Abstract
The diagnosis of human herpesvirus 6 (HHV-6) infection represents a complex issue because the most widely used diagnostic tools, such as immunoglobulin G antibody titer determination and qualitative DNA PCR with blood cells, are unable to distinguish between latent (clinically silent) and active (often clinically relevant) infection. We have developed a new, highly sensitive, quantitative PCR assay for the accurate measurement of HHV-6 DNA in tissue-derived cell suspensions and body fluids. The test uses a 5′ nuclease, fluorogenic assay combined with real-time detection of PCR amplification products with the ABI PRISM 7700 sequence detector system. The sensitivity of this method is equal to the sensitivity of a nested PCR protocol (lower detection limit, 1 viral genome equivalent/test) for both the A and the B HHV-6 subgroups and shows a wider dynamic range of detection (from 1 to 106 viral genome equivalents/test) and a higher degree of accuracy, repeatability, and reproducibility compared to those of a standard quantitative-competitive PCR assay developed with the same reference DNA molecule. The novel technique is versatile, showing the same sensitivity and dynamic range with viral DNA extracted from different fluids (i.e., culture medium or plasma) or from tissue-derived cell suspensions. Furthermore, by virtue of its high-throughput format, this method is well suited for large epidemiological surveys.
Human herpesvirus 6 (HHV-6) is a ubiquitous infectious agent isolated for the first time in 1986 from patients with lymphoproliferative disorders (39). Seroepidemiological surveys have demonstrated that HHV-6 is highly prevalent in the human population (30), with primary infection occurring between 6 months and 2 years of age (6, 8, 36). Primary infection is responsible for a febrile and eruptive disease of infancy, exanthem subitum (48), occasionally complicated by meningitis and meningoencephalitis (26, 33), fulminant or chronic hepatitis (5, 47), and idiophatic thrombocytopenic purpura (27). In immunocompetent adults, an HHV-6 etiology has been suggested for several pathological entities such as encephalitis (35; M. Ikusaka, K. Ota, Y. Honma, K. Shibata, S. Uchiyama, and M. Iwata, [Letter, Intern. Med. 36:157, 1997]), fulminant hepatitis (43) Epstein-Barr virus-negative infectious mononucleosis (4, 45), chronic fatigue syndrome (9, 16, 37), and, more recently, multiple sclerosis (2, 3, 11, 29, 44). A large body of evidence suggests that HHV-6 may act as an opportunistic agent in patients with immunodeficiencies, particularly those who have undergone bone marrow or organ transplantation (10, 15, 18, 19, 42) and human immunodeficiency virus-infected individuals (1, 17, 20, 28, 41). Moreover, a role of HHV-6 as a cofactor in the progression of human immunodeficiency virus infection toward full-blown AIDS has been proposed (30).
Despite an increasing number of clinical reports, however, the link between HHV-6 and human diseases other than exanthem subitum has not been definitively proved. Carefully controlled longitudinal studies are still missing, mostly due to the lack of reliable markers of active HHV-6 infection. Indeed, the serological determination of anti-HHV-6 immunoglobulin G (IgG) antibodies has only a limited value due to the high prevalence of HHV-6 infection in the human population (8) and the antigenic cross-reactivity with other beta-herpesviruses, such as human cytomegalovirus and HHV-7 (7, 25). Although the determination of titers of anti-HHV-6 IgM antibodies that are either broadly reactive (46) or that specifically recognize the p41 viral antigen (1) may identify active or reactivated infection, the specificity and sensitivity of such a determination are not optimal since up to 5% of healthy adults may be positive, and conversely, many culture-positive children fail to show detectable IgM titers (36, 38). Similar to IgG antibody titer determination, qualitative PCR assays for detection of viral DNA in peripheral blood leukocytes cannot discriminate between a latent infection, present in the vast majority of healthy individuals, and an active one (30). Only quantitative reverse transcriptase-based PCR assays (34) with blood cells or quantitative PCR techniques applied to the detection of free HHV-6 DNA in biological fluids may represent reliable assays for tracking of an active HHV-6 infection. Nevertheless, the routine application of these assays in large epidemiological surveys has been hampered by their inherent technical difficulties and labor-intensive nature.
Here, we describe the development of a new HHV-6 DNA-based PCR assay for reliable estimation of the HHV-6 load in plasma and cell suspensions. This assay, based on real-time quantitative PCR (24), combines in a single step a PCR amplification reaction with the detection and computer-assisted quantification of the amplified product, thus resulting in a reliable and practical PCR system well suited for the screening of large numbers of clinical samples.
MATERIALS AND METHODS
Oligonucleotide primers and TaqMan probe.
Primers TAQ6E (5′-CAAAGCCAAATTATCCAGAGCG-3′) and TAQ6A (5′-CGCTAGGTTGAGGATGATCGA-3′), which amplify a 133-bp fragment of the highly conserved U67 open reading frame of HHV-6, were selected by using Primer Express software (PE Biosystems, Foster City, Calif.) and were synthesized by Primm (Milan, Italy). A 25-bp oligonucleotide probe (5′-CACCAGACGTCACACCCGAAGGAAT-3′) complementary to an internal region 28 bp downstream of the forward primer was similarly selected and synthesized with a reporter dye, 6-carboxyfluorescein, and a quencher dye, 6-carboxytetramethylrhodamine, covalently linked to the 5′ and 3′ ends, respectively (PE Biosystems, Warrington, United Kingdom).
Preparation of HHV-6 standard and competitor DNA template.
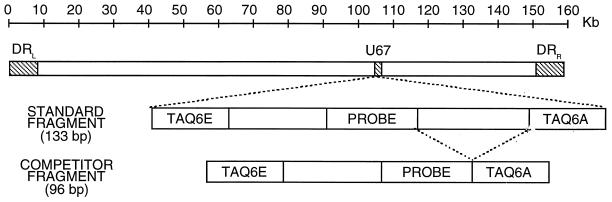
To obtain a construct to be used as a reference for the quantitation of HHV-6, we extracted the DNA from the culture supernatant of peripheral blood mononuclear cells (PBMCs) infected with HHV-6AGS and amplified a fragment of 133 bp derived from the U67 region (Fig. 1) with a PCR mixture containing 100 mM deoxynucleotides, 3 mM magnesium chloride, 1× TaqMan buffer A, primers TAQ6E and TAQ6A (each at 300 nM), 0.625 U of AmpliTaq Gold, and 40% (vol/vol) DNA template. The cycling profile consisted of a first denaturation step of 15 min at 95°C; a second step of 26 cycles with denaturation for 20 s at 95°C, annealing for 30 s at 58°C, and extension for 30 s at 72°C; and a final extension step of 10 min at 72°C, which closed the amplification protocol. The amplified 133-bp product was then cloned into the pCRII plasmid by using the TA cloning kit (Invitrogen Corp, San Diego, Calif.) according to the manufacturer's instructions.
FIG. 1.
Schematic representation of the HHV-6 sequence with the standard and competitor DNA constructs.
A competitor construct was obtained by amplifying the HHV-6 reference DNA molecule with the standard forward primer (TAQ6E) and the reverse primer 5′-CGCTAGGTTGAGGATGATCGAATTCCTTCGGGTGTGACGTCT-3′. This primer combines the first 21 nucleotides of the reverse primer TAQ6A with 21 additional nucleotides complementary to the probe; therefore, the amplification reaction yields a 96-bp fragment that corresponds to the standard fragment (133 bp) but that lacks the 37 bp that separate the probe from the reverse primer (Fig. 1). The inserts in the standard (pVU44) and the competitor (pVU45) constructs were first fully sequenced with the ThermoSequenase kit (Amersham Pharmacia Biotech AB, Uppsala, Sweden); then, the plasmids were expanded and accurately quantitated by evaluation with a spectrophotometer.
PCR assay conditions.
The TaqMan reaction was performed in a final volume of 25 μl containing 100 mM each dATP, dCTP, and dGTP; 200 mM dUTP; 5.5 mM magnesium chloride; 1× TaqMan buffer A; primers at 300 nM; probe at 50 nM; 0.625 U of AmpliTaq Gold; 0.25 U of uracil-N-glycosylase; and 40% (vol/vol) DNA template. The TaqMan PCR cycling conditions were 2 min of degradation of preamplified templates at 50°C, followed by 15 min of denaturation at 95°C and then 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 58°C for 60 s. The normalized fluorescent signal (ΔRn) generated by the degradation of the hybridized probe was automatically calculated by a computer algorithm that normalizes the reporter emission signal first by dividing it by the emission of a control dye (ROX) present in the PCR mixture and then by subtracting all the background signals generated in the first 15 cycles of the PCR. The algorithm then calculates the threshold cycle (Ct) at which each PCR amplification reaches a ΔRn threshold value (usually set at 10 times the standard deviation of the baseline signal) that is inversely proportional to the log number of target copies present in the sample (24).
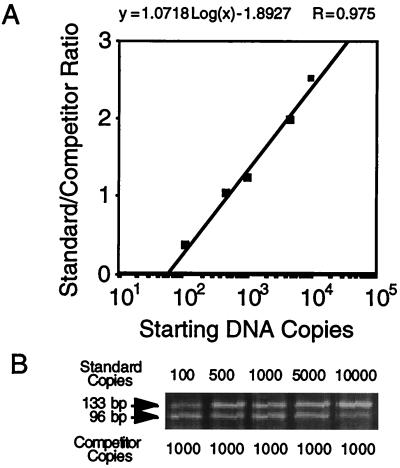
Quantitative-competitive PCR (QC-PCR) was performed with a fixed input of 1,000 copies of the synthetic competitor DNA molecule of 96 bp described above per reaction mixture. The reaction was carried out in a final volume of 50 μl, with 1× PCR buffer II (PE Biosystems, Foster City, Calif.), 5.5 mM magnesium chloride, 100 mM deoxynucleoside triphosphates, 2.5 U of AmpliTaq Gold, primers at 3 mM, and 20% (vol/vol) DNA template. The thermal cycler conditions were set as follows: 2 min at 50°C, 15 min at 95°C, and 45 cycles of 15 s at 95°C and 1 min plus 8 s per cycle at 58°C. The amplification products were stained with ethidium bromide and were analyzed by agarose gel electrophoresis. The intensity ratio of the two bands, corresponding to the DNA template and the competitor DNA amplicons, was determined by densitometric analysis with Image Quant software (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) The amount of HHV-6 genome equivalents present in the test samples was calculated by interpolation on a standard curve, obtained by plotting the value of the template/competitor ratio against the known initial copy number of standard DNA templates on a semilogarithmic scale (see Fig. 5).
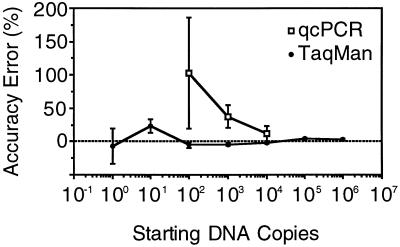
FIG. 5.
Accuracy error of the TaqMan and QC-PCR assays, computed as the average of the measured value after subtraction of the actual value. The error is reported as a function of the actual number of DNA copies to be measured. By both methods, accuracy improved with an increase in the amount of DNA, but the error was systematically lower by the TaqMan assay. In addition, QC-PCR yielded a marked overestimation of the number of copies at all levels.
The nested PCR assay was performed with two sets of primers, as described previously (40).
Sample preparation.
DNA was isolated from 100 μl of plasma or culture supernatant or from 5 × 104 cells derived from thymic grafts explanted from SCID-hu Thy/Liv mice inoculated with HHV-6AGS (21). Extraction of DNA from biological fluids was performed with 0.5 ml of a lysis buffer containing 10 mM Tris-HCl (pH 8), 5 mM EDTA, 0.5% (vol/vol) sodium dodecyl sulfate, and 0.1 μg of proteinase K per ml. DNA was extracted from the cells with 0.1 ml of a lysis solution containing 100 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl2, 0.5% (vol/vol) Tween 20, 0.5% (vol/vol) Nonidet P-40, and 0.5 μg of proteinase K per ml. In both instances, lysis was followed by phenol-chloroform extraction and high-salt isopropanol precipitation; the purified DNA was resuspended in 100 μl of a 5 mM Tris-HCl–0.5 mM EDTA buffer and was stored at −20°C until use.
To control for potential cross-contamination during sample manipulation, one negative control (plasma from a healthy volunteer or DNA extracted from uninfected thymic grafts) was included after each pair of test samples; the three different steps of the PCR procedure (DNA extraction, preparation of the PCR mixture, and addition of the DNA to the PCR mixture) were performed in three physically separated and dedicated rooms.
Preparation of viral stocks.
PBMCs were obtained from healthy donors by gradient centrifugation, activated in vitro with purified phytohemagglutinin for 2 days, and infected with HHV-6AGS or HHV-6BPL1 at a multiplicity of infection of 0.1 (i.e., 1 infectious particle per 10 cells). Two hours after infection, the cells were washed and incubated for several days at 37°C in a humidified CO2 incubator. The culture supernatant was harvested after 7 to 10 days of infection, and virus titration was performed by infecting triplicate cultures of phytohemagglutinin-activated PBMCs with serial 10-fold dilutions of the viral stock (31).
Statistical analysis.
Accuracy, defined as the level of approximation of a measured value to a reference value taken as a “gold standard,” was estimated by computing the arithmetic differences between the number of DNA copies evaluated by the two assays and the theoretical copy number expected according to UV spectroscopy. The significance of systematic biases was assessed by a paired-sample Student t test and was adjusted by covariance analysis, when needed.
Repeatability (i.e., the variability of a method when repeated measures are taken with the same material in a single experiment) and reproducibility (i.e., the variability of a method when repeated measures are taken in different experiments) were estimated by computing the coefficient of variation (CV; the ratio between the standard deviation and the mean of repeated measurements) under different conditions. The significance of the differences between methods was assessed by the F test.
The difference between reference curves was assessed by covariance analysis. The Duncan method was used to account for multiple tests.
RESULTS
Optimization of TaqMan assay conditions.
We first achieved reproducible detection of three doses of reference DNA (102, 104, and 106 copies/reaction mixture) by using the standard PCR conditions suggested by the 7700 ABI Prism sequence detector manufacturer (data not shown). Then, we exploited the conditions of the TaqMan reaction to ensure both the optimal kinetics of fluorescent signal accumulation and the earliest measurement of the Ct values over a wide range of known template DNA concentrations. As illustrated in Table 1, several parameters were optimized by varying each of them in the context of the standard PCR conditions; primer and probe concentrations were tested between 50 and 900 nM and between 10 and 200 nM, respectively, whereas the Mg2+ concentration was varied between 1.5 and 7 mM. The best amplification profiles were obtained with each primer at a concentration of 300 nM in conjunction with Mg2+ at a concentration of 4 mM or higher. A probe concentration of 50 nM was sufficient to ensure both the overall best Ct values (Table 1) and an optimal accumulation of the fluorescent signal (ΔRn); the latter was roughly proportional to the initial probe concentration (data not shown). The optimal AmpliTaq Gold activation time (Table 1) and the annealing-extension temperature were also experimentally determined; the highest sensitivity was achieved with an activation time of 15 min and an annealing-extension temperature of 58°C (data not shown), 3°C degrees under the predicted primer melting temperature.
TABLE 1.
Ct values under different PCR conditions
| Copy no. |
Ct valuea
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activation timeb (min)
|
TAQ6a-TAQ6E primer concnc (nM)
|
Probe concnc (nM)
|
Mg2+ concnc (mM)
|
||||||||||||||||||
| 5 | 10 | 15 | 50-50 | 50-300 | 50-900 | 300-50 | 300-300 | 300-900 | 900-50 | 900-300 | 900-900 | 10 | 25 | 50 | 100 | 200 | 2 | 4 | 6 | 7 | |
| 101 | 40 | 40 | 35 | 37 | 35 | 37 | 39 | 35 | 35 | 35 | 38 | 37 | 37 | 36 | 34 | 36 | 34 | 40 | 36 | 34 | 34 |
| 102 | 40 | 34 | 31 | 38 | 32 | 30 | 33 | 29 | 30 | 33 | 32 | 31 | 34 | 32 | 31 | 32 | 31 | 40 | 31 | 32 | 31 |
| 103 | 40 | 30 | 28 | 30 | 29 | 28 | 30 | 28 | 28 | 29 | 28 | 28 | 36 | 28 | 28 | 28 | |||||
| 104 | 40 | 26 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 27 | 25 | 25 | 25 | 25 | 33 | 24 | 24 | 24 |
| 105 | 40 | 22 | 21 | 31 | 20 | 20 | 21 | ||||||||||||||
The data represent the means of three independent experiments.
The optimal AmpliTaq Gold activation time was established by using the manufacturer-suggested PCR conditions.
The different primer, probe, and Mg2+ concentrations were tested by varying a single parameter at a time, using the optimal AmpliTaq Gold activation time (15 min).
Validation of reference curve.
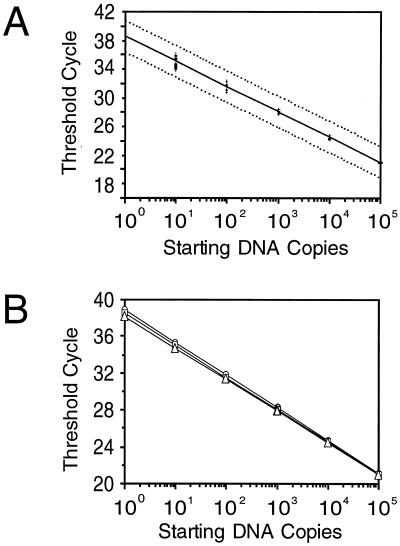
The 133-bp HHV-6 DNA fragment amplified by primers TAQ6A-TAQ6E was cloned into the pCRII plasmid, thus providing a reproducible source of standard DNA (Fig. 1). To generate the reference curve, the plasmid DNA was accurately quantitated by UV spectroscopy, and three distinct sets of 10-fold dilutions were prepared starting from a DNA concentration of 0.5 μg/μl (equivalent to 1011 copies per μl) and ending with a DNA concentration of 0.0005 fg/μl (equivalent to 10−1 copies per μl). The three series of samples were then amplified by PCR in the same run of the 7700 ABI Prism sequence detector. All the data collected were used to generate a log-linear regression plot (Fig. 2A) that showed a strong linear relationship (r = 0.99) between the log of the starting copy number and the Ct values. By covariance analysis, no significant differences were found among the plots generated by the three distinct sets of dilutions (F = 2.40 and P = 0.1 and F = 1.1 and p = 0.33 for the intercept and the slope of the plot, respectively), with the contribution of the dilution procedure to the overall error rate being not higher than 8% (Fig. 2B).
FIG. 2.
(A) Reference curve for TaqMan assay. The dotted lines represent the 95% confidence limits of the assay; the equation of the regression curve obtained by plotting the Ct values (y axis) against the indicated amounts of DNA inputs (x axis) is y = 38.534 − 3.495 log(x), with a value of fit (R) equal to 0.999. (B) Results obtained with three reference curves, constructed at different times, to assess reproducibility. In all the resulting equations [curve 1, y = 38.907 − 3.554 log(x); curve 2, y = 38.547 − 3.508 log(x); curve 3, y = 38.147 − 3.422 log (x)], both the slope coefficients and the intercept values were very similar, and no significant difference was found by covariance analysis.
Comparative analysis of TaqMan assay with other PCR techniques. (i) Assay sensitivity and dynamic range.
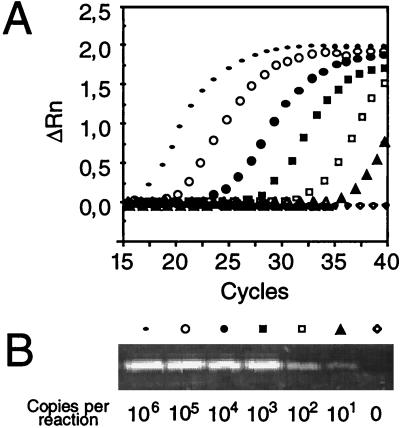
The sensitivity of the TaqMan assay was assessed in parallel by a highly sensitive nested PCR protocol (40); serial dilutions of the standard plasmid, as well as a crude HHV-6AGS stock, were used as templates. All the serial dilutions that yielded a detectable band by nested PCR generated a well-defined amplification plot (Fig. 3). The TaqMan assay was characterized by a very wide dynamic range, as it could discriminate between 100 and 106 HHV-6 genome equivalents in a single reaction. The sensitivity of the two systems was similar, since both required at least three replicates to reliably detect a single-copy input (data not shown). Greater starting copy numbers were measurable, but due to the premature detection of the fluorogenic signal, sample quantitation was possible only by decreasing the number of background measurements that allow threshold ΔRn calculation.
FIG. 3.
Comparison of assay sensitivity for HHV-6 DNA detection between TaqMan and nested PCR assays. The results are for serial 10-fold dilutions of the reference DNA (from 101 and 106 copies/reaction mixture) and one negative control sample, as detected by TaqMan assay amplification (A) or by nested PCR on an ethidium bromide-stained 2% agarose gel (B). The symbols shown at the top of the nested PCR bands identify the corresponding dilutions of the template. For each dilution, the normalized fluorescence signal (ΔRn) is plotted against the PCR cycle number.
(ii) Accuracy, repeatability, and reproducibility.
Next, we measured the accuracy of the TaqMan method and compared it with that of a newly developed QC-PCR assay which amplifies the same target sequence (Fig. 4) (see Materials and Methods). As shown in Fig. 5, in seven distinct experiments performed by the TaqMan assay, the error observed with serial dilutions of the pVU44 reference plasmid was minimal (4.9 to 1.5%) for DNA inputs above 100 copies/reaction mixture (102 to 106), whereas it was greater for inputs below 100 copies/reaction (7 and 23% for 100 and 101 copies/reaction, respectively). Moreover, we did not observe a significant bias of under- or overestimation even after adjusting for the different amounts of starting copy input (P = 0.75 by covariance analysis). By contrast, the degree of error measured in three independent experiments by the QC-PCR technique was significantly higher (11 to 102%) (Fig. 5). Moreover, a significant (P = 0.002) and systematic overestimation bias (values 56% higher than the expected values) was observed. Finally, comparison of the accuracies of the two assays showed a marked difference (P = 0.005) for the quantitation of the standard.
FIG. 4.
Standard curve for HHV-6 QC-PCR. A fixed amount (103 copies) of the pVU45 calibrator plasmid was amplified in the presence of increasing amounts of the pVU44 standard plasmid (A). Following ethidium bromide staining the relative amounts of amplified products were calculated by densitometric analysis, and ratios between the coamplified products were plotted on a semilogarithmic scale as a function of increasing amounts of the reference DNA (B). The corresponding equation is reported in the upper part of the figure.
The repeatability and reproducibility of the TaqMan assay were assessed by quantifying both the reference DNA molecule (Table 2) and viral DNA extracted from HHV-6-infected tissues obtained from SCID-hu Thy/Liv mice (Table 3) or human plasma. A progressive decrease of the CV (from 197 to 5%) was seen with an increase in the starting DNA amount in the intra-assay evaluation of the reference DNA. Similarly, the interassay CV decreased sharply (from 197 to 14% between 100 and 103 copies/reaction mixture) and then reached a plateau, with an average CV of 8% for all the remaining DNA concentrations (from 103 to 106 copies/reaction). No statistical difference was found between the intra- and the interassay CVs, confirming the reliability of the entire technical procedure over time. The CVs for repeatability and reproducibility of the QC-PCR method, measured with the same reference molecule, were significantly worse (decreases in the CV from 124 to 25% for repeatability and from 274 to 40% for reproducibility) than those measured by the TaqMan assay.
TABLE 2.
CV of standard plasmid pVU44 quantifications by TaqMan and QC-PCR
| Starting DNA copy no. | % CV
|
|||
|---|---|---|---|---|
| TaqMan assay
|
QC-PCR assay
|
|||
| Intra-assay | Interassay | Intra-assay | Interassay | |
| 100 | 197 | 197 | ||
| 101 | 67 | 67 | ||
| 102 | 30 | 30 | 124 | 274 |
| 103 | 12 | 14 | 25 | 59 |
| 104 | 8 | 8 | 36 | 40 |
| 105 | 7 | 8 | ||
| 106 | 5 | 8 | ||
TABLE 3.
CV for quantification of HHV-6 in infected tissue samples by TaqMan and QC-PCR assays
| Sample no. | TaqMan assay
|
QC-PCR assay
|
||||
|---|---|---|---|---|---|---|
| Quantification (no. of DNA copies) | Intra-assay CV (%) | Interassay CV (%) | Quantification (no. of DNA copies) | Intra-assay CV (%) | Interassay CV (%) | |
| 1 | 1,585 | 8 | 8 | 1,812 | 9 | 15 |
| 2 | 7,553 | 8 | 12 | 12,342 | 8 | 17 |
| 3 | 1,280 | 4 | 4 | 1,574 | 6 | 11 |
| 4 | 4,300 | 9 | 18 | 7,030 | 6 | 19 |
| 5 | 430 | 24 | 53 | 810 | 31 | 34 |
Quantification of the viral load in five samples derived from heterochimeric mice experimentally infected with HHV-6AGS showed that the QC-PCR assay significantly overestimated (P = 0.0001) the TaqMan assay measurements by an average of 40% (Table 3). The repeatability was similar by both methods (CVs, 4 to 24% and 6 to 31% for the TaqMan assay and the QC-PCR, respectively), whereas for three of five samples (samples 1 to 3) the reproducibility of the TaqMan assay was superior to that of the QC-PCR assay and it was significantly worse in only one case (sample 5).
To further verify the validity of the TaqMan assay for quantitation of biological samples, a set of human plasma samples previously assayed for the presence of HHV-6 DNA by nested PCR was tested. Three samples were chosen because they occasionally tested positive by nested PCR, three were chosen because they were consistently positive, albeit only after the second round of amplification, and the last three were chosen because they were already positive after the first round of amplification. By the TaqMan assay, marked differences in the viral loads in the three sets of samples were documented (range, 2 to 23,555 copies/ml); the repeatability and reproducibility for each sample were similar (intra-assay CV range, 12 to 135%; interassay CV range, 12 to 156%), with the CV values being not significantly different from the ones measured with the DNA reference standard.
Detection of HHV-6 subgroups.
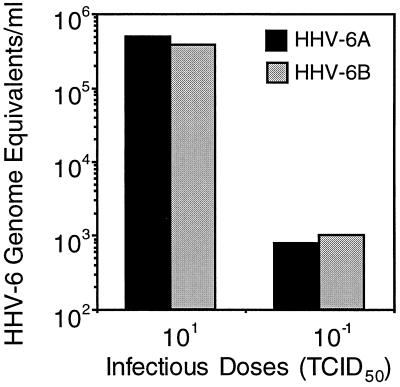
Lastly, we studied the ability of the TaqMan assay to quantitate the DNA loads of both HHV-6 subgroups (subgroups A and B). Titrated culture supernatants collected from PBMCs infected with HHV-6 strains GS (subgroup A) and PL1 (subgroup B) containing either 0.1 or 10 50% tissue culture infective doses (TCID50s) per μl were tested for the HHV-6 DNA load. As shown in Fig. 6, similar amounts of HHV-6 DNA of both subgroups were detected, with a consistent ratio (approximately 2 logs at the two dilutions tested) between the number of viral genome equivalents measured and the number of TCID50s used.
FIG. 6.
Detection of HHV-6 subtypes by TaqMan assay. HHV-6AGS (filled columns) and HHV-6B PL1 (shaded columns) genome equivalents measured in 1 ml of supernatant derived from cultured PBMCs infected with 0.1 and 10 TCID50s, respectively.
DISCUSSION
The laboratory diagnosis of active HHV-6 infection is a complex process that often requires the simultaneous assessment of multiple parameters. The reasons for such complexity are intrinsic to the HHV-6 life cycle: the virus enters the body during primary infection, often clinically manifested by exanthem subitum, followed by a variable period of clinical latency, during which the virus persists in selected anatomical sites from where it is probably never cleared. Subsequently, either reactivation or reinfection may occur, particularly in concomitance with episodes of immune dysregulation or deficiency; these can be followed, again, by variable periods of latency. As a consequence of this pattern of alternating latency and reactivation, the diagnostic value of anti-HHV-6 IgG antibody titers is limited. Similarly, qualitative DNA PCR with blood cells is unable to distinguish the maintenance of the viral genome in latently infected cells (cellular latency, clinically silent) from the presence of cells actively producing HHV-6 (often clinically relevant). Furthermore, quantitation of the viral load with blood cells (14, 15, 23, 40), although suggestive of actively replicating virus, does not provide direct proof of active or reactivated infection. Even isolation of virus from blood cells, even though it is generally successful during episodes of active virus replication, cannot formally distinguish between active and latent HHV-6 infection, as it requires the in vitro stimulation of the patient's cells, thereby potentially reactivating HHV-6 from latency. By contrast, the identification of cell-free viral products (either antigens or DNA) in body fluids can substantiate the diagnosis of active HHV-6 infection. To date, one HHV-6 antigen-capture test has been described (32), but its suitability for clinical use remains uncertain. The most practical test remains the detection of HHV-6 DNA by qualitative PCR techniques in serum, plasma, or other body fluids (13, 41). However, the assays developed to date present two major limitations: first, they are not extremely sensitive (12, 14); second, they cannot establish if the viral DNA is indeed derived from circulating virions or from latently infected cells accidentally damaged in vivo or ex vivo during sample manipulation. This problem can be circumvented by combining quantitative PCR techniques that measure the viral load and the cellular DNA content in the corpuscular and the liquid fraction of the same sample.
To reduce the marked variability of PCR results due to endpoint quantitation of the amplified products, the currently available QC-PCR assays must introduce a synthetic DNA competitor molecule that allows normalization for intra- and interreaction variability and that also reveals potential PCR artifacts due to Taq polymerase inhibitors, technical inaccuracies, etc. (13, 23, 40). Unfortunately, this introduces a significant degree of complexity into the PCR assay, which increases the time and attention required for determination of the correct result for a sample, thereby strongly limiting routine application. Conversely, the real-time detection of PCR amplification products measures the target DNA during the exponential phase of growth in the PCR, thus circumventing many of the problems connected with quantification at the plateau stage. This technological breakthrough combines a high level of accuracy and reproducibility with an extreme sensitivity and detection range simply by the use of an external reference curve.
Of importance, partial inhibition of the PCR, which may reduce the quantitation accuracy, can be revealed by alterations of the TaqMan assay detection curve in comparison with the amplification plots generated by the standards (G. Locatelli and M. S. Malnati, unpublished data). Qualitative alterations of the reference DNA can also be easily identified by virtue of the high interassay reproducibilities of the standard curve parameters. The only limitation of this assay, in comparison with QC-PCR, is represented by its inability to recognize PCR artifacts due to a complete failure of the PCR (false-negative results); conversely, the use of a specific probe to detect the amplified DNA permits, as in the Southern blot technique, limitation of the occurrence of false-positive results. Indeed, with over 300 measurements for a reference DNA molecule, the direct comparison of the TaqMan and QC-PCR assays developed with the same DNA sequence demonstrated that the real-time technique is a far more practical and reliable method of DNA quantitation. The design of our assay also guarantees the same level of efficiency and reproducibility for the quantitation of both the A and the B HHV-6 subgroups; furthermore, we showed that it is equally applicable to both liquid and cellular fractions of biological samples.
In conclusion, the real-time HHV-6 DNA detection assay described herein offers several advantages over the traditional QC-PCR method: first, the time required for labor, costs, and sample handling time are greatly reduced because a single PCR run without further postamplification steps is sufficient to accurately quantitate the target DNA; second, the absence of postamplification manipulation steps greatly reduces the risk of intersample contamination; lastly, the 96-well format provides a high-throughput system that makes this assay suitable for the large-scale evaluation of HHV-6 infection in human pathology (29), as well as in experimental animal models (21, 22).
ACKNOWLEDGMENTS
This work was supported by the European Union Biomed 2 (grant P1951301).
We thank C. A. Stoddart and J. M. McCune for kindly providing tissue samples from SCID-hu Thy/Liv mice and Stefania Laus for excellent editorial assistance.
REFERENCES
- 1.Ablashi D V, Marsh S, Kaplan M, Whitman J E, Jr, Pearson G R. HHV-6 infection in HIV-infected asymptomatic and AIDS patients. Intervirology. 1998;41:1–9. doi: 10.1159/000024909. [DOI] [PubMed] [Google Scholar]
- 2.Ablashi D V, Lapps W, Kaplan M, Whitman J E, Richert J R, Pearson GR. Human herpesvirus-6 (HHV-6) infection in multiple sclerosis: a preliminary report. Multiple Sclerosis. 1998;4:490–496. doi: 10.1177/135245859800400606. [DOI] [PubMed] [Google Scholar]
- 3.Ablashi D V, Eastman H B, Owen C B, Roman M M, Friedman J, Zabriskie J B, Peterson D L, Pearson G R, Whitman J E. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 4.Akashi K, Eizuru Y, Sumiyoshi Y, Minematsu T, Hara S, Harada M, Kikuchi M, Niho Y, Minamishima Y. Severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N Engl J Med. 1993;329:168–171. doi: 10.1056/NEJM199307153290304. [DOI] [PubMed] [Google Scholar]
- 5.Asano K, Yoshikawa T, Suga S, Yazaki T, Kondo Y, Yamanishi K. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet. 1990;335:862–863. doi: 10.1016/0140-6736(90)90983-c. [DOI] [PubMed] [Google Scholar]
- 6.Balachandra K, Ayuthaya P I, Auwanit W, Jayavasu C, Okuno T, Yamanishi K, Takahashi M. Prevalence of antibody to human herpesvirus 6 in women and children. Microbiol Immunol. 1989;33:515–518. doi: 10.1111/j.1348-0421.1989.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 7.Berneman Z N, Ablashi D V, Li G, Eger-Fletcher M, Reitz M S, Jr, Hung C L, Brus I, Komaroff A L, Gallo R C. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci USA. 1992;89:10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs M, Fox J, Tedder R S. Age prevalence of antibody to human herpesvirus 6. Lancet. 1998;i:1058–1059. doi: 10.1016/s0140-6736(88)91883-1. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald D, Cheney P R, Peterson D L, Henry B, Wormsley S B, Geiger A, Ablashi D V, Salahuddin S Z, Saxinger C, Biddle R, Komaroff A. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. 1992;116:103–113. doi: 10.7326/0003-4819-116-2-103. [DOI] [PubMed] [Google Scholar]
- 10.Carrigan D R, Drobyski W R, Russel S K, Tapper M A, Knox K K, Ash R C. Interstitial pneumosis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991;338:147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- 11.Challoner P B, Smith K T, Parker J D, MacLeod D L, Coulter S N, Rose T M, Schultz E R, Bennet L, Garber R L, Changf M, Schad P A, Steward P M, Nowinski R C, Brown J P, Burmer G C. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu S S, Cheung C Y, Tse C Y C, Peiris M. Early diagnosis of primary human herpesvirus 6 infection in childhood: serology, polymerase chain reaction, and virus load. J Infect Dis. 1998;178:1250–1256. doi: 10.1086/314432. [DOI] [PubMed] [Google Scholar]
- 13.Clark D A, Taled M, Kidd I M, McLaughlin J E, Johnson M A, Griffiths P D, Emery V C. Quantification of human herpesvirus 6 in immunocompetent persons and post-mortem tissues from AIDS patients by PCR. J Gen Virol. 1996;77:2271–2275. doi: 10.1099/0022-1317-77-9-2271. [DOI] [PubMed] [Google Scholar]
- 14.Clark D A, Kidd I M, Collingham K E, Tarlow M, Ayeni T, Riordan A, Griffiths P D, Emery V C, Pillay D. Diagnosis of primary human herpesvirus 6 and 7 infections in febrile infants by polymerase chain reaction. Arch Dis Child. 1997;77:42–45. doi: 10.1136/adc.77.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cone R W, Huang M L, Corey L, Zeh J, Ashley R, Bowden R. Human herpesvirus 6 infections after marrow transplantation: clinical and virologic manifestations. J Infect Dis. 1999;179:311–318. doi: 10.1086/314581. [DOI] [PubMed] [Google Scholar]
- 16.Di Luca D, Zorzenon M, Mirandola P, Colle R, Botta G A, Cassai E. Human herpesvirus 6 and human herpesvirus 7 in chronic fatigue syndrome. J Clin Microbiol. 1995;33:1660–1661. doi: 10.1128/jcm.33.6.1660-1661.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolcetti R, Di Luca D, Mirandola P, De Vita S, Carbone A, Tirelli U, Cassai E, Boiocchi M. Frequent detection of human herpesvirus 6 DNA in HIV-associated lymphadenopathy. Lancet. 1994;334:543. doi: 10.1016/s0140-6736(94)91931-3. [DOI] [PubMed] [Google Scholar]
- 18.Drobyski W R, Dunne W M, Burd E M, Knox K K, Ash R C, Horowitz M M, Flomenberg N, Carrigan D R. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipient: evidence of a marrow-suppressive role for HHV-6 in vivo. J Infect Dis. 1993;167:735–739. doi: 10.1093/infdis/167.3.735. [DOI] [PubMed] [Google Scholar]
- 19.Drobyski W R, Knox K K, Majewski D, Carrigan D R. Fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994;330:1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- 20.Fairfax M R, Schacker T, Cone R W, Collier A C, Corey L. Human herpesvirus 6 DNA in blood cells of human immunodeficiency virus-infected men: correlation of high levels with CD4 cell counts. J Infect Dis. 1994;169:1342–1345. doi: 10.1093/infdis/169.6.1342. [DOI] [PubMed] [Google Scholar]
- 21.Gobbi A, Stoddart C A, Malnati M S, Locatelli G, Santoro F, Abbey N W, Bare C, Linquist-Stepps V, Moreno M B, Herndier B G, Lusso P, McCune J M. Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice. J Exp Med. 1999;189:1953–1960. doi: 10.1084/jem.189.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobbi A, Stoddart C A, Locatelli G, Santoro F, Bare C, Linquist-Stepps V, Moreno M B, Abbey N W, Herndier B G, Malnati M S, McCune J M, Lusso P. Coinfection of SCID-hu Thy/Liv mice with human herpesvirus 6 and human immunodeficiency virus type 1. J Virol. 2000;74:8726–8731. doi: 10.1128/jvi.74.18.8726-8731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths P D, Ait-Khaled M, Beacroft C P, Clark D A, Qua S E, Burroughs A K, Rolles K, Kidd I M, Knight S N, Noibi Phillips A N, Emery V C. Human herpesvirus 6 and 7 as potential pathogens after liver transplant: prospective comparison with the effect of cytomegalovirus. J Med Virol. 1999;59:496–501. doi: 10.1002/(sici)1096-9071(199912)59:4<496::aid-jmv12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Heid C A, Stevens J, Livak K J, Williams P M. Real-time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 25.Irving W L, Cunningham A L, Keogh A, Chapman J R. Antibody to both human herpesvirus 6 and cytomegalovirus. Lancet. 1988;ii:630–631. doi: 10.1016/s0140-6736(88)90669-1. [DOI] [PubMed] [Google Scholar]
- 26.Ishiguro N, Yamada S, Takahashi T, Takahashi Y, Okuno T, Yamanishi K. Meningo-encephalitis associated with HHV-6 related exanthem subitum. Acta Pediatr Scand. 1990;79:987–989. doi: 10.1111/j.1651-2227.1990.tb11369.x. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura K, Ohta H, Ihara T, Kamiya H, Ochiai H, Yamanishi K, Tanaka K. Idiopathic thrombocytopenic purpura after human herpesvirus 6 infection. Lancet. 1994;344:830. doi: 10.1016/s0140-6736(94)92390-6. [DOI] [PubMed] [Google Scholar]
- 28.Knox K K, Carrigan D R. Active HHV-6 infection in the lymph nodes of HIV-infected patients: in vitro evidence that HHV-6 can break HIV latency. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:370–378. doi: 10.1097/00042560-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli, G., M. S. Malnati, D. Franciotta, R. Furlan, G. Comi, G. Martino, and P. Lusso. Detection of human herpesvirus 6 by quantitative real-time PCR in serum and cerebrospinal fluid of patients with multiple sclerosis. Multiple Sclerosis, in press.
- 30.Lusso P, Gallo R C. Human herpesvirus 6. Baillière's Clin. Haematol. 1995;8:201–223. doi: 10.1016/s0950-3536(05)80238-0. [DOI] [PubMed] [Google Scholar]
- 31.Malnati M S, Lusso P, Ciccone E, Moretta A, Moretta L, Long E O. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993;178:961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh S, Kaplan M, Asano Y, Hoekzema D, Komaroff A L, Whitman J E, Jr, Ablashi D V. Development and application of HHV-6 antigen capture assay for the detection of HHV-6 infections. J Virol Methods. 1996;61:103–112. doi: 10.1016/0166-0934(96)02075-7. [DOI] [PubMed] [Google Scholar]
- 33.McCullers J A, Lakeman F D, Whitley R J. Human herpesvirus 6 is associated with focal encephalititis. Clin Infect Dis. 1995;21:571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- 34.Norton R A, Caserta M T, Hall C B, Schnabel K, Hocknell P, Dewhurst S. Detection of human herpesvirus 6 by reverse transcription-PCR. J Clin Microbiol. 1999;37:3672–3675. doi: 10.1128/jcm.37.11.3672-3675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novoa L J, Nagra R M, Nakawatase T, Edwards-Lee T, Tourtellotte W W, Conford M E. Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult. J Med Virol. 1997;52:301–308. doi: 10.1002/(sici)1096-9071(199707)52:3<301::aid-jmv11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, Baba K. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27:651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patnaik M, Komaroff A L, Conley E, Ojo-Amaize E A, Peter J B. Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. J Infect Dis. 1995;172:1364–1367. doi: 10.1093/infdis/172.5.1364. [DOI] [PubMed] [Google Scholar]
- 38.Rudin C, Hirsch H. How often can the clinical diagnosis of exanthema subitum be confirmed with the HHV-6 serology test. Clin Pediatr. 1991;203:109–112. doi: 10.1055/s-2007-1025410. [DOI] [PubMed] [Google Scholar]
- 39.Salahuddin S Z, Ablashi D V, Markham P D, Josephs S F, Sturzennegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo R C. Isolation of a virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 40.Secchiero P, Zella D, Crowley R W, Gallo R C, Lusso P. Quantitative PCR in human herpesvirus 6 and 7. J Clin Microbiol. 1995;33:2124–2130. doi: 10.1128/jcm.33.8.2124-2130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Secchiero P, Carrigan D R, Asano Y, Benedetti L, Crowley R W, Komaroff A L, Gallo R C, Lusso P. Detection of HHV-6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J Infect Dis. 1995;171:273–280. doi: 10.1093/infdis/171.2.273. [DOI] [PubMed] [Google Scholar]
- 42.Singh N, Carrigan D R, Gayowsky T, Marino I R. Human herpesvirus-6 infection in liver transplant recipients: documentation of pathogenicity. Transplantation. 1997;64:674–678. doi: 10.1097/00007890-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 43.Sobue R, Miyazaki H, Okamoto M, Hirano M, Yoshikawa T, Suga S, Asano Y. Fulminant hepatitis in primary human herpesvirus-6 infection. N Engl J Med. 1991;324:1290. doi: 10.1056/NEJM199105023241818. [DOI] [PubMed] [Google Scholar]
- 44.Soldan S S, Berti R, Salem N, Secchiero P, Flamand L, Calabresi P A, Brennan M B, Maloni H W, McFarland H F, Lin H C, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 45.Stepper T A, Horwitz C A, Ablashi D V, Salahuddin S Z, Saxinger C, Saltzman R, Schwartz B. The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illness not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol. 1990;93:776–783. doi: 10.1093/ajcp/93.6.776. [DOI] [PubMed] [Google Scholar]
- 46.Suga S, Yoshikawa T, Asano Y, Nakashima T, Yazaki T, Fukuda M, Kojima S, Matsuyama T, Ono Y. IgM neutralizing antibody response to human herpesvirus-6 in patients with exanthema subitum or organ transplantation. Microbiol Immunol. 1992;36:495–506. doi: 10.1111/j.1348-0421.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 47.Tajiri H, Tanaka-Taya K, Ozaki Y, Okada S, Mushiake S, Yamanishi K. Chronic hepatitis in an infant, in association with human herpesvirus-6 infection. J Pediatr. 1997;131:473–475. doi: 10.1016/s0022-3476(97)80082-0. [DOI] [PubMed] [Google Scholar]
- 48.Yamanishi K, Okuna T, Shiraki K. Identification of human herpesvirus 6 as a causal agent for exhantem subitum. Lancet. 1988;i:1065. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]