Abstract
Objective:
To describe maxillary growth and maturation during infancy and early childhood.
Materials and Methods:
Serial cephalograms (N = 210) of 30 subjects (15 females and 15 males) from the Bolton-Brush Growth Study were analyzed. Each subject had a series of six consecutive cephalograms taken between birth and 5 years of age, as well as one adult cephalogram. Twelve maxillary measurements (eight linear and four angular) and seven landmarks were used to characterize maxillary growth. Maturation of the linear measures was described as a percentage of adult status.
Results:
Maxillary and anterior cranial base size increased in both sexes between 0.4 and 5 years of age. The linear anteroposterior (AP) measures (S-SE, SE-N, ANS-PNS) increased almost as much as the vertical measures (S-PNS, SE-PNS, N-A, N-ANS) over the first 5 years. After 5 years of age there was significantly more vertical than AP growth. The size and shape changes that occurred were greatest between 0.4 and 1 years; yearly velocities decelerated regularly thereafter. Overall linear growth changes that occurred between 0.5 and 5 years of age (a span of 4.5 years) were generally greater than the changes in maxillary growth that occurred between 5 and 16 years (a span of 11 years). The linear measures showed a gradient of maturation, with the AP measures being more mature than the vertical measures. Male maxillae were less mature than female maxillae at every age.
Conclusions:
The maxilla undergoes its greatest postnatal growth change during infancy and early childhood, when relative AP growth and maturation are emphasized.
Keywords: Maxilla, Infancy, Maturation, Growth, Cephalometrics
INTRODUCTION
Postnatal somatic growth is fastest and most intense during the first 5 years. Greater rates of somatic growth occur during infancy than at any other time postnatally.1 US children, for example, show marked deceleration of growth in recumbent length during the first 3 years.2 Based on the close associations between somatic and craniofacial growth and development,3–5 greater rates of craniofacial growth might also be expected during the first few postnatal years. Although limited, there is evidence of marked craniofacial growth during infancy and early childhood. The greatest amount of postnatal growth in facial depth occurs between 3 and 6 years of age.6,7 Farkas et al.8 showed that the greatest yearly growth increments in male head height and length occurred between 1 and 3 years of age. Based on large samples, Ohtsuki et al.9 reported greater cranial base growth during the first 5 years than during the remaining postnatal years, with the greatest anterior and posterior growth changes occurring during the first 2–3 postnatal years.
Understanding relative craniofacial growth is important because it provides an indirect measure of a structure's response potential. Relative growth provides an indication of a structure's growth response to growth hormone supplementation10 and alterations in masticatory function.11 Relative growth also makes it possible to directly compare structures, regardless of absolute size differences. Buschang et al.12 reported a growth maturity gradient between 4 and 16 years, with the maxilla being more mature than the mandible but less mature than the cranial base or vault. Farkas et al.8 showed that, by 1 year of age, head circumference (87.5%) and length (87.1%) were relatively more mature than other components of the craniofacial complex, approaching adult size by 5 years of age. Liu et al.13 found that corpus length was consistently the most mature measure, followed by overall length, then ramus height during the first 5 postnatal years. The relative growth of the maxilla during the first 5 years of life has not been well studied.
Longitudinal studies of maxillary growth are limited, especially during infancy and early childhood. Maxillary growth is important due to the substantial vertical dentoalveolar changes that occur14 and the potential role of the midface in coordinating the occlusal and mandibular relationship.15 Broadbent et al.16 reported that maxillary size (eg, N-ANS, S-N, ANS-PNS) increased during the first 5 years. While SNA decreased overall between 1 and 5 years, it did not change from 2 to 3 years, and it increased slightly from 4 to 5 years. A comprehensive longitudinal evaluation of maxillary growth and maturation has not previously been undertaken. The amount of maxillary growth that occurs and the sites where it is the most active during the various stages of early development remain largely unknown.
The purpose of the present study was to describe the early postnatal growth and maturation of the maxilla. To evaluate sources of variation explaining differences in maxillary growth, the effects of sex and class of occlusion were secondarily evaluated.
MATERIALS AND METHODS
Serial lateral cephalometric records of 30 normal, untreated, healthy Whites were drawn from the Bolton-Brush Growth Study.1 The sample included 15 males and 15 females, with equal numbers of Angle Class I or Class II division 1, as categorized by the Bolton study. Subjects with poor-quality cephalograms were excluded.
The subjects were chosen based on having good-quality, serial lateral cephalograms taken some time during the first year of life (0.4 ± 0.1 years), at approximately 1 year of age, and every year thereafter until approximately 5 years of age. Each subject also had to have an adult cephalogram taken at the minimum ages of 15 and 17 years for females and males, respectively. The adult female and male cephalograms were taken at 15.3 ± 0.60 and 17.2 ± 0.75 years of age, respectively.
Cephalometric Analysis
In total, 210 cephalograms were hand traced, scanned, and digitized by the primary author using Dolphin 3D Imaging 10.5 Premium Software (Dolphin Imaging, Chatsworth, Calif). The cephalograms were taken at the minimum midsagittal plane to film distance, producing average magnifications ranging from 7.4% to 8.4%.1 Differences due to magnification were not corrected in the present study.
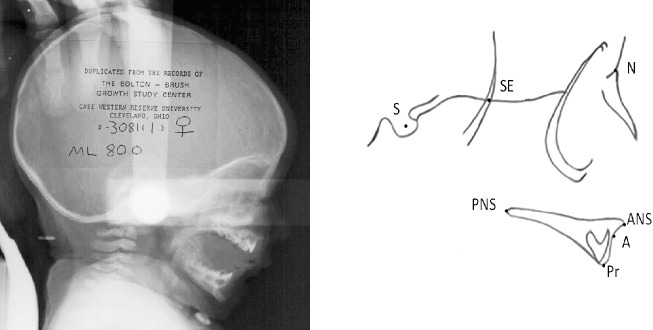
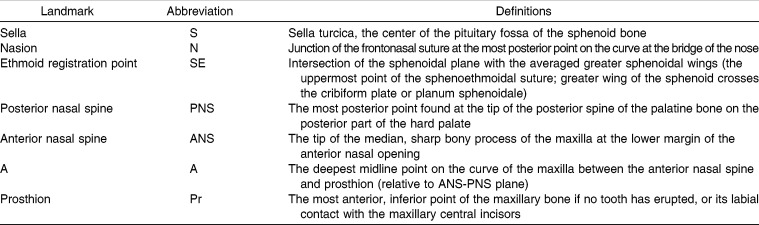
Seven landmarks were identified on the anterior cranial base and maxilla of each subject (Figure 1) using operational definitions (Table 1). Eight linear measurements were calculated to represent maxillary and cranial base growth, including presphenoid segment length (S-SE), fronto-ethmoid segment length (SE-N), posterior heights of the maxilla (SE-PNS and S-PNS), anterior heights of the maxilla (N-A and N-ANS), dentoalveolar height (ANS-Pr), and palatal plane length (ANS-PNS). Maxillary maturity during the first 5 postnatal years was calculated based on the percentage of each linear measure's adult size. Four angular measurements were calculated to describe the degree of maxillary prognathism (SNA), the direction of maxillary growth (N-S-A), the inclination of the palatal plane (SN/ANS-PNS, PPA), and the posterior position of palatal plane relative to cranial base (N-S-PNS). Reliability was enhanced by having each of the tracings checked for accuracy by one of the co-investigators.
Figure 1.
Cephalogram and cephalometric tracing along with the seven landmarks digitized.
Table 1.
Cephalometric Landmarks Along with Their Abbreviations and Definitions
Statistics
Descriptive and inferential statistics were calculated using SPSS version 18.0 (SPSS Inc., Chicago, Ill). Skewness and kurtosis statistics showed that the variables were normally distributed. Annual growth velocities were calculated by dividing the differences between measurements by the corresponding age differences. Analyses of variance were used to simultaneously evaluate sex and class effects, as well as their interactions. The relative maturity of each of the measures was calculated as the percentage of adult size.
RESULTS
Absolute Maxillary Growth Changes
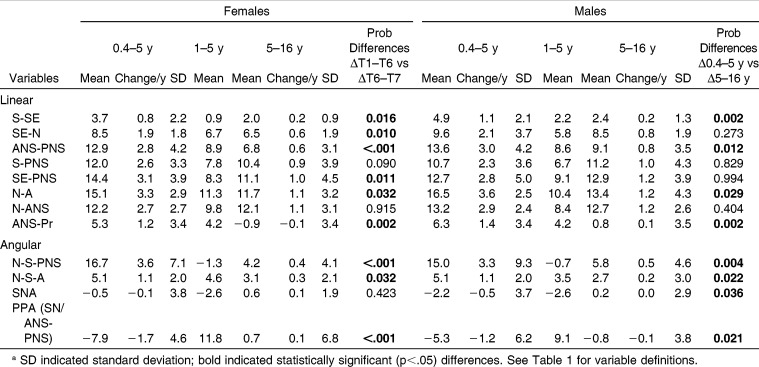
Repeated measures analyses of variance showed a statistically significant (P < .05) class difference for only one variable (N-S-PNS at 16 years), making it possible to combine the Class I and Class II subjects. There were, however, a number of statistically significant sex differences for ANS-PNS, N-A, and SE-N as well as for measures describing growth changes, including the variables SE-N and N-ANS.
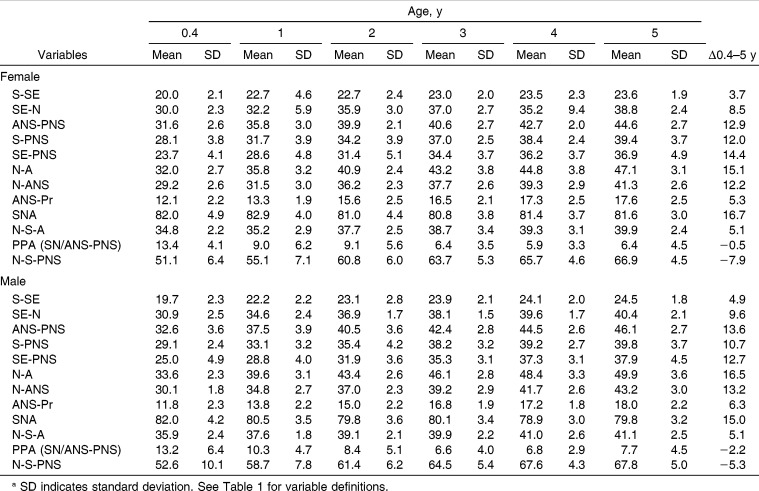
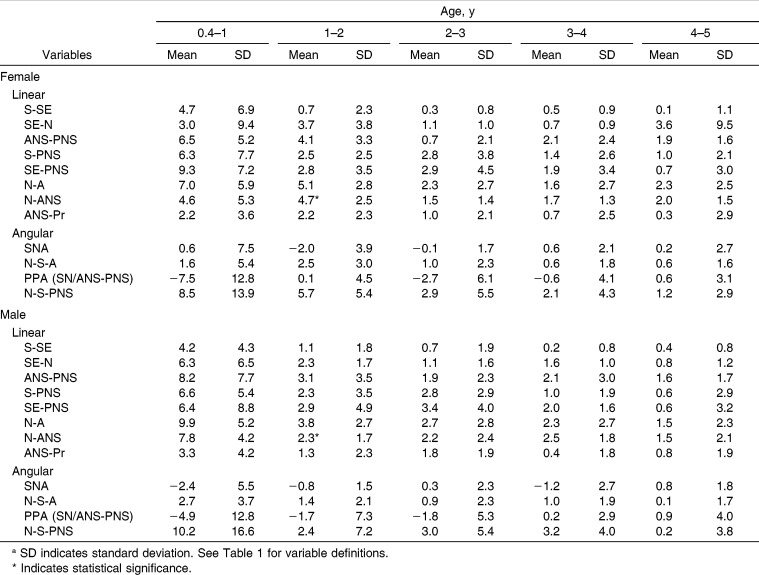
Maxillary size increased in both sexes between 0.4 and 5 years of age. The linear AP measures (S-SE, SE-N, ANS-PNS) increased almost as much as the vertical measures (S-PNS, SE-PNS, N-A, N-ANS) over the first 5 postnatal years (Table 2). The sphenoidal portion of the anterior cranial base (S-SE) and anterior dentoalveolar height (ANS-Pr) showed the smallest growth changes, while N-A showed the greatest. Males were generally larger than females, with the differences often attaining statistical significance. With the exception of S-PNS and SE-PNS, the linear measurements grew faster in males than females. The SNA and PPA angles decreased over time in both sexes. The N-S-A and N-S-PNS angles increased over time.
Table 2.
Maxillary Size (mm) and Shape (°) During Infancy and Early Childhooda
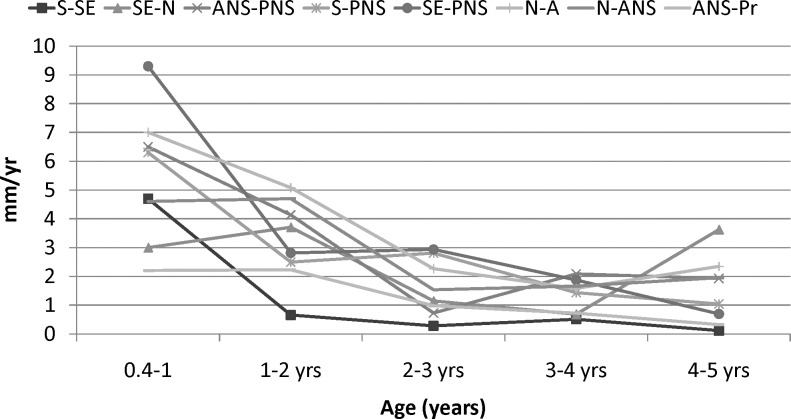
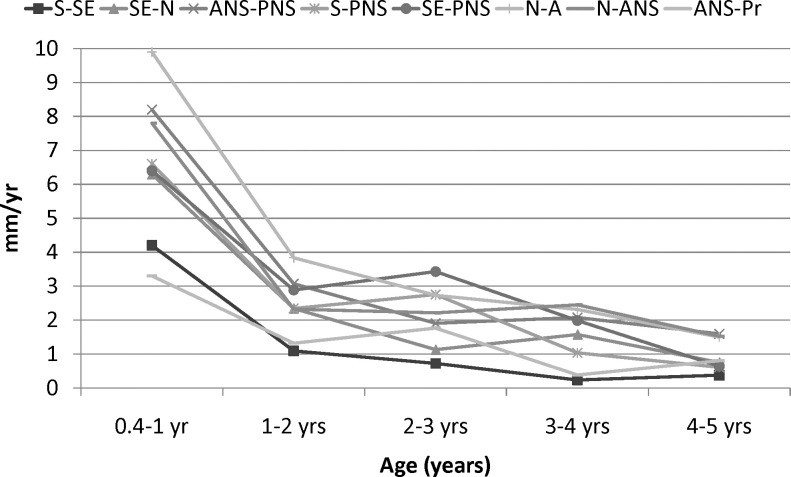
Yearly growth velocities of the linear measurements decelerated over the first 5 postnatal years (Figure 2; Table 3). They were greatest during the first year and decreased progressively through the fifth year. During the first year, several of the linear velocities decreased by more than 2 mm per year. S-SE showed the lowest rates of growth after the first year.
Figure 2A.
Yearly velocities of linear maxillary measurements of females.
Figure 2B.
Yearly velocities of linear maxillary measurements of males.
Table 3.
Year Growth Velocities of the Linear (mm/y) and Angular (°/y) Measures During Infancy and Early Childhooda
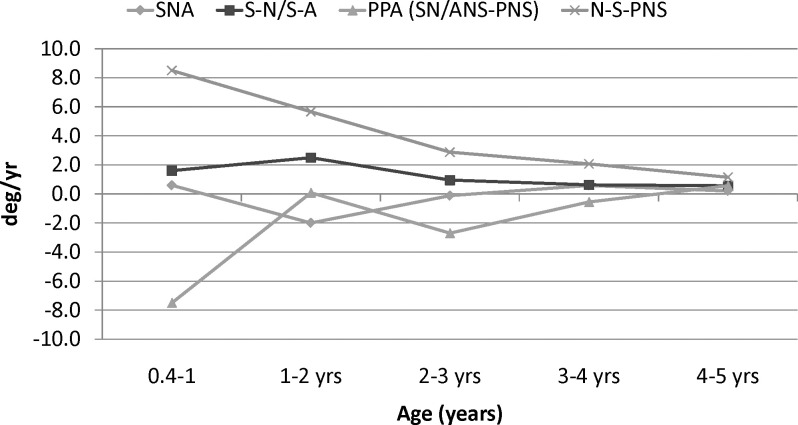
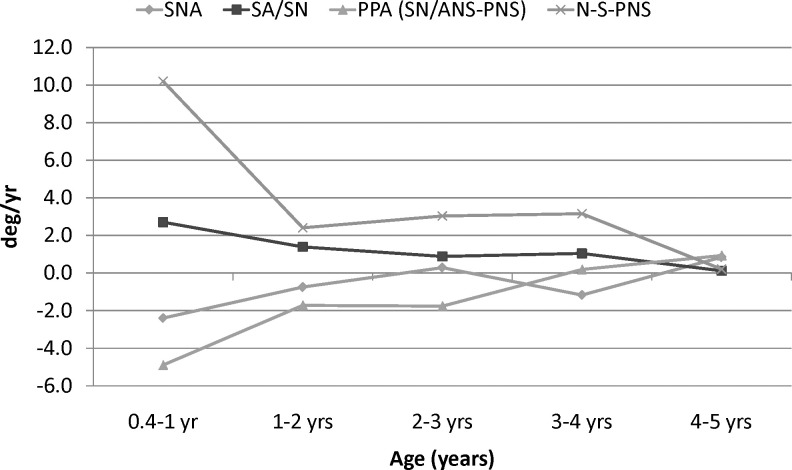
The angular measurements also showed the greatest rates of change during the first year (Figure 3). The N-S-PNS angle increased 8°–10° during the first year, 3° during the third year, and less than 1° during the fifth year. Similarly, the palatal plane angle decreased 3°–4° during the first year, approximately 2° during the third year, and increased slightly during the fifth year. The N-S-A angle showed progressively less change over time. The S-N-A showed small, inconsistent changes during the first 5 years.
Figure 3A.
Yearly velocities of angular maxillary measurements of females.
Figure 3B.
Yearly velocities of angular maxillary measurements of males.
The overall linear growth changes that occurred between 0.4 and 5 years of age (span of 4.5 years) were generally greater than the changes in maxillary growth that occurred between 5 and 16 years (a span of 11 years). The linear growth changes from 6 to 16 years were greater in males than females, but only the SE-N difference was statistically significant (Table 4). The angular changes were also generally greater during the first 4.5 years than the subsequent 11 years. The PPA, which underwent substantial changes initially, showed only minor changes after 5 years of age. Before 5 years of age, N-S-PNS increased approximately three times as much, and N-S-A increased approximately twice as much, as they did after 5 years of age. The S-N-A angle decreased during the first 5 years and increased slightly thereafter.
Table 4.
Comparison of Linear and Angular Measurements Between T1–T6 and T6–T7a
Relative Maxillary Maturity
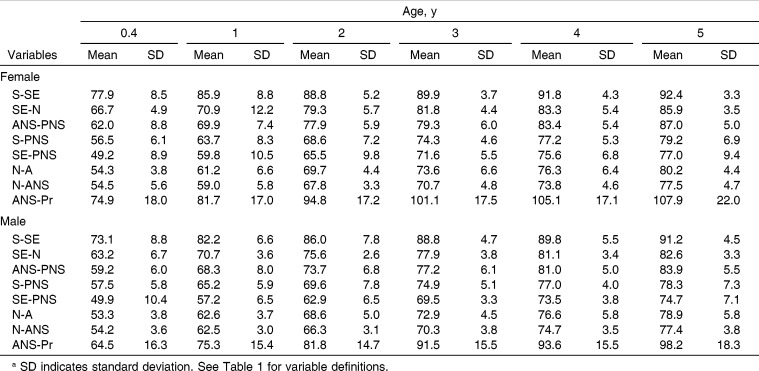
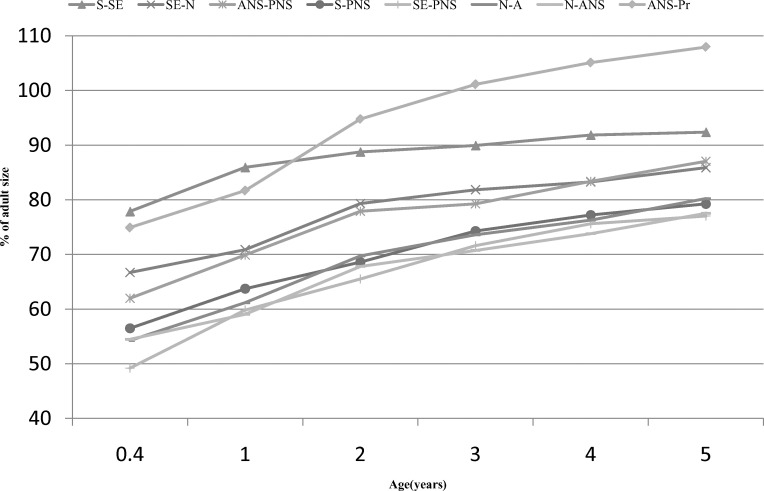
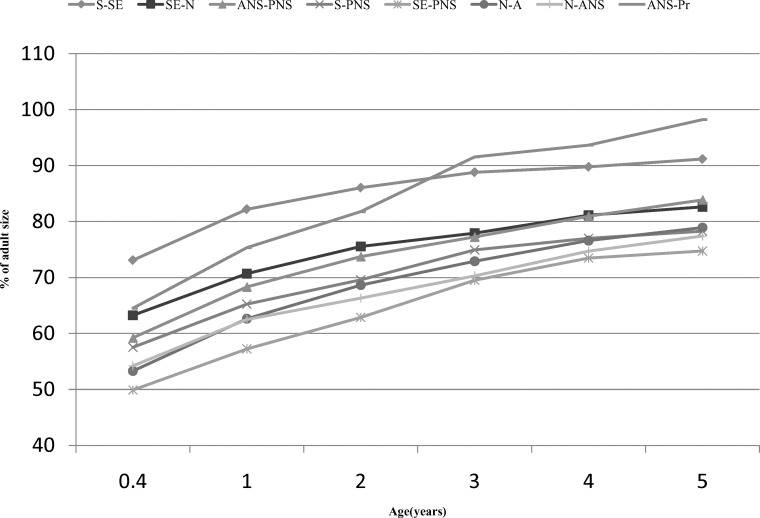
The maturity of the linear measures at 0.4 years ranged between 49% and 78% for females and between 50% and 73% for males (Table 5). The maxilla of males was 1%–10.4% less mature than the maxilla of females at 0.4 years and 0.1%–9.7% less mature at 5 years. S-SE was the most mature measure, having attained 77.9% (females) and 73.1% (males) of its adult size at 0.4 years of age. SE-PNS was the least mature measure at 0.4 years of age for both sexes, having attained 49.2% and 49.9% of its adult size in females and males, respectively (Figure 4). The other measures graded more or less regularly between these two. With the exception of ANS-Pr, the vertical measures were consistently less mature than the AP measures, regardless of sex. This graded pattern was maintained until 2 years of age in females and 3 years of age in males, at which point ANS-Pr became and remained the most mature measure.
Table 5.
Maxillary Maturity, as a Percentage of Adult Size, During Infancy and Early Childhooda
Figure 4A.
Percent adult status of females 0.4–5 years of age.
Figure 4B.
Percent adult status of males 0.4–5 years of age.
DISCUSSION
Growth rates were greatest during the first year and then decelerated over the next four years. Rapid deceleration of growth during the early years has been previously described for general somatic growth. For example, rates of growth in recumbent length for males decrease from approximately 25 cm/y during the first year to 0 cm/y during the third year.2 The greatest changes in cranial base growth also occur during the first 5 postnatal years, especially during the first 2–3 years.9 Liu et al.13 found that mandibular growth changes were also greatest during the first 6 months and decreased progressively thereafter. It appears that the decelerating pattern of rapid growth observed immediately after birth reflects a continuation of the even more rapid growth that occurs prenatally.
The overall growth changes that occurred during the first 5 postnatal years were generally greater than the changes that occurred between 5 and 16 years. Broadbent et al.16 found that the growth of ANS-PNS from 1 to 5 years was slightly less than the growth that occurred between 5 and 16 years, as were the changes of N-ANS. However, that study started at 1 year of age, while the present study started at 0.4 years, and the greatest growth changes occurred during the first year.
The relationship of the anterior maxilla to the anterior cranial base changed only slightly during infancy and early childhood. SNA decreased 0.4° and 2.2° in females and males, respectively. While using a smaller sample of subjects from the Bolton records, Broadbent et al.16 showed that SNA decreased 1.2° and 1.5° for females and males, respectively. Ohtsuki et al.18 reported greater decreases in the SNA angle between birth and 5 years of age. SNA decreases may represent a relative posterior repositioning of the maxilla associated with greater relative forward repositioning of the anterior cranial base or with the pronounced flexing of the cranial base the occurs during the first few postnatal years.9 This suggests that N is moving forward relatively more than A.18
Unlike the anterior region, the posterior aspect of the maxilla underwent substantial posterior repositioning relative to the cranial base between birth and 5 years of age. The N-S-PNS angle increased 15°–16°, 5°–6° of which occurred during the first year. Ohtsuki et al.18 also reported a substantial increase of this angle between birth and 5 years of age. In contrast, Brodie19 reported that PNS advances slightly relative to the S-N line (S was their stable references point) from birth to 1 year of age, and then maintains a straight forward growth direction. As previously suggested, the N-S-PNS angle might be expected to increase with the relative posterior repositioning of the maxilla.
During the first 5 years of life, absolute AP maxillary growth was similar to vertical growth, while vertical growth outpaced AP growth during later childhood and adolescence. Farkas et al.8 reported that the AP growth of the head was significantly greater than vertical growth before 5 years, while vertical growth was greater after 5 years. This difference explains why the AP maxillary measures are more mature than the vertical measures during infancy and early childhood. Similar patterns reported by Liu et al.13 found that the mandibular corpus length (Go-Gn) was consistently more mature than ramus height (Co-Go) during the first 5 years. Fields20 also reported that the vertical facial growth was the last dimension to be completed. On average, late vertical growth increments are greater in girls than in boys and occur in the maxilla.
The maxilla of males was 1% to 10.4% less mature than the maxilla of females at 0.4 years, and 0.1% to 9.7% less mature at 5 years. Buschang et al.,12 who also quantified craniofacial relative maturity, found that the maxilla of 4.5 years of males was 1%–2% less mature than the maxilla of females. Liu et al.13 reported that male mandibles were 3.3%–3.9% less mature than female mandibles at 0.4 years. The maturity differences indicate that females have less growth potential than males for birth onwards.
Anterior dentoalveolar height (ANS-Pr) showed greater increases in maturation than the other measures during the first 3 years, closely approaching its adult size by 4–5 years. Buschang et al.12 showed that anterior maxillary height had attained over 100% of adult size by 5.5 years of age, with size decreasing rapidly thereafter, followed by size increases. Savara and Singh21 and Singh and Savara22 reported the greatest increases in the growth of ANS-Pr during the first 5 years, followed by decreases between 6 and 8 years due to loss of primary central incisors. This indicates that appositional bone growth of the alveolar process occurs rapidly during the first 2–3 years to accommodate both the deciduous and permanent teeth prior to the early mixed dentition phase of development.
With the exception of ANS-Pr, which showed a different maturity pattern related to the developing dentition, the various measures showed a graded pattern of maturation, with the vertical measures being less mature than the AP measures. Buschang et al.12 were the first to report a maturity gradient for the entire craniofacial complex, showing that the vertical aspect of the maxilla (N-ANS) was also less mature than the AP (ANS-PNS) at 4.5 years of age, with percent maturity coinciding with the values obtained in the present study. It has also been shown that the AP dimensions of the mandible are more mature than its vertical dimensions.12,13
While it remained relatively unchanged after 5 years of age, the palatal plane angle (PPA) decreased substantially during the first 5 years. Although no descriptive statistics were provided, Brodie's19 illustration representing 21 white males also showed that the PPA decreases during the first 5 years of life. The decreases could be explained by the growth of the orbit, which grows rapidly during the first few years along with the rest of the nervous system, and contributes greatly to the vertical growth of the anterior part of the maxilla. The roof of the orbit, AP length of the orbital floor, orbital breadth, orbital height, and orbital volume grow most rapidly during the first year of life.23
CONCLUSIONS
Maxillary and anterior cranial base growth rates are the greatest during the first year, and then decelerated over the next 4 years.
Overall growth changes during the first 5 postnatal years are generally greater than the changes between 5 and 16 years.
The absolute growth of the AP measures is comparable to the growth of the vertical measures, whereas vertical growth outpaces AP growth during late childhood and adolescence.
ANS-Pr is the most mature during the first 3 years, closely approaching its adult size by 4–5 years.
The maxilla of males is 1%–10.4% less mature than females at 0.4 years, and 0.1% to 9.7% less mature at 5 years.
REFERENCES
- 1.Hägg U, Taranger J. Maturation indicators and the pubertal growth spurt. Am J Orthod. 1982;82:299–309. doi: 10.1016/0002-9416(82)90464-x. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Atlanta, Ga: Centers for Disease Control and Prevention; 2000. Clinical growth charts. Set 1: clinical charts with 5th and 95th percentiles. Available at: http://www.cdc.gov/growthcharts/clinical_charts.htm Accessed July 2, 2011. [Google Scholar]
- 3.Hunter CJ. The correlation of facial growth with body height and skeletal maturation at adolescence. Angle Orthod. 1966;36:44–54. doi: 10.1043/0003-3219(1966)036<0044:TCOFGW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Nanda RS. The rates of growth of several facial components measured from serial cephalometric roentgenograms. Am J Orthod. 1955;41:658–673. [Google Scholar]
- 5.Fishman LS. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod. 1979;49:181–189. doi: 10.1043/0003-3219(1979)049<0181:CVSAAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Hellman M. Changes in the human face brought about by development. Int J Orthod Oral Surg Radiog. 1927;13:475–516. [Google Scholar]
- 7.Goldstein M. Changes in dimensions and form of the face and head with age. Am. J Phys Anthropol. 1936;22:37–89. [Google Scholar]
- 8.Farkas LG, Posnick JC, Hreczko TM. Anthropometric growth study of the head. Cleft Palate Craniofac J. 1992;29:303–308. doi: 10.1597/1545-1569_1992_029_0303_agsoth_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsuki F, Mukherjee D, Lewis AB, Roche AF. A factor analysis of cranial base and vault dimensions in children. Am J Phys Anthropol. 1982;58:271–279. doi: 10.1002/ajpa.1330580305. [DOI] [PubMed] [Google Scholar]
- 10.Singleton DA, Buschang PH, Behrents RG, Hinton RJ. Craniofacial growth in growth hormone-deficient rats after growth hormone supplementation. Am J Orthod Dentofacial Orthop. 2006;130:69–82. doi: 10.1016/j.ajodo.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Abed GS, Buschang PH, Taylor R, Hinton RJ. Maturational and functional related differences in rat craniofacial growth. Arch Oral Biol. 2007;52:1018–1025. doi: 10.1016/j.archoralbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Buschang PH, Baume RM, Nass GG. A craniofacial growth maturity gradient for males and females between 4 and 16 years of age. Am J Phys Anthropol. 1983;61:373–381. doi: 10.1002/ajpa.1330610312. [DOI] [PubMed] [Google Scholar]
- 13.Liu YP, Behrents RG, Buschang PH. Mandibular growth, remodeling, and maturation during infancy and early childhood. Angle Orthod. 2010;80:97–105. doi: 10.2319/020309-67.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinmann J, Sicher H. Bone and Bones. St Louis, Mo: The CV Mosby Co; 1947. [Google Scholar]
- 15.Petrovic AG, Stutzmann JJ, Oudet CL. Control processes in the postnatal growth of the condylar cartilage of the mandible. In: McNamara JA, editor. Determinants of Mandibular Growth and Form. Ann Arbor, Mich: Center for Human Growth and Development, The University of Michigan; 1975. pp. 101–153. [Google Scholar]
- 16.Broadbent B, Sr, Broadbent B, Jr, Golden W. Bolton Standards of Dentofacial Developmental Growth. St Louis: The CV Mosby Co; 1975. [Google Scholar]
- 17.Zalel Y, Gindes L, Achiron R. The fetal mandible: an in utero sonographic evaluation between 11 and 31 weeks' gestation. Prenat Diagn. 2006;26:163–167. doi: 10.1002/pd.1363. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuki F, Mukherjee D, Lewis AB, Roche AF. A growth of cranial base and vault dimensions in children. J Anthrop Soc Nippon. 1982;90:239–258. doi: 10.1002/ajpa.1330580305. [DOI] [PubMed] [Google Scholar]
- 19.Brodie AG. On the growth pattern of the human head from the third month to the eighth year of life. Am J Anat. 1941;68:209–262. [Google Scholar]
- 20.Fields HW. Craniofacial growth from infancy through adulthood. Background and clinical implications. Pediatr Clin North Am. 1991;38:1053–1088. doi: 10.1016/s0031-3955(16)38189-5. [DOI] [PubMed] [Google Scholar]
- 21.Savara BS, Singh IJ. Norms of size and annual increments of seven anatomical measures of maxillae in boys from three to sixteen years of age. Angle Orthod. 1968;38:104–120. doi: 10.1043/0003-3219(1968)038<0104:NOSAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Singh IJ, Savara BS. Norms of size and annual increments of seven anatomical measures of maxillae in girls from three to sixteen years of age. Angle Orthod. 1966;36:312–324. doi: 10.1043/0003-3219(1966)036<0312:NOSAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Dixon AD, Hoyte DA, Ronning O. Fundamentals of Craniofacial Growth 1st ed. Boca Raton, Fla: CRC Press; 1997. pp. 229–233. [Google Scholar]