Abstract
Background
Point-of-care testing (POCT) is diagnostic testing performed at or near to the site of the patient. Understanding the current capacity, and scope, of POCT in this setting is essential in order to respond to new research evidence which may lead to wide implementation.
Methods
A cross-sectional online survey study of POCT use was conducted between 6th January and 2nd February 2020 on behalf of two United Kingdom (UK) and Ireland-based paediatric research networks (Paediatric Emergency Research UK and Ireland, and General and Adolescent Paediatric Research UK and Ireland).
Results
In total 91/109 (83.5%) sites responded, with some respondents providing details for multiple units on their site based on network membership (139 units in total). The most commonly performed POCT were blood sugar (137/139; 98.6%), urinalysis (134/139; 96.4%) and blood gas analysis (132/139; 95%). The use of POCT for Influenza/Respiratory Syncytial Virus (RSV) (45/139; 32.4%, 41/139; 29.5%), C-Reactive Protein (CRP) (13/139; 9.4%), Procalcitonin (PCT) (2/139; 1.4%) and Group A Streptococcus (5/139; 3.6%) and was relatively low. Obstacles to the introduction of new POCT included resources and infrastructure to support test performance and quality assurance.
Conclusion
This survey demonstrates significant consensus in POCT practice in the UK and Ireland but highlights specific inequity in newer biomarkers, some which do not have support from national guidance. A clear strategy to overcome the key obstacles of funding, evidence base, and standardising variation will be essential if there is a drive toward increasing implementation of POCT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12873-021-00556-7.
Keywords: Technology, Molecular biology, Health services research, Data collection
Background
There is a need for clinicians to make accurate and timely decisions regarding emergency management of their patients. Laboratory tests are often used, in conjunction with clinical findings, to determine the most appropriate care pathway. Delays in obtaining and reporting urgent samples can lead to department crowding, protracted discharge times, and failure to deliver optimal patient care in emergency and acute care settings [1–3]. Point-Of-Care Testing (POCT) has the potential to provide rapid and accurate results that reduce such delays [4–6]. Potential additional benefits include improved clinical management, treatment adherence, and patient satisfaction [5] which must be balanced against the clinical significance of time gained. For example, in the context of an over crowded department with long waiting times does a 15 min time to result improve outcomes for patients? Also there are concerns regarding reliability and cost of POCT compared with centralised laboratory testing, and appropriate governance of POCT. There is currently no clearly described consensus approach to procurement, implementation and governance of POCT in the UK and Ireland; similar lack of consensus is evident in medical literature internationally [7–11].
However emerging evidence and ongoing research continue to evaluate the potential impact of POCT. Should these tests prove to have good clinical utility, they are likely to be incorporated into national guidance, and clinical practice, more widely. Developing a framework for the clinical use, implementation, and governance of POCT is therefore essential within the healthcare system, especially those aiming to prioritise same day emergency care. It remains the case that many Emergency Departments serve both adult and paediatric populations, and POCT may serve the needs of both patient groups. This works well for test common to both age categories (such as glucose) but perhaps not so well for respiratory testing which is anecdotally more common place in paediatric practice. As no survey of POCT has previously been performed across Children’s Acute and Emergency Care, it is important to establish whether this is truly the case, across a widely inclusive range of POCTs.
The primary aim of this study was to describe POCT in current use in acute paediatric settings across the UK and Ireland. Secondary objectives were to examine implementation, maintenance, funding and governance and further opinions on the introduction of new POCT including obstacles and enablers.
Methods
This online cross sectional survey was conducted between 6th January and 2nd February 2020, and is reported in accordance with the Checklist for Reporting Results of Internet E-Surveys (CHERRIES [12]).
Existing literature on use of POCT in acute paediatric settings [9–11] informed the initial survey content, which was subsequently refined iteratively by the study team. In the absence of international guidance, content was finalised by consensus of the study team following external review, and prior to launch the survey underwent external piloting. This rationlised the number of questions and determined the range of POCTs to be surveyed. The survey was distributed to member sites of the General Adolescent and Paediatric Research in the UK & Ireland (GAPRUKI) and Paediatric Emergency Research in the UK & Ireland (PERUKI [13]) networks, with one response for each relevant emergency or acute paediatric unit requested from each network site (not all sites were members of both networks). Responses could be provided on behalf of Emergency Departments, Paediatric Assessment Units, Paediatric ward settings, and Urgent Care Centres.
For the purposes of this survey, POCT was defined as an investigative or diagnostic test utilised by staff in a clinical environment, for which results are available in a short time (within 30 min) to aid clinical decision making in that setting (i.e. not at a later date/time). Additional detail in this definition stated that they should be performed and interpreted by clinical staff caring for the patient, not sent elsewhere for other personnel to analyse and interpret, and should require no other interpretation (i.e. the result is binary, sequential or categorical).
The full survey, available in Appendix 1, included questions on availability of a range of POCT across clinical settings. Adaptive questioning was used, and where applicable, respondents were asked questions on personnel performing and interpreting tests, and POCT governance. All respondents were asked to provide the view of their site on the potential benefits and challenges presented by the concept of expanding POCT use, and were asked to describe any obstacles or enablers from previous experience of implementing POCT.
Responses were collected in Research Electronic Data Capture tools (REDCap [14, 15]), and were held on a secure University of Bristol server. We followed a standard framework; open for 4 weeks with non-responders sent reminders with 2 weeks to go, 1 week to go, and 48 h to go. Responses were analysed anonymously by the study team using Microsoft Excel version 16.42). Data are presented using descriptive statistics, including number and proportion for categorical variables.
As this survey study contained no patient level data, and was distributed using professional collaborative professional networks, ethical approval was unnecessary according to the Health Research Authority framework decision tool [16] .
Results
In total, 109 invites were sent, to which there were 91 (83.5%) responses across 139 units (Fig. 1, each clinician could respond for more than one unit on their site), contributing data regarding 72 Emergency Departments (including Observation Units), 28 Paediatric Assessment Units, 34 Paediatric Inpatient wards, and five Urgent Care Centres.
Fig. 1.
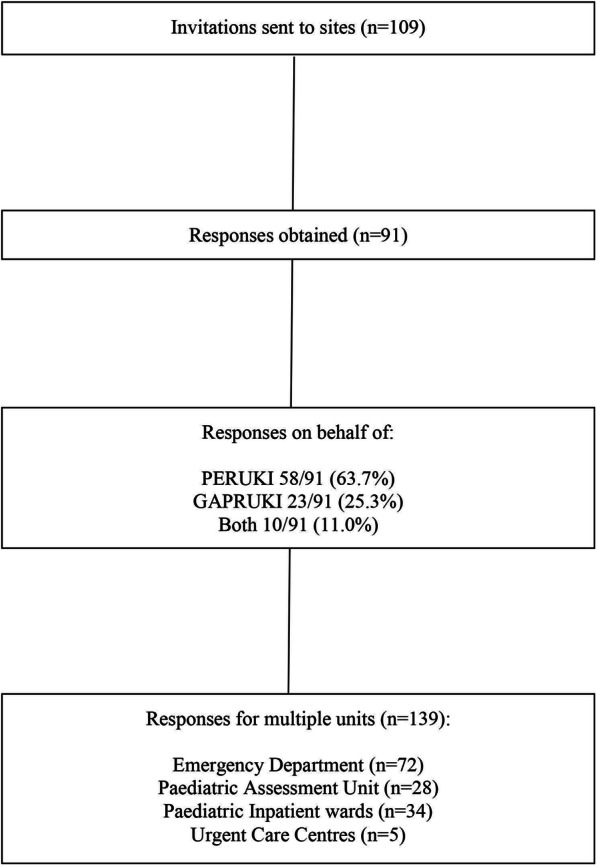
Flow diagram showing the flow of responses
There were a range of POCT available across sites, including those available in testing modalities analysing more than one marker simultaneously (for example, blood gas analysis variables) (Table 1). The most commonly performed POCT were blood sugar measurement (137/139; 98.6%), urinalysis (134/139; 96.4%) and blood gas analysis (132/139; 95%). In blood gas analysers, most sites had access to Lactate (127/139; 91.4%), pH/PaCO2/PaO2/Base Excess (126/139; 90.6%), Sodium/Potassium (126/139; 90.6%), Glucose (123/139; 88.5%) and Haemoglobin (116/139; 83.5%).
Table 1.
Point Of Care Tests (POCT) done and investigations available on blood gas analysers
| POCTs | Total (n = 139) | ED (n = 72) | PAU (n = 28) | UCC (n = 5) | Inpatient ward (n = 34) |
|---|---|---|---|---|---|
| Blood sugar | 137/139 (98.6%) | 72/72 (100%) | 27/28 (96.4%) | 5/5 (100%) | 33/34 (97.1%) |
| Urinalysis | 134/139 (96.4%) | 70/72 (97.2%) | 27/28 (96.4%) | 5/5 (100%) | 32/34 (94.1%) |
| Blood gas analysis | 132/139 (95%) | 72/72 (100%) | 25/28 (89.3%) | 3/5 (60%) | 32/34 (94.1%) |
| Blood ketones | 125/139 (89.9%) | 66/72 (91.7%) | 25/28 (89.3%) | 3/5 (60%) | 31/34 (91.2%) |
| Urinary Beta hCG | 115/139 (82.7%) | 66/72 (91.7%) | 20/28 (71.4%) | 5/5 (100%) | 24/34 (70.6%) |
| Influenza (any) | 45/139 (32.4%) | 29/72 (40.3%) | 7/28 (25%) | 1/5 (20%) | 8/34 (23.5%) |
| RSV | 41/139 (29.5%) | 24/72 (33.3%) | 7/28 (25%) | 1/5 (20%) | 9/34 (26.5%) |
| Other | 17/139 (12.2%) | 15/72 (20.8%) | 2/28 (7.1%) | 0/5 (0%) | 0/34 (0%) |
| CRP | 13/139 (9.4%) | 7/72 (9.7%) | 3/28 (10.7%) | 0/5 (0%) | 3/34 (8.8%) |
| Group A Streptococcus | 5/139 (3.6%) | 3/72 (4.2%) | 1/28 (3.6%) | 0/5 (0%) | 1/34 (2.9%) |
| Procalcitonin | 2/139 (1.4%) | 1/72 (1.4%) | 0/28 (0%) | 0/5 (0%) | 1/34 (2.9%) |
| Blood gas analyser investigations available among units doing blood gases | |||||
| Lactate | 127/139 (91.4%) | 69/72 (95.8%) | 25/28 (89.3%) | 3/5 (60%) | 30/34 (88.2%) |
| pH, PaCO2/PaO2, Base Excess | 126/139 (90.6%) | 69/72 (95.8%) | 25/28 (89.3%) | 3/5 (60%) | 29/34 (85.3%) |
| Sodium/Potassium | 126/139 (90.6%) | 69/72 (95.8%) | 24/28 (85.7%) | 3/5 (60%) | 30/34 (88.2%) |
| Glucose | 123/139 (88.5%) | 68/72 (94.4%) | 23/28 (82.1%) | 3/5 (60%) | 29/34 (85.3%) |
| Haemoglobin | 116/139 (83.5% | 67/72 (93%) | 21/28 (75%) | 3/5 (60%) | 25/34 (73.5%) |
| Calcium | 107/139 (77%) | 62/72 (86.1%) | 19/28 (67.9%) | 3/5 (60%) | 23/34 (67.6%) |
| Bilirubin | 46/139 (33.1%) | 25/72 (34.7%) | 11/28 (39.3%) | 1/5 (20%) | 9/34 (26.5%) |
| Phosphate | 27/139 (19.2%) | 18/72 (25%) | 2/28 (7.1%) | 1/5 (20%) | 6/34 (17.6%) |
| Other: | 23/139 (16.5%) | 18/72 (25%) | 3/28 (10.7%) | 0/5 (0%) | 2/34 (5.9%) |
ED Emergency Department, PAU Paediatric Assessment Unit, UCC Urgent Care Centre, hCG human Chorionic Gonadotrophin, RSV Respiratory Syncytial Virus, CRP C-reactive protein
The use of Influenza/Respiratory Syncytial Virus (RSV) POCT were available in approximately one-third of sites (45/139; 32.4%, and 41/139; 29.5%, respectively), whilst availability of POCT for other biomarkers including C - reactive protein (CRP) (13/139; 9.4%), Group A Streptococcus (5/139; 3.6%) and Procalcitonin (PCT) (2/139; 1.4%) was markedly lower.
A description of staff types performing and interpreting tests is provided in Table 2; this is further quantified by clinical area in Supplementary Tables 5 and 6. As multiple staff groups could be selected for each POCT for each unit, there was a total of 2132 responses relating to staff roles in performance of POCT, and 3097 relating to actioning of results. Clinical nurses were the staff group most commonly responsible for POCT performance (705/2132; 33.1%) followed by Emergency Nurse Practitioners (ENP)/Advanced Nurse Practitioners (ANP) (435/2132, 20.4%) and junior doctors (385/2132; 18.1%). POCT were mostly acted on by senior non-consultants (736/3097; 23.8%), consultants (736/3097; 23.8%) and junior trainees (702/3097; 22.7%).
Table 2.
Staff members responsible for performing and acting on POCT results
| Staff members who perform each POCT | ||||||||
| Clinical Nurse | Healthcare assistant | ENP/ACP | Junior Doctor | Consultant | Other | Total | ||
| Blood sugar | 135/325 (41.5%) | 2/325 (0.6%) | 71/325 (21.8%) | 61/325 (18.8%) | 54/325 (16.6%) | 2/325 (0.6%) | 325 (100%) | |
| Urinalysis | 134/444 (30.2%) | 87/444 (19.6%) | 85/444 (19.1%) | 74/444 (16.7%) | 59/444 (13.3%) | 5/444 (1.1%) | 444 (100%) | |
| Blood gas analysis | 107/471 (22.7%) | 46/471 (9.8%) | 86/471 (18.3%) | 121/471 (25.7%) | 109/471 (23.1%) | 2/471 (0.4%) | 471 (100%) | |
| Blood Ketones | 120/292 (41.1%) | 0/292 (0%) | 70/292 (24%) | 52/292 (17.8%) | 47/292 (16.1%) | 3/292 (1%) | 292 (100%) | |
| Urinary Beta HCG | 115/350 (32.9%) | 86/350 (24.6%) | 70/350 (20%) | 43/350 (12.3%) | 34/350 (9.7%) | 2/350 (0.6%) | 350 (100%) | |
| Influenza | 45/105 (42.9%) | 19/105 (18.1%) | 22/105 (21%) | 10/105 (9.5%) | 9/105 (8.6%) | 0/105 (0%) | 105 (100%) | |
| RSV | 39/84 (46.4%) | 14/84 (16.7%) | 17/84 (20.2%) | 7/84 (8.3%) | 5/84 (6%) | 2/84 (2.4%) | 84 (100%) | |
| CRP | 6/43 (14%) | 2/43 (4.7%) | 11/43 (25.6%) | 13/43 (30.2%) | 11/43 (25.6%) | 0/43 (0%) | 43 (100%) | |
| GAS | 4/14 (28.6%) | 3/14 (21.4%) | 3/14 (21.4%) | 2/14 (14.3%) | 2/14 (14.3%) | 0/14 (0%) | 14 (100%) | |
| Pct | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 2/4 (50%) | 2/4 (50%) | 0/4 (0%) | 4 (100%) | |
| Total | 705/2132 (33.1%) | 259/2132 (12.1%) | 435/2132 (20.4%) | 385/2132 (18.1%) | 332/2132 (15.6%) | 16/2132 (0.8%) | 2132 (100%) | |
| Staff who are responsible for acting on POCT results | ||||||||
| Clinical Nurse | Healthcare assistant | ENP/ACP | Junior Trainee (eg ST1–3) | Senior non-Consultant (eg ST4+) | Consultant | Other | Total | |
| Blood sugar | 97/601 (16.1%) | 10/601 (1.7%) | 93/601 (15.5%) | 132/601 (22%) | 135/601 (22.5%) | 132/601 (22%) | 2/601 (0.3%) | 601 (100%) |
| Urinalysis | 59/555 (10.6%) | 13/555 (2.3%) | 91/555 (16.4%) | 128/555 (23.1%) | 132/555 (23.8%) | 130/555 (23.4%) | 2/555 (0.4%) | 555 (100%) |
| Blood gas analysis | 33/493 (67.5%) | 4/493 (0.8%) | 80/493 (16.2%) | 120/493 (24.3%) | 127/493 (25.8%) | 127/493 (25.8%) | 2/493 (0.4%) | 493 (100%) |
| Blood Ketones | 62/522 (11.9%) | 8/522 (1.5%) | 86/522 (16.5%) | 118/522 (22.6%) | 124/522 (23.8%) | 123/522 (23.6%) | 1/522 (0.2%) | 522 (100%) |
| Urinary Beta hCG | 44/465 (9.5%) | 8/465 (1.7%) | 78/465 (16.8%) | 109/465 (23.4%) | 113/465 (24.3%) | 111/465 (23.9%) | 2/465 (0.4%) | 465 (100%) |
| Influenza | 34/206 (16.5%) | 7/206 (3.4%) | 35/206 (17%) | 40/206 (19.4%) | 45/206 (21.8%) | 44/206 (21.4%) | 1/206 (0.5%) | 206 (100%) |
| RSV | 32/181 (17.7%) | 3/181 (1.7%) | 29/181 (16%) | 36/181 (19.9%) | 40/181 (22.1%) | 40/181 (22.1%) | 1/181 (0.6%) | 181 (100%) |
| CRP | 0/48 (0%) | 0/48 (0%) | 10/48 (20.8%) | 12/48 (25%) | 13/48 (27.1%) | 13/48 (27.1%) | 0/48 (0%) | 48 (100%) |
| GAS | 0/19 (0%) | 0/19 (0%) | 4/19 (21.1%) | 5/19 (26.3%) | 5/19 (26.3%) | 5/19 (26.3%) | 0/19 (0%) | 19 (100%) |
| Pct | 0/7 (0%) | 0/7 (0%) | 1/7 (14.3%) | 2/7 (28.6%) | 2/7 (28.6%) | 2/7 (28.6%) | 0/7 (0%) | 7 (100%) |
| Total | 361/3097 (11.7%) | 53/3097 (1.7%) | 507/3097 (16.4%) | 702/3097 (22.7%) | 736/3097 (23.8%) | 727/3097 (23.5%) | 11/3097 (0.4%) | 3097 (100%) |
POCT Point-of-Care Test, ENP Emergency nurse practitioner, ANP Advanced nurse practitioner, ST Specialty trainee, hCG human Chorionic Gonadotrophin, RSV Respiratory Syncytial Virus, CRP C-reactive protein, GAS Group A Streptococcus, Pct Procalcitonin
Information regarding the non-clinical utilisation of POCT is provided in Table 3, with 561 responses related to governance, and 677 responses related to data storage.
Table 3.
Responsibility for POCT governance and documentation/storage
| Who is responsible for POCT governance? | How and where are POCT results stored? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory team take full responsibility for governance | Clinical staff take some responsibility for governance, in conjunction with laboratory teams | Other | Not applicable | Total | Handwritten in clinical record | Manual entry in electronic record | Printed out and stuck in record | Auto upload to electronic system | Other | Not applicable | Total | |
| Blood sugar | 25/99 (25.3%) | 69/99 (69.7%) | 5/99 (5.1%) | 0/99 (0%) | 99 (100%) | 54/111 (48.6%) | 32/111 (28.8%) | 12/111 (10.8%) | 13/111 (11.7%) | 0/111 (0%) | 0/111 (0%) | 111 (100%) |
| Urinalysis | 22/94 (23.4%) | 66/94 (70.2%) | 5/94 (5.3%) | 1/94 (1.1%) | 94 (100%) | 37/111 (33.3%) | 27/111 (24.3%) | 42/111 (37.8%) | 5/111 (4.5%) | 0/111 (0%) | 0/111 (0%) | 111 (100%) |
| Blood gas | 53/99 (53.5%) | 43/99 (43.4%) | 3/99 (3%) | 0/99 (0%) | 99 (100%) | 23/142 (16.2%) | 12/142 (8.5%) | 64/142 (45.1%) | 42/142 (29.6%) | 1/142 (0.7%) | 0/142 (0%) | 142 (100%) |
| Blood Ketones | 23/92 (25%) | 63/92 (68.5%) | 6/92 (6.5%) | 0/92 (0%) | 92 (100%) | 52/104 (52%) | 32/104 (30.8%) | 9/104 (8.7%) | 11/104 (10.6%) | 0/104 (0%) | 0/104 (0%) | 104 (100%) |
| Urinary Beta hCG | 17/82 (20.7%) | 58/82 (70.7%) | 4/82 (4.9%) | 3/82 (3.7%) | 82 (100%) | 36/91 (39.6%) | 25/91 (27.5%) | 27/91 (29.7%) | 3/91 (3.3%) | 0/91 (0%) | 0/91 (0%) | 91 (100%) |
| Influenza | 20/41 (48.8%) | 21/41 (51.2%) | 0/41 (0%) | 0/41 (0%) | 41 (100%) | 17/50 (34%) | 8/50 (16%) | 11/50 (22%) | 13/50 (26%) | 1/50 (2%) | 0/50 (0%) | 50 (100%) |
| RSV | 16/34 (47.1%) | 18/34 (52.9%) | 0/34 (0%) | 0/34 (0%) | 34 (100%) | 14/45 (31.1%) | 8/45 (17.8%) | 8/45 (17.8%) | 14/45 (31.1%) | 1/45 (2.2%) | 0/45 (0%) | 45 (100%) |
| CRP | 8/12 (66.7%) | 4/12 (33.3%) | 0/12 (0%) | 0/12 (0%) | 12 (100%) | 4/15 (26.7%) | 1/15 (6.7%) | 5/15 (33.3%) | 5/15 (33.3%) | 0/15 (0%) | 0/15 (0%) | 15 (100%) |
| Group A Streptococcus | 1/5 (20%) | 2/5 (40%) | 2/5 (40%) | 0/5 (0%) | 5 (100%) | 3/4 (75%) | 1/4 (25%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 4 (100%) |
| Procalcitonin | 1/3 (33.3%) | 2/3 (66.7%) | 0/3 (0%) | 0/3 (0%) | 3 (100%) | 1/4 (25%) | 0/4 (0%) | 2/4 (50%) | 1/4 (25%) | 0/4 (0%) | 0/4 (0%) | 4 (100%) |
| Total; n (n% of total for each section) | 186/561 (33.2%) | 346/561 (61.7%) | 25/561 (4.5%) | 4/561 (0.7%) | 561 (100%) | 241/677 (35.6%) | 146/677 (21.6%) | 180/677 (26.6%) | 107/677 (15.8%) | 3/677 (0.4%) | 0/677 (0%) | 677 (100%) |
POCT Point-of-Care Test, hCG human Chorionic Gonadotrophin, RSV Respiratory Syncytial Virus, CRP C-reactive protein
Most commonly, clinical staff took some responsibility for POCT governance in conjunction with laboratory teams (346/561; 61.7%), followed by laboratory teams taking full responsibility (186/561; 33.2%). The POCT most likely to come under shared responsibility were urinary human Chorionic Gonadotropin (hCG) (58/82; 70.7%), urinalysis (66/94; 70.2%), blood sugar (69/99; 69.7%) and blood ketones (63/92; 68.5%). POCT which give multiple results (such as blood gases) were primarily managed by laboratory teams (53/99; 53.5%,).
The most common method of data storage was handwritten notes in clinical records (241/677; 35.6%) followed by printouts attached to medical records (180/677; 26.6%) and manual entry (146/677; 21.6%). Automatic uploading to electronic systems occurred in only 15.8% of responses (107/677).
Obstacles to POCT introduction (158 responses), and nature of funding sources (98 responses), are displayed in Table 4. The most commonly reported obstacles were difficulties with funding (72/158; 45.6%), lack of evidence (33/158; 20.9%) and issues with POCT governance (20/158; 12.7%). POCT were typically funded as part of an ongoing service with sustainable long-term funding (81/98; 82.7%). Other methods of funding included temporary funds as part of a service evaluation (10/98; 10.2%), or charitable funding and/or donations (5/98; 5.1%).
Table 4.
Obstacles to introduction of POCT and sources of current POCT funding in units
| Obstacles currently existing to the use of POCT | n | % of 158 responses |
| Difficulties with funding | 72 | 45.6% |
| Evidence is lacking for POCT | 33 | 20.9% |
| Nobody will take responsibility for the governance of the test | 20 | 12.7% |
| Nobody has time to perform the quality control testing | 16 | 10.1% |
| Other | 12 | 7.6% |
| Nobody has time to run the test | 5 | 3.2% |
| Total | 158 | 100% |
| Sources of current POCT funding | % of 98 responses | |
| All funded as part of ongoing service with sustainable longterm funding | 81 | 82.7% |
| Some funded using temporary fund as part of a service evaluation | 10 | 10.2% |
| Some funded through charitable funding and/or donations | 5 | 5.1% |
| Some funded as part of an industry sponsored trial | 1 | 1% |
| Other | 1 | 1% |
| Total | 98 | 100% |
Discussion
We have demonstrated a diverse range of POCT in use in Children’s Emergency Departments and Assessment units. Some POCT, such as blood sugar testing, blood ketone testing, blood gas analysis and urinalysis are fairly commonplace, whilst “newer” POCT such as CRP and Procalcitonin were uncommon. Despite this penetrance of POCT into acute units, we have also identified wide variation in their governance, and usage processes. The challenge of identifying the optimal governance of POCT appears to hinder their implementation, as do a current lack of evidence, and cost; these elements are particularly important when considering introducing POCT to national guidance if emerging evidence supports their use.
Respiratory POCT were utilised in under half the responding units, with Respiratory Syncytial Virus (RSV) and influenza usage at 33.3 and 40.3% respectively. POCT for RSV has previously been evaluated and found to be a safe, cost-effective, and efficient way to improve bed management [17]. The lack of widespread utilisation is, therefore, perhaps surprising. However, some centres cohort infants with bronchiolitis based on symptoms, in which case POCT may conversely delay admission. A formal evaluation of these two approaches would help determine the utility of POCT in this situation. However, it must be recognised that determining viral aetiology is useful for public health surveillance and it is likely that ward based testing will still need to occur.
For influenza, one study of the use of POCT in febrile children showed no difference in physician management, cost, or length of stay in the paediatric Emergency Department (ED) [7]. However, a positive POCT for influenza was associated with a significant reduction in urine and blood cultures being sent for febrile children [18].
Some studies have recommended use of rapid streptococcal A infection testing for patients with a sore throat, citing reduction of antimicrobial use [19, 20]. However, in England, this is not routinely recommended by National Institute of Health and Care Excellence (NICE) guidance, as this approach is unlikely to be cost-effective. Their limited role in improving antimicrobial prescribing and stewardship, as well as patient outcomes, when compared to clinical scores alone [21] is the likely reason for the low use in surveyed sites.
In relation to biomarkers for infection, a systematic review and cost-effectiveness analysis evaluated whether Procalcitonin testing was helpful in guiding antibiotic therapy for sepsis in intensive care and ED settings [22, 23]. This concluded that addition of a Procalcitonin based algorithm to antibiotic guidance could be useful in reducing antibiotic exposure and length of hospital stay safely in adults [22]. Clinicians might infer that similar results may be seen in children, however high quality evidence is lacking, and further research is needed on the utility of Procalcitonin in this domain.
The most common source of funding for POCT was sustainable long term funding as part of an ongoing service commitment for well-established POCT; these included blood gas analysis, urinalysis and blood sugar testing, and are routinely used in > 90% of departments. The newer POCT were more likely to be funded using temporary funds as part of a service evaluation, through charitable funding and/ or donations or as part of an industry sponsored trial.
Given our findings regarding the challenges of implementing new POCT, robust evidence for patient benefit is required in order to provide a clear case to justify National Health Service (NHS) spending on the testing equipment and consumables required to use in routine clinical practice.
We acknowledge the limitations of a survey based qualitative study but believe our response rate and reach were sufficient to avoid significant bias. We acknowledge that some operator training and quality control have been identified as barriers to adoption and we didn’t ask focused questions on these. This was a point-in-time survey and it is likely that the situation in many departments may be different now than at the time the survey was undertaken. However we hope our identification of domains of interests will be useful for future evaluation and research.
In summary, if new POCT are to be introduced, it is vital that all stakeholders are involved in the decision making including clinical and laboratory teams, patients, regulatory authorities and insurers. Sustainability will depend on sound evidence base and financial viability [24].
Conclusions
The use of POCT for blood glucose, blood gas, urinalysis and blood ketones is widespread among UK Children’s Emergency Departments and Assessment units, however newer biomarkers tests are used less often, including those for pathogen identification. Variation exists both in unit practices, and the governance of POCT. A clear strategy to overcome the key obstacles of funding, evidence base, and standardising variation will be essential if there is a drive toward increasing implementation of POCT.
Supplementary Information
Acknowledgements
The following individuals acted as site study leads for PERUKI and/or GAPRUKI in their institution, as listed:
Adrian Boyle, Peter Heinz (Addenbrooke’s Hospital, Cambridge, England); Shrouk Messahel, Dan Hawcutt (Alder Hey Children’s Hospital NHS Foundation Trust, Liverpool, England); Caroline Ponmani (Barking, Havering & Redbridge University Hospitals NHS Trust, England); Chris Bird, Deepthi Jyothish (Birmingham Children’s Hospital, Birmingham, England); Catherine Williams (Bolton NHS Foundation Trust, Bolton, England); Ronan O’Sullivan (Bon Secours Hospital, Cork, Ireland); Elizabeth Jones (Bradford Royal Infirmary, Bradford, England); Mark Lyttle, Nwanneka Sargant (Bristol Royal Hospital for Children Bristol, England); James Ross (Chelsea & Westminster NHS Foundation Trust, London, England); Michael Barrett, Sinead Harty (Children’s Health Ireland at Crumlin, Dublin, Ireland); Turlough Bolger, David Coghlan (Children’s Health Ireland at Tallaght, Dublin, Ireland); Patrick Fitzpatrick, Conor Hensey (Children’s Health Ireland at Temple Street, Dublin, Ireland); Tim Hussan (County Durham & Darlington NHS Foundation Trust, England); Kate Charlick (Derriford Hospital, Plymouth, England); William Verling (Dorset County Hospital, Dorset, England); Peter Christian (East Kent Hospital, England); Matthew Clark (East Sussex NHS Health Trust, England); Bhavni Shah (Epsom General Hospital, Epsom, England); John Criddle, Ronny Cheung (The Evelina London Children’s Hospital, London, England); Roger Alcock (Forth Valley Hospital, Larbert, Scotland); Patrick Aldridge (Frimley Park Hospital, Frimley, England); Russell Peek (Gloucestershire Hospitals NHS Foundation Trust, Gloucester, England); Mark Anderson (Great North Children’s Hospital, Newcastle-upon-Tyne, England); Elizabeth Herrieven (Hull Royal Infirmary, Hull, England); Katherine Jerman, Arshid Murad (James Cook University Hospital, Middlesbrough, England); Charlotte Brown, Andy Marshall (John Radcliffe Hospital, Oxford, England); Fleur Cantle (Kings College Hospital, London, England); Gavin Wilson (Kingston Hospital NHS Foundation Trust, Kingston-upon-Thames, England); Alice Downes (Leeds General Infirmary, Leeds, England); Damian Roland, Srini Bandi (Leicester Royal Infirmary, Leicester, England); Adebayo Da-Costa (Medway Hospital NHS Foundation Trust, Gillingham, England); Ray Barry (Mercy University Hospital, Cork, Ireland); Natasha De Vere (The Mid Yorkshire Hospitals NHS Trust, England); Clare Dieppe (Morriston Hospital, Swansea, Wales); Jane Evans (Norfolk & Norwich University Hospital, Norwich, England); Gayle Hann, Clare Tipper (North Middlesex Hospital, London, England); Bengisu Bassay (Northampton General Hospital, England); Dermot Dalton (Northern Devon Healthcare NHS Trust, Barnstaple, England); Lauren Fraser (Northwick Park Hospital, London, England); Chris Gough (Nottingham University Hospitals NHS Trust, Nottingham, England); Sharryn Gardner (Ormskirk & District General Hospital, Ormskirk, England); Mark Tighe (University Hospitals Dorset, Dorset, England); Darren Ranasinghe, Simon Birch (Queen Alexandra Hospital, Portsmouth, England); Sharon Hall (Queen Elizabeth Hospital, Woolwich, England); Gareth Patton, Steve Turner (Royal Aberdeen Children’s Hospital, Aberdeen, Scotland); Emily Walton (Royal Alexandra Children’s Hospital, Brighton, England); Julie-Ann Maney, Tom Bourke (Royal Belfast Hospital for Sick Children, Belfast, Northern Ireland); Manish Thakker (Royal Berkshire NHS Foundation Trust, Reading, England); Gisela Robinson, Lizzie Starkey (Royal Derby Hospital, Derby, England); Andrew Appelboam (Royal Devon & Exeter Hospital, Exeter, England); Shye Wei Wong (Royal Free Hospital, London, England); Steven Foster, Louisa Pollock (Royal Hospital for Children, Glasgow, Scotland); Jen Browning (Royal Hospital for Children & Young People, Edinburgh, Scotland); Katherine Potier (Royal Manchester Children’s Hospital, Manchester, England); Kirsty Challen (Royal Preston Hospital, Preston, England); Elizabeth Gilby (Royal United Hospital, Bath, England); Lisa Kehler (Royal Wolverhampton NHS Trust, Wolverhampton, England); Sebastian Gray (Salisbury NHS Foundation Trust, Salisbury, England); Shammi Ramlakhan (Sheffield Children’s NHS Foundation Trust, Sheffield, England); Niall Mullen (South Tyneside & Sunderland NHS Foundation Trust, Sunderland, England); Jane Bayreuther, Katrina Cathie (Southampton Children’s Hospital, Southampton, England); Heather Jarman (St George’s University Hospitals NHS Foundation Trust, London, England); Neil Thompson (St Mary’s Hospital, Imperial College Healthcare NHS Trust, London, England); Ami Parikh (The Royal London Hospital, London, England); Siba Paul (Torbay Hospital, England); Sarah Trippick, Alastair Sutcliffe (University College Hospital London, England); Joanne Mulligan (University Hospital Crosshouse, Kilmarnock, Scotland); Sophie Keers (University Hospital Lewisham, London, England); Jeff Morgan (University Hospital of Wales, Cardiff, Wales) Michelle Jacobs (Watford General Hospital, Watford, England); Mike Linney (Western Sussex Hospitals NHS Trust, Chichester, England); Sarah Wilson (Wexham Park Hospital, Slough, England); Erum Jamall (Whittington Health NHS Trust, London, England).
In addition we thank Mai Baquedano (University Hospitals Bristol and Weston NHS Foundation Trust) for her assistance with construction of the study survey, and data management.
“What is already known on this topic”
• POCT has been explored as an adjunctive tool in clinical decision making for a number of acute conditions
• POCT can potentially help in earlier treatment initiation, improved patient outcomes, patient satisfaction, and patient flow through the emergency department but the evidence for benefit to patients is limited.
• Further larger research studies are required to evaluate the newer POCT in more detail.
“What this study adds”
• Among acute paediatric settings, commonly used POCT include blood gases, urinalysis and blood sugar testing, whilst newer POCT such as inflammatory biomarkers and pathogen identification are less frequently used
• POCT is mostly processed and interpreted by clinical teams, though there is wide variation in their governance
• The most commonly perceived obstacles to the use of POCT are lack of funding, evidence base, and infrastructure to support test performance and quality assurance
Abbreviations
- GAPRUKI
General and Adolescent Paediatric Research UK and Ireland
- PERUKI
Paediatric Emergency Research UK and Ireland
- PEMLA group
Paediatric Emergency Medicine Leicester Academic Group
- SAPPHIRE Group
Social Science Applied to Healthcare Improvement Research
- POCT
Point-Of-Care Testing
- UK
United Kingdom
- RSV
Respiratory Syncytial Virus
- CRP
C - Reactive Protein
- PCT
Procalcitonin
- CHERRIES
Checklist for Reporting Results of Internet E-Surveys
- REDCap
Research Electronic Data Capture tools
- ENP
Emergency Nurse Practitioners
- ANP
Advanced Nurse Practitioners
- hCG
human Chorionic Gonadotropin
- ED
Emergency Department
- NICE
National Institute of Health and Care Excellence
- NHS
National Health Service
- PAU
Paediatric Assessment Unit
- UCC
Urgent Care Centre
- ST
Specialty trainee
- GAS
Group A Streptococcus
- IP
Inpatient
Authors’ contributions
ML, KC, AM, TW and DR made substantial contributions to the conception/design of the work; MP performed the statistical analysis and wrote the initial draft; all authors were involved in the acquisition, analysis, and interpretation of data; all authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
As this survey study contained no patient level data, and was distributed using professional collaborative professional networks, ethical approval was unnecessary according to the Health Research Authority framework decision tool.
Patients were not included in the survey; therefore formal consent to participate was not needed. By agreeing to participate in the survey, the consent was presumed by the participating health professional.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Damian Roland, Email: dr98@leicester.ac.uk.
On behalf of GAPRUKI, PERUKI:
Adrian Boyle, Peter Heinz, Shrouk Messahel, Dan Hawcutt, Caroline Ponmani, Chris Bird, Deepthi Jyothish, Catherine Williams, Ronan O’Sullivan, Elizabeth Jones, Mark Lyttle, Nwanneka Sargant, James Ross, Michael Barrett, Sinead Harty, Turlough Bolger, David Coghlan, Patrick Fitzpatrick, Conor Hensey, Tim Hussan, Kate Charlick, William Verling, Peter Christian, Matthew Clark, Bhavni Shah, John Criddle, Ronny Cheung, Roger Alcock, Patrick Aldridge, Russell Peek, Mark Anderson, Elizabeth Herrieven, Katherine Jerman, Arshid Murad, Charlotte Brown, Andy Marshall, Fleur Cantle, Gavin Wilson, Alice Downes, Damian Roland, Srini Bandi, Adebayo Da-Costa, Ray Barry, Natasha De Vere, Clare Dieppe, Jane Evans, Gayle Hann, Clare Tipper, Bengisu Bassay, Dermot Dalton, Lauren Fraser, Chris Gough, Sharryn Gardner, Mark Tighe, Darren Ranasinghe, Simon Birch, Sharon Hall, Gareth Patton, Steve Turner, Emily Walton, Julie-Ann Maney, Tom Bourke, Manish Thakker, Gisela Robinson, Lizzie Starkey, Andrew Appelboam, Shye Wei Wong, Steven Foster, Louisa Pollock, Jen Browning, Katherine Potier, Kirsty Challen, Elizabeth Gilby, Lisa Kehler, Sebastian Gray, Shammi Ramlakhan, Niall Mullen, Jane Bayreuther, Katrina Cathie, Heather Jarman, Neil Thompson, Ami Parikh, Siba Paul, Sarah Trippick, Alastair Sutcliffe, Joanne Mulligan, Sophie Keers, Jeff Morgan, Michelle Jacobs, Mike Linney, Sarah Wilson, and Erum Jamall
References
- 1.Woo J, McCabe JB, Chauncey D, Schug T, Henry JB. The evaluation of a portable clinical analyzer in the emergency department. Am J Clin Pathol. 1993;100:599. doi: 10.1093/ajcp/100.6.599. [DOI] [PubMed] [Google Scholar]
- 2.Sands VM, Auerbach PS, Birnbaum J, Green M. Evaluation of a Portable Clinical Blood Analyzer in the Emergency Department. Acad Emerg Med. 1995;2:172. doi: 10.1111/j.1553-2712.1995.tb03190.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee-Lewandrowski E, Corboy D, Lewandrowski K, Sinclair J, McDermot S, Benzer TI. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center: impact on test turnaround time and patient emergency department length of stay. Arch Pathol Lab Med. 2003;127(4):456–460. doi: 10.5858/2003-127-0456-IOAPSL. [DOI] [PubMed] [Google Scholar]
- 4.Parvin CA, Lo SF, Deuser SM, Weaver LG, Lewis LM, Scott MG. Impact of point-of-care testing on patients’ length of stay in a large emergency department. Clin Chem. 1996;42:711. doi: 10.1093/clinchem/42.5.711. [DOI] [PubMed] [Google Scholar]
- 5.Larsson A, Greig-Pylypczuk R, Huisman A. The state of point-of-care testing: a european perspective. Ups J Med Sci. 2015;120(1):1–10. doi: 10.3109/03009734.2015.1006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. Br Med J. 1998;316(7137):1052–1057. doi: 10.1136/bmj.316.7137.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn AD, Dixon D, Meenan BJ. Barriers to hospital-based clinical adoption of point-of-care testing (POCT): a systematic narrative review. Crit Rev Clin Lab Sci. 2016;53(1):1–12. doi: 10.3109/10408363.2015.1054984. [DOI] [PubMed] [Google Scholar]
- 8.FitzGibbon F, Huckle D, Meenan BJ. Barriers affecting the adoption of point-of-care technologies used in chest pain diagnosis within the UK National Health Service: part 1-user issues. Point Care. 2010;9(2):70–79. doi: 10.1097/POC.0b013e3181d9d7f8. [DOI] [Google Scholar]
- 9.Turner PJ, van den Bruel A, Jones CHD, Plüddemann A, Heneghan C, Thompson MJ, Price CP, Howick J. Point-of-care testing in UK primary care: a survey to establish clinical needs. Fam Pract. 2016;33(4):388–394. doi: 10.1093/fampra/cmw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahm MR, McCaughey E, Li L, Westbrook J, Mumford V, Iles-Mann J, et al. Point-of-care testing across rural and remote emergency Departments in Australia: staff perceptions of operational impact. Stud Health Technol Inform. 2017;239:28. [PubMed] [Google Scholar]
- 11.Rasti R, Nanjebe D, Karlström J, Muchunguzi C, Mwanga-Amumpaire J, Gantelius J, et al. Health care workers’ perceptions of point-of-care testing in a low-income country - A qualitative study in Southwestern Uganda. PLoS One. 2017;12:e0182005. doi: 10.1371/journal.pone.0182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES). J Med Internet Res. 2004;6(3). 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed]
- 13.Lyttle MD, O’Sullivan R, Hartshorn S, Bevan C, Cleugh F, Maconochie I. Pediatric emergency research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child. 2014;99(6):602–603. doi: 10.1136/archdischild-2013-304998. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health Research Authority. Is my study research? Health Research Authority Web Page. 2017.
- 17.Mills JM, Harper J, Broomfield D, Templeton KE. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infect. 2011;77(3):248–251. doi: 10.1016/j.jhin.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Patel P, Laurich VM, Smith S, Sturm J. Point-of-care influenza testing in the pediatric emergency department. Pediatric Emergency Care [Internet]. 2020 Nov 1 [cited 2021 Oct 12];36(11):515–8. Available from: https://journals.lww.com/pec-online/Fulltext/2020/11000/Point_of_Care_Influenza_Testing_in_the_Pediatric.2.aspx [DOI] [PubMed]
- 19.Reinert R R. Rapid streptococcal antigen detection tests. Laboratoriums Medizin/J Lab Medicine 2007;31(6):280–93.
- 20.Stürenburg E, Junker R. Point-of-care testing in microbiology - The advantages and disadvantages of immunochromatographic test strips. Deutsches Arzteblatt. 2009;106:48. doi: 10.3238/arztebl.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner PJ, Heneghan C, Price CP, Yang Y, van den Bruel A, Plüddemann A. Point-of-care tests for group A streptococcus. Diagnostic Evidence Co-operative Oxford. 2015.
- 22.National Institute for Health and Care Excellence. Procalcitonin testing for diagnosing and monitoring sepsis (ADVIA Centaur BRAHMS PCT assay, BRAHMS PCT Sensitive Kryptor assay, Elecsys BRAHMS PCT assay, LIAISON BRAHMS PCT assay and VIDAS BRAHMS PCT assay). DG 18. 2015;(October):1–22.
- 23.Version R. Biomarker-guided duration of Antibiotic Treatment in Children Hospitalised with confirmed or suspected bacterial infection (BATCH Trial). 2020;0–67. Available from: https://www.isrctn.com/ISRCTN11369832
- 24.Luppa PB, Müller C, Schlichtiger A, Schlebusch H. Point-of-care testing (POCT): current techniques and future perspectives. Trends Analytical Chem. 2011;30(6):887–898. doi: 10.1016/j.trac.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].


