Abstract
Objectives:
To evaluate the in vivo effects of two acidic soft drinks (Coca-Cola and Sprite) on the shear bond strength of metal orthodontic brackets with and without resin infiltration treatment. In addition, the enamel surface was evaluated, after debonding, using a scanning electron microscope.
Materials and Methods:
Sixty noncarious maxillary premolars, scheduled for extraction in 30 orthodontic patients, were used. Patients were randomly divided into two groups according to the soft drink tested (Coca-Cola or Sprite). In each group, application of resin infiltration (Icon. DMG, Hamburg, Germany) was done on one side only before bonding of brackets. Patients were told to rinse their mouth with their respective soft drink at room temperature for 5minutes, three times a day for 3months. Shear bond strength was tested with a universal testing machine. After shearing test, a scanning electron microscope was used to evaluate enamel erosion. Statistical analysis was performed by twoway analysis of variance followed by the least significant difference test.
Results:
The Coca-Cola group without resin infiltration showed the lowest resistance to shearing forces. Scanning electron micrographs of both groups after resin application showed a significant improvement compared with results without resin use, as the enamel appeared smoother and less erosive.
Conclusion:
Pretreatment with the infiltrating resin has proved to result in a significant improvement in shear bond strength, regardless of the type of soft drink consumed.
Keywords: Resin infiltration, Soft drinks, Orthodontic brackets
INTRODUCTION
In recent years there has been an increase in the consumption of soft drinks among children and adolescents.1 Soft drinks are damaging not only because of the high levels of sugar they contain but also because most of them have pH levels below the critical limit for enamel demineralization (pH 5.5). Moreover, frequently consumed soft drinks have been shown to cause extreme dental erosion.2–5 Investigators have demonstrated that the erosive potential of soft drinks depends on the initial pH and buffering capacity of the drink. Carbonated soft drinks are potentially more erosive than noncarbonated beverages because of the additional carbonic acid present.6
Dental erosion is defined as the acidinduced loss of hard tissue, a chemical process in which bacteria play no part for this reason, it is not associated with dental plaque.7–9 In an in vivo study, Jensdottir et al.7 found that the prevalence of dental erosion increased as pH levels of the studied drinks decreased and as consumption increased. Other studies using scanning electron microscopy (SEM) have shown that soft drinks produce large areas of enamel decalcification.10–12 Decalcification of enamel around bonded orthodontic brackets has long been a concern for orthodontists. Investigators agree that decalcification is the first step in the breakdown of enamel. Decalcification is defined as loss of calcified tooth substance; it occurs when the pH of the oral environment favors diffusion of calcium and phosphate ions out of the enamel.13,14
A new approach in treating incipient caries lesions by an infiltration technique was introduced recently.15 Infiltration of caries lesions with lowviscosity lightcuring resins is a treatment option for noncavitated lesions not expected to arrest or remineralize. In contrast to the conventional sealing concept, wherein a resin layer is glued onto the lesion surface, caries infiltrants penetrate the porous body of initial caries lesions.16 Caries infiltrants are optimized for rapid capillary penetration and exhibit very low viscosity and high surface tension.6 Thus, laboratory experiments showed significantly deeper penetration of infiltrants into the lesion body than is seen with conventional adhesives.16–18 Clinical followup studies have proved this concept to be more effective than fluoridation measures in stopping progression of a carious lesion within 1.5years of observation.19 A recent study confirmed that the microinvasive therapy of caries by resin infiltration facilitates an early, virtually painless esthetic treatment and masking of postorthodontic white spot lesions on the enamel surface.20–22
Clearly, the subject of decalcification is complex and of great importance to the orthodontic specialty. Based on the fact that many of our patients routinely drink acidic soft drinks, the following study was conducted to evaluate the in vivo effects of two acidic soft drinks (Coca-Cola and Sprite) on the shear bond strength of metal orthodontic brackets with and without the application of resin infiltration technique and to evaluate the enamel surface after debonding using SEM.
MATERIALS AND METHODS
The present study was approved by the research ethical committee of Mansoura University. Sixty noncarious maxillary premolar teeth, scheduled for extraction in 30 orthodontic patients (12 to 17 years old), were used. All subjects were provided with verbal and written information concerning the study, and informed consents were signed. Patients were randomly divided into two groups according to the soft drink tested (Coca-Cola and Sprite, Atlanta, GA, USA; pH2.44 and 2.90 respectively). In each group, application of resin infiltration (Icon, DMG, Hamburg, Germany) was done on one side only, according to the manufacturers instructions, before bonding of brackets.23,24 Brackets were placed only on the teeth to be extracted and not on any other teeth in the mouth. Stainless steel premolar brackets (Victory series, 3M Unitek, Monrovia, Calif) with an average base area of 9.94mm2 were identically bonded on all 60 teeth. Patients in the two groups were told to rinse their mouth with their respective room temperature drink for 5minutes, three times a day, for 3months. They were told not to drink any acidic soft drinks apart from these. All volunteers who participated in this study brushed their teeth three times a day for 3 minutes. At the end of 3 months, the premolar teeth were extracted without damaging brackets. Accordingly, four subgroups of 15 premolars each were obtained group 1 (Icon, brackets, and Coca-Cola), group 2 Icon, brackets, and Sprite, group 3 (brackets and Coca-Cola), and group 4 (brackets and Sprite).
Application of Brackets
Teeth were etched (35% phosphoric acid gel for 30 seconds), washed with water, and dried by airblow. The primer of the Transbond XT (3M Unitek, Landsberg, Germany) bracket luting system was applied to the etched surface. The luting material was then applied to the bracket base, and the bracket was placed on the tooth with a standardized load of 500 g. Careful removal of excess material was performed. In all patients, light curing was performed for 60 seconds (15 seconds each in the cervical, incisal, mesial, and distal directions Epilar Freelight II LED, 1000mW/cm2, 3M ESPE, Seefeld, Germany).
Shear Bond Strength Testing
All teeth in the four groups were mounted vertically in acrylic blocks up to the clinical crown level. Shear bond strength was tested with a universal testing machine (Z010, Zwick, Ulm, Germany), ensuring consistency for the point of force application and the direction of the debonding force. The direction of the debonding force was parallel to enamel surface in an occlusogingival direction. A stainless steel rod with a chisel configuration was used for bracket debonding. Crosshead speed was 0.5 mm/min. Load at failure was recorded, and shear strength values were calculated according to the following equation S FA, where S is shear bond strength, F is load at failure (N), and A represents the adhesive area (mm2). Values gained from the tests were evaluated with the Students ttest using group and intergroup comparisons.
SEM
After shearing tests, a scanning electron microscope was used to determine the amount of erosion. Specimens were covered with gold and analyzed (2000× magnification) using a JEOL JSM-5200 scanning electron microscope. Photographs were also taken at this stage.
Statistical Analysis
A two–way analysis of variance test was used to detect if there was any significant effect of the two types of soft drinks with and without Icon resin infiltration. The least significant difference statistical test was then used to detect significant differences between groups at P ≤ .05.
RESULTS
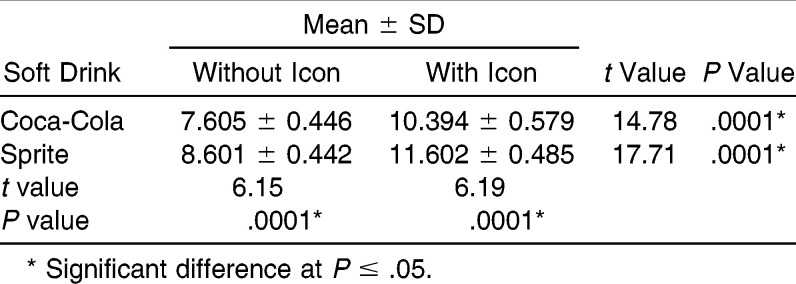
Means and standard deviations obtained from the shear test are shown in Table 1. The Coca-Cola group without Icon showed the lowest mean bond strength (7.605 MPa), and the Sprite group with Icon showed the highest (11.602 MPa). The Coca-Cola treated specimens showed a significantly reduced bond strength compared with that of Spritetreated specimens (P < .0001). There was also a significant difference in bond strength between specimens from the Coca-Cola group without Icon and those infiltrated with Icon (P < .0001), wherein the Icon-infiltrated specimens showed higher values. Similarly, a significant difference was observed between specimens of the Sprite group without Icon compared with those infiltrated with Icon (P < .0001).
Table 1.
Shear Bond Strength Results (in MPa)
SEM
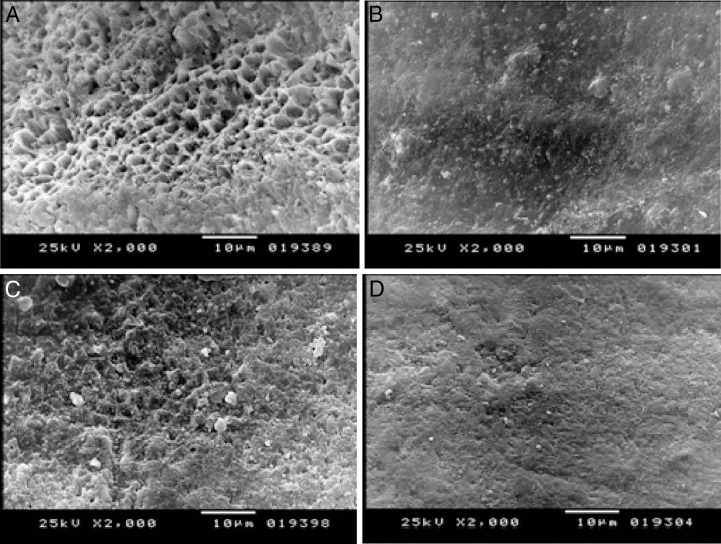
The enamel surfaces and adhesiveenamel borders of the teeth in the four groups were analyzed using SEM. In Figure 1A, areas of enamel defects caused by erosion are seen on the samples taken from the Coca-Cola group (2000× magnification). Areas of enamel defects of the Sprite group were not as extensive as those of the Coca-Cola group (2000× magnification) (Figure 1C). Figures1B and 1D show healthier enamel surfaces to which Icon resin infiltration was applied before exposure to Coca-Cola and Sprite, respectively.
Figure 1.
(A) Scanning electron micrograph showing the demineralization in the enamel surface in the Coca-Cola group (2000× magnification). (B) Scanning electron micrograph showing the demineralization in the enamel surface in the Coca-Cola group with Icon (2000× magnification). (C) Scanning electron micrograph showing the demineralization in the enamel surface in the Sprite group (2000× magnification). (D) Scanning electron micrograph showing the demineralization in the enamel surface in the Sprite group with Icon (2000× magnification).
DISCUSSION
In recent decades, soft drink consumption has steadily increased among children and adolescents in both Western and developing countries.1 Enamel white spot lesions not only develop during orthodontic treatment but might also be present even at the start of orthodontic treatment, especially in high softdrink consumers. Tufekci et al.21 and Gorelick et al.22 found that 11% and 24% of their orthodontic patients, respectively, had existing white spot lesions at the time of bracket fixation. Thus, preventive strategies are needed in orthodontics to remineralize previously demineralized enamel to allow for bracket fixation and improving shear bond strength.23
As a result of the sealing effect on sound enamel and the stabilization of demineralized enamel, it is conceivable that the caries infiltration technique may be beneficial as a form of pretreatment before bracket fixation.24 So the aim of this study was to evaluate the effects of Coca-Cola and Sprite on shear bond strength of orthodontic brackets with and without the application of resin infiltration and to evaluate the effects on enamel surface mineralization using SEM. These particular soft drinks were chosen for several reasons first, because of their high levels of consumption in Egypt. In developed countries, Coca-Cola has the largest segment within the carbonated sector, with a share approaching 50, followed by lemonflavor drinks (22%).25 Second, they have a pH level below the critical limit for demineralization of tooth enamel (pH < 5.5).4 Finally, Coca-Cola contains phosphoric acid and Sprite contains citric acid, both of which are used in acid etching for placement of orthodontic brackets. The study was conducted in vivo to confirm previous in vitro studies3,24 and to consider the defense mechanism of saliva against erosion26 and the bacterial functions of the oral environment. Steffen14 stated that the presence of bacteria in the mouth along with acidic soft drinks accelerates erosion.
The shear test was performed with a universal testing apparatus, similar to the procedure of others.27–29 Gillis and Redlich29 and Mascia and Chan30 also applied shearing to their samples with a stable speed of 0.5mmmin using the universal test apparatus. Results of this study indicated that the Coca-Cola group without resin infiltration application showed the lowest mean resistance to shearing forces, which agrees with a previous study.3 Significant statistical differences were observed between the Coca-Cola and Sprite groups with and without application of resin infiltration. On the other hand, resin infiltration has resulted in significant improvement in bond strength, regardless of the type of soft drink consumed. We believe the erosive defects caused by Coca-Cola and Sprite on enamel, as shown by SEM, has a negative effect on bracket retention.3 When the Coca-Cola and Sprite groups without resin infiltration were compared using SEM, the enamel defects in the Coca-Cola group were more extensive and more noticeable than those in the Sprite group. This may be due to the more extensive enamelerosive effect of the phosphoric acid (pH 2.44) in Coca-Cola.
Rugg–Gunn et al.31 compared the erosive capabilities of a citric acidbased orange juice drink and a phosphoric acidbased diet cola drink. They determined that the phosphoric acidbased diet cola had more erosive potential than the citric acidbased juice drink. This supports our results in the present study. Our findings, evaluated using SEM, were parallel to the results of Dinçer et al.4 Steffen,14 Gedalia,32 and Grando et al.33 The erosive effect may be attributed to the low pH value of the acidic soft drinks , which lowers pH of the oral cavity.2 The erosive potential depends on the acidic properties, which is the amount of acid available (titratable acidity) and the amount of the acid actually present (concentration of H+ ions—pKa). In addition, complex interactions between solid and soluble components of a beverage, such as the acid/ hydroxyapatite reaction, affect the erosive potential.31
The increase in bond strength of brackets with application of the resin coincides with the results of previous studies23,24 and was most likely the result of deeper penetration of the resin infiltrant into the body of the lesion compared with the primer of the orthodontic cements. Monomer formulations with an increased TEGDMA (triethyleneglycol dimethacrylate) content have a high penetration capability,33 which probably allows a chemical connection of the resin infiltrant to the monomers of the primer. Conventional adhesives, like Transbond XT, are able to penetrate carious lesions to some extent thus, it is assumed that its primer might also partly penetrate demineralized enamel and strengthen the outermost part of the infiltrated enamel when applied after Icon preconditioning.24
Scanning electron micrographs of both groups after resin application showed a significant improvement compared with groups without resin use, as enamel appears smoother and less eroded. With the resin infiltration technique, the unique lowviscosity resin is drawn deep into the pore system of a lesion, like a sponge draws up liquids. The resin completely fills the pores within the tooth, replacing lost tooth structure and stopping caries and erosion progression by blocking further introduction of any nutrients into the pore system.23,4
Although the results of this study are promising as a confirmation of the sealing effect of resin infiltrant and the stabilization of demineralized enamel in high consumers of soft drinks, further studies are needed to evaluate the longterm effect of this technique with orthodontics.
Acknowledgment
The authors would like to thank the Egyptian Ministry of Higher Education and Scientific Research for funding.
REFERENCES
- 1.West NX, Hughes JA, Addy M. Erosion of dentine and enamel in vitro by dietary acids the effects of temperature, acid character, concentration and exposure time. J Oral Rehabil. 2000;27:875–880. doi: 10.1046/j.1365-2842.2000.00583.x. [DOI] [PubMed] [Google Scholar]
- 2.West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001;28:860–864. doi: 10.1046/j.1365-2842.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Oncag G, Tuncer AV, Tosun YS. Acidic soft drinks effects on the shear bond strength of orthodontic brackets and a scanning electron microscopy evaluation of the enamel. Angle Orthod. 2005;75:247–253. doi: 10.1043/0003-3219(2005)075<0243:ASDEOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Diner B, Hazar S, Sen BH. Scanning electron microscope study of the effects of soft drinks on etched and sealed enamel. Am J Orthod Dentofacial Orthop. 2002;122:135–141. doi: 10.1067/mod.2002.124458. [DOI] [PubMed] [Google Scholar]
- 5.Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Erosion of deciduous and permanent dental hard tissue in the oral environment. J Dent. 2000;28:257–267. doi: 10.1016/s0300-5712(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 6.The 1997 Sucralose Drinks Report. Reading, UK: Tate Lyle Specialty Sweeteners; [Google Scholar]
- 7.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85:226–230. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 8.Eygen IV, Vande Vannet B, Wehrbein H. Influence of a soft drink with low pH on enamel surfaces an in vitro study. Am J Orthod Dentofacial Orthop. 2005;128:372–377. doi: 10.1016/j.ajodo.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Barbera E, Hernndez C, Maroto M, Miralles V. Efectos nocivos de la ingesta de zumos y bebidas carbonatadas sobre el esmalte dentario del nio. Gerencia Dent. 2007;14:38–45. [Google Scholar]
- 10.Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acidic drinks and other foodstuffs. Scand J Dent Res. 1988;96:324–333. doi: 10.1111/j.1600-0722.1988.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 11.Meurman JH, Frank RM. A scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991;25:1–6. doi: 10.1159/000261335. [DOI] [PubMed] [Google Scholar]
- 12.Grando LJ, Tames DR, Cardoso AC, Gabilan NH. In vitro study of enamel erosion caused by soft drinks and lemon juice in deciduous teeth analysed by stereomicroscopy and scanning electron microscopy. Caries Res. 1996;30:373–378. doi: 10.1159/000262345. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell L. Decalcification during orthodontic treatment with fixed appliancesan overview. Br J Orthod. 1992;19:199–205. doi: 10.1179/bjo.19.3.199. [DOI] [PubMed] [Google Scholar]
- 14.Steffen MJ. The effects of soft drinks on etched and sealed enamel. Angle Orthod. 1996;66:449–456. doi: 10.1043/0003-3219(1996)066<0449:TEOSDO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.MeyerLueckel H, Paris S. Improved resin infiltration of natural caries lesions. J Dent Res. 2008;87:1112–1116. doi: 10.1177/154405910808701201. [DOI] [PubMed] [Google Scholar]
- 16.Kielbassa AM, Muller J, Gernhardt CR. Closing the gap between oral hygiene and minimally invasive dentistry a review on the resin infiltration technique of incipient proximal enamel lesions. Quintessence Int. 2009;40:663–681. [PubMed] [Google Scholar]
- 17.Paris S, MeyerLueckel H, Colfen H, Kielbassa AM. Resin infiltration of artificial enamel caries lesions with experimental light curing resins. Dent Mater J. 2007;26:582–588. doi: 10.4012/dmj.26.582. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Lueckel H, Paris S. Progression of artificial enamel caries lesions after infiltration with experimental light curing resins. Caries Res. 2008;42:117–124. doi: 10.1159/000118631. [DOI] [PubMed] [Google Scholar]
- 19.Paris S, MeyerLueckel H. Infiltrants inhibit progression of natural caries lesions in vitro. J Dent Res. 2010;89:1276–1280. doi: 10.1177/0022034510376040. [DOI] [PubMed] [Google Scholar]
- 20.Hammad SM, El Banna M, El Zayat I, Mohsen MA. Effect of resin infiltration on white spot lesions after debonding orthodontic brackets. Am J Dent. 2012;25:3–8. [PubMed] [Google Scholar]
- 21.Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81:206–210. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod Dentofacial Orthop. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 23.Attin R, Stawarczyk B, Kecik D, Knosel M, Wiechmann D, Attin T. Shear bond strength of brackets to demineralize enamel after different pretreatment methods. Angle Orthod. 2012;82:56–61. doi: 10.2319/012311-48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidu E, Stawarczyk B, Tawakoli PN, Attin R, Attin T, Wiegand A. Shear bond strength of orthodontic resins after caries infiltrant preconditioning. Angle Orthod. doi: 10.2319/052112-409.1. InPress. doi:http://dx.doi.org/10.2319/052112-409.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ICH Topic 6 Guideline for Good Clinical Practice CPMPICH13595. http://ethikkommission.meduniwien.ac.atfileadminethikmediadokumenterechtsgrundlagenGCP.pdf . [Google Scholar]
- 26.O'Reilly MM, Featherstone J. Demineralization and remineralization around orthodontic appliances an in vivo study. Am J Orthod. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 27.Evans LJ, Peters C, Flickinger C, Taloumis L, Dunn W. A comparison of shear bond strengths of orthodontic brackets using various light sources, light guides and cure times. Am J Orthod. 2002;121:510–515. doi: 10.1067/mod.2002.121558. [DOI] [PubMed] [Google Scholar]
- 28.Bishara SE, Laffoon JF, Vonwald L, Warren JJ. The effect of repeated bonding on the shear bond strength of different orthodontic adhesives. Am J Orthod. 2002;121:521–525. doi: 10.1067/mod.2002.123042. [DOI] [PubMed] [Google Scholar]
- 29.Gillis I, Redlich M. The effect of different porcelain conditioning techniques on shear bond strength of stainless steel brackets. Am J Orthod. 1998;114:387–392. doi: 10.1016/s0889-5406(98)70183-0. [DOI] [PubMed] [Google Scholar]
- 30.Mascia VE, Chan SR. Shearing strengths of recycled direct bonding brackets. Am J Orthod. 1982;82:211–216. doi: 10.1016/0002-9416(82)90141-5. [DOI] [PubMed] [Google Scholar]
- 31.Rugg-Gunn AJ, Maguire A, Gordon PH, McCabe JF, Stephenson G. Comparison of erosion of dental enamel by four drinks using an intraoral appliance. Caries Res. 1998;32:337–343. doi: 10.1159/000016469. [DOI] [PubMed] [Google Scholar]
- 32.Gedalia I. Tooth enamel softening with a cola type drink and rehardening with hard cheese or stimulated saliva in situ. J Oral Rehabil. 1991;18:501–506. doi: 10.1111/j.1365-2842.1991.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 33.Paris S, Meyer–Lueckel H, Colfen H, Kielbassa AM. Penetration coefficients of commercially available and experimental composites intended to infiltrate enamel carious lesions. Dent Mater. 2007;23:742–748. doi: 10.1016/j.dental.2006.06.029. [DOI] [PubMed] [Google Scholar]