Abstract
Objectives
To examine the serum levels of interleukin (IL)-30 in patients with psoriasis and evaluate the correlations with the Psoriasis Area and Severity Index (PASI).
Methods
Serum was collected from 26 patients with psoriasis and 26 healthy controls in a case–control setting, and the level of IL-30 was determined using an enzyme-linked immunosorbent assay. Statistical analysis of the IL-30 levels among groups and further correlation analyses of IL-30 levels with PASI scores were performed.
Results
A significant increase in the level of IL-30 in patients with psoriasis compared with healthy controls was observed. In addition, a positive correlation between the IL-30 concentration and PASI scores was found in patients with psoriasis.
Conclusion
IL-30 is presumably involved in the proliferation of epidermal cells during the development of psoriasis. Further studies with a larger number of participants are required to comprehensively elucidate the biological roles of IL-30 in the pathogenesis of psoriasis.
Keywords: Psoriasis, interleukin-30, cytokine, psoriasis pathogenesis, Psoriasis Area and Severity Index, inflammation
Introduction
Psoriasis is a common chronic autoimmune and skin inflammatory condition characterized by erythematous-round lesions that occur due to rapid skin cell production and the presence of immature skin cells.1,2 Psoriasis causes the skin to regenerate at faster than normal rates, resulting in the build-up of skin cells.3,4
Psoriasis scales are commonly observed on large joints, such as elbows and knees. They may also develop on the back, hands, feet, neck, scalp, and face but rarely occur on the nails, mouth, and inguinal genitals.1,5 Psoriasis is generally associated with several other conditions that lead to reduced immunity, such as type 2 diabetes, inflammatory bowel disease, heart disease, psoriatic arthritis, anxiety, and depression.6,7 Psoriasis affects 2% of the population globally, of which 6.7 million are Americans. 8
Cytokines are small molecular weight proteins that activate immune cells. Therefore, cytokine evaluation is important for monitoring immune system responses to chronic inflammation and autoimmune diseases, such as psoriasis.9–11 Interleukin-30 (IL-30) is a 28 kDa protein belonging to the IL-6 cytokine family.12–14 IL-30 is mainly produced by immune cells of a myeloid origin, including macrophages, monocytes, microglia, and dendritic cells, activated by diverse microbial and immune stimuli. It is also produced by neutrophils, plasma cells, endothelial cells, and epithelial cells.13,15 IL-30 is involved in initiating both classic and trans-signaling pathways, suggesting that it may have various effects on different cell types.16,17
IL-30 independently plays a significant role in the development and progression of autoimmune diseases, such as psoriasis, by regulating both T helper (Th)1 and Th17 cells. 18 Moreover, a previous study reported that the measurement of serum cytokines in patients with psoriasis allows for a better understanding and prediction of the disease pathology. 19 Therefore, the evaluation of serum IL-30 levels in patients with psoriasis might be useful for monitoring the pathology and prognosis of psoriasis. This study aimed to examine the IL-30 level in the serum of patients with psoriasis and evaluate its correlations with the severity of the disease.
Materials and methods
Patients
Patients with a previously confirmed diagnosis of various degrees of plaque-type psoriasis were enrolled in this case-control study. Additionally, age- and sex-matched healthy, non-psoriatic volunteers with no family history of psoriasis were included as controls. All participants had been admitted to the dermatology inpatient and outpatient departments of Nanfang Hospital, Guangzhou, China, between 2018 and 2019.
Patients on systemic immunosuppressant therapy, such as methotrexate, systemic steroids, and other drugs, and patients with any type of cancer, systemic, or dermatological disease with a potentially compromised immune system were not included in the study. The patients with psoriasis were divided into three groups according to their psoriasis area severity index (PASI) score: mild (PASI < 10), moderate (PASI 10–29), and severe (PASI > 30). The study was approved by the Human Specimens Collection and Use Ethics Committee of the Southern Medical University, Guangzhou, China (Approval. No. NFEC-2018-01-6) and carried out following relevant rules and approved protocols. Signed informed consent was obtained from all patients and volunteers. This study was conducted according to the declaration of Helsinki principles 20 and reported following the STROBE guidelines for case-control studies. 21
IL-30 measurements
Whole venous blood was obtained from all participants in the study. Their serum was separated by centrifugation for 20 minutes at 1000 × g and stored at −80°C until examination. The serum IL-30 level was examined using an enzyme-linked immunosorbent assay kit for human IL-30 purchased from Elabscience (Wuhan, China), and all steps were performed in accordance with the manufacturer’s manual.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 22 (IBM Corp., Armonk, NY, USA) and Prism software (GraphPad 8 software, San Diego, CA, USA) and expressed as the mean ± standard deviation (SD). The comparison between two means was performed by student’s t-test, and more than two means were compared by one-way analysis of variance. Not normally distributed data were analyzed by Mann–Whitney U and Kruskal–Wallis tests. Spearman’s correlation analysis was performed to evaluate the relationship between IL-30 levels and PASI scores. A p-value of <0.05 was regarded as statistically significant.
Results
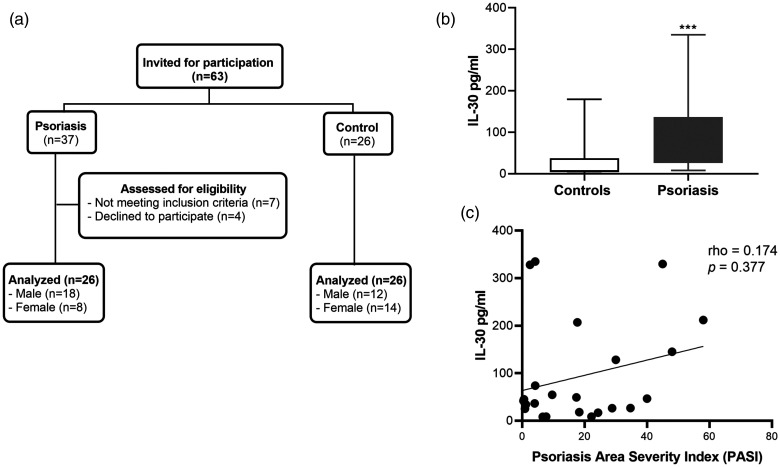
Twenty-six patients with psoriasis and 26 healthy controls were included in this study (Figure 1a). The mean age ± SD was 45.5 ± 12.8 years for patients with psoriasis and 40.1 ± 16.2 years for the healthy controls. Additionally, patients aged less than 40 years accounted for the highest proportion of participants (44.2%) (Table 1). No significant difference was observed between the two groups regarding the age distribution. More than two-thirds (69.2%) of the psoriasis cases were men compared with 46.2% for the control group. The statistical analysis of IL-30 levels in patients with psoriasis (111.96 pg/mL) and controls (33.76 pg/mL) revealed a significant difference in the mean IL-30 concentration between the groups (Table 1 and Figure 1b) (p < 0.001). Moreover, the results of the Mann–Whitney test showed that the mean rank of IL-30 in the serum of patients with psoriasis (890) was significantly higher than that of controls (488) (p < 0.001). However, no significant differences in the mean rank of IL-30 were found among the age groups (Table 2) of the psoriasis cases or controls. Additionally, a comparison of the mean rank of IL-30 among sexes revealed no significant differences in either the psoriasis or control groups (Table 2).
Figure 1.
IL-30 levels in patients with psoriasis and healthy controls. (a) Flow diagram of the study. (b). A comparison of the serum IL-30 levels in the psoriatic (N=26) and control groups (N=26) determined using an enzyme-linked immunosorbent assay kit. The results are shown as the mean ± standard deviation. ***p<0.001 by Student’s t-test. (c) Spearman’s correlation analysis of IL-30 levels and PASI scores in patients with psoriasis
IL-30, interleukin-30; PASI, Psoriasis Severity and Area Index.
Table 1.
The age, sex distributions, and IL-30 level in the psoriatic and control groups.
| Psoriasis | Control | Total | |||||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | p | |
| Age (years) | |||||||
| <40 | 10 | (38.5) | 13 | (50.0) | 23 | (44.2) | |
| 40–49 | 6 | (23.1) | 4 | (15.4) | 10 | (19.2) | |
| ≥50 | 10 | (38.5) | 9 | (34.6) | 19 | (36.5) | 0.656# |
| Mean (±SD) | 45.5 | (±12.8) | 40.1 | (±16.2) | 0.188† | ||
| Sex | |||||||
| Male | 18 | (69.2) | 12 | (46.2) | 30 | (57.7) | |
| Female | 8 | (30.8) | 14 | (53.8) | 22 | (42.3) | 0.092# |
| Total | 26 | (100.0) | 26 | (100.0) | 52 | (100.0) | |
| IL-30 | |||||||
| Mean (±SD) | 111.96 | (±149.66) | 33.76 | (±56.48) | <0.001‡ | ||
IL-30, interleukin-30; SD, standard deviation. †By t-test for two independent samples. #By chi-square test. ‡By Mann–Whitney test.
Table 2.
Mean IL-30 level by age and sex in the psoriatic and control groups.
| N | Mean IL-30 | (±SD) | Mean rank | p† | |
|---|---|---|---|---|---|
| Psoriasis | |||||
| Age (years) | |||||
| <40 | 10 | 72.94 | (±97.41) | 12.40 | |
| 40–49 | 6 | 104.73 | (±135.19) | 11.50 | |
| ≥50 | 10 | 155.32 | (±196.87) | 15.80 | 0.467 |
| Sex | |||||
| Male | 18 | 106.65 | (±159.96) | 12.72 | |
| Female | 8 | 123.90 | (±132.70) | 15.25 | 0.437 |
| Control | |||||
| Age (years) | |||||
| <40 | 13 | 40.77 | (±63.93) | 14.54 | |
| 40–49 | 4 | 6.06 | (±3.72) | 11.13 | |
| ≥50 | 9 | 35.95 | (±57.99) | 13.06 | 0.720 |
| Sex | |||||
| Male | 12 | 29.30 | (±51.95) | 13.75 | |
| Female | 14 | 37.59 | (±61.78) | 13.29 | 0.877 |
IL-30, interleukin-30; SD, standard deviation. †By Kruskal–Wallis test.
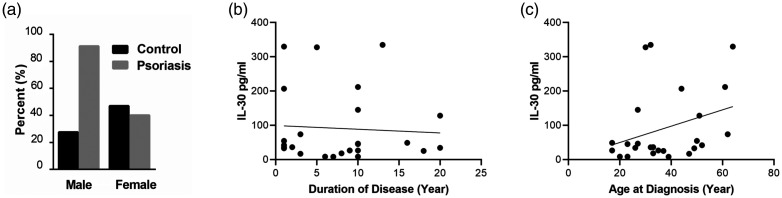
Regarding Spearman’s correlation analysis between IL-30 levels and PASI scores, a weak non-significant positive correlation was found in psoriasis cases (rho = 0.174) (Figure 1c). Moreover, no significant differences were observed in the mean rank of IL-30 among the three categories of psoriasis (mild, moderate, and severe) (Table 3). Our results showed a predominance of male patients with psoriasis (Figure 2a). Among patients with psoriasis, there was no significant association between the mean rank of IL-30 and the duration of their disease (Figure 2b) or their age at diagnosis (Figure 2c and Table 3).
Table 3.
Comparison of the mean and mean rank of IL-30 with the severity, duration, and age at diagnosis of patients with psoriasis.
| Psoriasis characteristics | N | Mean IL-30 | (±SD) | Mean rank | p |
|---|---|---|---|---|---|
| Severity‡ | |||||
| Mild | 14 | 121.23 | (±183.87) | 13.43 | |
| Moderate | 3 | 91.27 | (±101.24) | 14.00 | |
| Severe | 9 | 104.43 | (±110.07) | 13.44 | 0.993# |
| Duration (years) | |||||
| <10 | 14 | 86.96 | (±113.82) | 12.07 | |
| ≥10 | 12 | 141.12 | (±184.03) | 15.17 | 0.304† |
| Age at diagnosis | |||||
| <40 | 17 | 106.74 | (±170.96) | 12.00 | |
| ≥40 | 9 | 121.80 | (±106.55) | 16.33 | 0.169† |
| Total | 26 | 111.96 | (±149.66) |
IL-30, interleukin-30; SD, standard deviation. ‡Assessed by the Psoriasis Area and Severity Index. #By Kruskal–Wallis test. †By Mann–Whitney test.
Figure 2.
Association of sex, age at diagnosis, and duration of the disease to serum IL-30 levels in patients with psoriasis. (a) Predominance of male patients with psoriasis to female patients with psoriasis. The results are shown as the mean. No significant association was observed by Spearman’s correlation analysis between the mean rank of serum IL-30 and the duration of disease (b) or the age at diagnosis (c) in patients with psoriasis.
IL-30, interleukin-30.
Discussion
Psoriasis is a common chronic immune-mediated inflammatory skin disorder characterized by erythematous silvery scaling of the skin and severe itching. Reports indicate that psoriasis affects about 2% of the global population and reduces the quality of life for patients, limiting their activity and productivity in a community.8,22 With the advancement of knowledge regarding this condition, studying the possible contribution of cytokines to the pathogenesis of psoriasis has received increasing attention. To this end, several studies have reported that various cytokines are associated with psoriasis as their levels are increased in the serum of patients. These include IL-6, IL-8, IL-12, IL-17, IL-18, and IL-23.19,23–25 However, as evidenced via a PubMed search, no study has reported the serum levels of IL-30 in patients with psoriasis to date. To address this knowledge gap, the current study assessed the demographic and clinical laboratory characteristics of patients with psoriasis and controls, which interestingly revealed the possible contribution of IL-30 to psoriasis.
IL-30 is an independent cytokine produced by immune cells of a myeloid origin that mediates antineoplastic effects in several tumor models,26,27 presumably including psoriasis. This study determined that the serum IL-30 level in patients with psoriasis was significantly higher compared with that of controls. Moreover, our findings suggest a positive correlation between the serum levels of IL-30 and the severity of psoriasis indicated by the PASI score, although this result was not statistically significant. Despite the limitations of the PASI score, including its non-linear scale28,29 and lack of sensitivity, 30 the PASI score has been the most commonly recommended clinical index of psoriasis severity. 30 Of note, consistent with the results of previously reported studies on cytokines in psoriasis,23,25 the present study showed that the serum level of IL-30 was not associated with age or sex. Similarly, the age at diagnosis and duration of the disease in patients with psoriasis were not significantly associated with the mean rank of the serum IL-30 level. These results are consistent with the findings of previously reported studies on the association of related cytokines with psoriasis.25,31
Several studies have reported a similar incidence of psoriasis in men and women. 32 However, the current study showed a predominance of male patients with psoriasis (Figure 2a), consistent with the findings of Fernandez et al., 33 whereas others reported a higher prevalence of women. 34 The reported studies on psoriasis with an increased proportion of women suggest a possible contribution of sex to the quality of life based on the idea that women are more concerned about their health and treatment than men. 35 Nevertheless, the limited available data and the population awareness of psoriasis may have contributed to selection bias. Additionally, in contrast to the findings of several previous studies that have reported age and sex among the most contributing factors to the severity of psoriasis,36,37 the current study found no significant differences in the mean rank of serum IL-30 among the age or sex groups of patients with psoriasis.
Recent studies on cytokines have enabled a better understanding of the pathophysiology of psoriasis. 38 However, the mechanisms underlying the development of psoriasis are particularly complex. 39 Notably, the hyper-proliferation and abnormal differentiation of keratinocytes in psoriasis have been associated with impaired T-lymphocytes. 40 Consistently, several inflammatory cytokines found to be elevated in serum samples during psoriasis have been shown to be correlated with disease severity. Thus, determining the specific or combined cytokines that play a main role in the pathogenesis of psoriasis can revolutionize the diagnosis and treatment of psoriasis. The function of IL-30 in psoriasis lesions has not yet been fully elucidated and requires further in vitro and in vivo studies. However, the current study demonstrated a significant increase in the serum IL-30 level in patients with psoriasis compared with the controls and a positive correlation of the IL-30 level with PASI scores. These findings indicate that the downregulation of IL-30 expression may attenuate the abnormal keratinocyte proliferation and other related features during the pathogenesis of psoriasis.
Overall, consistent with the theoretical features of psoriasis, the current results demonstrate the involvement of IL-30 in the proliferation of epidermal cells in psoriasis, which further confirms that psoriasis represents an immune-mediated systemic disease. However, the origin of IL-30 in the serum and whether the changes in the serum IL-30 level in patients with psoriasis are a cause or a consequence of the disease remain unclear. The limitations of the study include a small number of participants, which may have affected the statistical significance of the results, and the absence of in vivo investigations in an animal model. Further in vitro and in vivo studies would be beneficial to fully elucidate the contributions of IL-30 to the pathogenesis of psoriasis.
Conclusion
The findings of the current study substantiate previously reported studies on the involvement of different cytokines in the pathogenesis of psoriasis.19,23–25 These cytokines involved in the interactions between keratinocytes and T-lymphocytes may contribute to the pathogenesis of psoriasis. However, the production and biological properties of these cytokines in psoriasis remain unknown. This study is the first to report on the serum levels of IL-30 in patients with psoriasis, which may improve the early diagnosis and comprehensive management of patients with this disease.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211004039 for Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: a case-control study by Nergez Sabah Omar, Xinxin Long, Jiayi Xian, Henok Kessete Afewerky, Saeed Ghulam Hussain and Xuebiao Peng in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211004039 for Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: a case-control study by Nergez Sabah Omar, Xinxin Long, Jiayi Xian, Henok Kessete Afewerky, Saeed Ghulam Hussain and Xuebiao Peng in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Henok Kessete Afewerky https://orcid.org/0000-0001-7030-3224
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386: 983–994. [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Zhang HY. The Immunogenetics of Psoriasis and Implications for Drug Repositioning. Int J Mol Sci 2017; 18: 2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang ST, Nijsten T, Elder JT. Recent Highlights in Psoriasis Research. J Invest Dermatol 2017; 137: 550–556. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Tsuji H, Hashimoto Y, et al. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol 2010; 35: 645–649. [DOI] [PubMed] [Google Scholar]
- 5.Golinska J, Sar-Pomian M, Rudnicka L. Dermoscopic features of psoriasis of the skin, scalp and nails - a systematic review. J Eur Acad Dermatol Venereol 2019; 33: 648–660. [DOI] [PubMed] [Google Scholar]
- 6.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol 2013; 149: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayala-Fontanez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 2016; 6: 7–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman SR, Goffe B, Rice G, et al. The Challenge of Managing Psoriasis: Unmet Medical Needs and Stakeholder Perspectives. Am Health Drug Benefits 2016; 9: 504–513. [PMC free article] [PubMed] [Google Scholar]
- 9.Coondoo A. Cytokines in dermatology - a basic overview. Indian J Dermatol 2011; 56: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouris A, Pistiki A, Katoulis A, et al. Proinflammatory cytokine responses in patients with psoriasis. Eur Cytokine Netw 2014; 25: 63–68. [DOI] [PubMed] [Google Scholar]
- 11.Krause ML, Davis JM, 3rd, Knutson KL, et al. Assessing immune function by profiling cytokine release from stimulated blood leukocytes and the risk of infection in rheumatoid arthritis. Clin Immunol 2011; 141: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalan-Dibene J, McIntyre LL, Zlotnik A. Interleukin 30 to Interleukin 40. J Interferon Cytokine Res 2018; 38: 423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Carlo E. Decoding the Role of Interleukin-30 in the Crosstalk Between Cancer and Myeloid Cells. Cells 2020; 9: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petes C, Mariani MK, Yang Y, et al. Interleukin (IL)-6 Inhibits IL-27- and IL-30-Mediated Inflammatory Responses in Human Monocytes. Front Immunol 2018; 9: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venerito V, Natuzzi D, Bizzoca R, et al. Serum sCD40L levels are increased in patients with psoriatic arthritis and are associated with clinical response to apremilast. Clin Exp Immunol 2020; 201: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baran P, Nitz R, Grotzinger J, et al. Minimal interleukin 6 (IL-6) receptor stalk composition for IL-6 receptor shedding and IL-6 classic signaling. J Biol Chem 2013; 288: 14756–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stumhofer JS, Tait ED, Quinn WJ, 3rd, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol 2010; 11: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Liu X, Huang N, et al. Soluble expression and purification of the functional interleukin-30 protein in Escherichia coli. Prep Biochem Biotechnol 2016; 46: 539–545. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Hamid MF, Aly DG, Saad NE, et al. Serum levels of interleukin-8, tumor necrosis factor-alpha and gamma-interferon in Egyptian psoriatic patients and correlation with disease severity. J Dermatol 2011; 38: 442–446. [DOI] [PubMed] [Google Scholar]
- 20.General Assembly of the World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014; 81: 14–18. [PubMed] [Google Scholar]
- 21.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 22.Salman A, Yucelten AD, Sarac E, et al. Impact of psoriasis in the quality of life of children, adolescents and their families: a cross-sectional study. An Bras Dermatol 2018; 93: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005; 2005: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asadullah K, Sterry W, Volk HD. Analysis of cytokine expression in dermatology. Arch Dermatol 2002; 138: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-alpha, IL-12/23p40, and IL-17 in plaque psoriasis and their correlation with disease severity. J Immunol Res 2014; 2014: 467541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garbers C, Hermanns HM, Schaper F, et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev 2012; 23: 85–97. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Wang Z, Ye N, et al. A protective role of IL-30 via STAT and ERK signaling pathways in macrophage-mediated inflammation. Biochem Biophys Res Commun 2013; 435: 306–312. [DOI] [PubMed] [Google Scholar]
- 28.Carlin CS, Feldman SR, Krueger JG, et al. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004; 50: 859–866. [DOI] [PubMed] [Google Scholar]
- 29.Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. J Invest Dermatol 2010; 130: 933–943. [DOI] [PubMed] [Google Scholar]
- 30.Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol 2010; 24: 10–16. [DOI] [PubMed] [Google Scholar]
- 31.Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF- alpha, IL-12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal 2014; 2014: 508178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol 2007; 25: 535–546. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Armenteros JM, Gomez-Arbones X, Buti-Sole M, et al. Epidemiology of Psoriasis. A Population-Based Study. Actas Dermosifiliogr 2019; 110: 385–392. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakou A, Patsatsi A, Sotiriadis D. Detailed analysis of specific nail psoriasis features and their correlations with clinical parameters: a cross-sectional study. Dermatology 2011; 223: 222–229. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb AB, Ryan C, Murase JE. Clinical considerations for the management of psoriasis in women. Int J Womens Dermatol 2019; 5: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagg D, Sundstrom A, Eriksson M, et al. Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am J Clin Dermatol 2017; 18: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Estebaranz JL, Sanchez-Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: Results from the ARIZONA study. J Dermatol 2016; 43: 395–401. [DOI] [PubMed] [Google Scholar]
- 38.Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine 2015; 73: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin 2015; 33: 13–23. [DOI] [PubMed] [Google Scholar]
- 40.Schon MP, Boehncke WH. Psoriasis. N Engl J Med 2005; 352: 1899–1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211004039 for Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: a case-control study by Nergez Sabah Omar, Xinxin Long, Jiayi Xian, Henok Kessete Afewerky, Saeed Ghulam Hussain and Xuebiao Peng in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211004039 for Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: a case-control study by Nergez Sabah Omar, Xinxin Long, Jiayi Xian, Henok Kessete Afewerky, Saeed Ghulam Hussain and Xuebiao Peng in Journal of International Medical Research