Abstract
Sleep can be seen as an environmentally determined, adaptive behavior.
Sleep has been viewed as a maladaptive behavior because it is incompatible with activity required to acquire food, defend against predation, and mate. Yet it appears to be nearly (1) universal among birds and mammals, leading to the assumption that sleep serves an unknown but vital physiological function. However, no function that can explain the huge variation in sleep times within and between species has yet been firmly identified, although many candidates, including reversal of oxidative stress, memory consolidation (2), extension of life span, and removal of various neurotoxins, have been proposed. On page 1654 of this issue, Lesku et al. (3) show that in one species of bird, those that sleep the least gain an advantage—they produce the most offspring.
Male polygynous pectoral sandpipers engage in complex courtship displays and aggressive defense of potential mates over 3-week periods. Lesku et al. observed that during this time, the males show no reduction in activity or degradation of performance despite little or no sleep. This finding and other recent work undermine the dominant paradigm that postulates a vital physiological function for sleep in birds and mammals. Killer whale and dolphin mothers and their calves are continuously active with eyes open for 6 or more weeks after birth. No rebound of inactive behavior follows (4). During this period, the neonates’ brain and body grow to their prodigious size and capacity without any apparent need for sleep-linked detoxification. Adult dolphins working for reward can accurately discriminate between visual stimuli presented at 30-s intervals on their left or right sides, 24 hours per day, for as long as 5 days. During this time, their performance shows no progressive decline. No rebound of inactivity follows the session (5). By contrast, humans whose sleep is interrupted on a similar schedule are dramatically impaired (6), demonstrating the variability of sleep regulation across species. Migrating birds greatly reduce sleep time with intact learning abilities and high rates of performance, with no subsequent sleep rebound (7).
If male sandpipers that are continuously active during the breeding season clearly leave more offspring than males who sleep, why hasn’t natural selection eliminated the sleepier males? An attractive explanation is that the active males are more likely to deplete caloric reserves. Under optimal conditions, this depletion can be prevented by eating more. However, in conditions of food scarcity, the birds that most greatly depleted caloric reserves during mating will be least likely to survive to the next mating season. Thus, in the long run, a dynamic balance should be achieved across a range of sleep times (8, 9).
A similar balance appears to exist in humans. People of similar age, sex, and body build can have very different sleep times. They can also vary in their response to sleep loss, with some being highly impaired, unable to resist sleep, and others showing high levels of functioning despite sleep loss. The effect of sleep deprivation on performance is not strongly related to baseline sleep duration (10). Furthermore, human sleep duration is not linearly related to health, with both high and low values being linked to shortened life span (11). Death of rats due to sleep deprivation may be related to stress rather than sleep loss. Sleep deprivation has not been reported to cause death in pigeons, mice, or in rats deprived by techniques that do not involve waking them frequently at sleep onset. Fatal familial insomnia can cause death in humans, but sleep loss does not appear to be responsible. This disease affects many body organs (8, 12).
So why do most of us feel so poorly when we reduce our sleep time? Natural selection has imposed a certain amount of sleep on us to restrict activity to appropriate times of day and to reduce long-term nonvital energy expenditure. The pressure to sleep operates by reducing brain activity. Although individuals with naturally short sleep times are not at elevated risk compared to those with naturally long sleep times, repeated sleep deprivation below the body’s programmed level is stressful and likely to impair health. Certain hormonal processes are linked to sleep. However, these are not universal, but rather are species and age specific (8, 12).
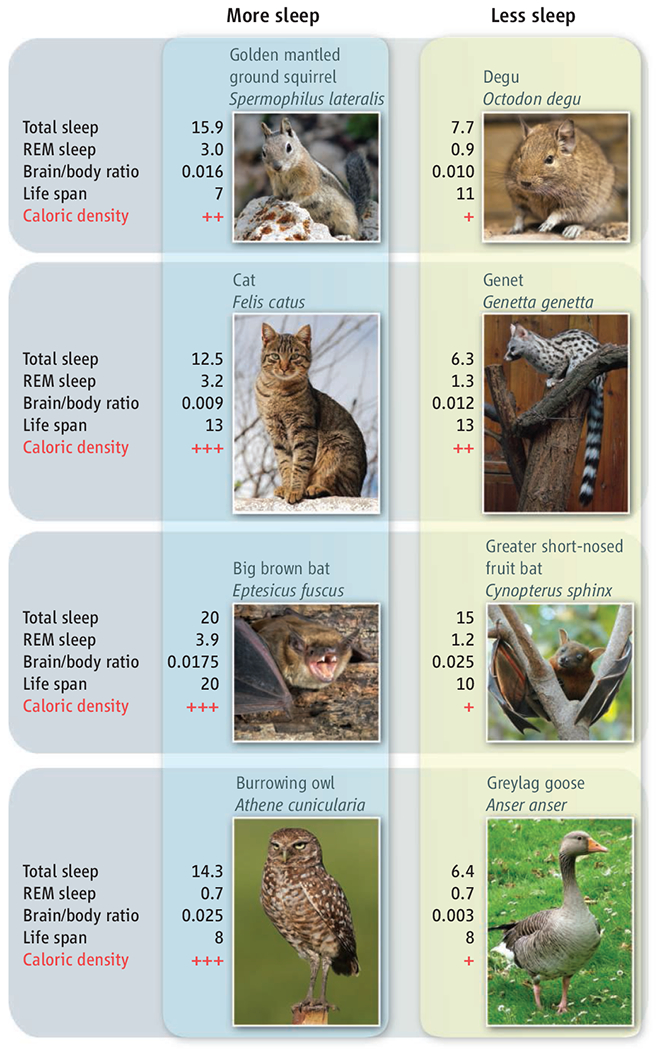
Sleep duration varies enormously across species, with total sleep amount ranging from 20 hours per day in the big brown bat to 2 hours per day in the horse. However, attempts to correlate sleep time with various parameters do not support any sleep physiology theory (8, 12). But species that eat food with low caloric density (e.g., herbivores) sleep less than those eating more nutritionally dense foods (e.g., carnivores) (12) (see the figure).
Quiet waking (with reduced motor activity) could serve the energy conservation functions attributed to sleep without the risks associated with the sleep state. However, the brain consumes an inordinate percentage of metabolic energy during quiet waking, as much as 25% of the body’s energy at rest. Brain energy consumption does not greatly differ between quiet and active waking, but it is greatly reduced in sleep (8). Animals will achieve a selective advantage in reducing brain energy consumption by sleep, but only if they have safe sleeping sites, such as underground burrows. Accordingly, large prey animals that do not have safe sleep sites do not sleep much and sleep very lightly (12).
In addition to mating and migration, sleep can also be reduced during food shortages, presumably to allow animals to invest more time in searching for the available food (13). Species whose environment has a severe seasonal variation in food availability have evolved to increase sleep during periods of food shortage and decrease sleep when food is available. Other species that have safe sites hibernate during periods of greatly reduced food availability, reducing energy expenditure even further (8).
The development of small devices that monitor sleep-related brain and muscle activity is likely to lead to major insights into the regulation of sleep duration under natural conditions (14). Darwin’s evolutionary monograph that underlies so much of our understanding of species survival does not consider the role of sleep in animal evolution. But understanding how the brain regulates sleep in response to behavioral requirements and environmental conditions across species will be a fruitful new paradigm for both sleep research and evolutionary biology.
Sleep across species.
The strongest correlate of sleep time across mammalian orders and avian class is diet. Animals that eat food with high caloric density do not need to spend as much time ingesting food. In zoos and laboratories, where most sleep studies have been done and animals are well fed, carnivores sleep more than omnivores who sleep more than herbivores (12). However, food deprivation increases waking and decreases sleep (13). Flexibility in sleep time increases the likelihood that energy input and output will be equalized and that other essential tasks, such as mating and care of young, will be successful (12). Caloric density of food: carnivores (+++); ominvores (++); herbivores (+). Sleep durations in hours/24; life span in years. REM, rapid eye movement.
Acknowledgments:
Supported by the Medical Research Service of the Department of Veterans Affairs, grants NS069640 and NSF0234687.
References and Notes
- 1.Siegel JM, Trends Neurosci. 31, 208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel JM, Science 294, 1058 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesku JA et al. , Science 337, 1654 (2012); 10.1126/science.1220939 [DOI] [PubMed] [Google Scholar]
- 4.Lyamin O, Pryaslova J, Lance V, Siegel J, Nature 435, 1177 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridgway S et al. , J. Exp. Biol 212, 1519 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH, Neurobiol. Aging 10, 21 (1989). [DOI] [PubMed] [Google Scholar]
- 7.Jones SG, Paletz EM, Obermeyer WH, Hannan CT, Benca RM, BMC Neurosci. 11, 87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel JM, Nat. Rev. Neurosci 10, 747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb WB, Sleep 11, 488 (1988). [DOI] [PubMed] [Google Scholar]
- 10.Van Dongen HPA, Bender AM, Dinges DF,Accid. Anal. Prev 45 (suppl.), 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR, Arch. Gen. Psychiatry 59, 131 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Siegel JM, Nature 437, 1264 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs BL, McGinty DJ, Exp. Neurol 30, 212 (1971). [DOI] [PubMed] [Google Scholar]
- 14.Rattenborg NC et al. , Biol. Lett 4, 402 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


