rate of recovery from organs varied from isolate to isolate. One isolate of human origin (Rio2) was recovered from EPB and induced inflammation of the TTJ in 100% of mice and secretion of IL-6 but was not recovered from internal organs, suggesting low disseminating capabilities in this model; considering this discrepancy, it was classified as having low pathogenicity. PV5 did not migrate through the skin and induced TTJ inflammation in only 25% of mice, was recovered only from the urinary bladder, and did not induce IL-6 secretion; it was also classified as having low pathogenicity. A third isolate, CL1, induced TTJ inflammation in 100% of mice and secretion of IL-6 and was recovered from EPB but was recovered only from the urinary bladder; it was therefore classified as pathogenic. For the rest of the strains, a positive EPB, inflammation of the TTJ in 100% of mice, secretion of IL-6, and recovery from at least two organs were seen; they were also classified as pathogenic. All the B. garinii strains showed a strong antibody reactivity to the homologous isolate by Western blotting (data not shown).
B. valaisiana is pathogenic for mice.
Of the two B. valaisiana isolates analyzed, one (PV7) did not show any sign of pathogenicity and the other (Rio6) was recovered from EPB, induced TTJ inflammation in 25% of mice, and was recovered from the urinary bladder and kidneys; it was classified as pathogenic. Both isolates showed a high reactivity with the 41-kDa protein and OspA by Western blotting to the homologous strain, and Rio6 was also reactive with a band in the range of the 22-kDa protein (Fig. 6).
In summary, the pathogenicity for mice was higher among the B. garinii isolates, one isolate of B. valaisiana was considered pathogenic, and all the B. burgdorferi isolates showed milder signs of pathogenicity. The isolates that were recovered from EPB were B. garinii and B. valaisiana strains. Also, recovery from the urinary bladder was considered of low significance since, even with no arthritogenicity or recovery from EPB, some isolates were found in this organ, suggesting that it was preferentially infected, with low pathogenic significance. The secretion of IL-6 at low level (1+) did not always correlate with inflammation of TTJ in our system, but secretion at 2+ to 3+ level was always associated with positive EPB.
DISCUSSION
Fifteen B. burgdorferi sensu lato isolates were recovered from Spanish I. ricinus ticks and biopsy specimens from EM patients. Of these, three were B. burgdorferi sensu stricto, nine were B. garinii, two were B. valaisiana, and one was B. lusitaniae. These findings indicate greater genospecies diversity of B. burgdorferi sensu lato in Spain than in other parts of Europe. The only genospecies not present was B. afzelii, even though this species is the second most frequently isolated throughout Europe (25, 54). Based on these results, B. afzelii may be absent at the southwestern margin of the continent. Accordingly, B. afzelii has not been detected in patients in Spain (1), and there has been an absence of descriptions of B. afzelii-related cutaneous manifestations in clinical series (2, 5, 20, 21). In contrast, B. lusitaniae is present in southern Europe and North Africa (40, 65) but it is not frequent in eastern Europe (49). Overlapping geographic areas in the Iberian peninsula with highly diverse B. burgdorferi populations as well as relapsing-fever borrelia (4) could create the necessary conditions for genetic exchanges and for the origin of new genospecies.
The high intraspecies variability detected on the basis of all the parameters studied is reflected in the behavior of the isolates in C3H mice (Table 3). Seven of the nine B. garinii isolates (CL1, Rio1, Rio3, Rio4, Rio5, PV4, and PV6) disseminated through the skin, induced severe TTJ inflammation in 20 of 20 mice, and caused disseminated infection in C3H mice. Organisms were recovered from a battery of internal organs (Table 3). The two remaining B. garinii strains (Rio2 and PV5) showed low pathogenicity, even though one of them was an isolate of human origin, which was the only one that disseminated through the skin of C3H mice. None of the three B. burgdorferi isolates were virulent to C3H mice (only strain PV1 was recovered from urinary bladder, and strain PV3 induced TTJ inflammation in 25% of mice). None of them were recovered from EPB. Interestingly, one of the two B. valaisiana isolates (Rio6) was able to disseminate through the skin and to induce severe TTJ inflammation in 25% of mice and was recovered from the kidneys and urinary bladder, suggesting that a tick-mouse cycle could maintain this isolate in nature.
Several authors have suggested that B. afzelii is preferentially or exclusively maintained in cycles involving small rodents and ticks (19, 24, 26, 27, 38). B. burgdorferi has been largely associated with small rodents (57). Associations for B. garinii appear to be more heterogeneous: a cycle involving sea birds has been well characterized for this species (41), and a mechanism of transient spirochetemia associated with migrating birds has been described (23). Several other studies have pointed out the existence of such cycles for B. garinii and B. valaisiana in different Eurasian countries (19, 23, 26, 33, 38). However, other descriptions have found B. garinii associated with small rodents (28, 51). Whether a bird-tick or rodent-tick cycle or perhaps both maintain local variants of B. garinii and B. valaisiana in nature remains to be elucidated, but, given the high frequency of B. garinii isolates in the areas studied and the data from the animal model, we have shown that at least some variants of B. garinii can infect mice and disseminate through the skin from day 7 after infection until at least day 90 (data not shown). Since B. garinii is a very heterogeneous species in terms of pulsotype (Fig. 2, Table 3), plasmid content (Fig. 3, Table 3) and OspA serotype (Fig. 5, Table 3), some of these differences could account for a distinct susceptibility of different hosts. Data about the transmission of the human isolate Rio1 from syringe-infected C3H mice to xenodiagnostic larval I. ricinus and the subsequent transmission of the organisms to mice via a tick bite from the derived nimphal ticks (M. M. Vitutia, unpublished data) support this hypothesis. We can assume that other B. garinii isolates that exhibited a full spectrum of pathogenicity could at least be equally and efficiently maintained in a tick-mouse cycle.
We did not find an association between OspA serotype and pathogenicity to mice. In fact, the only B. garinii strain that was not recovered from EPB was OspA serotype 5 (PV5), in common with four additional isolates that were cultured with this method (PV4, PV6, Rio1, and Rio3). Consequently, different degrees of pathogenicity to mice are found among serotype 5 B. garinii isolates. In summary, in this study, isolates belonging to OspA serotypes 5, 6, and 8 were pathogenic to mice and a serotype 3 isolate had low pathogenicity, suggesting that other factors seem to influence the behavior of B. garinii in mice.
These differences in pathogenicity to C3H mice found in this work for each isolate could be used, given the constraints of extrapolation to humans, to hypothesize about the risk for humans of contracting Lyme disease in a certain area. Given that the isolates represent a highly variable population, they could form the basis for a variable clinical spectrum in humans.
ACKNOWLEDGMENTS
Raquel Escudero participated in this study while supported by a contrast from the DGICYT (Dirección General de Investigación en Ciencia y Tecnología, Spanish Ministry of Education and Culture) program of “Incorporación de Doctores y Tecnólogos.” Ricela E. Sellek was supported by a Beca de Iniciación of the Instituto de Salud Carlos III (ref. 97/4181). This work was supported by Instituto de Salud Carlos III grants 98/0026-01 and 98/0026-02.
We are grateful to Angel del Pozo for the photographic work. We acknowledge the excellent technical work done by Isabel Rodríguez and Cati Chaparro. We also thank Gerardo Dominguez Peñafiel (zona de Salud de Soncillo, Burgos), Rufino Álamo Sanz (Consejería de Salud, Juntas de Castilla-León), and José Antonio Oteo (Servicio de Medicina Interna, Hospital de La Rioja) for providing ticks and patient samples for isolation.
REFERENCES
- 1.Alonso-Llamazares J, Persing D H, Anda P, Gibson L E, Rutledge B J, Iglesias L. No evidence for Borrelia burgdorferi infection in lesions of morphea and lichen sclerosus et atrophicus in Spain. A prospective study and literature review. Acta Dermatol Venereol. 1997;4:299–304. doi: 10.2340/0001555577299304. [DOI] [PubMed] [Google Scholar]
- 2.Anda P, Rodríguez I, de la Loma A, Fernández M V, Lozano A. A serological survey and review of clinical Lyme borreliosis in Spain. Clin Infect Dis. 1993;16:310–319. doi: 10.1093/clind/16.2.310. [DOI] [PubMed] [Google Scholar]
- 3.Anda P, Gebbia J A, Backenson P B, Coleman J L, Benach J L. A glyceraldehyde-3 phosphate dehydrogenase homolog in Borrelia burgdorferi and Borrelia hermsii. Infect Immun. 1996;64:262–268. doi: 10.1128/iai.64.1.262-268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anda P, Sánchez-Yebra W, Vitutia M M, Pérez-Pastrana E, Rodríguez I, Miller N, Backenson P B, Benach J L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. doi: 10.1016/s0140-6736(96)02332-x. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga F, García-Moncó J C. Association of Lyme disease with work and leisure activities. Enferm Infecc Microbiol Clin. 1998;16:256–258. [PubMed] [Google Scholar]
- 6.Balmelli T, Piffaretti T C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 7.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 8.Barral M, García-Pérez A L, Juste R A, Fernández de Luco D, Dehesa V. Estudio de las poblaciones de ixódidos sobre la vegetación del País Vasco. Acta Parasitol Port. 1993;1:170–174. [Google Scholar]
- 9.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Belfaiza J, Postic D, Bellenger E, Baranton G, Saint Girons I. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger B W, Johnson R C, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30:359–361. doi: 10.1128/jcm.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergström S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 13.Canica M M, Nato F, Du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp.nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Inf Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 14.Coleman J L, Benach J L. Isolation of antigenic components from the Lyme disease spirochaete: their role in early diagnosis. J Infect Dis. 1987;155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- 15.Comstock L E, Fikrig E, Shoberg R J, Flavell R A, Thomas D D. A monoclonal antibody to OspA inhibits association of Borrelia burgdorferi with human endothelial cells. Infect Immun. 1993;61:423–431. doi: 10.1128/iai.61.2.423-431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estrada-Peña A, Oteo J A, Estrada-Peña R, Gortazar C, Osacar J J, Moreno J A, Castella J. Borrelia burgdorferi sensu lato in ticks (Acari: Ixodidae) from two different foci in Spain. Exp Appl Acarol. 1995;19:173–180. doi: 10.1007/BF00046289. [DOI] [PubMed] [Google Scholar]
- 17.García-Moncó J C, Benach J L, Coleman J L, Galbe J L, Szczepanski A, Fernández Villar B, Norton Hughes C A, Johnson R C. Caracterización de una cepa española de Borrelia burgdorferi. Med Clin. 1992;98:89–93. [PubMed] [Google Scholar]
- 18.Gazumyan A, Schwartz J J, Liveris D, Schwartz I. Sequence analysis of the ribosomal operon of the Lyme disease spirochete, Borrelia burgdorferi. Gene. 1994;146:57–65. doi: 10.1016/0378-1119(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 19.Gern L, Humair P F. Natural history of Borrelia burgdorferi sensu lato. Wien Klin Wochenschr. 1998;110:856–858. [PubMed] [Google Scholar]
- 20.Guerrero A, Quereda C, Martí-Belda P, Escudero R. Borreliosis de Lyme: ¿cómo se manifiesta en España? Med Clin. 1993;101:5–7. [PubMed] [Google Scholar]
- 21.Guerrero A, Escudero R, Marti-Belda P, Quereda C. Frequency of the clinical manifestations of Lyme borreliosis in Spain. Enferm Infecc Microbiol Clin. 1996;14:72–79. [PubMed] [Google Scholar]
- 22.Guy E C, Stanek G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J Clin Pathol. 1991;44:610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gylfe A, Bergström S, Lundstróm J, Olsen B. Epidemiology: reactivation of Borrelia infection in birds. Nature. 2000;403:724–725. doi: 10.1038/35001663. [DOI] [PubMed] [Google Scholar]
- 24.Hu C M, Humair P F, Wallich R, Gern L. Apodemus sp. rodents, reservoir hosts for Borrelia afzelii in an endemic area in Switzerland. Int J Med Microbiol Virol Parasitol Infect Dis. 1997;285:558–564. doi: 10.1016/s0934-8840(97)80117-x. [DOI] [PubMed] [Google Scholar]
- 25.Hubálek Z, Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol. 1997;13:951–957. doi: 10.1023/a:1007426304900. [DOI] [PubMed] [Google Scholar]
- 26.Humair P F, Peter O, Wallich R, Gern L. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J Med Entomol. 1995;32:433–438. doi: 10.1093/jmedent/32.4.433. [DOI] [PubMed] [Google Scholar]
- 27.Humair P F, Rais O, Gern L. Transmission of Borrelia afzelii from Apodemus mice and Chletrionomys voles to Ixodes ricinus ticks: differential transmission patterns and overwintering maintenance. Parasitology. 1999;118:33–42. doi: 10.1017/s0031182098003564. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro F, Takada N, Nakata K, Yano Y, Suzuki H, Masuzawa T, Yanagihara Y. Reservoir competence of the vole, Clethrionomys rufocanus bedfordiae, for Borrelia garinii or Borrelia afzelii. Microbiol Immunol. 1996;40:67–69. doi: 10.1111/j.1348-0421.1996.tb03305.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson R C, Hyde F W, Schmid G P, Brenner D J. Borrelia burgdorferi sp.nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 30.Johnson S E, Klein G C, Schmid G P, Bowen G S, Feeley J C, Schulze T. Lyme disease: a selective medium for isolation of the suspected aetiological agent, a spirochaete. J Clin Microbiol. 1984;19:81–82. doi: 10.1128/jcm.19.1.81-82.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analyses of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 32.Korenberg E I, Gorelova N B, Postic D, Kovalevskii I V, Baranton G, VoroЬeva N N. The reservoir hosts and vectors of Borrelia—the causative organisms of ixodid tick-borne borreliosis in Russia. Zh Mikrobiol Epidemiol Immunobiol. 1997;6:36–38. [PubMed] [Google Scholar]
- 33.Kurtenbach K, Peacey M, Rijpkema S G T, Hoodless A N, Nuttall P, Randolf S E. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol. 1998;64:1169–1174. doi: 10.1128/aem.64.4.1169-1174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Le Fleche A, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 36.Marconi R T, Garon C F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992;30:2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism pattern, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakao M, Miyamoto K, Fukunaga M. Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, an Ixodes persulcatus ticks. J Infect Dis. 1994;170:878–882. doi: 10.1093/infdis/170.4.878. [DOI] [PubMed] [Google Scholar]
- 39.Nakao M, Miyamoto K. Mixed infections of different Borrelia species among Apodemus speciosus mice in Hokkaido, Japan. J Clin Microbiol. 1995;33:490–492. doi: 10.1128/jcm.33.2.490-492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuncio M S, Péter O, Alves M J, Bacellar F, Filipe A R. Isolamento e caracterizaçao de Borrélias de Ixodes ricinus L. em Portugal. Rev Port Doenças Infec. 1993;16:175–179. [Google Scholar]
- 41.Olsen B, Jaenson T G T, Noppa L, Bunikis J, Bergström S. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature. 1993;362:340–342. doi: 10.1038/362340a0. [DOI] [PubMed] [Google Scholar]
- 42.Olsen B, Jaenson T G T, Bergström S. Prevalence of B. burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oteo J A, Backenson P B, Vitutia M M, García-Moncó J C, Rodríguez I, Escudero R, Anda P. Use of the C3H/He Lyme disease mouse model for the recovery of a Spanish isolate of Borrelia garinii from erythema migrans lesions. Res Microbiol. 1998;149:39–46. doi: 10.1016/s0923-2508(97)83622-4. [DOI] [PubMed] [Google Scholar]
- 44.Oteo Revuelta J A, Estrada Peña A. Ixodes ricinus, vector comprobado de Borrelia burgdorferi en España. Med Clin. 1991;96:599. [PubMed] [Google Scholar]
- 45.Peter O, Bretz A G, Bee D. Occurrence of different genospecies of B. Burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur J Epidemiol. 1995;11:463–467. doi: 10.1007/BF01721234. [DOI] [PubMed] [Google Scholar]
- 46.Picken R N, Cheng Y, Han D, Nelson J A, Reddy A G, Hayden M K, Picken M M, Strle F, Bouseman J K, Trenholme G M. Genotypic and phenotypic characterization of Borrelia burgdorferi isolated from ticks ans small animals in Illinois. J Clin Microbiol. 1995;33:2304–2315. doi: 10.1128/jcm.33.9.2304-2315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picken R N, Cheng Y, Strle F, Picken M M. Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J Infect Dis. 1996;174:1112–1115. doi: 10.1093/infdis/174.5.1112. [DOI] [PubMed] [Google Scholar]
- 48.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of the rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 49.Postic D, Korenberg E, Gorelova N, Kovalevski Y V, Bellenger E, Baranton G. Borrelia burgdorferi sensu lato in Russia and neighbouring countries: high incidence of mixed isolates. Res Microbiol. 1997;148:691–702. doi: 10.1016/S0923-2508(99)80068-0. [DOI] [PubMed] [Google Scholar]
- 50.Postic D, Ras N M, Lane M, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of B. bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter D, Endepols S, Ohlenbusch A, Eiffert H, Spielman A, Matuschka F R. Genospecies diversity of Lyme disease spirochetes in rodents reservoirs. Emerg Infect Dis. 1999;5:291–296. doi: 10.3201/eid0502.990218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rijpkema S G T, Tazelaar D J, Molkenboer M, Noordhoek G T, Plantiga G, Schouls L M, Schellekens J F P. Detection of Borrelia afzelii, Borrelia burgdorferi, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect. 1997;3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 53.Ryffel K, Peter O, Rutti B, Suard A, Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J Clin Microbiol. 1999;37:4086–4092. doi: 10.1128/jcm.37.12.4086-4092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saint Girons I, Gern L, Gray J S, Guy E C, Korenberg E, Nuttall P A, Rijpkema S G T, Schönberg A, Stanek G, Postic D. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt I Orig. 1998;287:190–195. doi: 10.1016/s0934-8840(98)80120-5. [DOI] [PubMed] [Google Scholar]
- 55.Saz J V, Merino F J, Beltrán M. Situación actual de la enfermedad de Lyme en España: aspectos clínicos y epidemiológicos, Rev. Clin Esp. 1995;195:44–49. [PubMed] [Google Scholar]
- 56.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strle F, Picken N, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Ruzic-Sabljic E, Picken M M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- 59.Theisen M, Borre M, Mathiesen M J, Mikkelsen B, Lebeck A M, Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uruñuela Bernedo J, Díaz Sosa D. Eritema crónico migrans. Acta Dermosifilog. 1977;68:109–110. [Google Scholar]
- 61.Wang G, van Dam A P, Le Fleche A, Postic O, Peter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, van Dam A, Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J Clin Microbiol. 1999;37:3025–3028. doi: 10.1128/jcm.37.9.3025-3028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Will G, Jauris-Heipke S, Schwab E, Busch U, Röbler D, Soutschek S, Wilske B, Preac-Mursic V. Sequence analysis of ospA genes shows homogeneity within Borrelia burgdorferi sensu stricto and Borrelia afzelii strains but reveals major subgroups within the Borrelia garinii species. Med Microbiol Immunol. 1995;184:73–80. doi: 10.1007/BF00221390. [DOI] [PubMed] [Google Scholar]
- 64.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhioua E, Bouattour A, Hu C M, Gharbi M, Aeschlimann A, Ginsberg H, Gern L. Infections of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi sensu lato in North Africa (Tunisia), J. Med Entomol. 1999;36:216–218. doi: 10.1093/jmedent/36.2.216. [DOI] [PubMed] [Google Scholar]