Abstract
Immunotherapy revolutionized cancer treatment but has yet to elicit durable responses in the majority of patients with head and neck squamous cell carcinoma (HNSCC). HNSCC is generally characterized by a high tumor mutational burden, which has translated to a large neoantigen load that could prime the immune system to recognize and eliminate malignant cells. Studies are increasingly showing, however, that HNSCC is an “immune desert” tumor that can hijack multiple parts of the tumor immunity cycle in order to evade immune recognition and suppress immune system activation. Herein we will review how HNSCC tumors modulate their architecture, cellular composition, and cytokine milieu to maximize immunosuppression; as well as relevant therapeutic opportunities and emerging issues facing the field of HNSCC immuno-oncology.
Keywords: Head and neck cancer, squamous cell carcinoma, cancer immunology, tumor microenvironment, antigenicity, immunogenicity, immunotherapy, immune desert, immune evasion, immunosuppression
Introduction
The field of oncology was transformed with the discovery of immunotherapy, which leverages the body’s immune system to recognize malignant cells as foreign. Immunosurveillance involves the presentation of tumor proteins to antigen presenting cells (APCs), whose subsequent activation leads to immune cell killing of malignant cells and the attraction of T and B cells primed to the tumor’s specific antigens [1]. Head and neck squamous cell carcinomas (HNSCC) have a large number of mutations harbored by each tumor [2], which has in turn been associated with immunotherapy responsiveness in other malignancies [3]. Additionally, HNSCC tumors tend to have high tumor immune cell infiltrate, which has been linked to prognosis in various HNSCC subtypes [2]. While there have been some responses to immunotherapy, there is an overall low response rate in HNSCC [4, 5], with specific HNSCC subtypes that appear particularly resistant [5–7]. These tumors are thus considered poorly immunogenic or “immune deserts,” which is thought to result from either decreased detection by the immune system or suppression of the immune system’s response to the tumor. In this review, we will discuss how tumor microenvironment (TME) in HNSCC leads to immunosuppression; as well as opportunities and challenges for targeted immunotherapeutics.
Tumor architecture enhances immune evasion
Solid tumors tend to create an architecture that protects them from immune detection, as well as infiltration by and elimination from immune cells (Figure 1). For instance, tumors typically are surrounded by a dense extracellular matrix, limiting immune cell infiltration and through exclusion, their anti-tumor effect [8]. As solid tumors grow, their centers become increasingly hypoxic and potentially necrotic [9]. Hypoxia in the TME can lead to activation of angiogenesis, which can result in recruitment of immunosuppressive cells, tumor progression, and enhanced metastatic potential [10, 11]. Pro-angiogenesis molecules such as prostaglandin E2 and vascular endothelial growth factor (VEGF) are also overexpressed in HNSCC [10]. The abnormal and disorganized vasculature created are inherently leaky and contribute to increasing hypoxia, thus furthering the angiogenic-immunosuppressive cycle. Additionally, solid tumors lack normal lymph vessels, and the resultant increase in interstitial fluid pressure prevents lymphocyte extravasation [8]. Researchers have thus investigated ways to prevent or reverse hypoxic and vascular abnormalities, including using agents targeting VEGF. Recent evidence has demonstrated VEGF inhibition is a potent immunomodulatory agent that limits the immunosuppressive mechanisms of Treg mobilization and proliferation, decreases immunosuppressive cytokine release, and allows dendritic cell maturation and increased antigen presentation [12]. In fact, a phase II trial of the VEGF-inhibitor axitinib showed correlation with improved survival in unresectable recurrent or metastatic HNSCC [13]. Understanding the physical barriers imposed by the tumor is also vital for localizing drug delivery, which holds promise in limiting systemic effects of immunotherapies that currently have a narrow therapeutic window between anti-tumor effects and induction of autoimmune responses.
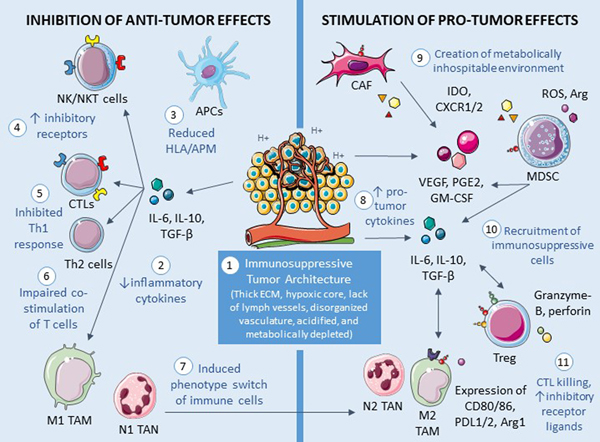
Figure 1. Immunosuppressive tumor microenvironment.
A variety of alterations in the tumor microenvironment have direct and indirect effects that suppress the innate and adaptive immune system. Anti-tumor effects begin with (1) an architecture that prevents entrance and subsequent function of effector immune cells. The tumor (2) secretes cytokines that further inhibit the immune system. Downstream effects include (3) suppressed antigen processing and presentation, (4) upregulated inhibitory receptors on effector cells, and (5) decreased Th1 response and (6) T cell co-stimulation. Furthermore, (7) multiple types of immune cells switch from a pro-inflammatory phenotype to a more pro-tumor state. The cancer also actively creates an environment that enhances tumor growth by (8) secreting pro-tumor cytokines. This ultimately leads to (9) metabolic depletion that hinders immune effector cells and (10) recruitment of immunosuppressive cells which subsequently (11) kill or inhibit immune effector cell functioning. NK=natural killer; NKT=natural killer-like; APC=antigen-presenting cell; HLA=human leukocyte antigen; APM=antigen processing machinery; CTL=cytotoxic T cell; IL=interleukin; TGF=transforming growth factor; TAM=tumor-associated macrophage; TAN=tumor-associated neutrophil; ECM=extracellular matrix; CAF=cancer-associated fibroblast; IDO=indoleamine-2,3-dioxygenase; CXCR=“C-X-C” chemokine receptor; MDSC=myeloid-derived suppressor cell; ROS=reactive oxygen species; Arg=arginine; VEGF=vascular endothelial growth factor; PGE=prostaglandin E; GM-CSF=granulocyte-macrophage colony-stimulating factor; Treg=T regulatory cell; PDL1/2=programmed death ligand 1/2. Cellular images are modified from Servier Medical Art (https://smart.servier.com), used under the terms of Creative Commons License 3.0.
Furthermore, angiogenesis and TME hypoxia are critical mediators of cancer stem cell (CSC) survival, with recent evidence linking CSC activity with direct and indirect immune avoidance. CSCs are a rare subset of cancer cells that are believed to initiate tumor growth, promote metastases, and allow for therapeutic resistance [14]. Part of their ability to survive and allow for long latency lies in their ability for immune escape. CD44+ CSCs have been shown to downregulate antigen presentation through depressed HLA-A2 and class II expression and limit T cell activity through altered immunomodulatory cytokine levels, specifically IL-8 and IL-4 [15, 16]. Overexpression of PD-L1 in CSCs has been shown to further enhance immune evasion through the endothelial to mesenchymal transition/β-catenin/STT3/PD-L1 signaling pathway [17]. This interaction of immune cells and mesenchymal cells creates a symbiotic relationship for immune evasion and maintenance of CSC. This mesenchymal support system has been well characterized in the role of cancer associated fibroblasts (CAFs) as critical mediators of CSC maintenance in immune-replete niches. Finally, a major component of CSC-associated immune escape likely arises from evolutionary selective pressures and CSC capacity for editing neoantigens, further enhancing immune evasion. While this work has been well-characterized in breast and lung cancer, CSCs as a mechanism for immune desert cancers represents a novel area of research in HNSCC.
Solid tumors can also create a metabolically inhospitable TME, as rapidly-dividing malignant cells quickly deplete the local nutrients available for infiltrating immune cells. Without adequate substrate, metabolic checkpoints and subsequent dysfunction of immune cells are triggered [18–20]. The growing tumor, meanwhile, can continue to thrive by switching from oxidative phosphorylation to a glycolytic pathway that leads to a more tumor-favorable TME via acidification [9, 21]. Metabolic reprogramming is thus thought to be a likely cancer treatment both for limiting cancer glycolysis and optimizing immune metabolism. Along these pathways, multiple studies have focused on epacadostat, an inhibitor of indoleamine 2,3-dioxygenase (IDO-1), an enzyme that depletes the essential nutrient tryptophan from the TME. In phase I/II trials of epacadostat administered in combination with pembrolizumab or nivolumab for advanced solid tumors, durable antitumor response was seen in the HNSCC subsets [22, 23]. Recent results from a phase III trial of epacadostat plus pembrolizumab in melanoma patients did not meet its primary end point of improving progression-free survival compared to pembrolizumab monotherapy, however [24]. Future studies may attempt subject selection based on IDO-1 tumor expression, use of other IDO-1 inhibitors, or combinatorial therapy with immunotherapies targeting other pathways.
Malignant cells can directly impair immune recognition of tumors
T cells
Cytotoxic CD8+ T lymphocytes (CTLs) are an important part of the body’s cancer-fighting immunological machinery. Reflecting a trend seen in other malignancies, higher numbers of CTLs have been correlated with improved HNSCC outcome [25–28]. Multiple oncogenic pathways have been implicated in impairing the T cell priming, activation, and infiltration required for a robust immune response however. Thus, the T cells that are present in immune desert tumors are often dysfunctional or “exhausted,” or T cells may be excluded from the TME altogether.
Immune checkpoints, or inhibitory receptors present on immune effector cells, normally prevent overreaction to self antigens. Tumors can hijack this system by upregulating immune checkpoint receptors and their ligands, thus hindering CTL activity. For example, binding of the immune checkpoint receptor PD-1 expressed on T cells to PD-L1 on malignant cells leads to T cell dysfunction via reduced T cell receptor (TCR) signaling, cytokine production, cell mobility, and differentiation into T regulatory (Treg) cells [29]. Importantly, PD-L1 is upregulated in some HNSCC tumors, and thus anti-PD-1 immune checkpoint inhibitors such as nivolumab and pembrolizumab are immunotherapies currently employed for HNSCC [30, 31]. Additional studies are underway to test other inhibitory receptors considered potential markers of T cell dysfunction, such as CTLA-4, LAG-3, and TIM-3 [6, 32] (Figure 2). Preliminary studies have identified TCR pathways involving the transcription factors NFAT and TOX for mediating T cell dysfunction, but much work remains to identify robust markers for exhaustion and to ascertain whether immunotherapy reinvigorates dysfunctional lymphocytes or primarily acts upon new effector cells [33]. T cell exhaustion is likely the major limitation to the long-term efficacy of treatments that activate the body’s immune system, and thus understanding its development is critical for advancing immunotherapy.
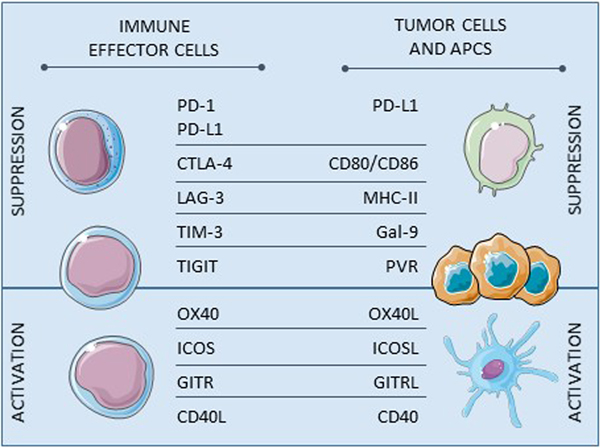
Figure 2. Major immunomodulatory T cell receptors and their ligands.
Tumor cells hijack T cell function by inducing inhibitory receptors or by suppressing stimulatory receptors, either directly or indirectly through antigen presenting cells. Thus these receptor:ligand axes represent therapeutic targets for immunosuppressive HNSCC. APC=antigen presenting cell; PD=programmed death; PD-L=programmed death-ligand; CTLA=cytotoxic T-lymphocyte-associated protein; LAG=lymphocyte activating gene; TIM=T cell immunoglobulin and mucin domain-containing; TIGIT=T cell immunoglobulin and ITIM domain; ICOS=inducible T cell co-stimulator; GITR=glucocorticoid-induced TNFR-related protein; CD40=cluster of differentiation; MHC-II=major histocompatibility complex 2; Gal=galectin; PVR=poliovirus receptor. Cellular images are modified from Servier Medical Art (https://smart.servier.com), used under the terms of Creative Commons License 3.0.
Immune escape can also occur via endogenous mutations in oncogenic pathways. In HNSCC, the WNT-β-catenin pathway appears to affect both T cell priming and trafficking into the TME via decreased recruitment of dendritic cells necessary for tumor antigen presentation to CTLs [34, 35]. Similarly, gain-of-function mutations in the MYC pathway, which are present in HPV-negative HNSCC [36], lead to decreased CTL recruitment and antigen-presenting cell (APC) activation through decreased PD-L1 and CD47 expression [35]. T cell activation is further limited by tumors with downregulation of co-stimulatory molecules like OX40 and CD137 required for T cell activation [37]. Thus, agonists to these receptors, as well as other co-stimulatory receptors such as CD27 and GITR, are now being tested as adjunctive treatments for a variety of malignancies [38].
B cells
B cell phenotypes in HNSCC vary between HPV and non-HPV-mediated cancers and stage of disease, likely reflecting why studies thus far have shown both positive and negative associations of B lymphocyte numbers and prognosis [39]. Similar to their T cell counterparts, B cells can participate in adaptive immunity or regulatory cells dampening the immune response. In comparison to tumor infiltrating T cells, HNSCC studies have not been as consistent in reporting B cell numbers or phenotypes, and thus future studies will be required to determine the impact of certain B cell profiles [40].
Of recent interest to the cancer immunology community more broadly, aggregates of B cells surrounded by T cells and specialized vasculature known as tertiary lymphoid structures have been identified in both autoimmune disease and across multiple cancers [41]. These formations lack capsules and thus experience direct antigenic stimulation and have been associated with a robust immune response and more favorable prognosis in multiple malignancies [42, 43]. In HNSCC, B cells and tertiary lymphoid structures have been identified [29, 40, 44], and HPV-specific antibody secreting cells in the TME were found to correlate with plasma IgG titers [44]. Additional research is needed to understand the mechanistic contribution of B cells to tumor immunity.
NK cells and NKT cells
Natural killer (NK) cells, potent mediators of innate immunity, can also be suppressed by HNSCC. While a recent systematic review in HNSCC revealed that generally numbers of NK cells are positively correlated with improved patient outcomes, tumors can escape NK cell-mediated killing with downregulation of downstream effectors of NK cell ligands [45]. Additionally, tumor-induced downregulation of Toll-like receptors (TLRs) on NK cells has been linked to a decreased innate immune response to HNSCC [46], and HLA mutations on tumor cells may also decrease NK-mediated elimination of HNSCC cells in the setting of resistance to cetuximab [47]. Meanwhile, a subpopulation of lymphoid cells called natural killer T cells (NKT cells) have also been implicated in adaptive immunity [48]. Preliminary evidence suggests that low numbers of peripheral NKT cells are associated with poor prognosis in HNSCC patients [49].
There has been increasing interest in therapies targeting NK cells. Shin et al identified that NK cells anti-tumor activity could be potentiated with multiple molecules through the aryl hydrocarbon receptor [50]. For instance, there are currently studies focused on combining PD-1/PD-L1:CTLA-4 axis inhibition with lirilumab, a monoclonal antibody targeting killer-cell immunoglobulin-like receptors (KIRs), which is thought to be a major inhibitory signal for NK-mediated cytotoxic cell immunity [6]. Additionally, NK cell checkpoint inhibitors have entered clinical trials [45]. Studies are still too premature, however, to determine the efficacy of these strategies.
Other cells within the tumor microenvironment can exert immunosuppressive effects
Stromal cells
CAFs, the predominant cell type in tumor stroma, secrete cytokines that often act on multiple immune cells simultaneously or otherwise alter the TME to influence immune cell infiltration. CAFs produce soluble factors such as IL-6 that exert influence on T-cells, NK cells, dendritic cells (DCs), macrophages, and neutrophils; in HNSCC, this mechanism correlates with worsened survival [51–54]. CAFs also secrete other growth factors, chemokines, and matrix-metalloproteinases (MMPs) that together induce tumor progression through TME remodeling [21, 55, 56]. In preclinical models, both CAFs and HNSCC tumor cells secrete paracrine factors that promote the proliferation of both cell types and overall tumor growth and invasion [56, 57]. Thus inhibitors of factors like fibroblast growth factor can be considered as a potential oncologic treatment [57].
MDSCs
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that are found in almost all individuals with cancer and have been shown to inhibit antitumor immunity while promoting cancer progression [58, 59]. Previous studies identified levels of MDSCs as well as granulocyte-macrophage colony-stimulating factor (GM-CSF) correlated with HNSCC advanced stages, recurrence, or metastasis [60]. Recently, Tsai et al used in vitro, in vivo, and clinical data to explore the positive correlation of IL-6 with recruitment of MDSCs and expression of PD-L1 in the induction of an immunosuppressive TME [51]. Furthermore, MDSCs encourage an antitumor environment through production of neoangiogenesis factors, MMPs, reactive oxygen species that prevent lymphocyte activation, sequestration of metabolites needed by effector cells, chemokine secretion, and cell-to-cell inhibitory contact with immune infiltrating cells [58]. A double-blinded placebo-controlled trial showed decreased MDSC numbers and improved CTL function with administration of tadalafil, an inhibitor of phosphodiesterase 5 that diminishes the immunosuppressive effects of MDSCs [61]. Preclinical studies also show promise in disrupting the MDSC trafficking mechanism through CXCR1/2, a chemokine that is often overexpressed in HNSCC [62–64].
Antigen-presenting cells
Professional APCs such as DCs, macrophages, and B cells are also affected by HNSCC cells. Tumor-associated macrophages (TAMs) can take an M1-like (antitumor) or M2-like (pro-tumor) phenotype. Polarization to “anti-inflammatory” M2-like macrophages can be induced in the acidic hypoxic TME [65]. In HNSCC, this is associated with production of pro-tumor factors like IL-6 and IL-10, leading to poorer patient outcomes [66–68]. Furthermore, TAMs also consume local nutrients needed by CTLs, express PD-L1, and recruit Tregs [59]. Use of an inhibitor of colony-stimulatory factor 1 receptor (CSF1R), which is necessary for TAM recruitment, is currently under study [59].
Malignant cells also can reduce their own antigenicity, or the ability for the body’s immune system to recognize tumor proteins as foreign to incite an immune response. Antigen processing and presentation is a complex pathway, and disruption of critical proteins can interfere with immune detection of the tumor. These disruptions can take the form of germline or somatic alterations, although they generally seem to be caused by transcriptional changes [69]. Mutations and differential expression of human leukocyte antigen (HLA) alleles [70, 71], β-2 microglobulin [72], antigen processing machinery (APM) [69, 73], and STAT1 signaling, as well as altered regulation of HLA expression [74, 75], have been found in the HNSCC TME.
Recent compelling evidence, including ours, suggests that type I interferon (IFN-I) signaling in the TME promotes CD8+ CTL production in melanomas and other cancer types. The induction of IFN-I is mediated by pattern recognition receptors, including DNA sensors such as cyclic GMP-AMP synthase (cGAS). DNA-bound cGAS generates a second messenger cyclic GMP-AMP to activate the adaptor protein stimulator of IFN genes (STING1), which promotes IFN-I. IFN-I targets genes including a number of Th1 chemokines, such as CXCL9 and CXCL10, which are critical for the tumor-homing of APCs, as well as cross-presentation and expansion of effectors, but STING signaling is often inhibited in cancers. SOX2 in HPV-negative HNSCC and HPV16 E7 can both dampen STING1-mediated immune activation, suppressing cancer immunogenicity [76].
Immunoediting has also been identified in some cancers, where the immune system eliminates malignant cells with “stronger” antigens early on in tumor development, thus selecting for fewer antigens that may inherently lead to weaker immune responses [77, 78]. These “weak” antigens can provide the tumor time to develop immune escape mechanisms or to benefit from immune effector cell dysfunction from chronic antigen signaling. Treatment approaches for correcting antigen presentation defects might include the use of co-stimulatory molecules for antigen presentation, modulation of epigenetic regulation for antigen processing genes, and even directly replacing mutated genes [79], as well as adoptive T cell transfer as discussed later.
T regulatory cells
Activated Tregs appear to suppress effector cells with inhibitory cytokines, metabolic competition, or direct inhibitory action on effector T cells and DCs [6, 80]. Frequency of circulating Tregs is higher in HNSCC patients than controls and generally are associated with lower numbers of CTLs [81]. Studies in HNSCC have revealed mixed results on the influence of Tregs on prognosis, perhaps based on the subtype of HNSCC studied, as well as the activation status and localization of Tregs in relation to the tumor [20, 29, 82, 83]. Targeting CCR4, a surface molecule predominantly seen on activated Tregs; TCR signaling; immune checkpoint receptors; and GITR, which modulates Treg immunosuppression; are some of the Treg-related treatment options currently being explored [84].
Cytokine milieu
As described above, HNSCC consists of malignant cells, host stromal cells sabotaged by tumor paracrine signaling, immune cells with suppressive phenotypes activated by the tumor, and immune effector cells either excluded from the tumor or rendered dysfunctional in the TME. The cytokines involved in creating this pro-tumor environment are multifold. For instance, malignant cells may directly secrete immunosuppressive and anti-inflammatory cytokines like IL-10 and TGF-β that negatively affect APCs and T cells [77, 85, 86]. Malignant cells can also secrete other cytokines that can polarize immune effector cells toward adopting an anti-inflammatory phenotype that leads to tumor progression [77], or they can produce chemokines that attract immunosuppressive cells into the tumor [87]. Meanwhile, HPV, a viral mediator of the majority of oropharyngeal HNSCC, is known to evade immune detection by interfering with normal immunostimulatory pathways of interferon production [76].
Thus, treatments focused on the TME cytokine milieu can either introduce pro-inflammatory cytokines or inhibit pro-tumor cytokines. In fact, administering pro-inflammatory cytokines such as IL-2, TNF-α, and IFN-α was the earliest form of immunotherapy in oncology [88, 89]. More recently, a phase II trial has found that a combination of immunostimulatory cytokines can increase tumor-infiltrating CTLs when administered as a neoadjuvant treatment [90], and a phase I/II study of an agonist for IL-15 with cetuximab in HNSCC is currently underway (NCT04136756). Other forms of immunotherapy in clinical trials include monoclonal antibodies targeted against pro-tumor cytokines [6]. Further studies are warranted to determine the appropriate manipulation of local cytokines to modulate the TME, particularly if other therapies are prescribed concurrently.
Emerging issues in immuno-oncology
Delineation of the myriad of mechanisms for immunosuppression induced by the HNSCC TME have led to creation of many novel immunotherapy approaches [6, 91]. At the time of writing this manuscript, there were 219 recruiting trials for HNSCC in the United States, of which 173 included inhibitors of the immunosuppressive TME (Table 1) or immunostimulatory agents (Table 2). Regardless of the type of immunotherapy utilized, additional research is required for understanding how to efficiently target, monitor, time, and combine these strategies.
Table 1.
Inhibitory immunotherapy agents included in recruiting clinical trials for head and neck cancer.
| Drug Target | Drugs | Trials* |
|---|---|---|
|
| ||
| Anti-PD-1; Anti-PD-L1 | ABBV151 (also targets TGF-β) | I-NCT03821935 |
| Abemaciclib | I/II-NCT03655444 | |
| Alisertib | I/II-NCT04555837 | |
| Atezolizumab | I-NCT03841110, I-NCT04096638, I/II-NCT03829501, I/II-NCT04471415, II-NCT03228667, II/III-NCT01810913, III-NCT03452137 | |
| Avelumab | I-NCT03498378, II-NCT02554812, II-NCT03228667 | |
| Bintrafusp Alpha / M7824 (also targets TGF-β) | I/II-NCT04220775, I/II-NCT04247282 | |
| Cemiplimab | I/II-NCT03684785, II-NCT03565783, II-NCT03916627, II-NCT04242173 | |
| GEN1046 | I/II-NCT03917381 | |
| Durvalumab | I-NCT03381183, I-NCT03618654, I-NCT03635164, I-NCT03739931, I/II-NCT03522584, I/II-NCT03618134, II-NCT02827838, II-NCT03174275, II-NCT03228667, II-NCT03529422, II-NCT03691714, I/II-NCT02643303, II/III-NCT03258554 | |
| Nivolumab | I-NCT02636036, I-NCT03565445, I-NCT03690986, I-NCT03758781, I-NCT03829436, I-NCT03841110, I-NCT03906526, I/II-NCT02955290, I/II-NCT03247712, I/II-NCT03311334, I/II-NCT03370276, I/II-NCT03435640, I/II-NCT03655444, I/II-NCT03735628, I/II-NCT04180215, I/II-NCT04349267, II-NCT03228667, II-NCT03341936, II-NCT03355560, II-NCT03521570, II-NCT03646461, II-NCT03715946, II-NCT03799445, II-NCT03829722, II-NCT03854032, II-NCT03878979, II-NCT03944915, II-NCT04080804, II-NCT04326257 | |
| PDR001 | I-NCT01351103 | |
| Pembrolizumab | I-NCT02376699, I-NCT02575404, I-NCT02783300, I-NCT03236935, I-NCT03245489, I-NCT03454451, I-NCT03565445, I-NCT03590054, I-NCT03647163, I-NCT03666273, I-NCT03799003, I-NCT03841110, I-NCT03849469, I-NCT04007744, I-NCT04187872, I-NCT04234113, I-NCT04344795, I-NCT04348916, I-NCT04429542, I-NCT04452214, I/II-NCT02799095, I/II-NCT02955290, I/II-NCT03138889, I/II-NCT03311334, I/II-NCT03474497, I/II-NCT03650764, I/II-NCT03674567, I/II-NCT03684785, I/II-NCT03684785, I/II-NCT03735290, I/II-NCT03789097, I/II-NCT04034225, I/II-NCT04060342, I/II-NCT04193293, I/II-NCT04555837, II-NCT02289209, II-NCT02641093, II-NCT02769520, II-NCT02777385, II-NCT02841748, II-NCT03049618, II-NCT03082534, II-NCT03085719, II-NCT03228667, II-NCT03383094, II-NCT03468218, II-NCT03546582, II-NCT03625323, II-NCT03645928, II-NCT03771820, II-NCT03823131, II-NCT03993353, II-NCT04144517, II-NCT04150900, II-NCT04220866, II-NCT04369937, II-NCT04414540, II-NCT04428151, III-NCT03765918, III-NCT04128696, III-NCT04199104, IV-NCT04489888 | |
| Sintilimab | III-NCT03748134 | |
| SL-279252 | I-NCT03894618 | |
| Tislelizumab | III-NCT03783442 | |
| TPST-1120 | I-NCT03829436, I-NCT04344795 | |
| XmAb20717 (also targets CTLA-4) | I-NCT03517488 | |
| XmAb23104 (also targets ICOS) | I-NCT03752398 | |
|
| ||
| Anti-CTLA-4 | Ipilimumab | I-NCT02812524, I-NCT03690986, I-NCT04290546, II-NCT03799445, II-NCT04080804, II-NCT04326257 |
| Tremelimumab | I/II-NCT02643303, I/II-NCT03522584, I/II-NCT03618134, II-NCT03693612 | |
| XmAb20717 (also targets PD-1) | I-NCT03517488 | |
| XmAb22841 (also targets LAG-3) | I-NCT03849469 | |
|
| ||
| Anti-LAG-3 | Eftilagimod alpha | II-NCT03625323 |
| INCAGN02385 | I-NCT03538028 | |
| Relatlimab | II-NCT04326257, II-NCT04080804 | |
| XmAb 22841 (also targets CTLA-4) | I-NCT03849469 | |
|
| ||
| Anti-TIM3 | INCAGN02390 | I-NCT03652077 |
|
| ||
| Anti-Galectin | GR-MD-02 | I-NCT02575404 |
|
| ||
| Anti-CD39/HLA-G | TTX-080 | I-NCT04485013 |
|
| ||
| Anti-CD94/NKG 2A | Monalizumab | I/II-NCT02643550 |
|
| ||
| Anti-ILDR2 | BAY1905254 | I-NCT03666273 |
|
| ||
| Anti-NRP1 | ASP1948 | I-NCT03565445 |
|
| ||
| Anti-KIR (NK cells) | Lirilumab | II-NCT03341936 |
|
| ||
| Anti-S15 (M2 macrophages) | NC318 | I/II-NCT03665285 |
|
| ||
| Anti-Semaphorin 4D | VX15/2503 | I-NCT03690986 |
|
| ||
| Anti-inhibitor of apoptosis proteins | Debio 1143 | III-NCT04459715 |
|
| ||
| Nitric oxide synthase inhibitor | L-NMMA | I-NCT03236935 |
|
| ||
| Anti-VEGF; Anti-PGE2 | Cabozantinib | I-NCT03667482, II-NCT03468218 |
| Lenvatinib | I-NCT03524326, II-NCT04428151, III-NCT04199104 | |
| Ramucirumab | I/II-NCT03650764 | |
| TPST-1495 | I-NCT04344795 | |
|
| ||
| Anti-IDO-1 | Epacadostat | II-NCT03823131 |
| BAY2416964 | I-NCT04069026 | |
| BMS-986205 | II-NCT03854032 | |
|
| ||
| Anti-TGF-β | ABBV151 (also targets PD-1) | I-NCT03821935 |
| BCA101 | I-NCT04429542 | |
| Bintrafusp Alpha (also targets PD-L1) | I/II-NCT04220775 | |
| PF-06940434 | I-NCT04152018 | |
|
| ||
| Colony-stimulating factor inhibitor | PD 0360324 | II-NCT02554812 |
|
| ||
| Anti-CCR4 | FLX475 | I/II-NCT03674567 |
Phase of study (I-IV) and National Clinical Trial (NCT) numbers as registered with www.clinicaltrials.gov. Only trials that were actively recruiting at the time of manuscript writing are included.
Table 2.
Stimulatory immunotherapy agents included in recruiting clinical trials for head and neck cancer.
| Drug Target | Drugs | Trials* |
|---|---|---|
|
| ||
| IL-1, IL-2, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, GM-CSF | ALKS4230 | I/II-NCT02799095, II-NCT04144517 |
| Bempegaldesleukin | I/II-NCT03435640 | |
| IRX-2 | I-NCT03381183, I-NCT03758781 | |
| IL-2 | I/II-NCT03474497 | |
|
| ||
| IL-15 agonist | N-803 | I-NCT04290546, I/II-NCT04247282, II-NCT03228667 |
| SO-C101 | I-NCT04234113 | |
|
| ||
| INF-β | VSV-IFNβ-NIS oncolytic virus | I-NCT03647163 |
|
| ||
| OX-40 agonist | ABBV-368 | I-NCT03818542, I-NCT04196283 |
| MEDI0562 | I-NCT03336606 | |
| PF-04518600 | II-NCT02554812 | |
|
| ||
| ICOS agonist | GSK3359609 | II-NCT03693612, III-NCT04128696 |
| KY1044 | I/II-NCT03829501 | |
| XmAb23104 (also targets PD-1) | I-NCT03752398 | |
|
| ||
| GITR agonist | ASP1951 | I-NCT03799003 |
|
| ||
| CD3 agonist | GEN1044 | I/II-NCT04424641 |
|
| ||
| CD11b agonist | GB1275 | I/II-NCT04060342 |
|
| ||
| CD40 agonist | ABBV-927 | I-NCT02988960, I-NCT03818542 |
|
| ||
| CD73 agonist | CPI-006 | I-NCT03454451 |
|
| ||
| CD94/NKG 2A agonist | BMS-986315 | I/II-NCT04349267 |
|
| ||
| CD122 agonist | NKTR-214 | I/II-NCT03138889, I/II-NCT03435640 |
|
| ||
| CD137 agonist | Utomilumab | II-NCT02554812 |
|
| ||
| TLR agonists | AST-008 | I/II-NCT03684785 |
| CMP-001 | II-NCT02554812 | |
| NKTR-262 | I/II-NCT03435640 | |
| Poly ICLC | I/II-NCT02643303, I/II-NCT03789097 | |
| Tilsotolimod | I-NCT04196283 | |
| VTX-2337 | I-NCT03906526 | |
|
| ||
| STAT3 agonist | NT219 | I/II-NCT04474470 |
| TTI-101 | I-NCT03195699 | |
|
| ||
| STING agonist | E7766 | I-NCT04144140 |
| MK1454 | II-NCT04220866 | |
| SB11285 | I-NCT04096638 | |
|
| ||
| Flt3L | Flt3L (dendritic cell growth factor) | I/II-NCT03789097 |
|
| ||
| Antigenic challenge | Ad-p53 (p53 antigen) | II-NCT03544723 |
| CUE-101 (HPV E7 protein) | I-NCT03978689 | |
| DSP-7888 (WT1 peptides) | I/II-NCT03311334 | |
| HB201 (HPV E6 and E7 proteins) | I/II-NCT04180215 | |
| PepCan (HPV E6 protein) | I/II-NCT03821272 | |
| SNS-301 (human aspartyl/asparaginyl beta-hydroxylase HAAH; DNA cpG motifs) | I/II-NCT04034225 | |
| YE-NEO-001 (patient-specific antigen) | I-NCT03552718 | |
|
| ||
| Antigen presenting cells | Ilixadencel (dendritic cells) | I/II-NCT03735290 |
|
| ||
| Immune effector cells | IMA201 (Adoptive T cell transfer) | I-NCT03247309 |
| LN-145 (Adoptive T cell transfer) | II-NCT03083873, II-NCT03645928 | |
| E6-specific T cell receptor T cells | I-NCT03578406 | |
| KITE-439 (E7 T cell receptor T cells) | I-NCT03912831 | |
| CIML NK cell infusion | I-NCT04290546 | |
| FT500 (NK cell) | I-NCT03841110 | |
|
| ||
| Oncolytic virus | Enadenotucirev | I-NCT02636036 |
| ONCR-177 | I-NCT04348916 | |
Phase of study (I-IV) and National Clinical Trial (NCT) numbers as registered with www.clinicaltrials.gov. Only trials that were actively recruiting at the time of manuscript writing are included.
Using signatures to target therapy
Precision immuno-oncology demands a reliable method for ascertaining the particular deficits of a patient’s anti-tumor immune response in order to deliver an efficacious therapy. Given the early predominance of PD-L1 inhibitors as HNSCC immunotherapy, PD-L1 expression by tumors was considered a predictive marker, although this has not been validated likely due to its poor specificity and association with immune infiltrate [7]. More recently, immunogenomic signatures show promise as a method for clustering cancers, allowing for rationalized decisions of what combination of immunotherapies to use for that particular tumor grouping [2, 47, 92–96]. Moving towards tumor-specific therapy is critical given that only a subset of patients respond to a given immunotherapy, subjecting the remaining treated patients to the financial burden, opportunity cost, and side effect profile of the chosen therapy. While immunogenomic signatures may prevent these consequences, prospective studies are needed to validate these strategies.
There has been recent recognition that even dysbiosis, or disruption to the gut microbiome, can lead to resistance to immune checkpoint inhibitor therapy [97]. Thus, microbiome signatures may also one day have implications for HNSCC treatment selection. Much work remains to understand the potential implications for the known microbiome diversity between anatomic subsites and patients [98].
Tumor-specific and tumor-associated antigen therapies
Advances in immunogenomics also hold promise in identifying antigens that may be tumor-specific (TSA) or tumor-associated (TAA). This is most immediately applicable to virally-mediated disease, with active investigations into targeting HPV proteins [6, 99]. For non-virally-mediated tumors, however, there is no consensus on a high-throughput reliable and specific methodology for antigen prediction, particularly for non-missense antigens such as structural variants [96, 100]. It is also unclear how to select which antigens are the most biologically relevant. What is evident is that antigens will vary between cancer subtypes, and may differ between patients or across time in the same patient [96]. There is also evidence that HPV-positive HNSCC may employ distinct immune evasion strategies as compared to HPV-negative disease [101]. Currently, there are trials studying vaccines involving HPV-specific peptides; p53, which is mutated in most HNSCC; and even patient specific antigens [91].
Another innovation has been the development of adoptive cell transfer, where a patient’s immune cells are removed and reintroduced after modification ex vivo to enhance their activity or specificity to a tumor [77]. Early success of these therapies in hematologic malignancies [102] have led to interest in extending this therapy to HNSCC (Table 2). The earliest adoptive cell transfer in HNSCC included expansion and activation of harvested tumor-associated TILs [103, 104]. More recently, chimeric antigen receptor (CAR) cells, which do not rely on major histocompatibility complex-mediated antigen presentation, have been applied to HSNCC for T cell [105–110] and NK cell [111] based therapies. Adoptive cell transfer will likely require combinatorial treatment to be efficacious in HNSCC, however, given the less robust responses seen in solid tumors, poor persistence of the transferred cells, and antigen modulation seen in vivo [112, 113].
Combination therapy
Promising preliminary results have been seen for combining immune checkpoint inhibitors, biologics, metabolic agents, chemotherapy, and radiotherapy [6, 7, 99]. Since immunological dysfunction can develop over time and during treatment [114], further work remains to determine optimal timing of immunotherapeutic intervention, including as neoadjuvant therapy [115], window of opportunity trials [116] for surgically resectable HNSCC, concurrently with traditional definitive treatment, or as adjuvant or salvage therapy. Regardless of which therapies are combined, caution and thoughtful studies are required to understand the effects of each added therapy on the original treatment, as well as the resultant adverse effects. Caution is warranted when targeting multiple points of the tumor immunity cycle (Figure 3) given serious potential adverse effects of even single agent immunotherapies that range from common reactions of fatigue or rash to severe, potentially life-threatening complications [117]. Beyond immediate complications of treatment, HNSCC survivors face significant quality of life effects from multimodality treatments [118] that must be balanced with the potential oncologic benefit for added therapies.
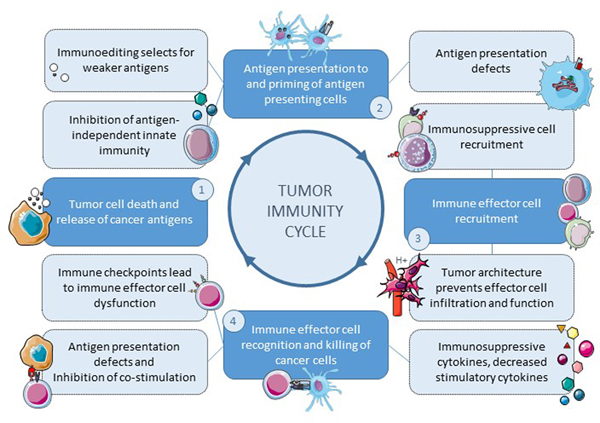
Figure 3. Immunosuppressive targets throughout the tumor immunity cycle.
Immunosurveillance involves (1) a process of released antigens from tumor cells, (2) presentation of tumor-associated antigens to antigen presenting cells, (3) chemotrafficking of immune effector cells into the tumor microenvironment, and (4) immune effector cell recognition and killing of the cancer cell. Tumors can evade the immune system throughout the tumor immunity cycle (dashed outlines).
Radiation, for instance, increases the antigenicity of tumors due to direct mutagenesis and induction of inflammatory cascades, thus multiplying immunotherapy’s potential effects [119, 120]. Chemotherapy, meanwhile, likely has synergistic effects with immunotherapy given its known disruption to tumor architecture with subsequent antigen presentation [4]. In recent years, there have been many trials of immunotherapy given before, with, or after radiation alone or chemoradiation in HNSCC, which are nicely summarized elsewhere [121]. These investigations have utilized neoadjuvant, concurrent, and adjuvant immunotherapy to take advantage of immune priming with intact lymphatic structures, synergism from chemoradiation-induced tumor architecture disruption and neoantigen production, and immune surveillance mechanisms for residual or recurrent disease [122].
Further potentiation of the immune response to tumors has focused on dual modality therapy with targeted therapies. Immune checkpoint inhibitors are being studied in combination with cetuximab, an anti-EGFR monoclonal antibody that remains the only targeted therapy approved for HNSCC to date. Cetuximab has been found to induce both innate and adaptive immune responses, as well as to alter expression of immune checkpoint receptors on TILs [6]. Additional targeted therapies acting upon the EGFR pathway are under investigation [123]. DNA Damage Repair (DDR) pathways have also been therapeutic targets. For instance, PARP and ATM inhibitors have shown a capability to increase antigenicity in several tumor types, thus priming an immune response when given in conjunction with PD-1/PD-L1 and CTLA-4 inhibitors [124–128]. Recent evidence in HNSCC suggests similar susceptibility to immune modulation through DDR-inhibition [129–131]. Whether other targeted therapies, reviewed elsewhere [132], potentiate tumor response to immunotherapy has yet to be seen.
Finally, simultaneously targeting multiple immune checkpoints, such as PD-1/PD-L1 and CTLA-4, has also shown some hopeful results in melanoma [133] and thus is now being investigated in HNSCC [134]. Combination therapy with newer strategies such as adoptive T cell transfer, cancer vaccines, and genetically-modified viruses are potential approaches as well [91, 110, 135].
Immune Modulation
In a recent phase II trial, neoadjuvant pembrolizumab was found to induce a partial response (>10%) in nearly half of treated patients (16/36) with a favorable response seen in those with specific pre-treatment immune profiles (i.e. PD-L1 overexpression, elevated TIL counts and IFNγ activity) [136]. This suggests that immune profiles may serve as a predictive biomarker for determination for immunotherapy. In immunosuppressed tumors, immune modulation to manipulate the TME to be more accommodating for immune infiltration is a novel approach. Only one trial in HNSCC has been done to assess the ability to immunomodulate immune desert malignancies. IRX-2, a primary tumor cell-derived biologic made up of Th 1 cytokines (IL-2, IL-1β, IFN-γ, and TNF-α) was shown in vitro to modulate and overcome tumor-mediated immunosuppression. In a phase II trial, IRX-2 was subsequently found to increase tumor infiltrating lymphocytes and reduce tumor size [137]. This is being further studied in a multicenter, randomized, phase IIB trial, INSPIRE (IRX-2 Neoadjuvant Therapy in HNSCC to Provide Immune Response Enhancement) trial (NCT02609386), but offers a novel way to improve immunotherapy efficacy.
Conclusion
The advent of immunotherapy has indelibly changed the landscape of oncology. Notable responses to immunotherapy have been seen in some; however, the vast majority of patients fail treatment. While HNSCC was initially considered ripe for immunotherapy due to high tumor mutational burden, a subset are “immune deserts” characterized by a failure of tumor detection or mounted response by the immune system. As the field understands more about the immunosuppressive TME of HNSCC and how the patient and malignancy’s underlying immunogenomics set the stage for immune evasion, tumor-specific therapeutics that promote tumor antigencity and immunogenicity can be chosen. Continuous assessment of a malignancy’s evolution and combination therapies will likely be required in the ongoing battle against a tumor’s immune evasion strategies.
Funding:
This work was supported by the National Institutes of Health (1K08CA226350-01A1, R01 DE026728), and the American Head and Neck Society Alando J. Ballantyne Resident Research Pilot Grant.
Footnotes
CONFLICTS OF INTEREST
None declared
REFERENCES
- [1].Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. [DOI] [PubMed] [Google Scholar]
- [2].Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- [5].Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33:3293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cavalieri S, Rivoltini L, Bergamini C, Locati LD, Licitra L, Bossi P. Immuno-oncology in head and neck squamous cell cancers: News from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev. 2018;65:78–86. [DOI] [PubMed] [Google Scholar]
- [8].Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. [DOI] [PubMed] [Google Scholar]
- [9].Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- [10].Seiwert TY, Cohen EE. Targeting angiogenesis in head and neck cancer. Semin Oncol. 2008;35:274–85. [DOI] [PubMed] [Google Scholar]
- [11].Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–74. [DOI] [PubMed] [Google Scholar]
- [12].Saada-Bouzid E, Le Tourneau C. Beyond EGFR Targeting in SCCHN: Angiogenesis, PI3K, and Other Molecular Targets. Front Oncol. 2019;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Swiecicki PL, Bellile EL, Brummel CV, Brenner JC, Worden FP. Efficacy of axitinib in metastatic head and neck cancer with novel radiographic response criteria. Cancer. 2021;127:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chinn SB, Darr OA, Owen JH, Bellile E, McHugh JB, Spector ME, et al. Cancer stem cells: mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck. 2015;37:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Volonte A, Di Tomaso T, Spinelli M, Todaro M, Sanvito F, Albarello L, et al. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol. 2014;192:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, et al. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin Cancer Res. 2016;22:3571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Puschel F, Favaro F, Redondo-Pedraza J, Lucendo E, Iurlaro R, Marchetti S, et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc Natl Acad Sci U S A. 2020;117:9932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laimer K, Troester B, Kloss F, Schafer G, Obrist P, Perathoner A, et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol. 2011;47:352–7. [DOI] [PubMed] [Google Scholar]
- [20].Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, et al. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer. 2013;132:E85–93. [DOI] [PubMed] [Google Scholar]
- [21].Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. 2019;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kao HF, Lou PJ. Immune checkpoint inhibitors for head and neck squamous cell carcinoma: Current landscape and future directions. Head Neck. 2019;41 Suppl 1:4–18. [DOI] [PubMed] [Google Scholar]
- [23].Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol. 2018;36:3223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–97. [DOI] [PubMed] [Google Scholar]
- [25].Hoesli R, Birkeland AC, Rosko AJ, Issa M, Chow KL, Michmerhuizen NL, et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol. 2018;77:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spector ME, Bellile E, Amlani L, Zarins K, Smith J, Brenner JC, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Quan H, Shan Z, Liu Z, Liu S, Yang L, Fang X, et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol Immunother. 2020;69:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang WF, Wong MCM, Thomson PJ, Li KY, Su YX. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018;86:81–90. [DOI] [PubMed] [Google Scholar]
- [32].Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942–50. [DOI] [PubMed] [Google Scholar]
- [33].Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Baruah P, Lee M, Odutoye T, Williamson P, Hyde N, Kaski JC, et al. Decreased levels of alternative co-stimulatory receptors OX40 and 4–1BB characterise T cells from head and neck cancer patients. Immunobiology. 2012;217:669–75. [DOI] [PubMed] [Google Scholar]
- [38].Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wondergem NE, Nauta IH, Muijlwijk T, Leemans CR, van de Ven R. The Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: on Subsets and Subsites. Curr Oncol Rep. 2020;22:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lechner A, Schlosser HA, Thelen M, Wennhold K, Rothschild SI, Gilles R, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology. 2019;8:1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25. [DOI] [PubMed] [Google Scholar]
- [42].Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5. [DOI] [PubMed] [Google Scholar]
- [43].Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wieland A, Patel MR, Cardenas MA, Eberhardt CS, Hudson WH, Obeng RC, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bisheshar SK, De Ruiter EJ, Devriese LA, Willems SM. The prognostic role of NK cells and their ligands in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology. 2020;9:1747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xie L, Pries R, Kesselring R, Wulff S, Wollenberg B. Head and neck cancer triggers the internalization of TLR3 in natural killer cells. Int J Mol Med. 2007;20:493–9. [PubMed] [Google Scholar]
- [47].Faden DL, Concha-Benavente F, Chakka AB, McMichael EL, Chandran U, Ferris RL. Immunogenomic correlates of response to cetuximab monotherapy in head and neck squamous cell carcinoma. Head Neck. 2019;41:2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. [DOI] [PubMed] [Google Scholar]
- [49].Schneiders FL, de Bruin RC, van den Eertwegh AJ, Scheper RJ, Leemans CR, Brakenhoff RH, et al. Circulating invariant natural killer T-cell numbers predict outcome in head and neck squamous cell carcinoma: updated analysis with 10-year follow-up. J Clin Oncol. 2012;30:567–70. [DOI] [PubMed] [Google Scholar]
- [50].Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, et al. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2013;110:12391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tsai MS, Chen WC, Lu CH, Chen MF. The prognosis of head and neck squamous cell carcinoma related to immunosuppressive tumor microenvironment regulated by IL-6 signaling. Oral Oncol. 2019;91:47–55. [DOI] [PubMed] [Google Scholar]
- [52].Bello IO, Vered M, Dayan D, Dobriyan A, Yahalom R, Alanen K, et al. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–8. [DOI] [PubMed] [Google Scholar]
- [53].Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–81. [DOI] [PubMed] [Google Scholar]
- [54].Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bagordakis E, Sawazaki-Calone I, Macedo CC, Carnielli CM, de Oliveira CE, Rodrigues PC, et al. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumour Biol. 2016;37:9045–57. [DOI] [PubMed] [Google Scholar]
- [56].Wheeler SE, Shi H, Lin F, Dasari S, Bednash J, Thorne S, et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head Neck. 2014;36:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sweeny L, Liu Z, Lancaster W, Hart J, Hartman YE, Rosenthal EL. Inhibition of fibroblasts reduced head and neck cancer growth by targeting fibroblast growth factor receptor. Laryngoscope. 2012;122:1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol. 2018;200:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. [DOI] [PubMed] [Google Scholar]
- [61].Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020;26:1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Clavijo PE, Friedman J, Robbins Y, Moore EC, Smith E, Zauderer M, et al. Semaphorin4D Inhibition Improves Response to Immune-Checkpoint Blockade via Attenuation of MDSC Recruitment and Function. Cancer Immunol Res. 2019;7:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Costa NL, Valadares MC, Souza PP, Mendonca EF, Oliveira JC, Silva TA, et al. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49:216–23. [DOI] [PubMed] [Google Scholar]
- [67].Kubota K, Moriyama M, Furukawa S, Rafiul H, Maruse Y, Jinno T, et al. CD163(+)CD204(+) tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci Rep. 2017;7:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Troiano G, Caponio VCA, Adipietro I, Tepedino M, Santoro R, Laino L, et al. Prognostic significance of CD68(+) and CD163(+) tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019;93:66–75. [DOI] [PubMed] [Google Scholar]
- [69].Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. [DOI] [PubMed] [Google Scholar]
- [70].Concha-Benavente F, Srivastava R, Ferrone S, Ferris RL. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016;58:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res. 2000;6:2794–802. [PubMed] [Google Scholar]
- [72].Chen CH, Su CY, Chien CY, Huang CC, Chuang HC, Fang FM, et al. Overexpression of beta2-microglobulin is associated with poor survival in patients with oral cavity squamous cell carcinoma and contributes to oral cancer cell migration and invasion. Br J Cancer. 2008;99:1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. [DOI] [PubMed] [Google Scholar]
- [74].Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-Induced HLA Class I Upregulation Enhances Immunogenicity and Clinical Response to Anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer Immunol Res. 2015;3:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020;130:1635–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Allen CT, Clavijo PE, Van Waes C, Chen Z. Anti-Tumor Immunity in Head and Neck Cancer: Understanding the Evidence, How Tumors Escape and Immunotherapeutic Approaches. Cancers (Basel). 2015;7:2397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- [79].Lampen MH, van Hall T. Strategies to counteract MHC-I defects in tumors. Curr Opin Immunol. 2011;23:293–8. [DOI] [PubMed] [Google Scholar]
- [80].Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chikamatsu K, Sakakura K, Whiteside TL, Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck. 2007;29:120–7. [DOI] [PubMed] [Google Scholar]
- [82].Weed DT, Walker G, De La Fuente AC, Nazarian R, Vella JL, Gomez-Fernandez CR, et al. FOXP3 subcellular localization predicts recurrence in oral squamous cell carcinoma. PLoS One. 2013;8:e71908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ihara F, Sakurai D, Horinaka A, Makita Y, Fujikawa A, Sakurai T, et al. CD45RA(−)Foxp3(high) regulatory T cells have a negative impact on the clinical outcome of head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Young MR, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–8. [DOI] [PubMed] [Google Scholar]
- [86].Bedi A, Chang X, Noonan K, Pham V, Bedi R, Fertig EJ, et al. Inhibition of TGF-beta enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol Cancer Ther. 2012;11:2429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Young MR, Petruzzelli GJ, Kolesiak K, Achille N, Lathers DM, Gabrilovich DI. Human squamous cell carcinomas of the head and neck chemoattract immune suppressive CD34(+) progenitor cells. Hum Immunol. 2001;62:332–41. [DOI] [PubMed] [Google Scholar]
- [88].Kirkwood JM, Ernstoff MS, Davis CA, Reiss M, Ferraresi R, Rudnick SA. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Ann Intern Med. 1985;103:32–6. [DOI] [PubMed] [Google Scholar]
- [89].Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. [DOI] [PubMed] [Google Scholar]
- [90].Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Heft Neal ME, Haring CT, Mann JE, Brenner JC, Spector ME, Swiecicki PL. Novel Immunotherapeutic Approaches in Head and Neck Cancer. J Cancer Metastasis Treat. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen YP, Wang YQ, Lv JW, Li YQ, Chua MLK, Le QT, et al. Identification and validation of novel microenvironment-based immune molecular subgroups of head and neck squamous cell carcinoma: implications for immunotherapy. Ann Oncol. 2019;30:68–75. [DOI] [PubMed] [Google Scholar]
- [93].Brooks JM, Menezes AN, Ibrahim M, Archer L, Lal N, Bagnall CJ, et al. Development and Validation of a Combined Hypoxia and Immune Prognostic Classifier for Head and Neck Cancer. Clin Cancer Res. 2019;25:5315–28. [DOI] [PubMed] [Google Scholar]
- [94].Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21:870–81. [DOI] [PubMed] [Google Scholar]
- [95].Li B, Cui Y, Nambiar DK, Sunwoo JB, Li R. The Immune Subtypes and Landscape of Squamous Cell Carcinoma. Clin Cancer Res. 2019;25:3528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–30 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- [98].Human Microbiome Project C. A framework for human microbiome research. Nature. 2012;486:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Heath BR, Michmerhuizen NL, Donnelly CR, Sansanaphongpricha K, Sun D, Brenner JC, et al. Head and Neck Cancer Immunotherapy beyond the Checkpoint Blockade. J Dent Res. 2019;98:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Richters MM, Xia H, Campbell KM, Gillanders WE, Griffith OL, Griffith M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Seliger B, Massa C, Yang B, Bethmann D, Kappler M, Eckert AW, et al. Immune Escape Mechanisms and Their Clinical Relevance in Head and Neck Squamous Cell Carcinoma. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].To WC, Wood BG, Krauss JC, Strome M, Esclamado RM, Lavertu P, et al. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: a phase 1 study. Arch Otolaryngol Head Neck Surg. 2000;126:1225–31. [DOI] [PubMed] [Google Scholar]
- [104].Ohtani T, Yamada Y, Furuhashi A, Ohmura Y, Nakamura S, Kato H, et al. Activated cytotoxic T-lymphocyte immunotherapy is effective for advanced oral and maxillofacial cancers. Int J Oncol. 2014;45:2051–7. [DOI] [PubMed] [Google Scholar]
- [105].Davies DM, Foster J, Van Der Stegen SJ, Parente-Pereira AC, Chiapero-Stanke L, Delinassios GJ, et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Haist C, Schulte E, Bartels N, Bister A, Poschinski Z, Ibach TC, et al. CD44v6-targeted CAR T-cells specifically eliminate CD44 isoform 6 expressing head/neck squamous cell carcinoma cells. Oral Oncol. 2021;116:105259. [DOI] [PubMed] [Google Scholar]
- [107].Papa S, Adami A, Metoudi M, Achkova D, Schalkwyk Mv, Pereira AP, et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC). Journal of Clinical Oncology. 2018;36:3046–. [Google Scholar]
- [108].Mei Z, Zhang K, Lam AK, Huang J, Qiu F, Qiao B, et al. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2020;9:640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Park YP, Jin L, Bennett KB, Wang D, Fredenburg KM, Tseng JE, et al. CD70 as a target for chimeric antigen receptor T cells in head and neck squamous cell carcinoma. Oral Oncol. 2018;78:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rosewell Shaw A, Porter CE, Watanabe N, Tanoue K, Sikora A, Gottschalk S, et al. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol Ther. 2017;25:2440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lee MY, Robbins Y, Sievers C, Friedman J, Abdul Sater H, Clavijo PE, et al. Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25:1341–55. [DOI] [PubMed] [Google Scholar]
- [113].Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017;171:934–49 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stafford M, Kaczmar J. The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers Head Neck. 2020;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Farlow JL, Birkeland AC, Swiecicki PL, Brenner JC, Spector ME. Window of opportunity trials in head and neck cancer. J Cancer Metastasis Treat. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hall ET, Singhal S, Dickerson J, Gabster B, Wong HN, Aslakson RA, et al. Patient-Reported Outcomes for Cancer Patients Receiving Checkpoint Inhibitors: Opportunities for Palliative Care-A Systematic Review. J Pain Symptom Manage. 2019;58:137–56 e1. [DOI] [PubMed] [Google Scholar]
- [118].Coca-Pelaz A, Halmos GB, Strojan P, de Bree R, Bossi P, Bradford CR, et al. The role of age in treatment-related adverse events in patients with head and neck cancer: A systematic review. Head Neck. 2019;41:2410–29. [DOI] [PubMed] [Google Scholar]
- [119].Demaria S, Coleman CN, Formenti SC. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer. 2016;2:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Plavc G, Strojan P. Combining radiotherapy and immunotherapy in definitive treatment of head and neck squamous cell carcinoma: review of current clinical trials. Radiol Oncol. 2020;54:377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Williamson CW, Sherer MV, Zamarin D, Sharabi AB, Dyer BA, Mell LK, et al. Immunotherapy and radiation therapy sequencing: State of the data on timing, efficacy, and safety. Cancer. 2021;127:1553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fasano M, Della Corte CM, Viscardi G, Di Liello R, Paragliola F, Sparano F, et al. Head and neck cancer: the role of anti-EGFR agents in the era of immunotherapy. Ther Adv Med Oncol. 2021;13:1758835920949418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front Oncol. 2020;10:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhang Q, Green MD, Lang X, Lazarus J, Parsels JD, Wei S, et al. Inhibition of ATM Increases Interferon Signaling and Sensitizes Pancreatic Cancer to Immune Checkpoint Blockade Therapy. Cancer Res. 2019;79:3940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep. 2018;25:2972–80 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin Cancer Res. 2017;23:3711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019;9:646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Glorieux M, Dok R, Nuyts S. Novel DNA targeted therapies for head and neck cancers: clinical potential and biomarkers. Oncotarget. 2017;8:81662–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jelinek MJ, Foster NR, Zoroufy AJ, Schwartz GK, Munster PN, Seiwert TY, et al. A phase I trial adding poly(ADP-ribose) polymerase inhibitor veliparib to induction carboplatin-paclitaxel in patients with head and neck squamous cell carcinoma: Alliance A091101. Oral Oncol. 2021;114:105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yasukawa M, Fujihara H, Fujimori H, Kawaguchi K, Yamada H, Nakayama R, et al. Synergetic Effects of PARP Inhibitor AZD2281 and Cisplatin in Oral Squamous Cell Carcinoma in Vitro and in Vivo. Int J Mol Sci. 2016;17:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ortiz-Cuaran S, Bouaoud J, Karabajakian A, Fayette J, Saintigny P. Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers. Front Oncol. 2021;11:614332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- [134].Zech HB, Moeckelmann N, Boettcher A, Muenscher A, Binder M, Vettorazzi E, et al. Phase III study of nivolumab alone or combined with ipilimumab as immunotherapy versus standard of care in resectable head and neck squamous cell carcinoma. Future Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- [135].Tan YS, Sansanaphongpricha K, Prince MEP, Sun D, Wolf GT, Lei YL. Engineering Vaccines to Reprogram Immunity against Head and Neck Cancer. J Dent Res. 2018;97:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin Cancer Res. 2020;26:5140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wolf GT, Moyer JS, Kaplan MJ, Newman JG, Egan JE, Berinstein NL, et al. IRX-2 natural cytokine biologic for immunotherapy in patients with head and neck cancers. Onco Targets Ther. 2018;11:3731–46. [DOI] [PMC free article] [PubMed] [Google Scholar]