Abstract
D-serine plays an important role in modulating N-methyl-D-aspartate receptor (NMDAR) neurotransmission in the mammalian brain by binding to the receptor’s glycine modulatory site (GMS). The cytosolic enzyme serine racemase (SR) converts L-serine to D-serine, while the peroxisomal enzyme D-amino acid oxidase (DAAO) catalyzes the breakdown of D-serine. Although it is important to understand how the activities of SR and DAAO regulate D-serine levels, very little is known about the mechanisms that regulate the expression of SR and DAAO. In this study, we investigated whether the different centrally active drugs affect the expression of SR and DAAO in adult mouse brain. We found that the NMDAR antagonist, MK801, and cocaine, psychotropic drugs that both augment glutamate release, reduce the expression of SR and DAAO. This regulation is brain region selective, and in the case of cocaine, is reversed in part byα-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX). However, D-serine and antipsychotics do not regulate SR and DAAO protein levels. In a genetic model of SR disruption, we found that DAAO expression was unaltered in SR conditional knockout mice, in which tissue D-serine content remains fairly stable despite marked reduction in SR expression. This study reveals a new mechanism by which AMPAR activity could regulate NMDAR function via D-serine availability.
Keywords: serine racemase, MK801, cocaine, NMDA receptor, clozapine, AMPA receptor
1. Introduction
Since the discovery of D-serine in the mammalian brain, it has been found to play an important role in modulating N-methyl-D-aspartate receptor (NMDAR) function (Hashimoto et al., 1992). The NMDAR is an ion channel receptor composed of two obligatory GluN1 subunits, which form the ion channel, and typically two GluN2 subunits. NMDAR activation requires glycine or D-serine binding at the glycine modulatory site (GMS) on GluN1, in addition to the binding of its agonist, glutamate, to the GluN2 subunit. D-serine binds to the GMS with three times higher affinity than glycine and serves as the primary endogenous NMDAR co-agonist in forebrain (Balu and Coyle, 2015; Matsui et al., 1995).
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPARs) is another ionotropic glutamate receptor, which does not require a co-agonist. These different receptors share glutamate as an agonist and have been shown to have interaction in some systems like long-term potentiation (LTP), cell proliferation or neurogenesis (Fachim et al., 2016; Herring and Nicoll, 2016; Huganir and Nicoll, 2013; Ritter, 2011). LTP is persistent enhancement of glutamatergic neurotransmission and is triggered by the synaptic activation of NMDARs (Collingridge et al., 1983), which is mediated by the recruitment of AMPARs to the synapse (Park et al., 2016). Brain levels of D-serine are determined by two enzymes, serine racemase (SR) and D-amino acid oxidase (DAAO) (Coyle et al., 2010; Nishikawa, 2011). D-serine is synthesized from L-serine by the cytosolic enzyme SR, which belongs to the family of pyridoxal 5’ phosphate (PLP) enzymes. The racemization reaction is reversible, and its activity requires several cofactors, including magnesium (Mg2+), ATP and pyridoxal 5’ phosphate (Goto et al., 2009; Martineau et al., 2014). SR also catalyzes the irreversible α,β-elimination of water from L- or D-serine to yield pyruvate and ammonia (De Miranda et al., 2002). SR is enriched in adult cortico-limbic regions of the brain, localized primarily to excitatory and a subset of inhibitory neurons (Balu et al., 2014; Takagi et al., 2020). DAAO is an astrocytic, peroxisomal flavin adenine dinucleotide (FAD)-containing enzyme that catalyzes the degradation of neutral D-amino acids, particularly D-serine (Pollegioni et al., 2007). In the adult brain, DAAO is highly expressed in the cerebellum and hindbrain (Schell et al., 1995). Notably, the D-serine levels in the neocortex and hippocampus remain in the wild-type range in mice with a mutant, inactive form of DAAO (Labrie et al., 2009).
Because SR and DAAO contribute respectively to the production and elimination of D-serine, they are important for modulating NMDAR function. For example, serine racemase-null mutant (SR−/−) mice generated by our laboratory, which by constitutive deletion of the first coding exon (exon 3) of the SR gene, show a 90% reduction in cortical D-serine and exhibit NMDAR hypofunction (Basu et al., 2009). Balan et al.(2009) found that NMDAR stimulation results in the translocation of SR from the cytosol to dendritic membranes and D-serine synthesis is inactivated in cultured neurons when NMDAR was activated (Balan et al., 2009). Several lines of investigation showed some proteins, such as glutamate receptor interacting protein (GRIP) and phosphatidylinositol (4, 5)- bisphosphate, control SR expression in cultured inflammatory (A1) astrocytes (Kim et al., 2005; Mustafa et al., 2009). In terms of DAAO, the gene product of G72 is found to be a putative activator of enzyme activity (Chumakov et al., 2002; Pollegioni et al., 2018). However, it remains largely unknown what regulates the expression and/or activity of these enzymes in vivo (Basu et al., 2009). In this study, we examined how several pharmacologic and genetic manipulations that affect glutamatergic transmission affect the expression of SR and DAAO, as well as D-serine levels in vivo.
2. Results
2.1. MK801 selectively reduces SR and DAAO expression in the frontal cortex
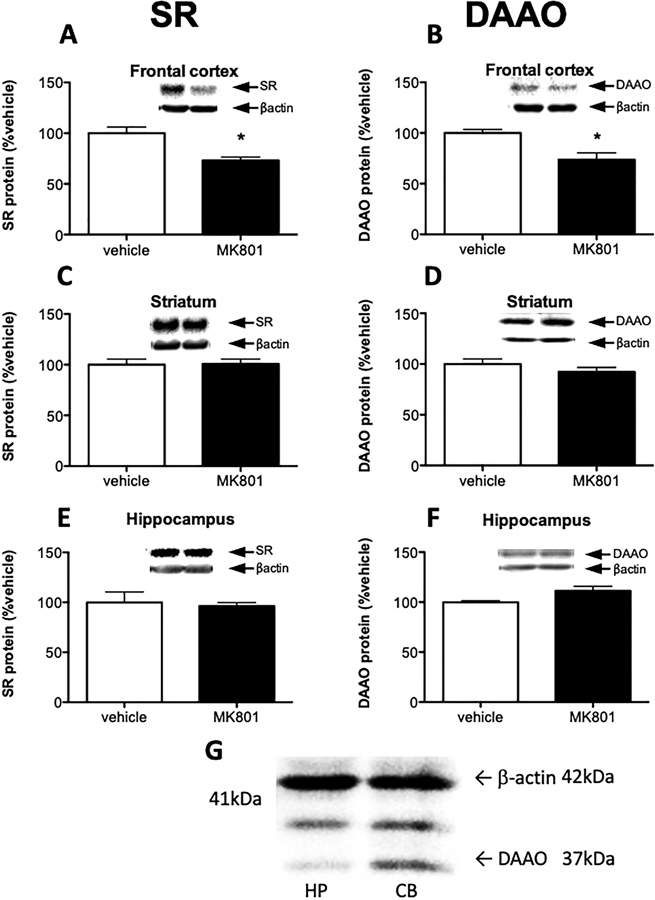
MK801 and other NMDAR antagonists have been shown to elevate extracellular glutamate in the frontal cortex (Adams and Moghaddam, 1998; Moghaddam et al., 1997; Roenker et al., 2012). Therefore, we were interested to see if NMDAR antagonism also interacts with the systems that regulate D-serine concentration. We found that sub-chronic (5 days) administration of MK801, an NMDAR antagonist, reduced both SR and DAAO protein levels in frontal cortex (Fig 1A–B), without significantly affecting D-serine tissue content in that region (Saline: 0.14 ± 0.06 μmol/g; MK801: 0.16 ± 0.04 μmol/g; p = 0.428). Interestingly, MK801 did not affect SR or DAAO in the striatum (Fig 1C–D) and hippocampus (Fig 1E–F).
Fig. 1.
MK801 selectively reduces SR and DAAO expression in the frontal cortex. Protein levels of serine racemase (SR; A, C, E) and D-amino acid oxidase (DAAO; B, D, F) were measured in the frontal cortex (A, B), striatum (C, D) and hippocampus (E, F) of vehicle treated (n = 5; white bars) and MK801 treated (n = 6; black bars) mice. (G) Representative Western blot images using DAAO antibody on cerebellar and hippocampal tissue lysate to test antibody specificity. Values are expressed as the optical density (OD) normalized to vehicle treated values (% vehicle). Unpaired Student t-test was used for analysis. Asterisk (*) indicates significant differences from the vehicle treated group (frontal cortex SR: 0.0029, DAAO: 0.0096). All values represent the mean ± SEM.
The specificity of the SR antibody has been unequivocally established using tissue from SR−/− mice as a control (Balu et al., 2014). The specificity of the DAAO antibody used here (Curcio et al., 2013) was determined by comparing the intensity of DAAO staining (Fig 1G) in cerebellum (high DAAO levels in adulthood) and hippocampus (low DAAO levels in adulthood). The antibody showed a band at the predicted molecular weight of DAAO (~37 kDa), that was much more intense in cerebellum than hippocampus (Horiike et al., 1994). This antibody also reacted with another protein between DAAO (~37 kDa) and β-actin (~42kDa) bands, but its intensity did not differ between brain regions. The results replicated those previously reported by Wang et al (Wang et al., 2020).
2.2. Cocaine regulation of SR and DAAO in the striatum is dependent on AMPA receptor activation
Similar to the effect of MK801 in the frontal cortex, repeated cocaine treatment increases extracellular glutamate in the striatum (Hotsenpiller and Wolf, 2003; McFarland et al., 2003; Rahman et al., 2005). As AMPARs are the primary mediators of fast excitatory neurotransmission, we determined whether extracellular glutamate via the AMPAR affects SR and DAAO expression. Mice received NBQX, a competitive AMPAR antagonist, 30 minutes prior to cocaine administration. In the striatum of cocaine treated mice, SR and DAAO protein levels were significantly reduced. The effect on SR was blocked by pretreatment with NBQX to vehicle treated group level (Fig 2 A). The effect on DAAO was significantly blocked by NBQX to vehicle treated group level from cocaine treated group level (Fig 2 B). However, cocaine or NBQX + cocaine did not modulate D-serine tissue content in the striatum (Saline: 0.11 ± 0.04 μmol/g; cocaine: 0.11 ± 0.04 μmol/g; cocaine/NBQX: 0.11 ± 0.06 μmol/g; p = 0.959). In the frontal cortex (Fig 2 C–D) and hippocampus (Fig 2 E–F), cocaine and NBQX did not affect SR or DAAO expression.
Fig. 2.
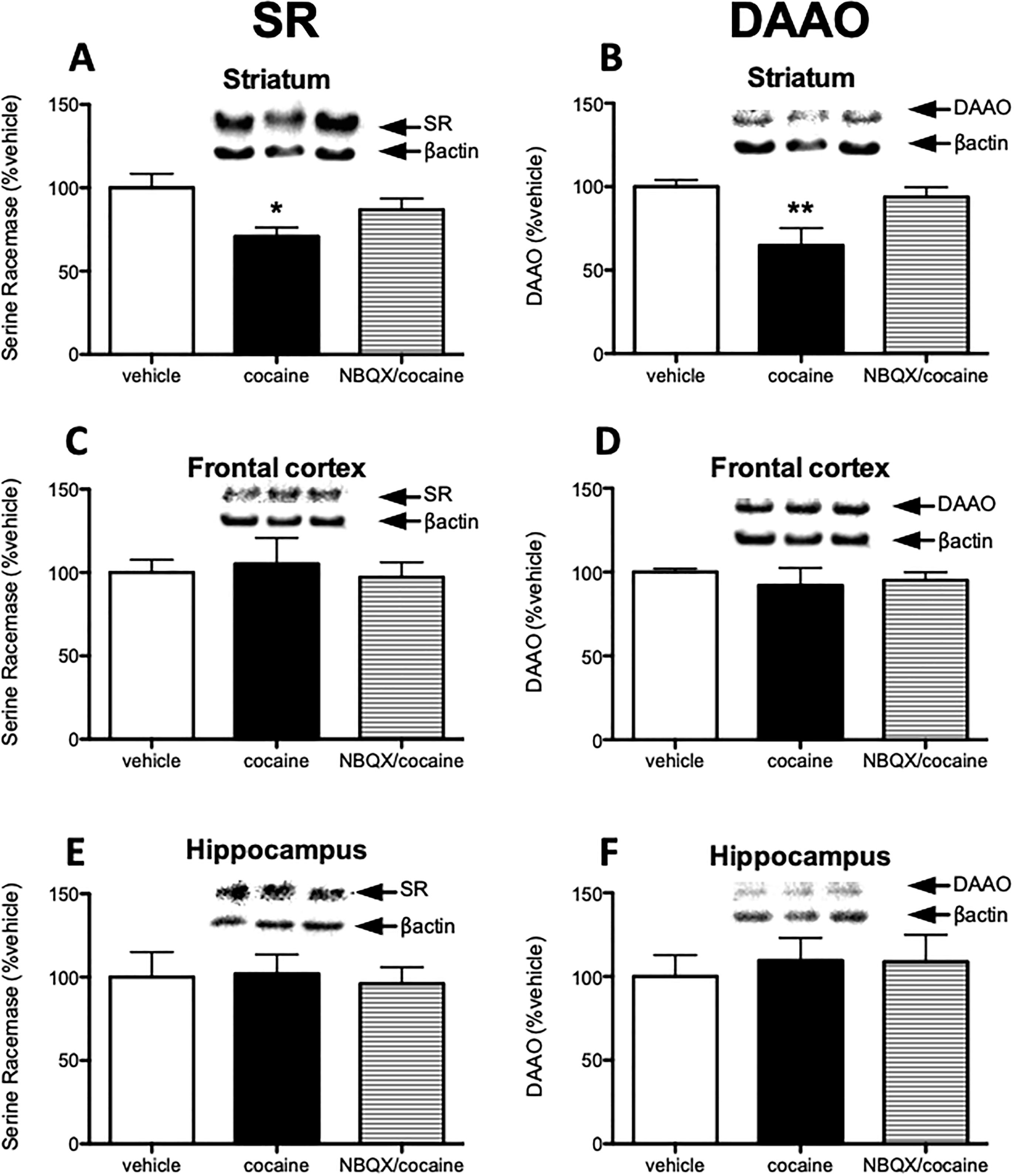
Cocaine regulation of SR and DAAO in the striatum is dependent on AMPA receptor activation. Animals were divided to 3 groups: vehicle (n = 4; two vehicle injections), cocaine (n = 5; cocaine injection after vehicle injection), and NBQX/cocaine group (n = 5; cocaine injection after NBQX injection). Protein levels of serine racemase (SR; A, C, E) and D-amino acid oxidase (DAAO; B, D, F) were measured in the striatum (A, B), frontal cortex (C, D) and hippocampus (E, F) from mice treated with vehicle (white bars), cocaine (black bars), and NBQX/cocaine (striped bars). Values are expressed as the optical density (OD) normalized to vehicle treated values (% vehicle). Significant one-way ANOVA was followed up by Tukey’s multiple comparison test. Asterisk (*) indicates significant differences from the vehicle treated group (F= 4.447, p=0.032). Double-asterisk (**) indicates significant differences from both the vehicle treated group and NBQX/cocaine treated group (F= 5.965, p=0.023, p=0.046, respectively). All values represent the mean ± SEM.
2.3. Haloperidol and Clozapine do not alter SR and DAAO expression.
Next, given the effects of dopamine enhancing action of cocaine on SR and DAAO expression, we examined whether two pharmacologically distinct antipsychotic medications affect SR and DAAO expression. Haloperidol, a potent dopamine type 2 (D2) receptor antagonist, is a representative of typical antipsychotic drugs (Kapur et al., 1999). Clozapine is a unique atypical antipsychotic that has been proven to have beneficial effects on negative symptoms through mechanisms that are poorly understood (Kapur et al., 1999). Interestingly, neither haloperidol nor clozapine affected SR and DAAO expression in any of the brain regions examined. (Fig 3 A–F).
Fig. 3.
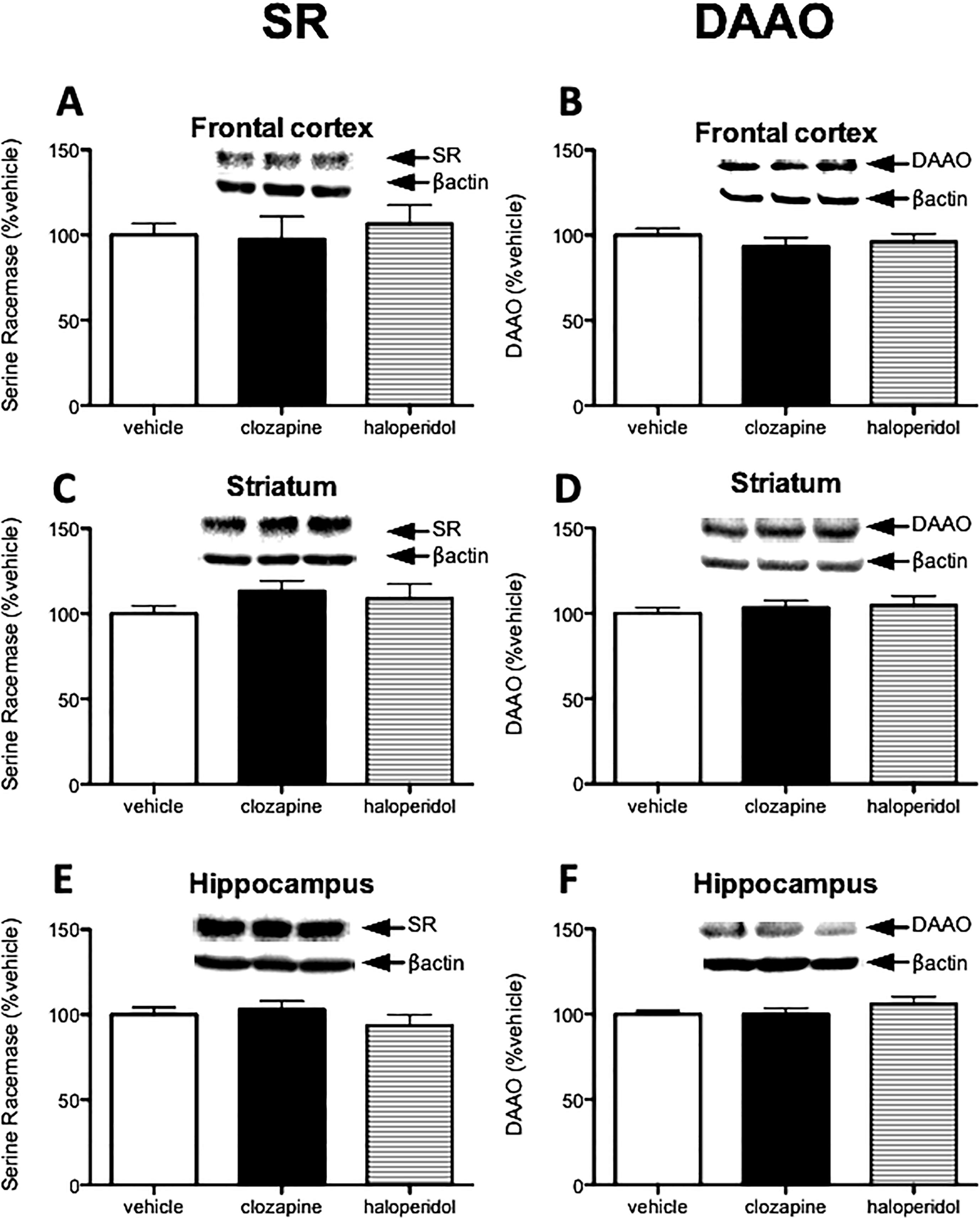
Haloperidol and clozapine do not alter SR and DAAO expression. Mice were divided into 4 groups (saline vehicle, acetic acid vehicle, haloperidol, and clozapine). Because saline vehicle (n = 4) and acetic acid vehicle (n = 5) group were not significantly different, we combined them into a single vehicle group. Protein levels of serine racemase (SR; A, C, E) and D-amino acid oxidase (DAAO; B, D, F) were measured in the frontal cortex (A, B), striatum (C, D) and hippocampus (E, F) from mice treated with vehicle (n = 9; white bars), clozapine (n = 8; black bars), or haloperidol (n = 7; stripe bars). Values are expressed as the optical density (OD) normalized to vehicle treated values (% vehicle). One-way ANOVA was used for analysis. All values represent the mean ± SEM.
2.4. D-serine treatment does not regulate SR and DAAO expression
Meta-analysis has shown that D-serine is effective in treating schizophrenia symptoms in conjunction with antipsychotic treatment, except clozapine (Tsai and Lin, 2010). However, it is not known whether D-serine administration affects the enzymes that regulate its concentration. We found that there was no change in SR or DAAO following either 1 day or 5 days of D-serine treatment (Fig 4 A–F), despite elevated D-serine levels in all brain regions, most notably in the hippocampus (Table 1).
Fig. 4.
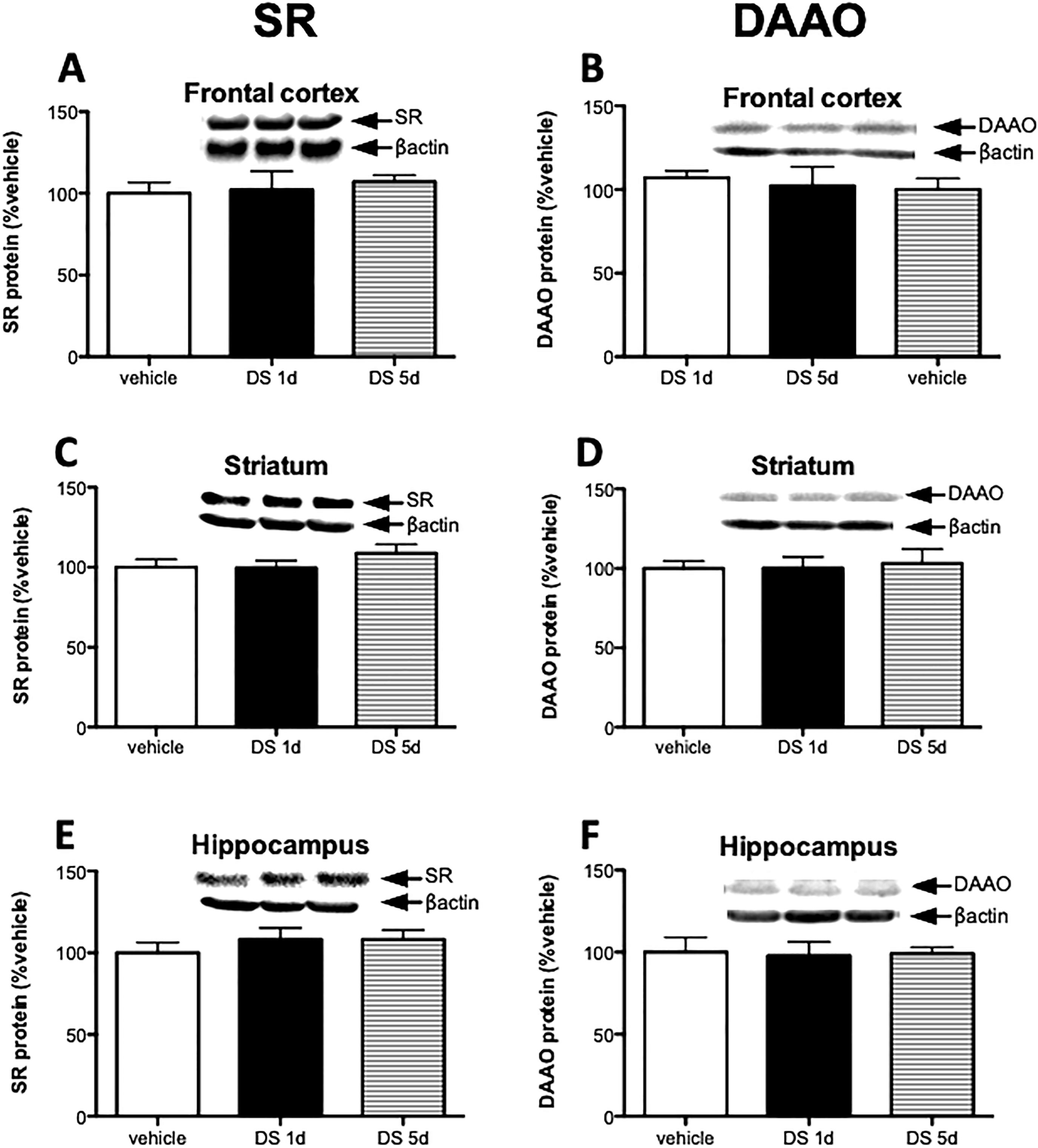
D-serine treatment does not regulate SR and DAAO expression. Mice were divided to 3 groups: vehicle (n = 6), 1 day of D-serine treatment (DS 1d; n = 5), and 5 days D-serine treatment (DS 5d; n= 5). Protein levels of SR (A, C, E) and DAAO (B, D, F) were measured in the frontal cortex (A, B), striatum (C, D) and hippocampus (E, F) of vehicle group (white bars), 1 day D-serine group (black bars) and 5 day D-serine group mice (stripe bars). Values are expressed as the optical density (OD) normalized to vehicle treated values (% vehicle). One-way ANOVA was used for analysis. All values represent the mean ± SEM.
Table 1.
Systemic d-serine administration increases d-serine levels in all examined brain regions. Mice were divided to 3 groups: vehicle (n = 6), 1 day of d-serine treatment (DS Id; n = 5), and 5 days d-serine treatment (DS 5d; n = 5). d-serine levels were measured in the hippocampus, frontal cortex, and striatum. Significant one-way ANOVA was followed up by Tukey’s multiple comparison test. Asterisk (*) indicates significant differences from the vehicle group (F = 17.32, DS Id: p = 0.00032, DS 5d: p = 0.00156). All values represent the mean ± SEM.
|
d-Serine (umol/g) |
|||
|---|---|---|---|
| Hippocampus | Frontal Cortex | Striatum | |
|
| |||
| Vehicle | 0.13 ± 0.07 | 0.23 ± 0.04 | 0.15 ± 0.05 |
| DS Id | 0.41 ± 0.12* | 0.31 ± 0.07 | 0.20 ± 0.09 |
| DS 5d | 0.36 ± 0.07* | 0.40 ± 0.24 | 0.19 ± 0.07 |
indicates significant differences from the vehicle treated group (frontal cortex SR: 0.0029, DAAO: 0.0096). All values represent the mean ± SEM.
2.5. DAAO expression is not altered in SR mutant mice
As brain D-serine levels are tightly regulated, we were interested in whether DAAO levels would be altered in mice with conditional SR deletions. Consistent with our previous findings (Benneyworth et al., 2012), nSRCKO had approximately a 50–70% reduction of SR in forebrain regions (Fig 5 A). In aSRCKO mice, we found a modest and selective 30% reduction in SR protein only in the hippocampus (our previous work measured SR in the total forebrain homogenate of aSRCKO, without region sub-dissection) (Fig 5 B), which was not associated with a reduction in hippocampal D-serine (WT: 0.18 ± 0.03 μmol/g; aSRCKO: 0.19 ± 0.05 μmol/g). We have found that in conditional SRKO mutants that tissue D-serine content remains fairly stable despite marked changes SR expression. Thus, we thought that DAAO might be reduced in these mutant mice as a mechanism to compensate for the reduced SR expression. However, we did not observe changes in DAAO protein in hippocampus of nSRCKO and aSRCKO mice, the brain region with the greatest magnitude of SR reduction (Fig 5 C, D).
Fig. 5.
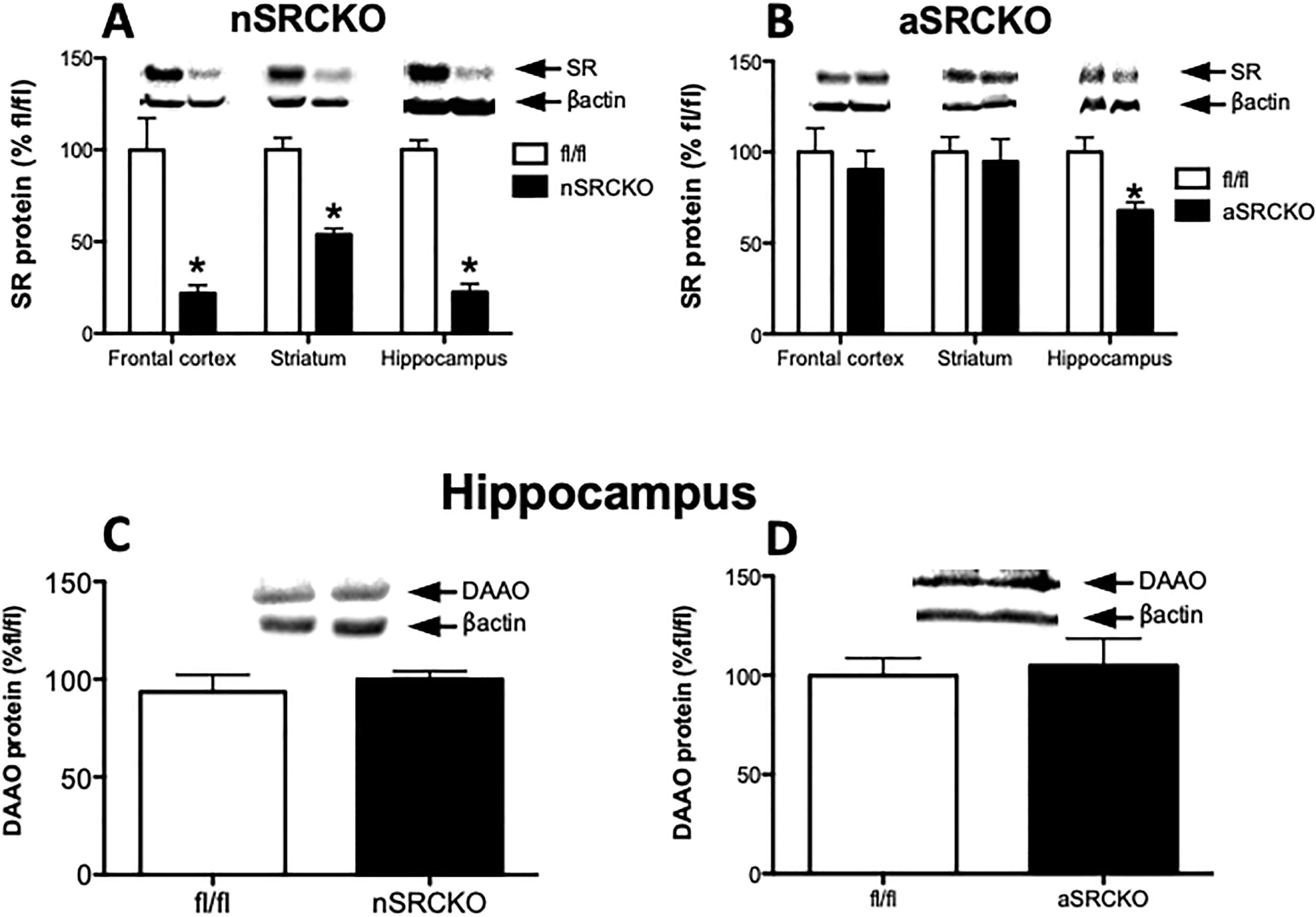
DAAO expression is not altered in conditional serine racemase mutant mice. Protein levels of SR in control fl/fl (n = 5 – 6; white bars), neuronal serine racemase knockout (A; nSRCKO; n = 5; black bars) and astrocyte serine racemase (B; aSRCKO; n = 5; black bars) were measured in the frontal cortex, striatum and hippocampus. Hippocampal DAAO protein was measured in control fl/fl (n = 5 – 6; white bars), neuronal serine racemase knockout (C; nSRCKO; n = 5; black bars) and astrocyte serine racemase (D; aSRCKO; n = 5; black bars). Values are expressed as the optical density (OD) normalized to fl/fl values (% fl/fl). Unpaired Student t-test was used for analysis. Asterisk (*) indicates significant differences from the control fl/fl group (p<0.05). All values represent the mean ± SEM.
3. Discussion
In this study, we were interested in determining what factors might regulate the expression of SR and DAAO in the brain, as these enzymes are important for regulating the levels of the NMDAR co-agonist, D-serine. We found that SR and DAAO expression were both reduced in a brain region specific manner following subchronic administration of the NMDAR antagonist, MK801, and pro-dopaminergic cocaine. Interestingly, the protein levels of these enzymes were not affected by antipsychotic drugs or D-serine treatment. Finally, DAAO protein was not changed in transgenic mice that lack SR in either CAMK expressing neurons or GFAP expressing astrocytes.
Regulation of SR and DAAO by psychotropic drugs has not been examined in previous studies. The dosage and the method of administration of the psychotropic drugs used in our study were based on the results from previous studies. MK801 treatment at a dosage of 0.15 mg/kg for 5 days was found to induce activation of neurotrophic signaling proteins in frontal cortex, hippocampus and striatum in our previous work (Takagi et al., 2015) and was found to regulate SR/DAAR in this study. D-serine with an initial dose of 300 mg for the first day and followed 150 mg for other days restored brain tissue’s D-serine level of SR −/− to the level of wild type mice and reversed the neurochemical, electrophysiologic and behavioral effects of the SR−/− phenotype (Balu et al., 2013). These prior results in the D-serine and MK801 experiments show the impact on proteins downstream of NMDARs. The dosing regimens of other drugs used in our study were determined according to the results of previous studies in which the doses of psychotropic drugs employed caused behavioral and neurochemical alterations. NBQX at 10 mg/kg for 5 days suppressed cocaine seeking behavior (Zavala et al., 2008), the dose employed in our study. Cocaine 20 mg/kg, Haloperidol 1 mg/kg and Clozapine 20 mg/kg are the typical doses for mice experiments (Bolden-Watson et al., 1993; Eisener-Dorman et al., 2011; Itzhak et al., 1999). While it is possible that higher doses could have elicited different results, the relevance of non-physiologic doses would be unclear.
We found that MK801 and cocaine selectively reduced SR and DAAO expression in the frontal cortex and striatum, respectively. It has been well established that MK801 increases extracellular glutamate in the frontal cortex (Adams and Moghaddam, 1998; Moghaddam et al., 1997; Roenker et al., 2012), while cocaine has the same effect in the striatum (Hotsenpiller and Wolf, 2003; McFarland et al., 2003; Rahman et al., 2005). Thus, we hypothesized that the brain region specific down regulation of SR and DAAO by MK801 and cocaine was due to the ability of these drugs to increase extracellular glutamate. One mechanism for glutamate signaling is through the ionotropic AMPARs, which drive excitatory post-synaptic potentials. To test whether extracellular glutamate regulates SR and DAAO expression via AMPAR activation, we treated mice with the AMPAR antagonist NBQX, prior to cocaine administration. Indeed, we found that NBQX blocked the reduction of both enzymes in the striatum induced by cocaine. Interestingly, NBQX did not increase SR or DAAO protein in the hippocampus or frontal cortex, brain regions where cocaine does not affect extracellular glutamate. Given that extracellular glutamate is tightly regulated by reuptake mechanisms and maintained at a very low concentration under normal conditions (Danbolt, 2001), the blockade of AMPAR by NBQX might not give further hypofunction of AMPAR and might not change its downstream. The results of our study suggest that the expression of SR is regulated by the AMPAR activity and the excessive activation of AMPARs might cause a compensatory reduction in D-serine availability to protect against NMDAR mediated excitotoxicity (Basu et al., 2009).
Contrary to what we found with SR and DAAO, neither MK801 nor cocaine administration reduced D-serine tissue content in the frontal cortex and striatum, respectively. AMPAR stimulation reduces extracellular D-serine content in the prefrontal cortex (Ishiwata et al., 2013). Therefore, although total D-serine content is not altered by MK801 and cocaine, it is likely that extracellular D-serine levels are reduced in the brain regions where these drugs cause an increase in glutamate release. Future in vivo microdialysis studies will be needed to test this hypothesis. In addition, more work will be needed to elucidate the significance of why both SR and DAAO protein are reduced by these drugs.
A substantial amount of genetic and pharmacologic evidence suggests that impaired glutamatergic signaling and altered expression of proteins associated with NMDAR function contribute to the pathophysiology of schizophrenia (Balu and Coyle, 2015). In particular, the NMDAR itself and proteins within two degrees of separation from it, including SR, have been genetically linked to schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics, 2014). Furthermore, D-serine is reduced in schizophrenia (Bendikov et al., 2007; Hashimoto et al., 2003) and D-serine treatment in conjunction with antipsychotics other than clozapine (Tsai et al., 1999; Tsai et al., 2006), attenuates schizophrenia symptoms (Tsai et al., 2006). Thus, we were interested whether the first-generation antipsychotic haloperidol, the second-generation antipsychotic clozapine, or D-serine regulates SR and/or DAAO expression. We found that administration of neither of these molecules affected SR and DAAO protein in the brain regions examined. It is possible that a longer duration of treatment or the use of an animal model of schizophrenia is needed for these drugs to elicit an effect. However, clozapine enhances the release of D-serine in PFC (Tanahashi et al., 2012) This line of researches suggests that D-serine release and/or re-uptake might be involved in the therapeutic effects of certain atypical antipsychotic medications.
We previously demonstrated no change in D-serine tissue content in our nSRKO and aSRKO mice, despite fairly robust reductions in SR levels (Benneyworth et al., 2012). Therefore, we hypothesized that a down-regulation in DAAO levels could be a compensatory mechanism by which these mice maintain D-serine levels within a normal range. However, there was no change in DAAO protein in the hippocampus of nSRCKO and aSRCKO mice. It should be noted that extracellular D-serine levels were lower in the CA1 region of the hippocampus nSRCKO mice in an in vivo dialysis study (Ishiwata et al., 2015). Thus, in forebrain where DAAO expression is quite low and limited to astrocytes separate from neuronal stores of D]-serine, DAAO’s influence on D-serine levels appears to be quite limited whereas in the cerebellum the levels of D-serine are inversely related to DAAO expression during development (Schell et al., 1995).
There are some conflicts in our study with previous studies. Some studies have reported that DAAO immunoreactivity is absent in forebrain, despite our study reporting its expression (Sasabe et al., 2014; Schell et al., 1995). However, other studies concur with our results showing DAAO expression in the same areas of the brain (Sacchi et al., 2008; Verrall et al., 2010; Yoshikawa et al., 2004b). Therefore, we consider that our western blot results in forebrain are consistent with most prior results showing low but measurable expression of DAAO in forebrain. Aother study reported SR and DAAO mRNA increases in various region of rat brain (Yoshikawa et al., 2004a) after MK801 administration in contrast to our results found in ouse frontal cortex. Timing, dose and duration of MK801 administration and species of animals might have caused these differences. Furthermore, it is a common finding that changes in mRNA levels do not correlate with protein levels after a particular intervention.
In summary, we found that psychotropic drugs that affect glutamate release, regulate the expression of SR and DAAO, enzymes that modulate the tissue concentration of D-serine. This regulation is brain region selective, and in the case of cocaine, is mediated in part by AMPAR activation. These results are intriguing because they uncover a potential new mechanism by which AMPAR activity could regulate NMDAR function via co-agonist availability.
4. Experimental Procedure
4.1. Animals
Wild-type (WT), constitutive SR −/−, Ca2+/calmodulin-dependent kinase II (CaMKII) promoter driven neuron-specific SR conditional knockout (nSRCKO) and glial fibrillary acidic protein (GFAP) promoter driven astrocyte-specific SR conditional knockout (aSRCKO) mice were generated as previously described (Basu et al., 2009; Benneyworth et al., 2012). The floxed (fl) SR construct was generated as previously described (Basu et al., 2009). All genetic constructs used in these experiments were backcrossed for over 10 generations onto a C57BL/6J background. SR+/− parents were bred to produce WT and SR−/− offspring. Mice homozygous for the floxed SR gene (SR fl/fl) were used as controls for nSRCKO and aSRCKO mice (adult male and female mice). Three-month old male mice were used for drug experiments. The animals were housed in a temperature (22 °C) and humidity-controlled facility with a 12/12 h light/dark cycle and provided with food and water ad libitum. All experimental procedures involving animals were approved by the McLean Hospital Institutional Animal Care and Use Committee.
4.2. Drugs
For all drug studies, WT mice were administered intraperitoneal (i.p.) injections for 5 days. For the D-serine study, mice were divided into 3 groups: vehicle only for 5 days, vehicle for 4 days and D-serine on last day (1 day D-serine treatment group), and D-serine for 5 days. The cocaine and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) study also had 3 groups: vehicle group (two vehicle injections), cocaine group (cocaine injection after vehicle injection), and cocaine/NBQX group (cocaine injection after NBQX injection). The two injections each day were 30 minutes apart. For the haloperidol and clozapine study, mice were divided to 4 groups (vehicle, acetic acid vehicle, haloperidol, and clozapine). These drugs were dissolved in sterile saline and given at the following doses: MK801 (0.15 mg/kg; Sigma-Aldrich, St. Louis MO), D-serine (200 mg/kg; Sigma-Aldrich, St. Louis MO), cocaine hydrochloride (20mg/kg; MEDISCA, Plattsburgh NY), NBQX disodium salt (10mg/kg; Abcam, Cambridge MA). Haloperidol HCl (Tocris, Ellisville MO; 1 mg/kg) was dissolved in sterile water. Clozapine (Tocris, Ellisville MO; 20 mg/kg) was dissolved in a small volume of acetic acid, adjusted to pH 5.2 with 10 N NaOH, and brought up to final volume with sterile water. The doses were calculated according to the base weight of each drug at a volume of 10 ml/kg body weight. In haloperidol and clozapine experiment, mice received injections at the dose of 20 ml/kg body weight. Drug-treated animals were sacrificed 90 minutes after the last injection, their brains were quickly dissected, flash frozen, and stored at −80 °C until analysis. Both hemispheres of the hippocampus, striatum and frontal cortex were collected and one hemisphere was used for western blot and the other for high-performance liquid chromatography (HPLC).
4.3. Western blot analysis
Brain tissue was briefly sonicated in extraction buffer (60-mM Tris buffer, 2% sodium dodecyl sulfate, 0.1% phosphatase inhibitor, pH 6.8). Protein content was determined by a colorimetric assay based upon the Bradford method using Bio-Rad dye reagent (Bio-Rad Life Sciences, Hercules, CA). Prior to gel loading, samples were heated to 95 °C for 5 min. Samples were electrophoretically separated on an SDS-12.5% polyacrylamide gel. Nitrocellulose membranes (Bio-Rad, Hercules, CA) were blocked with 5% nonfat dry milk (Shaw’s; Boise, ID) in 0.05% Tween-20/Tris-buffered saline and then incubated with primary antibody overnight at 4 °C with same blocking buffer. The primary antibodies were used at the following dilutions: goat anti SR (1:500; Santa Cruz Biotechnology, Santa Cruz CA), rabbit anti DAAO (1:500; Nordic Immunological, Netherlands), and rabbit anti β-actin (1:8000; Abcam, Cambridge MA). After incubation with goat anti rabbit (1:5000; Abcam, Cambridge MA) or rabbit anti goat (1:5000; Santa Cruz Biotechnology, Santa Cruz CA) horseradish peroxidase-conjugated secondary antibodies, immunocomplexes were visualized by chemiluminescence using Western Lightning-ECL (Perkin Elmer, Waltham MA). Semi-quantitative assessment of protein bands was executed by computerized densitometry using Image Labo Software (Bio-Rad, Hercule, CA). Chemiluminescent values of the protein of interest were divided by its corresponding β-actin chemiluminescent values.
4.4. HPLC
The specificity of the tissue assay for D-serine was validated by using brain tissue from SR−/− mice as the negative control (Wolosker et al., 2016). Amino acids were derivatized with o-phthaldialdehyde (Alfa Aesar, Ward Hill, MA) and N-tert-butyloxycarbonyl-L-cysteine (Novabiochem, Gibbstown NJ) as previously described (Benneyworth et al., 2012; Hashimoto et al., 1992). Derivatized samples were resolved by a C18 column (Alltima HP C18, 3 lm pore, Grace Discovery Sciences) and analyzed by fluorometric detection. The HPLC system consisted of a SCL- 10A controller, two LC-10AT VP pumps, a SIL-10AD auto injector, a DGU-20A5 degasser and a RF-551 fluorescence monitor (all instruments by Shimadzu Corporation, Kyoto, Japan). The separation was accomplished by application of a binary gradient of 25-mM sodium acetate (pH 6.5) and acetonitrile. The gradient progressed from 10 to 20% acetonitrile during a 20-min period at a flow rate of 1.0 mL/minute. The column was allowed to re-equilibrate to starting concentrations after each run. The detection and quantitation limits for D-serine were 0.16 and 0.53 lmol/g protein, respectively. Amino acid concentrations were calculated using the internal standard as a comparison to standard samples run at the beginning of each session, as previously described (Hashimoto et al., 1992).
4.5. Statistical analyses
The results of MK801 and genetic manipulated animals experiments were compared using unpaired Student t-test. Values of p < 0.05 were considered statistically significant. One-way ANOVA was used to analyze D-serine treatment, cocaine/NBQX and haloperidol/clozapine experiments. Significant F values were subject to post hoc analyses. Tukey’s multiple comparison test was used as a post hoc analysis after one-way ANOVA. Values of P < 0.05 were considered statistically significant.
Acknowledgements
We thank Alexandra Berg and Kendall Presti for animal colony maintenance and genotyping. JTC reports consulting with Concert Pharm and holding a patent on D-serine for the treatment of serious mental illness, which is owned by Massachusetts General Hospital. DTB served as a consultant for LifeSci Capital and received research support from Takeda Pharmaceuticals. ST reports no conflicts of interest.
Funding:
This research was supported by A Phyllis & Jerome Lyle Rappaport Mental Health Research Scholars Award and 1K99MH099252-01A1 (DTB); R01MH05190 and P50MH0G0450 (JTC). Shunsuke Takagi was supported in part by Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation from the Japan Society for the Promotion of Science (S2301).
Declarations:
S.T. declares that he has no conflict of interest. JTC reports consulting with Concert Pharm and holding a patent on D-serine for the treatment of serious mental illness, which is owned by Massachusetts General Hospital. DTB served as a consultant for LifeSci Capital and received research support from Takeda Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams B, Moghaddam B, 1998. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 18, 5545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, Ohno Y, Kihara A, Jensen ON, Radzishevsky IS, Wolosker H, 2009. Feedback inactivation of D-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc Natl Acad Sci U S A. 106, 7589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT, 2013. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A. 110, E2400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Takagi S, Puhl MD, Benneyworth MA, Coyle JT, 2014. D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol. 34, 419–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Coyle JT, 2015. The NMDA receptor ‘glycine modulatory site’ in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol. 20, 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT, 2009. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 14, 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G, 2007. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 90, 41–51. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT, 2012. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. 32, 613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden-Watson C, Watson MA, Murray KD, Isackson PJ, Richelson E, 1993. Haloperidol but not clozapine increases neurotensin receptor mRNA levels in rat substantia nigra. J Neurochem. 61, 1141–3. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D, 2002. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 99, 13675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H, 1983. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 334, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Balu D, Benneyworth M, Basu A, Roseman A, 2010. Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin Neurosci. 12, 359–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio L, Podda MV, Leone L, Piacentini R, Mastrodonato A, Cappelletti P, Sacchi S, Pollegioni L, Grassi C, D’Ascenzo M, 2013. Reduced D-serine levels in the nucleus accumbens of cocaine-treated rats hinder the induction of NMDA receptor-dependent synaptic plasticity. Brain. 136, 1216–30. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, 2001. Glutamate uptake. Prog Neurobiol. 65, 1–105. [DOI] [PubMed] [Google Scholar]
- De Miranda J, Panizzutti R, Foltyn VN, Wolosker H, 2002. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci U S A. 99, 14542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM, 2011. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav Brain Funct. 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachim HA, Pereira AC, Iyomasa-Pilon MM, Rosa ML, 2016. Differential Expression of AMPA Subunits Induced by NMDA Intrahippocampal Injection in Rats. Front Neurosci. 10, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Yamauchi T, Kamiya N, Miyahara I, Yoshimura T, Mihara H, Kurihara T, Hirotsu K, Esaki N, 2009. Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe. J Biol Chem. 284, 25944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K, 1992. The presence of free D-serine in rat brain. FEBS Lett. 296, 33–6. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M, 2003. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 60, 572–6. [DOI] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA, 2016. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol. 78, 351–65. [DOI] [PubMed] [Google Scholar]
- Horiike K, Tojo H, Arai R, Nozaki M, Maeda T, 1994. D-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 652, 297–303. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Wolf ME, 2003. Baclofen attenuates conditioned locomotion to cues associated with cocaine administration and stabilizes extracellular glutamate levels in rat nucleus accumbens. Neuroscience. 118, 123–34. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA, 2013. AMPARs and synaptic plasticity: the last 25 years. Neuron. 80, 704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S, Umino A, Umino M, Yorita K, Fukui K, Nishikawa T, 2013. Modulation of extracellular d-serine content by calcium permeable AMPA receptors in rat medial prefrontal cortex as revealed by in vivo microdialysis. Int J Neuropsychopharmacol. 16, 1395–406. [DOI] [PubMed] [Google Scholar]
- Ishiwata S, Umino A, Balu DT, Coyle JT, Nishikawa T, 2015. Neuronal serine racemase regulates extracellular D-serine levels in the adult mouse hippocampus. J Neural Transm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Ali SF, 1999. Effect of the dopaminergic neurotoxin MPTP on cocaine-induced locomotor sensitization. Pharmacol Biochem Behav. 63, 101–7. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G, 1999. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 156, 286–93. [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH, 2005. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A. 102, 2105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Duffy S, Wang W, Barger SW, Baker GB, Roder JC, 2009. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn Mem. 16, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M, Parpura V, Mothet JP, 2014. Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci. 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K, 1995. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 65, 454–8. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW, 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 23, 3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D, 1997. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 17, 2921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH, 2009. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci U S A. 106, 2921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, 2011. Analysis of free D-serine in mammals and its biological relevance. J Chromatogr B Analyt Technol Biomed Life Sci. 879, 3169–83. [DOI] [PubMed] [Google Scholar]
- Park P, Sanderson TM, Amici M, Choi SL, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL, 2016. Calcium-Permeable AMPA Receptors Mediate the Induction of the Protein Kinase A-Dependent Component of Long-Term Potentiation in the Hippocampus. J Neurosci. 36, 622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G, 2007. Physiological functions of D-amino acid oxidases: from yeast to humans. Cell Mol Life Sci. 64, 1373–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L, Piubelli L, Molla G, Rosini E, 2018. D-Amino Acid Oxidase-pLG72 Interaction and D-Serine Modulation. Front Mol Biosci. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Kwon NH, Won MS, Choe ES, Shim YB, 2005. Functionalized conducting polymer as an enzyme-immobilizing substrate: an amperometric glutamate microbiosensor for in vivo measurements. Anal Chem. 77, 4854–60. [DOI] [PubMed] [Google Scholar]
- Ritter RC, 2011. A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 105, 94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenker NL, Gudelsky GA, Ahlbrand R, Horn PS, Richtand NM, 2012. Evidence for involvement of nitric oxide and GABA(B) receptors in MK-801- stimulated release of glutamate in rat prefrontal cortex. Neuropharmacology. 63, 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi S, Bernasconi M, Martineau M, Mothet JP, Ruzzene M, Pilone MS, Pollegioni L, Molla G, 2008. pLG72 modulates intracellular D-serine levels through its interaction with D-amino acid oxidase: effect on schizophrenia susceptibility. J Biol Chem. 283, 22244–56. [DOI] [PubMed] [Google Scholar]
- Sasabe J, Suzuki M, Imanishi N, Aiso S, 2014. Activity of D-amino acid oxidase is widespread in the human central nervous system. Front Synaptic Neurosci. 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH, 1995. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 92, 3948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C., 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511, 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Balu DT, Coyle JT, 2015. Subchronic pharmacological and chronic genetic NMDA receptor hypofunction differentially regulate the Akt signaling pathway and Arc expression in juvenile and adult mice. Schizophr Res. 162, 216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Puhl MD, Anderson T, Balu DT, Coyle JT, 2020. Serine Racemase Expression by Striatal Neurons. Cell Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M, 2012. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 165, 1543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JT, 1999. D-serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 156, 1822–5. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chang YC, Chong MY, 2006. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 59, 230–4. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin PY, 2010. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 16, 522–37. [DOI] [PubMed] [Google Scholar]
- Verrall L, Burnet PW, Betts JF, Harrison PJ, 2010. The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. Mol Psychiatry. 15, 122–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang X, Yu H, Wang H, Qi Y, Geng M, 2020. Effects of arsenic exposure on D-serine metabolism in the hippocampus of offspring mice at different developmental stages. Arch Toxicol. 94, 77–87. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Balu DT, Coyle JT, 2016. The Rise and Fall of the d-Serine-Mediated Gliotransmission Hypothesis. Trends Neurosci. 39, 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Kobayashi T, Oka T, Kawaguchi M, Hashimoto A, 2004a. Distribution and MK-801-induced expression of serine racemase mRNA in rat brain by real-time quantitative PCR. Brain Res Mol Brain Res. 128, 90–4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Oka T, Kawaguchi M, Hashimoto A, 2004b. MK-801 upregulates the expression of d-amino acid oxidase mRNA in rat brain. Brain Res Mol Brain Res. 131, 141–4. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL, 2008. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacol. 18, 600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]