Abstract
Objective
The objective of this study was to validate the diagnosis of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) as the primary cause of end‐stage renal disease (ESRD) in the US Renal Data System (USRDS).
Methods
We identified patients with ESRD in the Mass General Brigham (MGB) health care system who were enrolled in the USRDS. The health records of those with AAV listed as the primary cause of ESRD in the USRDS were reviewed to confirm the diagnosis and estimate positive predictive value (PPV). Sensitivity was estimated by evaluating the primary cause of ESRD listed in the USRDS for patients with ESRD due to AAV in the MGB AAV cohort.
Results
We identified 89 MGB patients with ESRD due to AAV in the USRDS. Of these, 85 cases were confirmed to be true cases of AAV (PPV = 94%). Among the patients classified as having AAV, 84 (99%) had an ANCA test, which was predominantly myeloperoxidase/P‐ANCA (47 [55%]); 36 (42%) had a renal biopsy, and all biopsies were supportive of the diagnosis. The majority (81 [90%]) was identified as AAV by International Classification of Diseases Ninth Revision or International Classification of Diseases 10th Revision codes for granulomatosis with polyangiitis (446.4 or M313.1). Of the 77 MGB AAV cohort patients with ESRD who were linked to the USRDS, 41 (53%) had AAV listed as the cause of ESRD; in the remainder, ESRD was attributed to nonspecific nephritis.
Conclusion
The diagnosis of AAV as the cause of ESRD in the USRDS has a high PPV; sensitivity was moderate. These findings support the continued use of the USRDS to study ESRD due to AAV.
Significance & Innovations.
The US Renal Data System (USRDS) is a valuable data source for evaluating end‐stage renal disease (ESRD) outcomes, but the validity of antineutrophil cytoplasmic antibody–associated vasculitis (AAV) as the cause of ESRD is unknown.
AAV as the primary cause of ESRD in the USRDS has a high positive predictive value (94%) and moderate sensitivity (53%).
The use of the USRDS to study ESRD attributable to AAV is valid for past and future research.
INTRODUCTION
Glomerulonephritis and other renal manifestations are common in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV). Renal involvement in AAV is associated with adverse outcomes, including end‐stage renal disease (ESRD), in up to 25% of patients (1). Patients with ESRD due to AAV represent a unique population of patients with AAV whose management (eg, transplantation), comorbidity burden (eg, cardiovascular disease), and survival differs from that of patients with AAV without ESRD (2, 3). Although observational cohort studies and clinical trials have expanded our understanding of AAV outcomes, relatively small proportions of patients with ESRD are often included in such studies, limiting our ability to conduct health outcomes research in patients with ESRD attributable to AAV.
The US Renal Data System (USRDS), a national registry of patients with ESRD linked to the United Network for Organ Sharing, Medicare claims data, and other data sources, represents a unique nationwide data source for studying patients with AAV and ESRD. Indeed, the USRDS has been previously used to study patients with ESRD attributable to AAV, but the validity of AAV as the cause of ESRD in the USRDS is unknown (4, 5). To enable future studies of patients with AAV and ESRD using the USRDS, we sought to determine the positive predictive value (PPV) and sensitivity of AAV as the cause of ESRD in the USRDS using patient electronic health record data from the Mass General Brigham (MGB) health care system linked to the USRDS.
PATIENTS AND METHODS
Data source and study population
The USRDS is a national registry of patients with ESRD representing an estimated 94% of patients who receive dialysis or kidney transplantation. Patients who refuse replacement therapy, die prior to enrollment, or receive transient dialysis for acute renal failure may not be enrolled. Nephrologists are required by law to submit a Medical Evidence Report, which includes the cause of ESRD according to International Classification of Diseases Ninth Revision (ICD‐9) or International Classification of Diseases 10th Revision (ICD‐10) codes, within 45 days of a patient starting a new ESRD treatment. Further information may be found in the USRDS Annual Data Report (6).
We identified all patients in the MGB health care system, a large multicenter health care system in the greater Boston, MA, area, with an ICD‐9 or ICD‐10 code for either advanced chronic kidney disease or ESRD or a procedure code for dialysis or renal transplantation (Supplementary Table 1). We then linked all MGB patients fulfilling these criteria to their records in the USRDS, if possible, by name, sex, date of birth, and Social Security number. From this cohort of patients, we identified those with AAV or related diagnoses listed as the primary disease causing ESRD in the USRDS (ICD‐9: 446.0, 446.4; ICD‐10: M31.3X, M31.7). We included the ICD‐9 code for polyarteritis nodosa (446.0) because there is no specific ICD‐9 code for microscopic polyangiitis, and the polyarteritis nodosa code might have been used by some providers. Additionally, we linked patients with ESRD attributable to AAV in the MGB AAV cohort, a consecutive inception cohort of confirmed AAV cases in the MGB system (7, 8, 9), to their USRDS records, if possible, using the same methods.
Analysis
Two authors reviewed medical records to confirm whether a patient with ESRD attributable to AAV as the primary cause had a physician‐confirmed diagnosis of AAV. For each confirmed case, we extracted details of the patient's AAV history, including initial AAV manifestations and diagnosis date, renal and nonrenal biopsy results, and ANCA test results. Discrepancies were resolved through consensus between the two reviewers (this includes the expert reviewer). Details regarding initial ESRD onset date and initial ESRD treatment were obtained from the USRDS. Each AAV case was classified as granulomatosis with polyangiitis (GPA) or as microscopic polyangiitis (MPA) according to the methodology developed by Watts et al (10).
To estimate the PPV for AAV as the primary cause of ESRD, a physician diagnosis of AAV in the medical record by a nephrologist or rheumatologist, along with agreement by an author who was a board‐certified rheumatologist and vasculitis expert (ZSW), was used as the gold standard. Additionally, all patients fulfilled the criteria previously established by Watts et al (10) for use in epidemiologic studies of AAV. To confirm the diagnosis of AAV, the medical history, ANCA test results, and biopsy findings (as available) were reviewed. To estimate sensitivity, we identified patients with ESRD attributable to AAV in the MGB AAV cohort and evaluated the proportion in the USRDS who had ESRD attributable to AAV. A diagnosis code of AAV as the cause of ESRD was considered a true‐positive, and a diagnosis code for any other type of nephritis was considered a false‐negative. We excluded patients with AAV who had ESRD attributable to other causes (eg, diabetes mellitus, hypertension) from determination of sensitivity.
The USRDS does not permit reporting of any cell sizes with less than 11 individuals to maintain privacy and confidentiality. Owing to this limitation and the small number of non‐AAV cases identified, we do not report the results of the medical record review for cases not classified as AAV.
Data use
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data is the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
RESULTS
Of 8167 MGB patients identified in the USRDS, 90 had AAV listed as the primary cause of ESRD (Figure 1). Of these, 85 cases were confirmed to be true cases of AAV causing ESRD after medical record review (PPV: 94%, 95% confidence interval [CI]: 84%‐96%) (Table 1). The majority of cases (81 [90.0%]) were identified as AAV by ICD‐9 or ICD‐10 codes for GPA (446.4 or M313.1, respectively). Most cases were classified as GPA, as opposed to MPA (68.2% and 30.6%, respectively). The median (IQR) time to ESRD onset after AAV diagnosis was 1 (0‐6) year. In 42 (49.4%) patients, ESRD onset was greater than 1 year after the first AAV treatment initiation. A clinical course was available for review in 35 patients with ESRD onset more than 1 year after treatment, and 22 (63%) were found to have reached ESRD in the absence of active AAV but without an alternative etiology identified.
Figure 1.
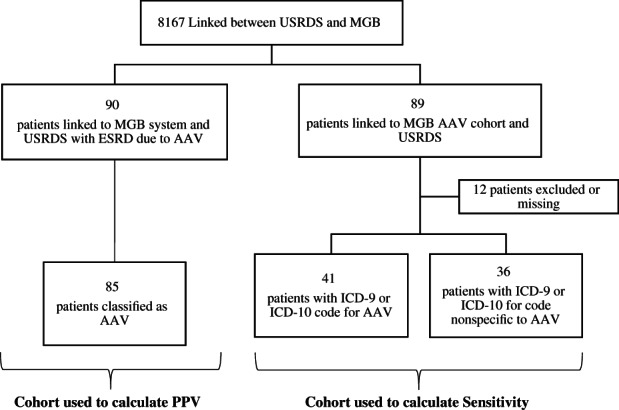
Validation of ESRD due to AAV in the USRDS inclusion and exclusion chart. AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ESRD, end‐stage renal disease; ICD‐9, International Classification of Diseases, Ninth Revision; ICD‐10, International Classification of Diseases, 10th Revision; MGB, Mass General Brigham; PPV, positive predictive value; USRDS, US Renal Data System.
Table 1.
Cases of ESRD attributable to AAV identified in the USRDS
| Physician‐diagnosed AAV (n = 85) | |
|---|---|
| Age, mean (SD), y | 61.3 (17.3) |
| Male sex, n (%) | 47 (55.3) |
| Race, n (%) | |
| White | 81 (95.3) |
| ANCA type, n (%) | 84 (98.8) |
| MPO/P‐ANCA+ | 47 (55.3) |
| PR3/C‐ANCA+ | 33 (38.8) |
| Clinical phenotype, n (%) | |
| MPA | 26 (30.6) |
| GPA | 58 (68.2) |
| Renal limited | 18 (21.2) |
| Renal biopsy, n (%) | 36 (42.4) |
| Pauci‐immune glomerulonephritis | 16 (44) |
| Years from AAV diagnosis to ESRD, median (IQR) | 1 (0‐6) |
| Principal diagnosis code (ICD‐9/ICD‐10), n (%) | |
| GPA (446.4 or M31.31) | 81 (95.3) |
Abbreviations: AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; C‐ANCA, diffuse cytoplasmic ANCA; ESRD, end‐stage renal disease; GPA, granulomatosis with polyangiitis; ICD‐9, International Classification of Diseases, Ninth Revision; ICD‐10, International Classification of Diseases, 10th Revision; IQR, interquartile range; MPA, microscopic polyangiitis; MPO, myeloperoxidase; P‐ANCA, perinuclear ANCA; PR3, peroxidase 3; USRDS, US Renal Data System.
Among the patients classified as having AAV, 84 (99%) had an ANCA test; the majority of results were myeloperoxidase (MPO)/P‐ANCA+ (47 [55%]). In 18 (21.2%) cases, glomerulonephritis was the only manifestation of AAV, but the clinical presentation and biopsy results, when obtained, supported the diagnosis of AAV. A minority (36 [42%]) of the 89 identified patients had a renal biopsy, but all biopsies were supportive of the diagnosis and did not suggest an alternative cause of renal disease. Pauci‐immune glomerulonephritis was reported in 18 (50%) cases in which a renal biopsy report was available for review. The remainder of the reports described crescentic or necrotizing glomerulonephritis, globally sclerotic glomeruli, and interstitial nephritis. Some reports were unavailable, but clinician notes reported that the findings were associated with AAV. Biopsies of nonrenal tissue (eg, pulmonary, skin), when available, supported a diagnosis of AAV and did not suggest an alternative explanation for the patient's presentation. Of the 85 patients with ESRD attributable to AAV, 22 (25.9%) ultimately underwent a renal transplant.
From the MGB AAV cohort (N = 692 at time of linkage) linked to the USRDS, 89 patients were identified in the USRDS. Among the 77 patients with ESRD attributable to AAV in the cohort, 41 (sensitivity = 53%, 95% CI = 41%‐65%) had an ICD‐9 or ICD‐10 code for AAV listed as the cause of ESRD; in the remainder, ESRD was attributed to nonspecific nephritis codes, or a code was missing. Non‐AAV causes of ESRD observed in the MGB AAV cohort linked to the USRDS included diabetes with renal manifestations and hypertensive chronic kidney disease.
DISCUSSION
The diagnosis of AAV as the primary cause of ESRD in the USRDS has a high PPV (94%), suggesting accurate classification of ESRD due to AAV in the USRDS. Additionally, we found moderate sensitivity (53%). These findings support the past and future use of the USRDS for studying outcomes among patients with ESRD attributable to AAV.
Practically, the vast majority of cases were identified by ICD‐9 and ICD‐10 codes specific for GPA (ie, 446.4, M313.1). There were a minimal number of cases identified by the ICD‐9 code for polyarteritis nodosa (446.0) or the ICD‐10 code for MPA (M311), limiting our ability to estimate the PPV associated with these codes. Because of restrictions related to the reporting of small cell counts by the USRDS, we are unable to report the exact number of cases identified by these codes. However, we expect that M311 would perform well because it is a code specifically associated with MPA, and the codes associated with GPA had excellent PPV.
Although the majority of patients were identified by codes specific for GPA, which is most often associated with proteinase 3 (PR3)‐ANCA positivity, it is notable that nearly half of the identified patients clinically had MPA, and 55% of patients were MPO‐ANCA+. Therefore, the ICD‐9 and ICD‐10 codes for GPA are commonly used by providers to classify patients as having AAV regardless of their clinical phenotype; this likely reflects that prior to the implementation of the ICD‐10 in the United States in 2015, there was no specific ICD code for MPA. There is increasing recognition that ANCA type (ie, PR3‐ANCA vs MPO‐ANCA) rather than clinical phenotype better categorizes AAV into clinically meaningful groups, suggesting that the significance of the specific ICD code used to identify patients with AAV in the USRDS has less relevance (11). Unfortunately, ANCA type is not collected in the USRDS.
Prior research has evaluated the validity of a diagnosis of systemic lupus erythematosus (SLE) in the USRDS (12). In that study, Broder et al (12) found similarly excellent PPV (93%) and good sensitivity (79%) for the diagnosis of SLE using USRDS data linked to their institution's electronic medical record system. Additionally, a prior study by Layton et al (13) evaluated the PPV of a diagnostic code for biopsy‐proven glomerulonephritis among patients with ESRD attributable to various causes of glomerulonephritis, including AAV. The investigators found that the diagnosis of AAV in the USRDS had a PPV of 38% and sensitivity of 30% for biopsy‐proven AAV (13). Indeed, in our study, we found that only 36 (42%) patients even had a renal biopsy. Notably, the estimates reported by Layton et al (13) were derived from a limited number of cases (eight to estimate PPV and 10 to estimate sensitivity). These observations speak to the fact that in the proper clinical context, renal biopsies are often not pursued in patients with AAV. With contemporary practice in mind, the present study did not consider a renal biopsy as the sole criteria for an AAV diagnosis, relying instead on a definitive physician diagnosis of AAV, confirmed by two chart reviews and an epidemiologic definition previously developed (10), as the gold standard.
In addition to confirming the validity of the diagnosis of AAV in the USRDS, we also observed that a large portion of patients who developed ESRD after their initial AAV presentation did so in the absence of clinically appreciable AAV activity. This is similar to a previous report by Lionaki et al (14) that approximately 43% of patients develop ESRD in the absence of active vasculitis. Although active vasculitis is not thought to play a role, it is challenging to determine specific factors driving progressive renal disease in patients with AAV with established renal injury from AAV. It is likely that a number of factors contribute, including comorbid conditions such as hypertension and diabetes, but also a potential smoldering AAV disease process that is difficult to detect clinically. Additional studies are needed to understand factors driving progressive renal disease in these patients.
There are several strengths to the current study, including the unique linkage of USRDS records to those of the MGB system to estimate PPV and to the MGB AAV cohort to estimate sensitivity. By linking these data sources, we were able to complement the data routinely collected in the USRDS with provider notes, biopsy reports, and ANCA test results available in the electronic health record. Despite these strengths, there are also certain limitations. First, because we did not limit our study to certain time periods, some older cases identified in the USRDS had to be excluded because the electronic medical record lacked sufficient detail to evaluate the accuracy of an AAV diagnosis. Second, there are no well‐developed classification criteria for a diagnosis of AAV that do not require or place high weight on biopsies. The forthcoming American College of Rheumatology/European League Against Rheumatism AAV classification criteria will be a significant advancement in the field, but in the absence of published criteria, we have used a previously developed algorithm, as well as physician‐confirmed diagnosis, as the gold standard for classifying true cases of AAV. Third, although ICD‐9 and ICD‐10 codes can be used to identify patients with AAV in the USRDS with a high PPV, this approach is likely unable to distinguish AAV phenotypes, such as GPA or MPA. Fourth, we validated the diagnosis of AAV using data linked to the MGB system, which includes a vasculitis and glomerulonephritis referral center. The PPV may differ when evaluated in other systems, although we think that is unlikely because the etiology of ESRD in the USRDS is assigned by the patient's attending nephrologist.
In summary, we linked USRDS and electronic health record data to validate the diagnosis of ESRD attributable to AAV in the USRDS. The USRDS may be leveraged as a valid tool for further epidemiologic research in ESRD due to AAV.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Wallace had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Cook, Stone, Choi, Wallace.
Acquisition of data
Cook, Fu, Wallace.
Analysis and interpretation of data
Cook, Fu, Zhang, Wallace.
Supporting information
Supplementary Table 1. ICD‐9 and ‐10 and procedure codes used to identify cases of ESRD in the MGB Healthcare System
Dr. Wallace's work was supported by grants from the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants K23‐AR‐073334 and L30‐AR‐070520).
Dr. Wallace reports research support from Bristol‐Myers Squibb and Principia and consulting fees from Viela Bio and Medpace. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Moiseev S, Novikov P, Jayne D, Mukhin N. End‐stage renal disease in ANCA‐associated vasculitis. Nephrol Dial Transplant 2017;32:248–53. [DOI] [PubMed] [Google Scholar]
- 2. Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long‐term patient survival in ANCA‐associated vasculitis. Ann Rheum Dis 2011;70:488–94. [DOI] [PubMed] [Google Scholar]
- 3. Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Höglund P, et al. Revisiting the classification of clinical phenotypes of anti‐neutrophil cytoplasmic antibody‐associated vasculitis: a cluster analysis. Ann Rheum Dis 2013;72:1003–10. [DOI] [PubMed] [Google Scholar]
- 4. Wallace ZS, Wallwork R, Zhang Y, Lu N, Cortazar FB, Niles JL, et al. Improved survival with renal transplantation for end‐stage renal disease due to granulomatosis with polyangiitis: data from the United States Renal Data System. Ann Rheum Dis 2018;77:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallace ZS, Zhang Y, Lu N, Stone JH, Choi HK. Improving mortality in end‐stage renal disease due to granulomatosis with polyangiitis (Wegener's) from 1995 to 2014: data from the United States Renal Data System. Arthritis Care Res (Hoboken) 2018;70:1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United States Renal Data System . 2019 USRDS annual data report: kpidemiology of kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2019.
- 7. Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All‐cause and cause‐specific mortality in ANCA‐associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford) 2020;59:2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDermott G, Fu X, Stone JH, Wallwork R, Zhang Y, Choi HK, et al. Association of cigarette smoking with antineutrophil cytoplasmic antibody‐associated vasculitis. JAMA Intern Med. 2020;180:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Miloslavsky E, Stone JH, Choi HK, Zhou L, Wallace ZS. Topic modeling to characterize the natural history of ANCA‐associated vasculitis from clinical notes: a proof of concept study. Semin Arthritis Rheum 2021;51:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watts RA, Mahr A, Mohammad AJ, Gatenby P, Basu N, Flores‐Suarez LF. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. Nephrol Dial Transplant 2015;30 Suppl 1:i14–22. [DOI] [PubMed] [Google Scholar]
- 11. Wallace ZS, Stone JH. Personalized medicine in ANCA‐associated vasculitis ANCA specificity as the guide? Front Immunol 2019;10:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Broder A, Mowrey WB, Izmirly P, Costenbader KH. Validation of systemic lupus erythematosus diagnosis as the primary cause of renal failure in the US Renal Data System. Arthritis Care Res (Hoboken) 2017;69:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, et al. Discrepancy between medical evidence form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 2010;5:2046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior BA, Jennette CE, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum 2012;64:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. ICD‐9 and ‐10 and procedure codes used to identify cases of ESRD in the MGB Healthcare System


