Abstract
Objectives
To compare the high-resolution computed tomography (HRCT)–derived severity score in COVID-19 patients between those who had earlier received the vaccine against the SARS-CoV-2 and those who did not.
Methods
A retrospective cross-sectional analysis of HRCT of the chest was done in correlation with the vaccination status of clinically diagnosed COVID-19 patients. The variable under evaluation was the CT severity score, whereby differential analysis of the variability on this parameter between incompletely (single dose) vaccinated, completely (both doses) vaccinated, and non-vaccinated individuals was the outcome.
Results
The analysis included 826 patients of which 581 did not receive any vaccination whereas 196 patients received incomplete (single dose) vaccination and 49 received complete vaccination. Mean CT severity score was lower in completely vaccinated patients (3.5 ± 6.3) vis-à-vis incompletely vaccinated (10.1 ± 10.5) and non-vaccinated (10.1 ± 11.4) individuals. The mean CT score was significantly lower in completely vaccinated patients of lower ages (≤ 60 years) compared to patients above that age. The incidence of severe disease (CT score ≥ 20) was significantly higher in the incompletely vaccinated and non-vaccinated patients compared to that in the completely vaccinated group.
Conclusions
CT severity scores in individuals receiving both doses of SARS-CoV-2 vaccination were less severe in comparison to those receiving a single dose of vaccine or no vaccine at all.
Key Points
• Patients who received complete two doses of vaccination had significantly low mean CT scores compared to the partially vaccinated patients and non-vaccinated patients.
• The mean CT scores were significantly lower in completely vaccinated patients of lower ages (< 60 years) while patients > 60 years did not show significantly different CT scores between the vaccinated and non-vaccinated groups.
• Consolidations and ground-glass opacities were significantly lower in the group receiving complete vaccination as compared to the unvaccinated and incompletely vaccinated patients.
Keywords: Tomography, X-ray computed; COVID-19; SARS-CoV-2; Vaccination
Introduction
The role of HRCT scan in making a diagnosis of COVID-19 has been emphasized as being a problem-solving modality in patients with complications, diagnostic dilemmas, and poor response to therapy [1–4]. Further, a more viable role may be in assessing the volume of lung involved in a confirmed patient and correlating the disease severity with a CT scan severity score [5–7]. Thirdly, with the upcoming of newer treatment modalities, drugs, and the vaccine, the same may be used to demonstrate the efficacy of a specific treatment on the actual morphological response in the pulmonary parenchyma. The fact remains that demonstration of less severe scores on CT scan following a specific intervention would offer a high level of confidence in favor of that modality. The aim of the present study was to compare the high-resolution computed tomography (HRCT)–derived severity scores in COVID-19 patients between those who had earlier received the vaccine against the SARS-CoV-2 and those who did not.
Patients and methods
Patients
This was a retrospective observational study done from 12 to 30 April 2021, using imaging data generated during routine clinical management with the informed consent from the patients, and approval from Institutional Ethical Committee has been taken. Non-disclosure of patients’ privacy has been ensured during the scripting. Patients with the diagnosis of COVID-19 based on clinical suspicion and a positive reverse transcriptase-polymerase chain reaction (RT-PCR) who underwent an HRCT scan of the chest in our hospital were included in the study. A single-point evaluation of the scan performed between the fifth and eighth day from the onset of clinical symptoms was done, while all preceding or follow-up scans were excluded from the study. HRCT was done for diagnostic workup where RT-PCR results were delayed or initial RT-PCR was negative but there was high suspicion of COVID 19 infection. In RT-PCR-positive patients, CT was performed to assess CT severity and guide therapeutic management. Scans showing significant background pre-existing/chronic pulmonary parenchymal diseases were excluded. The vaccinated patients had received either the inactivated virus vaccine BBV152 viz. Covaxin® (Bharat Biotech) or the non-replicating viral vector vaccine AZD1222 (ChAdOx1) viz. Covishield® (AstraZeneca, University of Oxford). The clinical severity of the disease was assigned at the time of CT scan and patients with SpO2 < 94% on room air, respiratory rate > 30 breaths/min, patients who were referred from ICU, and patients referred from ward who were on oxygen support were assigned as severe disease.
HRCT of chest
Non-contrast CT scans were performed using 128-slice CT (Lightspeed, GE Medical Systems) in a craniocaudal direction in a single breath-hold, from the lung apices to lateral costophrenic sulci. A helical scan was obtained in supine position with 120 kVp, 220–260 mA, rotation time 0.5 s, pitch 0.984, helical thickness 5 mm, and interslice gap 5 mm, and an additional high-resolution reconstruction was done with a thickness of 1.25 mm and interslice interval of 1.25 mm. Standard protocols for disinfection and sanitization of the scanning rooms and equipment were followed.
Image analysis
For assessing the COVID-19 disease load in the lungs, a 40-point scoring system described by Yang et al. [8] was followed. Based on cross-sectional anatomy, 18 segments of both lungs were divided into 20 sub-segmental regions (Table 1). Opacities in 20 lung regions were subjectively evaluated on HRCT images attributing scores of 0, 1, and 2 if parenchymal opacification involved 0%, less than 50%, and equal or more than 50% of each segmental region, respectively (Fig. 1).
Table 1.
CT severity score (40-point scoring system) for pulmonary involvement of COVID 19 on HRCT
| Right lung | Segment | Involvement | Score | Left lung | Segment | Involvement | Score |
|---|---|---|---|---|---|---|---|
| Right upper lobe | Apical | 0% | 0 | Left upper lobe | Apical | 0% | 0 |
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Anterior | 0% | 0 | Anterior | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Posterior | 0% | 0 | Posterior | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Right middle lobe | Medial | 0% | 0 | Lingular segments | Superior | 0% | 0 |
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Lateral | 0% | 0 | Inferior | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Right lower lobe | Superior | 0% | 0 | Left lower lobe | Superior | 0% | 0 |
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Anterior basal | 0% | 0 | Anterior basal | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Medial basal | 0% | 0 | Medial basal | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Lateral basal | 0% | 0 | Lateral basal | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Posterior basal | 0% | 0 | Posterior basal | 0% | 0 | ||
| < 50% | 1 | < 50% | 1 | ||||
| ≥ 50% | 2 | ≥ 50% | 2 | ||||
| Total scores (right lung) | 20 | Total scores (left lung) | 20 | ||||
Fig. 1.

HRCT thorax (a–f) images in a 36-year-old man with severe COVID-19 pneumonia. a CT images show areas of mixed ground-glass opacification and consolidations with segmental scores indicated in the bracket with each segments as follows. a, b Right upper lobe: apical (1), posterior (1), anterior (1) segments and Left upper lobe apical (0), anterior (0), posterior (0). b–f Right middle lobe medial (1) and lateral segments (1) and left upper lobe superior lingular (1) and inferior lingular (2) segments. b–f Right lower lobe superior (1), posterior basal (2), posterior lateral (2), anterior basal (1), and medial basal (1) and left lower lobe: superior (1), posterior basal (2), posterior lateral (2), anterior basal (1), and medial basal (1). A total CT severity score of 22/40 was assigned in this patient
The CT-SS (hence ranging between 0 and 40) was defined as the sum of scores of all the individual segmental regions. The image review and charting of data were done by two radiologists (A.V. and P.K.S.) each having 17 years of experience. Most entries done by the two observers did not show any difference, but in situations of minor differences, the entry was reviewed by a third senior observer (R.C.S.) with 35 years of experience. All three radiologists evaluated the radiological patterns of involvement and determined a predominant pattern of involvement in consensus as (i) ground-glass opacity, (ii) consolidation, (iii) fibrotic pattern, or (iv) mixed pattern. Radiologists were blinded to clinical data, laboratory data, and the vaccination status of patients. All the section CT images were reviewed in a “lung window” (window width of 1000 to 2000 Hounsfield units; window level of − 700 to − 500 Hounsfield units).
Statistical analysis
Statistics were performed on SPSS® version 22.0 (IBM Corp 2013) using an independent sample Mann–Whitney U test to compare mean values between the two groups and Kruskal–Wallis test to compare mean between more than two groups. The chi-square test was used for nominal data. A p value of < 0.05 was considered significant. A logistic regression was performed to ascertain the effects of age, gender, partial vaccination, complete vaccination, and vaccine type on the likelihood that the patients will have severe disease (CT score ≥ 20).
Results
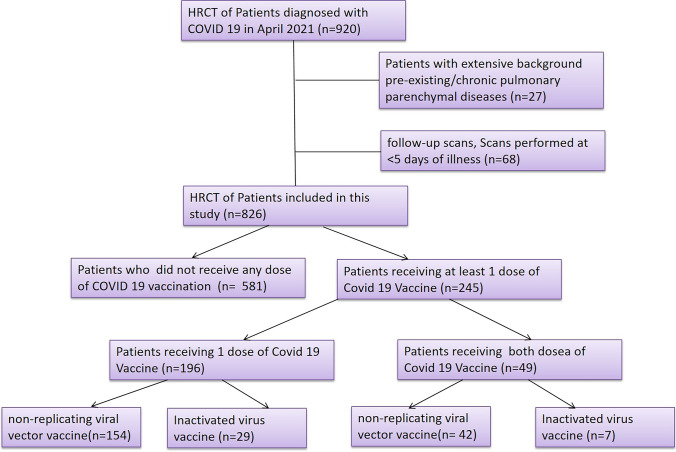
The final dataset consisted of 826 patients (Fig. 2) with a mean age of 44.6 ± 15.6 (median 44 years; range 11–87 years) and a male:female ratio of 13:7 (n = 534 and 292 respectively). The cohort was divided into three groups: (a) the completely vaccinated group receiving two doses of vaccine (n = 49), (b) partially vaccinated receiving one dose of vaccine (n = 196), and (c) the non-vaccinated group (n = 581). Overall, those who had received any vaccination (n = 245) had a mean age of 50.0 ± 14.7 years (median 52 years; range 21–87 years) and the non-vaccinated group (n = 581) had a mean age of 42.4 ± 15.4 years (median 40 years; range 11–88 years). Thirty-six patients had received inactivated viral vaccine (complete vaccination = 7; incomplete vaccination = 29) while 209 patients received non-replicating viral vector vaccine (complete vaccination = 42; incomplete vaccination = 167). Of the total 826 patients, 264 patients had clinically severe disease of which 191 were non-vaccinated, 68 were incompletely vaccinated, and 5 were completely vaccinated. Of these 5 patients in the completely vaccinated group, only one needed ICU management and ventilator support. Of these 264 patients, a CT score ≥ 20 was seen in 165 patients. The sensitivity and specificity of CT severity score equal to or greater than 20 in the prediction of clinically severe disease in our study participants were 62.5% and 98%, respectively, whereas positive and negative predictive values were 96.4% and 85.8% respectively.
Fig. 2.
Flow diagram showing patient enrolment in our study
The mean CT score of all the patients included in the study was 9.7 ± 11.1. The difference between the mean CT score between the vaccinated and non-vaccinated groups was not significant (p = 0.353) (Table 2). However, the patients who received complete vaccination had significantly low meant CT scores (3.5 ± 6.3) in comparison to the incompletely vaccinated patients (10.1 ± 10.5) and non-vaccinated (10.1 ± 11.4) patients. Patients who had received both vaccine doses more than 2 weeks ahead of falling ill (n = 35) showed a further lower mean CT severity score (2.1 ± 4.4) in comparison to those (n = 14) who had symptoms within 2 weeks of vaccination (6.8 ± 9.6). Comparison between the two vaccine types showed that the mean CT severity score was higher in patients receiving inactivated virus vaccine (14.5 ± 12.6) as compared to those receiving non-replicating viral vector vaccine (7.7 ± 9.4).
Table 2.
Multistage intergroup and subgroup analysis among the three groups
| Groups | No of patients (n) | CT severity score (mean ± SD) | p value |
|---|---|---|---|
| Non-vaccinated versus vaccinated patients | |||
| Non-vaccinated | 581 | 10.1 ± 11.5 | 0.3531 |
| Vaccinated | 245 | 8.8 ± 10.1 | |
| Intergroup analysis: non-vaccinated versus partially vaccinated versus completely vaccinated patients | |||
| Group A—non-vaccinated | 581 | 10.1 ± 11.4 |
< 0.0012 0.99 (A versus B) 0.001 (B versus C) < 0.001 (A versus C) |
| Group B—incomplete (1 dose) vaccination | 196 | 10.1 ± 10.5 | |
| Group C—complete (2 doses) vaccination | 49 | 3.5 ± 6.3 | |
| Subgroup analysis: group C | |||
| Within 2 weeks | 14 | 6.8 ± 9.6 | 0.1961 |
| More than 2 weeks | 35 | 2.1 ± 4.4 | |
| Subgroup analysis: vaccine sub-types (groups B and C) | |||
| Non-replicating viral vector vaccine | 209 | 7.7 ± 9.4 | 0.0121 |
| Inactivated virus vaccine | 36 | 14.5 ± 12.6 | |
| Subgroup analysis: vaccine sub-types (group C only) | |||
| Non-replicating viral vector vaccine | 42 | 2.4 ± 4.3 | 0.0471 |
| Inactivated virus vaccine | 7 | 7.7 ± 11.7 | |
1Mann-Whitney U test
2Kruskal-Wallis test
p values < 0.05 are considered significant (bold)
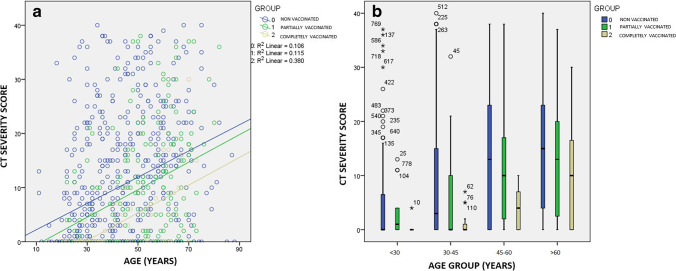
Age-based sub-classification and intergroup comparison (Table 3) of the mean CT scores between the three groups (non-vaccinated, incompletely vaccinated, and completely vaccinated) revealed the mean scores to be significantly lower in completely vaccinated patients of lower ages (< 60 years) while above that age group the difference was not significant (Table 3; Fig. 3b). The scatter plot (Fig. 3a) between the CT score and age in the three groups shows a linear relationship between the two variables. For patients, younger than 60 years old, the mean CT-SS was lower (1.3 ± 2.4) in patients receiving 2 doses more than 2 weeks ago (n = 30) compared to those who were vaccinated (n = 8) with 2 doses within 2 weeks (mean score 4.6 ± 4.5). There were no significant differences in mean CT scores between males and females in both the vaccinated and non-vaccinated groups.
Table 3.
Age and gender based sub-group analysis among the three groups
| Groups | No. of patients (n) | CT severity score (mean ± SD) |
p value |
|---|---|---|---|
| Age-based sub-group analysis | |||
| Age < 30 | |||
| Non-vaccinated | 131 | 4.6 ± 8.2 | 0.1811 |
| Vaccinated | 28 | 1.9 ± 3.7 | |
| Age 30–45 | |||
| Non-vaccinated | 204 | 8.5 ± 10.8 | 0.0031 |
| Vaccinated | 57 | 3.9 ± 6.8 | |
| Age 46–60 | |||
| Non-vaccinated | 151 | 14.1 ± 12.1 | 0.0341 |
| Vaccinated | 76 | 9.9 ± 9.9 | |
| Age > 60 | |||
| Non-vaccinated | 95 | 14.8 ± 11.6 | 0.3851 |
| Vaccinated | 84 | 13.1 ± 11.1 | |
| Gender-based sub-group analysis | |||
| Male | |||
| Non-vaccinated | 373 | 10.7 ± 11.9 | 0.1541 |
| Vaccinated | 161 | 8.5 ± 9.8 | |
| Female | |||
| Non-vaccinated | 208 | 9.2 ± 10.5 | 0.7081 |
| Vaccinated | 84 | 9.5 ± 10.8 | |
| Vaccinated group | |||
| Male | 161 | 8.5 ± 9.8 | 0.4501 |
| Female | 84 | 9.5 ± 10.8 | |
| Non-vaccinated group | |||
| Male | 373 | 10.7 ± 11.9 | 0.3591 |
| Female | 208 | 9.2 ± 10.5 | |
1Mann-Whitney U test
p values < 0.05 are considered significant (bold)
Fig. 3.
a Scatter plot showing correlation of age (in years) with the CT severity score. The central tendency in all three groups shows an ascending trend for the CT-SS as the age increases. Though all groups show a second-order linear correlation, a steeper slope with a higher central tendency is seen in the non-vaccinated group. b Box-whisker analysis of the CT-SS among the groups shows the non-vaccinated patients to have a higher median value in all sub-groups below 60 years of age. Note that the positive outliers are more in number in the non-vaccinated patients
An evaluation of various qualitative radiological findings noted in this cohort among groups with different vaccination statuses was also done. The presence of ground-glass opacities and consolidation was significantly lower in patients with complete vaccination compared to non-vaccinated or partially vaccinated patients. The presence of other findings such as fibrosis, nodules, discoid atelectasis, nodules with halo, and pleural effusion was similar between vaccinated and non-vaccinated patients. Enlarged mediastinal lymph nodes (more than 1 cm in short axis) were found in 7 non-vaccinated patients and were not seen in any patients receiving a vaccination (Table 4). The patients were divided between mild (CT score < 20) and severe disease (CT score ≥ 20). We found that only 1 patient who received both doses of vaccine had severe disease, whereas the incidence of severe disease (based on CT score) was significantly higher in the incomplete vaccine (n = 38) and non-vaccinated patients (n = 132) (p value = 0.003; Likelihood ratio = 17.1). There was no significant difference between various qualitative findings between completely vaccinated patients that become symptomatic more than 2 weeks after the 2nd dose, and those that become symptomatic within 2 weeks. We also evaluated the predominant radiological pattern in the three groups and found that predominant consolidation was most common in the non-vaccinated group (23%) and least common in the completely vaccinated group (6%). Predominant ground-glass opacity pattern was most common in the partially vaccinated group, and mixed pattern (both consolidation and ground-glass opacity) was the most common patterns in the non-vaccinated and completely vaccinated groups.
Table 4.
Distribution of qualitative CT scan features in each of the sub-groups with differential analysis of significance
| Radiological features | Non-vaccinated (n = 581) | Partially vaccinated (n = 196) | Completely vaccinated (n = 49) | p value |
|---|---|---|---|---|
| Ground-glass opacity | 339 | 130 | 18 | < 0.001 |
| Consolidation | 267 | 81 | 6 | < 0.001 |
| Fibrosis | 17 | 10 | 1 | 0.49 |
| Nodules | 11 | 3 | 0 | 0.6 |
| Halo around nodules | 2 | 2 | 0 | 0.42 |
| Pleural effusion | 19 | 2 | 1 | 0.24 |
| Discoid atelectasis | 44 | 18 | 2 | 0.44 |
| Mediastinal lymphadenopathy | 7 | 0 | 0 | 0.23 |
| Predominant radiological pattern of involvement | ||||
| Ground-glass opacity | 208 (36%) | 96 (49%) | 13 (27%) | 0.001 |
| Consolidation | 136 (23%) | 30 (15%) | 3 (6%) | |
| Fibrosis | 12 (2%) | 7 (4%) | 2 (4%) | |
| Mixed | 225 (39%) | 63 (32%) | 31(63%) | |
Chi-square test
p values < 0.05 are considered significant (bold)
Details of logistic regression analysis are presented in Table 5. Lower age, complete vaccination status, and administration of non-replicating viral vector vaccine were significantly associated with a decreased likelihood of getting a severe disease (CT score ≥ 20). Patients receiving 2 doses of vaccine were 14.29 times less likely to get a severe disease.
Table 5.
The results of binomial logistic regression to ascertain the effects of age, gender, partial vaccination, complete vaccination, and vaccine type to predict severe disease (CT-SS ≥ 20)
| Variables | Estimate | SE | Significance | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|
| Gender (male) | .108 | .190 | .569 | .89 | 0.61–1.31 |
| Age | .035 | .006 | < .001 | 1.04 | 1.03–1.05 |
| Partial vaccination | − 1.49 | 1.532 | .334 | 4.45 | 0.21–92.48 |
| Complete vaccination | − 2.66 | 1.151 | .021 | 14.29 | 1.49–136.38 |
| Inactivated virus vaccine | − 2.024 | 1.607 | 0.208 | 7.57 | 0.324–176.77 |
| Non-replicating viral vector vaccine | − 1.562 | .449 | .001 | 4.77 | 1.98–11.51 |
p values < 0.05 are considered significant (bold)
Discussion
In this study, we performed a retrospective analysis of a cohort of vaccinated COVID-19 patients and compared them to non-vaccinated patients scanned in the same time interval to observe the role of the vaccine on CT severity score. This study’s major findings were twofold: first, the fully vaccinated patients who did receive two doses of vaccine had a lower mean CT score than unvaccinated and incompletely vaccinated patients; secondly, the completely vaccinated patients who received both doses more than 2 weeks ago from the onset of clinical symptoms of COVID 19 had a lower CT score than those who were vaccinated less than 2 weeks ago.
Various studies have assessed the safety and efficacy of various vaccines that are being administered and [9–19] and have shown that the administration of vaccine scales down the pulmonary involvement in COVID along with an effect on the systemic inflammatory and coagulopathic responses. Our data provides substantial evidence of effectiveness of the vaccines against severe disease with CT severity score considered an imaging surrogate of the biological activity of the virus. The time interval between the second dose and clinical illness of more than 2 weeks was a significant factor resulting in lower CT severity scores in our study, similar to other studies which evaluated the patients of clinic-pathological parameters only [15–19].
Four stages of evolution of COVID have been identified on HRCT: early stage (0–4 days); progressive stage (5–8 days); peak stage (10–13 days); and absorption stage (≥ 14 days) [7]. Imaging was performed for assessment of severity in most of the patients on the 5th–8th day (progressive stage) as per our Institute’s protocol. We have included the scans performed in this stage in our study as the inclusion of the scans performed in other stages could have a potential confounding effect on interpretation.
The immune response in females has been considered to be superior to that in males both to internal and external antigens [20]. This physiological advantage reflects as much in recovery from diseases as the inefficacy of vaccines to multiple pathogens [21]. The present dataset however shows a paradoxical but insignificantly higher CT severity score in vaccinated females compared to the non-vaccinated females whereas in male vaccinated group showed a marginally lower mean CT score. Gender-based intergroup comparisons (Table 3) however did not yield any significant differences. This may be because the immunological response is known to be affected by the overall nutritional status of a person, which is poorer in females in this region as compared to the developed nations where studies advocating the above notion [20–22] have been conducted. Elderly patients are known to have a poorer outcome with multiple factors being held responsible for a more severe affliction of pulmonary parenchyma in them [23]. CT scan correlates of this clinical assertion have also confirmed the notion with milder and more atypical features seen at younger ages [24–27]. In the present dataset, a comparison between mean CT severity score at various age groups between completely vaccinated, incompletely vaccinated, and non-vaccinated patients was in confirmation to earlier studies with a lower mean score noted in patients less than 45 years. An inverse relation between the age and CT score was more prominent in the group which had received complete vaccination.
Our study also showed that acute air-space and interstitial inflammation characterized by consolidations and ground-glass opacities were significantly lower in the group receiving complete vaccination as compared to the unvaccinated and incompletely vaccinated patients. Accordingly, severe score (CT score ≥ 20) was observed in only a solitary patient who was completely vaccinated and was seen in a greater number of patients who were unvaccinated and incompletely vaccinated. No significant difference in other morphological (sub-acute and chronic) features was however noted among the groups. However, consolidation as a predominant radiological pattern was least common in the completely vaccinated group while it was most common in the non-vaccinated group. This finding may have a potential prognostic value as presence of a consolidation predominant radiological pattern on initial CT chest has been commonly observed in critically ill patients [28].
The present dataset did not chart certain other factors like the presence of co-morbidities, substance abuse, and daily activity levels, which could have possibly affected the severity of affliction of pulmonary parenchyma by SARS-CoV-2. Since all the patients included in this study belonged to same region, hence it seems unlikely that these factors would have been significantly different among individuals in the cohort. Further, in the interest of time and an urgent need to document information related to the SARS-CoV-2 vaccine, we did not consider the analysis of serial scans; this may be of interest to the scientific community especially for correlating the vaccination dose, interval, and type of vaccine with the CT severity score and morphological changes. The low number of patients is also a limitation of the study, especially in the group receiving inactivated virus vaccines. Another limitation of this study is that we did not evaluate the inter-observer agreement between the two radiologists in the study. Finally, we did not correlate the CT severity scores with the final outcome because the outcome was affected by many confounding factors such as limited availability of hospital beds and supply of medications during the pandemic.
Conclusion
In this retrospective study, patients receiving both doses of the SARS-CoV-2 vaccine showed lower CT severity scores in comparison to those who received only one dose or those who did not receive the vaccine at all. The time elapsed between vaccination and onset of clinical symptoms affects the pulmonary changes as seen on HRCT of the lung, with the patients who developed COVID symptoms 2 weeks or more after the second dose of vaccine showing lower CT severity scores. With CT severity score as the bio-surrogate, both vaccines analyzed together performed better in completely vaccinated patients less than 45 years of age in comparison to older patients. Finally, the pulmonary HRCT changes were more severe in non-vaccinated and incompletely vaccinated patients as compared to the completely vaccinated individuals.
Abbreviations
- COVID-19
Coronavirus disease 19
- HRCT
High-resolution computed tomography
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Prof. Ashish Verma.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Dr. Ishan Kumar, has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Footnotes
Ashish Verma and Ishan Kumar are first co-authors as their contribution is equal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pontone G, Scafuri S, Mancini ME, et al. Role of computed tomography in COVID-19. J Cardiovasc Comput Tomogr. 2021;15:27–36. doi: 10.1016/j.jcct.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollineni VR, Nieboer KH, Döring S, Buls N, de Mey J. The role of CT imaging for management of COVID-19 in epidemic area: early experience from a University Hospital. Insights Imaging. 2021;12:10. doi: 10.1186/s13244-020-00957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hope MD, Raptis CA, Shah A, Hammar MH, Henry TS. A role for CT in COVID-19? What data really tell us so far. Lancet. 2021;395(10231):P1189–1190. doi: 10.1016/S0140-6736(20)30728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elicker BM. What is the performance and role of CT in suspected COVID-19 infection? Radiology. 2021;298(2):E109–E111. doi: 10.1148/radiol.20202040130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Radiology (2020) ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-PositionStatements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed 23 Mar 2020
- 7.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging. 2020;2(2):e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson EJ, Rouphael NG, Widge AT et al (2020) for mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383(25):2427-2438 [DOI] [PMC free article] [PubMed]
- 14.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill EM, Keeling MJ. Comparison between one and two dose SARS-CoV-2 vaccine prioritization for a fixed number of vaccine doses. Medrxiv. 2021;03(15):21253542. doi: 10.1101/2021.03.15.21253542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaway E, Mallapaty S. What scientists do and don’t know about the Oxford– Astrazeneca covid vaccine- results confirming that the vaccine provides strong protection against COVID-19 were welcomed after a pause in roll-outs. Nature. 2021;592:15–17. doi: 10.1038/d41586-021-00241-6. [DOI] [PubMed] [Google Scholar]
- 18.Callaway E. Why Oxford’s positive COVID vaccine results are puzzling scientist’s: preliminary data suggest that the immunization was more effective in trial participants who received a lower dose. Nature. 2020;588:16–18. doi: 10.1038/d41586-020-03326-w. [DOI] [PubMed] [Google Scholar]
- 19.Voysey M, Clemens SAC, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 21.McCartney PR. Sex-based vaccine response in the context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020;49:405–408. doi: 10.1016/j.jogn.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fathi A, Addo MM, Dahlke C. Sex differences in immunity: implications for the development of novel vaccines against emerging pathogens. Front Immunol. 2020;11:601170. doi: 10.3389/fimmu.2020.601170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging. 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azadbakht J, Haghi-Aminjan H, Farhood B. Chest CT findings of COVID-19-infected patients, are there differences between pediatric and adult patients? A systematic review. Egypt J Radiol Nucl Med. 2020;51:145. doi: 10.1186/s43055-020-00261-8. [DOI] [Google Scholar]
- 27.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabatabaei SMH, Talari H, Moghaddas F, Rajebi H. CT features and short-term prognosis of COVID-19 pneumonia: a single-center study from Kashan. Radiol Cardiothorac Imaging. 2020;2(2):e200130. doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]