Abstract
Background
Despite the growing interest in dexmedetomidine as an adjunct to truncal blocks, little is known about the systemic absorption of dexmedetomidine after these blocks and its role in analgesia and in hemodynamics.
Objective
We investigated the pharmacokinetics and pharmacodynamics of dexmedetomidine as an adjunct to transversus abdominis plane (TAP) block in patients undergoing lower abdominal cancer surgery.
Methods
Twenty-four adult patients were randomized to receive a bilateral single-injection TAP block before surgery with 20 mL of bupivacaine 0.5% (TAP group, n = 12) or combined with 1 µg/kg dexmedetomidine (TAP-DEX group, n = 12) and diluted with saline to a volume of 40 mL (20 mL on each side). Plasma concentrations of dexmedetomidine and its pharmacokinetics were investigated using non-compartmental methods, postoperative analgesia, hemodynamics, and adverse events (nausea, vomiting, itching, hypotension, bradycardia, and respiratory depression).
Results
Dexmedetomidine was detected in the plasma of 11 patients in the TAP-DEX group. The mean dexmedetomidine peak plasma concentration (Cmax) was 0.158 ± 0.085 (range, 0.045–0.31) ng/mL. The median time to reach peak plasma concentration of dexmedetomidine (Tmax) was 15 (15–45) min. From 2 to 8 h postoperatively, visual analog pain scale (VAS) scores at rest and during movement were significantly lower in the TAP-DEX group. Analgesia time was (11.3 ± 3.12 vs 9.0 ± 4.69 h; P = 0.213) and postoperative morphine consumption was (7.4 ± 3.24 vs 11.5 ± 4.46 mg; P = 0.033) in TAP-DEX and TAP groups, respectively. Lower mean heart rate and mean blood pressure were recorded in the TAP-DEX group intraoperatively and 2 h postoperatively (P < 0.05). Except for mild nausea and vomiting, no adverse events were recorded in either group.
Conclusion
Systemic absorption of dexmedetomidine administered in a TAP block is common. Direct central effects on the locus coeruleus caused by this systemic absorption may play a role in the analgesia and hemodynamic effects produced by TAP-dexmedetomidine in addition to local mechanisms.
Trial Registration
ClinicalTrial.gov (identifier: NCT03328299).
Keywords: analgesia, transversus abdominis plane block, dexmedetomidine, pharmacokinetics, pharmacodynamics
Introduction
Since originally described by Rafi et al in 2001, the transversus abdominis plane (TAP) block has undergone various modifications in technique, approaches, and local anesthetic regimens.1 The main issue is that it provides somatic analgesia to the anterior abdominal wall through a non-dermatomal field block achieved by injecting a relatively large volume of local anesthetic into the fascial plane between the internal oblique and transversus abdominis muscles.2 It anesthetizes the spinal nerves from the 7th to 12th, the ilio-inguinal and ilio-hypogastric nerves.2
Compared to local anesthetic wound infiltration, TAP block significantly decreased pain scores and opioid consumption after abdominal surgery.3 Although not superior to epidural analgesia, TAP block has the advantage of being less invasive and is associated with a lower incidence of hypotension and shorter hospital stay.4,5 Clinicians frequently add different adjuvants to local anesthetics to prolong the duration of single-injection TAP block analgesia.6–8
Dexmedetomidine, a highly selective α-2 receptor agonist, is commonly used off-label as an adjuvant to single-injection perineural peripheral nerve blocks, such as the brachial plexus block,9 and truncal blocks, such as the TAP block.6 The addition of dexmedetomidine increased the duration of the block and reduced postoperative pain scores and opioid consumption, compared to local anesthetics alone.10,11 Intravenous and perineural dexmedetomidine has been found to also prolong the duration of analgesia after peripheral nerve blocks.12–14 The exact mechanism of action of perineural dexmedetomidine is unclear; it may occur partly due to peripheral perineural mechanisms or due to central effects on the locus coeruleus from systemic absorption of the drug.15,16
Despite the growing interest in dexmedetomidine as an adjunct to truncal and peripheral nerve blocks, little is known about the systemic concentrations of dexmedetomidine after these blocks. This study aimed to investigate the pharmacokinetics and pharmacodynamics of dexmedetomidine on postoperative analgesia, hemodynamics, and adverse events as an adjunct to single-injection TAP block in patients undergoing lower abdominal cancer surgery.
Patients and Methods
Ethical Considerations, Eligibility, and Study Design
This prospective randomized, double-blind clinical controlled study was approved by the Research Ethics Committee, South Egypt Cancer Institute, Assiut University, Assiut, Egypt (ID: SECI-IRB/IORG0006563/no. 402, date; 23/10/2017, Head of the Committee; Prof. Ashraf Zydan). Written informed consent was obtained from all study participants. The trial was registered on Clinical Trial. gov. (Identifier: NCT03328299, Principal investigator: Assistant Prof. Fatma Adel El Sherif, first posted: 1/11/2017, study start: 11/11/2017) before the enrollment of the first patient. We strictly followed the regulations and amendments of the Declaration of Helsinki. The patients enrolled in this study were aged 18–60 years, with body mass index (BMI) ≤30 kg/m2, American Society of Anesthesiologists (ASA) I–II, scheduled for lower abdominal cancer surgery (abdominal hysterectomy and radical cystectomy) under a low midline vertical incision, who were scheduled to receive bilateral single-injection TAP block for postoperative analgesia. Exclusion criteria included significant cardiac, respiratory, renal, central nervous system, or hepatic diseases; pregnancy; BMI ≥30 kg/m2; bleeding diathesis, allergy to study drugs, history of drug addiction, and patients with psychiatric illnesses that would interfere with the perception and assessment of pain.
Randomization and Blindness
Patient randomization was performed using computer-generated randomization tables and group allocation was hidden in sealed opaque envelopes. Twenty-four patients were enrolled in two groups of 12 patients each to receive a bilateral single-injection TAP block with 20 mL of bupivacaine 0.5% (Markyrene® Sigma-Tec, Egypt) (TAP group) or 20 mL of bupivacaine 0.5% combined with 1 µg/kg dexmedetomidine (Precedex® Hospira, Inc., Lake Forest, USA) (TAP-DEX group). A physician who was not involved in the study diluted the study drugs with normal saline 0.9% to a final volume of 40 mL and a bupivacaine concentration of 0.25% in identical sterile syringes with matching random codes that were opened before induction of anesthesia. The surgeon, attending anesthesiologists, data collection personnel, and patients were blinded to the group assignment.
Study Protocol
During the preoperative assessment, patients were instructed on how to express their pain using the visual analog pain scale (VAS) (scored 0–10; 0, no pain and 10, the worst pain imaginable), and how to use the intravenous patient-controlled analgesia (IV-PCA) device.
In the operating room, monitoring was attached to the patients, including noninvasive arterial blood pressure, electrocardiogram, end-tidal carbon dioxide capnography, and peripheral arterial oxygen saturation. Anesthesia was induced with propofol 2–3 mg/kg, fentanyl 1–2 μg/kg, lidocaine 1.5 mg/kg and 0.6 mg/kg rocuronium to facilitate endotracheal intubation. After induction of anesthesia and before surgery, all patients received a bilateral ultrasound-guided single-injection TAP block according to the group assignment. The surgery started 15 min after the TAP block was performed. Anesthesia was maintained with isoflurane in a 50% oxygen/air mixture, and the minimum alveolar concentration (MAC) was titrated in anticipation of the adjuvant effects of the TAP block. Patients were mechanically ventilated in volume cycle mode with ventilation parameters that achieved an ETCO2 of approximately 35–40 mmHg. At the end of the surgery, muscle paralysis was reversed with 2 mg/kg sugammadex IV. The patients were extubated awake and transferred to the surgical intensive care unit (SICU). All patients were connected to an IV-PCA analgesia device (B. Braun Melsungen, Melsungen, Germany) for postoperative analgesia. The IV-PCA solution contained 100 mg of morphine diluted in 100 mL of normal saline 0.9% (1 mg/mL). The IV-PCA program consisted of an initial bolus of morphine 0.1 mg/kg once the pain was conveyed by the patient or if VAS ≥3 was reported, followed by 1 mg boluses with a 5-min lockout period without continuous background infusion.
The Intervention
After induction of general anesthesia and before surgery, TAP block was performed by an experienced investigator under dynamic ultrasound guidance with a high-frequency linear ultrasound probe (Sonosite®, Inc. USA) and an in-plane 100 mm 20G needle (Pajunk Sono Plex Stim cannula USA). The ultrasound probe was placed transversely in the mid-axillary line between the anterior superior iliac spine and the costal margin. Using real-time ultrasound imaging, the external oblique, internal oblique, and transversus abdominis muscles were identified. After negative aspiration to exclude vascular puncture, a test dose of 1 mL of saline was injected to open the plane between the two muscles. If the test dose was demonstrated to be within the muscles rather than between them, needle repositioning was required, and the test was repeated. Subsequently, the full dose of the study drug was administered according to group assignment, and the detection of a hypoechoic layer on the ultrasound screen was confirmed. Then, the entire procedure was repeated on the other side. The success of the block was suspected intraoperatively if the patient’s hemodynamics did not change significantly with surgical incision and was accurately confirmed postoperatively after full recovery using the pinprick test. Using a sterile needle, the loss of sensation of the abdominal wall was tested through the anatomic distribution of the intercostal nerves from T6 to L1, depending on the standard chart of skin dermatomes. Failed cases were excluded from the study.
Blood Sampling
Seven whole venous blood samples (3 mL each) were collected in EDTA tubes. Samples were obtained from the patients at 15, 30, and 45 min and 1, 2, 4, and 6 h after drug administration. Centrifugation at 2500× g for 10 min was performed within 2 h of sample collection. The resultant plasma was stored at −80 °C until assayed.
Sample Extraction and Preparation for Liquid Chromatography-Tandem Mass Spectrometry
Five hundred microliters of plasma were thoroughly mixed with 1 mL of a mixture of methanol and acetonitrile (1:1, v/v) (Sigma-Aldrich, Steinheim, Germany), vortexes 60 s and centrifuged at 10,000× g for 15 min at 4 °C; 10 μL of the resultant clear supernatant was injected into the AB SCIEX LC/MS/MS system (AB SCIEX 3200 Q TRAP, Germany) equipped with an electrospray ionization source and an Agilent 1260 affinity high performance liquid chromatography (HPLC) system. The analytical column used was XBridge-C18 (150 mm × 2.1 mm × 5 μm, Waters, Ireland) at 25 °C. The mobile phase consists of two parts of 0.1% formic acid in water (solvent A) and one part of a mixture of methanol and acetonitrile (1:1, v/v) (solvent B), delivered at a flow rate of 0.3 mL/min. The Analyst 1.6 software was used for data acquisition and the Multiquant software was used for calculation. Typical chromatograms for dexmedetomidine were detected at 4.4 min retention time and following ion transition: m/z 201.2:95.1, for dexmedetomidine. The standard curve was prepared at concentrations ranging from 0.05 to 12.5 ng/mL of dexmedetomidine in drug-free plasma and extracted as mentioned in the sample preparation. The calibration curve showed a linear relationship (r2 ≥ 0.99).17
Outcome Measurements
In addition to the patients’ demographic and clinical data, including age, weight, and height, the following data were collected:
Pharmacokinetic Assessments
Pharmacokinetic parameters were determined using a non-compartmental method with WinNonlin professional version 2.1 software (Pharsight Corporation, Mountain View, CA, USA). The following parameters were calculated for plasma concentrations of dexmedetomidine: area under the concentration-time curve from time zero to the last measurable sampling time point (AUCall) (ng/mL*min), AUC from time zero to time infinity (AUCinf) (ng/mL*min), maximum plasma concentration (Cmax) (ng/mL), time to reach Cmax (Tmax) (min), apparent volume of distribution (V/f) (L), apparent clearance (CL/f) (L/min), and elimination half-life (t1/2) (min). Pharmacokinetic parameters were obtained for each patient in the TAP-DEX group.
Pharmacodynamic Assessments
Intraoperatively, hemodynamic vitals, including non-invasive blood pressure and heart rate, were recorded before the administration of TAP block (baseline) and at 3, 60, 90, 120, 150, and 180 min afterward.
Postoperative assessments included hemodynamic vitals (non-invasive blood pressure, heart rate, ventilatory frequency, and peripheral arterial oxygen saturation) recorded at admission to the SICU (baseline), and 2, 4, 6, 8, 12, 18, and 24 h postoperatively. The sedation score (0, awake; 1, easily aroused; 2, awake after verbal stimulation; 3, awake after tactile stimulation; and 4, not arousable) and the visual analog pain score at rest and during movement (on coughing) were recorded at the same time points. The time to first use of the IV-PCA device and the total consumption of the IV- PCA morphine dose in the first 24 h postoperatively were also recorded. Perioperative adverse events, such as nausea, vomiting, itching, hypotension, bradycardia, and respiratory depression, were recorded.
Statistics
Power of the Study
The primary outcome of this study was the plasma concentration of dexmedetomidine. Secondary outcomes were calculated pharmacokinetic parameters for dexmedetomidine, hemodynamic vitals, postoperative pain profile, sedation score, and perioperative adverse effects. Using the G-Power calculator 3.1.9.718 for the determination of sample size, 12 patients were needed in the TAP-DEX group to study dexmedetomidine pharmacokinetics based on a priori analysis with a type I error of 0.05, power of 0.8, and effect size of 0.8. Fourteen patients were enrolled in the TAP-DEX group to compensate for dropouts, and an equal number was enrolled in the TAP group to study the pharmacodynamics of dexmedetomidine.
Statistical Tests
Data were collected and processed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Continuous data were checked for normality by visual inspection of histograms and by the Shapiro–Wilk test. Continuous data are presented as mean (±SD) if normally distributed and as median (and range) if not normally distributed. Categorical data were expressed as numbers and frequencies (%) and analyzed using the chi-square or Fisher’s exact test, as appropriate. For normally distributed data, Student’s t-test and paired Student’s t-test were used for the analysis of independent and paired samples, respectively. For abnormally distributed and ordinal data, the Mann–Whitney U-test and Wilcoxon signed-rank test were used for the analysis of independent and paired samples, respectively. Statistical significance was set at P < 0.05.
Results
Participant Flow
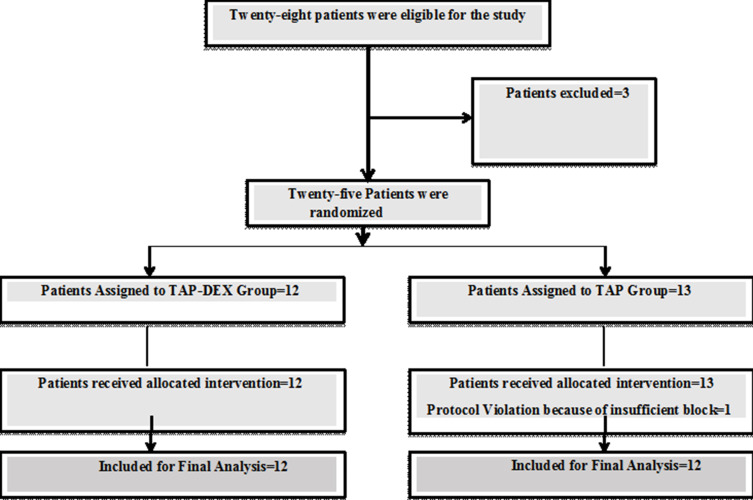
Twenty-eight patients were eligible for this study, of whom three did not meet our selection criteria, and one patient was excluded due to insufficient TAP block. Twenty-four patients (TAP-DEX group, n = 12; TAP group, n = 12) completed the blood sampling, pharmacokinetic, and pharmacodynamic assessments and were subjected to statistical analysis (Figure 1).
Figure 1.
Participant Flow chart showing that twenty-eight patients were eligible for this study, 3 patients did not meet selection criteria and a patient was excluded. Twenty-four patients (TAP-DEX group n=12, TAP group n=12) completed the study.
Abbreviations: TAP, transversus abdominis plane; DEX, dexmedetomidine.
Baseline Data
The demographic and operative characteristics of the patients were matched between the two groups (P > 0.05) (Table 1).
Table 1.
Patients’ Demographics and Clinical Data
| TAP-DEX Group | TAP Group | P-value | |
|---|---|---|---|
| (n= 12) | (n= 12) | ||
| Age (years) | 53.08 ± 9.6 | 52.58 ± 8.54 | 0.894 |
| Sex | |||
| Male/ Female | 5 /7 | 7/5 | 0.414 |
| Weight (Kg) | 76.92 ± 8.43 | 76.33 ± 7.98 | 0.863 |
| Height (cm) | 165.08 ± 6.71 | 168.00 ± 5.44 | 0.255 |
| BMI (Kg/m2) | 28.47 ± 4.81 | 27.09 ± 3.08 | 0.412 |
| Diagnosis | |||
| Cancer bladder | 6 | 7 | 0.682 |
| Cancer uterus | 6 | 5 | |
| ASA Class | |||
| ASA I/II | 9/3 | 8/4 | 1.000 |
| Serum albumin (g/L) | 44.47 ± 3.30 | 44.73 ± 2.98 | 0.844 |
| Duration of surgery (min.) | 173.00 ± 11.01 | 173.92 ± 17.89 | 0.881 |
| Duration of anesthesia (min.) | 182.00 ± 11.01 | 186.08 ± 21.13 | 0.559 |
| Time to first request for IV- PCA morphine (h). | 11.3±3.12 | 9.0±4.69 | 0.213 |
| Total IV- PCA morphine consumption dose (mg) | 7.4±3.24 | 11.5±4.46 | 0.033 |
| Postoperative adverse events: | |||
| -Nausea. | 2 | 4 | NA |
| -Vomiting. | 1 | 4 |
Notes: Data presented as mean (±SD) and number of patients. P-value is significant if <0.05.
Abbreviations: ASA, American Society of Anesthesiologists; PCA, patient controlled analgesia; NA, not applicable.
Primary Outcome
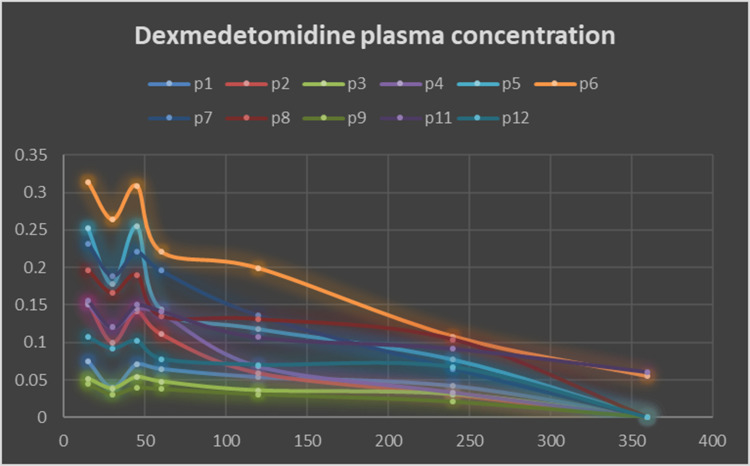
Dexmedetomidine was detected in the plasma of 11 patients after its administration as an adjuvant to bupivacaine in the TAP block in the TAP-DEX group. Repeated assessments did not detect dexmedetomidine in the plasma of patient no. 10. The dexmedetomidine plasma concentration versus time data for the studied patients are presented in Figure 2. The mean Cmax of dexmedetomidine was 0.158 ± 0.085 ng/mL (range, 0.045–0.31 ng/mL). The median of Tmax was 15 min (range, 15–45 min). The calculated pharmacokinetic variables of locally administered dexmedetomidine are shown in Table 2.
Figure 2.
Dexmedetomidine plasma concentration versus time after its administration in transversus abdominis plane block. The mean dexmedetomidine peak plasma concentration (Cmax) was 0.158± 0.085 (range: 0.045–0.31) ng/mL. Repeated assessments did not detect dexmedetomidine in the plasma of patient no. 10.
Abbreviation: P, patient.
Table 2.
Pharmacokinetics of Dexmedetomidine Administered in TAP Block
| Item | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P11 | P12 | Descriptive Statistics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmax (min.) | 15 | 15 | 45 | 15 | 45 | 15 | 15 | 15 | 15 | 15 | 15 | 15 (14–45) |
| Cmax (ng/mL) | 0.08 | 0.15 | 0.05 | 0.15 | 0.26 | 0.31 | 0.23 | 0.19 | 0.045 | 0.16 | 0.11 | 0.158 (0.086) |
| t1/2_Lambda_z (min) | 291.39 | 90.09 | 256.96 | 88.96 | 198.66 | 129.36 | 112.9873 | 456.89 | 209.96 | 257.81 | 893.19 | 209.96 (88.96–893.19) |
| AUCall (ng/mL*min) | 15.13 | 18.98 | 10.94 | 21.53 | 35.54 | 55.76 | 37.02 | 37.61 | 8.48 | 36.05 | 21.78 | 27.16 (14.35) |
| AUCINF (observed)(ng/mL*min) | 30.26 | 21.08 | 20.57 | 23.79 | 52.98 | 66.03 | 43.72 | 99.93 | 13.58 | 58.73 | 104.09 | 48.61 (31.44) |
| AUCINF (observed)/D (min/L) | 0.43 | 0.30 | 0.29 | 0.34 | 0.76 | 0.94 | 0.62 | 1.43 | 0.19 | 0.84 | 1.49 | 0.69 (0.45) |
| Vz (observed)/F (L) | 972.35 | 431.59 | 1261.72 | 377.67 | 378.65 | 197.86 | 261.02 | 461.76 | 1561.82 | 443.29 | 866.53 | 443.29 (197.86–1561.82) |
| Cl (observed)/F (L/min) | 2.31 | 3.32 | 3.40 | 2.94 | 1.32 | 1.06 | 1.60 | 0.70 | 5.16 | 1.19 | 0.67 | 2.15 (1.41) |
| Dose of DEX per patient (µg) | 80 | 70 | 80 | 90 | 75 | 70 | 70 | 68 | 90 | 80 | 80 | 76.92 ± 8.43 |
Note: Normally distributed data presented as mean (SD) and not normally distributed data presented as median (range).
Abbreviations: TAP, transversus abdominis plane; Cmax, maximum plasma concentration; tmax, time to reach Cmax; AUCall, area under the concentration-time curve from time zero to the last measurable sampling time point; AUCinf, AUC from time zero to time infinity maximum plasma concentration Cmax; V/f, apparent volume of distribution; CL/f, apparent clearance; t1/2, elimination half-life.
Secondary Outcomes
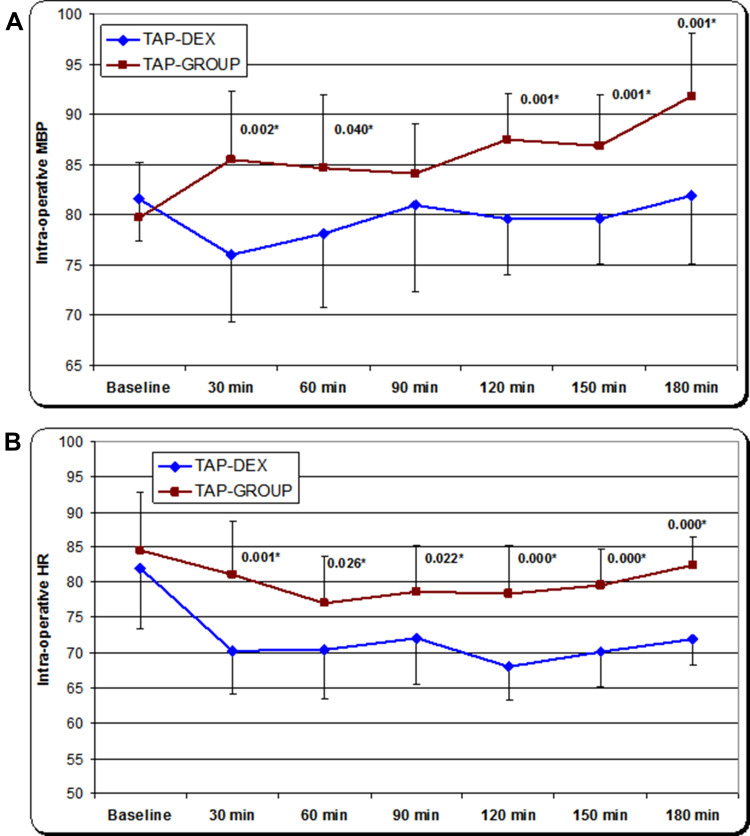
Intraoperatively, patients who received dexmedetomidine as an adjuvant to bupivacaine in the TAP block (TAP-DEX group) showed significantly lower mean arterial blood pressure and heart rate after the administration of dexmedetomidine and throughout the operative procedure compared to those who received bupivacaine alone (TAP group) (Figure 3A and B).
Figure 3.
(A) Mean intraoperative non-invasive arterial blood pressure. *Significant decrease in the mean arterial blood pressure throughout the procedure in the TAP-DEX group. (B) Mean intraoperative heart rate. *Significant decrease in the mean heart rate throughout the procedure in the TAP-DEX group.
Abbreviations: TAP, transversus abdominis plane; DEX, dexmedetomidine.
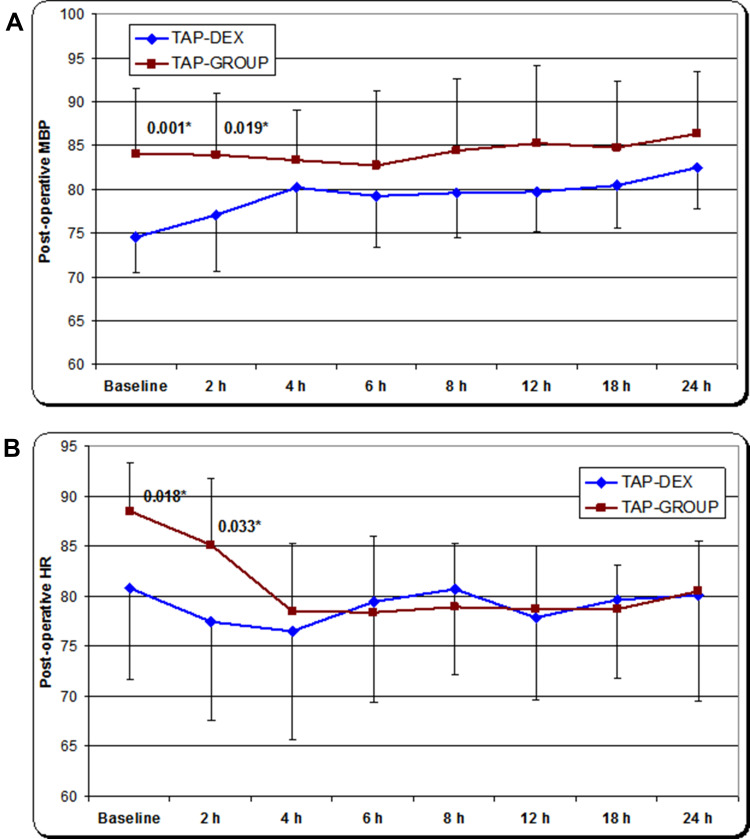
Postoperatively, upon admission to the SICU and at 2 h postoperatively, the mean arterial blood pressure and heart rate values were significantly lower in the TAP-DEX group than in the TAP group, with no significant differences between the two groups at other time points (Figure 4A and B). The postoperative sedation score, ventilatory frequency, and peripheral arterial oxygen saturation did not show significant differences between the two groups at any time point in this study (Tables 3–5).
Figure 4.
(A) Mean postoperative non-invasive arterial blood pressure. *Significant decrease in the mean postoperative arterial blood pressure in the TAP-DEX group. (B) Mean postoperative heart rate. *Significant decrease in the mean postoperative heart rate in the TAP-DEX group.
Abbreviations: TAP, transversus abdominis plane; DEX, dexmedetomidine.
Table 3.
Post-Operative Respiratory Rate (RR)
| Post-Operative RR (Breath/Min) | DEX (n= 12) | Control (n= 12) | P-value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Baseline | 12.33 ± 0.65 | 12.17 ± 0.39 | 0.455 |
| 2 h | 12.25 ± 0.45 | 12.25 ± 0.45 | 1.000 |
| 4 h | 12.50 ± 0.52 | 12.50 ± 0.52 | 1.000 |
| 6 h | 12.33 ± 0.65 | 12.33 ± 0.65 | 1.000 |
| 8 h | 12.58 ± 0.90 | 12.58 ± 0.90 | 1.000 |
| 12 h | 12.42 ± 0.79 | 12.42 ± 0.67 | 1.000 |
| 18 h | 12.25 ± 0.45 | 12.25 ± 0.45 | 1.000 |
| 24 h | 12.25 ± 0.45 | 12.25 ± 0.45 | 1.000 |
Notes: Data presented asMean ± SD. TAP-DEX Group, dexmedetomidine group; TAP group, Control; TAP, transversus abdominis plane.
Table 4.
Post-Operative Oxygen Saturation (SaO2%)
| Post-Operative SaO2 | TAP-DEX Group (n= 12) | TAP Group (n= 12) | P-value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Baseline | 98.75 ± 0.62 | 98.75 ± 0.62 | 1.000 |
| 2 h | 98.42 ± 0.51 | 98.42 ± 0.51 | 1.000 |
| 4 h | 98.75 ± 0.62 | 98.58 ± 0.90 | 0.603 |
| 6 h | 98.42 ± 0.51 | 98.42 ± 0.51 | 1.000 |
| 8 h | 98.75 ± 0.62 | 98.75 ± 0.45 | 1.000 |
| 12 h | 98.75 ± 0.62 | 98.75 ± 0.62 | 1.000 |
| 18 h | 98.75 ± 0.45 | 98.75 ± 0.45 | 1.000 |
| 24 h | 98.92 ± 0.29 | 98.92 ± 0.29 | 1.000 |
Notes: Data presented as Mean ± SD. TAP-DEX Group, dexmedetomidine group; TAP group, Control; TAP, transversus abdominis plane.
Table 5.
Postoperative Sedation Score
| Sedation Score | TAP-DEX Group (n= 12) | TAP Group (n= 12) | P-value |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| Baseline | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.000 |
| 2 h | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.623 |
| P-value2 | 0.011* | 0.003* | |
| 4 h | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 1.000 |
| P-value2 | 0.003* | 0.003* | |
| 6 h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| P-value2 | 0.001* | 0.001* | |
| 8 h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| P-value2 | 0.001* | 0.001* | |
| 12 h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| P-value2 | 0.001* | 0.001* | |
| 18 h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| P-value2 | 0.001* | 0.001* | |
| 24 h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| P-value2 | 0.001* | 0.001* |
Notes: Data presented as median (range). TAP-DEX Group, dexmedetomidine group; TAP group, Control; TAP, transversus abdominis plane. *Significant difference when comparing each time point to the base line.
From 2 to 8 h postoperatively, the VAS scores at rest and during movement were significantly lower in the TAP-DEX group, with no significant differences between the two groups at other time points (Figure 5A and B). The mean time to first request for IV-PCA morphine was (11.3 ± 3.12 vs 9.0 ± 4.69 h, P = 0.213) and the mean total dose of morphine consumption in the first 24 h postoperatively was (7.4 ± 3.24 vs 11.5 ± 4.46 mg, P = 0.033) in the TAP-DEX and TAP groups, respectively, (Table 1). Except for mild nausea and vomiting, no adverse events were recorded in either group in this study (Table 1).
Figure 5.
(A) Postoperative visual analogue pain scores (VASR) at rest. Data presented as median (range), *Significant decreased in the VAS in the DEX group compared to control group. (B) Postoperative visual analogue pain scores (VASM) during movement. Data presented as median (range), *Significant decreased in the VAS in the DEX group compared to control group.
Abbreviations: DEX, dexmedetomidine group; Control, bupivacaine group.
Discussion
In this study, we measured the plasma levels of dexmedetomidine locally administered as an adjuvant to bupivacaine in TAP block in patients undergoing lower abdominal cancer surgery. Systemic absorption of dexmedetomidine occurred in 11 out of the 12 patients investigated, and the plasma levels of dexmedetomidine detected ranged from 0.045 to 0.31 ng/mL (mean, 0.158 ± 0.085 ng/mL). The addition of dexmedetomidine to bupivacaine in the TAP block enlightened postoperative analgesia for the first 8 h postoperatively and reduced the postoperative IV-PCA morphine consumption in the first 24 h postoperatively (7.4 ± 3.24 vs 11.5 ± 4.46 mg; P = 0.033) compared with bupivacaine alone.
The only Food and Drug Administration FDA-approved indication for dexmedetomidine is sedation in adult ICU patients for <24 h duration; however, it has been widely used as an adjuvant to local anesthetics to truncal and peripheral nerve blocks.6,9,12–14 For TAP block, dexmedetomidine administration, in addition to local anesthetics targeting peripheral nociceptive receptors, has been used successfully for pain control after abdominal surgery.3–6 In this study, adding dexmedetomidine to bupivacaine in the TAP block significantly reduced pain scores in the first 8 h postoperatively and decreased postoperative consumption of IV-PCA morphine. These results are consistent with many similar previous studies, which demonstrated that when dexmedetomidine is used as an adjuvant to local anesthetics, it yields superior and prolonged analgesia.3–6
The analgesic effect of dexmedetomidine, a highly selective α-2 agonist, is mainly mediated by binding to central and spinal cord α2-receptors. Therefore, the transmission of pain is suppressed by interneuron hyperpolarization and diminished release of pronociceptive transmitters, including glutamate and substance P.19 In contrast to the sedative effects of α-2 agonists, the analgesic effects are still not fully understood and, in part, may be due to the altered perception and diminished levels of anxiety due to associated decreased release of norepinephrine and opioid-sparing effects.16
Angst et al conducted a study on healthy volunteers.20 They examined the analgesic effects of different doses of intravenous dexmedetomidine in direct comparison to alfentanil as a μ receptor agonist. At each level of sedation produced, the authors examined the analgesia level, accomplishing experimental models for heat and electrical pain. Four median step-up plasma concentrations of dexmedetomidine (0.09, 0.24, 0.54, and 1.23 ng/mL) and alfentanil (13.4, 33.8, 67.8, and 126.1 ng/mL) were administered via a computer-controlled infusion. They found that the sedative effects of both drugs were dose-dependent. The analgesic efficacy of alfentanil was also dose-dependent; however, dexmedetomidine did not. They concluded that dexmedetomidine causes mild to severe sedation, but it lacks analgesic efficacy for heat and pain in healthy volunteers.20 Moreover, comparing dexmedetomidine and remifentanil concerning the respiratory and analgesic effects showed that dexmedetomidine at plasma concentrations of up to 2.4 ng/mL produced less efficient analgesia than remifentanil.16 In this study, the detected dexmedetomidine plasma level ranged from 0.045 to 0.31 ng/mL (mean, 0.158 ± 0.085). These levels are not completely responsible for the analgesic effect that we recorded with locally administered dexmedetomidine, but it might potentiate the local mechanisms of TAP-DEX analgesia.
In contrast, Abdallah et al in their study concluded that both systemic and perineural dexmedetomidine produced comparable analgesia levels after interscalene brachial plexus block.12 Other studies reported that IV dexmedetomidine synergistically interacts with regional anesthesia, increasing the duration of sensory block by 34%, motor block by 17%, and the time to first request for postoperative analgesia by 53%.13,21 A limitation of this study is that we did not include an IV dexmedetomidine group for our comparisons. The presence of such a group could have enabled us to confirm or declare the role of systemic absorption of locally administered dexmedetomidine in the production of analgesia. Comparing the plasma levels of dexmedetomidine and associated analgesia produced after intravenous and local TAP-dexmedetomidine is deficient, and further studies on this topic are needed.
In this study, we confirmed that systemic absorption of TAP-dexmedetomidine is not uncommon. However, it is unclear whether the plasma levels we detected had the potential to further enhance the analgesia produced by local mechanisms.
As repeated assessments did not detect dexmedetomidine in the plasma of patient no. 10, we are looking forward to conduct a set of genetic studies to find an explanation for its consistent findings.
The plasma levels of dexmedetomidine detected in this study were sufficient to produce an intraoperative decrease in the mean arterial pressure and heart rate 30 min after its administration compared with patients who received TAP block with bupivacaine only. Consistent with many previous studies, the hemodynamic adverse effects of dexmedetomidine reported in this study were mild and easily managed.6,22–24
Systemic absorption of the drug can influence patients’ hemodynamics and analgesia and is important for optimum dosing strategies (interactions with other drugs).
Limitations of this study include the small sample size and the absence of an intravenous dexmedetomidine group. As mentioned before, the presence of such a group could have enabled us to estimate the degree and importance of systemic absorption of the locally administered dexmedetomidine.
Conclusion
In conclusion, systemic absorption of dexmedetomidine administered in TAP blocks is not uncommon. Direct central effects on the locus coeruleus caused by this systemic absorption may play a role in the analgesia produced by TAP-dexmedetomidine in addition to the local mechanisms.
Acknowledgment
We are grateful to the operating theatre and SICU staff for their co-operation in data collection.
Funding Statement
No funding was taken to accomplish this work.
Abbreviations
DEX, dexmedetomidine; HR, heart rate; MAP, mean arterial pressure; SaO2%, peripheral arterial oxygen saturation; VAS, visual analogue scale; SICU, surgical intensive care units; IV-PCA, intravenous patient controlled analgesia; Cmax, maximum plasma concentration; AUCall, area under the concentration-time curve from time zero to the last measurable sampling time point; AUCinf, AUC from time zero to time infinity maximum plasma concentration Cmax; tmax, time to reach Cmax; V/f, apparent volume of distribution; CL/f, apparent clearance; t1/2, elimination half-life.
Data Sharing Statement
We are not planning to share any of the data with anyone.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–1026. doi: 10.1046/j.1365-2044.2001.02279-40.x [DOI] [PubMed] [Google Scholar]
- 2.Carney J, Finnerty O, Rauf J, Bergin D, Laffey JG, Mc Donnell JG. Studies on the spread of local anaesthetic solution in transversus abdominis plane blocks. Anaesthesia. 2011;66:1023e30. [DOI] [PubMed] [Google Scholar]
- 3.Guo Q, Li R, Wang L, Zhang D, Ma Y. Transversus abdominis plane block versus local anaesthetic wound infiltration for postoperative analgesia: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(10):17343–17352. [PMC free article] [PubMed] [Google Scholar]
- 4.Baeriswyl M, Zeiter F, Piubellini D, Kirkham KR, Albrecht E. The analgesic efficacy of transverse abdominis plane block versus epidural analgesia: a systematic review with meta-analysis. Medicine. 2018;97(26):e11261. doi: 10.1097/MD.0000000000011261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai N, El-Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery - a systematic review, meta-analysis, and trial sequential analysis. Anaesthesia. 2020. doi: 10.1111/anae.15068 [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Liu S, Wu H, et al. Dexmedetomidine as an adjuvant to local anesthetics in transversus abdominis plane block: a systematic review and meta-analysis. Clin J Pain. 2019;35(4):375–384. doi: 10.1097/AJP.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhakar A, Lambert T, Kaye RJ, et al. Adjuvants in clinical regional anesthesia practice: a comprehensive review. Best Pract Res Clin Anaesthesiol. 2019;33(4):415–423. doi: 10.1016/j.bpa.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 8.Ammar AS, Mahmoud KM, Kasemy ZA. Comparison between adenosine and magnesium sulphate as adjuvants for transversus abdominis plane block: a prospective randomized controlled trial. Minerva Anestesiol. 2018;84(3):304–310. doi: 10.23736/S0375-9393.17.11931-0 [DOI] [PubMed] [Google Scholar]
- 9.Schnabel A, Reichl SU, Weibel S, et al. Efficacy, and safety of dexmedetomidine in peripheral nerve blocks: a meta-analysis and trial sequential analysis. Eur J Anaesthesiol. 2018;35(10):745–758. doi: 10.1097/EJA.0000000000000870 [DOI] [PubMed] [Google Scholar]
- 10.Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. doi: 10.1097/AAP.0000000000000033 [DOI] [PubMed] [Google Scholar]
- 11.Pan W, Liu G, Li T, et al. Dexmedetomidine combined with ropivacaine in ultrasound-guided tranversus abdominis plane block improves postoperative analgesia and recovery following laparoscopic colectomy. Exp Ther Med. 2020;19(4):2535–2542. doi: 10.3892/etm.2020.8508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah FW, Dwyer T, Chan VWS, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block. Anesthesiology. 2016;124:683–695. doi: 10.1097/ALN.0000000000000983 [DOI] [PubMed] [Google Scholar]
- 13.Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia. Anesth Analg. 2013;117:271–278. doi: 10.1213/ANE.0b013e318290c566 [DOI] [PubMed] [Google Scholar]
- 14.Kang R, Jeong JS, Yoo JC, et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med. 2018;43(5):488–495. doi: 10.1097/AAP.0000000000000773 [DOI] [PubMed] [Google Scholar]
- 15.Guo TZ, Tinklenberg J, Oliker R, Maze M. Central alpha 1-adrenoceptor stimulation functionally antagonizes the hypnotic response to dexmedetomidine, an alpha 2-adrenoceptor agonist. Anesthesiology. 1991;75:252–256. doi: 10.1097/00000542-199108000-00013 [DOI] [PubMed] [Google Scholar]
- 16.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed SA, Abdel-Ghaffar HS, Hassan NA, et al. Pharmacokinetics and pharmacodynamics of 3 doses of oral-mucosal dexmedetomidine gel for sedative premedication in women undergoing modified radical mastectomy for breast cancer. Anesth Analg. 2021;132(2):456–464. PMID: 32889844. doi: 10.1213/ANE.0000000000005108 [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 19.Vuyk J, Sitsen E, Reekers M, et al. Intravenous anesthetics. In: Miller RD, Cohen NH, Eriksson LI, editors. Miller’s Anesthesia. 8th ed. Amsterdam: Elsevier; 2015:854–859. [Google Scholar]
- 20.Angst MS, Ramaswamy B, Davies MF, Maze M. Comparative analgesic and mental effects of increasing plasma concentrations of dexmedetomidine and alfentanil in humans. Anesthesiology. 2004;101(3):744–752. doi: 10.1097/00000542-200409000-00024 [DOI] [PubMed] [Google Scholar]
- 21.Wu -H-H, Wang H-T, Jin -J-J, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamadia S, Pedemonte JC, Hobbs LE, et al. A pharmacokinetic and pharmacodynamic study of oral dexmedetomidine. Anesthesiology. 2020;133(6):1223–1233. doi: 10.1097/ALN.0000000000003568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal P, Garg S. Effect of adding dexmedetomidine to local anesthetic agents for transversus abdominis plane block: a meta-analysis. Clin J Pain. 2019;35(10):844–854. PMID: 31259745. doi: 10.1097/AJP.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 24.Aksu R, Patmano G, Biçer C, Emek E, Çoruh AE. Eficácia de bupivacaína e associação com dexmedetomidina em bloqueio do Plano transverso abdominal guiado por ultrassom na dor após cirurgia abdominal [Efficiency of bupivacaine and association with dexmedetomidine in transversus abdominis plane block ultrasound guided in postoperative pain of abdominal surgery]. Rev Bras Anestesiol. 2018;68(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]