In this review, Braschi et al. provide an updated consensus nomenclature for components of dynein motors and their assembly factors.
Abstract
Dyneins are highly complex, multicomponent, microtubule-based molecular motors. These enzymes are responsible for numerous motile behaviors in cytoplasm, mediate retrograde intraflagellar transport (IFT), and power ciliary and flagellar motility. Variants in multiple genes encoding dyneins, outer dynein arm (ODA) docking complex subunits, and cytoplasmic factors involved in axonemal dynein preassembly (DNAAFs) are associated with human ciliopathies and are of clinical interest. Therefore, clear communication within this field is particularly important. Standardizing gene nomenclature, and basing it on orthology where possible, facilitates discussion and genetic comparison across species. Here, we discuss how the human gene nomenclature for dyneins, ODA docking complex subunits, and DNAAFs has been updated to be more functionally informative and consistent with that of the unicellular green alga Chlamydomonas reinhardtii, a key model organism for studying dyneins and ciliary function. We also detail additional nomenclature updates for vertebrate-specific genes that encode dynein chains and other proteins involved in dynein complex assembly.
Introduction
Dynein family motor proteins form multiple different dynein complexes in mammals, with important roles in a wide range of cellular functions (King, 2017; Osinka et al., 2019; Roberts, 2018). Dyneins can be broadly classified into two groups: cytoplasmic and axonemal. Dynein complexes “walk” toward the minus ends of microtubules; while doing so, they can transport a variety of cargoes within cells (Trokter et al., 2012). The motor activity of these complexes allows them to play key roles in enabling motility of whole cells, generating fluid flow across cell surfaces, and transporting organelles and other components within the cytoplasm.
Dynein subunits are classified by mass into four categories: heavy (∼520 kD), intermediate (∼70 –140 kD), light intermediate (∼53–59 kD), and light (∼10–30 kD) chains (Pfister et al., 2006). The heavy and intermediate chains are specific to certain dynein complexes, while the light chains may be components of both cytoplasmic and axonemal dynein machinery, and in some cases, nondynein complexes. The light intermediate chains are present only in the cytoplasmic dynein class.
Dynein-based movement is powered by the ATP-driven dynein heavy chain subunits (Schmidt and Carter, 2018). 15 genes in the human genome encode dynein heavy chains: 1 for each of the 2 cytoplasmic dynein complexes and 13 that encode heavy chain components of the various axonemal dynein complexes. A dynein-related gene, DNHD1 (dynein heavy chain domain 1) has been referred to as a “ghost gene”: it may be a remnant of an earlier duplication that has not decayed at a normal rate, as a truncated version might poison cytoplasmic dynein heavy chain dimerization and thus be lethal (Gibbons, 2018; Schmidt and Carter, 2018). DNHD1 is currently classified as an “orphan” dynein heavy chain-encoding gene (Kollmar, 2016) but may be in the process of becoming a pseudogene (Wickstead, 2018).
Cytoplasmic dyneins
Dynein 1 complex
The cytoplasmic dynein 1 complex (Table S1) is present throughout eukaryotes, with some notable exceptions such as green plants and red algae (Wickstead and Gull, 2007). It is involved in a wide variety of intracellular transport activities, transporting cargoes including chromosomes, mRNA, and protein complexes (Reck-Peterson et al., 2018). The dynein 1 complex also acts in cell division, helping to form and orient the mitotic spindle (Torisawa and Kimura, 2020), establish cell polarity (Lu and Gelfand, 2017), and position organelles (Allan, 2014; Oyarzún et al., 2019; Palmer et al., 2009).
A dimer of DYNC1H1-encoded heavy chains forms the core of the cytoplasmic dynein 1 complex (Fig. 1 a) and acts as its ATPase motor (Palmer et al., 2009; Pfister et al., 2005). Each heavy chain contains six AAA+ domains, an antiparallel coiled-coil region with a microtubule-binding domain at its tip, and a C-terminal domain (Bhabha et al., 2016; Carter, 2013; Reck-Peterson et al., 2018; Roberts et al., 2013). Immediately N-terminal of the AAA1 domain is a linker that traverses the plane of the AAA ring and changes conformation during the ATPase cycle to drive motor activity. AAA1 exhibits ATP hydrolytic activity, acting as an ATPase and powering the dynein motor complex (Silvanovich et al., 2003), while nucleotide binding at several other AAA domains appears to modify how conformational change propagates through the AAA ring and affects microtubule-binding activity. The coordinated activity of both heavy chains within the dynein complex is required for processivity (Reck-Peterson et al., 2006).
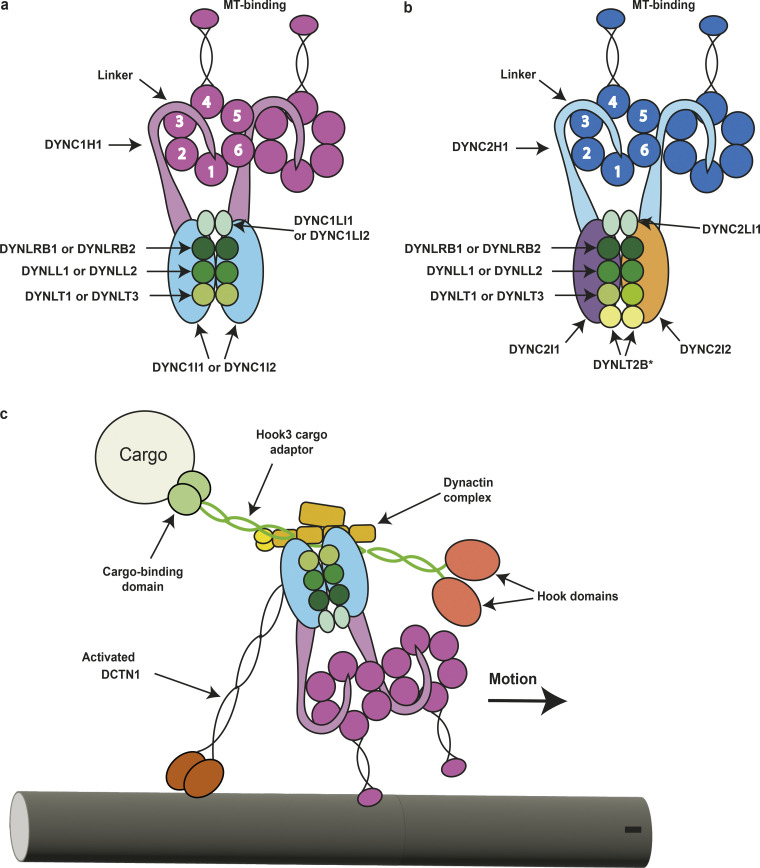
Figure 1.
Cytoplasmic dynein complexes. (a) The cytoplasmic dynein 1 complex. The DYNC1H1 protein heavy chains have large globular heads at the C-termini that are composed of a ring of six AAA+ domains. The microtubule-binding domains are located at the tips of antiparallel coiled coils that derive from AAA4. The linker/N-terminal domains connect the AAA rings and the intermediate and light chains. (b) The cytoplasmic dynein 2 complex. The DYNC2H1 protein heavy chains power retrograde IFT and have the same general domain organization as DYNC1H1. However, the tails of the two heavy chains fold differently due to an asymmetry imposed by the two different intermediate chains: one is straight while the other forms a zigzag shape and interacts with the IFT-B train (Toropova et al., 2019). The linker/N-terminal domain connects the AAA ring and the intermediate and light chains. *It remains unknown whether the DYNLT2B protein forms a homodimer or a heterodimer with another Tctex-type light chain. (c) Schematic showing the interaction between the dynein 1 and dynactin complexes. The adapter molecule affects the type of cargo bound; in this figure, the hook microtubule tethering protein 3 (HOOK3)–encoded protein is acting as a cargo adapter.
The intermediate chains of metazoan dynein 1 connect it to another multi-subunit complex known as dynactin (Loening et al., 2020). Dynactin is built around a filament of the protein encoded by ACTR1A (actin-related protein 1A). It activates dynein and regulates its binding to vesicles and organelles to be transported (Ketcham and Schroer, 2018). A coiled coil–containing cargo adaptor protein is required for dynein 1 activation (Fig. 1 c). A single adaptor protein sandwiches between dynactin and dynein, where it interacts with the dynein heavy chain tails and the light intermediate chain and along the length of the dynactin complex (Gonçalves et al., 2019; Reck-Peterson et al., 2018). There are currently ≥12 known cargo adaptor proteins, which are encoded by HOOK1, HOOK2, HOOK3, BICD2, BICDL1, BICDL2, RAB11FIP3, RASEF, CRACR2A, NIN, NINL, and SPDL1 (Barisic et al., 2010; Casenghi et al., 2005; Dona et al., 2015; Gonçalves et al., 2019; Horgan et al., 2010; Lee et al., 2018; Loening et al., 2020; Olenick et al., 2016; Vallee et al., 2021; Wang et al., 2019). Other protein cofactors may also be required for dynein recruitment to their cargoes. For example, the protein encoded by PAFAH1B1 (platelet activating factor acetylhydrolase 1b regulatory subunit 1; HUGO Gene Nomenclature Committee [HGNC] ID: 8574), also published using the alias LIS1 (lissencephaly 1) is required along with dynactin and BICD2 for dynein 1 to traffic many cargoes, such as nuclei, along microtubules (Faulkner et al., 2000; Splinter et al., 2012). LIS1 has most recently been suggested to stabilize the “open” conformation of cytoplasmic dynein 1 such that the heavy chains are able to undergo a mechanochemical cycle and cannot adopt the autoinhibited or “closed” state where movement of key mechanical elements is abrogated by interactions between heavy chains (Markus et al., 2020).
The dynein light chains can be divided into three subfamilies: the t-complex associated (Tctex)–type family (encoded by DYNLT1, DYNLT2, DYNLT2B, DYNLT3, DYNLT4, and DYNLT5), the LC8-type family (encoded by DYNLL1, DYNLL2, and DNAL4), and the roadblock-type family (encoded by DYNLRB1 and DYNLRB2; Bowman et al., 1999; King et al., 1998; King et al., 1996; King and Patel-King, 1995). Most of these protein chains can be found in both cytoplasmic dynein complexes: the exceptions are DYNLT2, which is an axonemal dynein subunit; DYNLT2B, which is found in the dynein 2 complex and the I1/f inner dynein arm (IDA); DYNLT4 and DYNLT5, which are not well characterized; and DNAL4, which is present only in outer dynein arms (ODAs).
Several proteins originally identified as dynein light chains are also found in numerous multimeric complexes unrelated to dyneins and appear to act as general dimerization engines or hubs (Williams et al., 2018). The LC8-type light chains (DYNLL1 and DYNLL2) are present in many enzymes including myosin V (Benashski et al., 1997; Espindola et al., 2000) and neuronal nitric oxide synthase (Jaffrey and Snyder, 1996). They also play a role in regulating apoptosis via an interaction with the BCL2 family protein encoded by BCL2L11 (Puthalakath et al., 1999). The DYNLT1 protein has reported roles in actin remodeling and neurite outgrowth (Chuang et al., 2005) and hypocretin signaling (Duguay et al., 2011). DYNLRB1 interacts with Rab6 family member proteins in the Golgi apparatus (Wanschers et al., 2008), and both roadblock-type dynein light chains are reportedly involved in a TGFβ signaling pathway (Jin et al., 2009).
Dynein 2 complex
Cilia are highly complex microtubule-based organelles that extend from the cell surface and can be classified as either primary (or nonmotile) or motile (Satir and Christensen, 2008). Most eukaryotic cells, excluding blood cells and those actively dividing, have an associated primary or nonmotile cilium. These act as sensory organelles, detecting a broad range of signaling molecules (Kopinke et al., 2021; Mykytyn and Askwith, 2017; Saternos et al., 2020).
The dynein 2 complex (also known as the intraflagellar transport [IFT] dynein or cytoplasmic dynein 1b in Chlamydomonas reinhardtii; Table S2) is found only in cells with associated cilia or flagella, where it locates within and around the base of these structures (Höök and Vallee, 2006). IFT trains are multiprotein complexes required for the assembly and function of cilia and flagella in eukaryotes (Dutcher, 2019; Wingfield et al., 2017). The anterograde IFT motor complex kinesin 2 moves IFT trains and associated cargoes plus the dynein 2 complex along microtubules, from the base to the tip of a cilium or flagellum (Toropova et al., 2019; Vuolo et al., 2020). The retrograde IFT motor complex dynein 2 transports IFT trains and associated factors from the tip back to the base (Hou and Witman, 2015; Pazour et al., 1998). The dynein 2 complex is required for the assembly of cilia and flagella (Pazour et al., 1999; Pfister et al., 2006) and also has key roles in ciliary signaling functions (Vuolo et al., 2020).
The core of dynein 2 is composed of a dimer of two DYNC2H1-encoded heavy chains (Fig. 1 b). The tails of these identical heavy chains are directed into two different conformations by the other subunits in the complex (Toropova et al., 2019). Each heavy chain is stabilized by its interaction with a DYNC2LI1-encoded protein subunit. The C-terminal helix of one of these light intermediate subunits associates with a DYNC2I1 (previously WDR60)-encoded protein with a DYNLRB-encoded subunit, to enforce a distinct conformation on one heavy chain (Toropova et al., 2019; Vuolo et al., 2020).
The DYNC2I1- and DYNC2I2-encoded intermediate chains bind the heavy chains via their C-terminal β-propeller domains. The N-terminal regions of these intermediate chains are dimerized by three DYNLL1/2 dimers and one of each of the other light chain dimers: DYNLT1/3, DYNLRB1/2, and DYNLT2B (Toropova et al., 2019). The DYNLT2B-encoded light chain is a unique accessory component of the dynein 2 complex. Whether it forms a homodimer or heterodimer with another light chain remains to be confirmed, although there is evidence to suggest that, unlike the other light chains, the DYNLT2B subunit may be monomeric (DiBella et al., 2001). Recent structural studies of Tetrahymena ODAs have revealed a Tctex-family heterodimer (Rao et al., 2021).
Axonemal dyneins
Motile cilia (sometimes termed flagella when they occur singly or in small numbers on a cell) are more restricted to certain cell types. Their movement enables sperm to swim (Linck et al., 2016), respiratory cilia on epithelial cells to sweep away mucus containing trapped pathogens (Hansson, 2019), and oviduct epithelial cells to waft an ovum along a fallopian tube toward the uterus (Spassky and Meunier, 2017). Multiciliated cells in the brain help move the cerebrospinal fluid and also influence neuronal migration (Brooks and Wallingford, 2014). In the male reproductive tract, the epithelial cells of the efferent ducts are densely covered with multiple motile cilia necessary for the transport of sperm cells (Aprea et al., 2021a). Motility of nodal cilia in the embryonic left–right organizer is necessary for the determination of correct left–right body asymmetry (Nonaka et al., 1998).
An axoneme is the microtubule superstructure core of the cilium and contains many tightly associated components. A motile cilium has a highly conserved “9 + 2” structure: 9 microtubule doublets that surround a central pair of 2 microtubule singlets (the “central apparatus”; Fig. 2). Axonemal dyneins are the motor complexes that drive a sliding motion between ciliary doublet microtubules, enabling movement. Motile cilia have IDAs and ODAs and radial spokes that are thought to be involved in signal transduction between the central pair and the outer microtubule ring (Ishikawa, 2017). Nonmotile cilia have only the outer doublet ring and have a 9 + 0 microtubule arrangement, although the number of outer doublets decreases and their arrangement changes beyond the proximal part of the cilium (Kiesel et al., 2020).
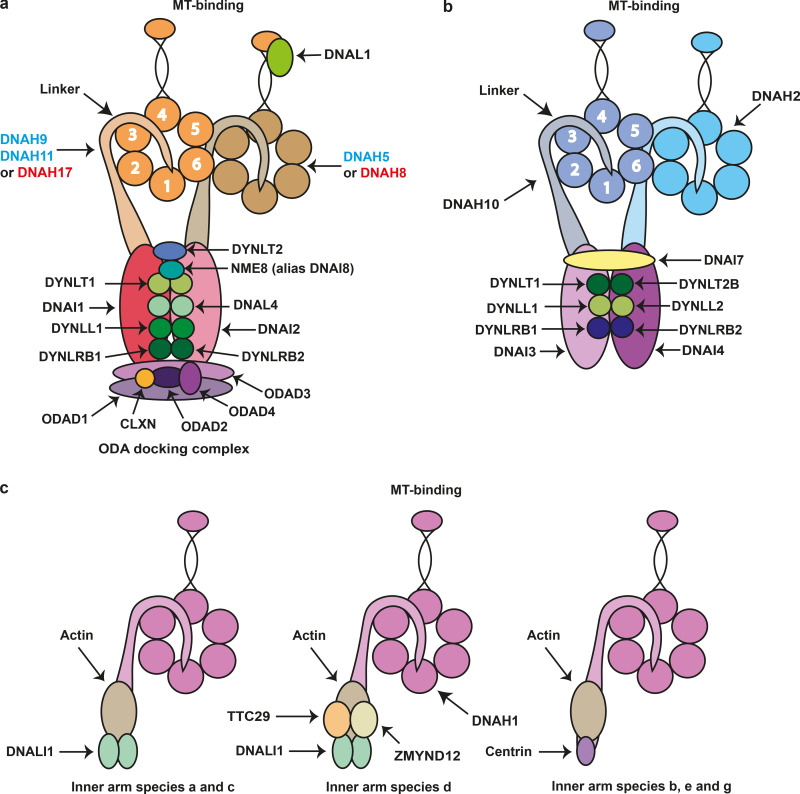
Figure 2.
Axonemal dynein complexes. (a) Axonemal ODA. The blue text denotes subunits found in ODA complexes in respiratory cilia, and red text denotes subunits found in ODA complexes in sperm flagella. (b) Axonemal inner arm I1/f complex subunits (IDA). (c) Monomeric IDAs. Each inner arm species is constructed around a distinct monomeric heavy chain associated with an actin monomer and either DNALI1 or centrin; species d contains two additional components. In most cases, the precise equivalence between the human and C. reinhardtii monomeric heavy chain species is uncertain.
The ODAs (Table S3 and Fig. 2 a) and IDAs (Table S4 and Table S5, and Fig. 2, b and c) in motile cilia are arranged in two rows with a complex 96-nm repeat organization. They are permanently attached to the A-tubule of one outer doublet microtubule (see Fig. 3, a and b) and transiently interact in an ATP-dependent manner with the B-tubule of the adjacent doublet to generate a sliding force (King, 2017). IDAs with a single heavy chain are termed monomeric (Table S4), while the I1/f IDA (Table S5) is dimeric, with two nonidentical heavy chains. These different types of dyneins vary in terms of their enzymatic and motor properties, likely reflecting their precise roles in the generation of ciliary motility (King, 2017).
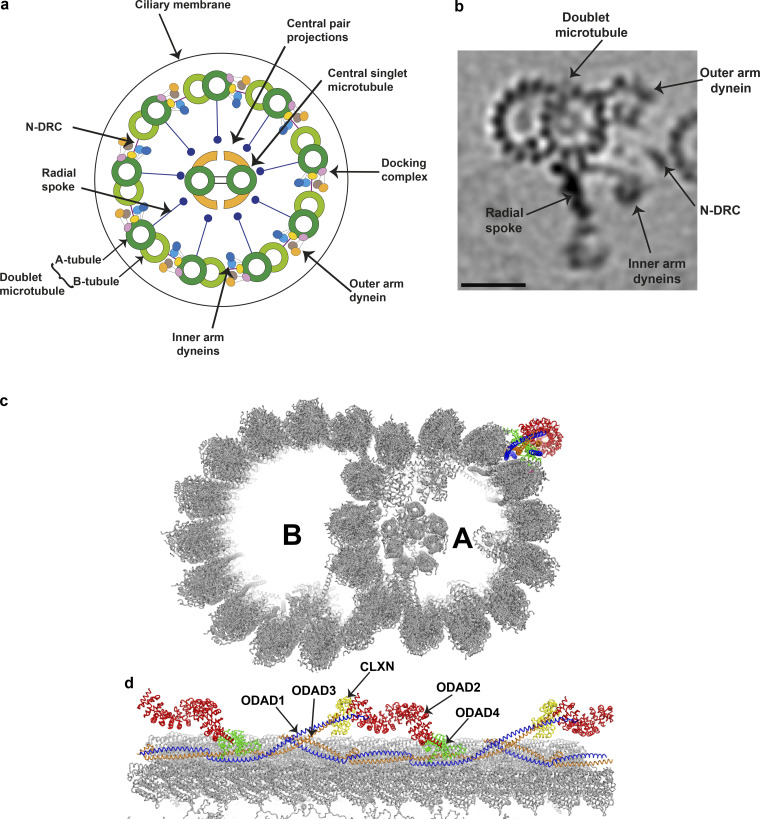
Figure 3.
Organization of a mammalian motile cilium. (a) The diagram illustrates the general 9 + 2 microtubule arrangement within the ciliary axoneme. The inner and outer rows of dynein arms generate the force required for ciliary beating. The N-DRC complex is a key regulatory structure that interconnects the doublet microtubules. The radial spokes regulate the beat of cilia by transducing signals between the doublets and the central microtubule pair. (b) Tomographic image of an averaged 96-nm repeat for a single human ciliary doublet microtubule, revealing the microtubule-associated dynein arms, N-DRC, and radial spoke. The scale bar represents 25 nm. This image was generated by Jason Schrad (Nicastro laboratory) using data from Lin et al. (2014). (c and d) Cross-section (c) and longitudinal (d) views of the 48-nm repeat organization of a bovine doublet microtubule. The components of the ODA-DC are individually colored and indicated. This ribbon diagram was generated with the PyMol molecular graphics system (Schrödinger) using Protein Data Bank accession no. 7RRO (Gui et al., 2021).
ODA docking complex (ODA-DC)
The correct functioning of cilia and flagella in most eukaryotes is dependent on the ODA chains attaching to the outer doublet microtubules at 24-nm intervals (Dean and Mitchell, 2015; King, 2017). The ODA-DC facilitates binding and may also play a role in regulating the activity of the ODAs (Takada et al., 2002). The ODA-DC in C. reinhardtii consists of three protein subunits, encoded by DCC1 (DC1), DCC2 (DC2), and DLE3 (DC3). In mammals, it consists of five protein subunits (Gui et al., 2021) encoded by five genes, now named ODAD1, ODAD2, ODAD3, ODAD4, and CLXN (calaxin; Fig. 2 a and Fig. 3, b and c). CLXN (previously EFCAB1) has been assigned the alias symbol ODAD5, and authors may refer to it as such in publications if they wish, referencing the approved gene symbol at least once to aid data retrieval. Only ODAD1 and ODAD3 have orthologues in C. reinhardtii (DCC2 and DCC1, respectively).
Dynein axonemal assembly factors (DNAAFs)
Genes encoding proteins that act as axonemal dynein assembly factors are named using the root symbol DNAAF. These proteins play an important role in the preassembly of IDAs and ODAs in the cytoplasm before their transport to cilia (Fabczak and Osinka, 2019; King, 2021).
Historically, the DNAAF root has been used only for proteins directly involved in the preassembly of axonemal dynein arms in the cytoplasm. We wrote to authors who have published on the genes that we are reporting in this publication as newly updated DNAAFs (see their symbols in bold in Table S6) and discussed this issue with our specialist advisors for this gene group (https://www.genenames.org/data/genegroup/#!/group/1627). This effort resulted in an agreement to use the term “DNAAF” more broadly. Therefore, a DNAAF symbol can now also be assigned to genes encoding proteins that play a role in trafficking dynein arms from the cytoplasm to cilia.
Association with human phenotypes
Humans have four described cilia types, and defects in all types are associated with various diseases: motile 9 + 2 cilia (e.g., respiratory cilia, ependymal cilia, sperm flagella); motile 9 + 0 cilia (e.g., nodal cilia); nonmotile 9 + 2 cilia (e.g., the kinocilium of hair cells and the proximal region of olfactory cilia); and nonmotile 9 + 0 cilia (e.g., renal monocilia and the connecting cilia of photoreceptor cells). Cilia are located on almost all polarized cell types of the human body; therefore, cilia-related disorders (ciliopathies) affect many organ systems (Fliegauf et al., 2007). Genetic mutations that impair cilia and/or flagella beating cause a heterogeneous group of rare disorders referred to as motile ciliopathies (Wallmeier et al., 2020). The pathogenic mechanisms, clinical symptoms, and severity of the diseases depend on the specific affected genes and the tissues in which they are expressed. Defects in ependymal cilia can result in hydrocephalus. Reduced fertility can be due to defective cilia in the fallopian tubes or the efferent ducts as well as sperm flagella. The malfunction of motile monocilia on the left–right organizer during early embryonic development can lead to laterality defects such as situs inversus and heterotaxy. Severe impairment of mucociliary clearance in the respiratory tract leads to chronic bronchial problems. Primary ciliary dyskinesia (PCD), which can present with a variety of these features, is the most common motile ciliopathy.
The genetic disorder PCD is heterogeneous and has been linked to variants in genes encoding dyneins, axonemal dynein assembly factors, and ODA-DC subunits (Table 1), as well as many other genes such as those encoding components of the molecular rulers that set up the core axonemal 96-nm repeat organization, nexin links, radial spokes, and the central apparatus. The most common ultrastructural defects observed in motile cilia of individuals with PCD affect axonemal structures (e.g., absence of IDAs or ODAs or both; Wallmeier et al., 2020). PCD-associated phenotypes include chronic respiratory problems, recurrent middle ear infections, male infertility, and subfertility in females (Leigh et al., 2019). Roughly 50% of PCD patients are diagnosed with Kartagener syndrome, a subtype defined by a triad of symptoms: chronic sinusitis, bronchiectasis, and situs inversus, where the positions of major body organs are reversed (Zariwala et al., 2011). Situs inversus totalis is observed when all thoracic and abdominal viscera are reversed; individuals with situs inversus or situs ambiguus show more variable organ positioning (seen in ≥6% of PCD cases; Kennedy et al., 2007; Sempou and Khokha, 2019).
Table 1. Human phenotypes associated with variants of genes encoding dyneins and dynein-associated proteins.
| Phenotype | Associated dynein or dynein-related gene variantsa | Selected associated publications (PubMed ID) | OMIM MIM number (phenotype subtype) |
|---|---|---|---|
| Primary ciliary dyskinesia (PCD): abnormal ciliary motility, respiratory distress, sinusitis, otitis media, bronchiectasis, laterality defects, infertility | DNAH1 | 11371505 20301301 24360805 | 617577 (CILD37) |
| DNAH5 | 11062149 11788826 | 608644 (CILD3) | |
| DNAH9 | 30471717 30471718 | 618300 (CILD40) | |
| DNAH11 | 12142464 | 611884 (CILD7) | |
| DNAI1 | 10577904 | 604366 (CILD1) | |
| DNAI2 | 18950741 | 612444 (CILD9) | |
| DNAL1 | 21496787 | 614017 (CILD16) | |
| NME8 (alias DNAI8 and TXNDC3) | 17360648 | 610852 (CILD6) | |
| ODAD1 | 23261302 23261303 23506398 30291279 32855706 | 615067 (CILD20) | |
| ODAD2 | 23849778 24203976 25186273 | 615451 (CILD23) | |
| ODAD3 | 24067530 25192045 25224326 30504913 31383820 | 616037 (CILD30) | |
| ODAD4 | 27486780 | 617092 (CILD35) | |
| DNAAF1 | 19944400 19944405 27261005 | 613193 (CILD13) | |
| DNAAF2 | 31107948 32638265 34785929 | 612518 (CILD10) | |
| DNAAF3 | 22387996 31186518 | 606763 (CILD2) | |
| DNAAF4 | 23872636 | 615482 (CILD25) | |
| DNAAF5 | 29358401 25232951 23040496 | 614874 (CILD18) | |
| DNAAF6 | 32170493 | 300991 (CILD36) | |
| ZMYND10 | 23604077 23891469 23891471 | 615444 (CILD22) | |
| LRRC6 | 23122589 | 614935 (CILD19) | |
| LRRC56 | 30388400 | 618254 (CILD39) | |
| SPAG1 | 24055112 26228299 | 615505 (CILD28) | |
| CFAP298 | 24094744 | 615500 (CILD26) | |
| CFAP300 | 29727692 29727693 | 618063 (CILD38) | |
| Spinal muscular atrophy (SMALED type 1): lower limb atrophy and weakness, mild to moderate cognitive impairment | DYNC1H1 | 24307404 25609763 32788638 | 158600 (SMALED) |
| BICD2 | 26998597 29353221 32709491 | 615290 (SMALED2A) 618291 (SMALED2B) | |
| Charcot-Marie-Tooth type 2: distal lower limb weakness, abnormal gait | DYNC1H1 | 24307404 20697106 22459677 22847149 33242470 | 614228 (CMT2O) |
| DNAH10 | 26517670 | Not listed in OMIM | |
| Asphyxiating thoracic dystrophies (including Jeune syndrome): skeletal abnormalities that may include short ribs and a chest wall deformity, shortened arm and leg bones, an unusually shaped pelvis, polydactyly, renal and hepatic disease (more rarely, retinal disease) | DYNC2H1 | 19442771 26874042 27925158 31935347 | 613091 (SRTD3) |
| DYNC2I1 | 23910462 26874042 29271569 | 615503 (SRTD8) | |
| DYNC2I2 | 24183449 24183451 | 615633 (SRTD11) | |
| DYNC2LI1 | 26130459 | 617088 (SRTD15) | |
| DYNLT2B | 25830415 26044572 28475963 | 617405 (SRTD17) | |
| Retinal degeneration | DYNC2H1 | 32753734 | Not listed in OMIM |
| Nonsyndromic rod-cone dystrophy | DYNC2I2 | 33124039 | Not listed in OMIM |
| Neurodevelopmental disorder with microcephaly and structural brain anomalies | DYNC1I2 | 31079899 | 618492 (NEDMIBA) |
| Mirror movements type 3: movements on one side of the body are involuntarily mirrored on the other side of the body | DNAL4 | 25098561 | 616059 (MRMV3) |
| Mental retardation autosomal dominant 13 | DYNC1H1 | 23603762 22368300 | 614563 (MRD13) |
| Spermatogenic failure | DNAH1 | 24360805 33989052 | 617576 (SPGF18) |
| DNAH2 | 30811583 | 619094 (SPGF45) | |
| DNAH8 | 32619401 | 619095 (SPGF46) | |
| DNAH17 | 31178125 31658987 31841227 | 618643 (SPGF39) | |
| Lissencephaly: developmental delay, myoclonic jerks and spasms, seizures, hypotonia, microcephaly, dysmorphic facies | PAFAH1B1 | 32692650 20301752 32341547 28886386 | 601545 (LIS) |
| Seckel syndrome: growth retardation, microcephaly, developmental delay | NIN | 27053665 22933543 | 614851 (SCKL7) |
Note that for some of these phenotypes, there are several variants with varying degrees of severity, and different genes may be associated with different types of these genetic conditions.
Mutations in DNAH5 encoding an axonemal ODA heavy chain are the most common genetic defect observed in PCD (Hornef et al., 2006). DNAH5 mutations result in dysmotility of respiratory as well as nodal cilia (Olbrich et al., 2002). Defective nodal cilia motility during early embryogenesis caused by mutations in genes encoding components essential for ciliary motility (e.g., due to DNAH5 mutations) result in situs inversus or situs ambiguus in approximately half of affected individuals due to the randomization of their left–right body asymmetry. Consistently, mice deficient for DNAH5 show immotility of respiratory cilia and embryonic nodal monocilia and exhibit ODA defects in both cilia types (Nöthe-Menchen et al., 2019). DNAH5 mutations also result in ODA defects and dysmotility of ependymal cilia (Ibañez-Tallon et al., 2004). DNAH5-deficient mice develop hydrocephalus during early postnatal life because the flow of cerebrospinal fluid around the brain is obstructed by the abnormal closure of the aqueduct of Sylvii connecting the third and fourth brain ventricles. Possibly due to the larger human brain size, the active propulsion of cerebrospinal fluid along the narrow passages of the ventricular system is not essential in most individuals with PCD; however, they still carry a slightly increased risk of developing hydrocephalus. This suggests that the non–motility-related functions of ependymal cilia might also be important (Wallmeier et al., 2020).
All known motile cilia types with DNAH5 loss-of-function mutations display aberrant motility, with the exception of sperm flagella. This is because the paralogous protein DNAH8 is present in sperm and exhibits functional overlap. The male reproductive tracts of mice deficient for DNAH5 have immotile efferent duct cilia, which results in severe stasis of sperm cell transport; this is due to disruption of the ODA composition. In human individuals with loss-of-function DNAH5 mutations, reduced sperm count in the ejaculate (oligozoospermia) and dilatations of the epididymal head were observed, consistent with DNAH5 in efferent duct cilia having an important role in sperm cell transport (Aprea et al., 2021a).
In females, the ODA composition of cilia in the Fallopian tube resembles that of respiratory cilia, with the ODA DNAH5 (dynein axonemal heavy chain 5) and DNAI1 (dynein axonemal intermediate chain 1) both being present (Raidt et al., 2015). The coordinated beating of the Fallopian tube ciliated cells produces a fluid flow from the distal site of the Fallopian tubes (ovaries), which transports the egg to the proximal end of the reproductive tract (uterine cavity; Lyons et al., 2006). Interestingly, some females with defective DNAH5 and DNAI1 are still able to conceive children. Thus, the motility of Fallopian tube cilia may not be essential for gamete transport, as Fallopian tube muscle contractions might aid in transporting the egg to the uterine cavity.
Mutations in genes encoding DNAAFs cause variable degrees of absence of ODAs and IDAs in respiratory cilia and sperm flagella (Aprea et al., 2021b), indicating that the process of cytoplasmic assembly of dynein arms is critical in both cell types. DNAAF mutant individuals consistently exhibit severely hampered motility of both sperm flagella and respiratory cilia. The sperm flagella of some DNNAF mutant males have shortened flagella axonemes, indicating that their length is also influenced by DNAAF function during dynein arm assembly.
Most defects of DNAAFs and axonemal dynein components affect motility of cilia and sperm flagella, contributing to motile ciliopathies (Leigh et al., 2019; Reiter and Leroux, 2017; Wallmeier et al., 2020). However, mutations in genes encoding cytoplasmic dynein subunits can affect the function of both motile and nonmotile cilia, as well other cellular processes. Thus, the clinical phenotype can vary enormously depending on the cell types that are affected. A variant of DYNC1H1 has been associated with a particular form of the ciliopathy SMALED (spinal muscular atrophy lower extremity dominant). This form of the condition mainly affects the lower limbs, causing progressive muscle weakness (Das et al., 2018). A different point mutation in DYNC1H1, also within the tail domain of the heavy chain protein, has been associated with the related neuropathy Charcot Marie Tooth disease. Dysfunction of the dynein heavy chains encoded by DYNC1H1 may also adversely affect maintenance of the morphology of mitochondria and may contribute to disease pathology (Eschbach et al., 2013).
Variants of several genes encoding dynein 2 subunits (Table 1) have been associated with a group of ciliopathies known as short-rib thoracic dysplasias, which include asphyxiating thoracic dystrophy, also known as Jeune syndrome. The association of a DYNC2H1 variant with these conditions suggests that the dynein 2 complex has a key role in endochondral bone formation during embryogenesis (Dagoneau et al., 2009). If retrograde IFT trafficking of cargoes from the tip to the base of the cilium is compromised, then so is hedgehog (Hh) signaling in the developing embryo, and the resulting incorrect embryonic patterning can produce a range of phenotypes (Goetz and Anderson, 2010). Patients with these conditions have skeletal abnormalities including a narrow thorax, short ribs, and bony spurs in a three-pronged formation observed at the hip joint; they may also display polydactyly.
Variants of some of the genes encoding dynein 2 subunits have also been linked to phenotypes affecting vision. The outer segment of photoreceptors is a modified cilium, and a constant turnover of outer segment constituents is required; IFT is key to this process. Four variants in DYNC2H1 in human are associated with nonsyndromic retinal degeneration (Vig et al., 2020). Some of these variants are suggested to affect the ciliary transport of the protein encoded by IFT88, an IFT component that is essential for the assembly and maintenance of vertebrate photoreceptors (Pazour et al., 2002).
Standardizing gene nomenclature
The HGNC (https://www.genenames.org) is the international authority assigning standardized nomenclature to human genes, and hence facilitating communication between researchers. We aim to assign unique, informative symbols and names to human genes that can be used in all domains, and across major biological and clinical databases and publications. Our sister project, the Vertebrate Gene Nomenclature Committee (VGNC; https://vertebrate.genenames.org), names genes across selected vertebrates in line with their human orthologues. VGNC species currently include chimp, macaque, cow, dog, horse, pig, and cat. We also work with other nomenclature committees responsible for naming genes in model vertebrates, such as mouse, rat, and Xenopus, to ensure consistency across species when possible (Tweedie et al., 2021).
Every named human gene has a symbol report on the HGNC website listing key data, including the approved nomenclature, published aliases, and locus type. An HGNC symbol report also contains links to multiple relevant sequence databases and clinical resources. It may additionally contain a link to a gene group page (see below), links to VGNC pages for orthologues in selected vertebrate species, and links to key publications in Europe PMC and PubMed. All data including our nomenclature guidelines (Bruford et al., 2020) can be accessed via our website.
The green alga C. reinhardtii is a key model organism for studying eukaryotic cilia and flagella and the dynein motor complexes that aid in their assembly and drive their movement. The alveolate Tetrahymena thermophila and sea urchins such as Strongylocentrotus purpuratus are also key model organisms for studying ciliary function. The nomenclature of human dyneins has been largely based on orthology with C. reinhardtii, but also partly based on sea urchin nomenclature. Unfortunately, there are inconsistencies in the naming of orthologues among these species due to historic numbering assignments based on protein migration in SDS/urea-polyacrylamide gels. We have brought mammalian dynein nomenclature more into line with that of C. reinhardtii where possible and have established a naming system for genes encoding dynein chains that are unique to vertebrate species.
While the stability of gene symbols, particularly those associated with phenotypes, is now a priority for the HGNC, we are still willing to consider updates for genes approved with placeholder symbols or for genes with domain-based nomenclature that may not give a clue to the function of the encoded protein, for example, genes named based on whether their encoded proteins contain transmembrane domains or coiled-coil regions (CCDC). Symbol changes are made only if an approved symbol has not become entrenched in the literature and if the community working on the gene in question is supportive of change to something more functionally informative.
In 2005, the nomenclature for the mammalian cytoplasmic dynein genes was revised (Pfister et al., 2005). The introduction of new DYNC1 and DYNC2 root symbols helped clarify whether genes encoded subunits that were components of the dynein 1 or dynein 2 complex. New root symbols were also introduced to subdivide the known human dynein light chains into three families: roadblock (DYNLRB), Tctex (DYNLT), and LC8 (DYNLL). A 2011 paper (Hom et al., 2011) reported updates made to C. reinhardtii dynein gene nomenclature based on the structural properties of their encoded protein products. This more systematic naming system helped to make the cross-species comparison of orthologues more straightforward and provided a framework for naming newly characterized dynein-encoding genes. Note that there are several human genes encoding dynein chains without orthologues in C. reinhardtii, as it lacks an equivalent of the cytoplasmic dynein 1/dynactin system, so some of the nomenclature is mammal specific.
Here we discuss our recent nomenclature updates for genes encoding dynein complex subunits, ODA-DC subunits, and axonemal dynein assembly factors in the human genome (Table 2). The previous nomenclature for these genes was less informative than it could be: some genes were assigned C#orf# placeholder symbols that are used for genes of unknown function, some symbols were based on domains within the encoded proteins, and others were based on homology with characterized genes in other species that were named without reference to the dynein complex subunits they encoded. As part of our VGNC project, these nomenclature updates will also apply to the orthologues of these genes across selected vertebrate species (Tweedie et al., 2021), as well as in the model organisms that follow HGNC nomenclature such as mouse, rat, and Xenopus.
Table 2. Summary table of nomenclature updates reported here.
| Approved HGNC Symbol | Name | Aliases (previously approved symbols in bold) | Chlamydomonas orthologuea (genes and proteins) | Protein present in |
|---|---|---|---|---|
| DYNLT2 | Dynein light chain Tctex-type 2 | TCTE3, TCTEX1D3, TCTEX2, Tctex4 | DLT2 (LC2) | Axonemal ODA complex |
| ODAD1 | Outer dynein arm docking complex subunit 1 | CCDC114, FLJ32926, CILD20 | DCC2 (ODA1) and DCC3 (ODA5)b | Axonemal ODA complex |
| ODAD2 | Outer dynein arm docking complex subunit 2 | ARMC4, FLJ10817, FLJ10376, DKFZP434P1735, CILD23, gudu | No orthologue | Axonemal ODA complex |
| ODAD3 | Outer dynein arm docking complex subunit 3 | CCDC151, MGC20983, ODA10 | DCC1 (ODA3) and ODA10 (ODA10)b | Axonemal ODA complex |
| ODAD4 | Outer dynein arm docking complex subunit 4 | TTC25, DKFZP434H0115 | No orthologue | Axonemal ODA complex |
| DNAI3 | Dynein axonemal intermediate chain 3 | WDR63, DIC3, FLJ30067, NYD-SP29 | DIC3 (IC140) | Axonemal IDA I1/f complex |
| DNAI4 | Dynein axonemal intermediate chain 4 | WDR78, DIC4, FLJ23129 | DIC4 (IC138) | Axonemal IDA I1/f complex |
| DNAI7 | Dynein axonemal intermediate chain 7 | CFAP94, CASC1, LAS1, FLJ10921, PPP1R54, IC97 | DII6 (FAP94) | Axonemal IDA I1/f complex |
| DYNLT2B | Dynein light chain Tctex-type 2B | TCTEX1D2, MGC33212 | DLT4 (Tctex2b) | Axonemal IDA I1/f complex |
| Cytoplasmic dynein 2 complex | ||||
| DYNC2I1 | Dynein 2 intermediate chain 1 | WDR60, FLJ10300, FAP163, CFAP163, DIC6 | DIC6 (FAP163) | Cytoplasmic dynein 2 complex |
| DYNC2I2 | Dynein 2 intermediate chain 2 | WDR34, DIC5, MGC20486, bA216B9.3, FAP133, CFAP133 | DIC5 (FAP133) | Cytoplasmic dynein 2 complex |
| DYNLT3 | Dynein light chain Tctex-type 3 | TCTE1L, TCTEX1L | DLT1 (LC9) | Cytoplasmic dynein 2 complex |
| DNAAF8 | Dynein axonemal assembly factor 8 | C16orf71, FLJ43261, DKFZp686H2240 | Axonemal dynein assembly factor | |
| DNAAF9 | Dynein axonemal assembly factor 9 | C20orf194, DKFZp434N061 | DNAAF9 | Axonemal dynein assembly factor |
| DNAAF10 | Dynein axonemal assembly factor 10 | WDR92, FLJ31741, Monad | DNAAF10 | Axonemal dynein assembly factor |
| DNAAF11 | Dynein axonemal assembly factor 11 | LRRC6, TSLRP, LRTP, CILD19, tilB | DNAAF11, MOT47, LRRC6, Seahorse | Axonemal dynein assembly factor |
| LRRC56 | Leucine rich repeat containing 56 | DNAAF12, FLJ00101, DKFZp761L1518 | DLU2 (ODA8) | Axonemal dynein assembly factor |
| SPAG1 | Sperm associated antigen 1 | DNAAF13, SP75, FLJ32920, HSD-3.8, TPIS, CT140, CILD28, | SPAG1 (SPAG1) | Axonemal dynein assembly factor |
| PIH1D1 | PIH1 domain containing 1 | DNAAF14, FLJ20643, Pih1, MOT48, | DAP2 (MOT48) | Axonemal dynein assembly factor |
| PIH1D2 | PIH1 domain containing 2 | DNAAF15 | Axonemal dynein assembly factor | |
| CFAP298 | Cilia and flagella associated protein 298 | FLJ20467, DAB2, FBB18, CILD26, Kur, C21orf48, C21orf59, DNAAF16 | DAB2 | Axonemal dynein assembly factor |
| CCDC103 | Coiled-coil domain containing 103 | FLJ13094, FLJ34211, PR46b, CILD17, DNAAF17c | CCDC103 | Axonemal dynein assembly factor |
| DAW1 | Dynein assembly factor with WD repeats 1 | FLJ25955, ODA16, WDR69, DNAAF18 | DAW1 | Axonemal dynein assembly factor |
Information about C. reinhardtii ciliary proteins, including dynein components, is curated and available at http://chlamyfp.org/.
Chlamydomonas encodes two paralogous proteins that both have the same human orthologue.
Reserved symbol/alias symbol. This gene will either be updated as a DNAAF or a DNAAF symbol will be added as an alias if further future publications support this.
Gene groups
HGNC gene groups are manually curated using data from publications and advice from our specialist advisors. The groups for genes encoding the subunits of human dynein complexes can be viewed here: https://www.genenames.org/data/genegroup/#!/group/537 and reflect the data shown in Table S1, Table S2, Table S3, Table S4, Table S5, and Table S6.
Discussion of HGNC nomenclature updates for dyneins and their cytoplasmic assembly factors
Dynein light chain nomenclature updates (dynein light chain Tctex-type [DYNLT])
Based on advice from experts in the field, we have updated the nomenclature of all the Tctex family genes to better reflect the function of their encoded proteins as dynein subunits. The six paralogs in this set now use the root symbol DYNLT in human.
DYNLT1 and DYNLT3
The gene currently approved as DYNLT1 (HGNC ID: 11697) was first approved using the symbol TCTEL1 based on homology with the mouse gene Tcte1 (t-complex associated testis expressed 1; Watanabe et al., 1996), which was reported to be specifically expressed in murine testes (Lader et al., 1989; Sarvetnick et al., 1989). The t-complex is a region of the mouse genome that shows non-Mendelian segregation, and some of the genes in it are associated with spermatogenesis (Castaneda et al., 2020). The alias symbol Tctex1 was also used to publish on this gene; it was characterized as encoding a cytoplasmic dynein light chain (Dedesma et al., 2006; King et al., 1998) and later also identified in axonemal inner arm I1/f (Harrison et al., 1998); in C. reinhardtii, a closely related protein is present in the ODA (DiBella et al., 2005).
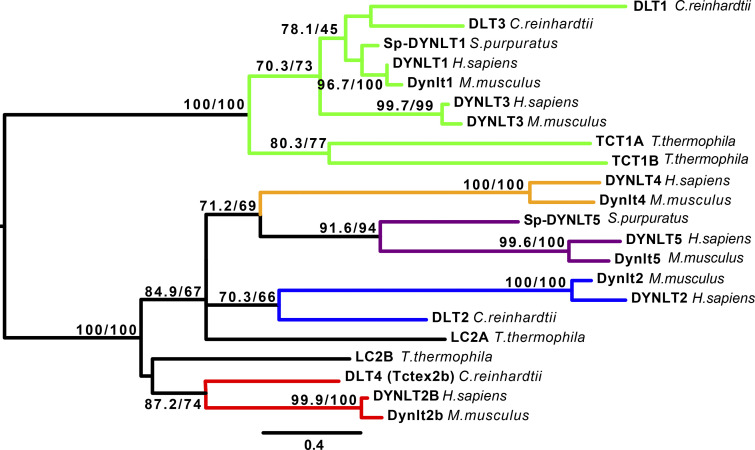
The most closely related paralogous gene to DYNLT1, now approved as DYNLT3 (HGNC ID: 11694), was originally assigned the symbol TCTE1L (Tcte1-like) in human, again to reflect its homology to mouse Tcte1. It was also published as a candidate for the retinitis pigmentosa RP3 locus (Roux et al., 1994), although this link was later disproven (Meindl et al., 1996) when RPGR (retinitis pigmentosa GTPase regulator) was identified as the causative gene for this phenotype (Ferrari et al., 2011). DYNLT3 was reported to encode a cytoplasmic dynein light chain in 1998 (King et al., 1998) and was later published as also playing a role in regulating primary cilium length (Palmer et al., 2011). We have constructed a phylogenetic tree (Fig. 4) that shows there is no clear 1:1 orthology relationship for either human DYNLT1 or DYNLT3 with respect to invertebrate species.
Figure 4.
Maximum-likelihood phylogenetic tree to show the relationship of Tctex-type dynein light chains in selected species. This tree is shown with a midpoint rooting. The figures on the nodes show the Shimodaira–Hasegawa likelihood ratio test and the Ultrafast bootstrap support values for the branches (SH-aLRT %/UFBoot %). Bootstrap values of ≥70% only are shown. The scale bar represents the expected number of amino acid substitutions per site. M. musculus has multiple Dynlt1 and Dynlt2 paralogs, but as these are identical at the amino acid level, only one sequence has been included in each case. The colors highlight supported clades: green for DYNLT1 and DYNLT3 and their orthologues, blue for DYNLT2 and its orthologues, red for DYNLT2B and its orthologues, yellow for DYNLT4 and its orthologue, and purple for DYNLT5 and its orthologues.
DYNLT2 and DYNLT2B
We have updated the nomenclature of the gene previously approved as TCTE3 (HGNC ID: 11695) to DYNLT2, and that of its closely related paralog previously approved as TCTEX1D2 (Tctex1 domain containing 2; HGNC ID: 28482) to DYNLT2B. These new symbols are more functionally informative, and this update brings the human nomenclature into line with that of C. reinhardtii, S. purpuratus, and T. thermophila (see Fig. 4). The phylogeny (Fig. 4) shows the paralogous relationship between DYNLT2 and DYNLT2B and that their 1:1 orthologues in the other species fall into two separate subclades.
Although DYNLT2 and DYNLT2B are paralogs, their protein products are components of distinct dynein complexes. DYNLT2 encodes an axonemal dynein subunit, required for outer arm assembly (Patel-King et al., 1997), and has not been reported as being part of any cytoplasmic dynein complex. The DYNLT2B-encoded protein is part of the cytoplasmic dynein 2 complex (Hamada et al., 2018; Schmidts et al., 2015) and is also an axonemal inner arm I1/f complex subunit (DiBella et al., 2004).
DYNLT4 and DYNLT5
We have updated the nomenclature of the gene previously approved as TCTEX1D4 (HGNC ID: 32315) to DYNLT4. This gene encodes a dynein light chain protein that belongs to the TCTEX1 family. Freitas et al. (2014) discussed its role in sperm motility and IFT.
While discussing the update for TCTEX1D4 with experts, we also proposed a nomenclature update for TCTEX1D1 (HGNC ID: 26882). This gene could not be updated to DYNLT1 in line with the TCTEX1D1 numbering, as this symbol was already in use, so we proposed an update to DYNLT5. There is currently a single paper published on this human gene (Spitali et al., 2020), linking a variant of it with the phenotype Duchenne muscular dystrophy. Although it seems likely that, as a paralog of the other DYNLT genes, DYNLT5 will be found to encode a dynein light chain, we have included the term family member in its current gene name to indicate that although it is related to the other DYNLT genes, a shared function has not yet been established. The phylogeny (Fig. 4) reveals that S. purpuratus has a 1:1 orthologue of DYNLT5, while C. reinhardtii and T. thermophila do not.
DNAI nomenclature updates
DNAI3 and DNAI4
We have updated the nomenclature of the human orthologues of C. reinhardtii DIC3, encoding IC140 (alias IDA7); and DIC4, encoding IC138 (alias BOP5), to DNAI3 (HGNC ID: 30711) and DNAI4 (HGNC ID: 26252), respectively. These genes were previously approved as WDR63 (WD repeat domain 63) and WDR78. In C. reinhardtii, IC140 and IC138 have been well characterized as intermediate chain subunits of an IDA complex (I1 dynein complex, also known as dynein-f; Hendrickson et al., 2004; Yang and Sale, 1998). Updating WDR63 and WDR78 using the DNAI root brings their nomenclature in line with the other human genes encoding axonemal dynein intermediate chains, DNAI1 and DNAI2. It also keeps the numbering system used equivalent to that of the C. reinhardtii orthologues.
The DNAI3-encoded protein is not essential for fertility in male mice, as other intermediate chains of the IDA I1/f complex may compensate for this role in mouse sperm motility (Young et al., 2015). The mouse orthologue of DNAI4 encodes a dynein intermediate chain in vertebrates. The DNAI4 protein interacts with multiple subunits of the axonemal inner arm I1/f dynein complex and is essential for the ciliary assembly of this complex in vertebrates (Zhang et al., 2019).
DNAI7 and NME8 (alias DNAI8)
We originally considered updating the nomenclature of the gene previously approved as CASC1 (cancer susceptibility 1; HGNC ID: 48939) to DNAI5. However, after discussion with experts, we realized this could be confusing, as it is not the orthologue of C. reinhardtii DIC5, and all the other human DNAI genes are numbered in line with their C. reinhardtii orthologues. There is also a DIC6 gene in C. reinhardtii, and its orthologue is the human gene now approved as DYNC2I1 (dynein 2 intermediate chain 1).
We were also reluctant to reassign CASC1 as DII6, the symbol used for the C. reinhardtii orthologue of this gene (Hom et al., 2011). We do not have an established DII# (dynein inner arm interacting) root approved in human, and most of the orthologues of the DII# C. reinhardtii genes are already approved and published using alternative symbols. These genes include DNALI1 (dynein axonemal light intermediate chain 1), the orthologue of DII1; ACTG1 (actin γ1), the orthologue of DII4; and ANK2 (ankyrin 2), the orthologue of DII7. In addition, with the exception of DNALI1, it is possible that one or more of these genes may not necessarily encode proteins that are dynein-arm interacting in vertebrates. Therefore, we updated CASC1 as DNAI7, reflecting that its protein product is a dynein intermediate chain in human. The mouse orthologue of DNAI7 encodes an intermediate chain in vertebrates that forms part of the inner arm I1/f dynein complex required for ciliary beating (Zhang et al., 2019).
This leaves NME8 (NME/NM23 family member 8) as the only remaining human gene known to encode a dynein intermediate chain but not named as such. This gene was previously approved as TXNDC3 (thioredoxin domain containing 3; Duriez et al., 2007) and has also been published using the alias symbol SPTRX2 (sperm-specific thioredoxin 2; Sadek et al., 2001).
There are 10 genes in the human NME/NM23 family, at least five of which encode active nucleoside diphosphate kinases (Ćetković et al., 2015). NME8 (HGNC ID: 16473) is the human orthologue of the sea urchin IC1 gene (Duriez et al., 2007), which encodes a sea urchin ODA intermediate chain and, like its human orthologue, contains an N-terminal thioredoxin-like domain (Ogawa et al., 1996). In C. reinhardtii, the ODA contains two paralogous thioredoxin-like light chains (LC3 and LC5) but lacks a nucleoside diphosphate kinase (Patel-King et al., 1996).
NME8 encodes a protein with a ciliary role, and its gene product is suggested to be bifunctional, with isoforms expressed at varying levels in different tissues (Duriez et al., 2007). The TXNDC3d7 protein isoform can bind microtubules, plays a role in ciliary function, and may be a component of ODAs (Duriez et al., 2007). As NME8 is already named as part of a gene group, is a functionally informative symbol, and has been used in the literature, we have decided to retain this nomenclature. However, this gene has been assigned the alias symbol DNAI8 and added to our dynein axonemal outer arm complex subunits gene group page (https://www.genenames.org/data/genegroup/#!/group/2031).
DYNC2I1 and DYNC2I2
We have updated the nomenclature of the human orthologue of C. reinhardtii DIC6 encoding D1bIC1 (alias FAP163) from WDR60 to DYNC2I1 (HGNC ID: 28296). We have also updated the nomenclature of the human orthologue of C. reinhardtii DIC5, encoding D1bIC2 (alias FAP133) from WDR34 to DYNC2I2 (HGNC ID: 21862). The numbering was assigned in this way so that the human gene nomenclature corresponds to that of the C. reinhardtii proteins.
DIC5/FAP133 in C. reinhardtii is associated with the IFT dynein motor (dynein 2, usually known as dynein 1b in C. reinhardtii) complex (Rompolas et al., 2007). DIC6/FAP163 encodes a C. reinhardtii intermediate chain that is closely related to DIC5/FAP133 and is also a component of the dynein 2 complex (Patel-King et al., 2013). Previous studies linked these two genes to ciliopathies including short rib polydactyly and Jeune syndrome (McInerney-Leo et al., 2013; Schmidts et al., 2013) and suggested that these orthologues of C. reinhardtii dynein intermediate chains may also encode components of the dynein 2 complex. Indeed, it was confirmed that both human genes encode dynein 2 intermediate chains (Asante et al., 2014).
ODA-DC (ODAD) nomenclature updates
ODAD1, ODAD2, ODAD3, ODAD4, and CLXN (ODAD5)
The ODA-DC has only recently been characterized in human (Hjeij et al., 2014; Onoufriadis et al., 2013; Wallmeier et al., 2016), and it became apparent that the nomenclature of the genes encoding the constituent proteins was not as functionally informative as it could be. The nomenclature of four of the ODA-DC subunits was initially based on the presence of structural domains in the encoded proteins: ARMC4 (armadillo repeat containing 4), CCDC114 and CCDC151 (coiled-coil domain containing 114 and 151, respectively), and TTC25 (tetratricopeptide repeat domain 25), as there was no functional information published when they were initially named.
These four genes have now been reassigned using the root symbol ODAD (ODA-DC subunits). The ODAD genes have been assigned numbers in the order in which they were characterized as encoding ODA-DC subunits in human and in line with the ODA numbering in C. reinhardtii where possible. We could not use the DCC root in human for these genes, as it clashed with the approved symbol for an unrelated gene, DCC (DCC netrin 1 receptor; HGNC ID: 2701).
ODAD1 is the orthologue of C. reinhardtii DCC2 (encoding DC2, alias ODA1), which encodes a docking complex subunit, and of its paralog DCC3, which encodes the ODA5 assembly factor (Takada et al., 2002). ODAD3 is the orthologue of DCC1, which encodes the protein DC1 (alias ODA3; Koutoulis et al., 1997), a docking complex subunit in C. reinhardtii, and of its paralog ODA10, which encodes a dynein assembly factor in C. reinhardtii (Dean and Mitchell, 2013). ODAD2 and ODAD4 have no known C. reinhardtii orthologues.
A fifth gene has recently been published in a study examining mammalian tracheal cilia as encoding an ODA-DC subunit (Gui et al., 2021; Fig. 3, c and d). Its encoded protein, calaxin, is a member of a neuronal calcium sensor family and was originally identified in ODAs from the sea squirt Ciona intestinalis; subsequent studies revealed it is required for normal ciliary motility in mice (Mizuno et al., 2009; Mizuno et al., 2012; Sasaki et al., 2019). We have updated its approved nomenclature from the previously approved but less frequently used EFCAB1 (EF-hand calcium binding domain 1) to CLXN (calaxin), aliasing it as ODAD5 after discussion with authors.
The symbol ODAD6 is reserved for the gene currently approved as CCDC63, a closely related paralog of ODAD1. We will continue to monitor the literature and may update the nomenclature of this gene, either approving ODAD6 or adding it as an alias if CCDC63 is shown to encode an ODA-DC subunit. The ODA-DC gene group page can be seen on our website (https://www.genenames.org/data/genegroup/#!/group/2019).
DNAAFs
We have updated the nomenclature of four genes as DNAAFs, including two previously assigned using placeholder C#orf# symbols (see Table S6). There are now 18 genes included in our axonemal dynein assembly factor gene group set (https://www.genenames.org/data/genegroup/#!/group/1627).
We have updated the nomenclature of the gene previously approved using the placeholder symbol C16orf71 (chromosome 16 open reading frame 71; HGNC ID: 25081) to DNAAF8. The Xenopus orthologue was recently published using the alias symbol Daap1 (dynein axonemal-associated protein 1; Lee et al., 2020), but following discussion, this gene has been approved as dnaaf8 in line with its human orthologue.
We have also updated the nomenclature of the gene previously approved as C20orf194 (chromosome 20 open reading frame 194; HGNC ID: 17721) to DNAAF9. The Tetrahymena orthologue of this gene was published using the alias name “shulin” (Mali et al., 2021). Those authors’ work showed that the encoded protein has a role in keeping the axonemal ODAs in a nonfunctional state before delivery to cilia. With these authors, our experts, and all researchers who had previously published on this gene, we discussed assigning this gene as DNAAF9, and they were supportive of this update. A gene (Cre11.g467556) exhibiting some similarity to DNAAF9 is present in C. reinhardtii; this is in a potentially poorly assembled genomic region, and further characterization will be required to determine whether it is the true orthologue of this human gene.
Two other genes, previously approved as WDR92 and LRRC6 (leucine rich repeat containing 6), have also been updated to DNAAF10 and DNAAF11, respectively. Both have been shown to encode proteins that are involved in axonemal dynein assembly (Patel-King and King, 2016; Fabczak and Osinka, 2019; Liu et al., 2019; Patel-King et al., 2019; Li et al., 2021; Zur Lage et al., 2018). The DNAAF10 protein product interacts with the protein encoded by SPAG1 (sperm associated antigen 1; see below) during dynein preassembly (Zur Lage et al., 2018). The DNAAF11 protein product interacts with the protein encoded by ZMYND10 (zinc finger MYND-type containing 10), which is aliased as DNAAF7 (Zariwala et al., 2013). ZMYND10 has been retained as the approved symbol because it has been well used in publications, and the current nomenclature reflects the fact that the encoded protein contains a MYND-type zinc finger domain.
We also assigned four other genes (LRRC56, SPAG1, PIH1D1, and PIH1D2) with DNAAF aliases to reflect the roles of their encoded proteins in dynein assembly (Bonnefoy et al., 2018; Knowles et al., 2013; Yamaguchi et al., 2018). These were assigned the alias symbols DNAAF12, DNAAF13, DNAAF14, and DNAAF15, respectively. Although it seems very likely based on two publications (Bonnefoy et al. [2018] and Desai et al. [2015]) that LRRC56 encodes a DNAAF, we are continuing to monitor the literature and could consider updating the nomenclature of this gene to DNAAF12 if there is sufficient evidence published to support this.
The SPAG1 and PIH1D1 symbols are already well established in the literature, and SPAG1, PIH1D1, and PIH1D2 all encode proteins that are subunits of complexes with many other functions as well as being involved in dynein assembly (von Morgen et al., 2015). The PIH1D2- and SPAG1-encoded proteins are part of the R2SP complex (Chagot et al., 2019), and the PIH1D1-encoded protein is part of the R2TP complex (Rodríguez and Llorca, 2020). Therefore, we have chosen to retain their currently approved symbols but have added them to our DNAAF gene group page. While we always ask that authors reference the approved gene symbols at least once in all publications, they can of course also use the DNAAF aliases.
We also discussed a DNAAF symbol update for the orthologue of C. reinhardtii DAB2 with authors and our expert advisors. DAB2 accumulates in cilia, and their motility is impaired (Austin-Tse et al., 2013). Variants of the Danio rerio orthologue of this gene, Kurly, are found in zebrafish mutants that display abnormalities in their development and have dynein arm defects, suggesting that the Kurly protein plays a role in ciliary motility but is also involved in regulating planar cell polarity (Jaffe et al., 2016). The human orthologue, previously approved as C21orf59, encodes a protein that has been shown to interact with known DNAAFs, including proteins encoded by ZYMND10 and DNAAF11 (previously LRRC6; Cho et al., 2018), and has been associated with the human phenotype PCD (Bolkier et al., 2021). Discussion with authors and our specialist advisors for the DNAAFs and cilia- and flagella-associated proteins (CFAPs) revealed community support for assigning a more general CFAP symbol for this gene. Its association with cilia and flagella is clear, and it also has a wider function beyond its role as an axonemal dynein arm assembly factor. However, while we have updated this gene as CFAP298 (HGNC ID: 1301), we have also assigned it the alias symbol DNAAF16. We also updated another cilia-associated gene, the orthologue of C. reinhardtii FBB5, as CFAP300 (previously approved as C11orf70) and have assigned it the alias symbol DNAAF17. Phylogenetic analysis strongly suggests that this gene is specific to organisms with motile cilia (being part of the MotileCut grouping; Merchant et al., 2007), and our CFAP nomenclature specialist advisor supported this change. As more becomes known about the function of the CFAP300 protein, we can consider whether a further symbol change would be helpful for this gene.
We are retaining the symbol DAW1 (dynein assembly factor with WD repeats 1), as it is the orthologue of C. reinhardtii DAW1 and its current nomenclature is functionally informative. However, we have aliased it as DNAAF18 and added it to the DNAAF gene group. We have also reserved the gene symbol DNAAF19 for the gene currently approved as CCDC103. The CCDC103 protein affects dynein assembly (King and Patel-King, 2020; Panizzi et al., 2012), but its exact role has still to be defined.
Conclusion
In total, we have updated the nomenclature of nine genes encoding human dynein chains, four genes encoding proteins that form the ODA-DC, and four genes encoding axonemal dynein assembly factors. Several other genes have retained their current symbols but have been aliased as ODADs or DNAAFs and added to the appropriate HGNC gene group pages. All updates were made following consultation with experts from the community, and these changes were widely supported among the authors publishing in this field. While we aim to limit changes in gene nomenclature, especially when the genes are linked to a phenotype, these updates have largely replaced uninformative placeholder or domain-based symbols, and we view the new informative symbols as stable. As such, users should regard these new symbols as the permanent gene symbols for these human genes.
We hope that all researchers will use the new nomenclature in their future publications to aid communication and data retrieval within the field. Approved symbols should be mentioned at least once in publications, along with the associated HGNC ID if possible.
Materials and methods
Dynein light chain phylogenetic tree
Amino acid protein sequences for dynein light chains were obtained for each of the six selected species from NCBI. A multiple alignment was built using the MUSCLE online tool (https://www.ebi.ac.uk/Tools/msa/muscle/; Madeira et al., 2019) and edited using AliView 1.20 (Larsson, 2014). The ends of the alignment were trimmed, and all indels were removed. The IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/) was used to construct a maximum-likelihood tree. The substitution model was autoselected with ultrafast bootstrapping and SH-aLRT branch test methods applied.
Online supplemental material
The supplementary tables show HGNC approved nomenclature for genes encoding subunits of dynein complexes alongside their known published alias symbols and their orthologs in C. reinhardtii. Table S1 shows cytoplasmic dynein 1 subunits. Table S2 shows cytoplasmic dynein 2 subunits. Table S3 shows axonemal ODA subunits. Table S4 shows monomeric dynein heavy chains and their accessory subunits. Table S5 shows axonemal inner arm dynein I1/f subunits. Table S6 shows axonemal dynein assembly factors (DNAAFs).
Supplementary Material
shows cytoplasmic dynein 1 subunits.
shows cytoplasmic dynein 2 subunits.
shows axonemal ODA subunits.
shows monomeric dynein heavy chains and their accessory subunits.
shows axonemal IDA I1/f subunits.
shows axonemal dynein assembly factors (DNAAFs).
Acknowledgments
We thank Jason Schrad (Nicastro laboratory) for the image shown in Fig. 3 b.
The work of the HGNC is supported by the National Human Genome Research Institute (grant U24HG003345) and the Wellcome Trust (grant 208349/Z/17/Z). S.M. King is supported by the National Institutes of Health (grant R35-GM140631). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interests.
Author contributions: B. Braschi, S.M. King, and H. Omran wrote the first draft of the manuscript, with H. Omran contributing to the "Association with human phenotypes" section. B. Braschi and S.M. King created the figures. B. Braschi, G.B. Witman, G.J. Pazour, K.K. Pfister, E.A. Bruford, and S.M. King edited the manuscript.
References
- Allan, V. 2014. Cell biology. One, two, three, cytoplasmic dynein is go! Science. 345:271–272. 10.1126/science.1257245 [DOI] [PubMed] [Google Scholar]
- Aprea, I., Nothe-Menchen T., Dougherty G.W., Raidt J., Loges N.T., Kaiser T., Wallmeier J., Olbrich H., Strunker T., Kliesch S., Pennekamp P., and Omran H.. 2021a. Motility of efferent duct cilia aids passage of sperm cells through the male reproductive system. Mol. Hum. Reprod. 27:gaab009. [DOI] [PMC free article] [PubMed]
- Aprea, I., Raidt J., Höben I.M., Loges N.T., Nöthe-Menchen T., Pennekamp P., Olbrich H., Kaiser T., Biebach L., Tüttelmann F., et al. 2021b. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet. 17:e1009306. 10.1371/journal.pgen.1009306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante, D., Stevenson N.L., and Stephens D.J.. 2014. Subunit composition of the human cytoplasmic dynein-2 complex. J. Cell Sci. 127:4774–4787. 10.1242/jcs.159038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin-Tse, C., Halbritter J., Zariwala M.A., Gilberti R.M., Gee H.Y., Hellman N., Pathak N., Liu Y., Panizzi J.R., Patel-King R.S., et al. 2013. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am. J. Hum. Genet. 93:672–686. 10.1016/j.ajhg.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisic, M., Sohm B., Mikolcevic P., Wandke C., Rauch V., Ringer T., Hess M., Bonn G., and Geley S.. 2010. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol. Biol. Cell. 21:1968–1981. 10.1091/mbc.e09-04-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benashski, S.E., Harrison A., Patel-King R.S., and King S.M.. 1997. Dimerization of the highly conserved light chain shared by dynein and myosin V. J. Biol. Chem. 272:20929–20935. 10.1074/jbc.272.33.20929 [DOI] [PubMed] [Google Scholar]
- Bhabha, G., Johnson G.T., Schroeder C.M., and Vale R.D.. 2016. How dynein moves along microtubules. Trends Biochem. Sci. 41:94–105. 10.1016/j.tibs.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkier, Y., Barel O., Marek-Yagel D., Atias-Varon D., Kagan M., Vardi A., Mishali D., Katz U., Salem Y., Tirosh-Wagner T., et al. 2021. Whole-exome sequencing reveals a monogenic cause in 56% of individuals with laterality disorders and associated congenital heart defects. J. Med. Genet. 10.1136/jmedgenet-2021-107775 [DOI] [PubMed] [Google Scholar]
- Bonnefoy, S., Watson C.M., Kernohan K.D., Lemos M., Hutchinson S., Poulter J.A., Crinnion L.A., Berry I., Simmonds J., Vasudevan P., et al. ; Care4Rare Canada Consortium . 2018. Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am. J. Hum. Genet. 103:727–739. 10.1016/j.ajhg.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A.B., Patel-King R.S., Benashski S.E., McCaffery J.M., Goldstein L.S., and King S.M.. 1999. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146:165–180. 10.1083/jcb.146.999.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, E.R., and Wallingford J.B.. 2014. Multiciliated cells. Curr. Biol. 24:R973–R982. 10.1016/j.cub.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruford, E.A., Braschi B., Denny P., Jones T.E.M., Seal R.L., and Tweedie S.. 2020. Guidelines for human gene nomenclature. Nat. Genet. 52:754–758. 10.1038/s41588-020-0669-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, A.P. 2013. Crystal clear insights into how the dynein motor moves. J. Cell Sci. 126:705–713. 10.1242/jcs.120725 [DOI] [PubMed] [Google Scholar]
- Casenghi, M., Barr F.A., and Nigg E.A.. 2005. Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J. Cell Sci. 118:5101–5108. 10.1242/jcs.02622 [DOI] [PubMed] [Google Scholar]
- Castaneda, J.M., Miyata H., Archambeault D.R., Satouh Y., Yu Z., Ikawa M., and Matzuk M.M.. 2020. Mouse t-complex protein 11 is important for progressive motility in sperm. Biol. Reprod. 102:852–862. 10.1093/biolre/ioz226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćetković, H., Perina D., Harcet M., Mikoč A., and Herak Bosnar M.. 2015. Nme family of proteins--clues from simple animals. Naunyn Schmiedebergs Arch. Pharmacol. 388:133–142. 10.1007/s00210-014-1017-x [DOI] [PubMed] [Google Scholar]
- Chagot, M.E., Dos Santos Morais R., Dermouche S., Lefebvre D., Manival X., Chipot C., Dehez F., and Quinternet M.. 2019. Binding properties of the quaternary assembly protein SPAG1. Biochem. J. 476:1679–1694. 10.1042/BCJ20190198 [DOI] [PubMed] [Google Scholar]
- Cho, K.J., Noh S.H., Han S.M., Choi W.I., Kim H.Y., Yu S., Lee J.S., Rim J.H., Lee M.G., Hildebrandt F., and Gee H.Y.. 2018. ZMYND10 stabilizes intermediate chain proteins in the cytoplasmic pre-assembly of dynein arms. PLoS Genet. 14:e1007316. 10.1371/journal.pgen.1007316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, J.Z., Yeh T.Y., Bollati F., Conde C., Canavosio F., Caceres A., and Sung C.H.. 2005. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev. Cell. 9:75–86. 10.1016/j.devcel.2005.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau, N., Goulet M., Geneviève D., Sznajer Y., Martinovic J., Smithson S., Huber C., Baujat G., Flori E., Tecco L., et al. 2009. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am. J. Hum. Genet. 84:706–711. 10.1016/j.ajhg.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, J., Lilleker J.B., Jabbal K., and Ealing J.. 2018. A missense mutation in DYNC1H1 gene causing spinal muscular atrophy - Lower extremity, dominant. Neurol. Neurochir. Pol. 52:293–297. 10.1016/j.pjnns.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Dean, A.B., and Mitchell D.R.. 2013. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol. Biol. Cell. 24:3689–3696. 10.1091/mbc.e13-06-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, A.B., and Mitchell D.R.. 2015. Late steps in cytoplasmic maturation of assembly-competent axonemal outer arm dynein in Chlamydomonas require interaction of ODA5 and ODA10 in a complex. Mol. Biol. Cell. 26:3596–3605. 10.1091/mbc.E15-05-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedesma, C., Chuang J.Z., Alfinito P.D., and Sung C.H.. 2006. Dynein light chain Tctex-1 identifies neural progenitors in adult brain. J. Comp. Neurol. 496:773–786. 10.1002/cne.20958 [DOI] [PubMed] [Google Scholar]
- Desai, P.B., Freshour J.R., and Mitchell D.R.. 2015. Chlamydomonas axonemal dynein assembly locus ODA8 encodes a conserved flagellar protein needed for cytoplasmic maturation of outer dynein arm complexes. Cytoskeleton (Hoboken). 72:16–28. 10.1002/cm.21206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella, L.M., Benashski S.E., Tedford H.W., Harrison A., Patel-King R.S., and King S.M.. 2001. The Tctex1/Tctex2 class of dynein light chains. Dimerization, differential expression, and interaction with the LC8 protein family. J. Biol. Chem. 276:14366–14373. 10.1074/jbc.M011456200 [DOI] [PubMed] [Google Scholar]
- DiBella, L.M., Smith E.F., Patel-King R.S., Wakabayashi K., and King S.M.. 2004. A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum. J. Biol. Chem. 279:21666–21676. 10.1074/jbc.M313540200 [DOI] [PubMed] [Google Scholar]
- DiBella, L.M., Gorbatyuk O., Sakato M., Wakabayashi K., Patel-King R.S., Pazour G.J., Witman G.B., and King S.M.. 2005. Differential light chain assembly influences outer arm dynein motor function. Mol. Biol. Cell. 16:5661–5674. 10.1091/mbc.e05-08-0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona, M., Bachmann-Gagescu R., Texier Y., Toedt G., Hetterschijt L., Tonnaer E.L., Peters T.A., van Beersum S.E., Bergboer J.G., Horn N., et al. 2015. NINL and DZANK1 co-function in vesicle transport and are essential for photoreceptor development in zebrafish. PLoS Genet. 11. e1005574. 10.1371/journal.pgen.1005574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguay, D., Bélanger-Nelson E., Mongrain V., Beben A., Khatchadourian A., and Cermakian N.. 2011. Dynein light chain Tctex-type 1 modulates orexin signaling through its interaction with orexin 1 receptor. PLoS One. 6:e26430. 10.1371/journal.pone.0026430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez, B., Duquesnoy P., Escudier E., Bridoux A.M., Escalier D., Rayet I., Marcos E., Vojtek A.M., Bercher J.F., and Amselem S.. 2007. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA. 104:3336–3341. 10.1073/pnas.0611405104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher, S.K. 2019. Dynein tails: how to hitch a ride on an IFT train. Nat. Struct. Mol. Biol. 26:760–761. 10.1038/s41594-019-0285-z [DOI] [PubMed] [Google Scholar]
- Eschbach, J., Sinniger J., Bouitbir J., Fergani A., Schlagowski A.I., Zoll J., Geny B., René F., Larmet Y., Marion V., et al. 2013. Dynein mutations associated with hereditary motor neuropathies impair mitochondrial morphology and function with age. Neurobiol. Dis. 58:220–230. 10.1016/j.nbd.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola, F.S., Suter D.M., Partata L.B.E., Cao T., Wolenski J.S., Cheney R.E., King S.M., and Mooseker M.S.. 2000. The light chain composition of chicken brain myosin-Va: calmodulin, myosin-II essential light chains, and 8-kDa dynein light chain/PIN. Cell Motil. Cytoskeleton. 47:269–281. [DOI] [PubMed] [Google Scholar]
- Fabczak, H., and Osinka A.. 2019. Role of the novel Hsp90 co-chaperones in dynein arms’ Preassembly. Int. J. Mol. Sci. 20:6174. 10.3390/ijms20246174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner, N.E., Dujardin D.L., Tai C.-Y., Vaughan K.T., O’Connell C.B., Wang Y., and Vallee R.B.. 2000. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2:784–791. 10.1038/35041020 [DOI] [PubMed] [Google Scholar]
- Ferrari, S., Di Iorio E., Barbaro V., Ponzin D., Sorrentino F.S., and Parmeggiani F.. 2011. Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics. 12:238–249. 10.2174/138920211795860107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf, M., Benzing T., and Omran H.. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8:880–893. 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- Freitas, M.J., Korrodi-Gregório L., Morais-Santos F., Cruz e Silva E., and Fardilha M.. 2014. TCTEX1D4 interactome in human testis: unraveling the function of dynein light chain in spermatozoa. OMICS. 18:242–253. 10.1089/omi.2013.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons, I.R. 2018. Discovery of dynein and its properties: a personal account. In Dyneins: structure, biology and disease. Volume 1- the Biology of Dynein Motors. King S.M., editor. Elsevier, Academic Press, Oxford, UK; 5–87. [Google Scholar]
- Goetz, S.C., and Anderson K.V.. 2010. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11:331–344. 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, J.C., Dantas T.J., and Vallee R.B.. 2019. Distinct roles for dynein light intermediate chains in neurogenesis, migration, and terminal somal translocation. J. Cell Biol. 218:808–819. 10.1083/jcb.201806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, M., Farley H., Anujan P., Anderson J.R., Maxwell D.W., Whitchurch J.B., Botsch J.J., Qiu T., Meleppattu S., Singh S.K., et al. 2021. De novo identification of mammalian ciliary motility proteins using cryo-EM. Cell. 184:5791–5806.e19. 10.1016/j.cell.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, Y., Tsurumi Y., Nozaki S., Katoh Y., and Nakayama K.. 2018. Interaction of WDR60 intermediate chain with TCTEX1D2 light chain of the dynein-2 complex is crucial for ciliary protein trafficking. Mol. Biol. Cell. 29:1628–1639. 10.1091/mbc.E18-03-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, G.C. 2019. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 285:479–490. 10.1111/joim.12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, A., Olds-Clarke P., and King S.M.. 1998. Identification of the t complex-encoded cytoplasmic dynein light chain tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J. Cell Biol. 140:1137–1147. 10.1083/jcb.140.5.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson, T.W., Perrone C.A., Griffin P., Wuichet K., Mueller J., Yang P., Porter M.E., and Sale W.S.. 2004. IC138 is a WD-repeat dynein intermediate chain required for light chain assembly and regulation of flagellar bending. Mol. Biol. Cell. 15:5431–5442. 10.1091/mbc.e04-08-0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjeij, R., Onoufriadis A., Watson C.M., Slagle C.E., Klena N.T., Dougherty G.W., Kurkowiak M., Loges N.T., Diggle C.P., Morante N.F.C., et al. UK10K Consortium . 2014. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 95:257–274. 10.1016/j.ajhg.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom, E.F., Witman G.B., Harris E.H., Dutcher S.K., Kamiya R., Mitchell D.R., Pazour G.J., Porter M.E., Sale W.S., Wirschell M., et al. 2011. A unified taxonomy for ciliary dyneins. Cytoskeleton (Hoboken). 68:555–565. 10.1002/cm.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höök, P., and Vallee R.B.. 2006. The dynein family at a glance. J. Cell Sci. 119:4369–4371. 10.1242/jcs.03176 [DOI] [PubMed] [Google Scholar]
- Horgan, C.P., Hanscom S.R., Jolly R.S., Futter C.E., and McCaffrey M.W.. 2010. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J. Cell Sci. 123:181–191. 10.1242/jcs.052670 [DOI] [PubMed] [Google Scholar]
- Hornef, N., Olbrich H., Horvath J., Zariwala M.A., Fliegauf M., Loges N.T., Wildhaber J., Noone P.G., Kennedy M., Antonarakis S.E., et al. 2006. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 174:120–126. 10.1164/rccm.200601-084OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Y., and Witman G.B.. 2015. Dynein and intraflagellar transport. Exp. Cell Res. 334:26–34. 10.1016/j.yexcr.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Tallon, I., Pagenstecher A., Fliegauf M., Olbrich H., Kispert A., Ketelsen U.P., North A., Heintz N., and Omran H.. 2004. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13:2133–2141. 10.1093/hmg/ddh219 [DOI] [PubMed] [Google Scholar]
- Ishikawa, T. 2017. Axoneme Structure from Motile Cilia. In Cold Spring Harbor Perspectives in Biology: Cilia. Vol. 9. Marshall W. and Basto R., editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, K.M., Grimes D.T., Schottenfeld-Roames J., Werner M.E., Ku T.S.J., Kim S.K., Pelliccia J.L., Morante N.F.C., Mitchell B.J., and Burdine R.D.. 2016. c21orf59/kurly Controls Both Cilia Motility and Polarization. Cell Rep. 14:1841–1849. 10.1016/j.celrep.2016.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey, S.R., and Snyder S.H.. 1996. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 274:774–777. 10.1126/science.274.5288.774 [DOI] [PubMed] [Google Scholar]
- Jin, Q., Gao G., and Mulder K.M.. 2009. Requirement of a dynein light chain in TGFbeta/Smad3 signaling. J. Cell. Physiol. 221:707–715. 10.1002/jcp.21910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, M.P., Omran H., Leigh M.W., Dell S., Morgan L., Molina P.L., Robinson B.V., Minnix S.L., Olbrich H., Severin T., et al. 2007. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 115:2814–2821. 10.1161/CIRCULATIONAHA.106.649038 [DOI] [PubMed] [Google Scholar]
- Ketcham, S.A., and Schroer T.A.. 2018. Role of dynactin in dynein-mediated motility. In Dyneins: structure, biology and disease. Volume 1- the Biology of Dynein Motors. King S.M., editor. Elsevier, Academic Press, Oxford, UK; 503–515. 10.1016/B978-0-12-809471-6.00017-6 [DOI] [Google Scholar]
- Kiesel, P., Alvarez Viar G., Tsoy N., Maraspini R., Gorilak P., Varga V., Honigmann A., and Pigino G.. 2020. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 27:1115–1124. 10.1038/s41594-020-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M. 2017. Axonemal dynein arms. In Marshall W. and Basto R., editors. Cold Spring Harbor Perspectives in Biology: Cilia. Vol. 9. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- King, S.M. 2021. Cytoplasmic factories for axonemal dynein assembly. J. Cell Sci. 134:jcs258626. 10.1242/jcs.258626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M., and Patel-King R.S.. 1995. The M(r) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J. Biol. Chem. 270:11445–11452. 10.1074/jbc.270.19.11445 [DOI] [PubMed] [Google Scholar]
- King, S.M., and Patel-King R.S.. 2020. The outer dynein arm assembly factor CCDC103 forms molecular scaffolds through multiple self-interaction sites. Cytoskeleton (Hoboken). 77:25–35. 10.1002/cm.21591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M., Barbarese E., Dillman J.F. III, Patel-King R.S., Carson J.H., and Pfister K.K.. 1996. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J. Biol. Chem. 271:19358–19366. 10.1074/jbc.271.32.19358 [DOI] [PubMed] [Google Scholar]
- King, S.M., Barbarese E., Dillman J.F. III, Benashski S.E., Do K.T., Patel-King R.S., and Pfister K.K.. 1998. Cytoplasmic dynein contains a family of differentially expressed light chains. Biochemistry. 37:15033–15041. 10.1021/bi9810813 [DOI] [PubMed] [Google Scholar]
- Knowles, M.R., Ostrowski L.E., Loges N.T., Hurd T., Leigh M.W., Huang L., Wolf W.E., Carson J.L., Hazucha M.J., Yin W., et al. 2013. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 93:711–720. 10.1016/j.ajhg.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar, M. 2016. Fine-tuning motile cilia and flagella: evolution of the dynein motor proteins from plants to humans at high resolution. Mol. Biol. Evol. 33:3249–3267. 10.1093/molbev/msw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke, D., Norris A.M., and Mukhopadhyay S.. 2021. Developmental and regenerative paradigms of cilia regulated hedgehog signaling. Semin. Cell Dev. Biol. 110:89–103. 10.1016/j.semcdb.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutoulis, A., Pazour G.J., Wilkerson C.G., Inaba K., Sheng H., Takada S., and Witman G.B.. 1997. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J. Cell Biol. 137:1069–1080. 10.1083/jcb.137.5.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader, E., Ha H.S., O’Neill M., Artzt K., and Bennett D.. 1989. tctex-1: a candidate gene family for a mouse t complex sterility locus. Cell. 58:969–979. 10.1016/0092-8674(89)90948-3 [DOI] [PubMed] [Google Scholar]
- Larsson, A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 30:3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I.G., Olenick M.A., Boczkowska M., Franzini-Armstrong C., Holzbaur E.L.F., and Dominguez R.. 2018. A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity. Nat. Commun. 9:986. 10.1038/s41467-018-03412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C., Cox R.M., Papoulas O., Horani A., Drew K., Devitt C.C., Brody S.L., Marcotte E.M., and Wallingford J.B.. 2020. Functional partitioning of a liquid-like organelle during assembly of axonemal dyneins. eLife. 9:e58662. 10.7554/eLife.58662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh, M.W., Horani A., Kinghorn B., O’Connor M.G., Zariwala M.A., and Knowles M.R.. 2019. Primary Ciliary Dyskinesia (PCD): A genetic disorder of motile cilia. Transl. Sci. Rare Dis. 4:51–75. 10.3233/TRD-190036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Jiang C., Zhang X., Liu M., Sun Y., Yang Y., and Shen Y.. 2021. The effect of a novel LRRC6 mutation on the flagellar ultrastructure in a primary ciliary dyskinesia patient. J. Assist. Reprod. Genet. 38:689–696. 10.1007/s10815-020-02036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J., Yin W., Smith M.C., Song K., Leigh M.W., Zariwala M.A., Knowles M.R., Ostrowski L.E., and Nicastro D.. 2014. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat. Commun. 5:5727. 10.1038/ncomms6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck, R.W., Chemes H., and Albertini D.F.. 2016. The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J. Assist. Reprod. Genet. 33:141–156. 10.1007/s10815-016-0652-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G., Wang L., and Pan J.. 2019. Chlamydomonas WDR92 in association with R2TP-like complex and multiple DNAAFs to regulate ciliary dynein preassembly. J. Mol. Cell Biol. 11:770–780. 10.1093/jmcb/mjy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening, N.M., Saravanan S., Jespersen N.E., Jara K., and Barbar E.. 2020. Interplay of disorder and sequence specificity in the formation of stable dynein-dynactin complexes. Biophys. J. 119:950–965. 10.1016/j.bpj.2020.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, W., and Gelfand V.I.. 2017. Moonlighting motors: kinesin, dynein, and cell polarity. Trends Cell Biol. 27:505–514. 10.1016/j.tcb.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, R.A., Saridogan E., and Djahanbakhch O.. 2006. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update. 12:363–372. 10.1093/humupd/dml012 [DOI] [PubMed] [Google Scholar]
- Madeira, F., Park Y.M., Lee J., Buso N., Gur T., Madhusoodanan N., Basutkar P., Tivey A.R.N., Potter S.C., Finn R.D., and Lopez R.. 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47(W1):W636–W641. 10.1093/nar/gkz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, G.R., Ali F.A., Lau C.K., Begum F., Boulanger J., Howe J.D., Chen Z.A., Rappsilber J., Skehel M., and Carter A.P.. 2021. Shulin packages axonemal outer dynein arms for ciliary targeting. Science. 371:910–916. 10.1126/science.abe0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus, S.M., Marzo M.G., and McKenney R.J.. 2020. New insights into the mechanism of dynein motor regulation by lissencephaly-1. eLife. 9:e59737. 10.7554/eLife.59737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney-Leo, A.M., Schmidts M., Cortés C.R., Leo P.J., Gener B., Courtney A.D., Gardiner B., Harris J.A., Lu Y., Marshall M., et al. UK10K Consortium . 2013. Short-rib polydactyly and Jeune syndromes are caused by mutations in WDR60. Am. J. Hum. Genet. 93:515–523. 10.1016/j.ajhg.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl, A., Dry K., Herrmann K., Manson F., Ciccodicola A., Edgar A., Carvalho M.R., Achatz H., Hellebrand H., Lennon A., et al. 1996. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat. Genet. 13:35–42. 10.1038/ng0596-35 [DOI] [PubMed] [Google Scholar]
- Merchant, S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Maréchal-Drouard L., et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 318:245–250. 10.1126/science.1143609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, K., Padma P., Konno A., Satouh Y., Ogawa K., and Inaba K.. 2009. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca(2+)-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol. Cell. 101:91–103. 10.1042/BC20080032 [DOI] [PubMed] [Google Scholar]
- Mizuno, K., Shiba K., Okai M., Takahashi Y., Shitaka Y., Oiwa K., Tanokura M., and Inaba K.. 2012. Calaxin drives sperm chemotaxis by Ca2+-mediated direct modulation of a dynein motor. Proc. Natl. Acad. Sci. USA. 109:20497–20502. 10.1073/pnas.1217018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn, K., and Askwith C.. 2017. G-Protein-Coupled Receptor Signaling in Cilia. In Marshall W. and Basto R., editors. Cold Spring Harbor Perspectives in Biology: Cilia. Vol. 9. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 10.1101/cshperspect.a028183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., and Hirokawa N.. 1998. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 95:829–837. 10.1016/S0092-8674(00)81705-5 [DOI] [PubMed] [Google Scholar]
- Nöthe-Menchen, T., Wallmeier J., Pennekamp P., Höben I.M., Olbrich H., Loges N.T., Raidt J., Dougherty G., Hjeij R., Dworniczak B., and Omran H.. 2019. Randomization of left-right asymmetry and congenital heart defects: the role of DNAH5 in humans and mice. Circ. Genom. Precis. Med. 10.1161/CIRCGEN.119.002686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, K., Takai H., Ogiwara A., Yokota E., Shimizu T., Inaba K., and Mohri H.. 1996. Is outer arm dynein intermediate chain 1 multifunctional? Mol. Biol. Cell. 7:1895–1907. 10.1091/mbc.7.12.1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich, H., Häffner K., Kispert A., Völkel A., Volz A., Sasmaz G., Reinhardt R., Hennig S., Lehrach H., Konietzko N., et al. 2002. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 30:143–144. 10.1038/ng817 [DOI] [PubMed] [Google Scholar]
- Olenick, M.A., Tokito M., Boczkowska M., Dominguez R., and Holzbaur E.L.. 2016. Hook adaptors induce unidirectional processive motility by enhancing the dynein-dynactin interaction. J. Biol. Chem. 291:18239–18251. 10.1074/jbc.M116.738211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis, A., Paff T., Antony D., Shoemark A., Micha D., Kuyt B., Schmidts M., Petridi S., Dankert-Roelse J.E., Haarman E.G., et al. ; UK10K . 2013. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am. J. Hum. Genet. 92:88–98. 10.1016/j.ajhg.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinka, A., Poprzeczko M., Zielinska M.M., Fabczak H., Joachimiak E., and Wloga D.. 2019. Ciliary proteins: filling the gaps. Recent advances in deciphering the protein composition of motile ciliary complexes. Cells. 8:730. 10.3390/cells8070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún, J.E., Lagos J., Vázquez M.C., Valls C., De la Fuente C., Yuseff M.I., Alvarez A.R., and Zanlungo S.. 2019. Lysosome motility and distribution: Relevance in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 1865:1076–1087. 10.1016/j.bbadis.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Palmer, K.J., Hughes H., and Stephens D.J.. 2009. Specificity of cytoplasmic dynein subunits in discrete membrane-trafficking steps. Mol. Biol. Cell. 20:2885–2899. 10.1091/mbc.e08-12-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, K.J., MacCarthy-Morrogh L., Smyllie N., and Stephens D.J.. 2011. A role for Tctex-1 (DYNLT1) in controlling primary cilium length. Eur. J. Cell Biol. 90:865–871. 10.1016/j.ejcb.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzi, J.R., Becker-Heck A., Castleman V.H., Al-Mutairi D.A., Liu Y., Loges N.T., Pathak N., Austin-Tse C., Sheridan E., Schmidts M., et al. 2012. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat. Genet. 44:714–719. 10.1038/ng.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King, R.S., and King S.M.. 2016. A prefoldin-associated WD-repeat protein (WDR92) is required for the correct architectural assembly of motile cilia. Mol. Biol. Cell. 27:1204–1209. 10.1091/mbc.E16-01-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King, R.S., Benashki S.E., Harrison A., and King S.M.. 1996. Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonas outer dynein arm. J. Biol. Chem. 271:6283–6291. 10.1074/jbc.271.11.6283 [DOI] [PubMed] [Google Scholar]
- Patel-King, R.S., Benashski S.E., Harrison A., and King S.M.. 1997. A Chlamydomonas homologue of the putative murine t complex distorter Tctex-2 is an outer arm dynein light chain. J. Cell Biol. 137:1081–1090. 10.1083/jcb.137.5.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King, R.S., Gilberti R.M., Hom E.F., and King S.M.. 2013. WD60/FAP163 is a dynein intermediate chain required for retrograde intraflagellar transport in cilia. Mol. Biol. Cell. 24:2668–2677. 10.1091/mbc.e13-05-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King, R.S., Sakato-Antoku M., Yankova M., and King S.M.. 2019. WDR92 is required for axonemal dynein heavy chain stability in cytoplasm. Mol. Biol. Cell. 30:1834–1845. 10.1091/mbc.E19-03-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., Wilkerson C.G., and Witman G.B.. 1998. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141:979–992. 10.1083/jcb.141.4.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., Dickert B.L., and Witman G.B.. 1999. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144:473–481. 10.1083/jcb.144.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., Baker S.A., Deane J.A., Cole D.G., Dickert B.L., Rosenbaum J.L., Witman G.B., and Besharse J.C.. 2002. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 157:103–113. 10.1083/jcb.200107108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, K.K., Fisher E.M., Gibbons I.R., Hays T.S., Holzbaur E.L., McIntosh J.R., Porter M.E., Schroer T.A., Vaughan K.T., Witman G.B., et al. 2005. Cytoplasmic dynein nomenclature. J. Cell Biol. 171:411–413. 10.1083/jcb.200508078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, K.K., Shah P.R., Hummerich H., Russ A., Cotton J., Annuar A.A., King S.M., and Fisher E.M.. 2006. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2:e1. 10.1371/journal.pgen.0020001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath, H., Huang D.C., O’Reilly L.A., King S.M., and Strasser A.. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3:287–296. 10.1016/S1097-2765(00)80456-6 [DOI] [PubMed] [Google Scholar]
- Raidt, J., Werner C., Menchen T., Dougherty G.W., Olbrich H., Loges N.T., Schmitz R., Pennekamp P., and Omran H.. 2015. Ciliary function and motor protein composition of human fallopian tubes. Hum. Reprod. 30:2871–2880. 10.1093/humrep/dev227 [DOI] [PubMed] [Google Scholar]
- Rao, Q., Han L., Wang Y., Chai P., Kuo Y.W., Yang R., Hu F., Yang Y., Howard J., and Zhang K.. 2021. Structures of outer-arm dynein array on microtubule doublet reveal a motor coordination mechanism. Nat. Struct. Mol. Biol. 28:799–810. 10.1038/s41594-021-00656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., Yildiz A., Carter A.P., Gennerich A., Zhang N., and Vale R.D.. 2006. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 126:335–348. 10.1016/j.cell.2006.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., Redwine W.B., Vale R.D., and Carter A.P.. 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 19:382–398. 10.1038/s41580-018-0004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter, J.F., and Leroux M.R.. 2017. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18:533–547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A.J. 2018. Emerging mechanisms of dynein transport in the cytoplasm versus the cilium. Biochem. Soc. Trans. 46:967–982. 10.1042/BST20170568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A.J., Kon T., Knight P.J., Sutoh K., and Burgess S.A.. 2013. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 14:713–726. 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, C.F., and Llorca O.. 2020. RPAP3 C-terminal domain: a conserved domain for the assembly of R2TP co-chaperone complexes. Cells. 9:1139. 10.3390/cells9051139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas, P., Pedersen L.B., Patel-King R.S., and King S.M.. 2007. Chlamydomonas FAP133 is a dynein intermediate chain associated with the retrograde intraflagellar transport motor. J. Cell Sci. 120:3653–3665. 10.1242/jcs.012773 [DOI] [PubMed] [Google Scholar]
- Roux, A.F., Rommens J., McDowell C., Anson-Cartwright L., Bell S., Schappert K., Fishman G.A., and Musarella M.. 1994. Identification of a gene from Xp21 with similarity to the tctex-1 gene of the murine t complex. Hum. Mol. Genet. 3:257–263. 10.1093/hmg/3.2.257 [DOI] [PubMed] [Google Scholar]
- Sadek, C.M., Damdimopoulos A.E., Pelto-Huikko M., Gustafsson J.A., Spyrou G., and Miranda-Vizuete A.. 2001. Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells. 6:1077–1090. 10.1046/j.1365-2443.2001.00484.x [DOI] [PubMed] [Google Scholar]
- Sarvetnick, N., Tsai J.Y., Fox H., Pilder S.H., and Silver L.M.. 1989. A mouse chromosome 17 gene encodes a testes-specific transcript with unusual properties. Immunogenetics. 30:34–41. 10.1007/BF02421467 [DOI] [PubMed] [Google Scholar]
- Sasaki, K., Shiba K., Nakamura A., Kawano N., Satouh Y., Yamaguchi H., Morikawa M., Shibata D., Yanase R., Jokura K., et al. 2019. Calaxin is required for cilia-driven determination of vertebrate laterality. Commun. Biol. 2:226. 10.1038/s42003-019-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saternos, H., Ley S., and AbouAlaiwi W.. 2020. Primary cilia and calcium signaling interactions. Int. J. Mol. Sci. 21:7109. 10.3390/ijms21197109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir, P., and Christensen S.T.. 2008. Structure and function of mammalian cilia. Histochem. Cell Biol. 129:687–693. 10.1007/s00418-008-0416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, H., and Carter A.P.. 2018. Mechanism and regulation of dynein motors. In Dyneins: Structure, Biology and Disease. Volume 2 - Dynein Mechanics, Dysfunction and Disease. King S.M., editor. Elsevier Inc, Academic Press, Oxford, UK. [Google Scholar]
- Schmidts, M., Vodopiutz J., Christou-Savina S., Cortés C.R., McInerney-Leo A.M., Emes R.D., Arts H.H., Tüysüz B., D’Silva J., Leo P.J., et al. UK10K . 2013. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am. J. Hum. Genet. 93:932–944. 10.1016/j.ajhg.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts, M., Hou Y., Cortés C.R., Mans D.A., Huber C., Boldt K., Patel M., van Reeuwijk J., Plaza J.M., van Beersum S.E., et al. UK10K . 2015. TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport. Nat. Commun. 6:7074. 10.1038/ncomms8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempou, E., and Khokha M.K.. 2019. Genes and mechanisms of heterotaxy: patients drive the search. Curr. Opin. Genet. Dev. 56:34–40. 10.1016/j.gde.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Silvanovich, A., Li M.G., Serr M., Mische S., and Hays T.S.. 2003. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol. Biol. Cell. 14:1355–1365. 10.1091/mbc.e02-10-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky, N., and Meunier A.. 2017. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18:423–436. 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- Spitali, P., Zaharieva I., Bohringer S., Hiller M., Chaouch A., Roos A., Scotton C., Claustres M., Bello L., McDonald C.M., et al. CINRG Investigators . 2020. TCTEX1D1 is a genetic modifier of disease progression in Duchenne muscular dystrophy. Eur. J. Hum. Genet. 28:815–825. 10.1038/s41431-019-0563-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter, D., Razafsky D.S., Schlager M.A., Serra-Marques A., Grigoriev I., Demmers J., Keijzer N., Jiang K., Poser I., Hyman A.A., et al. 2012. BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol. Biol. Cell. 23:4226–4241. 10.1091/mbc.e12-03-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., Wilkerson C.G., Wakabayashi K., Kamiya R., and Witman G.B.. 2002. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol. Biol. Cell. 13:1015–1029. 10.1091/mbc.01-04-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisawa, T., and Kimura A.. 2020. The generation of dynein networks by multi-layered regulation and their implication in cell division. Front. Cell Dev. Biol. 8:22. 10.3389/fcell.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toropova, K., Zalyte R., Mukhopadhyay A.G., Mladenov M., Carter A.P., and Roberts A.J.. 2019. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 26:823–829. 10.1038/s41594-019-0286-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokter, M., Mücke N., and Surrey T.. 2012. Reconstitution of the human cytoplasmic dynein complex. Proc. Natl. Acad. Sci. USA. 109:20895–20900. 10.1073/pnas.1210573110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie, S., Braschi B., Gray K., Jones T.E.M., Seal R.L., Yates B., and Bruford E.A.. 2021. Genenames.org: the HGNC and VGNC resources in 2021. Nucleic Acids Res. 49(D1):D939–D946. 10.1093/nar/gkaa980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee, R.B., Yi J., Quintremil S., and Khobrekar N.. 2021. Roles of the multivalent dynein adaptors BicD2 and RILP in neurons. Neurosci. Lett. 752:135796. 10.1016/j.neulet.2021.135796 [DOI] [PubMed] [Google Scholar]
- Vig, A., Poulter J.A., Ottaviani D., Tavares E., Toropova K., Tracewska A.M., Mollica A., Kang J., Kehelwathugoda O., Paton T., et al. Genomics England Research Consortium . 2020. DYNC2H1 hypomorphic or retina-predominant variants cause nonsyndromic retinal degeneration. Genet. Med. 22:2041–2051. 10.1038/s41436-020-0915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Morgen, P., Hořejší Z., and Macurek L.. 2015. Substrate recognition and function of the R2TP complex in response to cellular stress. Front. Genet. 6:69. 10.3389/fgene.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo, L., Stevenson N.L., Mukhopadhyay A.G., Roberts A.J., and Stephens D.J.. 2020. Cytoplasmic dynein-2 at a glance. J. Cell Sci. 133:jcs240614. 10.1242/jcs.240614 [DOI] [PubMed] [Google Scholar]
- Wallmeier, J., Shiratori H., Dougherty G.W., Edelbusch C., Hjeij R., Loges N.T., Menchen T., Olbrich H., Pennekamp P., Raidt J., et al. 2016. TTC25 deficiency results in defects of the outer dynein arm docking machinery and primary ciliary dyskinesia with left-right body asymmetry randomization. Am. J. Hum. Genet. 99:460–469. 10.1016/j.ajhg.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier, J., Nielsen K.G., Kuehni C.E., Lucas J.S., Leigh M.W., Zariwala M.A., and Omran H.. 2020. Motile ciliopathies. Nat. Rev. Dis. Primers. 6:77. 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Huynh W., Skokan T.D., Lu W., Weiss A., and Vale R.D.. 2019. CRACR2a is a calcium-activated dynein adaptor protein that regulates endocytic traffic. J. Cell Biol. 218:1619–1633. 10.1083/jcb.201806097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanschers, B., van de Vorstenbosch R., Wijers M., Wieringa B., King S.M., and Fransen J.. 2008. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil. Cytoskeleton. 65:183–196. 10.1002/cm.20254 [DOI] [PubMed] [Google Scholar]
- Watanabe, T.K., Fujiwara T., Shimizu F., Okuno S., Suzuki M., Takahashi E., Nakamura Y., and Hirai Y.. 1996. Cloning, expression, and mapping of TCTEL1, a putative human homologue of murine Tcte1, to 6q. Cytogenet. Cell Genet. 73:153–156. 10.1159/000134329 [DOI] [PubMed] [Google Scholar]
- Wickstead, B. 2018. The evolutionary biology of dyneins. In Dyneins: Structure, Biology and Disease. Volume 1 - the Biology of Dynein Motors. King S.M., editor. Elsevier, Academic Press, Oxford, UK; 101–138. 10.1016/B978-0-12-809471-6.00003-6 [DOI] [Google Scholar]
- Wickstead, B., and Gull K.. 2007. Dyneins across eukaryotes: a comparative genomic analysis. Traffic. 8:1708–1721. 10.1111/j.1600-0854.2007.00646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J.C., Siglin A.E., Lightcap C.M., and Dawn A.. 2018. Structural analysis of dynein intermediate and light chains. In Dyneins: Structure, Biology and Disease. Volume 2 - Dynein mechanics, dysfunction and disease. King S.M., editor. Elsevier Academic Press, Cambridge, MA; 53–87. 10.1016/B978-0-12-809470-9.00003-5 [DOI] [Google Scholar]
- Wingfield, J.L., Mengoni I., Bomberger H., Jiang Y.Y., Walsh J.D., Brown J.M., Picariello T., Cochran D.A., Zhu B., Pan J., et al. 2017. IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife. 6:e26609. 10.7554/eLife.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, H., Oda T., Kikkawa M., and Takeda H.. 2018. Systematic studies of all PIH proteins in zebrafish reveal their distinct roles in axonemal dynein assembly. eLife. 7:e36979. 10.7554/eLife.36979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P., and Sale W.S.. 1998. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol. Biol. Cell. 9:3335–3349. 10.1091/mbc.9.12.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S.A., Miyata H., Satouh Y., Kato H., Nozawa K., Isotani A., Aitken R.J., Baker M.A., and Ikawa M.. 2015. CRISPR/Cas9-mediated rapid generation of multiple mouse lines identified Ccdc63 as essential for spermiogenesis. Int. J. Mol. Sci. 16:24732–24750. 10.3390/ijms161024732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala, M.A., Omran H., and Ferkol T.W.. 2011. The emerging genetics of primary ciliary dyskinesia. Proc. Am. Thorac. Soc. 8:430–433. 10.1513/pats.201103-023SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala, M.A., Gee H.Y., Kurkowiak M., Al-Mutairi D.A., Leigh M.W., Hurd T.W., Hjeij R., Dell S.D., Chaki M., Dougherty G.W., et al. 2013. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am. J. Hum. Genet. 93:336–345. 10.1016/j.ajhg.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Chen Y., Zheng J., Wang J., Duan S., Zhang W., Yan X., and Zhu X.. 2019. Vertebrate dynein-f depends on Wdr78 for axonemal localization and is essential for ciliary beat. J. Mol. Cell Biol. 11:383–394. 10.1093/jmcb/mjy043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Lage, P., Stefanopoulou P., Styczynska-Soczka K., Quinn N., Mali G., von Kriegsheim A., Mill P., and Jarman A.P.. 2018. Ciliary dynein motor preassembly is regulated by Wdr92 in association with HSP90 co-chaperone, R2TP. J. Cell Biol. 217:2583–2598. 10.1083/jcb.201709026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
shows cytoplasmic dynein 1 subunits.
shows cytoplasmic dynein 2 subunits.
shows axonemal ODA subunits.
shows monomeric dynein heavy chains and their accessory subunits.
shows axonemal IDA I1/f subunits.
shows axonemal dynein assembly factors (DNAAFs).