Abstract
OBJECTIVE
There are few studies testing the amount of weight loss necessary to achieve initial remission of type 2 diabetes mellitus (T2DM) following bariatric surgery and no published studies with use of weight loss to predict initial T2DM remission in sleeve gastrectomy (SG) patients.
RESEARCH DESIGN AND METHODS
With Cox proportional hazards models we examined the relationship between initial T2DM remission and percent total weight loss (%TWL) after bariatric surgery. Categories of %TWL were included in the model as time-varying covariates.
RESULTS
Of patients (N = 5,928), 73% were female; mean age was 49.8 ± 10.3 years and BMI 43.8 ± 6.92 kg/m2, and 57% had Roux-en-Y gastric bypass (RYGB). Over an average follow-up of 5.9 years, 71% of patients experienced initial remission of T2DM (mean time to remission 1.0 year). With 0–5% TWL used as the reference group in Cox proportional hazards models, patients were more likely to remit with each 5% increase in TWL until 20% TWL (hazard ratio range 1.97–2.92). When categories >25% TWL were examined, all patients had a likelihood of initial remission similar to that of 20–25% TWL. Patients who achieved >20% TWL were more likely to achieve initial T2DM remission than patients with 0–5% TWL, even if they were using insulin at the time of surgery.
CONCLUSIONS
Weight loss after bariatric surgery is strongly associated with initial T2DM remission; however, above a threshold of 20% TWL, rates of initial T2DM remission did not increase substantially. Achieving this threshold is also associated with initial remission even in patients who traditionally experience lower rates of remission, such as patients taking insulin.
Introduction
Bariatric surgery is the most effective treatment for severe obesity and metabolic disorders such as type 2 diabetes mellitus (T2DM) (1). However, the response to bariatric surgery is not uniform, with most patients regaining some weight in the long-term and experiencing relapse of T2DM (2–4). There has been a long-standing debate about what constitutes “successful” treatment of severe obesity and metabolic disease following surgery (5). Some standards for weight loss have been discussed over the last decades including achieving and maintaining ≥50% excess weight loss and more recently ≥30% total weight loss (TWL) (6). This definition matters, as conclusions about factors related to weight loss after surgery are different depending on thresholds of “successful” weight loss (7). A clinically meaningful weight loss for nonsurgical interventions is generally at least 5% TWL (8); however, most experts agree that this is an unreasonably low target for bariatric surgery. A recent long-term large population-based study of >60,000 bariatric operations suggested that an average of 25% TWL and 18% TWL could be expected after 5 years for Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), respectively (3). There are no standards for “successful” rates of T2DM remission, and rates vary widely (1,9,10).
Although bariatric surgery may “reset” metabolic processes through mechanisms that are not weight related, especially for T2DM, it is widely believed that the effect of bariatric surgery on metabolic disease is closely tied to weight loss (11). Until recently this was an untested assumption. Recent studies suggest that there is a strong association between weight loss and T2DM remission (12) and that there could be a threshold of 25% TWL necessary to resolve metabolic syndrome (13). This area is still relatively unexplored, especially for SG operations, and is critically important to understand because physicians may have unrealistic expectations about the surgical weight loss necessary for T2DM remission (14), which could be communicated to patients and their families, limiting the uptake of the most effective long-term treatment we have for severe obesity. There are very few studies in real-world U.S. clinical practice settings to provide physicians with recommendations about the effectiveness of bariatric surgery that reflect the very diverse patient populations with T2DM having surgery.
The current study was designed to test the relationship between surgical weight loss and initial T2DM remission in a large (N = 5,928), diverse (65% non-White) real-world clinical care sample of patients having the most common bariatric operations in the world: RYGB and SG (15). We specified the following a priori hypotheses and analyses: 1) initial T2DM remission rates would increase with each increase in clinically meaningful categories of percent TWL (%TWL) and 2) this relationship would depend on patient-level factors at the time of surgery that have been shown to impact weight loss and T2DM in the literature (3,10,16). Our sample reflects common decision-making about T2DM monitoring by physicians in large adult primary care practices (17), and these findings could be used to change how bariatric surgery is viewed as a treatment for T2DM as well as severe obesity by primary care physicians.
Research Design and Methods
Participants
Data for this study came from an integrated health care system serving 4.2 million members living in the Southern California region of the U.S. The study was reviewed and approved by the institutional review board of this health care system. The details of how patients are prepared in this setting for weight loss surgery (18) and the Effectiveness of Gastric Bypass versus Gastric Sleeve for Cardiovascular Disease (ENGAGE CVD) study cohort (19) included for the current study have previously been published. In general, eligibility for weight loss surgery is based on national recommendations (20): having a BMI ≥40 kg/m2 or having a BMI of 35–39 kg/m2 with at least one major obesity-related comorbidity.
For this study, we focused on patients with T2DM at the time of surgery, defined according to the methods of the Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) study, where both the capture and accuracy of information regarding patients with diabetes were maximized with use of electronic medical records (EMR) (21,22). The SUPREME-DM study included extensive validation of definitions for T2DM using only EMR across multiple health care systems in the U.S. The protocol for validation, which was used in the current study, is available from the study website and is explained in more detail in Supplementary Material (21). A patient was considered to have T2DM at the time of surgery for the current study if one of the following criteria was met: 1) one inpatient diagnosis within 2 years of surgery or 2) any combination of two of the following criteria: the latest HbA1c value within 2 years of surgery was ≥6.5%, prescription for T2DM medications at the time of surgery, latest fasting plasma glucose >126 mg/dL (7.0 mmol/L), a random plasma glucose >200 mg/dL (11.1 mmol/L) within 2 years of surgery, and an outpatient diagnosis code within 2 years of surgery. A frequency count of the types of diabetes medications used to define our T2DM cohort as well as the medications used after surgery are shown in Supplementary Table 1.
Supplementary Fig. 1 provides a visual representation of the patient selection for the study. The initial ENGAGE CVD cohort consisted of 22,095 patients who underwent SG or RYGB from 2009 to 2016 (19). Of this cohort, 6,633 (30%) had T2DM at the time of surgery based on the definition detailed above. From the 6,633, the patients were excluded before analyses for the following reasons: weight was never measured (n = 7), no weight measurement available from ±6 months of T2DM remission (N = 205), and no HbA1c measurement at the time of surgery (n = 392). In addition, 80 patients who gained weight after surgery were removed because of their inherent clinical differences from people who lost weight. We also removed 21 patients with >50% TWL, who were eliminated based on chart review that indicated these were data entry errors by clinical personnel. This resulted in a final analytic sample of 5,928 patients (3,382 RYGB and 2,546 SG) with T2DM at the time of surgery.
Data
All data for the study were obtained from patient EMR and electronic billing claims for outside services. Medical records and billing claims were examined from the time a patient became a member of the health plan up until the patient’s bariatric operation, and the follow-up period was after each patient’s bariatric operation until 31 December 2018. The ENGAGE CVD cohort was designed to ensure that all patients had at least 2 years of time after the operation. Details of the validation of bariatric cases can be found in Supplementary Material.
Outcome
The primary outcome was remission of T2DM. Remission was determined after a “washout” period of at least 90 days following either the date of surgery or the end of all T2DM medication prescriptions (if the prescriptions overlapped the day of surgery). Ninety days was chosen because any HbA1c measures taken prior to 90 days would have been influenced by the medication. T2DM remission was defined as the first date for which a patient met both of the following criteria: 1) no diabetes medications filled and 2) either HbA1c <6.5% or fasting glucose <126 mg/dL. This definition was chosen to reflect the clinical practice guidelines of treatment of T2DM in large integrated health care systems at the time of the study (22,23), as well as to remain consistent with our previous work in this area (4,10,16). Random glucose values were not used to determine remission, and laboratory values were not obtained during or up to 90 days following pregnancy/birth, hospitalization, or use of oral steroids.
Exposure
The primary exposure of interest was %TWL, calculated as [(weight at the time of surgery – weight at the time of remission or censor)/weight at the time of surgery] from surgery to first instance of T2DM remission or to censorship if no remission was observed. Censoring occurred upon loss of membership, death, last weight measurement, or end of study period (31 December 19). %TWL was categorized into seven groups (0 to ≤5%, >5 to ≤10%, >10 to ≤15%, >15 to ≤20%, >20 to ≤25%, >25 to ≤30%, and >30%). We categorized %TWL to allow flexibility in the relationship between %TWL and T2DM remission and to examine the possibility that certain thresholds of %TWL could be meaningful for T2DM remission. A continuous variable of %TWL would not provide us with the information we would need to provide clinical recommendations about a minimal threshold of weight loss to achieve for T2DM remission. The seven categories were chosen based on feedback from our bariatric surgical partners regarding what would be considered “successful” weight loss following bariatric surgery (19) and recent U.S. population-based studies demonstrating the proportion of patients achieving different thresholds of weight loss after surgery (3).
Covariates
Table 1 provides a list of all the covariates in the analyses measured before or at the time of surgery. Because the parent study, ENGAGE CVD, was focused on comparative effectiveness of RYGB and SG, these were chosen based on collaboration with our advisory board of bariatric surgeons regarding how they made decisions between operations for certain patients (e.g., T2DM patients are more likely to get RYGB) (19) and previous literature on EMR-based determinants of bariatric surgery outcomes (3,4,7,10,16). Several continuous covariates were categorized to allow flexibility in the functional forms of association with T2DM remission: weight, BMI, inpatient and emergency department visits in the prior year, outpatient appointment attendance rate, weight change in the prior year, comorbidity burden with use of the Elixhauser score (24), and Diabetes Remission (DiaRem) score, which is a specific composite score developed for predicting diabetes remission in bariatric patients (25). A list can be found of the precise form of all covariates included in the models in the legends of Figures 1 to 3.
Table 1.
Descriptive statistics for all patients with T2DM (N = 5,928) and the covariates included in all statistical models
| All | 0–5% TWL | >5–10% TWL | >10–15% TWL | >15–20% TWL | >20% TWL | |
|---|---|---|---|---|---|---|
| n | 5,928 | 115 | 356 | 684 | 1,157 | 3,616 |
| T2DM remission (yes), n (%) | 4,216 (71) | 29 (25) | 125 (35) | 400 (58) | 815 (70) | 2,847 (79) |
| Years to T2DM remission | 1.0 (0.92) | 1.5 (1.24) | 1.1 (1.13) | 1.0 (0.99) | 0.8 (0.68) | 1.0 (0.95) |
| Age (years) | 49.8 (10.3) | 51.4 (10.8) | 50.3 (10.9) | 50.8 (10.2) | 50.4 (10.2) | 49.3 (10.3) |
| Age ≥65 years, n (%) | 397 (7) | 11 (10) | 34 (10) | 59 (9) | 88 (8) | 205 (6) |
| RYGB, n (%) | 3,382 (57) | 41 (36) | 114 (32) | 251 (37) | 579 (50) | 2,397 (66) |
| Hispanic, n (%) | 2,572 (43) | 47 (41) | 130 (37) | 281 (41) | 475 (41) | 1,639 (45) |
| Non-Hispanic White, n (%) | 2,059 (35) | 35 (30) | 118 (33) | 225 (33) | 399 (34) | 1,282 (35) |
| Non-Hispanic Black, n (%) | 1,054 (18) | 27 (23) | 90 (25) | 152 (22) | 227 (20) | 558 (15) |
| Other races or unknown, n (%) | 243 (4) | 6 (5) | 18 (5) | 26 (4) | 56 (5) | 137 (4) |
| BMI (kg/m2) | 43.8 (6.9) | 42.5 (6.4) | 42.5 (6.6) | 42.9 (6.8) | 43.4 (6.9) | 44.2 (6.95) |
| BMI ≥50 kg/m2, n (%) | 1,016 (17) | 18 (16) | 47 (13) | 95 (14) | 173 (15) | 683 (19) |
| Insulin use, n (%) | 1,982 (33) | 42 (37) | 120 (34) | 195 (29) | 335 (29) | 1,290 (36) |
| DiaRem score (range 2–22) | 8.4 (5.4) | 8.9 (5.7) | 8.5 (5.4) | 8.0 (5.1) | 7.9 (5.2) | 8.6 (5.5) |
| DiaRem score ≥8, n (%) | 2,208 (37) | 45 (39) | 132 (37) | 227 (33) | 369 (32) | 1,435 (40) |
| Female, n (%) | 4,326 (73) | 82 (71) | 263 (74) | 493 (72) | 872 (75) | 2,616 (72) |
| HbA1c | 7.5 (1.1) | 7.6 (1.2) | 7.5 (1.1) | 7.5 (1.1) | 7.4 (1.0) | 7.5 (1.1) |
| Weight (lb) | 269.6 (54.4) | 262.6 (52.0) | 261.4 (49.8) | 265.1 (52.9) | 265.7 (52.9) | 272.7 (54.8) |
| Hypertension, n (%) | 3,793 (64) | 82 (71) | 234 (66) | 434 (63) | 758 (66) | 2,285 (63) |
| Cirrhosis, n (%) | 71 (1) | 0 (0) | 4 (1) | 13 (2) | 12 (1) | 42 (1) |
| Sleep apnea, n (%) | 922 (16) | 20 (17) | 42 (12) | 114 (17) | 175 (15) | 571 (16) |
| Chronic kidney disease, n (%) | 1,138 (19) | 22 (19) | 70 (20) | 127 (19) | 206 (18) | 713 (20) |
| Serious mental illness, n (%) | 318 (5) | 7 (6) | 17 (5) | 28 (4) | 60 (5) | 206 (6) |
| Severe anxiety or depression, n (%) | 387 (7) | 9 (8) | 21 (6) | 42 (6) | 75 (6) | 240 (7) |
| Mild/moderate anxiety or depression, n (%) | 2,529 (43) | 50 (43) | 149 (42) | 295 (43) | 504 (44) | 1,531 (42) |
| Aspirin use 12 months before, n (%) | 2,582 (44) | 53 (46) | 170 (48) | 302 (44) | 506 (44) | 1,551 (43) |
| Aspirin use 3 months before, n (%) | 1,737 (29) | 36 (31) | 128 (36) | 203 (30) | 339 (29) | 1,031 (29) |
| NSAID use 12 months before, n (%) | 2,546 (43) | 44 (38) | 137 (38) | 292 (43) | 490 (42) | 1,583 (44) |
| NSAID use 3 months before, n (%) | 920 (16) | 17 (15) | 62 (17) | 110 (16) | 186 (16) | 545 (15) |
| Dyslipidemia medication use, n (%) | 3,337 (56) | 73 (63) | 190 (53) | 397 (58) | 654 (57) | 2,023 (56) |
| Number of T2DM medication | 1.3 (0.98) | 1.4 (0.98) | 1.3 (0.96) | 1.2 (0.93) | 1.3 (0.93) | 1.3 (1.0) |
| Elixhauser score | 3.8 (10.6) | 5.0 (10.1) | 3.8 (9.9) | 3.4 (9.96) | 3.3 (10.0) | 4.0 (10.96) |
| Hospital days 12 months before | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.35) | 0.1 (0.3) | 0.1 (0.4) |
| Emergency visits 12 months before | 0.4 (0.9) | 0.3 (0.6) | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.4 (0.97) |
| % attendance outpatient visits 12 months before | 75.7 (12.1) | 76.4 (11.9) | 74.4 (12.1) | 75.2 (12.2) | 76.5 (11.8) | 75.6 (12.2) |
| Weight loss (lb) 12 months before | −16.4 (13.62) | −21.5 (15.21) | −19.8 (15.65) | −17.1 (12.99) | −16.8 (13.70) | −15.6 (13.36) |
Data are presented as n (%) for categorical variables and mean (SD) for continuous variables. All variables except T2DM remission and %TWL were measured at the time of surgery.
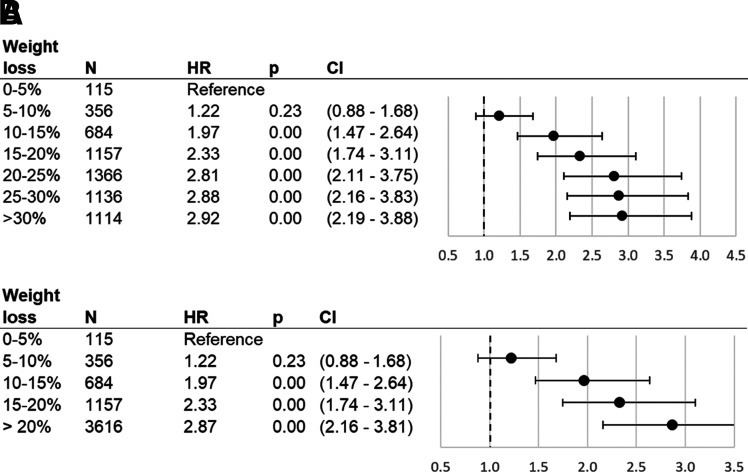
Figure 1.
Association between T2DM remission and %TWL after bariatric surgery: all a priori categories of %TWL (A) and upper categories collapsed into >20% TWL (B). Cox proportional hazards model results for T2DM remission as related to percentage of weight lost from surgery date until date of remission or censoring, as compared with patients who lost 0–5% of their surgery weight. %TWL was modeled as a time-varying covariate. Sample is comprised of ENGAGE CVD recipients of bariatric surgery who had T2DM at time of surgery and subsequently had their weight measured, had their weight measured at time of T2DM remission (if they remitted), and were not outliers (weight gain of >0% or weight loss of >50%). HRs: adjustment for surgery type, sex, race/ethnicity, age, age squared, HbA1c (quartiles), DiaRem score (quartiles), Elixhauser score (quartiles), BMI (quartiles), weight at surgery (quartiles), BMI ≥50 kg/m2 at surgery indicator, indicators for drug use in year and 3 months prior to surgery (aspirin, nonsteroidal anti-inflammatory drugs), number of diabetes drugs at surgery (indicators for 0, 1, and 2), drug use at surgery (dyslipidemia drugs, insulin), inpatient visits in year presurgery above median, emergency department visits in year presurgery above median, presurgery appointment attendance rate above median, presurgery weight change above median, and prior diagnosis indicators (hypertension, cirrhosis, sleep apnea, chronic kidney disease, serious mental condition, severe anxiety, mild-to-moderate anxiety).
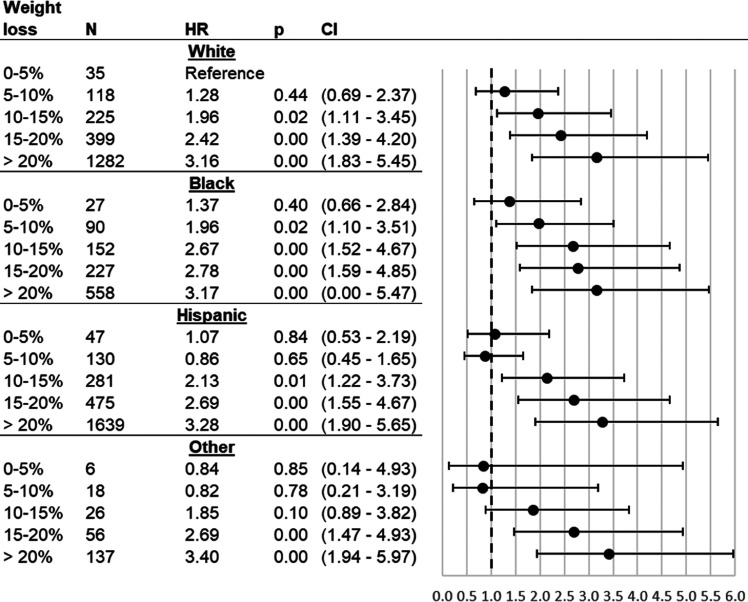
Figure 3.
Association between T2DM remission and %TWL after bariatric surgery, stratified by race/ethnicity. Cox proportional hazards model results for T2DM remission as related to total weight loss (%TWL) from surgery date until date of remission or censoring, as compared with patients who lost 0–5% of their surgery weight. %TWL was modeled as a time-varying covariate and interacted with race/ethnicity. Sample is comprised of ENGAGE recipients of bariatric surgery who had T2DM at time of surgery and subsequently had their weight measured, had their weight measured at time of T2DM remission (if they remitted), and were not outliers (weight gain of >0% or weight loss of >50%). HRs: adjustment for surgery type, sex, age, age squared, HbA1c (quartiles), DiaRem score (quartiles), Elixhauser score (quartiles), BMI (quartiles), weight at surgery (quartiles), BMI ≥50 kg/m2 at surgery indicator, indicators for drug use in year and 3 months prior to surgery (aspirin, nonsteroidal anti-inflammatory drugs), number of diabetes drugs at surgery (indicators for 0, 1, and 2), drug use at surgery (dyslipidemia drugs, insulin), inpatient visits in year presurgery above median, emergency department visits in year presurgery above median, presurgery appointment attendance rate above median, presurgery weight change above median, and prior diagnosis indicators (hypertension, cirrhosis, sleep apnea, chronic kidney disease, serious mental condition, severe anxiety, mild-to-moderate anxiety).
Analyses
Cox proportional hazards models with adjustments for all covariates (see Table 1) were used. Indicator variables for each category of %TWL were included in the model as time-varying covariates. We also examined heterogeneity of treatment effects (HTE) for this association using the following a priori categories of patients, determined at the time of surgery: T2DM remission likelihood (DiaRem ≥8 [high, corresponding with <50% chance of remission] vs. ≤7 [low, >64% chance of remission]), insulin use (yes vs. no), race/ethnicity (White vs. Black, Hispanic, or other), BMI (≥50 vs. <50 kg/m2), age (≥65 vs. <65 years), and bariatric operation (RYGB vs. SG).
Because the initial outcome findings (see Results) suggested that there was an upper threshold for the association of weight loss (>20% TWL) with initial T2DM remission, we also examined the following categories of %TWL to improve the stability of the HTE models: 0 to ≤5%, >5 to ≤10%, >10% to ≤15%, >15% to ≤20%, and >20%. In a sensitivity analysis, we examined an alternative specification with combination of the ≤5% group and the >5 to ≤10% group. We also ran sensitivity analyses adjust for covariates, adjusting for a parsimonious set of covariates, and including all five categories of DiaRem risk. To test the hypothesis that there was a heterogeneous relationship between %TWL and initial T2DM remission, we used Wald tests of the joint hypothesis that the interaction coefficients for categories of %TWL among members of the aforementioned groups (e.g., users of insulin), were equal to the same %TWL categories in the complementary group (e.g., those not using insulin). As a supplementary analysis, we examined the relationship between %TWL and changes in HbA1c, using linear regressions with individual fixed effects. Full details of all analyses are available in Supplementary Material.
Results
Participants
The analytic sample of 5,928 patients with T2DM was followed for an average of 5.9 years/person. Over this time, 71% of the sample experienced an initial T2DM remission (72% for RYGB and 70% for SG). The average time to remission was 1.0 years (0.98 years for RYGB and 1.01 years for SG). The 5-year retention rate was 82.9%. Follow-up was defined as the amount of time from date of surgery to the date of last weight measurement. Loss to follow-up was due to missing weight data (64.0%), loss of membership in the health plan (30.1%), and death (5.9%). In comparison with patients who did not have at least 5 years of follow-up, those who did were more likely to experience T2DM remission (60% vs. 74%, respectively; P < 0.001), less likely to have a BMI ≥50 kg/m2 (20% vs. 17%; P = 0.04), more likely to be women (68% vs. 73%; P < 0.001), and less likely to be non-Hispanic White (45% vs. 35% vs. P < 0.001), be older (49.2 vs. 50.2 years old; P = 0.02), have a greater number of comorbid conditions at surgery (3.7 vs. 4.7; P = 0.04), have lower %TWL in the year before surgery (17.0% vs. 15.6; P = 0.02), and weigh less (278.6 vs. 270.0 lb; P < 0.001) at surgery.
Weight Loss Mediation of T2DM Remission
Results for the mediation of T2DM remission rates by amounts of weight loss were adjusted for all covariates shown in Table 1. The number of initial T2DM remission events in each %TWL category are included in Table 1, and the unadjusted remission rates per person-year by %TWL categories are shown in Supplementary Table 2. Compared with patients who had 0% to ≤5% TWL, patients who lost successively more weight were more likely to remit (see Fig. 1A), beginning with patients having >10% to ≤15% TWL and continuing through patients with >20 to ≤25% TWL: >10% to ≤15% TWL 1.97 times more likely (hazard ratio [HR] 1.97; CI 1.47–2.64), >15 to ≤20% TWL 2.33 times more likely (HR 2.33; CI 1.74–3.11), and >20% to ≤25% TWL 2.81 times more likely (HR 2.81; CI 2.11–3.75). Although patients with >25% to ≤30% TWL or >30% TWL were also more likely to remit compared with patients with 0 to ≤5% TWL, these patients had likelihood of remission (HR 2.88, CI 2.16–3.83, and HR 2.92, CI 2.19–3.88 respectively) similar to that of patients with >20% to ≤25% TWL. Fig. 1B presents results with the >20% TWL category consolidated. Compared with patients with 0% to ≤5% TWL, those with >20% TWL were 2.87 times more likely to remit (HR 2.87; CI 2.16–3.81).
HTE for %TWL and T2DM Remission
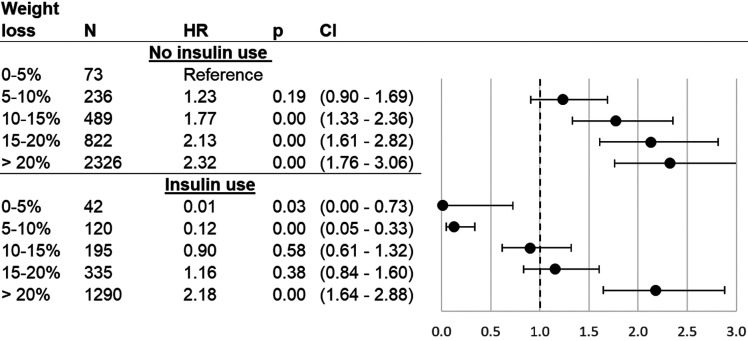
Results for HTE analyses were adjusted for all covariates shown in Table 1. The association of %TWL with initial T2DM remission was different depending on use of insulin at the time of surgery (P < 0.001) (see Fig. 2 and Supplementary Table 3). In general, use of insulin was associated with a lower probability of remission. However, patients with insulin use who had >20% TWL were 2.18 times more likely to experience initial T2DM remission than a patient who was not taking insulin but only had 0 to ≤5% TWL (HR 2.18; CI 1.64–2.88). An increase in T2DM remission likelihood when taking insulin before surgery was only present for those who lost >20% TWL.
Figure 2.
Association between T2DM remission and %TWL after bariatric surgery, stratified by insulin use at surgery. Cox proportional hazards model results for T2DM remission as related to percentage of weight lost from surgery date until date of remission or censoring, as compared with patients who lost 0–5% of their surgery weight. %TWL was modeled as a time-varying covariate and interacted with binary insulin use. Sample is comprised of ENGAGE CVD recipients of bariatric surgery who had T2DM at time of surgery and subsequently had their weight measured, had their weight measured at time of T2DM remission (if they remitted), and were not outliers (weight gain of >0% or weight loss of >50%). HRs: adjustment for surgery type, sex, race/ethnicity, age, age squared, HbA1c (quartiles), DiaRem score (quartiles), Elixhauser score (quartiles), BMI (quartiles), weight at surgery (quartiles), BMI ≥50 kg/m2 at surgery indicator, indicators for drug use in year and 3 months prior to surgery (aspirin, nonsteroidal anti-inflammatory drugs), number of diabetes drugs at surgery (indicators for 0, 1, and 2), drug use at surgery (dyslipidemia drugs), inpatient visits in year presurgery above median, emergency department visits in year presurgery above median, presurgery appointment attendance rate above median, presurgery weight change above median, and prior diagnosis indicators (hypertension, cirrhosis, sleep apnea, chronic kidney disease, serious mental condition, severe anxiety, mild-to-moderate anxiety).
The overall relationship between %TWL and initial T2DM remission was different for non-Hispanic Black compared with White patients (P < 0.001) and Hispanic patients (P < 0.001) but not between any other race/ethnicity dyad (see Fig. 2 and Supplementary Table 3). Regardless of race/ethnicity, all patients had the same likelihood of initial T2DM remission if they only lost 0 to ≤5% TWL. However, Non-Hispanic Black patients who lost >5% TWL were 1.96 times more likely to experience initial T2DM remission than White patients who lost ≤5% TWL (HR 1.96; CI 1.10–3.51) with increases in likelihood of remission as Black patients lost more weight. Hispanic patients who lost >10% TWL compared with White patients who lost ≤5% TWL were 2.13 times more likely to experience initial T2DM remission (HR 2.13; CI 1.22–3.73).
In additional analyses we found that the relationship between %TWL and T2DM remission was heterogeneous across DiaRem scores (high vs. low; P < 0.001) and surgical operation type (RYGB vs. SG; P < 0.001) but not across age-groups (≥65 vs. <65 years) or BMI categories (≥50 vs. <50 kg/m2) (see Supplementary Figs. 2–5 and Supplementary Table 3). The effect for DiaRem scores was similar to that found for insulin use (see Supplementary Fig. 2). Although statistically significant, different rates of initial T2DM remission across operations were small in all categories of %TWL (see Supplementary Fig. 5).
Sensitivity Analyses
Combining the 0–5% group and the 5–10% group did not meaningfully change the results (Supplementary Fig. 6). Sensitivity analyses without adjustment for covariates, and with only adjustment for a parsimonious set of covariates, did not substantively change the results (Supplementary Figs. 7 and 8). Findings for five categories of DiaRem risk (Supplementary Fig. 9) showed were similar to those for the two-category model (Supplementary Fig. 2B). A supplementary analysis examined the relationship between %TWL and changes in HbA1c and showed that compared with individuals with 0–5%TWL, those who lost more weight had significantly larger decreases in HbA1c (Supplementary Table 4).
Conclusions
Our findings suggest that the probability of achieving T2DM remission with bariatric surgery increased as %TWL increased from 0–5% up to 20%. However, above a threshold of 20% TWL, there was little additional gain in remission of T2DM. To our knowledge, this is the first study to test statistical models of the relationship between specific surgical weight loss thresholds and T2DM remission. In a recent study investigators examined the relationship between categories of %TWL and metabolic syndrome remission and found that a threshold of 25% TWL was necessary to resolve at least three of the five components of metabolic syndrome (13). This is similar to our findings; however, these investigators did not examine diabetes remission per se, had a homogenous sample of Chinese patients, and had only 214 SG patients. Our findings are much more relevant to contemporary bariatric practices in the U.S. (15). Most patients regain some amount of the weight they lost after surgery (1–3,26), and our findings support the benefit of surgery for T2DM remission even for modest amounts of surgical weight loss. Our findings are also supported by some of the recent literature in nonsurgical weight loss in patients with severe obesity. In the Diabetes Remission Clinical Trial (DiRECT), the highest remission rates (86%) were found when patients achieved 15% TWL (27).
In addition, the benefit existed even when patients were taking insulin or had a BMI ≥50 kg/m2 at the time of surgery. These same subsets of patients also tended to lose less weight following surgery (3,4); however, it may not matter for T2DM remission if they achieve a threshold of at least 20% TWL. The current study did not examine the relationship between weight regain and T2DM relapse, which would provide a more complete picture for the effect of % TWL on T2DM. This was beyond the scope of the current study but should be addressed with future research.
This study had several limitations, most notably the retrospective observational design. To mitigate confounding, we worked closely with our advisory board of bariatric surgeons and our patient and provider coinvestigators to identify factors related to why patients would choose/undergo SG or RYGB (19). We were not able to account for all factors that are known to impact diabetes remission, such as the duration of T2DM before surgery, because these data are not available for automated abstraction from the medical record for thousands of patients. Our models did include adjustment for factors such as number of diabetes medications, insulin use, and HbA1c values, which can also affect the rates of T2DM remission.
We also had a 17% attrition rate at 5 years. People who were lost to follow-up were less likely to experience initial T2DM remission, were heavier and had a higher comorbidity burden at the time of surgery, and were more likely to be men compared with those included in our analyses, potentially limiting the generalizability of our findings. However, an 83% 5-year retention rate is comparable with some of the highest retention rates reported in the literature (26) and we recently demonstrated that loss of patients to follow-up was unlikely to impact the overall findings of our observational studies (28).
Diabetes and its initial remission were determined with use of electronic data sources only. It is possible that a prospective study with physician examinations of patients could have found a different remission rate, as the newest randomized clinical trial results of SG and RYGB for 5-year T2DM outcomes have included lower rates of durable T2DM remission, between 20% and 68% (9,29,30). The 71% that we report is similar to the percentages of other observational studies (1,4,10,16); however, if we had accounted for relapse of T2DM, as did the clinical trials and other large observational studies, our remission rates would have been lower (4).
Another limitation of our study was that it was focused on initial T2DM remission and not durable T2DM remission. The complexity of modeling multiple measurements over time and accounting for patterns of T2DM remission and relapse as well as weight loss and regain across years of follow-up was beyond the scope of this study. We did show that compared with individuals with 0–5% TWL, those with greater %TWL had significantly larger decreases in HbA1c (Supplementary Table 4), suggesting that weight maintenance over time might also result in durable T2DM; however, we could not test this assumption in our analyses. Future studies should focus on weight regain and durable T2DM.
Finally, although we had a large sample of both SG and RYGB, the results with stratification by surgical operation (see Supplementary Fig. 5) should be interpreted with caution because of selection by patients/physicians between these two operations, which likely confounds the comparative analyses (e.g., more patients with T2DM receive RYGB). Our variable selection process was designed to account for these differences between operations (19); however, as mentioned previously some of these factors were not observable and could therefore bias our analyses. Despite this limitation, our findings are comparable with those of other studies in this area. For example, a large population-based comparative effectiveness study for T2DM outcomes was recently published, which included the ENGAGE CVD cohort patients (4), and the investigators found that there were not large differences in the remission rates for T2DM between RYGB (86.1%) and SG (83.5%) patients after 5 years.
However, this conclusion may be tempered by findings in the same cohort that 70% of RYGB patients regardless of T2DM status lost at least 20% TWL after 5 years compared with 45% of SG patients (3). This suggests that RYGB patients will more often achieve the 20% TWL threshold necessary for T2DM remission. In combination, these findings point to the preferential use of RYGB when T2DM remission is the main clinical outcome of interest for the patient and provider, particularly among patients on insulin at the time of surgery.
Our study also had several strengths including a large (N = 5,928), diverse (65% non-White) sample of patients receiving care from 23 surgeons and 9 surgical practices in a real-world setting. Our definition of diabetes prevalence and remission was designed to reflect real-world clinical practice in large health care settings (21,22) to maximize the generalizability of our results. We also have one of the largest samples of SG patients in the literature (n = 2,546), which is now the most common bariatric operation in the world (15).
Because of these strengths, our findings can be used to help providers and patients discuss realistic expectations for weight loss following bariatric surgery and how this will affect their T2DM remission. Our findings also suggest that patients using insulin and having a BMI ≤50 kg/m2 (2,4,10,16), factors shown to reduce the effectiveness of bariatric surgery for T2DM remission, will still have substantial benefits from surgery as long as they achieve at least 20% TWL. This information should be used by providers, in combination with other recent studies about nonsurgical treatments for T2DM and severe obesity, in a shared decision-making approach to treatment such that each patient receives the treatment that will most likely benefit them.
Article Information
Acknowledgments. The authors acknowledge the bariatric patients who contributed data for this study, without whom the work would not be possible.
Funding. Support for this study was provided by the National Heart, Lung, and Blood Institute (5R01HL130462). K.J.C., D.E.A., A.C., A.B., K.R., and D.B. all have funding from the National Institutes of Health for this manuscript and other unrelated projects. D.E.A. and A.C. have funding from the Patient-Centered Outcomes Research Institute unrelated to the current manuscript.
The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Duality of Interest. K.R. has funding from Merck & Co., Novartis, and CSL Behring, LLC, unrelated to the current manuscript. D.E.A. has conference travel support from International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Latin American Chapter and World Congress for Interventional Therapies for Type 2 Diabetes unrelated to the current manuscript. A.B. received consulting fees through Salutis Consulting LLC unrelated to the current manuscript. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.B. designed all analyses and assisted with conducting them, wrote the original draft of the study, and is the main person responsible for the content of the manuscript. K.J.C. obtained the funding for the manuscript, designed the overall study, obtained the data, interpreted the data, wrote the original draft of the manuscript, and is responsible for the overall conduct of the study. E.B. performed all analyses and provided interpretation of the findings. L.J.B., H.F., and T.K.Y. obtained and processed all data for the study and contributed to the design of the cohort, covariates, and outcome measures. D.E.A., A.B., and K.R. contributed to the design of the cohort, covariates, and outcome measures and assisted in the interpretation of and presentation of the findings. A.C., C.L.C., P.N.F., B.B.K., E.C.M., S.B.M., and R.E.Z. served as content experts in the design of the cohort, covariates and outcome measures, assisted in the interpretation of and presentation of the findings, and provided guidance for recommendations that could be used by surgeons, providers, and patients. All authors read and approved all versions of the manuscript before publication. K.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying article, p. 28.
This article contains supplementary material online at https://doi.org/10.2337/figshare.15113349.
References
- 1. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA 2020;324:879–887 [DOI] [PubMed] [Google Scholar]
- 2. King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA 2018;320:1560–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arterburn D, Wellman R, Emiliano A, et al.; PCORnet Bariatric Study Collaborative . Comparative effectiveness and safety of bariatric procedures for weight loss: A PCORnet cohort study. Ann Intern Med 2018;169:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McTigue KM, Wellman R, Nauman E, et al.; PCORnet Bariatric Study Collaborative . Comparing the 5-year diabetes outcomes of sleeve gastr ectomy and gastric bypass: the National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA Surg 2020;155:e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van de Laar AWJM, Acherman YIZ. Weight loss percentile charts of large representative series: a benchmark defining sufficient weight loss challenging current criteria for success of bariatric surgery. Obes Surg 2014;24:727–734 [DOI] [PubMed] [Google Scholar]
- 6. van de Laar AW, van Rijswijk AS, Kakar H, Bruin SC. Sensitivity and specificity of 50% excess weight loss (50%EWL) and twelve other bariatric criteria for weight loss success. Obes Surg 2018;28:2297–2304 [DOI] [PubMed] [Google Scholar]
- 7. Coleman KJ, Toussi R, Fujioka K. Do gastric bypass patient characteristics, behavior, and health differ depending upon how successful weight loss is defined? Obes Surg 2010;20:1385–1392 [DOI] [PubMed] [Google Scholar]
- 8. Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess 2011;15:1–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med 2020;383:721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pessoa BM, Browning MG, Mazzini GS, et al. Factors mediating type 2 diabetes remission and relapse after gastric bypass surgery. J Am Coll Surg 2020;230:7–16 [DOI] [PubMed] [Google Scholar]
- 13. Tu Y, Pan Y, Han J, et al. A total weight loss of 25% shows better predictivity in evaluating the efficiency of bariatric surgery. Int J Obes 2021;45:396–403 [DOI] [PubMed] [Google Scholar]
- 14. Sherf-Dagan S, Schechter L, Lapidus R, Sakran N, Goitein D, Raziel A. Perceptions of success in bariatric surgery: a nationwide survey among medical professionals. Obes Surg 2018;28:135–141 [DOI] [PubMed] [Google Scholar]
- 15. Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg 2020;271:201–209 [DOI] [PubMed] [Google Scholar]
- 16. Coleman KJ, Haneuse S, Johnson E, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care 2016;39:1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thereaux J, Lesuffleur T, Czernichow S, et al. Association between bariatric surgery and rates of continuation, discontinuation, or initiation of antidiabetes treatment 6 years later. JAMA Surg 2018;153:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis 2014;10:396–403 [DOI] [PubMed] [Google Scholar]
- 19. Coleman KJ, Fischer H, Arterburn DE, et al. Effectiveness of gastric bypass versus gastric sleeve for cardiovascular disease: protocol and baseline results for a comparative effectiveness study. JMIR Res Protoc 2020;9:e14936. DOI: https://doi.org/10.2196/14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute of Diabetes and Digestive and Kidney Disorders . Potential Candidates for Bariatric Surgery. Accessed 1 October 2019. Available from https://www.niddk.nih.gov/health- information/weight-management/bariatric-surgery/ potential-candidates
- 21. SUPREME-DM - Home . Accessed 15 November 2016. Available from https://www.supreme-dm.org/index.html
- 22. Nichols GA, Schroeder EB, Karter AJ, et al.; SUPREME-DM Study Group . Trends in diabetes incidence among 7 million insured adults, 2006-2011: the SUPREME-DM project. Am J Epidemiol 2015;181:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selby JV, Peng T, Karter AJ, et al. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care 2004;10:163–170 [PubMed] [Google Scholar]
- 24. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47:626–633 [DOI] [PubMed] [Google Scholar]
- 25. Aminian A, Brethauer SA, Kashyap SR, Kirwan JP, Schauer PR. DiaRem score: external validation. Lancet Diabetes Endocrinol 2014;2:12–13 [DOI] [PubMed] [Google Scholar]
- 26. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health out comes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg 2018;153:427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–551 [DOI] [PubMed] [Google Scholar]
- 28. Koffman L, Levis AW, Arterburn D, et al. Investigating bias from missing data in an electronic health records-based study of weight loss after bariatric surgery. Obes Surg 2021;31:2125–2135 [DOI] [PubMed] [Google Scholar]
- 29. Wölnerhanssen BK, Peterli R, Hurme S, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS). Br J Surg 2021;108:49–57 [DOI] [PubMed] [Google Scholar]
- 30. Sha Y, Huang X, Ke P, et al. Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for type 2 diabetes mellitus in nonseverely obese patients: A systematic review and meta-analysis of randomized controlled trials. Obes Surg 2020;30:1660–1670 [DOI] [PubMed] [Google Scholar]