Abstract
Parkinson's disease (PD) is the second most frequent neurodegenerative disorder following Alzheimer's disease. Advanced stages of PD, 4 or 5 of the Hoehn and Yahr Scale, are characterized by severe motor complications, limited mobility without assistance, risk of falling, and non-motor complications. The aim of this review was to provide a practical overview on specific artificial intelligence (AI) systems for the management of advanced stages of PD, as well as relevant technological limitations. The authors conducted a systematic search on PubMed and EMBASE with predefined keywords searching for studies published until December 2020. Full articles that satisfied the inclusion criteria were included in the systematic review. To minimize results bias, the reference list was manually searched for pertinent articles to identify any additional relevant missed publications. Exclusion criteria included the following: Other stages of PD than 4 and 5 of the Hoehn and Yahr Scale, case reports, reviews, practice guidelines, commentaries, opinions, letters, editorials, short surveys, articles in press, conference abstracts, conference papers, and abstracts published without a full article. The search identified 21 studies analyzing AI-based applications and robotic systems used for the management of advanced stages of PD, out of which 6 articles analyzed AI-based applications for autonomous management of pharmacologic therapy, 5 articles analyzed home-based telemedicine systems and 10 articles analysed robot-assisted gait training systems. The authors identified significant evidence demonstrating that current AI-based technologies are feasible for automatic management of patients with advanced stages of PD. Improving the quality of care and reducing the cost for patients and healthcare systems are the most important advantages.
Keywords: advanced Parkinson's disease, artificial intelligence, telemedicine, deep learning, machine learning, future therapies, Parkinson's disease, future of medicine, automated management, levodopa-carbidopa, home titration
1. Introduction
Parkinson's disease (PD) is the second most frequent neurodegenerative disorder following Alzheimer's disease, affecting almost 1% of the population over 60 years of age and 5% in individuals up to 85 years (1). Advanced stages of PD, 4 or 5 of the Hoehn and Yahr Scale, are characterized by severe motor complications, limited mobility without assistance, risk of falling, and non-motor complications (1). PD affects individuals differently and not all the patients may experience the full range of non-motor symptoms. Patients with stage 4 are unable to live an independent life and require permanent assistance throughout the day and are not capable of walking or standing unassisted, but they frequently use a walker to help them. When patients reach the final stage of PD, stage five, they require a wheelchair or may be immobilized. An around-the-clock care team is mandatory to help the patient which is overwhelmed by critical non-motor symptomatology. Furthermore, the intensification of critical symptomatology is frequently a consequence of insufficient optimization of levodopa-carbidopa therapy. Since dementia also occurs in up to 75% of individuals with stage 5 and the side effects of pharmacological agents outweigh their benefits, caring for these patients represents a real challenge for their families and for the healthcare personnel (1-3).
The number of patients with advanced stages of PD has increased as a direct consequence of a greater life expectancy and an improved clinical management. Although in the last decade considerable progress has been made in PD pharmacological therapy, particularly in the advanced stages, over the years, the treatment loses its efficacy, thereby reducing the quality of life (QoL) and lifespan of the patient (1). Pharmacological therapy of the advanced stages of PD is completely different from treatment of the earlier stages and is focused on a multidisciplinary team that could help overcome the critical non-motor symptomatology consisting of hallucinations, psychosis, dysphagia, urinary incontinence, orthostatic hypotension, postural instability, and multiple fractures (1-3).
Notwithstanding the fact that current therapeutic guidelines are comprehensive and accurate, tailored therapy is still recommended for each individual patient (1-3). This approach implies a careful assessment of a considerable number of resources, including professional healthcare systems, specialized personnel, interdisciplinary collaborations and synchronized medical procedures. Unfortunately, the unprecedented shortage of healthcare workers is worsening constantly worldwide, with artificial intelligence (AI)-based medical technologies emerging as one of the most promising solutions to this problem (4,5).
At present, only a limited range of specific AI-based medical systems are available in clinical practice, including the automated detection of atrial fibrillation, seizures, or automated imaging computer-aided diagnosis (4). Additionally, as the global population ages, the economic burden of neurodegenerative disorders is significantly increasing, thereby any measures or technologies that prevent, delay, or improve PD evolution may contribute to reduced healthcare system costs (5). The present review therefore aimed to provide a practical overview of specific AI systems for the management of advanced stages of PD and to discuss future directions of implementation of AI systems in clinical practice, as well as relevant technologic limitations.
2. Methods
To identify potentially eligible observational studies evaluating AI applications and robotic systems used in the management of PD patients with advanced stages, the authors conducted a systematic search of PubMed and EMBASE from inception until the 4th of December 2020 without restrictions. The search strategy applied in these two databases included the following search string for PubMed [(‘advanced PD’(MeSH)] or (‘AI’) or [‘AI’(MeSH)] or (‘automated’) or [‘telemedicine’(MeSH)] or (‘machine learning’) or (‘deep learning’) or [‘home-based telemedicine’(MeSH)] and the following search string for EMBASE (‘advanced PD’/exp or ‘AI’ or ‘AI’/exp or ‘telemedicine’ or ‘deep learning’/exp or ‘home-based telemedicine’).
Furthermore, to minimize results bias, the reference list was manually searched for pertinent articles to identify any additional relevant missed publications. Exclusion criteria included the following: Other stages of PD than 4 and 5 of the Hoehn and Yahr Scale, case reports, reviews, practice guidelines, commentaries, opinions, letters, editorials, short surveys, articles in press, conference abstracts, conference papers, and abstracts published without a full article.
According to the aforementioned eligibility criteria, two investigators (LPD and LCP) performed a screening evaluation independently by scrutinizing titles and abstracts excluding any apparently irrelevant studies. Subsequently, selected articles fulfilling the inclusion and exclusion criteria were further evaluated by carefully reviewing the entire text. A mutual consensus was reached by discussing any discrepancies regarding study eligibility. One investigator (LPD) extracted the data through an electronic spreadsheet, and then the other investigator (LCP) reviewed the extracted data for accuracy. Discrepancies regarding the results of extracted data were settled by discussion. Extracted data was then entered into three tables, while final data was collated and presented in the following sections of the review.
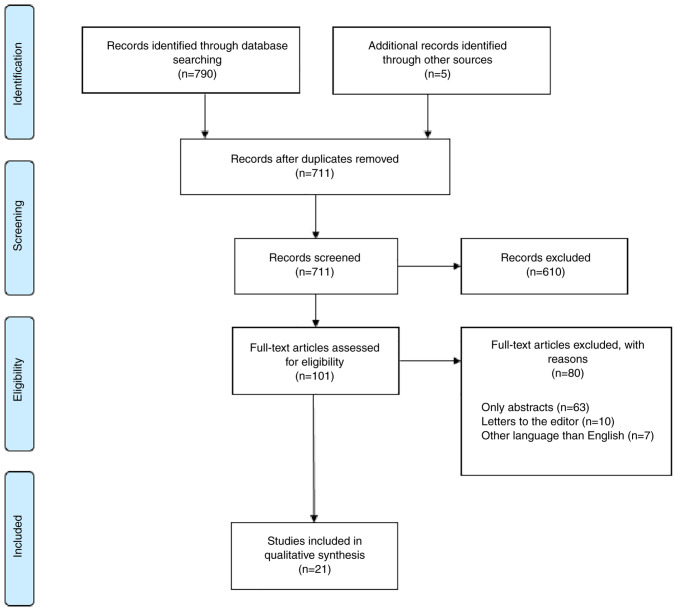
The search algorithm identified 21 studies that were included in the qualitative synthesis (Fig. 1).
Figure 1.
PRISMA flow diagram for study selection.
3. AI applications for autonomous management of pharmacologic therapy
The search identified 6 articles regarding autonomous management of pharmacologic PD therapy (Table I). A study performed by Li et al revealed how extracting movement information from 2D videos using deep-learning-based pose estimation algorithm (Convolution Pose Machines) could detect disease severity or levodopa-induced dyskinesia (LID) (6). They used nine participants diagnosed with PD and LID and evaluated those regularly using standardized scales such as the Unified Dyskinesia Rating Scale (UDysRS) and Unified Parkinson's Disease Rating Scale (UPDRS). This information was corroborated with movement trajectories of individual joints extracted from videos. Specific features of movement trajectories were used to train random forests to detect and estimate PD severity and LID presence. The tapping task presented satisfactory results for PD detection, while leg agility detected PD severity. Based on the communication task, authors were capable of estimating LID presence (6).
Table I.
Artificial intelligence applications for autonomous management of pharmacologic therapy used in advanced stages of PD.
| First author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|---|---|---|---|---|---|
| Li et al | 2018 | Observational study | Extracting movement information from 2D videos using a deep-learning-based pose estimation algorithm (Convolution Pose Machines) | Levodopa | Communication task-evaluation detected levodopa-induced dyskinesia. Toe-tapping detected parkinsonism, while leg agility detected parkinsonism severity | (6) |
| Shamir et al | 2015 | Observational study | Creation of a CDSS that retrieves patient information, visualizes drug treatment, and recommends deep brain stimulation and drug dosages based on 3 machine-learning methods (support vector machines, Naïve Bayes and random forest) | Levodopa Deep brain stimulation | A CDSS that uses appropriate parameters and has the potential of improving the clinical management of PD patients | (7) |
| Tucker et al | 2015 | Clinical observational study | Data mining using non-wearable sensors to model and predict adherence to drug treatment outside the hospital, based on gait variations | PD medication Levodopa | Whole-body movement data allow cost-effective, remote monitoring of drug treatment adherence in PD | (8) |
| Turner et al | 2016 | Clinical observational study | EpiNet, a novel artificial gene regulatory network, with dynamic analysis transition between different states of PD | Levodopa | EpiNet can discriminate between different movement patterns indicative of levodopa including optimal, insufficient, or excessive treatment | (9) |
| Yue et al | 2017 | Case study | WGCNA was used to identify two over-represented PD-specific gene co-expression network modules and using drug-protein regulatory relationship databases (DMAP), developed a DESS for candidate drugs that could restore gene expression in the identified modules | Novel PD drugs that could restore gene expression in the identified modules | Integrating gene co-expression modules with biomolecular interaction network analysis can lead to the identification of network modules important in PD pathways This approach can help identify drugs useful in polygenic diseases such as PD | (10) |
| Przybyszewski et al | 2016 | Clinical experimental study | Rough set theory was used to integrate reflexive saccades (latency, amplitude, and duration) to develop predictions of neurological symptoms in patients | Deep brain stimulation Levodopa | Reflexive saccades can be a powerful biomarker in the assessment of symptom progression in PD | (11) |
PD, Parkinson's disease; CDSS, clinical decision support system; weighted gene correlation network analysis; DESS, drug effect sum score.
Shamir et al created a complex clinical decision support system (CDSS) based on support vector machines, Naïve Bayes and random forest machine learning methods (7). The CDSS was created using diagnosis information and drug treatment from 10 patients following 89 postoperative visits post-deep brain stimulation (DBS) to make information retrieval, visualization of treatment and recommendations either for deep brain stimulation or for drug treatment adjustments. The CDSS was capable of predicting 86% of the motor improvement scores at one year following DBS (7).
Lack of adherence to drug treatment is a widespread problem, but in PD it is even more prevalent (more than 61% of patients) due to the cognitive impairment associated with old-age and to disease progression, particularly in the lack of adherence (8). As adherence to drug treatment is usually assessed by self-reporting throughout short clinical visits, non-adherence may be easily missed. Tucker et al used non-wearable sensors to model and predict adherence to drug treatment outside the hospital, based on gait variations, using data mining (8). The authors used seven voluntary patients first off their dopaminergic drugs (overnight, >12 h) and subsequently on therapy (at least 1 h following drug therapy administration). Each time, they were subjected to a walking trial. The Microsoft Kinect multimodal off-the-shelf sensor was used to capture 3D skeletal images. Recorded data was processed and revealed a high correlation between adherence and non-adherence identification and whole-body movement patterns (8). Thus, remote monitoring of PD adherence to drug treatment may increase drug treatment effectiveness, disease control and patient QoL.
Apart from movement evaluation, Turner et al used epiNet, a novel artificial gene regulatory network, with dynamic analysis transition between different states of PD (9). First, they acquired data from 25 patients with confirmed PD diagnosis that were being treated with levodopa (9). Patients were subjected to movement monitoring using light-weight sensors and UDysRS. Secondly, movement data was corroborated with gene expression data to reveal patterns in LID or insufficient levodopa administration. Authors revealed that epiNet was able to differentiate between states of under- or over-dosage of levodopa (9).
Regarding drug discovery for PD, Yue et al used a weighted gene correlation network analysis (WGCNA) and identified two over-represented PD-specific gene co-expression network modules, namely the Brown module (comprising 544 genes) and the Turquoise module (comprising 1,190 genes) (10). Furthermore, using drug-protein regulatory relationship databases (DMAP) they developed a Drug Effect Sum Score (DESS) for candidate drugs that could restore gene expression in the identified modules. Authors identified 5 potential new treatments for PD and 6 drugs with potential repositioning applications (10). Thus, integrating gene co-expression modules with biomolecular interaction network analysis may lead to the identification of network modules important in PD pathways. This approach could help identify drugs useful in polygenic diseases such as PD (10).
Furthermore, being able to assess symptom progression in PD could mean rapid dosing changes or referral to deep brain stimulation, resulting in a higher disease control and QoL. Przybyszewski et al used a rough set theory to integrate reflexive saccades (latency, amplitude and duration) to develop predictions of neurological symptoms in patients (11). Authors evaluated 10 patients, integrating the age of patients and reflexive saccades. They were able to accurately predict neurological symptoms in ~80% of patients (11).
4. Home-based telemedicine systems
The search strategy returned 5 articles related to the management of advanced stages of PD using home-based telemedicine systems, out of which 4 were population-based cohort studies and 1 was a randomized controlled trial (Table II).
Table II.
Home-based telemedicine systems used in advanced stages of Parkinson's disease.
| Author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|---|---|---|---|---|---|
| Willows et al | 2017 | Observational study | Nasojejunal tube placed during fluoroscopic passive/active positioning, with radiological confirmation of placement | LCIG delivery initiated through telemedicine over a 16-h period: total morning 5-10 ml (100-200 mg levodopa) to 20 ml max; median time for titration 2.8 days | Technically achievable, a well-tolerated alternative method | (12) |
| Evans et al | 2020 | Pilot Study | Phone consultations in virtual clinic combined with a report from a Parkinson's KinetiGraph | - | Acceptable for most patients, timesaving, in need of further cost analysis | (13) |
| Hssayeni et al | 2019 | Comparative Study | Wearable sensors combined with gradient tree boosting or with a deep learning model based on LSTM networks | - | Highest correlation for gradient tree boosting; solid approach for assessing tremor severity | (14) |
| Cilia et al | 2020 | Observational Study | Remote telenursing assistance service ‘Parkinson Care’ and video-consultations on Microsoft teams® platform | - | Introduction of a new element (case managers, not initially part of patient's care team); development of triage algorithm | (15) |
| Beck et al | 2017 | Randomized controlled trial | Usual care by a physician compared to 4 virtual consults by a remote neurologist added to usual care | - | Virtual care was achievable with no major differences in quality of life and burden | (16) |
LCIG, levodopa-carbidopa intestinal gel; LSTM, long short-term memory.
Home-based telemedicine systems are defined as the process of providing healthcare remotely using communication devices and AI applications for the diagnosis and management of a particular disorder. Telemedicine systems follow a hierarchical tiered structure which includes three distinct levels: Rural/remote center; city/district hospital center; speciality center. The system provides real-time, high quality audio-video interactions between home-based patients and nurses, physicians and occasionally a multidisciplinary team (12).
A study performed by Willows et al analyzed the feasibility of telemedicine for levodopa-carbidopa intestinal gel (LCIG) home titration, optimization and assessed patient, physician, and nurse satisfaction (12). A total of 15 patients with advanced stages of PD were enrolled from 4 clinics and telemedicine-assisted LCIG home management period was defined as the start of the pump to the decision for permanent percutaneous endoscopic transgastric jejunostomy (PEG-J) surgery or termination of LCIG treatment (12). To assess technical feasibility and also procedural limits, all technical events were reported and further analyzed. The results revealed that the system was highly efficient, technically feasible and well-accepted (12). Patients, neurologists and nurses were satisfied and considered the system to consume less time than required for a patient admitted to the hospital for the management of LCIG (12). Technical problems associated with the novel home-based telemedicine system were extremely rare, correlated with internet outage and rapidly resolved. No severe adverse events were present, with the exception of only one event: A tube occlusion. The authors of the study emphasized that the home-based telemedicine system for LCIG management may reduce healthcare costs by reducing in-ward bed occupancy as well as hospitalization and travel costs (12).
A pilot study by Evans et al on 61 PD patients analyzed a new concept called PD virtual clinic to develop an efficient system combining home-based telemedicine systems and digital wearable technology. The Parkinson's KinetiGraph (a wrist-worn device providing objective motor assessment) was used by clinicians for management and optimization of medication (13). The results of the study demonstrated that a PD virtual clinic is safe and efficient, and the majority of consultations are equivalent to face-to-face clinic in terms of treatment outcome (13).
Notwithstanding the fact that home-based telemedicine systems are a feasible solution for the management of advanced stages of PD, a constant difficulty is represented by the absence of a precise motor dysfunction quantification method in advanced stages of PD. However, a study by Hssayeni et al (14) revealed that a novel ensemble model based on gradient tree boosting with an automated interpretation by a deep learning algorithm is able to provide a full spectrum of motor dysfunction by analyzing the tremor of the patients. The system performs a continuous monitoring of the movement of individuals in their own homes, to record the results, and finally to estimate total Parkinsonian tremor. The system represents an efficient method for automated PD motor dysfunction assessment and could be properly used in future home-based telemedicine applications (14).
Since the outbreak of the COVID-19 pandemic in December 2019, it was demonstrated that patients with pre-existing neurodegenerative disorders and COVID-19 may develop an exacerbation of the neurological symptomatology and severe, potentially life-threatening forms of COVID-19 pneumonia. In this new paradigm for managing crises throughout and following the curfew, home-based telemedicine systems represent a safe, cost-efficient solution for patients, physicians, nurses, and healthcare systems. A study by Cilia et al proposes a two-pronged model to optimize the management of PD patients, based on a successful experience of telemedicine throughout the COVID-19 crisis in Italy (15), which was operated in two distinct steps. The first step was a novel remote telemedicine platform for nursing assistance service, named ‘ParkinsonCare’, which currently remains available in Milan since February 2019(15). Notably, the whole telemedicine service was made available free of charge for all patients. A subset of data obtained between the 12th of March and the 14th of May 2020 was analyzed and the results revealed that the telemedicine system managed 2,021 interactions (telephone calls) between nurses and 525 patients and only one third of PD patients required a neurologist and out of the 194 video-consultations (using Zoom® platform) performed by a neurologist, only 18 failed, mostly due to inability of the patient to deal with the software (15). The second step was implemented using Microsoft Teams® platform for regulation-compliant video-consultations with experienced neurologists (15). Similar with the first step, between the 30th of March and the 14th of May 2020, video-consultations increased steadily to become over two-thirds of the total number of outpatient assessments and only 21.8% of patients required a visit to the hospital. The analysis of the questionnaires of the patients revealed that more than two-thirds of the patients had provided positive feedbacks, comments were not provided in 28% of cases and 2% of PD patients had a negative feedback (15). The authors concluded that for the future a pre-existing multidisciplinary medical team could overcome difficulties in treating non-motor complications of PD using the aforementioned home-based telemedicine applications (15).
A randomized controlled trial compared usual care with usual care supplemented by 4 virtual visits (16) in order to determine whether home-based telemedicine is a feasible and efficient method of providing specialized neurologic care for PD patients. The results revealed that each virtual house call saved patients a median of 88 min and 38 miles, demonstrating that virtual consultations are feasible and provide substantial convenience. The virtual visit was neither more nor less effective than usual in-person care visits (16).
5. Robotic systems and AI applications for gait management
The search identified 10 articles concerning robotic systems and AI applications for gait management in PD (Table III).
Table III.
Robot-assisted gait training systems and their outcomes.
| Author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|---|---|---|---|---|---|
| Lo et al | 2010 | Prospective study | Lokomat | 10 sessions of 30 min each | Reduction in FOG (self-report and clinician-rated scoring) | (17) |
| Nardo et al | 2014 | Prospective study | Lokomat | Daily 45 min sessions for 5 weeks | Possible improvement of gait performance | (18) |
| Picelli et al | 2012 | Randomized controlled trial | Robotic stepper training (using the Gait Trainer) vs. physiotherapy | 12 sessions of 45 min each (3/week, 4 consecutive weeks) | Statistically significant improvement in walking speed and walking distance | (19) |
| Galli et al | 2016 | Prospective study | Commercially available G-EO system vs. intensive treadmill therapy (Gait trainer™) | 20 sessions of 45 min each (5 days a week for 4 weeks) | (20) | |
| Kang et al | 2019 | Prospective, single-blind, single-center, randomized controlled trial | Walkbot-S™ vs. treadmill training | 12 sessions in 4 weeks each with an actual training time of 30 min | Changes in gait speed | (21) |
| Fundarò et al | 2019 | Retrospective study | Lokomat System® vs. conventional training program under the supervision of a physiotherapist | 20 sessions of 30 min each (5 days/week for 4 weeks) | Significant improvement of total UPDRS | (22) |
| Capecci et al | 2019 | Multicentre single-blind prospective randomized controlled study | End-effector robotic device G-EO system vs. treadmill training | 20 sessions of 45 min each (5 days/week for 4 weeks) | Significantly lower frequency of daily episodes of gait freezing | (23) |
| Arami et al | 2019 | Comparative study | Two-class approach vs. three class approach for predicting freezing of gait | - | Superior to the conventional approach | (24) |
| Shalin et al | 2020 | Observational study | Use of foot plantar pressure data for predicting freezing of gait | - | Removal of the need for feature extraction and selection | (25) |
| Bevilacqua et al | 2020 | Single-blinded randomized controlled trial | Traditional therapy vs. Tymo system vs. Walker view | 50 min traditional training vs. 30 min traditional training + 20 min RAGT 10 sessions (2 per week) | Still in progress | (26) |
UPDRS, Unified Parkinson's Disease Rating Scale; RAGT, robot-assisted gait training.
Maintenance of independent motor function and ability to walk is the primary goal of therapy for the 4th stage of PD. Such a personalized approach enables the patient to remain independent for as long as possible, improves QoL and delays entering the 5th stage of PD where the patient is immobilized, and therefore, gait management is impossible. For this reason, in our review only the articles referring to robotic systems and AI-based applications for gait management exclusively for advanced stages of PD were included.
A pilot study that investigated the influence of robot-assisted gait training (RAGT) on patients diagnosed with PD and suffering from gait freezing, revealed encouraging results (17) and recommended further follow-up evaluations of the long-term effects of RAGT (17). When performed on patients with previous deep brain stimulation, therapy sessions with the same system as in the aforementioned study, were considered possibly constructive only regarding space-temporal gait parameters and motor score (UPDRS), but not concerning kinetic and kinematic gait parameters (18).
The starting point for trials comparing conventional gait training with RAGT was encouraging, as the trial conducted by Picelli et al identified a statistically significant difference in favour of robotic stepper training (when compared to physiotherapy with active joint mobilization) (19). The positive effect lasted up to 1 month. The authors suggested the future comparison between RAGT and treadmill training or the same amount of overground walking (19).
The first study to perform a quantitative comparison of the effects of RAGT and treadmill training in PD, evaluating gait kinematics as well as spatiotemporal parameters identified improved gait kinematics, particularly in the frontal plane at the pelvic and hip joint level in patients who underwent robotic training (20). This study was followed by further research concerning the comparison between the two treatment options, such as the one by Kang et al (21). This team designed a study to specifically evaluate the effects of RAGT (Walkbot-S™) on gait speed, compared with treadmill training (21), in addition to also revealing what pathways apply for these effects to occur, by monitoring fluctuations in brain functional networks and gait automaticity (21).
When it comes to the effect on self-selected speed gait training, a previous study did not identify any differences between RAGT (with the Lokomat plus VR), and conventional gait training overground (22). The same study raised the need to further investigate and adjust other parameter settings such as body weight support and guidance force for a tailored use of RAGT, which could significantly add to the benefits of this type of treatment (22).
Another comparison of RAGT with treadmill training took walking endurance, speed, and number of episodes of gait freezing into consideration, but also the general attitude towards the disease (23). The frequency of daily episodes of gait freezing appeared to be significantly decreased in the group using RAGT. Medium and long-term follow-up could prove valuable, as motor learning retention may benefit from prolonged exercise (23).
As freezing of gait is a frequently encountered phenomenon in parkinsonian disorders certain studies have attempted to predict its occurrence, such as the novel algorithm submitted by Arami et al (24). It inferred the use of a binary classification preceded by a feature time series prediction, and it demonstrated an improvement compared with the conventional three-class prediction approach. Therefore, it was suggested as a standard (24).
Another method that proved useful for predicting the aforementioned incident involves plantar pressure data represented in the form of 2D images and evaluated by a convolutional neural network (CNN) (25). The newest research designed by Bevilacqua et al included 195 subjects divided into three groups: A control group that followed traditional therapy sessions with a duration of 50 min, and two technological intervention groups, one that used the Tymo system, and another that used the Walker View (in addition to a 30-min traditional rehabilitation session, and another 20 min of treatment with a robotic system) (26). Its aim was to analyze the improvement of balance and gait in older PD patients at 3 follow-ups (6 months, 1 year and 2 years following the 5-week rehabilitation programme) (26).
The present study has several strengths. First, to the best of our knowledge, this is the first systematic review to evaluate the feasibility of the automatized management of advanced stages of PD by AI-based technologies and robotic systems. Second, the subject of this systematic review is of major relevance due to the rapid increase of prevalence of PD worldwide (27), the coronavirus COVID-19 outbreak, and the serious global human resources shortage (28). Third, AI algorithms are more cost-efficient than conventional methods and the effect of complete autonomous management on healthcare systems and on the QoL of PD patients is underestimated (29).
In advanced stages of the disease, patients frequently experience an enhanced sensitivity to small changes in pharmacological therapy, particularly in levodopa-carbidopa and present more severe adverse reactions to antiparkinsonian drugs (30). Consequently, the number of consultations, the time of transportation and the general economic burden, further increase (31). All this makes an integrated autonomous AI-based system unavoidable in the near future.
Despite levodopa effectiveness in PD, long-term use may lead to an either ‘on-off’ syndrome or to motor complications such as LID (32-34). Prompt detection of the lack of effectiveness or overexposure to levodopa may have beneficial effects for patients and be cost-effective for the healthcare system (35). The use of deep learning methods may enable a faster and more accurate detection of drug therapy problems as well as informing patients and physicians regarding symptoms and disease evolution (6-9).
Using computer vision and deep learning, including the deep-learning-based pose estimation algorithm (Convolution Pose Machines) that detect disease severity or LID, may facilitate more frequent patient-clinician interactions and an improved treatment personalization and quality of care (6). In addition, complex CDSS may help the clinician provide a DBS recommendation or drug treatment adjustment (7-9).
Other sensor-based devices aid in identifying non-adherence to drug treatment and help decrease its prevalence (8). Movement assessment devices, coupled with gene expression data from PD patients may help to improve differentiation between different states of levodopa treatment (under- or over-treatment) (9). As in advanced PD, levodopa treatment may no longer be effective and DBS is not always an option for every PD patient, drug discovery is an important part of PD research and future perspectives of AI-based technologies focus on new methods for identifying drug candidates, which are represented by WGCNA systems that use gene expression analysis to identify key dysregulated modules and suggest drugs that could restore homeostasis in these systems (10).
This review has five major limitations. The first limitation is the small number of participants in the majority of the studies included in our review. Secondly, the limited number of trials analyzing AI-based technologies exclusively for advanced stages of PD. Thirdly, it was not possible to analyze every possible lifestyle factor which alters the results, nor the way those technologies were applied by patients, relatives and healthcare professionals. Thereby, the observational nature of this approach leaves the possibility of residual confounding. Fourth, studies differed in their outcomes, design, and measurements, and this heterogeneity reduced our capacity to formulate a precise evaluation. Fifth, no studies which included all types of technology available for the automatization process of the management of advanced stages of PD were identified; therefore, it was not possible to analyze the feasibility of all AI-based systems applied as one integrated structure. Thus, international cooperation is recommended between a multidisciplinary team of healthcare professionals composed of neurologists, gastroenterologists, psychiatrists, and nurses on one hand, and a multidisciplinary team of hardware engineers and software developers, on the other hand.
6. Conclusions
Significant evidence demonstrating that current AI-based technologies are feasible for automatic management of patients with advanced stages of PD was identified. Improving the quality of care and reducing the cost for patients and healthcare systems are the most important advantages.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LPD suggested the methodology, searched the literature and made substantial contributions to the writing of the manuscript. MB contributed to the writing of the robotic systems and AI applications for gait management section. DCL made contributions to the methodology of the manuscript. CP contributed to the writing of the AI-based applications for autonomous management of pharmacologic therapy section. LCP searched the literature and revised the manuscript. MB and SLP confirm the authenticity of all the raw data. NT revised the manuscript for important intellectual content. SLP made contributions to the writing of non-motor complications management and analyzed the results. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pedersen SW, Suedmeyer M, Liu LW, Domagk D, Forbes A, Bergmann L, Onuk K, Yegin A, van Laar T. The role and structure of the multidisciplinary team in the management of advanced Parkinson's disease with a focus on the use of levodopa-carbidopa intestinal gel. J Multidiscip Healthc. 2017;10:13–27. doi: 10.2147/JMDH.S111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimes D, Fitzpatrick M, Gordon J, Miyasaki J, Fon EA, Schlossmacher M, Suchowersky O, Rajput A, Lafontaine AL, Mestre T, et al. Canadian guideline for Parkinson disease. CMAJ. 2019;191:E989–E1004. doi: 10.1503/cmaj.181504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amjad F, Bhatti D, Davis TL, Oguh O, Pahwa R, Kukreja P, Zamudio J, Metman LV. Current practices for outpatient initiation of levodopa-carbidopa intestinal gel for management of advanced Parkinson's disease in the United States. Adv Ther. 2019;36:2233–2246. doi: 10.1007/s12325-019-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran BX, Vu GT, Ha GH, Vuong QH, Ho MT, Vuong TT, La VP, Ho MT, Nghiem KP, Nguyen HLT, et al. Global evolution of research in artificial intelligence in health and medicine: A bibliometric study. J Clin Med. 2019;8(360) doi: 10.3390/jcm8030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, Ray Dorsey E, Dahodwala N, Cintina I, Hogan P, Thompson T. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. 2020;6(15) doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li MH, Mestre TA, Fox SH, Taati B. Vision-based assessment of parkinsonism and levodopa-induced dyskinesia with pose estimation. J Neuroeng Rehabil. 2018;15(97) doi: 10.1186/s12984-018-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamir RR, Dolber T, Noecker AM, Walter BL, McIntyre CC. Machine learning approach to optimizing combined stimulation and medication therapies for Parkinson's disease. Brain Stimul. 2015;8:1025–1032. doi: 10.1016/j.brs.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker CS, Behoora I, Nembhard HB, Lewis M, Sterling NW, Huang X. Machine learning classification of medication adherence in patients with movement disorders using non-wearable sensors. Comput Biol Med. 2015;66:120–134. doi: 10.1016/j.compbiomed.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner AP, Lones MA, Trefzer MA, Smith SL, Jamieson S, Alty JE, Cosgrove J, Tyrrell AM. Using epigenetic networks for the analysis of movement associated with levodopa therapy for Parkinson's disease. Biosystems. 2016;146:35–42. doi: 10.1016/j.biosystems.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Yue Z, Arora I, Zhang EY, Laufer V, Bridges SL, Chen JY. Repositioning drugs by targeting network modules: A Parkinson's disease case study. BMC Bioinformatics. 2017;18 (Suppl 14)(S532) doi: 10.1186/s12859-017-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przybyszewski AW, Kon M, Szlufik S, Szymanski A, Habela P, Koziorowski DM. Multimodal learning and intelligent prediction of symptom development in individual Parkinson's patients. Sensors (Basel) 2016;16(1498) doi: 10.3390/s16091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willows T, Dizdar N, Nyholm D, Widner H, Grenholm P, Schmiauke U, Urbom A, Groth K, Larsson J, Permert J, Kjellander S. Initiation of levodopa-carbidopa intestinal gel infusion using telemedicine (video communication system) facilitates efficient and well-accepted home titration in patients with advanced Parkinson's disease. J Parkinsons Dis. 2017;7:719–728. doi: 10.3233/JPD-161048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans L, Mohamed B, Thomas EC. Using telemedicine and wearable technology to establish a virtual clinic for people with Parkinson's disease. BMJ Open Qual. 2020;9(e001000) doi: 10.1136/bmjoq-2020-001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hssayeni MD, Jimenez-Shahed J, Burack MA, Ghoraani B. Wearable sensors for estimation of parkinsonian tremor severity during free body movements. Sensors (Basel) 2019;19(4215) doi: 10.3390/s19194215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cilia R, Mancini F, Bloem BR, Eleopra R. Telemedicine for parkinsonism: A two-step model based on the COVID-19 experience in Milan, Italy. Parkinsonism Relat Disord. 2020;75:130–132. doi: 10.1016/j.parkreldis.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck CA, Beran DB, Biglan KM, Boyd CM, Dorsey ER, Schmidt PN, Simone R, Willis AW, Galifianakis NB, Katz M, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89:1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo AC, Chang VC, Gianfrancesco MA, Friedman JH, Patterson TS, Benedicto DF. Reduction of freezing of gait in Parkinson's disease by repetitive robot-assisted treadmill training: A pilot study. J Neuroeng Rehabil. 2010;7(51) doi: 10.1186/1743-0003-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardo A, Anasetti F, Servello D, Porta M. Quantitative gait analysis in patients with Parkinson treated with deep brain stimulation: The effects of a robotic gait training. NeuroRehabilitation. 2014;35:779–788. doi: 10.3233/NRE-141173. [DOI] [PubMed] [Google Scholar]
- 19.Picelli A, Melotti C, Origano F, Waldner A, Fiaschi A, Santilli V, Smania N. Robot-assisted gait training in patients with Parkinson disease: A randomized controlled trial. Neurorehabil Neural Repair. 2012;26:353–361. doi: 10.1177/1545968311424417. [DOI] [PubMed] [Google Scholar]
- 20.Galli M, Cimolin V, De Pandis MF, Le Pera D, Sova I, Albertini G, Stocchi F, Franceschini M. Robot-assisted gait training versus treadmill training in patients with Parkinson's disease: A kinematic evaluation with gait profile score. Funct Neurol. 2016;31:163–170. doi: 10.11138/fneur/2016.31.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang MG, Yun SJ, Shin HI, Kim E, Lee HH, Oh BM, Seo HG. Effects of robot-assisted gait training in patients with Parkinson's disease: Study protocol for a randomized controlled trial. Trials. 2019;20(15) doi: 10.1186/s13063-018-3123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson's disease: Robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55:456–462. doi: 10.23736/S1973-9087.18.05368-6. [DOI] [PubMed] [Google Scholar]
- 23.Capecci M, Pournajaf S, Galafate D, Sale P, Le Pera D, Goffredo M, De Pandis MF, Andrenelli E, Pennacchioni M, Ceravolo MG, Franceschini M. Clinical effects of robot-assisted gait training and treadmill training for Parkinson's disease. A randomized controlled trial. Ann Phys Rehabil Med. 2019;62:303–312. doi: 10.1016/j.rehab.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Arami A, Poulakakis-Daktylidis A, Tai YF, Burdet E. Prediction of gait freezing in parkinsonian patients: A binary classification augmented with time series prediction. IEEE Trans Neural Syst Rehabil Eng. 2019;27:1909–1919. doi: 10.1109/TNSRE.2019.2933626. [DOI] [PubMed] [Google Scholar]
- 25.Shalin G, Pardoel S, Nantel J, Lemaire ED, Kofman J. Prediction of freezing of gait in Parkinson's disease from foot plantar-pressure arrays using a convolutional neural network. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:244–247. doi: 10.1109/EMBC44109.2020.9176382. [DOI] [PubMed] [Google Scholar]
- 26.Bevilacqua R, Maranesi E, Di Rosa M, Luzi R, Casoni E, Rinaldi N, Baldoni R, Lattanzio F, Di Donna V, Pelliccioni G, Riccardi GR. Rehabilitation of older people with Parkinson's disease: An innovative protocol for RCT study to evaluate the potential of robotic-based technologies. BMC Neurol. 2020;20(186) doi: 10.1186/s12883-020-01759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi JYJ, Collado-Mateo D, et al. Global, regional, and national burden of Parkinson's disease,1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Raeve P, Adams E, Xyrichis A. The impact of the COVID-19 pandemic on nurses in Europe: A critical discussion of policy failures and opportunities for future preparedness. Int J Nurs Stud Adv. 2021;3(100032) doi: 10.1016/j.ijnsa.2021.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesario A, D'Oria M, Calvani R, Picca A, Pietragalla A, Lorusso D, Daniele G, Lohmeyer FM, Boldrini L, Valentini V, et al. The role of artificial intelligence in managing multimorbidity and cancer. J Pers Med. 2021;11(314) doi: 10.3390/jpm11040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash N, Simuni T. Infusion therapies for Parkinson's disease. Curr Neurol Neurosci Rep. 2020;20(44) doi: 10.1007/s11910-020-01062-2. [DOI] [PubMed] [Google Scholar]
- 31.Keränen T, Kaakkola S, Sotaniemi K, Laulumaa V, Haapaniemi T, Jolma T, Kola H, Ylikoski A, Satomaa O, Kovanen J, et al. Economic burden and quality of life impairment increase with severity of PD. Parkinsonism Relat Disord. 2003;9:163–168. doi: 10.1016/s1353-8020(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 32.Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson's disease. J Neurol. 1999;246:1127–1133. doi: 10.1007/s004150050530. [DOI] [PubMed] [Google Scholar]
- 33.Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- 34.Nutt JG. Motor fluctuations and dyskinesia in Parkinson's disease. Parkinsonism Relat Disord. 2001;8:101–108. doi: 10.1016/s1353-8020(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Olanow CW. Levodopa: Effect on cell death and the natural history of Parkinson's disease. Mov Disord. 2015;30:37–44. doi: 10.1002/mds.26119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.