Abstract
OBJECTIVE
To evaluate associations between gestational diabetes mellitus (GDM) and various incident cardiovascular disease (CVD) end points, considering the effects of the mediating role of type 2 diabetes and shared environmental/familial factors.
RESEARCH DESIGN AND METHODS
This population-based cohort study included 10,02,486 parous women in Denmark during 1978–2016. We used Cox regression to 1) examine the associations of GDM with overall and type-specific CVDs using full-cohort and sibling-matched analysis, 2) quantify the impact of type 2 diabetes after GDM using mediation analysis, and 3) assess whether these associations were modified by prepregnancy obesity or maternal history of CVD.
RESULTS
Women with a history of GDM had a 40% increased overall CVD risk (hazard ratio [HR] 1.40, 95% CI 1.35–1.45). Sibling-matched analyses yielded similar results (HR 1.44, 95% CI 1.28–1.62). The proportion of association between GDM and overall CVD explained by subsequent type 2 diabetes was 23.3% (15.4–32.8%). We observed increased risks of specific CVDs, including 65% increased stroke risk and more than twofold risks for myocardial infarction, heart failure, and peripheral artery disease. The elevated overall risks were more pronounced among women with GDM and prepregnancy obesity or maternal history of CVD.
CONCLUSIONS
A history of GDM was associated with increased risks of overall and specific CVDs. Increased risks were partly explained by subsequent type 2 diabetes, and the need to identify other pathways remains important. Continuous monitoring of women with a history of GDM, especially those with prepregnancy obesity or maternal history of CVD, may provide better opportunities to reduce their cardiovascular risk.
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that first occurs during pregnancy (1). In the short-term, GDM is associated with pregnancy complications, such as preeclampsia, preterm birth, stillbirth, macrosomia, and cesarean birth (2,3). While GDM usually resolves after giving birth, the influence of GDM extends beyond pregnancy (1). Studies have shown that women with a history of GDM have a higher risk of developing type 2 diabetes, metabolic syndrome, chronic kidney disease, and cardiovascular disease (CVD) later in life (2,4,5).
Although a link between GDM and subsequent CVD has been reported, most of the research has stemmed from cross-sectional, case-control, or retrospective cohort studies (4,6–12), except for one prospective cohort in which only self-reported GDM was used as the exposure (13). In addition, evidence on the association of GDM with specific CVDs is also lacking. Furthermore, few studies have taken into consideration the interaction of various environmental factors and genetic susceptibility, which potentially influence the association between GDM and CVD (5,13,14). Finally, GDM is strongly associated with the development of type 2 diabetes, and both GDM and subsequent type 2 diabetes also predispose affected women to CVD (15). However, studies assessing the impact of type 2 diabetes on the association between GDM and CVD have often produced inconsistent findings (5,7,13,16). No studies have quantified the mediating role of type 2 diabetes in this association.
In this nationwide Danish cohort study with a follow-up of up to 39 years, we investigated the associations of a history of GDM with overall and specific types of CVD by using both population analysis and sibling-matched analysis, aiming to take into consideration shared stable unmeasured environmental factors within families and genetic susceptibility (17). We further quantified the mediating role of type 2 diabetes in the associations using mediation analysis and assessed whether the relationships differed by the presence of prepregnancy obesity or maternal history of CVD (18,19).
Research Design and Methods
Study Population
All Danish residents are assigned a unique central personal register number (CPR), and high-quality data at the individual level from national registries can be linked using the CPR (Supplementary Text 1) (20,21). Based on data from several national registers, we conducted a population-based cohort study that included all adult women who had their first pregnancy during 1978–2016 (n = 1,098,962). After excluding 1) 10,005 who were <18 years of age at the date of first delivery (i.e., adolescent mothers), 2) 12,952 with preexisting type 1 diabetes or type 2 diabetes, 29,009 with CVD, and 39,045 with cancer before the first pregnancy, and 3) 5,465 with congenital heart disease before a diagnosis of CVD, our final cohort comprised 1,002,486 parous women. Follow-up started at the date of first giving birth and ended at the date of the first CVD event, death, emigration, or 31 December 2016, whichever came first. Women who emigrated or died of non-CVD causes during follow-up were censored at the time of emigration or death.
GDM
History of GDM was identified at the date of the first delivery and updated at every pregnancy. GDM exposure was a time-varying variable; thus, a woman with a pregnancy without GDM and a later pregnancy with GDM would be considered as both unexposed and then exposed over the course of follow-up. Information on the diagnosis of GDM was obtained from the Danish National Patient Registry (DNPR) using the International Classification of Disease (ICD) codes (Supplementary Text 1 and 2) (20). The DNPR contains hospital discharge diagnoses from 1977 and outpatient and emergency diagnoses since 1995.
CVD Incidence
The outcome of interest was CVD incidence, defined as the first occurrence of CVD in the DNPR or the Danish Register of Causes of Death (20). The outcome was identified using the ICD codes for CVD or surgery codes for coronary artery bypass graft surgery (CABG) and percutaneous coronary intervention (PCI). With the large study sample and a long follow-up, we were able to investigate the following specific types of CVD: ischemic heart disease, myocardial infarction, cerebrovascular disease, stroke, heart failure, atrial fibrillation, hypertensive disease, deep vein thrombosis, pulmonary embolism, CABG or PCI, and other types of CVD (specific codes are provided in Supplementary Table 1).
Mediator
A potential mediator was type 2 diabetes diagnosed before the CVD diagnosis. Information on type 2 diabetes diagnosis was obtained from the Danish National Diabetes Register, the DNPR, and the Danish National Prescription Registry (Supplementary Text 1 and 2) (20).
Covariates
Potential confounders were selected based on our directed acyclic graph (Supplementary Fig. 1). This included parity (one, two, or three or more) and the following covariates at the time of first delivery: age (<20, 20–24, 25–29, 30–34, or ≥35 years), cohabitation (single, cohabitating), education (0–9, 10–14, or ≥15 years), country of origin (Danish, non-Danish origin), residence (Copenhagen, cities with ≥100,000 inhabitants, or other), smoking during pregnancy (yes, no), prepregnancy obesity (yes, no), maternal and paternal CVD history (yes, no), and time period of first delivery (≤1980, 5-year intervals during 1981–2010, or 2011–2016). Missing covariate values were treated as a separate category. The covariates were defined on the date of the first delivery. If a woman reported a non-GDM pregnancy and a subsequent GDM pregnancy, information on the covariates was updated accordingly. We also used complete case analysis and multiple imputations, with 10 imputations to handle missing values.
Statistical Analyses
Cox regression with follow-up time as the time scale was used to compute hazard ratios (HRs) with 95% CIs to assess the association of history of GDM with overall and specific CVDs. The evaluation of a log-minus-log plot suggested that the proportional hazard assumption was not violated. Considering non-CVD deaths as competing events, we estimated the cumulative incidence function among women with and without a history of GDM averaged over the distribution of covariates using inverse probability of treatment weighting (22). We evaluated whether the presence of prepregnancy obesity or maternal history of CVD further increased CVD risk by examining their multiplicative and additive interactions (23). The relative excess risk due to interaction (RERI) was used to examine additive interactions (23). Additive and multiplicative interactions can both reveal whether the presence of effect modifiers changes the association between the exposure and outcome, but they differ in public health and clinical implications. The additive interaction measures the absolute change in risk and has more public health significance, while the multiplicative interaction measures the relative change in risk and has more etiological significance, which might be instructive in revealing the underlying mechanisms of disease.
We performed mediation analysis to examine how type 2 diabetes might mediate the effect of history of GDM on CVD risk (24). Under a counterfactual framework, the total effect of history of GDM on CVD risk can be decomposed into a controlled direct effect (CDE) and portion eliminated (PE) by eliminating the impact by type 2 diabetes (24). The CDE captures the influence of history of GDM on CVD if the link between a history of GDM and type 2 diabetes was hypothetically prevented or removed. To estimate CDE, we controlled for type 2 diabetes, time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, maternal CVD history, paternal CVD history, and considered the interaction of history of GDM and type 2 diabetes. PE was obtained by dividing the CDE by the total effect. The PE measures the proportion of the total effect that would be eliminated by removing the mediation and interaction effects involving subsequent type 2 diabetes. The bootstrapped CIs for mediation analysis were obtained using 100 replicates. Sensitivity analysis was performed to evaluate the impact of violations of the assumption of no uncontrolled confounding for mediation analysis (Supplementary Text 3).
To evaluate the influence of uncontrolled confounding due to shared familial characteristics, we used a sibling-matched design. This entailed analyzing both half-sibling pairs from the same mothers and full-sibling pairs from the same mothers and fathers (17,25). We used stratified Cox regression with a separate stratum for each half-sibling pair identified by the mother’s unique identification number and for each full-sibling pair identified by both the mother’s and the father’s unique identification numbers in which only sibling pairs discordant for both GDM and CVD are informative and contribute to the effect estimate. Stratified Cox regression allowed each sibling pair to have its own baseline rate function, which reflected shared familial characteristics. Thus a sibling-matched design using stratified Cox regression inherently controlled for unmeasured familial factors shared by sibling pairs (25). Moreover, we restricted analyses to women without preeclampsia/eclampsia, with only one pregnancy, at least 1 year of follow-up, without a stillbirth pregnancy, or who gave birth after 1980, 1985, 1991 (the year that smoking data became available), or 1994 (the year that the ICD-10 was adopted), 2001, or 2005. As the DNPR was established in 1977, the analysis was also restricted to women whose first pregnancy was after 1980 to allow for a 3-year window to sufficiently evaluate the exclusion criteria of no previous diabetes, CVD, or cancer. Restricted cubic splines were used to fit the potential nonlinear relation between continuous covariates (age at first pregnancy, calendar year) and CVD risk. We also performed analyses stratified by the time period of first delivery and used age as the time scale. We performed analyses with additional adjustment for gestational age, or prepregnancy hypercholesterolemia, or the prepregnancy Comorbidity Index score. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Stata 15 (StataCorp, College Station, TX) software.
Results
Of 1,002,486 parous women, 21,353 (2.1%) had a history of GDM. The median age at the time of the first delivery was 27 years (interquartile range 24–30 years). The proportion of women with a history of GDM increased over time, reaching 3.1% during 2011–2016. (Supplementary Fig. 2). A total of 37,339 women (3.7%) were censored at the end of follow-up due to noncardiovascular death (n = 9,989) or emigration (n = 27,350). Compared with women without a history of GDM, women with a history of GDM were more likely to have a higher parity, be older at first delivery, have a lower education level, cohabitate, be of non-Danish origin, have a higher prevalence of prepregnancy obesity, and develop type 2 diabetes (Supplementary Table 2).
During up to 39 years of follow-up (median follow-up 16.2 years, interquartile range 7.7–25.4 years), 3,015 women with a history of GDM and 182,805 women without a history of GDM were diagnosed with CVD. Women with a history of GDM had a higher overall risk of CVD than women without a history of GDM (HR 1.40, 95% CI 1.35–1.45; standardized cumulative incidence among unexposed women at the follow-up of 35 years: 39.1%, 95% CI 38.9–39.3%; standardized cumulative incidence difference: 18.0%, 95% CI 15.2–20.9%) (Table 1 and Supplementary Fig. 3). The increased risks were also observed for specific types of CVD for women with a history of GDM, in particular, a 65% increased risk for stroke and more than twofold risks for myocardial infarction, heart failure, hypertensive disease, peripheral artery disease, and CABG or PCI (Table 1).
Table 1.
HR for the associations between history of GDM and overall CVD and specific CVD types among 1,002,486 Danish women, 1978–2016
| History of GDM | No. of CVD cases | Rate per 1,000 person-years | HR (95% CI) model 1 | HR (95% CI) model 2 | |
|---|---|---|---|---|---|
| Overall CVDs | No GDM | 182,05 | 10.82 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 3,015 | 16.84 | 1.73 (1.67–1.80) | 1.40 (1.35–1.45) | |
| Type-specific CVDs | |||||
| Ischemic heart disease | No GDM | 23,537 | 1.27 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 508 | 2.51 | 2.61 (2.39–2.85) | 2.02 (1.85–2.21) | |
| Myocardial infarction | No GDM | 5,431 | 0.29 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 121 | 0.59 | 2.74 (2.29–3.29) | 2.35 (1.95–2.82) | |
| Cerebrovascular disease | No GDM | 17,091 | 0.92 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 256 | 1.26 | 1.65 (1.46–1.86) | 1.47 (1.30–1.67) | |
| Stroke | No GDM | 11,958 | 0.64 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 190 | 0.93 | 1.77 (1.53–2.04) | 1.65 (1.43–1.91) | |
| Ischemic stroke | No GDM | 9,328 | 0.50 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 162 | 0.79 | 2.00 (1.71–2.34) | 1.73 (1.48–2.02) | |
| Heart failure | No GDM | 3,804 | 0.20 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 84 | 0.41 | 2.80 (2.25–3.48) | 2.20 (1.76–2.74) | |
| Atrial fibrillation | No GDM | 7,083 | 0.38 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 95 | 0.46 | 1.68 (1.37–2.06) | 1.40 (1.14–1.72) | |
| Hypertensive disease | No GDM | 51,344 | 2.81 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 1,358 | 6.93 | 3.31 (3.14–3.50) | 2.63 (2.49–2.78) | |
| Deep vein thrombosis | No GDM | 8,621 | 0.46 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 166 | 0.81 | 1.92 (1.65–2.24) | 1.46 (1.25–1.70) | |
| Pulmonary embolism | No GDM | 4,349 | 0.23 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 76 | 0.37 | 1.81 (1.44–2.27) | 1.33 (1.06–1.68) | |
| Peripheral artery disease | No GDM | 2,763 | 0.15 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 51 | 0.25 | 2.52 (1.91–3.33) | 2.19 (1.65–2.90) | |
| CABG or PCI | No GDM | 3,569 | 0.19 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 88 | 0.43 | 3.33 (2.69–4.11) | 2.89 (2.33–3.59) | |
| Other CVDs | No GDM | 117,375 | 6.73 | 1.0 (Reference) | 1.0 (Reference) |
| GDM | 15,78 | 8.27 | 1.27 (1.21–1.33) | 1.06 (1.00–1.11) |
Model 1: Follow-up time as time scale. Model 2: Follow-up time as time scale, controlled for time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, maternal CVD history, and paternal CVD history.
CVD was diagnosed in 830 women (0.1%) with a history of GDM and subsequent type 2 diabetes, 1,976 women (0.2%) with a history of GDM but without type 2 diabetes, 5,587 women (0.6%) without a history of GDM but with type 2 diabetes, and 177,218 women (17.7%) without a history of GDM or type 2 diabetes. We found that history of GDM was strongly associated with the development of type 2 diabetes (crude risk ratio 7.26, 95% CI 6.99–7.54), and type 2 diabetes was related to a higher risk of developing CVD (crude risk ratio 1.71, 95% CI 1.67–1.74). Mediation analyses showed that the estimated proportion of total effect between history of GDM and overall CVD eliminated by hypothetically preventing type 2 diabetes was 23.3% (95% CI 15.4–32.8; HRPE 1.07, 95% CI 1.05–1.10). The mediating role of type 2 diabetes was also observed for most specific types of CVD, particularly myocardial infarction, heart failure, hypertensive disease, atrial fibrillation, peripheral artery disease, and having CABG or PCI (Table 2).
Table 2.
The mediating role of type 2 diabetes in the association between history of GDM, overall CVD, and specific CVD types
| Outcome | HRTE* | HRCDE† | HRPE† | Proportion eliminated (%)† |
|---|---|---|---|---|
| Overall CVD | 1.40 (1.35–1.45) | 1.31 (1.25–1.36) | 1.07 (1.05–1.10) | 23.3 (15.4–32.8) |
| Type-specific CVDs | ||||
| Ischemic heart disease | 2.02 (1.85–2.21) | 1.77 (1.57–1.99) | 1.14 (1.07–1.24) | 25.0 (13.7–37.1) |
| Myocardial infarction | 2.35 (1.95–2.82) | 1.83 (1.42–2.36) | 1.28 (1.05–1.53) | 38.3 (8.9–65.0) |
| Cerebrovascular disease | 1.47 (1.30–1.67) | 1.46 (1.26–1.70) | 1.01 (0.94–1.10) | 2.1 (0.0–30.7) |
| Stroke | 1.65 (1.43–1.91) | 1.59 (1.33–1.89) | 1.04 (0.97–1.14) | 10.1 (0.0–34.1) |
| Ischemic stroke | 1.73 (1.48–2.02) | 1.58 (1.30–1.93) | 1.09 (0.98–1.24) | 19.7 (0.0–51.6) |
| Heart failure | 2.20 (1.76–2.74) | 1.43 (1.02–2.00) | 1.54 (1.26–2.02) | 64.2 (38.9–100.0) |
| Atrial fibrillation | 1.40 (1.14–1.72) | 1.24 (0.96–1.61) | 1.13 (0.96–1.34) | 39.5 (0.0–100.0) |
| Hypertensive disease | 2.63 (2.49–2.78) | 2.08 (1.93–2.24) | 1.26 (1.21–1.33) | 33.8 (27.7–39.2) |
| Deep vein thrombosis | 1.46 (1.25–1.70) | 1.47 (1.23–1.75) | 0.99 (0.92–1.08) | — |
| Pulmonary embolism | 1.33 (1.06–1.68) | 1.37 (1.06–1.78) | 0.97 (0.88–1.10) | — |
| Peripheral artery disease | 2.19 (1.65–2.90) | 1.28 (0.81–2.02) | 1.71 (1.16–2.96) | 76.3 (23.4–100.0) |
| CABG or PCI | 2.89 (2.33–3.59) | 1.76 (1.24–2.48) | 1.65 (1.32–2.09) | 60.0 (38.3–88.7) |
| Other CVDs | 1.06 (1.00–1.11) | 1.07 (1.02–1.14) | 0.98 (0.96–1.00) | — |
Data are presented with the 95% CI.
Follow-up time as time scale, controlled for time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, maternal CVD history, and paternal CVD history.
Follow-up time as time scale, controlled for type 2 diabetes, time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, maternal CVD history, and paternal CVD history.
HRPE = (HRTE/HRCDE). Proportion eliminated = (HRTE − HRCDE)/(HRTE − 1), only present if the direction of CDE and PE was the same. The bootstrapped CIs for HRPE and proportion eliminated were obtained using 100 replicates.
A higher incidence of CVD was found in women with both a history of GDM and obesity (HR 1.76, 95% CI 1.59–1.95) compared with women with a history of GDM alone (HR 1.43, 95% CI 1.38–1.49), and only a multiplicative interaction was detected (P = 0.001 for multiplicative interaction) (Table 3). Women with a history of GDM and maternal history of CVD also had a higher risk of CVD (HR 1.75, 95% CI 1.66–1.84) compared with women with a history of GDM alone (HR 1.31, 95% CI 1.23–1.40) (Table 3). Our data suggested both multiplicative interaction (P = 0.042 for multiplicative interaction) and additive interaction (RERI 0.21, 95% CI 0.09–0.34) for these two factors.
Table 3.
Effect modification by prepregnancy obesity and maternal history of CVD on the association between history of GDM and subsequent CVD in Danish women
| Effect modifier | Exposure | No. of CVD cases | Rate per 1,000 person-years | HR (95% CI) model 1 | HR (95% CI) model 2 |
|---|---|---|---|---|---|
| Prepregnancy obesity* | No history of GDM and no prepregnancy obesity | 179,385 | 10.78 | 1.0 (Reference) | 1.0 (Reference) |
| History of GDM only | 2,651 | 16.85 | 1.69 (1.63–1.76) | 1.43 (1.38–1.49) | |
| Prepregnancy obesity only | 3,420 | 13.17 | 1.81 (1.75–1.87) | 1.49 (1.44–1.54) | |
| History of GDM and prepregnancy obesity | 364 | 16.76 | 2.29 (2.07–2.54) | 1.76 (1.59–1.95) | |
| Multiplicative interaction (GDM × obesity) | 0.75 (0.67–0.84) | 0.82 (0.74–0.93) | |||
| P for multiplicative interaction | <0.001 | 0.001 | |||
| RERI | −0.21 (−0.46 to 0.04) | −0.16 (−0.36 to 0.03) | |||
| P for additive interaction | 0.099 | 0.104 | |||
| Maternal history of CVD† | No history of GDM and no maternal CVD | 53,741 | 9.25 | 1.0 (Reference) | 1.0 (Reference) |
| History of GDM only | 864 | 13.46 | 1.59 (1.49–1.70) | 1.31 (1.23–1.40) | |
| Maternal CVD history only | 98,837 | 11.67 | 1.17 (1.16–1.18) | 1.22 (1.21–1.23) | |
| History of GDM and maternal CVD | 1,572 | 18.77 | 2.04 (1.94–2.14) | 1.75 (1.66–1.84) | |
| Multiplicative interaction (GDM × CVD) | 1.10 (1.01–1.19) | 1.09 (1.00–1.19) | |||
| P for multiplicative interaction | 0.032 | 0.042 | |||
| RERI | 0.28 (0.13–0.43) | 0.21 (0.09–0.34) | |||
| P for additive interaction | <0.001 | 0.001 |
Model 1: Follow-up time as time scale.
Model 2: Follow-up time as time scale, controlled for time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, country of origin, maternal CVD history, and paternal CVD history.
Model 2: Follow-up time as time scale, controlled for time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, and paternal CVD history.
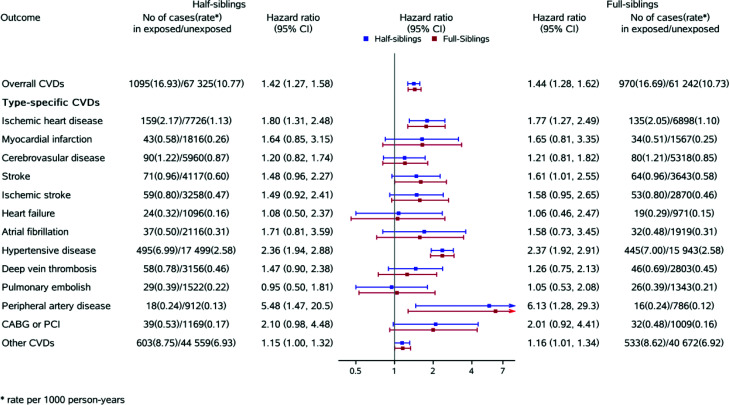
The associations for overall CVD using a sibling-matched design in both half-sibling (HR 1.42, 95% CI 1.27–1.58) and full-sibling cohorts (HR 1.44, 95% CI 1.28–1.62) were similar to that of the full unmatched population. Similar patterns were also observed for type-specific CVDs (Fig. 1).
Figure 1.
Sibship design for the association between history of gestational diabetes, overall CVD, and specific CVD types. Follow-up time as time scale controlled for time period of first delivery, parity, age at first delivery, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, and country of origin.
Results from analyses restricted to women without preeclampsia/eclampsia or stillbirth, with only one lifetime pregnancy, with at least 1 year of follow-up, with a first pregnancy since a specific calendar time (1980, 1985, 1991, 1994, 2000, or 2005), and with complete data were similar to those obtained in the primary analyses. Analyses using multiple imputations, restricted cubic splines for continuous covariates, or with additional adjustment for gestational age, or prepregnancy hypercholesterolemia, or prepregnancy Charlson Comorbidity Index score also were similar to those obtained in the primary analyses. Finally, analyses using stratified Cox regression by time period of the first delivery yielded results similar to those obtained in the primary analyses (Supplementary Table 3). Sensitivity analyses to assess the potential impact of unmeasured confounding in the mediation analysis found that even if the unmeasured confounder was strong enough to increase CVD risk by twofold, we would still observe the mediating effect of type 2 diabetes for most type-specific CVDs (Supplementary Text 3 and Supplementary Table 4).
Conclusions
We found that women with a history of GDM had increased risks of overall CVDs and varied increased risks for most common specific types of CVD, in particular, myocardial infarction, heart failure, hypertensive disease, peripheral artery disease, and CABG or PCI, even after accounting for prepregnancy sociodemographic, lifestyle, familial factors, and conventional CVD risk factors. Approximately 23% of the increased risks could be explained by the subsequent type 2 diabetes. The strongest associations were observed among women who had prepregnancy obesity or maternal history of CVD.
For the first time, we were able to investigate the association between GDM and a number of specific CVD outcomes, taking advantage of large study sample and long-term follow-up. Our findings of potential long-term effects of severe GDM on the risk of CVDs later in life are in line with the findings from studies examining a history of GDM and cardiovascular outcomes (6,8,9,11,13,16,26–28). A U.K. study (6) reported 85% and 178% increased risks for hypertension and ischemic heart disease related to GDM, respectively, but only for a relatively short-term effect over a median follow-up of 2.9 years. Self-reported GDM was associated with a 59% increased risk of myocardial infarction in the Nurses’ Health Study II (n = 89,479) over a median of 25.7 years of follow-up (13). While we observed a 65% higher risk of stroke associated with a history of GDM, the corresponding risk in the Nurses’ Health Study II (13) was 22%, but their estimate might not be informative due to the small number of cases (n = 33). Conversely, other studies in the U.K. and the Netherlands reported no association between history of GDM and stroke (6,27). These differences may be due to misclassification bias from self-reported GDM, the small number of events, or too short a follow-up time to evaluate stroke incidence. On the other hand, the development of CVD is a long process also influenced by both various environmental exposures and genetic factors (14), which may play a role in the pathway from GDM to CVD and has not been adequately examined. In addition to the inclusion of a wide range of potential confounders in our full population analysis, we further used a sibling-matched analysis to take into consideration factors, such as stable family environmental factors and genetic susceptibility, for which we do not have the information. Therefore, our study provided further evidence on the positive association of a history of GDM with subsequent CVDs and varied increased risks for type-specific CVD, in particular, myocardial infarction, heart failure, hypertensive disease, and peripheral artery disease.
The mechanisms linking a history of GDM and CVD risk remain to be studied (4). The mediating pathway of subsequently developed type 2 diabetes in the association of GDM with CVD has been widely discussed (5). However, whether elevated CVD risk following GDM depends on the development of type 2 diabetes (5,8,10,11) is still unclear. A Canadian study of >1 million women reported an increased risk of CVD for women with a history of GDM regardless of the presence of subsequent type 2 diabetes (7). However, the Nurses’ Health Study II suggested an elevated CVD risk only for women with both a history of GDM and progression to type 2 diabetes (13). Another Canadian cohort of 8,191 women with a history of GDM and 81,262 women without a history of GDM concluded that much of the increased risk of CVD among women with a history of GDM was attributed to the subsequent development of type 2 diabetes (16). The findings from our mediation analysis suggest that type 2 diabetes development plays a mediating role in the association between a history of GDM and CVD risk, but this elevated CVD risk is only partly (one-fifth) attributable to type 2 diabetes, indicating the need to explore other pathways linking GDM and CVD. Previous studies have suggested that weight change from prepregnancy to postpregnancy, dyslipidemia, and high blood pressure may be associated with later adverse cardiovascular health (29). However, information on weight change, lipid level, lipoprotein level, and blood pressure was not available in our study. Further investigation is warranted to explore the roles of these factors in the association between GDM and subsequent CVD.
The differences in the association of GDM with risk of subsequent type-specific CVDs and corresponding mediating effects of type 2 diabetes may be due to complex pathophysiology and the impacts of different risk factors on the development of type-specific CVDs (30). Further research is needed to elucidate underlying mechanisms and to explore the effects of other risk factors for specific CVDs.
GDM may also increase CVD risk via changes in cardiac structure (7,13,31,32). The Coronary Artery Risk Development in Young Adults (CARDIA) study found that a history of GDM was associated with impaired left ventricular relaxation, lower left ventricular systolic function, and increased left ventricular mass (32). Of note, women with a history of GDM had a higher prevalence or level of CVD risk factors, such as metabolic syndrome, prepregnancy HbA1c, and hypoadiponectinemia, compared with women without a history of GDM (7,13,33). Various studies have reported that some CVD risk factors may present in the early postpartum period or even prior to pregnancy (4,7,33,34). These data suggest that the diagnosis of GDM may be considered as a precursor that signifies a high risk of CVD later in life. Additionally, some unrecognized CVD risk factors before or during pregnancy may contribute to the observed association of history of GDM with CVD. However, our analysis, which also examined women without prepregnancy obesity and without preeclampsia, still found an increased CVD risk among women with a history of GDM compared with their peers. These findings suggest that the observed associations are less likely due to complete confounding by unrecognized CVD risk factors before or during pregnancy and that a history of GDM may predispose women to CVD later in life.
Noticeably, we observed a 76% higher risk of developing CVD among women with a history of GDM and prepregnancy obesity compared with women without a history of GDM and prepregnancy obesity. Previous evidence has reported that being moderately overweight during pregnancy can lead to a higher likelihood of developing GDM (18). Our previous study also found that the offspring of mothers with a history of CVD had a 75% higher risk of developing CVD themselves (35). Maternal history of CVD was associated with an increased risk of dyslipidemia, other CVD risk factors, myocardial infarction, stroke, and cardiovascular mortality in offspring (19,36,37), and thus, familial aggregation may suggest genetic influences on the development of CVD (19,37). This finding is consistent with prior observations that the degree of insulin resistance among women may be affected by their obesity and genetic inheritance (31). The added influence of obesity and maternal history of CVD suggests that more attention should be paid to these women who already have a high CVD risk when implementing intervention programs.
Strengths and Limitations of This Study
This study has several strengths. First, this prospective registry-based cohort study comprised almost all pregnant women in Denmark, thus minimizing the potential influence of selection bias, referral, and recall bias.
Second, a large sample size allowed for the examination of a wide range of specific CVDs and the use of a sibling design to reduce concerns of shared genetic or early life environmental confounding (17,25).
Third, substantial misclassification of CVD is unlikely because the validity of cardiovascular diagnoses and related procedures in the DNPR is high (38,39).
Finally, we used quantitative mediation analysis to explore the mechanism of the influence of a history of GDM on CVD risk. Elucidating the link between a history of GDM and CVD through type 2 diabetes might help inform the design and implementation of public health interventions aimed at reducing CVD risk.
Several limitations must also be noted. Although we adjusted for a wide range of potential confounders, we cannot entirely exclude uncontrolled confounding by unmeasured genetic or familial characteristics. Our sibship design from both full-sibling pairs and half-sibling pairs yielded results consistent with that of the unpaired study design of the whole cohort. These findings suggest that the observed associations are not entirely attributable to confounding by genetics and familial environment.
Although our study was able to adjust for several socioeconomic and lifestyle factors, such as education, smoking during pregnancy, and prepregnancy BMI, data on other factors (diet, sleep, alcohol consumption, etc.) were not available. The observed exposure-outcome associations could change if those factors were considered. Further research is warranted encompassing a broad range of socioeconomic and lifestyle factors.
Moreover, causal mediation analysis is subject to strict and untestable assumptions of no unmeasured confounding of exposure-mediator, exposure-outcome, and mediator-outcome links. As mentioned previously, not all potential confounders could be measured in our large-scale observational study. Sensitivity analyses exploring potential mediator-outcome confounding suggested that part of the estimated direct effects could be explained by high uncontrolled mediator-outcome confounding, using our assumed moderate to strong bias parameters and directionality of the mediator-confounder association. For instance, the mediation effect was reduced substantially for some specific CVD types when an unmeasured confounder was strong enough to increase CVD risk twofold (under the assumed scenario that we missed measuring and adjusting for such a strong confounder).
Also, the ascertainment of GDM solely based on hospital records is subject to potential misclassification bias, but we suspect that such misclassification in our prospective study design would be nondifferential.
Last, our findings suggest that the association is weaker in recent calendar periods than in the early beginning of our study period. This may be due to a mixture of increased use of coding for GDM and type 2 diabetes in the ICD-10 system after 1994, a broadening of the GDM term after 1999, generally increased screening and thus detection of milder GDM and type 2 diabetes cases from the early 2000s, and a relatively young population with a lower number of CVD events in later periods. Further research is needed to explore the underlying mechanisms.
Conclusion and Implications
Our study showed that a history of GDM was associated with increased risks of CVD in general and several major types of CVD. The associations were stronger among those with prepregnancy obesity or maternal history of CVD. The increased risks of CVD due to GDM were only partially explained by subsequent type 2 diabetes. The history of GDM and subsequent type 2 diabetes should be taken into account when designing a low-cost screening test for future CVD in women (40). Continuous monitoring of women with a history of GDM, especially those with prepregnancy obesity or maternal history of CVD, might provide important opportunities to reduce their cardiovascular risk.
Article Information
Funding. This study was supported by unrestricted grants from the Independent Research Fund Denmark (DFF-6110-00019B, DFF 9039-00010B and DFF-1030-00012B), a grant from the Nordic Cancer Union (R275-A15770), a grant from the Karen Elise Jensens Fond (2016), a grant from the Novo Nordisk Foundation (NNF18OC0052029), a grant from Shanghai Rising-Star Program (21QA1401300), a grant from the National Natural Science Foundation of China (82073570), a National Institute of Health National Center for Advancing Translational Science grant (UL1TR001881), and the facilities and resources provided by the California Center for Population Research (CCPR) at University of California, Los Angeles, which receives core support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R24HD041022) at the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.Y. drafted the manuscript. Y.Y., M.S., and O.A.A. undertook the statistical analysis. Y.Y., H.T.S., J.L., and O.A.A. conceived and designed the study. All authors provided critical input to the analyses. All authors interpreted the data and revised the manuscript critically. All authors had final responsibility for the decision to submit for publication. Y.Y. and J.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16826482.
This article is featured in a podcast available at https://diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373:1789–1797 [DOI] [PubMed] [Google Scholar]
- 2. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. [DOI] [PubMed] [Google Scholar]
- 3. Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 4. McKenzie-Sampson S, Paradis G, Healy-Profitós J, St-Pierre F, Auger N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol 2018;55:315–322 [DOI] [PubMed] [Google Scholar]
- 5. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905–914 [DOI] [PubMed] [Google Scholar]
- 6. Daly B, Toulis KA, Thomas N, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med 2018;15:e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care 2017;40:101–108 [DOI] [PubMed] [Google Scholar]
- 8. Goueslard K, Cottenet J, Mariet AS, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol 2016;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaul P, Savu A, Nerenberg KA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med 2015;32:164–173 [DOI] [PubMed] [Google Scholar]
- 10. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol 2014;180:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG 2014;121:1530–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 2013;99:1118–1121 [DOI] [PubMed] [Google Scholar]
- 13. Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 2017;177:1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benjamin EJ, Virani SS, Callaway CW, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 15. Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 16. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008;31:1668–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Y, Cnattingius S, Olsen J, et al. Prenatal maternal bereavement and mortality in the first decades of life: a nationwide cohort study from Denmark and Sweden. Psychol Med 2017;47:389–400 [DOI] [PubMed] [Google Scholar]
- 18. Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr 2000;71(Suppl.):1242S–1248S [DOI] [PubMed] [Google Scholar]
- 19. Palinski W. Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation 2014;129:2066–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–549 [DOI] [PubMed] [Google Scholar]
- 22. Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–686 [DOI] [PubMed] [Google Scholar]
- 23. VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods 2014;3:33–72 [Google Scholar]
- 24. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;25:749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cnattingius S, Kramer MS, Norman M, Ludvigsson JF, Fang F, Lu D. Keep it in the family: comparing perinatal risks in small-for-gestational-age infants based on population vs within-sibling designs. Int J Epidemiol 2019;48:297–306 [DOI] [PubMed] [Google Scholar]
- 26. Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation 2019;139:1069–1079 [DOI] [PubMed] [Google Scholar]
- 27. Heida KY, Franx A, van Rijn BB, et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension 2015;66:1116–1122 [DOI] [PubMed] [Google Scholar]
- 28. Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006;29:2078–2083 [DOI] [PubMed] [Google Scholar]
- 29. Grieger JA, Hutchesson MJ, Cooray SD, et al. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther Adv Reprod Health 2021;15:2633494120986544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiano C, Benincasa G, Franzese M, et al. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol Ther 2020;210:107514. [DOI] [PubMed] [Google Scholar]
- 31. Di Cianni G, Ghio A, Resi V, Volpe L. Gestational diabetes mellitus: an opportunity to prevent type 2 diabetes and cardiovascular disease in young women. Womens Health (Lond) 2010;6:97–105 [DOI] [PubMed] [Google Scholar]
- 32. Appiah D, Schreiner PJ, Gunderson EP, et al. Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA Study. Diabetes Care 2016;39:400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Retnakaran R, Shah BR. Divergent trajectories of cardiovascular risk factors in the years before pregnancy in women with and without gestational diabetes mellitus: a population-based study. Diabetes Care 2020;43:2500–2508 [DOI] [PubMed] [Google Scholar]
- 34. Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am J Epidemiol 2010;172:1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu Y, Arah OA, Liew Z, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ 2019;367:l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weijmans M, van der Graaf Y, Reitsma JB, Visseren FL. Paternal or maternal history of cardiovascular disease and the risk of cardiovascular disease in offspring. A systematic review and meta-analysis. Int J Cardiol 2015;179:409–416 [DOI] [PubMed] [Google Scholar]
- 37. Lloyd-Jones DM, Nam BH, D’Agostino RB Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA 2004;291:2204–2211 [DOI] [PubMed] [Google Scholar]
- 38. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adelborg K, Sundbøll J, Munch T, et al. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population-based validation study. BMJ Open 2016;6:e012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Retnakaran R, Shah BR. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol 2019;7:378–384 [DOI] [PubMed] [Google Scholar]