Abstract
Talaromyces marneffei is a rare dimorphic pathogenic fungus that can induce severe infections in human immunodeficiency virus (HIV)-infected patients. However, such infections have also been reported in non-HIV hosts. This current case report describes a very rare case of a T. marneffei pulmonary infection in an HIV-negative patient with a mutation in the tuberous sclerosis complex subunit 2 (TSC2) gene. A 24-year-old male patient presented with cough and expectoration for 6 months. Computed tomography showed multiple ground-glass opacities and cystic and cavitated lesions in both lungs. Next generation sequencing (NGS) of the bronchoalveolar lavage fluid was performed to confirm T. marneffei pulmonary infection. The results were further verified using bronchoscopy specimen cultures. This was an HIV-negative patient without a travel history to endemic zones and his blood exon sequencing results showed a mutation in the TSC2 gene. To date, he has recovered well with voriconazole therapy. In summary, patients with TSC2 mutations that induce bronchopulmonary dysplasia may be potential hosts for T. marneffei. Early and timely diagnosis is important for improving prognosis. NGS plays a critical role in the diagnosis of T. marneffei pulmonary infection.
Keywords: Talaromyces marneffei, pulmonary infection, TSC2, HIV-negative
Introduction
Talaromyces marneffei (also known as Penicillium marneffei) is a rare dimorphic fungus that was first reported in 1956. 1 It is known to induce severe infection in human immunodeficiency virus (HIV)-positive patients or other immunocompromised patients in the epidemic region of Southeast and South Asia.2,3 It can develop into yeast-like cells at 37 °C and mycelia at 25 °C. 4 In China, T. marneffei is mainly prevalent in Guangdong, Guangxi, Yunnan and other southern provinces.5,6 In recent years, the incidence of T. marneffei infection has decreased following treatment with antiretroviral therapy. 7 However, the rate of T. marneffei infection in HIV-negative patients has increased.8,9 This current case report describes a patient with T. marneffei infection in a non-prevalent region of South China. The patient was immunocompetent and presented with diffuse ground-glass, cystic and cavitated lesions in both lungs. In addition, a mutation in the tuberous sclerosis complex subunit 2 (TSC2) gene was identified in the patient.
Case report
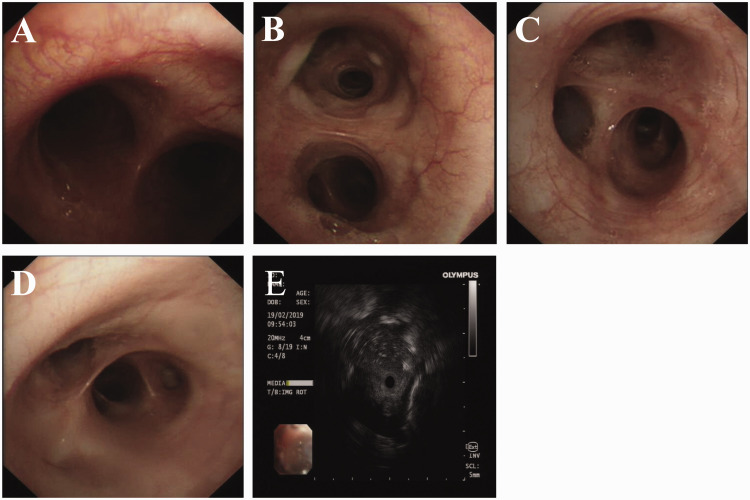
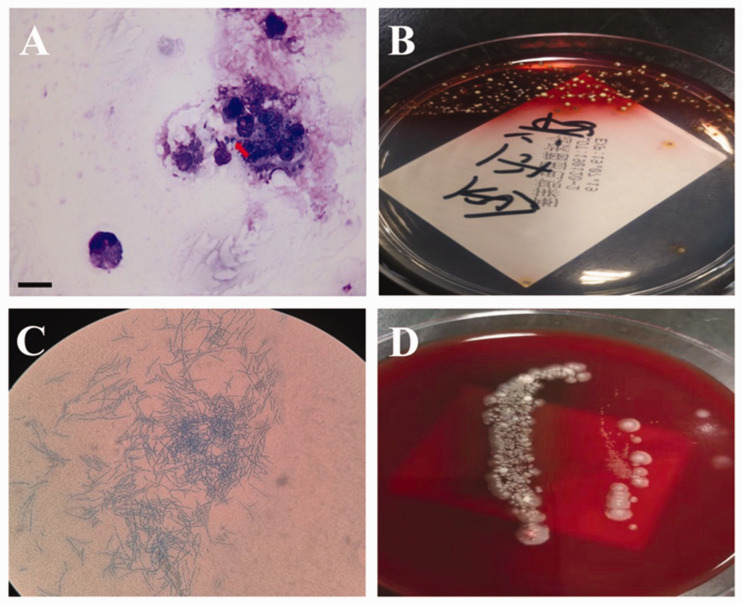
A 24-year-old male patient, native of Zhejiang Province of Southeast China and without a travel history to endemic zones, was admitted to the Department of Respiratory Disease, Thoracic Disease Centre, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China in February 2019. The patient presented with a 6-month history of recurrent cough and expectoration without fever, shortness of breath or chest pain. He had been treated with the antibiotic latamoxef at a local hospital. However, the symptoms of cough and expectoration did not improve. His HIV test results were negative. His serum immunoglobulin (Ig) G was 2907 mg/dl (reference range, 800–1800 mg/dl) and the CD8+ T cell count was 12.3 (reference range, 15–44). The serum levels of cancer antigen 125 and neuron-specific enolase were both elevated, at 133.6 U/ml (reference range, 0–35 U/ml) and 47.3 ng/ml (reference range, 0–30 ng/ml), respectively. Other routine laboratory tests, such as white blood count, and levels of creatinine, aminotransferases, C-reactive protein and vasculitis antibodies were normal. The cryptococcal capsular antigen and galactomannan test were negative. Sputum cultures for both fungi and bacteria were also negative. Chest computed tomography (CT) imaging showed multiple ground-glass opacities, and cystic and cavitated lesions in both lungs (Figure 1A). Bronchoscopic examination revealed clear bronchi and no neoplasms. A mass at the subbranch of the right lower lobe bronchial dorsal area was explored using endobronchial ultrasound (Figure 2). Cultures of the bronchoalveolar lavage fluid (BALF) for bacteria, fungi and acid-fast bacilli were all negative. The cell classification in BALF was as follows: neutrophils, 92%; lymphocytes, 4%; mesothelial, 1%; and fungal spores were observed in phagocytes (Figure 3A). Histological examination revealed chronic granulomatous inflammation in the right lower dorsal bronchus. Periodic acid-Schiff and Giemsa staining of bronchoscopic tissue were negative. Next generation sequencing (NGS) of the BALF was performed to confirm T. marneffei pulmonary infection with 456 sequence numbers. Approximately 1 week later, based on the temperature-dependent growth characteristics of the pathogen and the production of soluble red pigment, the patient was finally diagnosed with T. marneffei pulmonary infection (Figures 3B–3D).
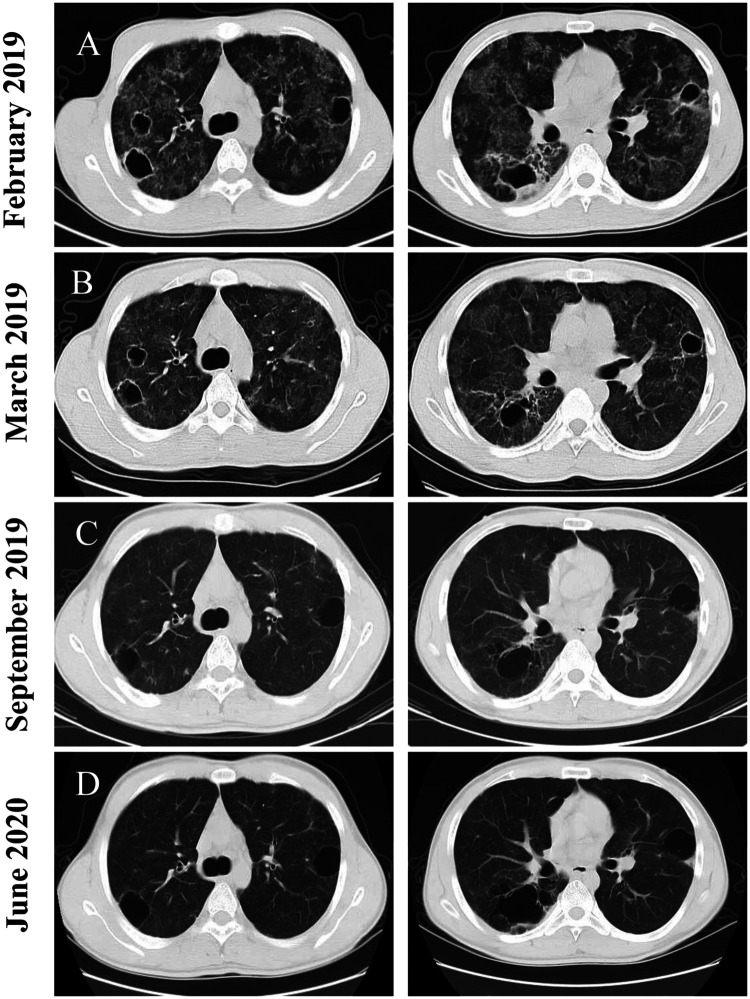
Figure 1.
Representative computed tomography (CT) imaging scans of a 24-year-old male patient that presented with a 6-month history of recurrent cough and expectoration without fever, shortness of breath or chest pain. (A) CT scan taken in February 2019 showing multiple ground glass opacities, cystic changes and cavitated lesions in both lungs. (B) CT scan taken in March 2019 showing the ground glass shadows were absorbed. (C & D) CT scans taken during follow-up in September 2019 and June 2020, respectively, showing distinct resolution of lesions in both lungs, except for the cystic lesions.
Figure 2.
Representative bronchoscopic examinations of a 24-year-old male patient that presented with a 6-month history of recurrent cough and expectoration without fever, shortness of breath or chest pain demonstrated that both bronchi were clear (A–D). Endobronchial ultrasound showing a mass at the subbranch of the right lower lobe bronchial dorsal (E). The colour version of this figure is available at: http://imr.sagepub.com.
Figure 3.
Cultures of the bronchoalveolar lavage fluid (BALF) from a 24-year-old male patient that presented with a 6-month history of recurrent cough and expectoration without fever, shortness of breath or chest pain. (A) Fungal spores seen in phagocytes from BALF (red arrow) (scale bar 15 µm). (B) Culture of BALF revealed Talaromyces marneffei, which showed temperature-dependent dimorphic growth characteristics and the cells produced a soluble red pigment at 25 °C. (C) A mycelium was produced at temperatures between 25 °C and 30 °C. (D) Fungus growing as yeast-like cells at 37 °C. The colour version of this figure is available at: http://imr.sagepub.com.
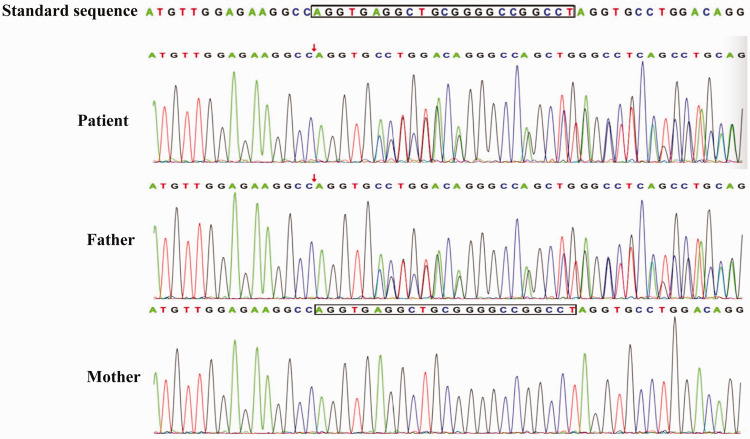
When considering that the patient was young and his HIV test was negative, it was not possible to exclude congenital immunodeficiency. Blood exon sequencing was performed to identify possible TSC2 gene variations. The patient’s father had a similar gene mutation to that identified in the patient. However, his mother presented with the wild-type gene sequence (Figure 4 and Table 1). Finally, the patient was diagnosed with T. marneffei infection and a TSC2 mutation. Consequently, the patient received 200 mg voriconazole every 12 h for 2 weeks via intravenous administration. The CT re-examination in March 2019 showed that the ground glass shadows were absorbed when compared with the results observed in February 2019, while the cavities in the upper lobes of both lungs still existed (Figure 1B). The patient was then prescribed 200 mg voriconazole orally every 12 h until September 2019. The CT performed in September 2019 (Figure 1C) showed a clear resolution of the lesions in both lungs. However, cystic lesions still existed in both lungs. His cough and expectoration symptoms were significantly alleviated. Consequently, the patient stopped voriconazole treatment in September 2019. Follow-up CT examination showed a stable pulmonary lesion in June 2020 (Figure 1D).
Figure 4.
Sanger sequencing verification results for a 24-year-old male patient and his parents. The patient and his father carried a mutation in the tuberous sclerosis complex subunit 2 (TSC2) gene and his mother was wild-type. The colour version of this figure is available at: http://imr.sagepub.com.
Table 1.
Heterozygous mutation in exome regions of the tuberous sclerosis complex subunit 2 (TSC2) gene identified by Sanger sequencing a 24-year-old male patient and his father.
| Patient and the family genetic mutation | ||||
|---|---|---|---|---|
| Gene | Inheritance mode | Patient* | Father* | Mother* |
| TSC2 | Unknown | Heterozygous 135/361 | Heterozygous 152/329 | Wild-type 0/200 |
| Detailed gene information | ||||
| Gene | Chromosomal location | Nucleic acid changes (exon number) | Amino acid changes (variant number) | ACMG |
| TSC2 | Chr16:2135322-2135344 | c.4661(exon36)_c.4662 +21(IVS36)del AGGTGAG GCTGCGGGGC CGGCCT | p.Q1554Qfs 11(p.Gln15 54Glnfs11) (NM_000548) | Pathogenic |
*The carrying state of TSC2 and the ratio of variation depth to total depth.
ACMG, American College of Medical Genetics and Genomics.
This study was approved by the Ethical Review Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (no. 2021IIT174). The patient provided written informed consent for publication of this case report.
Discussion
Talaromyces marneffei is prevalent in Southern China and Southeast Asia.2,5 It is an opportunistic fungal infection most commonly reported in immunocompromised hosts that are HIV-positive, with malignancies, post-organ transplantation and autoimmune disease. 10 It is rarely reported in immunocompetent patients. 11 However, the incidence of T. marneffei has been increasing in immunocompetent hosts.8,9,12 A previous study reported that 22 out of 34 non-HIV patients infected with T. marneffei had no previously known diseases and no immunodeficiencies. 13 This current case report describes a relatively rare case of an immunocompetent patient with T. marneffei lung infection and a TSC2 mutation. The application of NGS in BALF assisted in the rapid diagnosis of T. marneffei.
The typical clinical manifestations of T. marneffei infection include fever, skin lesions, hepatosplenomegaly and respiratory symptoms. However, the severity of the disease depends on the immune status of the patients.2,14 The imaging changes in T. marneffei vary and can include ground glass shadows, cavitated lesions, inflammatory exudates, nodules and cystic changes. 9 In the current case, the CT imaging showed glass shadows with cavitated lesions and cystic changes. After treatment with voriconazole for 7 months, the ground glass shadows were significantly absorbed while the cystic lesions remained. The change in the cavitation caused by the T. marneffei pulmonary infection was a long process of absorption. The relationship between the TSC2 mutation and cystic changes in both lungs in this patient remains unclear.
In this current case, a mutation in the TSC2 gene was identified by Sanger sequencing. The inactivation of TSC1 and TSC2 leads to tuberous sclerosis. 15 The TSC1 gene, which encodes hamartin (130 kDa), is located on chromosome 9q34, encompassing 55 kb of DNA. 16 The TSC2 gene is located on chromosome 16p13.3, encompasses 40 kb of DNA, and encodes tuberin (200 kDa).16,17 More than 900 TSC2 mutations have been recorded in the Human Gene Mutation database.18–21 Most mutations are missense mutations, gross deletions and splicing mutations.22,23 The genotypes of tuberous sclerosis are highly heterogeneous and no common TSC2 gene mutation has been found. 24 In this current case, TSC2 exon 36 was important for T. marneffei pulmonary infection. It is well known that disruption of hamartin and tuberin induces upregulation of signal transduction through mTOR. 25 The mTOR signalling pathway is a highly conserved intracellular signalling pathway in evolution, which is involved in the conduction of multiple signalling pathways, including the PI3K/AKT/mTOR pathway, AKT/TSC1-TSC2/Rheb/mTOR pathway, LKB1-AMPK-TSC-MTOR pathway and FGF-10-Spry2-MtorC1-Stat3/HIF-1-VEGF-A pathway.26,27 These signalling pathways may be involved in lung development and the regulation of a variety of lung diseases.28,29 Therefore, abnormalities in the mTOR signalling pathway, which are mediated by TSC2 mutations, may induce bronchopulmonary dysplasia (BPD, such as bronchiectasis and cystic changes in the lung), leading to T. marneffei invasion and lung infection in the host. 30 One limitation of the current case study was that it was not possible to determine how important the role of the TSC2 mutation was in this current case, but the results suggested that the TSC2 mutation increased susceptibility to BPD and microbial infections in the lungs, a direction that will be explored in future research.
In conclusion, an increased incidence of T. marneffei infection in non-HIV patients has been observed in recent years. Patients with TSC2 mutations that induce BPD may be potential hosts for T. marneffei infection. Rapid diagnosis at an early stage is important for improving prognosis. The application of NGS played a critical role in the diagnosis of T. marneffei pulmonary infection, showing the clinical importance of this technology in the rapid identification of the aetiology. However, the pathogenesis and mechanism of T. marneffei pulmonary infection in HIV-negative patients with TSC2 mutations remain to be investigated. For HIV-negative patients, the possibility of co-existing genetic mutations should be considered and screened for.
Acknowledgements
We thank the relatives of the patient for allowing us to share his medical history and clinical course.
Author contributions: Q.S. and J.Y.Z. provided patient information; Q.S. collected the data; and Q.S. and J.Y.Z. were responsible for study conception and design and acquisition of financial support. L.Y.S. critically revised the manuscript for intellectual content. All authors contributed to the manuscript writing and editing and gave it final approval.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This work was supported by a grant from the Medicine and Health Project of Zhejiang Province, China (no. 2018KY061).
ORCID iD: Jianying Zhou https://orcid.org/0000-0002-8924-935X
References
- 1.Lau SKP, Tsang CC, Woo PCY. Talaromyces marneffei Genomic, Transcriptomic, Proteomic and Metabolomic Studies Reveal Mechanisms for Environmental Adaptations and Virulence. Toxins (Basel ) 2017; 9: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175: 57–67. [DOI] [PubMed] [Google Scholar]
- 3.Vanittanakom N, Cooper CR, Jr, Fisher MC, et al. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev 2006; 19: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasricha S, MacRae JI, Chua HH, et al. Extensive Metabolic Remodeling Differentiates Non-pathogenic and Pathogenic Growth Forms of the Dimorphic Pathogen Talaromyces marneffei. Front Cell Infect Microbiol 2017; 7: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li HR, Cai SX, Chen YS, et al. Comparison of Talaromyces marneffei Infection in Human Immunodeficiency Virus-positive and Human Immunodeficiency Virus-negative Patients from Fujian, China. Chin Med J (Engl) 2016; 129: 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 2019; 184: 709–720. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Qin F, Meng S, et al. Effects of cotrimoxazole prophylaxis on Talaromyces marneffei infection in HIV/AIDS patients receiving antiretroviral therapy: a retrospective cohort study. Emerg Microbes Infect 2019; 8: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y. A Talaromyces marneffei infection with osteolytic lesions in an HIV-negative patient at non-endemic areas: A case report. SAGE Open Med Case Rep 2020; 8: 2050313X20938242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Ye J, Qiu C, et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: a case report. Ther Adv Respir Dis 2020; 14: 1753466620929225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Bi X, Zou X, et al. Retrospective analysis of 15 cases of Penicilliosis marneffei in a southern China hospital. Mycopathologia 2014; 177: 271–279. [DOI] [PubMed] [Google Scholar]
- 11.Cheng D, Ding X. Rare misdiagnosed case of penicilliosis marneffei in an immunocompetent patient. J Int Med Res 2020; 48: 300060520959484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu Y, Liao H, Zhang J, et al. Differences in clinical characteristics and prognosis of Penicilliosis among HIV-negative patients with or without underlying disease in Southern China: a retrospective study. BMC Infect Dis 2015; 15: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y, Huang X, Chen H, et al. Burden of Talaromyces marneffei infection in people living with HIV/AIDS in Asia during ART era: a systematic review and meta-analysis. BMC Infect Dis 2020; 20: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salussolia CL, Klonowska K, Kwiatkowski DJ, et al. Genetic Etiologies, Diagnosis, and Treatment of Tuberous Sclerosis Complex. Annu Rev Genomics Hum Genet 2019; 20: 217–240. [DOI] [PubMed] [Google Scholar]
- 16.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997; 277: 805–808. [DOI] [PubMed] [Google Scholar]
- 17.van Slegtenhorst M, Nellist M, Nagelkerken B, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet 1998; 7: 1053–1057. [DOI] [PubMed] [Google Scholar]
- 18.The Human Gene Mutation Database, http://www.hgmd.org (available since 2007, accessed May 2021).
- 19.Krawczak M, Cooper DN. The human gene mutation database. Trends Genet 1997; 3: 121–122. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Pesini E, Lott MT, Procaccio V, et al. An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Res 2007; 35: D823–D828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenson PD, Mort M, Ball EV, et al. The Human Gene Mutation Database (HGMD®): optimizing its use in a clinical diagnostic or research setting. Hum Genet 2020; 139: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosset C, Netto CBO, Ashton-Prolla P. TSC1 and TSC2 gene mutations and their implications for treatment in Tuberous Sclerosis Complex: a review. Genet Mol Biol 2017; 40: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Zhou W, Shi J, et al. TSC1 and TSC2 Gene Mutations in Chinese Tuberous Sclerosis Complex Patients Clinically Characterized by Epilepsy. Genet Test Mol Biomarkers 2020; 24: 1–5. [DOI] [PubMed] [Google Scholar]
- 24.van Eeghen AM, Black ME, Pulsifer MB, et al. Genotype and cognitive phenotype of patients with tuberous sclerosis complex. Eur J Hum Genet 2012; 20: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northrup H, Krueger DA. and International Tuberous Sclerosis Complex Consensus G . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer 2018; 18: 744–757. [DOI] [PubMed] [Google Scholar]
- 27.Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 2015; 25: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac Cancer 2020; 11: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Xu Q, Wan H, et al. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab Invest 2020; 100: 801–811. [DOI] [PubMed] [Google Scholar]
- 30.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol 2005; 16: 29–37. [DOI] [PubMed] [Google Scholar]