ABSTRACT
Purpose
Although several mechanisms have been proposed for the tumor-suppressive effect of exercise, little attention has been given to myokines, even though skeletal muscle is heavily recruited during exercise resulting in myokine surges. We measured resting serum myokine levels before and after an exercise-based intervention and the effect of this serum on prostate cancer cell growth.
Methods
Ten prostate cancer patients undertaking androgen deprivation therapy (age, 73.3 ± 5.6 yr) undertook a 12-wk exercise-based intervention including supervised resistance training, self-directed aerobic exercise, and protein supplementation. Body composition was assessed by dual-energy x-ray absorptiometry and muscle strength by the one-repetition maximum method. Fasting blood was collected at baseline and postintervention, and serum levels of myokines—secreted protein acidic and rich in cysteine, oncostatin M (OSM), decorin, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 (IGFBP-3)—were measured. The growth of the prostate cancer cell line DU145 with baseline and postintervention serum was measured.
Results
Body weight (P = 0.011), fat mass (P = 0.012), and percent body fat (P = 0.033) were reduced, whereas percent lean mass (P = 0.001) increased, as did strength (leg press, P = 0.006; chest press, P = 0.020) across the intervention. Serum OSM levels (P = 0.020) and relative serum OSM levels (P = 0.020) increased compared with baseline. A significant reduction in DU145 Cell Index (P = 0.012) and growth rate (P = 0.012) was observed after applying postintervention serum compared with baseline serum.
Conclusions
This study provides evidence for enhanced myokine expression and tumor-suppressive effects of serum from chronically exercise-trained prostate cancer patients on androgen deprivation therapy.
Key Words: EXERCISE ONCOLOGY, EXERCISE, MYOKINE, PROSTATE CANCER
Exercise oncology, the application of exercise medicine in cancer, has been rapidly evolving in the clinical oncology field and has provided a strong basis for cancer patients to be engaged in physical activity or exercise (1). Moreover, epidemiological studies in the field have consistently shown an inverse association between physical activity and disease progression as well as cancer-specific mortality in patients, including those with prostate cancer, which is the most common nonskin cancer in men (2–4). As such, multiple studies have been undertaken to investigate the potential of exercise in providing benefits for prostate cancer patients, including the potential direct tumor-suppressive effect of exercise (5).
Among the exercise-induced physiological alterations, systemic alteration of cell-free/soluble content has been suggested as a potential mechanism for providing clinical benefits by retarding prostate cancer growth. Rundqvist and colleagues (6) demonstrated a 31% reduction in prostate cancer cell line LNCaP viability with an application of serum acquired after 60 min of aerobic exercise by healthy men compared with serum acquired before exercise. In addition, a study investigating the effect of serum acquired from healthy subjects after 11 d of intense exercise plus dietary intervention also demonstrated a 30% reduction of prostate cancer cell line (LNCaP) growth when postintervention serum at rest was applied to prostate cancer cell lines (7). Similarly, the direct application of serum acquired from active, healthy subjects also reduced prostate cancer cell line LNCaP growth (27%) compared with sedentary subjects (8). However, the exercise-conditioned serum samples, both immediately after the exercise bout and resting serum after exercise intervention, were acquired from healthy subjects, and the authors did not report the effect of potential physiological differences that prostate cancer patients may experience by disease or treatments, such as androgen deprivation therapy (ADT).
ADT is an effective therapy for preventing prostate cancer growth and is a commonly prescribed treatment for prostate cancer patients at all stages (9). However, prolonged ADT may promote castrate-resistant prostate cancer (10), which dramatically reduces patient survival (11). The benefits of exercise for prostate cancer patients undergoing ADT have been demonstrated in multiple studies investigating the efficacy of exercise in attenuating treatment toxicities (2,12–14). For instance, we (13,15–18) and others (12,14) have demonstrated enhancement of physical outcomes such as muscle strength, lean mass (LM), and physical function, and patient-reported outcomes such as cancer-related fatigue and quality of life in these patients as a result of an exercise intervention. However, no study has investigated the potential of exercise-induced physiological changes in direct tumor suppression in prostate cancer patients undergoing ADT.
The role of skeletal muscle as a secretory organ has been proposed since the early 2000s (19,20). The beneficial roles of skeletal muscle secretomes called myokines (also known as exerkines) in muscle hypertrophy and ameliorating metabolic disease have been studied extensively in exercise medicine (19,21–24). Furthermore, preclinical studies that have applied myokines, such as secreted protein acidic and rich in cysteine (SPARC) (25–27), oncostatin M (OSM) (28,29), decorin (30,31), IL-6 (32), IL-15 (33), and irisin (34,35), to various types of cancer cell lines have also provided evidence for the potential direct tumor-suppressive role of myokines, including in prostate cancer. However, because of the lack of clinical studies investigating myokine expression among prostate cancer patients, these myokines are only a candidate molecular player for tumor suppression (5). Thus, the purposes of the current exploratory study were to investigate the effect of a 12-wk exercise-based intervention on serum levels of myokines in prostate cancer patients undertaking ADT and to examine the growth-suppressive effect of the exercise-conditioned serum.
METHODS
Participants
Recruitment for the study has been previously described (36). Briefly, 54 men with prostate cancer were contacted initially from February 2018 to June 2019. The initial contact was made through clinical referral, prostate cancer support groups, and advertisements and progressed through the study as shown in Figure 1. The inclusion criteria were as follows: undertaking ADT for at least 6 months, remaining on ADT for the entire study period, and having a body fat percentage ≥25%. Patients with bone metastases, a secondary cancer diagnosis, and musculoskeletal or uncontrolled medical conditions preventing patients from undertaking moderate- to high-intensity exercise were excluded from the study. As a result, 11 patients completed the intervention with data from 10 patients analyzed for this report, as one patient with a very high body mass index (outlier ≥45 kg·m−2) was excluded. The study was approved by the Edith Cowan University Human Research Ethics Committee (ID: 18832). Informed consent was obtained from all subjects involved in the study before inclusion.
FIGURE 1.
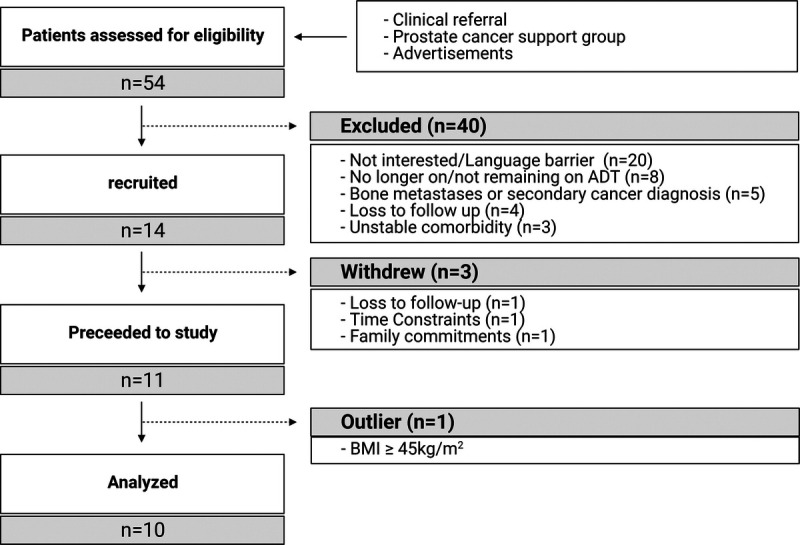
CONSORT diagram. BMI, body mass index.
Twelve-week exercise intervention
The 12-wk intervention was designed so that patients accumulated 300 min of exercise weekly and has been previously described (36). Briefly, patients undertook three clinic-based supervised resistance exercise sessions per week for 12 wk. The program was progressive in nature and comprised five to nine resistance exercises targeting the major muscle groups of the upper and lower body, with one to four sets per exercise at a 6- to 12-repetition maximum intensity. Each exercise session was ~1 h in duration, including a 5- to 10-min aerobic warm-up and cool-down. The patients were also asked to undertake daily self-directed moderate to vigorous-intensity aerobic exercise (rating of perceived exertion, 3–8 on the 0–10 Borg scale). In addition, patients were provided with two consultations with an Accredited Practicing Dietician (week 1 and week 3 of intervention) to modify food intake to induce a caloric deficit of 500–1000 kcal·wk−1. To support muscle protein synthesis, patients consumed 40 g of whey protein supplement (Whey Protein Concentrate; Bulk Nutrients, Tasmania, Australia) immediately after each clinic-based exercise session.
Body composition and muscle strength
Body composition was assessed at baseline and postintervention by dual-energy x-ray absorptiometry (Horizon A; Hologic, Washington, DC). Values derived were whole-body LM (in kilograms), upper body LM (in kilograms), lower body LM (in kilograms), whole-body fat mass (FM; in kilograms), LM percent, FM percent, and appendicular LM, which is the sum of upper and lower limb LM. Neuromuscular strengths for the chest press, leg press, and seated row exercises were determined using the one-repetition maximum (1RM) method at baseline and postintervention (35). Each patient’s upper body and lower body relative strength was also calculated by dividing muscle strength (1RM for chest press, seated row, and leg press) by the upper body (upper limb LM + trunk LM) or lower body LM (lower limb LM).
Blood assessment and analysis
Resting fasting venous blood samples (at least 10 h of overnight fasting) were collected before any physical tests on the day of baseline and postintervention body composition assessments using serum separating tubes. After blood collection, the blood was left at room temperature for 30 min and then immediately centrifuged at 1600g for 10 min. The centrifuged blood samples were transported to the Exercise Medicine Research Institute (Joondalup, WA, Australia), and the supernatant was carefully aliquoted. The serum samples were then stored in a −80°C freezer at the Exercise Medicine Research Institute until further analysis. Serum levels of SPARC, OSM, IL-6, IL-15, and irisin were analyzed using a multiplexed magnetic bead–based immunoassay (MILLPLEX human myokine magnetic bead panel; Millipore, Burlington, MA), and serum levels of decorin, insulin-like growth factor-1 (IGF-1), and insulin-like growth factor binding protein-3 (IGFBP-3) were analyzed using an enzyme-linked immunosorbent assay (Human decorin ELISA kit, Human IGF1 SimpleStep ELISA kit, Human IGFBP-3 SimpleStep ELISA kit; Abcam, Cambridge, United Kingdom).
Cell culture and real-time cellular analysis
The human prostate cancer cell line, DU145 (ATCC HTB-81), was obtained from The Harry Perkins Institute, Nedlands, WA, Australia. DU145 cells were used because they are androgen insensitive and would minimize the potential effect of androgen in serum samples. The cells were cultured in RPMI-1640 media containing 10% fetal bovine serum and incubated in a 37°C, 5% CO2 incubator and routinely passaged at ~80% confluence. The growth of DU145 cells was assessed using a Real-Time Cellular Analysis system, xCELLigence DP unit (ACEA Bioscience, San Diego, CA), in the presence of human serum. The xCELLigence DP unit allows for assessment of the cell number in the unit of the Cell Index in real time by recording electrical impedance passing through the bottom of the specialized well plates in a specific time frame. Wells were seeded with 15,000 cells onto the E-plate (ACEA Bioscience) with 100 μL of serum-free RPMI-1640. After 24 h of starvation, 100 μL of growth media (RPMI-1640) containing 20% human serum (final concentration of 10%) was added to each well of the E-plate. The plates were incubated in the xCELLigence DP unit for 3 d (72 h) while recording the Cell Index every hour.
Statistical analysis
The data were analyzed using R software (Version 4.0.2; The R Foundation), with the rstatix (v0.7.0, Kassambra, 2021) and ggplot2 (v3.3.3, Wickham, 2020) packages for statistics and graphical visualization. All values are presented as the mean ± SD, with change values presented as the mean and 95% confidence intervals (CI). The normality of the distribution for outcome measures was tested using the Shapiro–Wilk test and Q–Q plots. A paired-sample t-test and Wilcoxon signed-rank test were used, as appropriate, to investigate the mean difference between baseline and postintervention outcomes, including body composition, physical outcomes, serum myokine levels, Cell Index at each incubation time point, and growth rate of the cells. Spearman’s rank-order correlation was used to investigate the association between physical outcomes and the serum level of myokines. All tests were two-tailed, with statistical significance set at P < 0.05.
RESULTS
Body composition and strength
The mean age of the patients was 73.3 ± 5.6 yr, with an average of 21.2 ± 15.9 months since ADT was initiated. All patients had a Gleason score ≥7, with four patients reporting lung or lymph node metastases. The change in body composition across the 12-wk period was significant for total body weight (Δtotal body weight = −3.65 kg; 95% CI, −6.22 to −1.09; P = 0.011), percent LM (Δpercent LM = +2.02%; 95% CI, 1.04 to 2.99; P = 0.001), total FM (Δtotal FM = −2.55 kg; 95% CI, −4.39 to −0.71; P = 0.012), and percent FM (Δpercent FM = −1.25%; 95% CI, −2.37 to −0.12; P = 0.033; Table 1, Fig. 2). In contrast, there was no significant change in total LM or appendicular LM. A significant increase in leg press (Δleg press = +25.84 kg; 95% CI, 6.07 to 45.60; P = 0.006) and chest press (n = 9, Δchest press = +7.43 kg; 95% CI, 1.53 to 13.34; P = 0.020) muscle strength was observed after 12-wk training, with a trend for an increase in seated row strength (P = 0.081; Table 1, Fig. 2). Relative strength also increased significantly over the 12-wk intervention when the 1RM for leg press was divided by lower body LM (Δrelative lower body strength = +1.60; 95% CI, 0.40 to 2.80; P = 0.002) and the 1RM for chest press was divided by upper body LM (Δrelative upperbody strength = +0.23; 95% CI, 0.03 to 0.42; P = 0.027; Table 1, Fig. 2). A trend of an increase was also shown in relative upper body strength when the 1RM for seated row (P = 0.064) was divided by upper body LM (Table 1, Fig. 2).
TABLE 1.
Changes in physical outcomes and serum myokines levels before and after 12 wk of exercise intervention.
| Baseline, Mean ± SD | Postintervention, Mean ± SD | P | |
|---|---|---|---|
| Body composition | |||
| Weight (kg) | 95.89 ± 13.51 | 92.24 ± 12.74 | 0.011 |
| Total LM (kg) | 55.97 ± 6.85 | 55.66 ± 6.50 | 0.503 |
| LM percent (%) | 58.56 ± 2.77 | 60.58 ± 3.33 | 0.001 |
| Appendicular LM (kg) | 23.13 ± 3.42 | 23.20 ± 3.33 | 0.817 |
| Appendicular LM index (kg·m−2) | 8.01 ± 1.20 | 8.02 ± 1.01 | 0.930 |
| Total FM (kg) | 37.56 ± 7.40 | 35.01 ± 7.04 | 0.012 |
| FM percent (%) | 38.97 ± 3.27 | 37.72 ± 3.21 | 0.033 |
| Body mass index (kg·m−2) | 33.15 ± 3.69 | 31.81 ± 3.13 | 0.007 |
| Strength (1RM) | |||
| Leg press (kg)* | 84.4 (69.71–115.12) | 96.8 (92.12–144.44) | 0.006 |
| Chest press (kg)† | 45.26 ± 13.18 | 52.69 ± 13.55 | 0.020 |
| Seated row (kg) | 65.02 ± 7.64 | 67.52 ± 8.07 | 0.081 |
| Relative strength | |||
| Leg press/lower body LM* | 5.45 (4.36–6.91) | 6.28 (5.94–8.52) | 0.002 |
| Chest press/upper body LM | 1.30 ± 0.51 | 1.51 ± 0.49 | 0.027 |
| Seated row/upper body LM* | 1.75 (1.73–1.97) | 1.91 (1.76–2.13) | 0.064 |
| Serum myokine and growth hormone level | |||
| OSM (pg·mL−1)* | 3.06 (5.46–7.55) | 5.57 (3.80–18.61) | 0.020 |
| SPARC (ng·mL−1)* | 471.13 (434.15–585.31) | 514.96 (583.28–724.10) | 0.065 |
| Decorin (pg·mL−1) | 47.91 ± 8.74 | 49.55 ± 12.56 | 0.527 |
| IGF-1 (pg·mL−1) | 975.24 ± 426.04 | 985.71 ± 613.69 | 0.774 |
| IGFBP-3 (pg·mL−1) | 15827.08 ± 5863.93 | 13820.48 ± 5369.70 | 0.033 |
| IGF-1:IGFBP-3 ratio | 0.059 ± 0.020 | 0.064 ± 0.032 | 0.680 |
The P < 0.05 is indicated as bold text.
*P values are obtained by the Wilcoxon signed-rank test, and the results are presented as median (interquartile range).
†The result from only nine patients because 1 patient was not able to perform the chest press.
FIGURE 2.
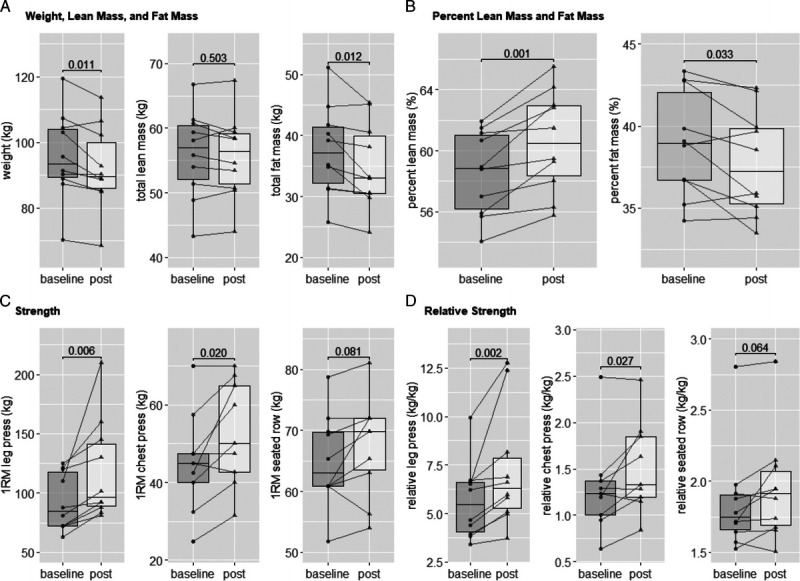
Graphical illustration of individual data of body composition and strength. A, Whole body weight, total LM, and FM. B, Total LM and FM percent. C, 1RM strength test of leg press, chest press, and seated row. D, 1RM strength divided by upper or lower body part LM. Lines and dots indicate individual data, with box-and-whisker representation of the whole group; P values are presented for each comparison. The chest press results were only available for nine patients.
Serum Myokine, IGF-1, and IGFBP-3 levels
Serum concentrations of six different myokines (SPARC, OSM, decorin, IL-6, IL-15, and irisin) and IGF-1 and IGFBP-3 were analyzed from serum acquired at baseline and postintervention. However, only OSM, SPARC, decorin, IGF-1, and IGFBP-3 were able to be analyzed because of the low recovery of other myokines. The serum levels of IL-6, IL-15, irisin, and myostatin were not detectable above the threshold using the commercially available multiplexed magnetic bead–based immunoassay. A significant increase was observed in serum OSM levels (ΔOSM = + 4.70 ng·mL−1; 95% CI, 0.49 to 8.91; P = 0.027) and body mass relative OSM levels (serum OMS levels/total body mass; Δrelative OSM = + 0.06 ng·mL−1·kg−1; 95% CI, 0.01 to 0.10; P = 0.020) before to after the 12-wk intervention (Table 1, Fig. 3). A trend for an increase in serum SPARC levels was also observed (P = 0.065), but postintervention relative SPARC levels (serum SPARC levels/total body mass) were not changed compared with baseline (Table 1, Fig. 3). Serum levels of decorin and relative decorin levels did not significantly change as a result of the intervention (Table 1, Fig. 3). For growth hormone, serum IGFBP-3 levels were significantly reduced after the 12-wk intervention (ΔIGFBP-3 = −2006.60 ng·mL−1·kg−1; 95% CI, −3807.70 to −205.51; P = 0.033); however, serum IGF-1 and the IGF-1:IGFBP-3 ratio did not significantly change (Table 1). Furthermore, there was a positive correlation between change of relative OSM levels (ΔOSM serum levels/Δtotal body mass) and change of relative LM (Δtotal LM/Δtotal body mass; R = 0.67, P = 0.039), but not for relative SPARC (ΔSPARC levels/Δtotal body mass) or decorin (Δdecorin levels/Δtotal body mass) levels with a change of relative LM (ΔLM/Δtotal body mass (Fig. 3).
FIGURE 3.
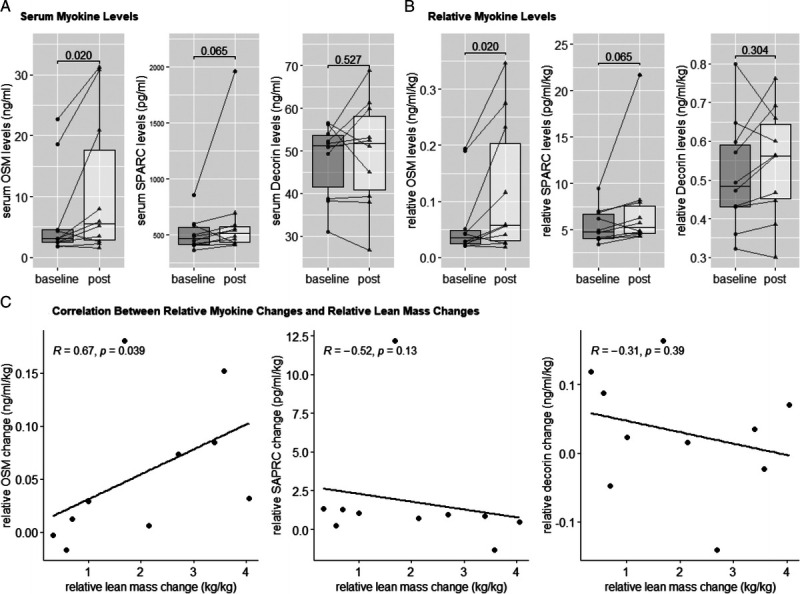
Serum myokine levels. A, Serum levels of OSM, SPARC, and decorin. B, Relative serum levels of OSM, SPARC, and decorin (serum myokine levels/total body mass). *Significant changes over a 12-wk exercise intervention. C, Correlation analysis of change values of relative myokine (OSM, SPARC, and decorin) levels and change values of relative LM over a 12-wk exercise intervention. Lines and dots indicate individual data, with box-and-whisker representation of the whole group; P values are presented for each comparison. Spearman R and P values are indicated for each correlation.
Prostate cancer cell line growth
After 24 h of the starvation period (−23 to 0 h in Fig. 4A), the growth media containing 10% serum acquired from 10 prostate cancer patients undertaking ADT before and after the 12-wk intervention was applied to the DU145 prostate cancer cell lines and incubated for 72 h. However, the serum from one participant was contaminated; therefore, only sera from nine patients were analyzed. The Cell Index was recorded every 15 min, and the time-course changes of the Cell Index are presented every hour in Figure 4. Cell growth presented a particular pattern with similar growth during the first 24 h of culture (period (a) in Fig. 4A) after the addition of the patient’s serum from before and after the exercise intervention. Curves separate during thereafter (period (b) in Figure 4A). Cell Index at 72 h was significantly lower for cells cultured with serum acquired after the 12-wk intervention compared with cells treated with baseline serum (Table 1, Fig. 4B). Overall, the mean Cell Index at 72 h was reduced by 21.3% (baseline: 5.829 ± 1.112, postintervention: 4.566 ± 1.515; P = 0.012). The average growth rate of the cells over 72 h was significantly reduced by 22.5% (baseline: 0.080 ± 0.016, postintervention: 0.062 ± 0.021; P = 0.012) when the postintervention serum was applied (Fig. 4C). This reduction in cell growth was particularly apparent after 25 h of incubation (Figs. 4A, D), becoming significantly different after 45 h (Fig. 4A).
FIGURE 4.
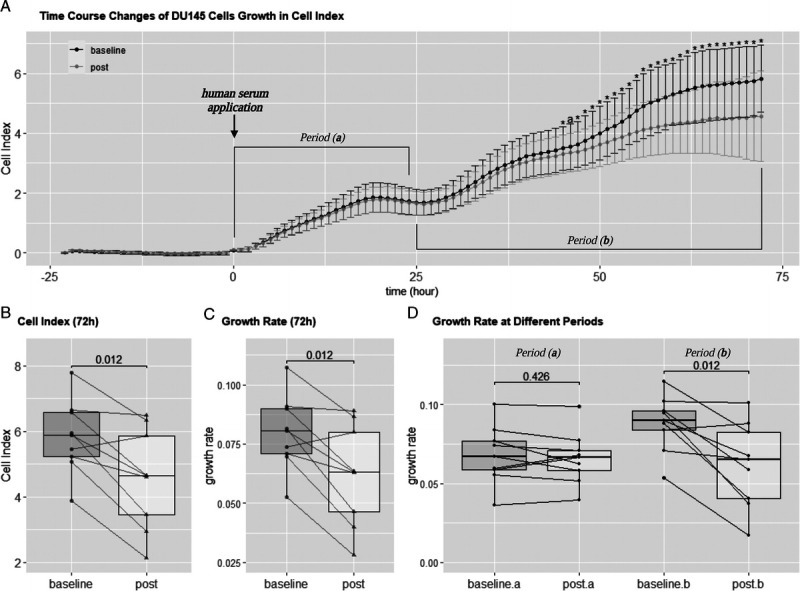
Real-Time Cellular Analysis. A, Time-course changes of DU145 cell growth in the Cell Index after application of serum acquired from prostate cancer patients undertaking ADT before and after a 12-wk exercise-based intervention (mean ± SD). DU145 cells were starved for 24 h, and growth media containing 10% serum from baseline and postintervention was applied at time point 0 h. *Significant difference (P < 0.05). aA strong trend of difference (P < 0.07) at the same incubation time point. B, Cell Index at 72-h point. C, Average growth rate over 72 h. D, Average growth rate at periods (a) and (b) from the time-course change of DU145 cell growth. Lines and dots indicate individual data; significance levels are presented. Period (a): 0–24 h; period (b): 25–72 h.
DISCUSSION
The aim of this exploratory study was to examine the effect of a 12-wk exercise-based intervention on potential muscle-induced candidates for tumor suppression in prostate cancer patients undertaking ADT. We confirmed that the exercise program was accompanied by an increase in myokine expression, specifically OSM, with a change in relative LM positively associated with myokine expression. Moreover, we examined the effect of serum conditioned by 12 wk of exercise training on the prostate cancer cell line DU145. Although we could not signify a direct relationship between muscle-induced myokines and prostate cancer cell line growth, the postintervention serum drawn from patients in a rested state significantly reduced DU145 cancer cell growth.
The benefit of exercise for prostate cancer patients undertaking ADT is well established. Hormone suppression has been shown to result in numerous treatment-related adverse effects and significantly reduces LM and increases FM, especially in the first year of treatment (37). Reduction of LM and increase in FM can result in sarcopenic obesity, alteration in cell-free/soluble blood contents, and metabolic imbalance resulting in poorer disease outcomes (9–11,38,39). However, in patients with prostate cancer on ADT, exercise has been shown to enhance body composition and muscle strength, and improve patient-reported outcomes (13,15–18). Although the current study was a single group design, our findings confirm the benefits of exercise in improving physical outcomes, such as body composition and muscle strength of patients undertaking ADT.
The current study is the first to examine myokine expression before and after an exercise training intervention in prostate cancer patients undertaking ADT. Although multiple studies in exercise oncology have investigated the effect of exercise on the systemic milieu in prostate cancer patients, these studies only focused on immune markers and adipokines (40,41). However, it should be noted that, although skeletal muscle is now recognized as having an extensive endocrine function and is the organ involved most during exercise, limited studies have explored the role of myokines in direct tumor suppression (5). In addition, blood was collected after complete rest, that is, no exercise or vigorous exercise within 48 h before blood collection. This was undertaken to eliminate the acute physical arousal as a result of the last exercise session in order to examine the effect of exercise adaptation on the systemic milieu in patients with localized prostate cancer.
We sought to examine six different myokines, SPARC, OSM, decorin, IL-6, IL-15, and irisin, that have previously been shown in preclinical studies a exert a direct tumor-suppressive effect on cancer cell lines (5). Furthermore, a higher volume and intensity of exercise was prescribed for participants in this trial than in previous (42) and current exercise guidelines for cancer patients (43). This might have resulted in a sufficient stimulus for myokine expression in the circulatory system (5). Although IL-6, IL-15, and irisin were not able to be analyzed because of the low recovery rate of the magnetic bead–based immunoassay analysis, we were able to analyze serum OSM, SPARC, and decorin levels and showed a significant elevation of serum OSM levels and a trend for an elevation of serum SPARC levels after the 12-wk intervention. Furthermore, a positive correlation was shown between the relative change in LM and serum OSM levels. Our findings are encouraging, but there is a need for a larger randomized trial to investigate the effect of exercise on serum myokine levels and also to examine the threshold exercise intensity/volume required for chronic myokine adaptations in prostate cancer patients undertaking ADT. Although it was very important to demonstrate altered myokine levels and tumor suppression from blood collected from trained patients with prostate cancer at rest, the next logical step is to evaluate the effect of an acute bout of exercise in these patients.
It has been demonstrated from multiple studies that acute or chronic alteration of cell-free/soluble content might reduce growth in a range of cancer cells (6–8,44–46). Research by Rundqvist et al. (6) and Hwang et al. (44) showed the potential of exercise to directly suppress prostate cancer cell growth by demonstrating the reduction of LNCaP growth after directly applying serum acquired after acute exercise. In addition, these studies also demonstrated alteration in levels of EGF, IGFBP-1, and the myokines OSM and SPARC. Although the physiological and cellular response to acute exercise-induced alteration of cell-free/soluble contents may differ in prostate cancer patients compared with serum content alteration induced by adaptation to chronic exercise, there is increasing evidence that activated skeletal muscle secretes factors highly influential in tumor suppression. Moreover, the studies by Barnard et al. (46), Leung et al. (8), and Ngo et al. (7) reported the potential of the tumor-suppressive effect of cell-free/soluble components in blood by showing a significant reduction in LNCaP growth and an increase of p53 protein after direct application of serum acquired from a population with regular exercise or after a short, intense exercise–diet intervention. However, the blood from these studies was collected from young, healthy subjects, and the studies did not report the physiological difference between prostate cancer patients undertaking ADT and the healthy population.
Our examination of the effect of exercise-induced serum content alteration on prostate cancer cell lines follows the framework of previous studies (5). As such, we collected serum from patients before and after the 12-wk exercise-based intervention and applied it directly to the prostate cancer cell line DU145. The Cell Indexes and growth rates were significantly reduced when serum acquired after the training period was applied to DU145 cells compared with when preintervention serum was applied. Our results are in contrast with those of Dethlefsen et al. (45) and Devin et al. (47), who reported no tumor-suppressive effect after applying serum acquired from breast cancer patients and colon cancer survivors, respectively, to breast and colon cancer cell lines before compared with after an exercise training intervention. The difference in patient population and cancer cell type in the studies by Dethlefsen et al. (45) and Devin et al. (47) compared with our study may have resulted in disparate outcomes. However, outcomes from the studies conducted by Barnard et al. (46), Leung et al. (8), and Ngo et al. (7) were similar to the current study, indicating serum content alteration via exercise training may have a role in suppressing disease progression by directly reducing prostate cancer cell growth.
The current exploratory study has some limitations. Because this was a 12-wk trial, we were unable to examine the effect of exercise on disease progression or regression in our patient cohort. Moreover, the program contained a dietary component aiming to reduce body fat. As such, we can not conclude that elevations in serum myokines and physical improvements were solely due to exercise training. For instance, we have also provided the two nutrition consultations and protein supplements throughout intervention; we could not analyze how these dietary components affected the results independently to the exercise, although the previous report from Wilson et al. (36) have demonstrated no significant changes in the caloric intakes during a 12-wk weight loss program in obese prostate cancer patients undertaking ADT. In addition, although we reported an increase in serum myokine levels and a significant reduction of DU145 cell growth after applying exercise-conditioned-human serum, the current study is limited to in-depth intercellular mechanistic measures to signify the tumor-suppressive role of exercise-induced myokines.
Despite these limitations, the current study has strengths that are worthy of comment. We report the effect of exercise adaptation after 12 wk of supervised exercise intervention with dual-energy x-ray absorptiometry used for the whole body and regional body composition assessment providing accurate measures of fat and lean tissue. Importantly, the study provided clinically relevant evidence for tumor suppression using serum acquired from prostate cancer patients undertaking ADT. The data to date for myokine expression research have been limited to either healthy cohorts or those with metabolic disease (5). However, here we report myokine expression in prostate cancer patients on ADT after exercise training for the first time, demonstrating that these patients retain the capacity to alter myokine expression through exercise and that the altered serum contents may be directed to prostate cancer cell growth suppression.
CONCLUSIONS
The current study provides evidence for enhanced myokine expression and a resulting tumor-suppressive effect of exercised-conditioned serum collected at rest in prostate cancer patients on hormone suppression after a short-term exercise-based intervention. Importantly, myokine expression was correlated with the relative change in LM, emphasizing the importance of maintaining or increasing muscle mass in these patients. Future randomized controlled trials are needed to further elucidate the influence of exercise on myokine expression, particularly specifics of exercise prescription such as threshold exercise intensity, volume, and mode for optimizing myokine expression. Although we have demonstrated highly beneficial chronic effects of exercise, the acute responses and implications for cancer biology of a single bout (dose) of exercise in patients with cancer undergoing different treatments need to be explored. More in-depth intercellular mechanistic research involving the application of both acute and chronically exercise-conditioned human serum is also required to enhance our understanding of the direct tumor-suppressive role of myokines in prostate cancer patients undertaking ADT.
Acknowledgments
J.-S. K. is supported by a National Health and Research Council Centre of Research Excellence (NHMRC-CRE) in Prostate Cancer Survivorship Scholarship. D. A. G. and R. U. N. are funded by an NHMRC-CRE in Prostate Cancer Survivorship. R. L. W. is supported by an Australian Government Research Training Program Scholarship. NHMRC-CRE funding supported this research.
The authors declare no conflict of interest. The results of the study are presented clearly, honestly, without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sport Medicine.
J.-S. K., D. R. T., D. A. G., E. G., and R. U. N. conceptualized the study. J.-S. K. and R. L. W. designed the study. J. -S. K. and R. L. W. collected the data. D. R. T., D. A. G., E. G., and R. U. N. supervised the study. J.-S. K. wrote the original draft. J.-S. K., D. R. T., D. A. G., E. G., and R. U. N. reviewed and edited the manuscript before submission.
Contributor Information
REBEKAH L. WILSON, Email: rebekahl_wilson@dfci.harvard.edu.
DENNIS R. TAAFFE, Email: d.taaffe@ecu.edu.au.
DANIEL A. GALVÃO, Email: d.galvao@ecu.edu.au.
ELIN GRAY, Email: e.gray@ecu.edu.au.
ROBERT U. NEWTON, Email: r.newton@ecu.edu.au.
REFERENCES
- 1.Schmitz KH Campbell AM Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunnell AS Joyce S Tomlin S, et al. Physical activity and survival among long-term cancer survivor and non-cancer cohorts. Front Public Health. 2017;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71(11):3889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J-S, Galvão DA, Newton RU, Grey E, Taaffe DR. Exercise-induced myokines and their effect on prostate cancer. Nat Rev Urol. 2021;18(9):519–42. Epub ahead of print, June 22, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Rundqvist H Augsten M Stromberg A, et al. Effect of acute exercise on prostate cancer cell growth. PLoS One. 2013;8(7):e67579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144(6):2319–24. [DOI] [PubMed] [Google Scholar]
- 8.Leung P-S, Aronson WJ, Ngo TH, Golding LA, Barnard RJ. Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol (1985). 2004;96(2):450–4. [DOI] [PubMed] [Google Scholar]
- 9.Datta D, Aftabuddin M, Gupta DK, Raha S, Sen P. Human prostate cancer hallmarks map. Sci Rep. 2016;6:30691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget. 2016;7(39):64447–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. [DOI] [PubMed] [Google Scholar]
- 12.Bourke L Smith D Steed L, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 13.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–7. [DOI] [PubMed] [Google Scholar]
- 14.Shephard RJ. Physical activity and prostate cancer: an updated review. Sports Med. 2017;47(6):1055–73. [DOI] [PubMed] [Google Scholar]
- 15.Galvão DA Nosaka K Taaffe DR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–52. [DOI] [PubMed] [Google Scholar]
- 16.Galvão DA Spry N Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856–64. [DOI] [PubMed] [Google Scholar]
- 17.Taaffe DR Newton RU Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–9. [DOI] [PubMed] [Google Scholar]
- 18.Wall BA Galvão DA Fatehee N, et al. Exercise improves V˙O2max and body composition in androgen deprivation therapy-treated prostate cancer patients. Med Sci Sports Exerc. 2017;49(8):1503–10. [DOI] [PubMed] [Google Scholar]
- 19.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda). 2013;28(5):330–58. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen BK Steensberg A Fischer C, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24(2–3):113–9. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BK. The physiology of optimizing health with a focus on exercise as medicine. Annu Rev Physiol. 2019;81:607–27. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen BK, Akerström TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007;103(3):1093–8. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Casado A, Martin-Ruiz A, Perez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3(6):423–41. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol. 2019;10:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoi W Naito Y Takagi T, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62(6):882–9. [DOI] [PubMed] [Google Scholar]
- 26.Said N, Frierson HF, Jr, Chernauskas D, Conaway M, Motamed K, Theodorescu D. The role of SPARC in the TRAMP model of prostate carcinogenesis and progression. Oncogene. 2009;28(39):3487–98. [DOI] [PubMed] [Google Scholar]
- 27.Shin M Mizokami A Kim J, et al. Exogenous SPARC suppresses proliferation and migration of prostate cancer by interacting with integrin β1. Prostate. 2013;73(11):1159–70. [DOI] [PubMed] [Google Scholar]
- 28.Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301(3):E504–10. [DOI] [PubMed] [Google Scholar]
- 29.Hutt JA, DeWille JW. Oncostatin M induces growth arrest of mammary epithelium via a CCAAT/enhancer-binding protein delta-dependent pathway. Mol Cancer Ther. 2002;1(8):601–10. [PubMed] [Google Scholar]
- 30.Hu Y Sun H Owens RT, et al. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 2009;11(10):1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W Neill T Yang Y, et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther. 2015;22(3):247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen L Idorn M Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6–dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC Ramlogan-Steel CA Yu P, et al. Vaccination with tumor cells expressing IL-15 and IL-15Rα inhibits murine breast and prostate cancer. Gene Ther. 2014;21(4):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136(4):E197–202. [DOI] [PubMed] [Google Scholar]
- 35.Tekin S, Erden Y, Sandal S, Yilmaz B. Is Irisin an anticarcinogenic peptide? Med-Sci. 2015;4(2):2172–80. [Google Scholar]
- 36.Wilson RL, Newton RU, Taaffe DR, Hart NH, Lyons-Wall P, Galvão DA. Weight loss for obese prostate cancer patients on androgen deprivation therapy. Med Sci Sports Exerc. 2021;53(3):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63(4):742–5. [DOI] [PubMed] [Google Scholar]
- 38.Faris JE, Smith MR. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013;189(1 Suppl):S34–42; discussion S3–4. [DOI] [PubMed] [Google Scholar]
- 40.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galvao DA Nosaka K Taaffe DR, et al. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008;11(2):160–5. [DOI] [PubMed] [Google Scholar]
- 42.Campbell KL Winters-Stone KM Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes SC, Newton RU, Spence RR, Galvão DA. The Exercise and Sports Science Australia Position Statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–99. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JH McGovern J Minett GM, et al. Mobilizing serum factors and immune cells through exercise to counteract age-related changes in cancer risk. Exerc Immunol Rev. 2020;26:80–99. [PubMed] [Google Scholar]
- 45.Dethlefsen C Lillelund C Midtgaard J, et al. Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat. 2016;159(3):469–79. [DOI] [PubMed] [Google Scholar]
- 46.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56(3):201–6. [DOI] [PubMed] [Google Scholar]
- 47.Devin JL, Hill MM, Mourtzakis M, Quadrilatero J, Jenkins DG, Skinner TL. Acute high intensity interval exercise reduces colon cancer cell growth. J Physiol. 2019;597(8):2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


