Abstract
Syndromes of cardiac ischemia with nonobstructive coronary arteries have been increasingly recognized as a clinical entity with heterogenous clinical presentations, commonly encountered in women. Knowledge of pathophysiology and clinical risk factors is key to ensure appropriate diagnostic evaluation and management for these often-neglected patients. In this review, we discuss the epidemiology, risk factors, and clinical presentations of these syndromes. We provide algorithms for diagnosis and management of these entities based on current scientific knowledge and highlight some of the key knowledge gaps and ongoing trials in this emerging field.
Keywords: Angina, Coronary microvascular dysfunction, Ischemia, Myocardial infarction with nonobstructive coronary arteries, Nononstructive coronary artery disease, Vasospastic angina
INTRODUCTION
The syndrome of symptoms and/or signs of myocardial ischemia with nonobstructive coronary arteries (NOCA) is increasingly recognized as an emerging heterogenous clinical entity most often encountered among women.1–6 Studies have shown that myocardial infarction with nonobstructive coronary arteries (MINOCA) accounts for 6%−10% of acute myocardial infarctions, and women have ~5-fold higher odds of MINOCA compared with men presenting with acute myocardial infarction.7,8 Moreover, these patients have heterogenous clinical presentations further adding to the complexity—elevated cardiac biomarkers in MINOCA, evidence of ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries (INOCA), or angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries (ANOCA).1,5–10 With advancements and increasing use of cardiac imaging, these syndromes are more frequently recognized.9–18 In this review, we discuss the definitions of these entities, epidemiology and risk factors, role of cardiac imaging and high-sensitivity troponin in diagnosis, management and prognosis, and some key knowledge gaps in this field.
DEFINITIONS
MINOCA
MINOCA was first documented >80 years ago on autopsy showing myocardial necrosis without significant coronary artery atherosclerosis.5,6 An American Heart Association (AHA) scientific statement defined MINOCA by the following features: (1) presence of the fourth universal acute myocardial infarction criteria with an elevated cardiac biomarker, typically a cardiac troponin >99th percentile of the upper reference level with a rise or fall in the level on serial assessment; (2) absence of obstructive coronary artery disease (≥50% stenosis); and (3) no overt cause for the clinical presentation at the time of angiography (cardiac trauma or features for Takotsubo cardiomyopathy).3,5
INOCA
In another scientific report by the AHA9, INOCA was defined as (1) stable chronic (several weeks or longer) symptoms suggesting ischemia such as chest discomfort with both classic (angina pectoris) and atypical features in terms of location, quality, and inciting factors; (2) objective evidence for myocardial ischemia from electrocardiography or cardiac imaging (echocardiography, nuclear imaging, magnetic resonance imaging [MRI], or spectroscopy) at rest or during stress (exercise, mental, or pharmacological); and (3) absence of flow-limiting obstruction by coronary angiography (invasive or computed tomographic angiography) as defined by any epicardial coronary artery diameter reduction ≥50% or fractional flow reserve <0.8.9
A similar definition was proposed by a consensus statement by the European Society of Cardiology on Coronary Pathophysiology & Microcirculation that was endorsed by the Coronary Vasomotor Disorders International Study (COVADIS) group, highlighting that INOCA is a demand–supply mismatch of coronary artery blood flow leading to transient or recurrent cardiac chest pain related to myocardial ischemia. The mismatch between blood supply and myocardial oxygen demand may be caused by coronary microvascular dysfunction and/or epicardial coronary artery spasm, typically in the setting of nonobstructive coronary atherosclerosis. In this setting, they provided diagnostic criteria for microvascular angina to include all of the following: (1) symptoms of myocardial ischemia, (2) absence of obstructive coronary arteries (<50% diameter reduction or fractional flow reserve >0.80), (3) objective evidence of myocardial ischemia, and (4) evidence of impaired coronary microvascular function.1
Elevated troponin levels are related to the extent of myocardial involvement and blood flow (wash-out). Thus, widespread use of high-sensitivity troponin potentially may lead to overdiagnosis of MINOCA due to the ability to detect even minimal troponin efflux with reversible ischemic cardiomyocyte injury.16
ANOCA
ANOCA refers to symptoms and/or signs suggestive of ischemic heart disease, including anginal chest pain or exertional dyspnea, with non-obstructive coronary arteries. Such patients may not have clear evidence of ischemia on cardiac evaluation using current available diagnostic modalities or may not have been appropriately tested for ischemia.10 Studies have shown that approximately 60% of women and 30% of men presenting with angina for coronary angiography have otherwise nonobstructive lesions.10,11 Figure 1 and Table 1 summarize definitions of each entity.
Figure 1:
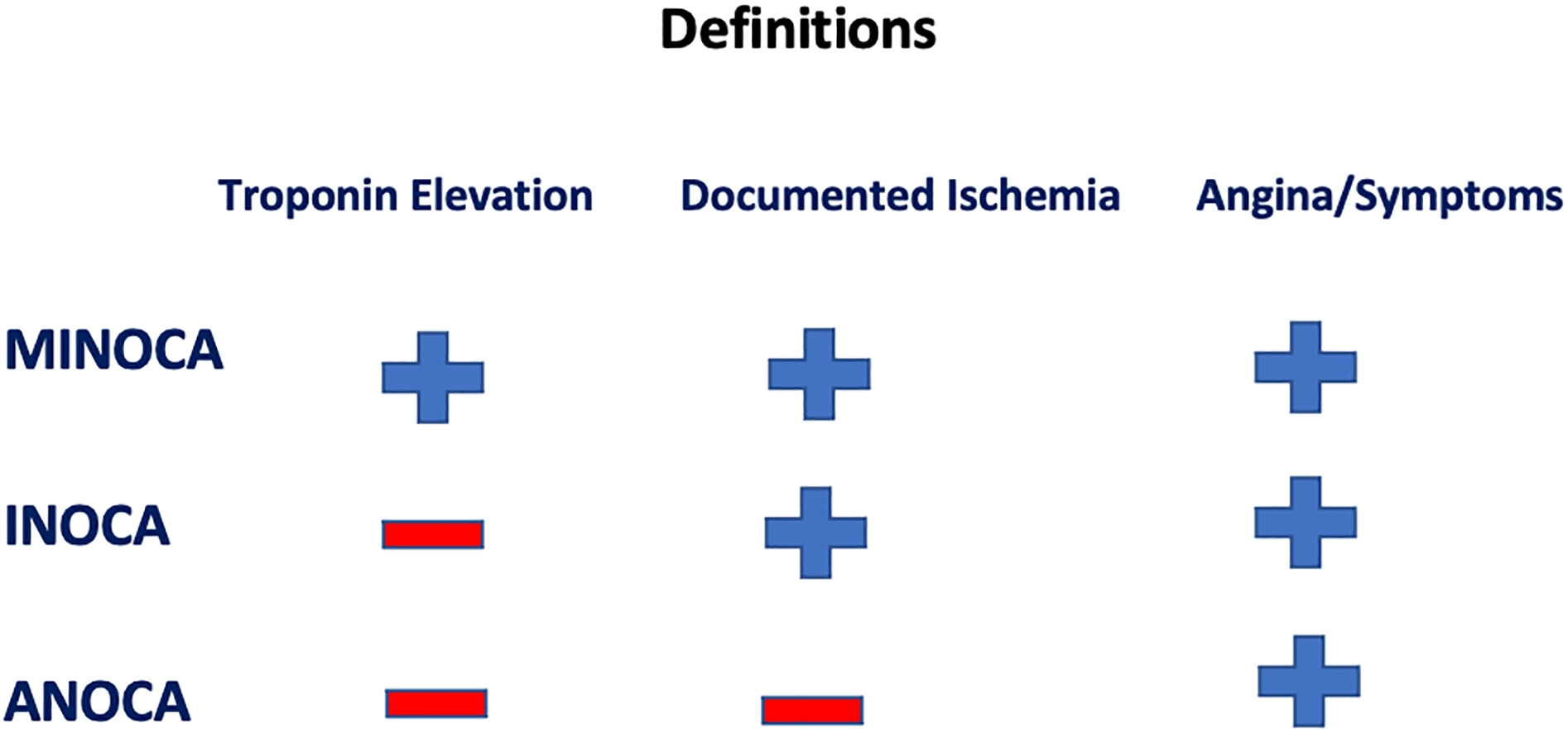
Summary of MINOCA, INOCA and ANOCA definitions based on elevated troponin, documented ischemia, and presence of symptoms/angina. ANOCA = angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries; INOCA = ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries; MINOCA = myocardial infarction with nonobstructive coronary arteries.
Table 1.
Summary of definitions of non-obstructive coronary artery syndromes.
| Syndrome | Definition Criteria |
|---|---|
| MINOCA | 1) Presence of the universal acute myocardial infarction criteria with an elevated cardiac biomarker, typically a cardiac troponin >99th percentile of the upper reference level with a rise or fall in the level on serial assessment 2) Absence of obstructive coronary artery disease (≥50% stenosis) 3) Exclude overt cause for the clinical presentation at the time of angiography (cardiac trauma or classic features for Takotsubo cardiomyopathy) |
| INOCA | 1) Stable chronic (several weeks or longer) symptoms suggesting ischemic heart disease such as chest discomfort with both classic (angina pectoris) and atypical features in terms of location, quality, and inciting factors 2) Objective evidence of myocardial ischemia on electrocardiography or a cardiac imaging study (echocardiography, nuclear imaging, magnetic resonance imaging, or spectroscopy) at rest or during stress (exercise, mental, or pharmacological) 3) Absence of flow-limiting obstruction by coronary angiography (invasive or computed tomographic angiography) as defined by any epicardial coronary artery diameter reduction ≥50% or fractional flow reserve <0.8 |
| ANOCA | 1) Symptoms and signs suggestive of ischemic heart disease, including anginal chest pain or exertional dyspnea 2) Either non-obstructive coronary arteries without clear evidence of ischemia on cardiac work-up using the current available diagnostic modalities or no clear diagnosis for symptoms due to incomplete testing (cardiac imaging and invasive tests for microvascular dysfunction). |
ANOCA = angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries; INOCA = ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries; MINOCA = myocardial infarction with nonobstructive coronary arteries.
CURRENT LIMITATIONS
It is important to understand that we have no optimal test for ischemia among patients without obstructive coronary artery disease who have microvascular dysfunction. This is because essentially all ischemia testing has been validated using obstructive coronary artery disease by angiography as the benchmark where there is a relatively large malperfused region compared with a relatively normally perfused region. At present, cardiac positron emission tomography and MRI have been useful but it is unclear whether either has sufficient resolution, and neither is very useful for detection of either endothelial dysfunction or microvascular spasm, both key mechanisms for microvascular dysfunction.
Also important is that coronary spasm can be reliably detected only by invasive coronary angiography during a spontaneous or a provoked episode, which is relieved either spontaneously or by nitroglycerin. Furthermore, microvascular spasm is not possible to assess in the presence of epicardial spasm.
EPIDEMIOLOGY AND RISK FACTORS
Studies suggest that MINOCA is present in 3.5%−15% of patients with acute myocardial infarction.5,7 MINOCA patients are usually younger and disproportionately women.8,12 A study from Europe showed that a large proportion of patients (up to 70%) undergoing coronary angiography because of angina and myocardial ischemia did not have obstructive epicardial coronary arteries.13 Similar to MINOCA, INOCA and ANOCA patients tend to be younger, more likely women, and usually have lower rates of traditional cardiovascular risk factors such as diabetes mellitus, compared with patients with obstructive disease.1,7,9,12,14,17
PATHOPHYSIOLOGY
MINOCA and INOCA/ANOCA share some common pathophysiologic mechanisms. These syndromes may be considered part of a disease spectrum of different severity and acuity. MINOCA patients, in general, present acutely, while the presentation of INOCA/ANOCA is mainly chronic and indolent.1,5,7 The potential underlying mechanisms for MINOCA include: 1) coronary causes such as plaque disruption, spontaneous coronary thrombosis or emboli, dissection, coronary spasm, and microvascular dysfunction; 2) myocardial injury due to myocardial disorders, including myocarditis and cardiomyopathies; and 3) noncardiac causes such as sepsis and pulmonary embolism.3,18
Coronary plaque disruption, which includes plaque rupture, plaque erosion, and calcific nodules, is frequently observed when intravascular imaging is performed among patients with MINOCA.4 This can lead to thrombus formation resulting in myocardial infarction through distal embolization, superimposed vasospasm, or transient complete thrombosis with spontaneous thrombolysis and recanalization.1,4 Similarly, spontaneous coronary artery dissection is a common cause of MINOCA, especially in young pregnant or early postpartum women.19 Coronary thrombosis due to hypercoagulable disorders is another cause of MINOCA.20
Coronary artery spasm is defined as marked constriction that reduces myocardial blood flow and may occur at the epicardial level, the microvascular level, or both. It may occur either in response to drugs, toxins, cold exposure, or emotional or exercise stress, or be spontaneously caused by heightened coronary vasomotor tone of unknown cause.3,4,21,22
Microvascular dysfunction is defined as microvascular endothelial or nonendothelial vascular smooth muscle dysfunction that limits myocardial perfusion. It is most often detected as reduced coronary flow reserve in absence of upstream obstructive coronary arteries.4,7 Patients with coronary microvascular dysfunction typically present with chest discomfort as stable ischemic heart disease, but it might also be a potential cause of MINOCA.4,9
There are two main endotypes of microvascular dysfunction. One is functional, characterized by elevated resting flow that is related to enhanced nitric oxide synthase activity. The other endotype is structural, where endothelial dysfunction leads to diminished coronary blood flow augmentation at rest or during increased demands like emotional or exercise stress.23 An example of structural type is microvascular spasm, in which dysfunctional vessels appear to be hypersensitive to acetylcholine and other vasoconstrictors. As with epicardial coronary spasm, microvascular spasm may occur more frequently in women.1,21 Unfortunately, when epicardial spasm is present, it becomes impossible to assess the microcirculation.
On the other hand, structural remodeling of the coronary microvasculature, whether caused by inward remodeling of coronary arterioles with increased wall-to-lumen ratio or loss of myocardial capillary density (e.g., rarefication) or both, is associated with decreased microcirculatory conductance and oxygen delivery capacity.1 These changes explain findings of low coronary flow reserve and elevated microvascular resistance in patients with microvascular dysfunction.1,9
Additionally, studies have shown that elevated pro-inflammatory mediators are associated with lower coronary flow reserve and thus, inflammation could potentially play a role in the pathophysiology of microvascular dysfunction.1,17,24,25 As the inflammatory process is usually systemic, microvascular dysfunction may affect other organs in the body in addition to the coronary vessels.26,27 However, it may be “patchy” in severity (more prominent) in some coronary segments or arteries of other organs.
DIAGNOSIS
Diagnosis of open coronary artery syndromes depends on the clinical presentation and diagnostic work-up.28 We divide the work-up of MINOCA (Figure 2A) and INOCA/ANOCA (Figure 2B) and discuss each separately.
Figure 2:
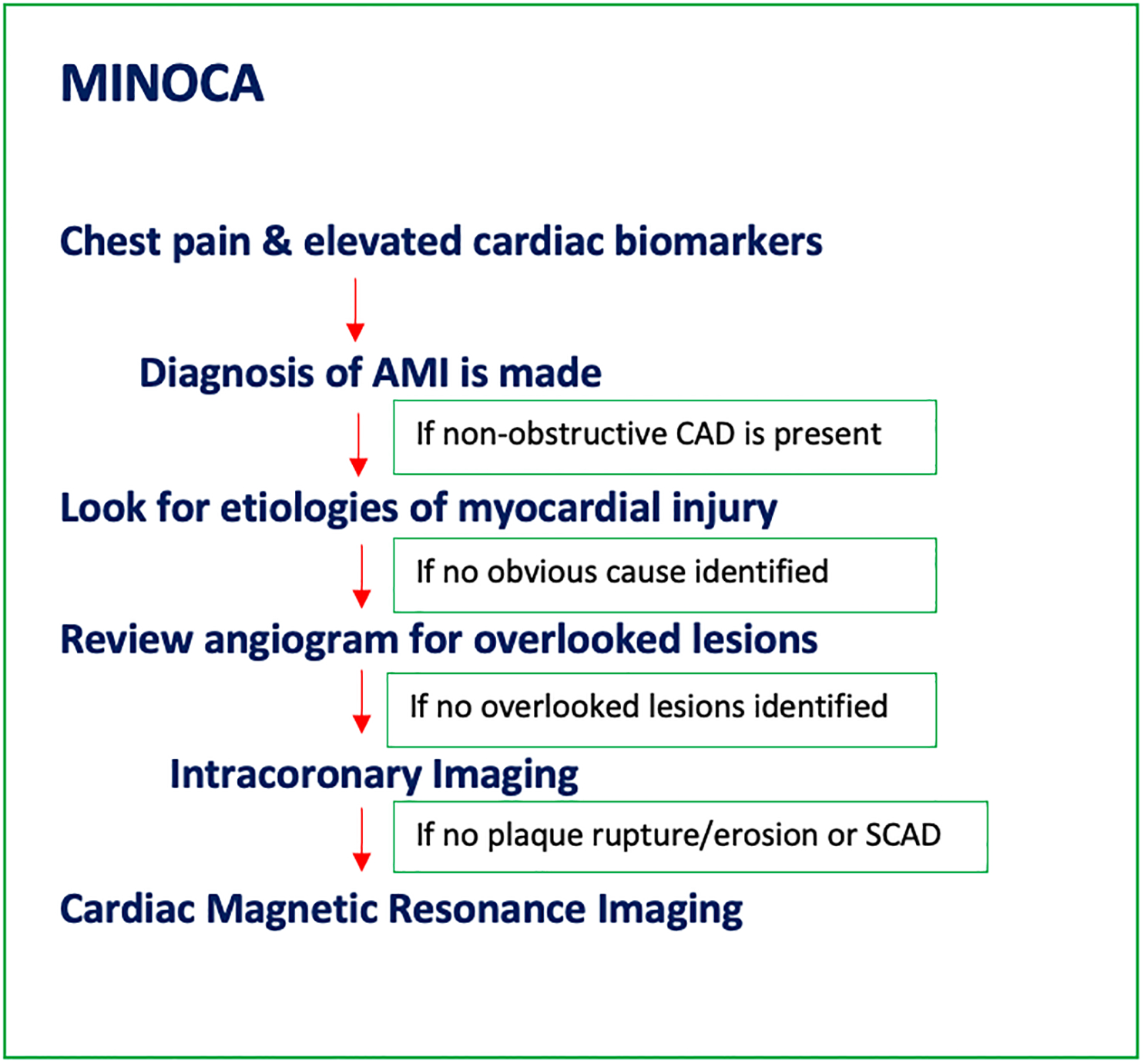
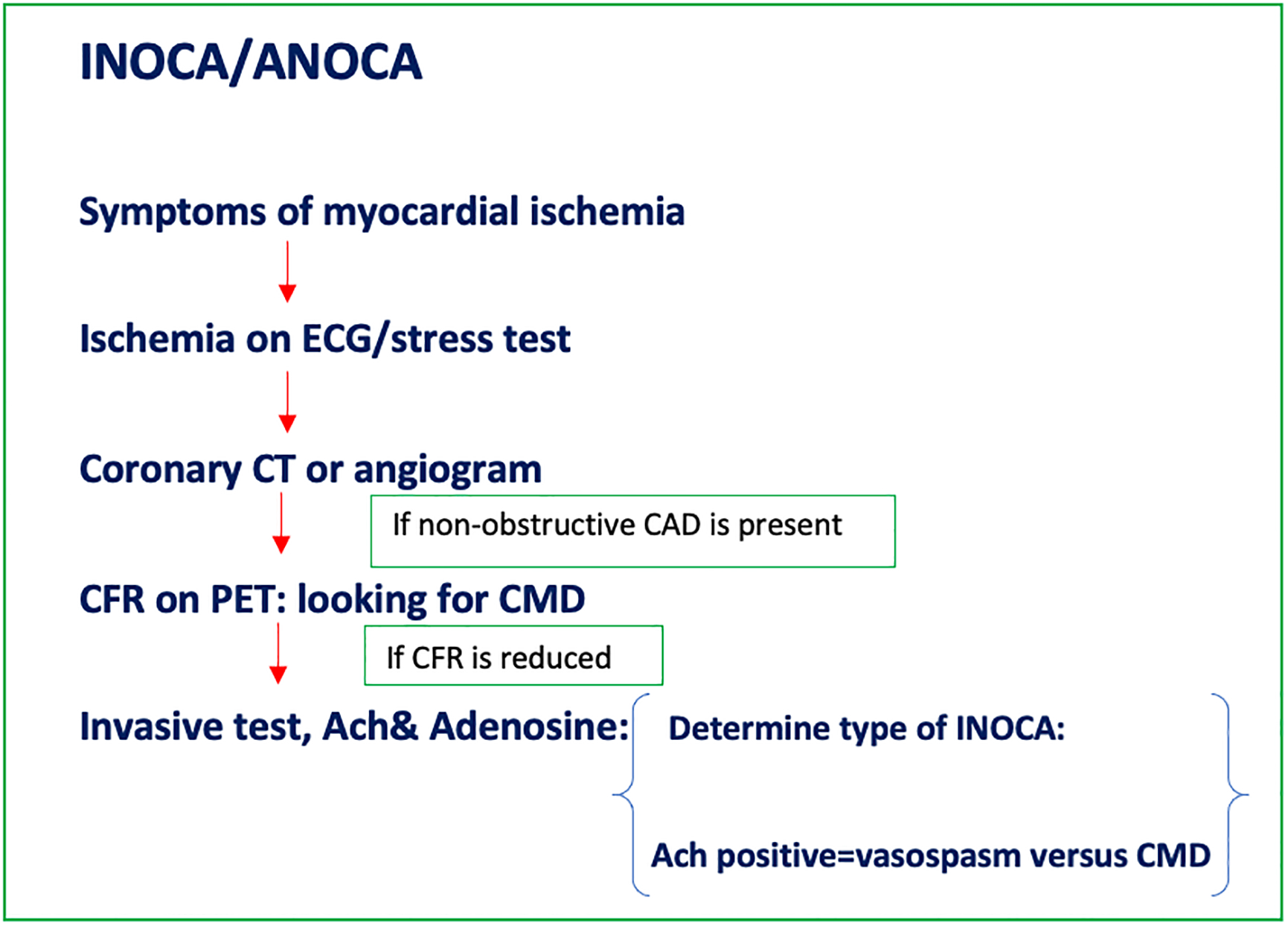
(A) Flow diagram summarizing the diagnostic evaluation for MINOCA patients; (B) Flow diagram summarizing the diagnostic work-up for INOCA/ANOCA patients. AMI = acute myocardial infarction; ANOCA = angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries; CAD = coronary artery disease; CFR = coronary flow reserve; CMD = coronary microvascular dysfunction; CT = computed tomography; ECG = electrocardiogram; INOCA = ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries; MINOCA = myocardial infarction with nonobstructive coronary arteries; PET = positron emission tomography; SCAD = spontaneous coronary artery dissection.
MINOCA
The initial evaluation in patients with suspected acute myocardial infarction and nonobstructive coronary arteries involves careful consideration of the clinical context and the exclusion of clinically overt causes for a myocardial injury.5 After this step, the clinician should exclude potentially overlooked obstructed coronary arteries by re-reviewing the angiogram and consideration of additional invasive testing. Because it can exclude myocarditis, Takotsubo syndrome, and cardiomyopathies, as well as provide imaging confirmation of myocardial infarction, cMRI is recommended in the evaluation of MINOCA patients.3,5,29 Other investigations might include assessment of coronary flow reserve by noninvasive and/or invasive testing, invasive provocative spasm testing, and intracoronary imaging.3
It is also important to understand that in many patients the acute rise in cardiomyocyte injury markers may be missed because the patient had only mild and or nonspecific symptoms and did not seek acute care. Yet, later during evaluation for other reasons, evidence of myocardial scarring is found on cardiac imaging. If the scar is subendocardial or in the distribution of a major epicardial branch the diagnosis of ischemic heart disease is made. However, scars related to microvascular disease may not follow such distributions. Thus, in. the chronic situation, MINOCA is difficult to adequately evaluate.
Cardiac biomarkers.
The limit of detection of cardiac troponin T and I assays has historically been above the 99th percentile upper limit and typically undetectable in the healthy population. With the new highly sensitive cardiac troponin T and I assays, the limit of detection is now lower than the 99th percentile upper limit.30 Even more recently, the latest generation of the cardiac troponin assay, the ultra-high–sensitivity troponin, has the ability to detect even lower concentrations of troponin.31
Intracoronary imaging.
Two-dimensional angiography is oftentimes limited in assessing the atherosclerotic burden of coronary arteries, and this is especially true for patients with MINOCA. Studies have shown that intravascular ultrasound and optical coherence tomography identified atherosclerosis or plaque rupture in a significant proportion of these patients.32–37
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging identifies the underlying cause in as many as 87% of patients with MINOCA, whether late gadolinium enhancement or myocardial edema occurs in regional patterns, consistent with infarction or ischemic injury or in a pattern consistent with myocarditis.3,30,37,38
INOCA/ANOCA
INOCA/ANOCA patients have a wide array of clinical presentations, varying from typical anginal symptoms to atypical and nonspecific symptoms, including indigestion, nausea, vomiting, weakness, and fatigue.1,39
Non-invasive testing.
Noninvasive diagnostic work-up for INOCA/ANOCA include tests evaluating for ischemia and anatomy, with electrocardiography, coronary computed tomography (CTA), stress imaging modalities, and echocardiography. Stress imaging modalities include stress nuclear studies and stress echocardiography among others.1
Coronary CTA has been increasing utilized to assess patients in the low-intermediate risk zone presenting with chest pain.40 Moreover, in the ISCHEMIA trial, most enrolled patients had coronary CTA to exclude left main disease and non-obstructive coronary arteries. This led to less invasive testing of patients with possible microvascular dysfunction and underdiagnosis of this entity.41
Coronary flow reserve is a surrogate for microvascular dysfunction and can be calculated using positron emission tomography imaging as the ratio of hyperemic blood flow in response to various vasoactive stimuli divided by resting blood flow.1 The maximal hyperemia is usually achieved through intravenous administration of vasodilators such as adenosine or regadenoson.1
Echocardiography can help assess coronary flow velocity by pulsed wave Doppler of the left anterior descending coronary artery at rest and after dipyridamole or adenosine, although this is not a common test in clinical practice in the USA.9
Invasive testing.
Measurement of coronary vascular function includes measurements of coronary blood flow and coronary artery diameter with endothelium-dependent probes, e.g., acetylcholine, and predominantly endothelium-independent probes, e.g., adenosine.1,9 While non-endothelial dependent dysfunction may be assessed by non-invasive tests, acetylcholine can only be administered during invasive testing. Thus, a complete diagnostic assessment for INOCA/ANOCA usually requires invasive angiography.1,39 Although some have suggested cold pressor stress testing with noninvasive imaging (PET or cMRI), we have not found this useful for evaluating endothelial function.
Comprehensive invasive testing for INOCA/ANOCA is consistent with the diagnostic protocol used in the CorMicA project (NCT 0319294), a landmark trial in microvascular dysfunction assessment in INOCA patients, It showed that complete assessment including fractional flow reserve, flow reserve, microvascular resistance, and acetylcholine vasoreactivity testing with stratified medical therapy improves angina scores over 6 months in INOCA patients.39 The investigators later documented sustained angina improvement and better quality of life at 1 year. In addition, the 2019 ESC guidelines provide a class IIa recommendation for guidewire-based measurement of flow reserve and/or microcirculatory resistance in patients with persistent symptoms, despite normal coronary arteries or nonobstructive disease on angiography.41 Intracoronary acetylcholine testing is supported by class IIb recommendation to assess coronary microvascular spasm and vasospastic angina, as well as class IIa recommendation to clarify endothelium-dependent and endothelium-independent mechanisms of microvascular dysfunction.42
Others: 31-Phosphorus Magnetic Resonance Spectroscopy (31P-MRS).
Cardiac spectroscopy is perhaps one of the better methods to evaluate such patients, as we first documented in the WISE project.56,57 as it can identify viable, stunned or hibernating myocardium, in which adenosine triphosphate levels remain normal, from nonviable and scarred myocardium where adenosine triphosphate is reduced or completely absent.43
MANAGEMENT
MINOCA
Although data are limited on the optimal management approach for patients with MINOCA, the AHA recommends a clinical approach, which includes the following: (1) emergency supportive care; (2) a working diagnostic approach; (3) cardioprotective therapies irrespective of the underlying cause of MINOCA; and (4) cause-targeted therapies (Figure 3A).5
Figure 3:
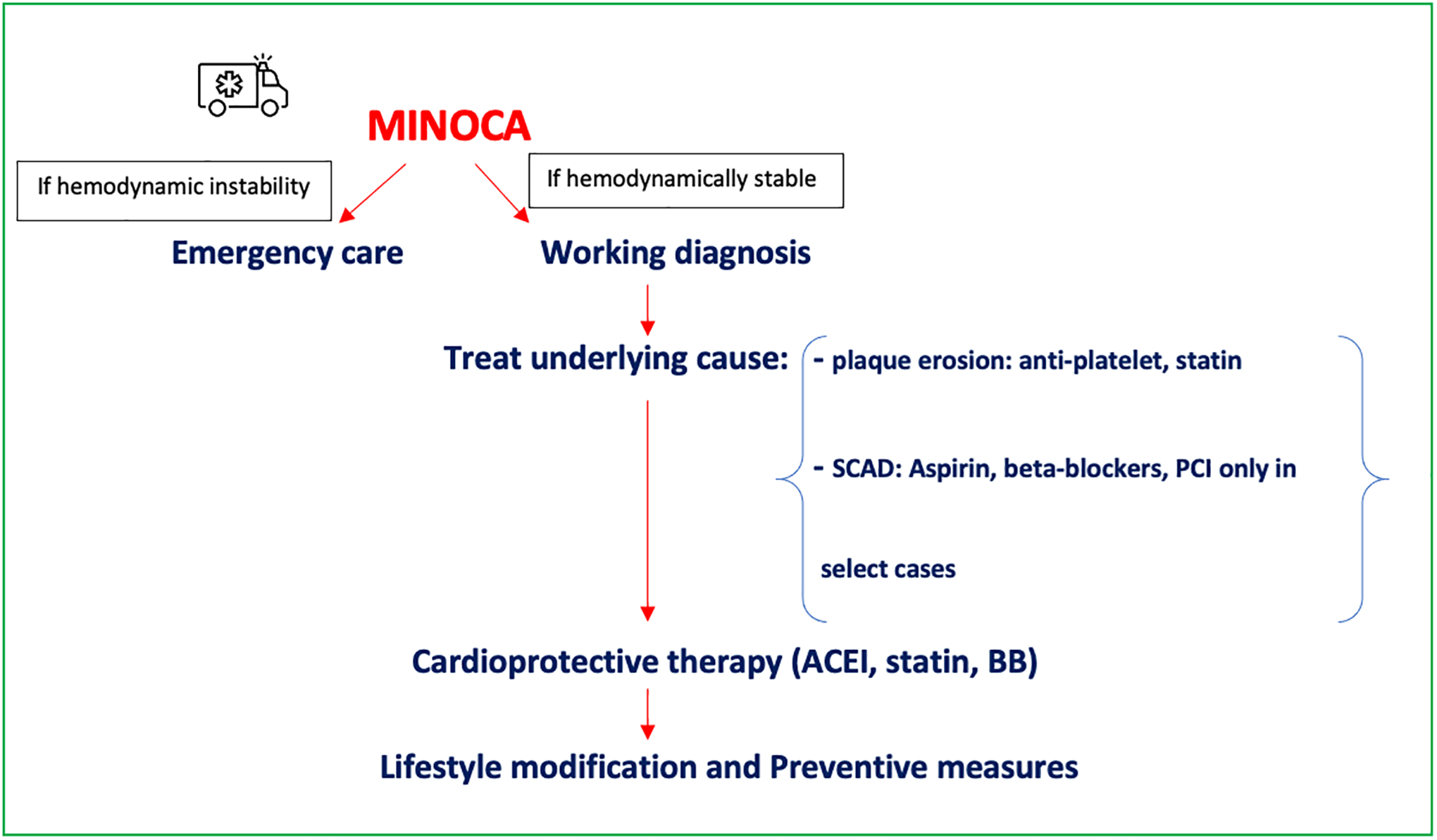

(A) Flow diagram summarizing the management algorithm for MINOCA patients; (B) Flow diagram summarizing the management algorithm for INOCA/ANOCA patients. ACEI = angiotensin-converting enzyme inhibitor; ANOCA = angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries; BB = beta blocker; CCB = calcium channel blocker; CMD = coronary microvascular dysfunction; INOCA = ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries; MINOCA = myocardial infarction with nonobstructive coronary arteries.
Cardioprotective Medications in MINOCA.
Studies have shown that angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) medications have a potential benefit in MINOCA.1,5,44–46 Statin use not only lowers cholesterol levels, but also has anti-inflammatory properties, making it particularly useful in MINOCA. In an analysis of the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy (SWEDEHEART) Registry including 9466 patients with MINOCA, statins (hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.68–0.87) and ACEI/ARBs (HR 0.82; 95% CI 0.73–0.93) were associated with lower major adverse cardiac events. There was a trend toward lower events with beta-blocker therapy (HR 0.86; 95% CI 0.74–1.01).45
INOCA/ANOCA
Management should be patient-centered with a multidisciplinary care approach focusing on lifestyle modification and aggressive risk factor control, including hypertension, diabetes, and hyperlipidemia management, smoking cessation, and exercise (Figure 3B).1 Once the diagnosis is confirmed, treatment of the underlying cause should be pursued.1 The CorMicA trial showed that stratified therapy based on the type of microvascular dysfunction led to improvement in angina and quality of life; patients with coronary vasospasm showed benefit from vasodilator therapy with calcium channel blockers,1,39,47 while patients with microvascular angina experienced improvement in their symptoms with beta-blockers and consideration of ACEI/statin therapy.1,37,48–50 In patients with diabetes mellitus, the addition of spironolactone has been shown to improve coronary microvascular function.50
Similar to MINOCA, the benefit from statin not only comes from its cholesterol-lowering effect, but also from the anti-inflammatory properties it provides.1,47,48–50 These findings are to be confirmed by the ongoing Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD (WARRIOR), which is designed to assess the benefit of statin and ACEI/ARB therapy on major adverse cardiovascular events in symptomatic women with INOCA.51
PROGNOSIS
MINOCA
In the Variation in Recovery: Role of Gender on Outcomes of Young myocardial infarction Patients (VIRGO) study, patients with MINOCA had similar mortality at 1 month and 1 year and similar quality-of-life measures compared with patients with myocardial infarction due to obstructive disease.5,12 The Korean Infarct Registry showed that MINOCA patients had a similar risk of major adverse events as myocardial infarction due to obstructive disease in single- or double-vessel angiographic disease.5,52
INOCA/ANOCA
Studies have shown that INOCA patients have relatively higher cardiac events compared with the general population.52,53 Women with INOCA had significant symptom burden with major adverse cardiac events (death, nonfatal myocardial infarction, nonfatal stroke, and heart failure hospitalization) of more than 2.5% yearly by 5 years, as well as elevated readmission and repeat angiography rates.9,53
KNOWLEDGE GAPS AND ONGOING TRIALS
As knowledge gaps exist with regard to pathophysiology and potential disease mechanisms, interactions of different risk factors, utility of the diagnostic tools and algorithms, and optimal management options with their impact on prognosis of these syndromes, ongoing trials are set to investigate some of these unanswered questions (Table 2). The Randomized Evaluation of β‐Blocker and ACEI/ARB Treatment in MINOCA Patients (MINOCA BAT; ClinicalTrials.gov Identifier: NCT03686696) study will randomize 3500 patients with MINOCA to treatment with ACEI or ARB and beta‐blockers or placebo. The primary end point of the study is time to mortality of any cause or readmission due to myocardial infarction, ischemic stroke, or heart failure.46 The WARRIOR trial (NCT03417388) is a multi-center, randomized, blinded outcome trial to evaluate the benefit of statin and ACEI/ARB therapy on major adverse cardiovascular events in symptomatic women with INOCA.51 The CorCTCA (NCT03477890) is an ongoing trial aiming to clarify the prevalence and outcomes of INOCA when standard care is based on coronary computed tomography angiography.54 The Precision Medicine With Zibotentan in Microvascular Angina (PRIZE) trial (ClinicalTrials.gov Identifier: NCT04097314) is assessing the benefit of an oral endothelin A receptor antagonist in patients with microvascular dysfunction.55
Table 2.
Summary of ongoing trials in the field of non-obstructive coronary artery syndromes.
| Trial name | Brief description |
|---|---|
| MINOCA-BAT (NCT03686696) | The study will randomize 3500 patients with MINOCA to treatment with ACEI or ARB and beta‐blockers or matching placebo. The primary end point of the study is time to mortality of any cause or readmission due to myocardial infarction, ischemic stroke, or heart failure. |
| WARRIOR (NCT03417388) | This study will evaluate the benefit of intensive statin and ACEI/ARB therapy on major adverse cardiovascular events in symptomatic women with INOCA. |
| CorCTCA trial (NCT03477890) | The study will clarify the prevalence and clinical significance of INOCA when standard care is based on coronary computed tomography angiography |
| PRIZE (NCT04097314) | The study will assess the benefit of Zibotentan, an oral endothelin A receptor antagonist, which may help counteract the hypersensitivity to vasoconstriction seen in coronary microvascular dysfunction. |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CorCTCA = Coronary Microvascular Function and CT Coronary Angiogram; INOCA = ischemia without elevated biomarkers of cardiomyocyte injury in patients with nonobstructive coronary arteries; MINOCA = myocardial infarction with nonobstructive coronary arteries; MINOCA-BAT = Randomized Evaluation of Beta Blocker and ACEI/ARB Treatment in MINOCA patients; PRIZE = Precision Medicine With Zibotentan in Microvascular Angina; WARRIOR = Women’s IschemiA TRial to Reduce Events In Non-ObstRuctive CAD.
CONCLUSION
Ischemic syndromes associated with nonobstructive epicardial coronary arteries have been increasingly recognized as a clinical entity, and they have heterogenous clinical presentations. Knowledge of the appropriate evaluation is key in the diagnosis of these clinical entities. Importantly, medical stratification based on the type of condition has been shown to be beneficial in improving angina scores in these patients and should be pursued whenever possible. As evidence-based therapies for these syndromes exist, careful attention to diagnosis is pivotal. Finally, it is important to understand that there are many knowledge gaps in the “open artery ischemia” story.
Clinical Significance.
Syndromes of nonobstructive coronary arteries represent a heterogenous clinical entity.
Myocardial infarction with nonobstructive coronary arteries (MINOCA) patients presents acutely, while ischemia with nonobstructive coronary arteries (INOCA)/angina pectoris highly suspect for but without ischemia or biomarker positivity in nonobstructive coronary arteries (ANOCA) patients have more indolent patients.
Intracoronary imaging and cardiac magnetic resonance imaging are useful diagnostic tools to evaluate MINOCA patients.
Coronary flow reserve on positron emission tomography and additional invasive testing are useful to evaluate INOCA/ANOCA patients.
Treating the underlying pathology and risk factor control is key; while cardioprotective medications are potentially beneficial in certain patients.
Funding:
Dr. Pepine receives funding from grants R01-HL146158 (WISE HFpEF) from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute; U54-AG065141 (MAE-WEST) from the NIH/National Institute on Aging; and W811XWH-17-2-0300 (WARRIOR) from the US Department of Defense.
Footnotes
Disclosures: Authors have nothing to disclose
References
- 1.Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021;16:1049–69. doi: 10.4244/EIJY20M07_01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J 2006;27(12):1408–15. doi: 10.1093/eurheartj/ehl040 [DOI] [PubMed] [Google Scholar]
- 3.Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation 2017;135(16):1490–3. doi: 10.1161/CIRCULATIONAHA.117.027666 [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee D Myocardial infarction with nonobstructive coronary arteries: a call for individualized treatment. J Am Heart Assoc 2019;8(14):e013361. doi: 10.1161/JAHA.119.013361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamis‐Holland JE, Jneid H, Reynolds HR, et al. ; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019; 139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 6.Gross H, Steinberg WH. Myocardial infarction without significant lesions of coronary arteries. Arch Int Med (Chic) 1939;64:2. [Google Scholar]
- 7.Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry‐GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry‐Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 8.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries [published correction appears in Circulation 2015;131:e475]. Circulation 2015;131:861–70. doi: 10.1161/CIRCULATIONAH [DOI] [PubMed] [Google Scholar]
- 9.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135(11):1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bairey Merz CN, Shaw LJ, Reis SE, et al. ; WISE Investigators. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study, part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47(Suppl):S21–S29. doi: 10.1016/j.jacc.2004.12.084 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary R, Sukhi A, Chaudhary R, et al. Gender differences in thrombogenicity among patients with angina and non-obstructive coronary artery disease. J Thromb Thrombolysis 2019;48(3):373–81. doi: 10.1007/s11239-019-01901-1. [DOI] [PubMed] [Google Scholar]
- 12.Safdar B, Spatz ES, Dreyer RP, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7:e009174. doi: 10.1161/JAHA.118.009174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeh J, Therming CB, Heitmann M, et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J 2019;40(18):1426–35. doi: 10.1093/eurheartj/ehy806 [DOI] [PubMed] [Google Scholar]
- 14.Wessel TR, Arant CB, McGorray SP, et al. ; NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin Cardiol 2007;30:69–74. doi: 10.1002/clc.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra L, Kowlgi NG. Low incidence of diabetes mellitus in coronary microvascular dysfunction: an intriguing association. JACC Cardiovasc Interv 2016;9(4):395–6. doi: 10.1016/j.jcin.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 16.Hjort M, Lindahl B, Baron T, et al. Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am Heart J 2018;200:60–6. doi: 10.1016/j.ahj.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 17.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging 2013;6:660–7. doi: 10.1016/j.jcmg.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’ Eur Heart J 2015;36:2984–7. [DOI] [PubMed] [Google Scholar]
- 19.Hayes SN, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection: JACC State-of-the-Art review. J Am Coll Cardiol 2020;76(8):961–84. doi: 10.1016/j.jacc.2020.05.084 [DOI] [PubMed] [Google Scholar]
- 20.Tomaiuolo R, Bellia C, Caruso A, et al. Prothrombotic gene variants as risk factors of acute myocardial infarction in young women. J Transl Med 2012;10:235. doi: 10.1186/1479-5876-10-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Kaikita K, Nakayama N, et al. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc 2013;2(4):e000227. doi: 10.1161/JAHA.113.000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobuyoshi M, Abe M, Nosaka H, et al. Statistical analysis of clinical risk factors for coronary artery spasm: identification of the most important determinant. Am Heart J 1992;124(1):32–8. doi: 10.1016/0002-8703(92)90917-k [DOI] [PubMed] [Google Scholar]
- 23.Rahman H, Demir OM, Khan F, et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol 2020;75(20):2538–49. doi: 10.1016/j.jacc.2020.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakr SA, Abbas TM, Amer MZ, et al. Microvascular angina: the possible role of inflammation, uric acid, and endothelial dysfunction. Int Heart J 2009;50:407–41. [DOI] [PubMed] [Google Scholar]
- 25.Schroder J, Mygind ND, Frestad D, et al. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int J Cardiol Heart Vasc 2019;24:100370. doi: 10.1016/j.ijcha.2019.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford TJ, Rocchiccioli P, Good R, et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39(46):4086–97. doi: 10.1093/eurheartj/ehy529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 2009;40(5):e322–30. doi: 10.1161/STROKEAHA.108.542266 [DOI] [PubMed] [Google Scholar]
- 28.Morris PD, Gosling R, Zwierzak I, et al. A novel method for measuring absolute coronary blood flow & microvascular resistance in patients with ischaemic heart disease. Cardiovasc Res 2020. Jul 14:cvaa220. doi: 10.1093/cvr/cvaa220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021;143(7):624–40. doi: 10.1161/CIRCULATIONAHA.120.052008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatia PM, Daniels LB. Highly sensitive cardiac troponins: the evidence behind sex-specific cutoffs. J Am Heart Assoc 2020;9(10):e015272. doi: 10.1161/JAHA.119.015272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AlBadri A, Wei J, Quesada O, et al. Coronary vascular function and cardiomyocyte injury: a report from the WISE-CVD. Arterioscler Thromb Vasc Biol 2020;40(12):3015–21. doi: 10.1161/ATVBAHA.120.314260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scalone G, Niccoli G, Crea F. Editor’s choice- Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care 2019;8(1):54–62. doi: 10.1177/2048872618782414 [DOI] [PubMed] [Google Scholar]
- 35.Gerbaud E, Arabucki F, Nivet H, et al. OCT and CMR for the diagnosis of patients presenting with MINOCA and suspected epicardial causes. JACC Cardiovasc Imaging 2020;13(12):2619–31. doi: 10.1016/j.jcmg.2020.05.045 [DOI] [PubMed] [Google Scholar]
- 36.Opolski MP, Spiewak M, Marczak M, et al. Mechanisms of myocardial infarction in patients with nonobstructive coronary artery disease: results from the Optical Coherence Tomography Study. JACC Cardiovasc Imaging 2019;12(11 Pt 1):2210–21. doi: 10.1016/j.jcmg.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 37.Pustjens TFS, Appelman Y, Damman P, et al. Guidelines for the management of myocardial infarction/injury with non-obstructive coronary arteries (MINOCA): a position paper from the Dutch ACS working group. Neth Heart J 2020;28:116–30. doi: 10.1007/s12471-019-01344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tornvall P, Gerbaud E, Behaghel A, et al. Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: a meta-analysis of individual patient data. Atherosclerosis 2015;241:87–91. doi: 10.1016/j.atherosclerosis.2015.04.816 [DOI] [PubMed] [Google Scholar]
- 39.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. J Am Coll Cardiol 2018;72(23 Pt A):2841–55. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Herscovici R, Sedlak T, Wei J, et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA): What is the risk?. J Am Heart Assoc 2018;7(17):e008868. doi: 10.1161/JAHA.118.008868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maron DJ, Hochman JS, Reynolds HR, et al. ; ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382(15):1395–1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knuuti J, Wijns W, Saraste A, et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41(3):407–77. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 43.Hudsmith LE, Neubauer S. Magnetic resonance spectroscopy in myocardial disease. JACC Cardiovasc Imaging 2009;2(1):87–96. doi: 10.1016/j.jcmg.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 44.Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long‐term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017; 135:1481–9. [DOI] [PubMed] [Google Scholar]
- 45.Choo EH, Chang K, Lee KY, et al. ; KAMIR‐NIH Investigators. Prognosis and predictors of mortality in patients suffering myocardial infarction with non‐obstructive coronary arteries. J Am Heart Assoc 2019;8:e011990. doi: 10.1161/JAHA.119.011990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordenskjöld AM, Agewall S, Atar D, et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): rationale and design. Am Heart J 2021;231:96–104. doi: 10.1016/j.ahj.2020.10.059 [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Li Q, Zhao J, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis 2014;25:40–4. doi: 10.1097/MCA.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 48.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X: role of superoxide dismutase activity. Circulation 2004;109:53–8. doi: 10.1161/01.CIR.0000100722.34034.E4 [DOI] [PubMed] [Google Scholar]
- 50.Garg R, Rao AD, Baimas-George M, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–42. doi: 10.2337/db14-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116(4):856–870. doi: 10.1093/cvr/cvaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang WY, Jeong MH, Ahn YK, et al. ; Korea Acute Myocardial Infarction Registry Investigators. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol 2011;146:207–12. doi: 10.1016/j.ijcard.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 53.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009; 169:843–50. doi: 10.1001/archinternmed.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidik NP, McEntegart M, Roditi G, et al. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study. Am Heart J 2020;221:48–59. doi: 10.1016/j.ahj.2019.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow AJ, Ford TJ, Mangion K, et al. Rationale and design of the Medical Research Council’s Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J 2020;229:70–80. doi: 10.1016/j.ahj.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342(12):829–35. doi: 10.1056/NEJM200003233421201 [DOI] [PubMed] [Google Scholar]
- 57.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109(24):2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2 [DOI] [PubMed] [Google Scholar]


