Abstract
Background
Inhaled bronchodilators form the mainstay of treatment for acute exacerbations of COPD. Two types of agent are used routinely, either singly or in combination: anticholinergic agents and beta2‐sympathomimetic agonists.
Objectives
To assess the effect of anti‐cholinergic agents on lung function and dyspnea in patients with acute exacerbations of COPD, compared with placebo or short‐acting beta‐2 agonists.
Search methods
We searched the Cochrane Airways Group Specialised Register using pre‐specified terms. We also searched references listed in each included trial for additional trial reports. Searches were current as of October 2005 and will be updated annually.
Selection criteria
We included studies if the participants were adult patients with a known diagnosis of COPD and had symptoms consistent with criteria for acute exacerbation of COPD. All randomized controlled trials that compared inhaled ipratropium bromide or oxitropium bromide to appropriate controls were considered. Appropriate control treatments included placebo, other bronchodilating agents, or combination therapies. Studies of acute asthma or ventilated patients were excluded.
Data collection and analysis
Two reviewers assessed all trials that appeared to be relevant who independently selected trials for inclusion. Differences were resolved by consensus.
Main results
Four trials compared the short‐term effects of ipratropium bromide versus a beta2‐agonist. Short‐term changes in FEV1 (up to 90 minutes) showed no significant difference between beta2‐agonist and ipratropium bromide treated patients. The differences were similar among the studies and when combined: Weighted Mean Difference (WMD) 0.0 liters (95% Confidence Interval (95% CI) ‐0.19 to 0.19). There was no significant additional increase in change in FEV1 on adding ipratropium to beta2‐agonist: WMD 0.02 liter (95% CI ‐0.08 to 0.12). Long‐term effects (24 hours) of the ipratropium bromide and beta2‐agonist treatment combination were similar: WMD 0.05 liters (95% CI ‐0.14 to 0.05).
Neither of two studies found significant changes in PaO2, either short‐ or long‐term, with ipratropium versus beta‐agonist, although one showed an increase in PaO2 in subjects receiving ipratropium bromide at 60 minutes. Adverse drug reactions included dry mouth and tremor.
Authors' conclusions
There was no evidence that the degree of bronchodilation achieved with ipratropium bromide was greater than that using a short‐acting beta2‐agonist. The combination of a beta2‐agonist and ipratropium did not appear to increase the effect on FEV1 more than either used alone.
Plain language summary
Anticholinergic bronchodilators versus beta2‐sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease
Shortness of breath is the main complaint of persons with chronic obstructive pulmonary disease (COPD). This symptom worsens during an exacerbation or 'flare' of COPD. Trials comparing ipratropium bromide versus beta‐agonists showed no significant difference in short‐term or long‐term effects (24 hours) on ease of breathing. Side effects of these drugs were reported by only a minority of patients and include dry mouth and tremor, and a 'strange feeling' after drug administration.
Background
Dyspnea secondary to airflow limitation is the main complaint of persons with chronic obstructive pulmonary disease (COPD). The cause of chronic airflow limitation is a combination of the accumulation of mucous in the airways and hypertrophy/constriction of bronchial smooth muscle. Additionally, inflammatory cells infiltrate the airways and contribute to mucous secretion and airway edema. Mucous accumulation in COPD is due to the increased number and size of mucous glands and goblet cells in the airway wall. These cell types are stimulated to increase the production and release of mucous through mediators of inflammation. Smooth muscles surround the bronchi; when they are stimulated, their contraction causes physical narrowing of the airways. Hypertrophy of the smooth muscle further decreases the diameter of the effective airway. Control of these functions (or dysfunction) is the central airway muscarinic receptor. It is here that the mediators bind and create the narrowed/obstructed airway.
The natural history of moderate to severe COPD is punctuated by acute exacerbations, in which worsening symptoms of dyspnea and an increase in amount or purulence of sputum may be accompanied by chest discomfort, fever, and other constitutional symptoms. The increasing dyspnea of acute exacerbations is due to an increase in mucous production, inflammation and broncho‐constriction, leading to further compromising of airflow limitation. Acute exacerbations are often the reason that those with mild disease seek medical care. Treatment is often multifaceted, with antibiotics, corticosteroids and bronchodilators.
Anti‐cholinergic receptor blockers act by preventing the stimulation of muscarinic receptors. By blocking the activity of the receptor, the level of bronchoconstriction and mucous production is lessened. This review will evaluate the evidence for using anti‐cholinergic medications to achieve relief of dyspnea during COPD exacerbations. Currently available inhaled anticholinergic bronchodilators include ipratropium, oxitropium and tiotropium bromide (only ipratropium bromide is available in the US at this time).
Objectives
To describe and assess the effect of anti‐cholinergic agents such as ipratropium and oxitropium bromide, administered by inhalation, on ventilatory function and dyspnea in patients with acute exacerbation of COPD.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials comparing inhaled ipratropium bromide. oxitropium bromide or tiotropium bromide with appropriate control where other co‐interventions are standardized. Studies of acute asthma were excluded. Appropriate control treatments include placebo, other bronchodilating agents, or combination therapies.
Types of participants
Studies were included if the participants are adult patients with a known diagnosis of COPD and have symptoms of acute exacerbation increased shortness of breath, increased sputum production, or sputum purulence consistent with criteria for defining/diagnosing acute exacerbation of COPD (Anthonisen 1987, Murphy 1992).
Studies of patients receiving ventilatory assistance were excluded. We assumed that most cases of exacerbation were due to infection, but did not limit the target population to this suspected etiology. We expected that patients with acute exacerbations of COPD could have various co‐morbidities (such as congestive heart failure or pulmonary embolus) or complications of COPD (e.g. pneumothorax) as the etiology of exacerbation of their symptoms. We required that studies in some way assured that patients with other reasons for these symptoms were not studied.
Types of interventions
Any studies employing ipratropium or oxitropium bromide administered by inhalation via nebulizer, or metered dose inhaler.
Types of outcome measures
Outcome measures included: (1) Lung function measurements, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) or peak expiratory flow rate (PEFR) (2) Arterial blood gas measurements (3) Symptom scores The above short term outcomes will be assessed as post‐treatment data or change from pre‐ to post‐treatment in two time frames ‐ within 90 minutes and greater than one day.
Outcomes also to be assessed: (4) Health status or quality of life assessments (5) Functional capacity ‐ timed walking tests, endurance tests (6) Health services utilization measures, duration of hospitalization or time to emergency room discharge (7) Hospital readmission or return to emergency room (8) Mortality (9) Adverse drug reactions.
Search methods for identification of studies
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'COPD' were searched using the following terms:
(Anticholinerg* and inhal*) or (ipratropium or oxitropium or tiotropium or darifenacin or rispenzipine or revatropate or bronchodilat* or atrovent or combivent)
The most recent search was run in October 2005 and will be updated annually.
We then searched references listed in each included trial for additional trial reports.
Data collection and analysis
Selection of studies
Two reviewers assessed all trials that appear to be relevant were who independently selected trials for inclusion. Differences were resolved by consensus.
Assessment of risk of bias in included studies
All trials were entered and scored using the following principles: Grade A ‐ Double blind Grade B ‐ Single blind Grade C ‐ Obviously not blinded or not known
The internal validity of individual trials was assessed using the scale devised by Jadad 1996, which was operationalized as follows: (1) Was the study described as randomized? (1 = yes; 0 = no) (2) Was the method of randomization well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate) (3) Was the study described as double‐blind? (1 = yes; 0 = no) (4) Was the method of double‐blinding well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate) (5) Was there a description of withdrawals and dropouts sufficient to determine the number of patients in each treatment group entering and completing the trial? (1 = yes; 0 = no).
The external validity of the trials was described using the following features:
(1) Validity of the underlying COPD diagnosis: (a) Is COPD diagnosis based on spirometry‐based criteria (e.g. ATS (ATS 1995) or Siafakas (Siafakas 1995))? (b) Is the baseline stable FEV1 of study population described?
(2) Validity of diagnosis of AECB (Acute exacerbation of chronic bronchitis): Does the definition of AECB include at least two of the following: increased sputum production, increased sputum volume, and increased dyspnea?
(3) Characterization of severity of AECB: Does the study describe the severity of AECB at enrollment based upon at least two of the following domains: (a) Mental status change (b) Work of breathing (e.g. respiratory rate or use of accessory muscles)? (c) Ventilatory status (e.g. FEV1 or PEFR, O2 saturation or pO2 and pCO2)? (4) Duration of follow‐up: Is there an outcome assessment at 24 hours or longer?
Unit of analysis issues
For crossover studies, we only analyzed first arm data (before the change over). This was to prevent the possibility of effects from the previously administered drug contaminating the second set of results. Lloberes 1988 used a paired t‐test to compare the change in FEV1 seen between drugs administered in each arm of the protocols. Because we did not use information from the second arm, the paired t‐tests were not of value. We utilized the raw data (actual change in FEV1 +/‐ a calculated standard deviation) in our analyses.
Assessment of heterogeneity
We tested the size of the treatment effects for homogeneity. If we found that the study estimates were homogeneous, they were combined using a fixed effect model.
Data synthesis
We combined trials using Review Manager software.
Subgroup analysis and investigation of heterogeneity
When significant heterogeneity was present, we made an attempt to explain the differences based on the clinical characteristics of the included studies. Clinically dissimilar studies could not be statistically combined. However, when a group of studies with heterogeneous results appeared clinically similar, we combined the study estimates using a random effects model.
Planned subgroup analyses included:
(1) A comparison of studies using concurrent medication vs those that did not. Such co‐interventions may include beta2 agonists or other bronchodilating agents, mucus clearing therapies, or corticosteroids. (2) A comparison of studies by severity of exacerbation of included patients or degree of airways obstruction. (3) A comparison of studies utilizing delivery of bronchodilators by metered dose inhaler versus nebulizer.
Results
Description of studies
Results of the search
A combined database of 1267 entries was created from the results of MEDLINE and COPD‐RCT register literature searches. After excluding articles that focused on pediatric patients and/or asthma there were 342 entries for further evaluation. We then excluded studies in which the study population had stable COPD. Twenty‐two studies were selected for full‐text evaluation for inclusion in the review. Of those 22 reports, only nine were RCTs that met the criteria for inclusion (See characteristics of included/excluded studies). An updated search in October 2005 did not identify any further studies for inclusion.
We identified no placebo‐controlled comparisons of ipratropium bromide. Four studies permitted a direct comparison of ipratropium to an inhaled beta2‐agonist (Backman 1985; Karpel 1990; Lloberes 1988; Rebuck 1987). Five studies compared a combination of ipratropium and a short‐acting beta2‐agonist to use of a beta2‐agonist alone (Lloberes 1988; Moayyedi 1995; Patrick 1990; O'Driscoll 1989; Rebuck 1987; Shretha 1991). Details of these studies are given in Characteristics of included studies.
Risk of bias in included studies
All but one of the studies (Lloberes 1988) was randomized. Instead, they tested the same 13 participants on three different occasions when admitted for AECB. Each time, the subjects received drugs by a different protocol and the protocols were compared to each other. The participants were blinded as to the medications being administered. Rebuck 1987 used a computer generated randomization schedule, while Cydulka 1995 used a block‐design scheme. Patrick 1990 used a random number sequence known only to the pharmacist dispensing the drugs. A random drawing of pieces of paper was the method employed by Shretha 1991, while O'Driscoll 1989 used the subjects year of birth to determine which drug a study enrollee would receive. In those studies that added ipratropium to a beta‐agonist, placebos were used for those not randomized to the study drug (ipratropium). The remaining studies did not detail the method of randomization in their methods.
Three studies were either single‐blinded (Lloberes 1988, Moayyedi 1995) or not blinded at all (Rebuck 1987). The remaining six studies were designed in a double‐blind format. ATS criteria were utilized for the diagnosis of COPD and COPD exacerbations in almost all of the included studies. In the two studies that did not employ ATS criteria, Backman 1985 used the criteria set by the British MRC and Rebuck 1987 did not state the diagnostic criteria used in its evaluation. Severity of disease was described using FEV1 and/or PEFR at the time of entry into the study. Spirometry was overwhelmingly the outcome measurement of choice.
The following parameters were used to measure efficacy: change in FEV1, PEFR, PaO2, and PaCO2. Because all the participants were admitted to hospitals for care of severe exacerbations, the participant population tended to include those patients with more severe disease (i.e. FEV1 < 1 liter). There was also a lack of standardization of co‐interventions in the studies. All allowed the use of additional medications, such as corticosteroids, theophylline, and antibiotics; however, use of these interventions was left to the discretion of the admitting physician.
Effects of interventions
The most common outcome reported was FEV1. All but two studies reported short‐term (up to 90 minutes) changes in FEV1. The exceptions, Moayyedi 1995, and Patrick 1990, described changes in FEV1 at 24 hours after starting treatment. Both of these studies tested the potential benefits of adding ipratropium bromide to a treatment regimen already including a beta2‐agonist.
Four trials compared the short‐term effects of ipratropium bromide versus beta2‐agonists. Short‐term changes in FEV1 (up to 90 minutes) showed no significant difference between beta‐agonists and ipratropium bromide treated patients. The differences were similar among the studies and when combined resulted in a Weighted Mean Difference of 0.0 liters with a 95% confidence interval range from ‐0.19 liters to +0.19 liters.
There were five studies describing the change in FEV1 after adding ipratropium to a beta2‐agonist treatment regimen. Three studies, looking at short‐term effects, found no advantage to adding ipratropium to beta2‐agonist treatment. The 95% confidence range for the Weighted Mean Difference in this group is ‐0.08 liters to 0.12 liters. Long‐term effects (24 hours) of the ipratropium bromide and beta2‐agonist treatment combination confirm this finding with a Weighted Mean Difference of ‐0.05 liters (95%CI ‐0.14, 0.05).
The data on changes in blood gases were limited. Only two of the nine studies (Backman 1985, Karpel 1990) reported changes in hypoxia measures. Neither found significant changes in PaO2, either short‐ or long‐term. Karpel 1990 showed an increase in PaO2 in subjects receiving ipratropium bromide at 60 minutes. At the same time, those receiving metaproterenol showed a decrease in PaO2. However, this difference was gone after 90 minutes, when levels returned to baseline.
Adverse drug reactions were reported by only a minority of the included studies (n = 4). The reaction described dry mouth and tremor (Karpel 1990, Rebuck 1987), and a 'strange feeling' after drug administration (Backman 1985). O'Driscoll 1989 and Karpel 1990 specifically looked at hemodynamic changes and found no differences between the treatment groups.
Discussion
There was no evidence that the degree of bronchodilation achieved with ipratropium bromide was greater than that using a short‐acting beta2‐agonist. The combination of a beta2‐agonist and ipratropium did not appear to increase the effect on FEV1 more than either used alone.
Only a few studies have been conducted in populations of patients with acute exacerbations of COPD. The studies available for this analysis describe both short‐ and long‐term impact on ventilatory function. The analysis of these studies does not show that ipratropium bromide has a greater effect than beta2‐agonists. The results showed similar effects and the combined estimate of no difference in the studies looking at short term effects of ipratropium versus beta2‐agonist. Studies looking at the effects of combination treatment also found no statistically significant differences.
A major limitation of this analysis is the small number of studies performed in this patient population. The few studies that met criteria for inclusion had a scarcity of subjects. Also, two studies reported variances only for pre‐and post‐treatment scores, rather than the observed pre‐post changes (Karpel 1990, Lloberes 1988). We then had to estimate the variance of changes with the sum of the pre‐ and post‐treatment variances, which overestimates the true variance and decreases the power of our analysis.
Authors' conclusions
Implications for practice.
Ipratropium bromide appears to result in short‐term improvement of FEV1 and PEFR. The effect was at least equivalent to beta2‐agonists and combined treatment appears to be no more effective than the use of either as a single agent. In all studies, the use of a bronchodilator improved FEV1 and PEFR. Symptomatic benefits were not assessed.
Implications for research.
Few studies of bronchodilating drugs have been performed in populations with acute exacerbation of COPD. Most studies included one or more additional medications, usually a corticosteroid, an antibiotic, or both, which may confound the results. In order to determine the true effect of anti‐cholinergic drugs on the short and long term outcomes of patients with acute exacerbations of COPD, we would ideally conduct a large, multi‐center RCT with strict control of co‐interventions . Studies should measure both the objective changes (i.e. FEV1, PEFR, PaO2) and the more subjective patient‐oriented outcomes such as quality of life, dyspoena, symptom scores. This may not be possible because of ethical constraints in trying to perform research on therapies that have already become a part of standard and accepted patient care.
Feedback
Included studies
Summary
It is said in the review that 9 studies were included. However only eight were characterized in the table (Backman 1985 was not), 1 study concerning glycopyrrolate not ipratropium (Cydulka 1995) was not included into the analysis (the authors did not mention it), the study of O'Driscoll 1989 was included only into the analysis of safety and not of efficacy. The objective was to compare the effect of anti‐cholinergic agents with beta‐2 agonists, in Karpel 1990 metaproterenol was used which is beta‐agonist (not beta‐2 agonist).
Reply
Dr. Lesniak raises a number of issues:
1. The exclusion of the Backman (1985) study from the "characteristics of included studies" table was an oversight on our part. Thank you for pointing this out. It will be remedied at the time of the next update. [This has now been addressed]
2. Although our inclusion criteria state that only ipratropium or oxitropium interventions are included in the review we describe the Cydulka (1995) study because its intervention glycopyrrolate, is an anticholinergic drug which is the scope implied by the review's title "Anti‐cholinergic bronchodilators versus beta2‐sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease". We will update the inclusion criteria in the next update of the review.
3. The O'Driscoll study (1989) was included only in the safety analysis because the efficacy results did not include any variance data (standard deviation or SEM). The graph depicted a standard error bar, however, it was too poorly resolved to provide an accurate estimate of the SEM. Had variance data been available, the peak flow outcome from O'Driscoll could have bee combined with those from Backman (1985) which also included peak flow changes. Our safety analysis was based on the number of patients having an event, which did not require mean deviation data.
4. Karpel 1990: Metaproterenol is not a strict beta‐2 agonist; it has both beta‐1 and beta‐2 agonist properties. It has been used as a bronchodilator for a long time. Many of the more currently used short‐acting beta‐2 agonists were given approval for use based on efficacy studies versus metaproterenol. This is why we felt comfortable including the study in our analysis. When this study is removed from the analysis, the results do not change. (WMD ‐0.01 (95% CI ‐0.24 to 0.22 without Karpel 1990).
Contributors
Lesniak W
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 31 August 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the following persons, without whom this project would not be possible: Ms. Karen Blackhall for obtaining all articles reviewed for this analysis; Dr. John White for his editing skills, and Mr. Steve Milan for his patience. Thanks also to Kirsty Olsen who has copy edited this review.
Data and analyses
Comparison 1. Ipratropium vs. Beta‐2 Agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV‐1 at 90 minutes | 4 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.19, 0.19] |
1.1. Analysis.
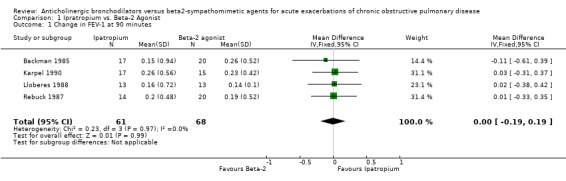
Comparison 1 Ipratropium vs. Beta‐2 Agonist, Outcome 1 Change in FEV‐1 at 90 minutes.
Comparison 2. Addition of Ipratropium to Beta‐2 agonist treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV‐1at 90 minutes | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.08, 0.12] |
| 2 Change in FEV‐1 at 24 hours | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.14, 0.05] |
2.1. Analysis.
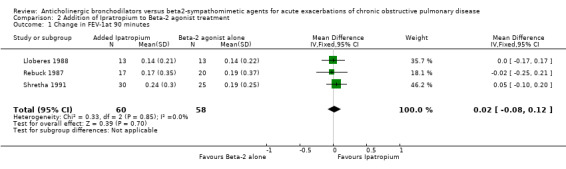
Comparison 2 Addition of Ipratropium to Beta‐2 agonist treatment, Outcome 1 Change in FEV‐1at 90 minutes.
2.2. Analysis.
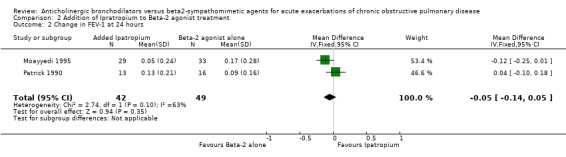
Comparison 2 Addition of Ipratropium to Beta‐2 agonist treatment, Outcome 2 Change in FEV‐1 at 24 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Backman 1985.
| Methods | Design: SPPG
Dates: N/S
Location: Finland
Assessment period: 7 days External validity: 2/5 COPD dx‐ COPD bl‐ AECB dx‐ AECB sev+ Tx duration+ Internal validity: 3/5 r, db, dd |
|
| Participants | N = 40
Setting: Inpatient (hospital)
Inclusion (COPD): Chronic bronchitis, according to criteria of the British Medical Council [no ref given] Inclusion (AcEx): Acute exacerbation of chronic bronchitis (no diagnostic criteria given) Exclusion: N/S Smoking history: N/S Baseline stable FEV1: N/S FEV1 before treatment on day 1: Ipratropium, 0.73 ± 0.35 (SD) L; fenoterol, 0.93 ± 0.56 L (n.s.; p‐value not reported) Age: Range: 48 to 77 Sex: 36 M, 4 F Race: N/S Dropouts: 1/40 pts (2.5%), treatment group N/S |
|
| Interventions | Experimental: Nebulized ipratropium 0.2 mg, 3x/day Control: Nebulized fenoterol 0.5 mg, 3x/day Co‐interventions: Pts with signs of an acute infection (fever, pneumonic infiltration, and positive sputum bacteriology) were treated with antibiotics before the start of the trial; pts taking beta2‐receptor stimulants (n = 16) stopped them at least 8 hrs before starting the trial; all pts received daily physical treatment; otherwise, pts continued their usual regimens, which included oral theophyllamine (n = 7), combination treatment with ephedrine and hydroxyzine (n = 4), prednisolone (n = 2), and no continuous treatment (n = 7) |
|
| Outcomes | FEV1: Measured on the 1st and 7th day immediately before and 5, 10, 15, 30, 60, 120, and 240 min after the morning study drug administration. Investigators compared mean FEV1 scores for identical timepoints on days 1 and 7. Blood gases: Arterial PaO2 and PaCO2 measured on 1st and 7th day. Investigators analyzed change from day 1 to 7. There were no significant differences between the two treatment groups for any of the outcomes measured (no between‐group p‐values reported). Dropouts: 1/40 pts (2.5%), treatment group N/S | |
| Notes | FEV1 improvements were small with both interventions. In the ipratropium group (n = 17), mean scores at 15 min (p < 0.02) and 30 min (p < 0.05) post‐treatment were significantly higher on the 7th day than on the 1st; no significant differences were noted between the 1st and 7th day at 0, 5, 10, 60, 120, or 240 min. In the fenoterol group (n = 20), mean scores at 60 min post‐treatment were significantly higher on the 7th day than on the 1st (p < 0.05); otherwise, no significant differences were observed between the 1st and 7th day. Mean pre‐treatment FEV1 scores on days 1 and 7 were: (a) Ipratropium (n = 17): Day 1, 0.73 ± 0.35 (SD) L; Day 7, 0.82 ± 0.34 L (b) Fenoterol (n = 20): Day 1, 0.93 ± 0.56 L; Day 7, 1.02 ± 0.66 L Blood gases/oximetry: Investigators stated that there were no significant changes in PaO2 or PaCO2 from day 1 to day 7 in either group, but did not report any data. Adverse events: 1/19 pts (5%) taking ipratropium and 1/20 (5%) taking fenoterol complained of a "strange feeling" Two pts in the ipratropium group who did not withdraw prematurely were unable to perform spirometry and so were not included in the FEV1 analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cydulka 1995.
| Methods | Design: SPPG Dates: Feb '91 to Feb '92 Location: US Assessment period: 3 hrs from administration of study med External validity: 2/5 COPD dx+ COPD bl‐ AECB dx‐ AECB sev+ Tx duration‐ Internal validity: 4/5 r+,db+, dnd | |
| Participants | N = 57 Setting: Pts presenting to ED (urban teaching hospital) Inclusion (COPD): Pts with self‐reported diagnosis of COPD or histories consistent with COPD, as defined by ATS Inclusion (AcEx): Acute respiratory distress plus cough, wheezing, or both Exclusion: Personal history of asthma; initial FEV1 readings > 75% of predicted normal value; clinical or radiographic evidence of pneumothorax, pneumonia, decompensated CHF, or history of lung cancer; inability to perform spirometry; having received prehospital aerosol treatment; contraindications to study med; age < 18; pregnant; nursing mothers; prisoners Smoking history: 46 pack‐yrs (n = 51 current or past smokers) Baseline stable FEV1: N/S FEV1 at admission: Glycopyrrolate grp, 0.89 ± 0.36 (SD) L; placebo grp, 0.93 ± 0.42 L (p = 0.05) Age: 61.5 Sex: N/S Race: N/S | |
| Interventions | Experimental: Single dose of aerosolized glycopyrrolate 2 mg, administered as first aerosol Control: Placebo Co‐interventions: Nebulized albuterol every hr for 3 aerosols; oxygen by nasal cannula | |
| Outcomes | Spirometry performed at baseline and 1 hr after first and third aerosolsFEV1: (a) Mean absolute values at baseline, 1 hr, and 3 hrs(b) Mean % change in absolute value from baseline to 1 and 3 hrs. Percentage of pts admitted to hospital Note: No. of pts in each treatment grp unclear. FEV1: (a) No significant differences between mean pre‐ and post‐treatment scores (± SD) for either grp: Glycopyrrolate grp, 0.89 ± 0.36 and 1.31 ± 0.61 (p = 0.11); placebo grp, 0.93 ± 0.42 and 1.09 ± 0.57 (p = 0.19). No between‐grp results reported.(b) Repeated‐measures analysis showed glycopyrrolate significantly better than placebo for mean % change (increase) in absolute FEV1 values from pre‐ to post‐treatment: Glycopyrrolate grp, 56.0% ± 56.1%; placebo grp, 19.3% ± 40.9% (p < 0.01).Percentage of pts admitted to hospital: Overall, 30/57 pts (53%) admitted. No significant differences between grps: Glycopyrrolate grp, 50% admitted vs. 57% in placebo grp (p = 0.05) Adverse events: Glycopyrrolate + albuterol grp (27% of pts): Dry mouth, headache, hypotension, palpitations. Albuterol‐alone (placebo) grp (15% of pts): Tremors, anxiety, palpitations. Dropouts: Not described | |
| Notes | FEV1 results accompanied by large SDs Small sample size yielded low power to detect differences in AEs between grps Within‐pt analysis found difference missed by comparison of grp means | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Karpel 1990.
| Methods | Design: CrOv Dates: N/S Location: US Assessment period: 90 min for each phase of crossover External validity: 3/5 COPD dx+ COPD bl‐ AECB dx+ AECB sev+ Tx duration‐ Internal validity: 5/5 r+, db+, dd | |
| Participants | N = 32 Setting: ED and pulmonary clinic Inclusion (COPD): Chronic bronchitis or emphysema as defined by the ATS Committee on Diagnostic Standards for Nontuberculous Respiratory Disease (1962) Inclusion (AcEx): Increasing dyspnea and/or change in sputum production newly occurring in previous 24 hrs; FEV1 < 60% of predicted Exclusion: Asthma; baseline pulmonary function tests which failed to show persistent airway obstruction; any acute concomitant medical problem; respiratory acidosis with pH < 7.3; need for continuous oxygen therapy; need for medications other than those included in study protocol; use of inhaled b‐agonist or ipratropium in previous 6 hrs Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: Ipratropium first: 0.62 ± 0.08 (SEM) L; metaproterenol first: 0.69 ± 0.06 L Age: 61.6 Sex: N/S Race: N/S | |
| Interventions | Experimental: Ipratropium, single dose of 3 puffs (54 mg) administered by MDI Control: Metaproterenol, single dose of 3 puffs (1.95 mg) administered by MDI Co‐interventions: None Crossover design: Pts treated with one of the two study meds initially (phase 1), then crossed over to treatment with the other after 90 min (phase 2); no washout; no information on possible carry‐over effect | |
| Outcomes | FEV1: Measured at entry and at 30, 60, and 90 min after administration of study med in both phases of crossover; investigators analyzed change in mean FEV1 from 0 to 90 min in phase 1Blood gases: Recorded only for the first 20 pts randomized into the study; measured at entry and at 30, 60, and 90 min after administration of study med in phase 1 only; investigators analyzed mean changes in PaO2, PaCO2, and pH from 0 to 30 min and 0 to 90 min
Percentage of pts admitted to hospital FEV1: In phase 1 of the trial, mean FEV1 (± SEM) improved significantly in both treatment groups from 0 to 90 min. For ipratropium (n = 17), scores improved 25%, from 0.62 ± 0.08 to 0.88 ± 0.11 (p< 0.01). For metaproterenol (n = 15), the improvement was 18%, from 0.69 ± 0.06 to 0.92 ± 0.09 (p < 0.01). There was no significant difference between the two groups for this outcome (p > 0.05).For phase 2 of the trial, investigators reported only that there was no further improvement in FEV1 in either group. Blood gases: At 30 min, there was a significant (p < 0.05) rise in PaO2 with ipratropium (n = 10) (5.8 ± 3.0 [SEM]), and a significant (p < 0.05) decine in PaO2 with metaproterenol (n = 10) (‐6.2 ± 1.2). At 90 min, these changes were no longer significant.There were no other significant changes in PO2, PCO2, or pH during phase 1. Percentage of pts admitted to hospital: 3/17 pts (18%) from the ipratropium‐first group, 2/15 pts (13%) from the metaproterenol‐first group. Adverse events: Recorded for phase 1 only. Ipratropium: Nervousness (2 pts), tremors (2), dry mouth (1), palpitations (1), headache (1). Metaproterenol: Palpitations (3 pts), nervousness (3), tremors (2), dry mouth (1), headache (1).Dropouts: 0 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lloberes 1988.
| Methods | Design: CrOv Dates: N/S Location: Spain Assessment period: 4 days; 3‐day treatment period after an initial 24‐hr washout period; 3 crossover periods (protocols), with 2 different treatment phases in each period (see "crossover design") External validity: 4/5 COPD dx+ COPD bl+ AECB dx+ AECB sev+ Tx duration‐ Internal validity: 0/5 nr, ndb, dnd | |
| Participants | N = ? (see "Notes" column) Setting: Hospitalized pts drawn from outpt clinic where they received regular bronchodilator treatment (including beta2‐adrenergics and oral theophylline) Inclusion (COPD): Chronic bronchitis, emphysema, or both, and evidence of severe airflow obstruction, with FEV1 < 1 L (determined by clinical diagnosis) Inclusion (AcEx): 'Acute exacerbation' defined as "an increase of symptoms (breathlessness and mucus secretion) and gasometric deterioration, attributed to a bronchial infection" Exclusion: Episodic attacks of wheezing indicative of bronchial asthma; lung function reversibility of > 15% after inhalation of 2 puffs of albuterol while clinically stable Smoking history: N/S Baseline stable FEV1: 0.82 ± 0.19 (SD) L (range, 0.54‐1.1) FEV1 at admission: 0.64 ± 0.11 (SD) L (range, 0.35‐1.01) Age: 65 (range, 50‐75) Sex: N/S Race: N/S | |
| Interventions | Interventions:
Crossover Design: After the initial AcEx phase had been stabilized, bronchodilator treatment was withheld for 24 hrs (washout); pts (n = 13) then completed 3 crossover periods (protocols), 1 per day, with 2 phases per period. During phase 1, pts took an initial treatment every 30 min until a bronchodilatation phase was reached; during phase 2, pts were randomized to 1 of 2 different treatments for 45 min. Time between protocols was not specified. Description of Protocols: Protocol A: Inhaled albuterol 200 :g (2 puffs), administered every 30 min until bronchodilatation plateau reached (n = 13); then pts randomized to receive either ipratropium bromide 40 mg (n = 5), using a metered‐dose inhaler, or a loading dose of aminophylline IV (n = 8), 5.6 mg/kg in 30 min followed by a continuous infusion of 0.9 mg/kg‐hr; both treatments assessed at 45 min Protocol B: Inhaled ipratropium bromide 40 :g (n = 13), administered every 30 min, using a metered‐dose inhaler, until bronchodilatation plateau reached; then pts randomized to receive either a single dose of albuterol (amount and route of administration N/S) (n = 7) or aminophylline IV (n = 6), administered as described above; both treatments assessed at 45 min Protocol C: Aminophylline IV (n = 13), administered using loading dose of 5.6 mg/kg in 30 min followed by a continuous infusion of 0.9 mg/kg‐hr; administered every 30 min until bronchodilatation plateau reached; then pts randomized to receive either a single dose of albuterol (amount and route of administration N/S) (n = 5) or a single dose of ipratropium (amount and route of administration N/S) (n = 8); both treatments assessed at 45 min Co‐interventions: Corticosteroids and antibiotics were permitted |
|
| Outcomes | Forced spirometric measurements (by Vitalograph) were taken on each of 3 days before initial study drug was administered and at 45 min after 2nd study drug was administered FEV1: (a) Absolute (mean ± SEM) and percentage pre‐ to post‐treatment changes in baseline FEV1 for each protocol; 3‐way ANOVA used to analyze changes among the 3 protocols (b) Absolute and percentage changes in FEV1 after second drug was administered, compared with maximal bronchodilatation FEV1 obtained with the first treatment (for each protocol); paired t‐test used to analyze changes from first to second drug FEV1: (a) There were no significant differences in baseline FEV1 values among the three drugs (no p‐values reported). All three agents improved significantly from baseline to the maximal treatment effect achieved at end of phase 1 of each protocol (no p‐values reported), but there were no signficant differences between the increases achieved by ipratropium, albuterol, or aminophylline alone (no p‐values reported). Absolute and percentage change increases in FE |
|
| Notes | Low quality score (0) Not clear how many pts entered trial or if those selected (n = 13) were chosen randomly; during phase 2 of each protocol, however, pts were randomized to receive 2 different treatments during each protocol Not clear whether order of protocols was randomized |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Moayyedi 1995.
| Methods | Design: SPPG Dates: N/S Location: UK Assessment period: To discharge (mean time to discharge, 11.1 days; 19 pts discharged on day 14 or later) External validity: 3/5 COPD dx+ COPD bl‐ AECB dx‐ AECB sev+ Tx duration+ Internal validity: 4/5 r, db+, dd | |
| Participants | N = 70 Setting: Hospital Inclusion (COPD): Age > 45 yrs; smoking history > 10 pack‐yrs; baseline stable FEV1 < 65% of predicted; > 3‐yr history of exertional dyspnea resulting from respiratory disease; previous diagnosis of non‐asthmatic COPD Inclusion (AcEx): Clinical diagnosis of acute exacerbation of COPD (no details provided) Exclusion: Regular home use of nebulized bronchodilators; history suggestive of asthma; peripheral eosinophilia of > 10%; > 20% (at least 200 ml) reversibility of FEV1 to 400 mg of inhaled albuterol at discharge Smoking history: 51.7 pack‐yrs Baseline stable FEV1: N/S FEV1 on Day 1: 0.78 ± 0.41 (SD) L in the albuterol + ipratropium group and 0.77 ± 0.34 in the albuterol group Age: 69.2 Sex: N/S Race: N/S | |
| Interventions | Interventions: Experimental: Nebulized albuterol 5 mg + ipratropium 500 mg, 4x/day, given as a mixture Control: Nebulized albuterol 5 mg, 4x/day Co‐interventions: Prescribed at discretion of attending physician; included aminophylline IV, steroids IV, and antibiotics | |
| Outcomes | FEV1: Spirometric values were measured once on days 1, 3, and 7, and then weekly and on day of discharge. All readings were taken just before the nebulizer treatment at 18.00 hrs. NB: Not measured immediately on admission ‐ day 1 reading either from 18.00 hrs on day of admission (if pt admitted before 18.00 hrs) or 18.00 hrs of next day (if pt admitted after 18.00 hrs). Investigators analyzed mean change in FEV1 from day 1 to (a) day 3, (b) day 7, (c) day 14, and (d) day of discharge.
Length of stay in hospital: Mean no. of days FEV1: No significant differences between the two groups for mean change in FEV1 from day 1 to (a) day 3, (b) day 7, (c) day 14, and (d) day of discharge (p > 0.05, all time points). (a)Albuterol + ipratropium (n = 29), 0.05 ± 0.24 (SD); albuterol (n = 33), 0.17 ± 0.28 (b)Albuterol + ipratropium (n = 29), 0.15 ± 0.26; albuterol (n = 33), 0.21 ± 0.42 (c)Albuterol + ipratropium (n = 10), 0.26 ± 0.29; albuterol (n = 9), 0.06 ± 0.11 (d) Albuterol + ipratropium (n = 29), 0.15 ± 0.32; albuterol (n = 33), 0.23 ± 0.32 Length of stay in hospital: No significant difference between the two groups for mean no. of days in hospital: Albuterol + ipratropium (n =29), 11.8 ± 4.4 (SD); albuterol (n = 33), 10.5 ± 4.7 (p > 0.05).Adverse events: Two pts withdrawn due to AEs, 1 from albuterol + ipratropium group (wheezing) and 1 from albuterol group (chest pain). No further data reported on AEs. Dropouts: 8/70 pts (11%) overall; 4/33 (12%) in the combination therapy group, 4/37 (11%) in the albuterol group. |
|
| Notes | No significant difference between the two groups in the mean number of days on aminophylline IV, steroids IV, or antibiotics No significant difference between the two groups in the average time of day at which pts admitted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
O'Driscoll 1989.
| Methods | Design: SPPG Dates: N/S Location: UK Assessment period: 1 hr External validity: 1/5 COPD dx+ COPD bl‐ AECB dx‐ AECB sev‐ Tx duration‐ Internal validity: 2/5 r‐, db, dd | |
| Participants | N = 47 COPD pts and 56 asthma pts completed trial; total of 125 randomized to treatment (not broken down by diagnosis) Setting: ED Inclusion (COPD): Criteria of ATS (1987) Inclusion (AcEx): Acute airflow obstruction (no further details provided) Exclusion: Use of home nebulizer or nebulizer treatment en route to ED; history of heart disease or renal disease Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: N/S Age: 66.6 (range, 47‐81) (COPD pts) Sex: N/S Race: N/S | |
| Interventions | Experimental: Nebulized albuterol 10 mg + ipratropium 0.5 mg Control: Nebulized albuterol 10 mgCo‐interventions: Aminophylline IV, hydrocortisone IV administered to some pts | |
| Outcomes | Peak flow rate (PFR): Improvement from pre‐treatment to 1 hr PFR: No significant difference between the two treatment groups (p = 0.55). Mean pre‐treatment and 1‐hr PFR scores were as follows (no variance data reported):(a)Albuterol alone (n = 21): Pre‐treatment, 109 l/min; 1 hr, 134(b)Albuterol + ipratropium (n = 26): Pre‐treatment, 116; 1 hr, 135. Adverse events: Tremor reported by 2/44 pts (5%) receiving albuterol alone and by 5/59 (8%) receiving the combined therapy (not broken down by diagnosis). No other AEs reported. Dropouts: 22/125 pts (18%), 11 from each treatment group (not broken down by diagnosis) |
|
| Notes | For entire patient population, no significant differences between treatment groups in number of pts receiving aminophylline or hydrocorti‐sone; not broken down by diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Patrick 1990.
| Methods | Design: SPPG Dates: N/S Location: Canada Assessment period: 24 hrs External validity: 1/5 COPD dx‐ COPD bl‐ AECB dx‐ AECB sev‐ Tx duration+ Internal validity: 4/5 r, db+, dd | |
| Participants | N = 68; of 50 pts included in analysis, 21 had admission diagnosis of asthma, 29 acute exacerbation of COPD Setting: ED Inclusion (general): (1) Presented at ED because of dyspnea, cough, wheeze or chest discomfort, and (2) FEV1 < 60% predicted and FEV1/FVC < 60% predicted, and (3) clinical diagnosis by ED physician compatible with airflow limitation requiring hospital admission but not mechanical ventilation, and (4) nebulized b‐agonist, aminophylline IV, and corticosteroids IV administered on admissionInclusion (AcEx): No indication of criteria used to distinguish pts with acute exacerbation of COPD from those with asthma Exclusion: N/S Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: COPD pts only: Ipratropium, 0.62 L ± 0.33 (SD); placebo, 0.44 L ± 0.14 (no p‐value reported) Age: 57.6 (all pts) Sex: 23 M, 27 F (all pts) Race: N/S | |
| Interventions | Interventions: Experimental: Ipratropium 80 mg by MDI at 0 and 2 hrs, then every 3 hrs (immediately after each albuterol treatment) Control: Placebo Co‐interventions: Nebulized albuterol at 0 and 2 hrs, then every 3 hrs; aminophylline IV by continuous infusion; methylprednisolone IV every 6 hrs; broad spectrum antibiotic (ampicillin, tetracycline, or erythromycin), dosing schedule N/S | |
| Outcomes | FEV1: Measured before and 15 min after nebulized albuterol treatment on entry and at 24 hrs. Investigators analyzed mean change in pre‐bronchodilator FEV1 from 0 to 24 hrs FEV1: Among pts with acute exacerbation of COPD, there was no significant difference between the two treatment groups for mean change in pre‐bronchodilator FEV1 from 0 to 24 hrs: Ipratropium (n = 13), 0.15 L ± 0.24 (SD); placebo (n = 16), 0.10 L ± 0.18 (no p‐value reported). Adverse events: No data reported. Dropouts: 18/68 pts (26%), treatment group and diagnosis N/S |
|
| Notes | Trial included both pts with asthma and pts with acute exacerbation of COPD. FEV1 results were reported separately for the two types of patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rebuck 1987.
| Methods | Design: SPPG Dates: N/S Location: Canada Assessment period: 90 min External validity: 2/5 COPD dx+ COPD bl‐ AECB dx‐ AECB sev+ Tx duration‐ Internal validity: 5/5 r+, db+, dd | |
| Participants | N = 52 with acute exacerbation of COPD (see Notes) Setting: 4 EDs Inclusion (COPD): ATS criteria (1962) Inclusion (AcEx): Age > 18 yrs; FEV1 < 70% of predicted Exclusion: Complicating medical illnesses (e.g., pneumonia, pulmonary edema, acute myocardial infarction, frequent ventricular ectopic beats); pregnancy or breastfeeding; use of nebulized bronchodilator in previous 6 hrs; need for medications other than those specified in study protocol Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: 0.67 ± 0.29 (SD) L (28% of predicted) (COPD pts only) Age: 66.2 (COPD pts only) Sex: 28 M, 23 F Race: N/S | |
| Interventions | Interventions: Experimental: Nebulized ipratropium 0.5 mg + fenoterol 1.25 mg, single dose Control 1: Nebulized ipratropium 0.5 mg, single dose Control 2: Nebulized fenoterol 1.25 mg, single dose Co‐interventions: Aminophylline IV or corticosteroids IV could be used at discretion of attending physician; all other drugs proscribed | |
| Outcomes | FEV1: Mean change from pre‐treatment to 45 and 90 min FEV1: Each of the three treatment regimens produced a significant improvement in FEV1 from 0 to 90 min (p < 0.05). There was no significant difference among the three treatments for this outcome (p < 0.2). Mean improvement scores (± SEM) were reported in graphic form only. Pre‐treatment and 90‐min mean FEV1 scores (± SD) for the three groups were:Fenoterol: Pre‐: 0.69 ± 0.30; 90‐min: 0.88 ± 0.44 Ipratropium: Pre‐: 0.69 ± 0.26; 90‐min: 0.89 ± 0.41Ip + Fen: Pre‐: 0.63 ± 0.31; 90‐min: 0.80 ± 0.40. Adverse events: Pts were questioned about specific AEs. Results were not reported separately for pts with asthma and COPD. The most commonly reported AEs were dry mouth (10.6% of pts taking combination treatment, 19.1% taking fenoterol alone, 7.4% taking ipratropium alone); tremor (16.7% of pts taking combination treatment, 13.2% taking fenoterol alone, 2.9% taking ipratropium alone); and bad taste (8.4% overall). Eye irritation, sweating, and dizziness were each reported by < 3% of pts overall. Dropouts: 1/52 pts (2%), from ipratropium group. |
|
| Notes | Trial included both pts with acute asthma (n = 150) and pts with acute exacerbation of COPD (n = 52). Most results were reported separately for the two types of patients. No significant difference in the proportion of COPD pts receiving aminophylline IV and cortico‐steroids IV in the three treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shretha 1991.
| Methods | Design: SPPG Dates: April ‐Sept '88 Location: US Assessment period: To discharge from ED (range, 80‐530 min) External validity: 1/5 COPD dx‐ COPD bl‐ AECB dx‐ AECB sev+ Tx duration‐ Internal validity: 5/5 r+, db+, dd | |
| Participants | N = 68 Setting: ED Inclusion (COPD): Age > 40 yrs Inclusion (AcEx): Clinical diagnosis of COPD exacerbation (ATS [= ATS Committee on Diagnostic Standards for Nontuberculous Respiratory Disease], 1962); FEV1 < 40% of predicted Exclusion: N/S Smoking history: N/S Baseline stable FEV1: N/S FEV1 at admission: Ipratropium, 0.68 ± 0.27 (SD); placebo, 0.69 ± 0.25 (p > 0.05) Age: Ipratropium grp, 62 ± 9.3 (median ± SD); placebo grp, 63 ± 8.0 Sex: 35 M, 20 F Race: 33 Black, 16 White, 6 Latin American | |
| Interventions | Interventions: Experimental: Ipratropium by MDI; 3 puffs (54 mg) given w/in 5‐10 min of initial isoetharine dose; further doses of 2 puffs (36 mg) each given at 1 and 3 hrs, just after isoetharine Control: Placebo Co‐interventions: Nebulized isoetharine on presentation and every hr thereafter; aminophylline IV given if serum theophylline level not adequate; methylprednisolone IV administered "if there was no contraindication and if the physician thought that the patient would benefit from corticosteroid treatment" | |
| Outcomes | Outcomes:Percentage of pts admitted to hospital
Length of stay in ED: Mean (in min) Percentage of pts admitted to hospital: 2/30 pts (7%) receiving ipratropium, 3/25 pts receiving placebo (12%); not analyzed by investigators (too few pts). Length of stay in ED: Mean length of stay significantly (p < 0.05) shorter for pts receiving ipratropium (n = 30) than for pts receiving placebo (n = 25):Ipra: 226 ± 102 (SD) minPlac: 317 ± 135 min. Adverse events: Specific AEs were not sought, and none were reported during trial. Dropouts: 13/68 (19%) total, 8/33 (24%) in the placebo group, 5/35 (14%) in the ipratropium group. |
|
| Notes | No significant differences between treatment grps in the proportion of pts receiving aminophylline and methyl‐prednisolone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
1. External validity score (5 points possible): COPD dx = COPD diagnosis based on spirometry (+ = yes = 1; ‐ = no = 0); COPD bl = baseline stable ventilatory status of population described (+ = yes = 1; ‐ = no = 0); AECB dx = diagnosis of AECB includes at least two of following: increased sputum purulence, increased sputum volume, increased dyspnea (+ = yes = 1; ‐ = no = 0); AECB sev = severity of AECB at enrollment described based on at least two of following: mental status change, work of breathing, ventilatory status (+ = yes = 1; ‐ = no = 0); Tx duration = outcomes assessed at 24 hours or later (+ = yes = 1; ‐ = no = 0).
2. Internal validity score (5 points possible): Randomization: r = study described as randomized, method of randomization not described (1 point); r+ = method of randomization described and adequate (2 points); r‐ = method of randomization described but inadequate (0 points). Double‐blinding: ndb = not double‐blind (0 points); db = study described as double‐blind, method of blinding not described (1 point); db+ = method of blinding described and adequate (2 points); db‐ = method of blinding described but inadequate (0 points). Dropouts: dd = dropouts described (1 point); dnd = dropouts not described (0 points).
3. Abbreviations of study designs: SPPG ‐ single period parallel group (2 groups of patients, each recieving a particular treatment for a specified time); CrOv ‐ crossover (each group of patients are assigned to a treatment and then switched to the alternate treatment after a specified time).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alabaster 1997 | Review article |
| Anonymous 1992 | Not a RCT |
| Anonymous 1997a | Not a RCT |
| Anonymous 1997b | Not a RCT |
| Anzueto 2004 | Not acute exacerbations |
| Arsokski 2004 | Not acute exacerbations |
| Balkinssoon 2005 | Not acute exacerbations |
| Bleecker 1991 | Stable COPD, not acute exacerbation |
| Blosser 1995 | Stable COPD, not acute exacerbation |
| Celikel 2004 | Not acute exacerbations |
| Chapman 1990 | Not a RCT |
| de Guia 2004 | Not acute exacerbations |
| Dejaegher 1984 | Stable COPD, not acute exacerbation |
| Disse 1999 | Not a RCT |
| Dusser 2004 | Not acute exacerbations |
| Fernandez 1994 | All patients on mechanical ventilation |
| Gross 1987 | Stable COPD, not acute exacerbation |
| Ikeda 1998 | Not a RCT |
| Kalberg 2004 | Not acute exacerbations |
| Limauro 1995 | Study does not separate results by diagnosis; lumps data on asthmatic, cystic fibrosis and COPD patients. |
| Lode 1999 | Not a RCT |
| Madison 1998 | Not a RCT |
| Matera 1996 | Stable COPD, not acute exacerbation |
| Miravitlles 1999a | Not a RCT |
| Miravitlles 1999b | Not a RCT |
| Musil 1998a | Dosage study |
| Musil 1998b | Not a RCT |
| Nair 2005 | Correct population but does not have the correct intervention and comparison groups |
| Nakanishi 1997 | Not a RCT. Stable COPD, not acute exacerbation |
| Nardini 1996 | Not a RCT |
| O'Connor 1996 | Patients are asthmatic, not acute exacerbation of COPD |
| Rees 1998 | Not a RCT |
| Rennard 1996 | Stable COPD, not acute exacerbation |
| Rennard 1997 | Review |
| Sabradillo 1997 | Stable COPD |
| Sawa 1997 | Stable COPD, not acute exacerbation |
| Shioya 1996 | Not a RCT |
| Tashkin 2005 | Not acute exacerbations |
| Tzelepis 1996 | Stable COPD, not acute exacerbation |
Contributions of authors
DM: Collection of articles, writing/editing of review. CB: Collection of articles, writing/editing of review.
Sources of support
Internal sources
No sources of support supplied
External sources
Agency for Health Care Policy and Research (Contract No. 290‐97‐0014), USA.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Backman 1985 {published data only}
- Backman R, Hellstrom PE. Fenoterol and ipratropium in respiratory treatment of patients with chronic bronchitis. Current Therapeutic Research, Clinical and Experimental 1985;38(1):135‐40. [Google Scholar]
Cydulka 1995 {published data only}
- Cydulka RK, Emerman CL. Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Annals of Emergency Medicine 1995;25(4):470‐3. [DOI] [PubMed] [Google Scholar]
Karpel 1990 {published data only}
- Karpel JP, Pesin J, Greenberg D, Gentry E. A comparison of the effects of ipratropium bromide and metaproterenol sulfate in acute exacerbations of COPD. Chest 1990;98(4):835‐9. [DOI] [PubMed] [Google Scholar]
Lloberes 1988 {published data only}
- Lloberes P, Ramis L, Montserrat JM, Serra J, Campistol J, Picado C, et al. Effect of three different bronchodilators during an exacerbation of chronic obstructive pulmonary disease. European Respiratory Journal 1988;1:536‐9. [PubMed] [Google Scholar]
Moayyedi 1995 {published data only}
- Moayyedi P, Congleton J, Page RL, Pearson SB, Muers MF. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax 1995;50(8):834‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Driscoll 1989 {published data only}
- O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulized salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989;1(8652):1418‐20. [DOI] [PubMed] [Google Scholar]
Patrick 1990 {published data only}
- Patrick DM, Dales RE, Stark RM, Laliberte G, Dickinson G. Severe exacerbations of COPD and asthma. Incremental benefit of adding ipratropium to usual therapy. Chest 1990;98(2):295‐7. [DOI] [PubMed] [Google Scholar]
Rebuck 1987 {published data only}
- Rebuck AS, Chapman KR, Abboud R, Pare PD, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. The American Journal of Medicine 1987;82:59‐64. [DOI] [PubMed] [Google Scholar]
Shretha 1991 {published data only}
- Shrestha M, O'Brien T, Haddox R, Gourlay HS, Reed G. Decreased duration of emergency department treatment of chronic obstructive pulmonary disease exacerbations with the addition of ipratropium bromide to beta‐agonist therapy. Annals of Emergency Medicine 1991;20(11):1206‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alabaster 1997 {published data only}
- Alabaster VA. Discovery and development of selective M3 antagonists for clinical use. Life Sciences 1997;Vol 60(13‐14):1053‐60. [DOI] [PubMed] [Google Scholar]
Anonymous 1992 {published data only}
- Anonymous. Bronchodilation for COPD?. Emergency Medicine 1992;24(11):62. [Google Scholar]
Anonymous 1997a {published data only}
- Anonymous. Combivent. Hospital Formulary 1997;32(2):119. [Google Scholar]
Anonymous 1997b {published data only}
- Anonymous. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. The COMBIVENT Inhalation Solution Study Group. Chest 1997;112(6):1514‐21. [DOI] [PubMed] [Google Scholar]
Anzueto 2004 {published data only}
- Anzueto A, Kesten S. Effects of tiotropium in COPD patients only treated with PRN albuterol [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, Orlando. 2004:C47, Poster F81.
Arsokski 2004 {published data only}
- Arsovki Z, Dokic D, Boskovska M, Goseva Z, Kaeva B, Gliugorovski L, et al. The effect of combined B2‐agonist and anticholinergic inhaled therapy in patients with COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):341s. [Google Scholar]
Balkinssoon 2005 {published data only}
- Balkinssoon RC, Murphy JR, Make B. Safety and efficacy of albuterol vs aulbuterol/ipratropium bromide in COPD subjects on fluticasone/salmeterol 500/50 [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:Poster F8.
Bleecker 1991 {published data only}
- Bleecker ER, Britt EJ. Acute bronchodilating effects of ipratropium bromide and theophylline in chronic obstructive pulmonary disease. The American Journal of Medicine 1991;91(4A):24A‐27S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blosser 1995 {published data only}
- Blosser SA, Maxwell SL, Reeves‐Hoche MK, Localio AR, Zwillich CW. Is an anticholinergic agent superior to a beta2‐agonist in improving dyspnea and exercise limitation in COPD?. Chest 1995;108(3):730‐5. [DOI] [PubMed] [Google Scholar]
Celikel 2004 {published data only}
- Celikel S, Yilmaz A, Seyfikli Z, Etikan I. Evaluation of bronchodiltor response to single dose tiotropium bromide [Abstract]. European Respiratory Journal 2004;24(Suppl 48):504s. [Google Scholar]
Chapman 1990 {published data only}
- Chapman KR. The role of anticholinergic bronchodilators in adult asthma and chronic obstructive pulmonary disease. Lung 1990;168(Suppl):295‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Guia 2004 {published data only}
- Guia T, Punzal P, Canizares L, Sy‐Naval S, Salonga R, Tan D, et al. Evaluation of the response of Filipino COPD patients to Tiotropium (EVEREST STUDY) [Abstract]. American Thoracic Society 100th International Conference, May 21‐26; Orlando. 2004:Poster F78.
Dejaegher 1984 {published data only}
- Dejaegher P, Demedts M, Rochette F, Veken J. Effects of 40 mcg. and 400 mcg. of ipratropium bromide in obstructive lung disease. Acta Clinica Belgica 1984;39(3):136‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Disse 1999 {published data only}
- Disse B, Speck GA, Rominger KL, Witek TJ Jr, Hammer R. Tiotropium (spiriva(TM)): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sciences 1999;64(6‐7):457‐64. [DOI] [PubMed] [Google Scholar]
Dusser 2004 {published data only}
- Dusser D, Bravo ML, Iacono P. Tiotropium reduces health resource utilization associated with COPD exacerbations [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
- Dusser D, Bravo ML, Iacono P, MISTRAL Study Group. Tiotropium reduces COPD exacerbations: the MISTRAL study. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
Fernandez 1994 {published data only}
- Fernandez A, Muñoz J, Calle B, Alia I, Ezpeleta A, Cal MA, et al. Comparison of one versus two bronchodilators in ventilated COPD patients. Intensive Care Medicine 1994;20:199‐202. [MEDLINE: ; EMBASE 94097968] [DOI] [PubMed] [Google Scholar]
Gross 1987 {published data only}
- Gross NJ, Bankwala Z. Effects of an anticholinergic bronchodilator on arterial blood gases of hypoxemic patients with chronic obstructive pulmonary disease. Comparison with a beta‐adrenergic agent. American Review of Respiratory Disease 1987;136(5):1091‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ikeda 1998 {published data only}
- Ikeda A, Nishimura K, Izumi T. Pharmacological treatment in acute exacerbations of chronic obstructive pulmonary disease. Drugs & Aging 1998;12(2):129‐37. [DOI] [PubMed] [Google Scholar]
Kalberg 2004 {published data only}
- Kalberg C, Emmett A, Bourne E, Merchant K, Knobil K. Fluticasone propionate/salmeterol provides greater relief of dyspnea than Ipratropium/Albuterol in patients with COPD [Abstract]. American Thoracic Society 100th International Conference, May 21‐26; Orlando. 2004:Poster 528.
Limauro 1995 {published data only}
- Limauro J, Mahler DA, Olmstead EM, Tosteson ANA. Responses to inhaled bronchodilator in patients with acute airflow obstruction. Respiratory Care 1995;40(8):815‐9. [EMBASE 1995258156] [Google Scholar]
Lode 1999 {published data only}
- Lode H. Inhalational anticholinergic versus inhaled beta‐mimetic drugs in COPD. Internist 1999;40(1):114. [PubMed] [Google Scholar]
Madison 1998 {published data only}
- Madison JM, Irwin RS. Chronic obstructive pulmonary disease. Lancet 1998;352(9126):467‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matera 1996 {published data only}
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90(8):497‐9. [DOI] [PubMed] [Google Scholar]
Miravitlles 1999a {published data only}
- Miravitlles M, Murio C, Guerrero T, Segu JL. Treatment of chronic bronchitis and chronic pulmonary obstructive disease in primary care. Archivos de bronchopneumologia 1999;35(4):173‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miravitlles 1999b {published data only}
- Miravitlles M, Mayordomo C, Artes M, Sanchez‐Agudo L, Nicolau F, Segu JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Respiratory Medicine 1999;93(3):173‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Musil 1998a {published data only}
- Musil J, Hirsch V, Vondra V, Reisova M. Dosage of drugs in combined nebulisation therapy of patients with chronic obstructive pulmonary disease (COPD). Studia Pneumologica et Phtiseologica 1998;57(6):257‐9. [Google Scholar]
Musil 1998b {published data only}
- Musil J, Hirsch V, Vondra V, Reisova M. What is the adequate dose in combined inhalation treatment of patients with exacerbations of chronic obstructive pulmonary disease?. Vnitrni Lekarstvi 1998;44(7):415‐7. [PubMed] [Google Scholar]
Nair 2005 {published data only}
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005;128(1):48‐54. [DOI] [PubMed] [Google Scholar]
Nakanishi 1997 {published data only}
- Nakanishi N, Ueda N, Kitade M, Moritaka T, Kamei T. Effects of oxitropium bromide on exercise and lung function in patients with pulmonary emphysema. Japanese Journal of Thoracic Diseases. 1997;35(1):38‐42. [PubMed] [Google Scholar]
Nardini 1996 {published data only}
- Nardini S. Inhaled antimuscarinic agents and COPD. Monaldi Archives for Chest Disease 1996;51(1):52‐3. [PubMed] [Google Scholar]
O'Connor 1996 {published data only}
- O'Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine‐induced bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 1996;154(4 Pt 1):876‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rees 1998 {published data only}
- Rees PJ. Bronchodilators in the therapy of chronic obstructive pulmonary disease. European Respiratory Monograph 1998;3(7):135‐49. [Google Scholar]
Rennard 1996 {published data only}
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD: A retrospective analysis of data from seven clinical trials. Chest 1996;110(1):62‐70. [DOI] [PubMed] [Google Scholar]
Rennard 1997 {published data only}
- Rennard SI. Choice of bronchodilator therapy in chronic obstructive pulmonary disease. Respirology 1997;2(Suppl 1):S11‐5. [PubMed] [Google Scholar]
Sabradillo 1997 {published data only}
- Sabradillo PV. The outpatient and hospital use of anticholinergics in COPD. Archivos De Bronconeumologia 1997;33(Suppl 2):34‐9. [PubMed] [Google Scholar]
Sawa 1997 {published data only}
- Sawa T, Yasuda N, Gotoh K, et al. Effect of additional high dose inhalation of oxitropium bromide on respiratory function in the patients with COPD. Therapeutic Research 1997;18(3):389‐95. [Google Scholar]
Shioya 1996 {published data only}
- Shioya T, Kagaya M, Sano M, Itaba M, Shindo T, Miura M. Antimuscarinic effect of tiquizium bromide in vitro and in vivo. European Journal of Clinical Pharmacology 1996;50(5):375‐80. [DOI] [PubMed] [Google Scholar]
Tashkin 2005 {published data only}
- Tashkin D, Celli B, Decramer M, Pauwels R, Menjoge S, Burkhart D, et al. Bronchodilator responsiveness in COPD patients enrolled in the UPLIFT trial [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:Poster J102.
Tzelepis 1996 {published data only}
- Tzelepis G, Komanapolli S, Tyler D, Vega D, Fulambarker A. Comparison of nebulized glycopyrrolate and metaproterenol in chronic obstructive pulmonary disease. European Respiratory Journal 1996;9(1):100‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106(2):196‐204. [MEDLINE: ; EMBASE 87077377] [DOI] [PubMed] [Google Scholar]
ATS 1995
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1995;152(5 (Pt 2)):S77‐121. [MEDLINE: ] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 1992
- Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;146(4):1067‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Siafakas 1995
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. European Respiratory Journal 1995;8(8):1398‐420. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


